Abstract
Objective
The study was conducted to develop and validate a high performance thin-layer chromatography (HPTLC)-densitometric method for the quantitative analysis of morin in Maclura cochinchinensis heartwood collected from different locations in Thailand.
Methods
HPTLC analysis was performed on an aluminium sheet of silica gel 60 F254 using toluene: ethyl acetate: formic acid (36:12:7, volume percent) as a mobile phase. The densitometric scanning was performed at the wavelength 410 nm. HPTLC method was validated according to ICH guideline.
Results
The proposed HPTLC method showed acceptable validation parameters. The content of morin in M. cochinchinensis heartwood collected from eight different provinces in Thailand were in the ranges of 1.53%−2.73%.
Conclusion
The simple and sensitive HPTLC method was successfully developed and validated for determination of morin in M. cochinchinensis heartwood. The proposed HPTLC method was found to be simple, fast and inexpensive, and can be used for the routine quality control of raw materials.
Keywords: HPTLC, Maclura cochinchinensis (Lour.) Corner, morin
1. Introduction
Maclura cochinchinensis (Lour.) Corner, a spiny scandent shrub or woody climber, belongs to a family Moraceae (Chotchoungchatchai, Saralamp, Jenjittikul, Pornsiripongse, & Prathanturarug, 2012). It is widely distributed in many countries in Asia such as China, Japan, Malaysia, Myanmar, India, Sri Lanka, Vietnam, and Thailand (eFloras, 2008; Hien, Hughes, & Cherry, 1997). In Thai traditional medicine, the M. cochinchinensis heartwood has been used for fever, tonic effect, diarrhea, fainting, abnormality of lymph node, skin infection and diabetes (Mokkhasmit, Ngarmwathana, Sawasdimongkol, & Permphiphat, 1971; Bunyapraphatsara, Dechsree, Yoosook, Herunsalee, & Panpisutchai, 2000). According to the National Lists of Essential Medicine of Thailand, M. cochinchinensis heartwood was a component of Thai herbal preparation as a blood tonic in postnatal period (National Drug Committee, 2006). There are various polyherbal formulations containing M. cochinchinensis heartwood available in the market for blood tonic, accelerating involution of uterus and lochia discharge in postnatal period. M. cochinchinensis heartwood promotes several biological activities including antioxidant, antiviral, antibacterial, anti-inflammatory, and hepatoprotective effect (Bunyapraphatsara et al., 2000; Swargiary and Ronghang, 2013; Yoosook, Bunyapraphatsara, Boonyakiat, & Kantasuk, 2000; Kummee and Intaraksa, 2008; Lin, Lee, Chang, & Yang, 1999). Chemical bioactive constituents in M. cochinchinensis heartwood including morin, β-sitosterol, resveratrol, oxyresveratrol have been reported. Among these compounds, a flavonol morin was determined as a major component (Kongkiatpaiboon et al., 2017).
The variation of chemical constituents in herbal extract poses a great challenge to control the quality of herbal products. The qualitative and quantitative analyses are useful techniques to assess the safety and efficacy of plant material which mostly related to the content of marker compounds present in the plant. Morin, the major compound, is regarded as a marker compound to assess the quality of M. cochinchinensis heartwood extract. Several analytical methods were previously reported to isolate and determine morin such as thin layer chromatography (National Drug Committee, 2006; Medic-Saric, Jasprica, Smolcic-Bubalo, & Mornar, 2004) and high performance liquid chromatography (HPLC) (Kongkiatpaiboon et al., 2017; Hsiu, Tsao, Tsai, Ho, & Chao, 2001). HPLC is the most widely used method to identify and quantify the compounds from plant extracts and biological samples. Although HPLC provides rapid and reliable result with high sensitivity and high reproducibility, but it seems to have some disadvantages over other analytical methods such as complexity, large quantities of solvents, and high operation cost. An alternative method is required to facilitate and reduce time of analysis with relatively few costs for routine analysis. Accordingly, this study was aimed to develop and validate a simple, rapid and sensitive HPTLC method for determination of morin content in M. cochinchinensis heartwood extract.
2. Materials and methods
2.1. Chemicals and reagents
Morin hydrate was purchased from Sigma, USA. All reagents used were of analytical grade.
