Abstract
Allergic diseases, mainly mediated by T helper type 2 (Th2) immunity, have become a worldwide public health problem. Traditional Chinese medicine (TCM) has long been used in treating and preventing allergic symptoms. As the new target of anti-allergy TCM, basophils, after approximately 140 years since their discovery, are just now gaining respect as important contributors in the pathogenesis underlying allergic inflammation and disease. In addition to their role as effector cells, basophils can release early IL-4, migrate from circulatory system into draining lymph nodes, present antigen to naive CD4+T cells, and promote the differentiation of Th2 cells. Herein, we briefly summarized the recent research advances of the essential contributions of basophils in the initiation of Th2 immune responses.
Key words: basophils, IL-4, immune response, T helper type 2, traditional Chinese medicines
1. Introduction
In modern medicine, allergy is defined as a hypersensitivity response induced by an immune mechanism. Although there is no exact concept of allergy in traditional Chinese medicine (TCM), it has records about allergic diseases as early as in huang di nei jing (the Canon of Internal Medicine). According to TCM theory, allergic diseases caused by the invasion of the body by external pathogens, belongs to the category of exogenous pathogenic diseases. For example, wind or cold can cause allergic rhinitis, dryness can cause cough variant asthma, and wind chill and wind heat can cause hives (Wang, 2008). Allergic diseases caused by wind characterized by itches symptom are usually treated with TCM for preventing wind and relieving itching, as well as promoting lung and relieving symptoms. TCM of heat-clearing, damp-clearing and phlegm-reducing can remove the pathological products produced in the pathogenesis of allergic diseases. Furthermore, TCM of regulating qi and activating blood circulation, tonic and insect are also used in the treatment of allergic diseases (Shu & Zeng, 2013). More recently, it was found that TCM had potential in treating food allergy via inhibitory effect on basophils, which shed light on the exploration of molecular mechanisms of anti-allergy TCM and the explanation of TCM theories with modern medicine.
In recent years, the incidence of type I allergy has been rising rapidly in China (Tan, 2016; Zhi, 2016), and even reaches 30% in some other countries (Wills-Karp, Nathan, Page & Karp, 2010). As anti-allergy drugs, mast cell stabilizers and histamine receptor blockers are commonly used in clinic. They can relieve allergy symptoms by blocking the release of allergy mediators or preventing the interaction of histamine and its effector cells. However, these two strategies both act on the late phase when the allergic responses have already been triggered. Effective intervention on or before the initiation phase will possibly break the chain between the initiation phase and the late phase to greatly relieve type I allergy symptoms, which is mediated by T helper type 2 (Th2) cells via secretion of cytokines, differentiation of B cells to plasma cells, and production of IgE (Zhu & Paul, 2010). Therefore, if the initiation of Th2 immunity can be prevented or suppressed, the incidence of allergy will be reduced.
Derived from granulocyte-mononuclear progenitor cells in the bone marrow, basophils enter the peripheral blood after differentiation and maturation, which is obviously distinct from mast cells that only mature after migration into the peripheral tissue. It has been 140 years since the discovery of basophils in 1879 (Ehrlich, 1879). Basophils comprise less than 1% of all peripheral blood leukocytes and have long been regarded as mast cell-like cells lacking unique functions. Owing to the phenotypic similarity with mast cells and their low abundance in blood, basophils have been considered to play minor and redundant roles in the body's immune system for a long time. The typical example is American Journal of Immunobiology edited by Charles et al. (2008). Of the six kinds of myeloid cells displayed in the schematic diagram of “Medullary cells in innate and acquired immunity”, only basophils were labeled as “unknown function”. However, recent studies have shown that basophils are pathophysiologically non-redundant and indispensable. They not only contribute to the development of allergic responses (Tsujimura et al., 2008), but also play a crucial role in innate immunity and immune regulation (Wynn, 2009). Some researchers even used “Basophils are back!” as the title to evaluate their findings (Galli & Franco, 2008), and described it as “A neglected minority gains new respect” (Karasuyama, Mukai, Tsujimura & Obata, 2009). Recent researchers have highlighted the latest advances in understanding of the nonnegligible contributions of basophils in Th2 immunity. In the present review, we focus on the recent findings of the interaction between basophils and Th2 cells, and the mechanisms of basophils in initiating Th2 immunity. Based on these discussions, we review and propose the molecular mechanisms of anti-allergy TCM.