2.2. Plant materials
The heartwood of M. cochinchinensis was collected from eight different provinces in Thailand as follows: (1) Bangkok; (2) Nakhon Ratchasima; (3) Surin; (4) Kanchanaburi; (5) Suphan Buri; (6) Chanthaburi; (7) Sa Kaeo; (8) Chiang Mai in August, 2016. The samples were identified by comparing with an authentic sample. The voucher specimens (MC001–MC008) have been deposited at Department of Food Chemistry, Faculty of Pharmacy, Mahidol University. M. cochinchinensis heartwoods were grounded to coarse powder, sieved through a 18# mesh and kept at −20 °C for further studies.
2.3. Preparation of standard solution
Morin hydrate 5.27 mg (equal to morin 5.00 mg) was accurately weighed and dissolved in 5 mL of methanol for preparing stock solution (1 mg/mL). Standard working solution of morin was prepared by diluting the stock solution with methanol to obtain the concentration of 200 μg/mL.
2.4. Preparation of sample solution
Each powdered sample of M. cochinchinensis heartwood was accurately weighed (0.1 g), dissolved in methanol and adjusted to 10 mL in a volumetric flask. Each sample was sonicated in an ultrasonic bath for 30 min to enable a complete dissolution. Prior to analysis, each solution was filtered through a 0.45 μm nylon membrane filter. The sample solution of 2 µL/band was applied (n = 3).
2.5. Instrument and chromatographic condition
HPTLC separation was performed on silica gel HPTLC plates F254 (20 cm × 10 cm with 0.2 mm thickness; Merck, Darmstadt, Germany). Samples and morin standard solution were applied as band width 7 mm, distance from lower edge 10 mm by Linomat 5 automatic sample spotter (CAMAG, Switzerland). A constant application rate of 150 nL/s was used. The plate was developed in CAMAG twin trough glass chamber, presaturated 30 min with developing solvent consisting of toluene: ethyl acetate: formic acid (36:12:7, volume percent). The plate was developed to a distance of 80 mm. Densitometric scanning was performed at 410 nm using TLC scanner 3 (CAMAG, Switzerland). The slit dimension was 6.00 mm × 0.45 mm, with a scanning speed of 20 mm/s. Densitograms were analyzed by WinCATs software.
2.6. Method validation
The proposed method was validated in terms of linearity, precision, accuracy, robustness, limit of detection (LOD), and limit of quantitation (LOQ) according to the International Conference on Harmonization guideline (ICH 1996/2005).
2.6.1. Linearity
From a standard working solution of morin (200 μg/mL), 1.5−3.5 μL, corresponding to the concentration of morin 300−700 ng/band, were separately applied on the HPTLC plate. The calibration curves were obtained by plotting between the peak areas versus the concentration of standard morin.
2.6.2. Precision
For the study of repeatability and intermediate precision, three concentrations of standard solution including 300, 400, and 500 ng/band were applied on the HPTLC plate (n = 3). Repeatability was determined by analysis of morin at three different time intervals within one day, while the intermediate precision was determined on three consecutive days using the proposed method. The precision was expressed as percentage of relative standard deviation (% RSD)
2.6.3. Accuracy
Accuracy of the method was confirmed by measurement of recovery. The heartwood of M. cochinchinensis from Supanburi Province was used for recovery test. Three different concentrations of morin (approximately 50%, 100%, and 150% of the pre-quantified extract) were added to the M. cochinchinensis heartwood extract. Spiked samples were prepared in triplicate. Data were calculated and expressed as percentage of recovery as follows:
Recovery (%) = 100 × (detected amount−original amount)/spike amount.
2.6.4. Limit of detection and limit of quantitation
LOD and LOQ were determined based on the standard deviation of y-intercepts of regression lines (SD) and the slope of the calibration curve (S) of the sample in the range of LOD, LOQ using the following formula: LOD = 3.3(SD/S) and LOQ = 10(SD/S).
2.6.5. Robustness
The chromatographic conditions were slightly modified for the robustness test of morin (300 ng/band). The time from spotting of morin on the HPTLC plate to development and the time from development to densitometric scanning were varied to 5, 15, and 30 min. Mobile phase composition was slightly changed to 36:12:7, 38.5:12:7, 33:12:7, volume percent, of toluene: ethyl acetate: formic acid, respectively. The % RSD of the peak areas of morin reference standard was calculated for robustness variations.