2. Basophils are a dominant source of early IL-4 required for Th2 differentiation
Th2 immunity, which develops in response to antigens such as allergens and parasites, is characterized by high levels of immunoglobulin E (IgE), differentiation of epithelial cells to mucous secreting cells and infiltration of Th2 cells, basophils and eosinophils. Early IL-4 plays a crucial role in initiating Th2 immunity, which prompts naive CD4+T lymphocytes (Th0) to differentiate into Th2 cells through T cell receptor (TCR) signaling pathway (Gros, Ben-Sasson, Seder, Finkelman & Paul, 1990; Swain, Weinberg, English & Huston, 1990; Yamane, Zhu & Paul, 2005). In the IL-4 knockout mice, their ability to resist parasitic infection was weakened significantly, but restored after exogenous injection of IL-4 (Li, Pan & Liu, 2015). To our knowledge, many types of cells can produce and secrete IL-4, including natural killer T cells, mast cells, eosinophils and basophils, etc. But which is the source cell of early IL-4 in the microenvironment of Th2 differentiation? Studies employing the mice transferred with IL-4 green fluorescent protein (GFP) reporter gene demonstrated that basophils were the main source cells under the worm infection after ruling out the possibility of other cells (Min et al., 2004). The similar conclusion was also obtained by Voehringer et al. who found that basophils-derived IL-4 played a key role in the murine models of Nippostrongylus brasiliensis infection and OVA-induced lung allergy (Voehringer, Reese, Huang, Shinkai & Locksley, 2006). Van Panhuys et al. assessed the IL-4 production from CD4+T cells and basophils using a murine IL-4-enhanced GFP reporter system. It was found that the source of IL-4 in the peripheral tissues was completely different in the primary and the subsequent secondary infection responses to N. brasiliensis. Basophils were the main cellular source of IL-4 during the primary infection, while Th cells were identified as the main source of IL-4 during the secondary infection. However, unlike Th0 cells, which have to differentiate into Th2 cells before they can secrete IL-4, basophils can secrete immediately a large amount of IL-4 after activation (Panhuys et al., 2011). Taken together, these studies indicate that basophils are the major producers of initial IL-4 in the differentiation of Th0 cells to Th2 cells (Miyake & Karasuyama, 2017).
3. Basophils can be recruited into lymph nodes by antigen to activate Th0 cells
The differentiation of Th0 cells mainly occurs in peripheral lymphoid tissues, while basophils exist in the circulatory system. Then how do basophils promote the development of Th0 cells differentiation into Th2 cells? It turned out that within 3 − 4 d after protein-antigen immunization or parasitic infection, basophils migrated into the immune-activated draining lymph nodes and were located next to the zone of Th0 cells (Gerner, Casey, Kastenmuller & Germai, 2017; Min et al., 2004; Sokol, Barton, Farr & Medzhitov, 2008). House dust mite antigen (Der p1), known as an activated protease, can directly activate basophils in the absence of specific IgE (sIgE) and its activation can be significantly inhibited by protease inhibitors (Phillips, Coward, Pritchard & Hewitt, 2003). Protease-activated receptors (PARs) can sense protease, so innate or adaptive immunity cells are activated (Shpacovitch, Feld, Hollenberg, Luger & Steinhoff, 2008), but published work failed to confirm the PARs expression on basophils (Falcone, Morroll & Gibbs, 2005). Instead, papain was found to directly activate Th0 cells through protease-activated receptor 2 (PAR2) on the Th0 cell membrane and promote the release of chemokines CCL17 and CCL22. Basophils, which are chemotactic, are accordingly expressed CCR4 on the cell membrane. Then, basophils were recruited into lymph nodes to initiate the Th0 cell differentiation to Th2 cell (Liang et al., 2012). CCL7, released by dendritic cells (DCs) that are recruited into lymph nodes due to reactive oxygen species (ROS) signal, can also promote the chemotaxis of basophils into the lymph nodes (Tang et al., 2010). In addition, IL-3 and IL-3Rβ were also involved in this process (Kim et al., 2010).
4. Roles of basophils in differentiation of Th2 immunity
Besides the innate immune responses, the adaptive immune responses also play a key role in eliminating pathogens or foreign substances. They complement each other in contributing to the immune defense of the body (Iwasaki & Medzhitov, 2010). Basophils play an important role in linking innate immunity and adaptive immunity. Especially when regulating Th2 differentiation as the immune regulatory cells, they play diverse roles depending on the type of antigen.