3. Results and discussion
3.1. Method development
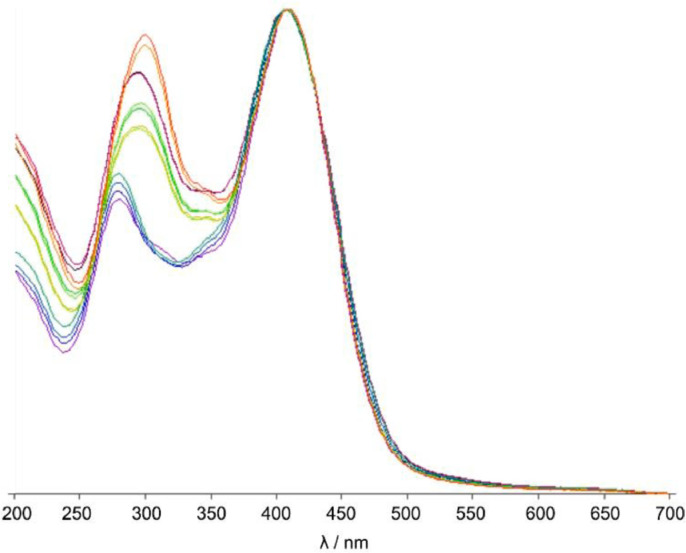
HPTLC-densitometric method was developed to analyze morin content in M. cochinchinensis heartwood samples. Optimization of the mobile phase was done using various solvents. The mobile phase consisting of toluene: ethyl acetate: formic acid (36:12:7, volume percent) showed acceptable resolution and separation of the component of the sample. The specificity of morin in M. cochinchinensis heartwood extract was confirmed by overlay UV spectra between morin reference standard and sample (Fig. 1). Purity is calculated by measuring three spectra within one peak (one spectrum at the rising flank, one at the peak max and one at the falling flank) and comparing these statistically. Peak purity test showed high degree of correlation between spectra scanned at peak start, peak apex, and peak end positions [r(S, M) > 0.999 and r(M, E) > 0.999] of morin peak. From the UV spectra overlay, the wavelength higher than 400 nm indicated no interference of other compounds. Therefore, the maximum absorption of morin at 410 nm was chosen for the analysis.
Fig. 1.
Overlay UV spectra of morin reference standard and M. cochinchinensis heartwood extract scanning from 200 to 700 nm.
3.2. Method validation
The developed method was validated for the analysis of morin content in M. chochinchinensis heartwood extracts. Linearity, precision, accuracy, LOD and LOQ were parameters that were examined (ICH 1996/2005). The proposed HPTLC method showed acceptable validation parameters (Table 1). The calibration curves of morin were found to be linear across the range of 300−700 ng/band. The correlation coefficient (r2) was greater than 0.995, indicating good linearity of the method. The percentage of relative standard deviation (% RSD) for both repeatability and intermediate precision were less than 2%. The LOD and LOQ were found to be 50.33 and 152.50 ng/band, respectively. The LOD and LOQ were the lowest concentration of morin in sample which can be detected and quantified under the experimental conditions, respectively. The recovery of morin in M. cochinchinensis heartwood extract was in the range of (98.39 ± 3.14)%−(100.43 ± 3.49) %, indicating the high accuracy of the method. The results of robustness study were shown in Table 2. The slightly change of chromatographic conditions did not affect detection of morin content. The % RSD values were less than 5%. Therefore, the developed method was considered to be robust.
Table 1.
Validation parameters by proposed HPTLC method
| Parameters | Morin |
|---|---|
| Range of linearity | 300−700 ng/band |
| Regression equation, (n = 6) | Y = 14.9677X + 377.4341 |
| Correlation coefficient (r2) | 0.9964 ± 0.0016 |
| Repeatability (% RSD) | 0.49%−1.46% |
| Intermediate precision (% RSD) Recovery (%), (n = 3) | 0.36%−1.90% (98.39 ± 3.14)%−(100.43 ± 3.49)% |
| Limit of detection (LOD) | 50.33 ng/band |
| Limit of quantitation (LOQ) | 152.50 ng/band |
X = concentration of morin in ng/mL, Y = peak area
Table 2.