4.1. Basophils function as antigen-presenting cells (APCs) to produce early IL-4 in response to haptens or peptide antigens
Before discovering that basophils can independently serve as APCs in Th2 differentiation, it is generally believed that it is mature DCs who respond to antigen, and promote the Th0 cell proliferation and differentiation to Th2 cell (Lambrecht, 2005). Until 2009, three consecutive papers published in the same issue of Nature Immunology (from three independent laboratories) simultaneously reported that basophils were able to migrate into draining lymph nodes and take up or process antigens as APCs in response to papain antigen and helminth infection (Perrigoue et al., 2009; Sokol et al., 2009; Yoshimoto et al., 2009). In addition, basophils could express MHC-II proteins and costimulatory molecules such as CD40, CD80 and CD86, meanwhile could secrete the cytokines IL-4 and thymic stromal lymphopoietin (TSLP) necessary for Th2 cell differentiation. Under certain conditions, basophils alone could promote Th0 cell differentiation to Th2 cell without DCs. One year later, the conclusion that basophils alone function as APCs was questioned since the bone marrow-derived basophils (BM-Bas) used in several of the above experiments actually contained FcɛRI-expressing inflammatory DCs (Hammad et al., 2010). However, using CD11c-deleted BM-Bas to exclude the confounding inflammatory DCs, Otsuka et al. (2013) found that although basophils were inefficient in taking up particulate antigens, they did express MHC class II, costimulatory molecules and IL-4 upon exposure to haptens or peptide antigens, thus functioning as both APCs and IL-4 producing cells (Otsuka & Kabashima, 2015).
4.2. Basophils prompt Th2 differentiation together with DCs in response to protein antigens
When DCs work as APCs, basophils only serve as IL-4 provider. Researchers found that the pattern that basophils alone serve as APCs to initiate Th2 immunity could not explain some phenomena in the study. For example, Th2 immunity in response to N. brasiliensis infection depended on dermal CD301b+ DCs, while depletion of CD301b+ DCs prior to infection significantly reduced the number of IL-4-producing CD4+ T cells (the Th2 cells) (Gao et al., 2013; Kumamoto et al., 2013). Further studies suggested that CD301b+ DCs could also express programmed death ligand 2 (PDL2) and interferon regulatory factor 4 (IRF4), the latter was exactly one of the cytokines necessary for Th2 differentiation in vivo (Kumamoto et al., 2013; Na, Cho & Chung, 2016). Consistent with this finding, dermal PDL2-expressing DCs does express MHC-II proteins necessary for antigen-presenting (Gao et al., 2013). However, CD301b+ DCs themselves could not induce Th2 immunity whatever in vitro (Kumamoto et al., 2013) or in vivo (Gao et al., 2013). The research results of Otsuka et al. (2013) reasonably explained this phenomenon. Basophils could not directly take up or process protein antigens, but they could assist DCs to induce Th2 immunity by producing IL-4 necessary for Th2 differentiation (Otsuka & Kabashima, 2015).
Basophils can also function as APCs to produce IL-4 via cell contact-dependent trogocytosis. Miyake et al. (2017) provided a new compelling evidence of basophils serving as APCs in Th2 differentiation. They found that under certain experimental conditions, the expression of MHC-II related to Th2 differentiation was clearly detected on the basophils. Surprisingly, basophils indeed express MHC-II on the cell surface, but with little transcription of corresponding genes, which implied that the expression was detected only at protein but not at transcription level. Hence, it is highly suggested that MHC-II detected previously on the basophils was likely to be obtained from other cells (such as DCs). Subsequently, the coculture of basophils and DCs was examined and analyzed with confocal microscope and flow cytometry. After 15 min, fragments of DC plasma membrane together with MHC-II proteins were found to transfer to the basophil membrane, while the transfer of MHC-II from DCs to the basophil membrane was significantly suppressed by cellular (or intercellular) adhesion molecule (such as ICAM-1 or CD11a), which suggested that the effect depends on the direct contact between cells. These results indicated that the interaction between DCs and basophils completely conforms to the characteristics of trogocytosis (Campana, De, Carrega, Ferlazzo & Bonaccorsi, 2015; Joly & Hudrisier, 2003). Consistent with these studies in vitro, this conclusion was also confirmed by experiments in vivo. The acquired peptide-MHC-II complexes enabled basophils to induce antigen-specific T cells producing IL-4 in the model of atopic dermatitis-like inflammation. While in mice model, if DCs-derived MHC-II was specifically deficient, basophils in draining lymph nodes would no longer be able to express MHC-II. Thus, trogocytosis, through which basophils acquired MHC-II complexes from DCs, can also be identified in vivo (Miyake et al., 2017). This important finding objectively mediates the dispute of whether DCs or basophils are Th2-inducing APCs (Yamanishi, Miyake, Iki, Tsutsui & Karasuyama, 2017). Nevertheless, the distinct non-redundant function of basophils in initiating Th2 immunity is no longer in doubt.
5. Functions of differentiated Th2 cells in lymph nodes
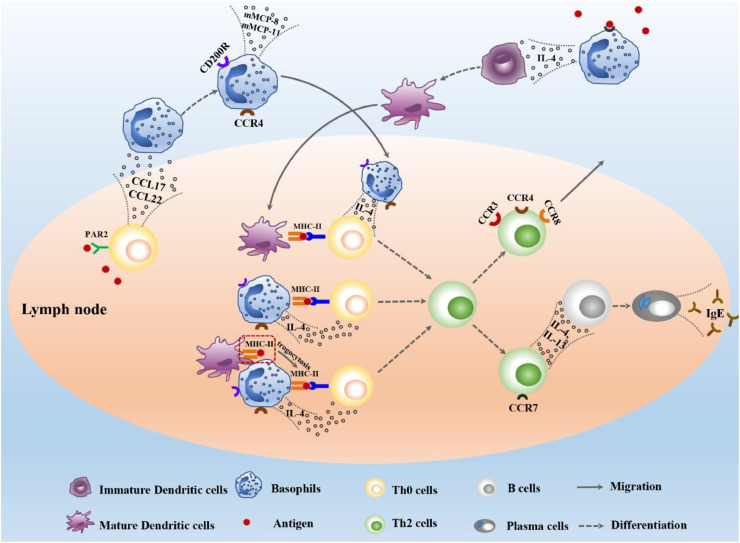
C—C chemokine receptor type 7 (CCR7) can be expressed on the membrane of differentiated Th2 cells, which promotes the migration of Th2 from paracortex zone of lymph nodes to primary lymphoid follicles where initial B cells locate. Then basophils exert their effect on B cells through the production of IL-4 and IL-13, which drive B cells to differentiate into plasma cells and to produce various antibody isotypes including IgE. Meanwhile, CCR3, CCR4 and CCR8 are expressed by Th2 cells, which led Th2 cells out of lymph nodes and into the corresponding lesion location (Take, Mali & Wu, 2012). In short, basophil-mediated Th2 immunity starts with the recruitment of basophils into lymph nodes, and end with the flowing of differentiated Th2 cells and IgE out of lymph nodes. Various cells and cytokines are involved in this process (Fig. 1).
Fig. 1.
Mechanism of basophils in initiating Th2 immune response.
6. Discussion
Several types of cells other than basophils are known to contribute to the regulation of Th2 immune responses, including dendritic cells, epithelial cells and mast cells, et al. Previously, DCs were known to be dominant APCs for Th2 differentiation. However, studies over the last decade have suggested the essential function of basophils in primary Th2 cell responses. The outcomes of such further studies will not only broaden our understanding of initiation of Th2 cell responses, but may also facilitate the development of new therapeutic approaches for human allergic diseases and the modernization of anti-allergy TCM.
6.1. A novel crucial target of Th2 related allergic diseases
For the patients allergic to only one or a few allergens, the preventive treatment is often used clinically, including avoiding exposure to specific antigens or using the desensitization therapy. However, these methods are not suitable for those who are susceptible to multifarious environmental antigens. The reason may be that their initiation threshold of Th2 immunity is relatively lower than that of normal people. Thus, the most effective strategy may be the up-regulation of their Th2 differentiation threshold and inactivating their sensitivity to allergens. Based on recent discoveries that uncovered previously unrecognized functions of basophils in the initiation of Th2 immunity, it is likely that directly targeting basophils may be beneficial in the treatment of allergic disease states.