Results of robustness study.
| Chromatographic conditions | RSD/% |
|---|---|
| Mobile phase composition ratio | |
| (toluene: ethyl acetate: formic acid, 36:12:7, 38.5:12:7, 33:12:7, volume percent) | 3.74 |
| Time from spotting on the HPTLC plate to development (5, 15, and 30 min) | 1.18 |
| Time from development to densitometric scanning (5, 15, and 30 min) | 1.55 |
3.3. Method application
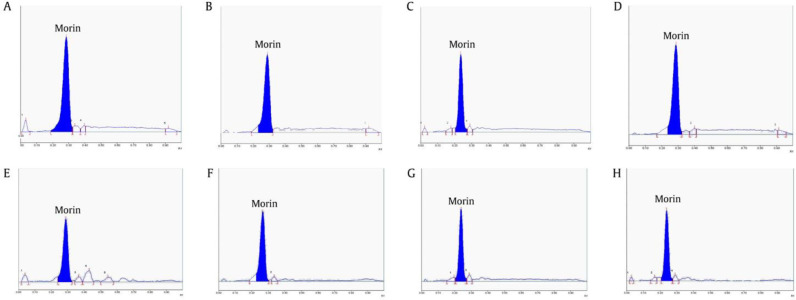
The developed method was applied for the analysis of morin content in M. chochinchinensis heartwood extracts. According to the HPTLC analysis, morin contents in M. cochinchinensis heartwoods collected from eight provinces in Thailand varied from (1.53 ± 0.01)% to (2.73 ± 0.07)% (Table 3). HPTLC densitogram of M. cochinchinensis heartwood extract revealed a peak at Rf of (0.29 ± 0.01) as shown in Fig. 2. The sample from Surin Province contained the highest morin content (2.73 ± 0.07)%, while sample from Bangkok contained the lowest morin content (1.53 ± 0.01)%. The variation of morin contents could be due to various factors such as area of plantation, environment, humidity, and age of the plant. Previous stability study of morin hydrate revealed that pH and light are the major factors affecting stability of morin hydrate rather than temperature. The solutions and formulations of morin hydrate could be highly stable at room temperature and dark conditions (Jangid, Pooja, & Kulhari, 2018). From our previous HPLC methods for the quantification of morin in M. cochinchinensis heartwood (Kongkiatpaiboon et al., 2017), a reversed-phase HPLC was performed on isocratic solvent system composed of 0.5% acetic acid in water: acetonitrile (80:20). The amounts of morin in M. cochinchinensis heartwood determined by the HPLC method were in the range of 0.74%−1.57% which in accordance with our study.
Table 3.
Morin content in M. cochinchinensis heartwoods and polyherbal formulations (mean ± SD, n = 3).
| Sample location regions | Morin content/% |
|---|---|
| Bangkok (Central) | 2.53 ± 0.04 |
| Nakhon Ratchasima (Northeastern) | 2.24 ± 0.04 |
| Surin (Northeastern) | 2.73 ± 0.07 |
| Kanchanaburi (Western) | 2.63 ± 0.02 |
| Suphan Buri (Western) | 1.53 ± 0.01 |
| Chanthaburi (Eastern) | 2.22 ± 0.02 |
| Sa Kaeo (Eastern) | 2.13 ± 0.01 |
| Chiang Mai (Northern) | 2.63 ± 0.04 |
Fig. 2.
HPTLC densitograms of M. cochinchinensis heartwood from eight different provinces: (A) Bangkok; (B) Nakhon Ratchasima; (C) Surin; (D) Kanchanaburi; (E) Suphan Buri; (F) Chanthaburi; (G) Sa Kaeo; (H) Chiang Mai; mobile phase: toluene: ethyl acetate: formic acid (36:12:7, volume percent).
In comparison to the HPLC method, although HPTLC provided lower resolution than HPLC technique. TLC is widely used in the analysis of pharmaceuticals, botanicals, food-stuffs, and environmental and clinical samples (Ranger, Vegh, & Ferenczi-Fodor, 2011). It is a preferred analytical tool for fingerprint analysis and quantification of marker compounds in herbal drugs because of its simplicity, accuracy and suitability for high-throughput screening. Therefore, HPTLC is suitable as an alternative method for the routine analysis and quality control of M. cochinchinensis heartwood raw material and polyherbal formulation containing M. cochinchinensis heartwood product.
4. Conclusions
The simple and sensitive HPTLC method was successfully developed and validated for determination of morin in M. cochinchinensis heartwood. The proposed HPTLC method showed acceptable validation parameters. This method offered several advantages including simplicity, rapid, multiple sample handling, less solvent used, less time of analysis, and less cost per analysis when compared to HPLC method. Consequently, this validated HPTLC method could be used as an alternative method for quantitative analysis of morin content in heartwood extracts and herbal preparation of M. cochinchinensis.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgments
The authors thank Faculty of Pharmacy, Mahidol University, Thailand for the laboratory facilities. Partial of this work was financial supported by Thammasat University Research Fund under the TU Research Scholar, Contract No. 2/29/2561.