6.2. Exploration of molecular mechanism of anti-allergy TCM
In fact, several researchers have suggested the utility of Chinese herbal medicine acting on basophils for the treatment of a variety of allergic diseases, such as allergic rhinitis and asthma, etc. Derived from Wumei Pills, Food Allergy Herbal Formula-2 (FAHF-2) is an extract of nine herbs, which is commonly used to treat parasite infection and food allergy-like symptoms. FAHF-2 protected against peanut-induced anaphylactic symptoms in the peanut allergic murine model via the reduction of allergen stimulated basophil activation, hyper-releasability and percentage of circulating basophils (Patil et al., 2011). Another example is Shuanghuanglian Oral Liquid, a three-herb antimicrobial formula containing Lonicerae Japonicae Flos, Scutellariae Radix and Forsythiae Fructus; it is commonly used for relieving type I hypersensitivity via its suppressive effect on Th2 immunity. It attenuated shrimp tropomyosin induced Th2 cytokine release (e.g., IL-4, IL-5, IL-10 and IL-13) and decreased serum IgE production due to the inhibition of basophil activation, including decreasing IL-4 release in basophil-rich splenocytes and suppressing CD200R surface expression in peripheric basophils (Fei et al., 2018). These researches undoubtedly help to explain the mechanism of anti-allergic TCM, a safe, well-tolerated and effective immunotherapeutic option for allergic patients, and are beneficial for the modernization and globalization of TCM.
Taken together, despite being a minor population of peripheral blood leukocytes, basophils are a key player in the initiation of Th2 immunity. It is now appreciated that activated basophils are capable of providing initial IL-4 that promotes the differentiation of Th0 cells to Th2 cells and their migration to the lymph nodes where they function as APCs independently or prompt Th2 differentiation along with DCs. Collectively, basophils contribute to multiple components of the innate and adaptive immune responses. Further studies of basophil phenotype, activation, and function may yield significant insights into the new therapeutic intervention strategies for allergic diseases.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
This paper was supported by the National Natural Science Foundation of China (NSFC) (No.81873066).
References
- Campana S., De P.C., Carrega P., Ferlazzo G., Bonaccorsi I. Cross-dressing: An alternative mechanism for antigen presentation. Immunology Letters. 2015;168(2):349–354. doi: 10.1016/j.imlet.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. Beiträge zur Kenntniss der granulirten Bindegewebszellen und der eosinophilen Leukocythen. Archives Anatomie Physiology (Leipzig) 1879;3:166. [Google Scholar]
- Falcone F.H., Morroll S., Gibbs B.F. Lack of protease activated receptor (PAR) expression in purified human basophils. Inflammation Research. 2005;54(Suppl. 1):S13–S14. doi: 10.1007/s00011-004-0405-y. [DOI] [PubMed] [Google Scholar]
- Fei Q.L., Han Y.X., Qi R.J., Gao Y., Fang L., Hou R., et al. Shuang-Huang-Lian prevents basophilic granulocyte activation to suppress Th2 immunity. BMC Complementary and Alternative Medicine. 2018;18(1):2. doi: 10.1186/s12906-017-2071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S.J., Franco C.B. Basophils are back! Immunity. 2008;28(4):495–497. doi: 10.1016/j.immuni.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Gao Y., Nish S.A., Jiang R., Hou L., Licona-Limón P., Weinstein J.S. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39(4):722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner M.Y., Casey K.A., Kastenmuller W., Germai R.N. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. Journal of Experimental Medicine. 2017;214(10):3105–3122. doi: 10.1084/jem.20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros G.L., Ben-Sasson S.Z., Seder R., Finkelman F.D., Paul W.E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. Journal of Experimental Medicine. 1990;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Plantinga M., Deswarte K., Pouliot P., Willart M.A., Kool M., et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. Journal of Experimental Medicine. 2010;207(10):2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., et al. Science Press; Beijing: 2008. Immunobiology (Fifth edition) p7. [Google Scholar]
- Joly E., Hudrisier D. What is trogocytosis and what is its purpose? Nature Immunology. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Mukai K., Tsujimura Y., Obata K. Newly discovered roles for basophils: A neglected minority gains new respect. Nature reviews. Immunology. 2009;9(1):9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- Kim S., Prout M., Ramshaw H., Lopez A.F., LeGros G., Min B. Cutting edge: Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. Journal of Immunology. 2010;184(3):1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto Y., Linehan M., Weinstein J.S., Laidlaw B.J., Craft J.E., Iwasaki A. CD301b⁺ dermal dendritic cells drive t helper 2 cell-mediated immunity. Immunity. 2013;39(4):733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B.N. Dendritic cells and the regulation of the allergic immune response. Allergy. 2005;60(3):271–282. doi: 10.1111/j.1398-9995.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- Li L., Pan Q.J., Liu H.F. Functions of basophils cells in Th2 immune response. Journal of Immunology. 2015;31(2):176–180. [Google Scholar]
- Liang G., Barker T., Xie Z., Charles N., Rivera J., Druey K.M. Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. Journal of Allergy and Clinical Immunology. 2012;129(5):1377–1386. doi: 10.1016/j.jaci.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., Prout M., Hu-Li J., Zhu J.F., Jankovic D., Morgan E.S. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. Journal of Experimental Medicine. 2004;200(4):507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Karasuyama H. Emerging roles of basophils in allergic inflammation. Allergology International. 2017;66(3):382–391. doi: 10.1016/j.alit.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Miyake K., Shiozawa N., Nagao T., Yoshikawa S., Yamanishi Y., Karasuyama H. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(5):1111–1116. doi: 10.1073/pnas.1615973114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H., Cho M., Chung Y. Regulation of Th2 cell immunity by dendritic cells. Immune Network. 2016;16(1):1–12. doi: 10.4110/in.2016.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A., Kabashima K. Contribution of basophils to cutaneous immune reactions and Th2-mediated allergic responses. Frontiers in Immunology. 2015;6:393. doi: 10.3389/fimmu.2015.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A., Nakajima S., Kubo M., Egawa G., Honda T., Kitoh A., et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nature Communications. 2013;4:1739. doi: 10.1038/ncomms2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhuys N., Prout M., Forbes E., Min B., Paul W.E., Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. Journal of Immunology. 2011;186(5):2719–2728. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S.P., Wang J., Song Y., Noone S., Yang N., Wallenstein S., et al. Clinical safety of food allergy herbal formula-2 (FAHF-2) and inhibitory effect on basophils from patients with food allergy: Extended phase I study. Journal of Allergy and Clinical Immunology. 2011;128(6):1259–1265. doi: 10.1016/j.jaci.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nature Immunology. 2009;10(7):697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C., Coward W.R., Pritchard D., Hewitt C.R. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. Journal of Leukocyte Biology. 2003;73(1):165–171. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- Shpacovitch V., Feld M., Hollenberg M.D., Luger T.A., Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. Journal of Leukocyte Biology. 2008;83(6):1309–1322. doi: 10.1189/jlb.0108001. [DOI] [PubMed] [Google Scholar]
- Shu X.M., Zeng Q.X. Discussing the etiology and pathogenesis and treatment based on syndrome differentiation of diseases related with allergic constitution. Journal of Sichuang of Traditional Chinese Medicine. 2013;31(8):17–19. [Google Scholar]
- Sokol C.L., Barton G.M., Farr A.G., Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunology. 2008;9(3):310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol C.L., Chu N., Yu S., Nish Si.A., Laufer T.M., Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nature Immunology. 2009;10(7):713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.L., Weinberg A.D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. Journal of Immunology. 1990;145(11):3796–3806. [PubMed] [Google Scholar]
- Take, M., Mali, S., & Wu, Y.Z. (2012). An introduction to the immune response. (pp. 163).
- Tan J. China have hundreds of millions of patients with allergic diseases. Health News. 2016 [Google Scholar]
- Tang H., Cao W., Kasturi S.P., Ravindran R., Nakaya H.I., Kundu K., et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nature Immunology. 2010;11(7):608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura Y., Obata K., Mukai K., Shindou H., Yoshida M., Nishikado H., et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28(4):581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Voehringer D., Reese T.A., Huang X., Shinkai K., Locksley R.M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. Journal of Experimental Medicine. 2006;203(6):1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.K. Preliminary understanding and clinical experience of allergic diseases. Journal of Shanxi Traditional Chinese Medicine. 2008;24(4):5–7. [Google Scholar]
- Wills-Karp M., Nathan A., Page K., Karp C.L. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunology. 2010;3(2):104–110. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. Basophils trump dendritic cells as APCs for T(H)2 responses. Nature Immunology. 2009;10(7):679–681. doi: 10.1038/ni0709-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H., Zhu J., Paul W.E. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. Journal of Experimental Medicine. 2005;202(6):793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi Y., Miyake K., Iki M., Tsutsui H., Karasuyama H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunological Reviews. 2017;278(1):237–245. doi: 10.1111/imr.12548. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Yasuda K., Tanaka H., Nakahira M., Imai Y., Fujimori Y., et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nature Immunology. 2009;10(7):706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- Zhi Y.X. Minutes of Chinese college of allergy and asthma & the 10th international summit on allergic disease meeting. Chinese Journal of Allergy & Clinical Immunology. 2016;10(3):303. [Google Scholar]
- Zhu J., Paul W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunological Reviews. 2010;238(1):247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]