References
- Bunyapraphatsara N., Dechsree S., Yoosook C., Herunsalee A., Panpisutchai Y. Anti-herpes simplex virus component isolated from Maclura cochinchinensis. Phytomedicine. 2000;6(6):421–424. doi: 10.1016/S0944-7113(00)80069-0. [DOI] [PubMed] [Google Scholar]
- Chotchoungchatchai S., Saralamp P., Jenjittikul T., Pornsiripongse S., Prathanturarug S. Medicinal plants used with Thai traditional medicine in modern healthcare services: A case study in Kabchoeng hospital, Surin province, Thailand. Journal of Ethnopharmacology. 2012;141:193–205. doi: 10.1016/j.jep.2012.02.019. [DOI] [PubMed] [Google Scholar]
- eFloras. (2008). Flora of China, http://www.efloras.org(accessed March 2019).
- Hien T.V., Hughes M.A., Cherry G.W. In vitro studies on the antioxidant and growth stimulatory activities of a polyphenolic extract from Cudrania cochinchinensis used in the treatment of wounds in Vietnam. Wound Repair and Regeneration. 1997;5(2):159–167. doi: 10.1046/j.1524-475X.1997.50208.x. [DOI] [PubMed] [Google Scholar]
- Hsiu S.L., Tsao C.W., Tsai Y.C., Ho H.J., Chao P.D. Determinations of morin, quercetin, and their conjugate metabolites in serum. Biological and Pharmaceutical Bulletin. 2001;24(8):967–969. doi: 10.1248/bpb.24.967. [DOI] [PubMed] [Google Scholar]
- ICH Q2(R1) Validation of analytical procedures: Text and methodology. International Conference on Harmonization; Geneva; 1996/2005. [Google Scholar]
- Jangid A.K., Pooja D., Kulhari H. Determination of solubility, stability, and degradation kinetics of morin hydrate in physiological solutions. RSC Advances. 2018;8:28836–28842. doi: 10.1039/c8ra04139c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongkiatpaiboon S., Tungsukruthai P., Sriyakool K., Pansuksan K., Tunsirikongkon A., Pandith H. Determination of morin in Maclura cochinchinensis heartwood by HPLC. Journal of Chromatographic Science. 2017;55(3):346–350. doi: 10.1093/chromsci/bmw191. [DOI] [PubMed] [Google Scholar]
- Kummee S., Intaraksa N. Antimicrobial activity of Desmos chinensis leaf and Maclura cochinchinensis wood extracts. Songklanakarin Journal of Science and Technology. 2008;30(5):635–639. [Google Scholar]
- Lin C.C., Lee H.Y., Chang C.H., Yang J.J. The anti-inflammatory and hepatoprotective effects of fractions from Cudrania cochinchinensis var. gerontogea. The American Journal Chinese Medicine. 1999;27(2):227–239. doi: 10.1142/S0192415X99000264. [DOI] [PubMed] [Google Scholar]
- Medic-Saric M., Jasprica I., Smolcic-Bubalo A., Mornar A. Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croatica Chemica Acta. 2004;77(1-2):361–366. [Google Scholar]
- Mokkhasmit M., Ngarmwathana W., Sawasdimongkol K., Permphiphat U. Pharmacological evaluation of Thai medicinal plants. Journal of the Medical Association of Thailand. 1971;54(7):490–503. [PubMed] [Google Scholar]
- National Drug Committee . The agricultural co-operative federation of Thailand Limited Printing; Bangkok: 2006. List of herbal medicinal products A. D. [Google Scholar]
- Ranger B., Vegh Z., Ferenczi-Fodor A. Validation of thin layer, high performance thin layer chromatographic methods. Journal of Chromatography A. 2011;1218:2712–2721. doi: 10.1016/j.chroma.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Swargiary A., Ronghang B. Screening of phytochemical constituents, antioxidant and antibacterial properties of methanolic bark extracts of Maclura cochinchinensis (Lour.) Corner. International Journal of Pharma and Bio Sciences. 2013;4(4):449–459. [Google Scholar]
- Yoosook C., Bunyapraphatsara N., Boonyakiat Y., Kantasuk C. Anti-herpes simplex virus activities of crude water extracts of Thai medicinal plants. Phytomedicine. 2000;6(6):411–419. doi: 10.1016/S0944-7113(00)80068-9. [DOI] [PubMed] [Google Scholar]