Abstract
Objective
Chrysophanol (Chry) displays potent anticancer activity in human cancer cells and animal models, but the cellular targets of Chry have not been fully defined. Herein, we speculated whether mitochondria were a target involved in Chry-induced cytotoxicity.
Methods
Human liver cancer cell line HepG2 was incubated. The cytotoxicity was evaluated by MTT assay. Mitochondria localization was evaluated by a confocal microscopy. Mitochondrial membrane potential ΔΨm was detected by TMRE staining and determined by the flow cytometer. The levels of ATP, mitochondrial superoxide anions, and GSH/GSSG were determined according to the assay kits. The apoptosis were evaluated through Hoechst33342/PI and Annexin V/PI staining, respectively. The expression of cyclophilin D (CyPD) was determined by immunoblot method, and the interaction between CyPD and Chry was analyzed by molecule docking procedure.
Results
Chry itself mainly localized in mitochondria to cause mitochondrial dysfunction and cell death in HepG2 cells. As regard to the mechanism, cyclosporin A as the inhibitor for the formation of mitochondrial permeability transition pore (mPTP) moderately suppressed cell death, indicating mPTP involved in the process of cell death. Further, Chry enhanced the protein expression of Cyclophilin D (CyPD) which is a molecular componentry and a modulator of mPTP, while antioxidant N-acetyl-L-cysteine inhibited the expression of CyPD. Molecule docking procedure disclosed two hydrogen-bonds existed in CyPD-Chry complex with −11.94 kal/mol of the binding affinity value. Besides, the mtDNA-deficient HepG2-ρ0 cells were much resistant to Chry-induced cell death, indicating mtDNA at least partly participated in cell death. A combination of Chry and VP-16 produced the synergism effect toward cell viability and ΔΨm, while Chry combined with Cis-Pt elicited the antagonism effect.
Conclusion
Taken together, enrichment in mitochondria and actions on mPTP, CyPD and mtDNA provides an insight into the anticancer mechanism of Chry. The combination therapy for Chry with clinical drugs may deserve to further explore.
Keywords: chrysophanol, combination therapy, mitochondria, mitochondrial permeability transition pore, mtDNA
1. Introduction
Chrysophanol (Chry) is a naturally occurring anthraquinone that distributes across the plant and animal kingdoms as well as in the microbial world (Malik and Müller, 2016, Prateeksha et al., 2019, Xie et al., 2019). Extraction from Rheum palmatum L. gave 6.030 mg/g Chry by reflux in water for 30 min after hydrolysis anthraquinones (Wei, Yao, Ji, Wei, & Peng, 2013). The pharmacological effects of Chry have been extensively explored, including anticancer, antioxidant, neuroprotection, antibacterial, antiviral and antiaging, which suggested that Chry might be used against various diseases (Malik and Müller, 2016, Prateeksha et al., 2019, Xie et al., 2019). Specific to anticancer activity, Chry could suppress malignant cell growth, induce apoptosis and necrosis, trigger endoplasmic reticulum stress, arrest cell cycle progression, and inhibit cell invasion, metastasis (Lim et al., 2018, Lu et al., 2010, Ni et al., 2012). Some molecular targets in various types of human cancer cells have been reported to be affected by Chry, including phosphatidylinositol 3-kinase (PI3K)/Akt (Lim, Yang, Bazer, & Song, 2017), extracellular signal-regulated kinase (ERK) (Lim et al., 2017), mitogen-activated protein kinase (MAPK) signal pathway (Lim et al., 2018, Park et al., 2018), EGFR/mTOR pathway (Lee, Cha, Sul, Song, & Kim, 2011), NF-κB pathway (Ren et al., 2018), serine/threonine-protein kinase 1 (RIP1) (Choi, 2016) and DNA (Yang, Li, Huang, Li, & Yan, 2012). Because of its undoubted anticancer efficacy, the other cellular targets and modes of action of Chry in cells still need to be further explored.
Mitochondrial matrix-specific protein cyclophilin D (CypD), as the only isomer of human mitochondria cyclophilins, participates in the formation of mitochondrial permeability transition pore (mPTP) which leads to both apoptosis and necrosis (Li et al., 2004, Gutiérrez-Aguilar and Baines, 2015). Evidently, isolated mitochondria from a neuronal B50 cells stably overexpressing CypD was far more susceptible to Ca2+-elicited mPTP opening and oxidative stress (Li et al., 2004); And mitochondria from CypD-null mice were more resistant to Ca2+- and oxidative stress-induced mPTP opening than wild-type mice (Baines et al., 2005). Additionally, the cellular redox environment has a certain effect on the structure and functional characteristics of CypD. CypD in its reduced form might be responsible for refolding of the newly imported proteins in mitochondria and maintaining organelle integrity. Oxidized CypD might induce the opening of mPTP and lead to mitochondria-mediated cell death (Linard et al., 2009). For instance, it has demonstrated that the higher expression of CypD was detected in necrocytosis caused by drugs such as salinomycin (Qin, Jia, Zhang, & Zhang, 2015), cisplatin (Chen et al., 2013), adriamycin (Lu, Shi, & Xu, 2014), and gemcitabine (Chen et al., 2014).
Additionally, human mitochondrial genome contains 16.5 kb DNA which encode 13 respiratory chain subunits. Respiratory chain regulates mitochondrial ATP generation, so drugs that damage mitochondrial DNA (mtDNA) probably will remarkably affect cell growth and cellular physiological function (Fliss, 2000). Moreover, because of the lack of protection by histones and the relatively weak DNA repair capacity in mitochondria, mtDNA is more susceptible to DNA damaging agents (Singh et al., 1999). Failure to repair mtDNA damage might facilitate to initiate cell death (Grishko, Rachek, Musiyenko, LeDoux, & Wilson, 2005).
In the present study, we observed uptake of Chry in mitochondria and investigated how Chry affected mitochondrial components to trigger HepG2 cell death. And the combination efficacies of Chry with VP-16 or cisplatin (Cis-Pt) on HepG2 cell viability and mitochondrial membrane potential (ΔΨm) were also evaluated.
2. Materials and methods
2.1. Materials
Chrysophanol (Chry), Cyclosporin A (CsA), N-acetyl-L-cysteine (NAC), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) were from Sigma Aldrich Chemical Co. (Beijing, China). Fetal bovine serum (FBS) was purchased from Hyclone (Shanghai, China). Tetramethylrhodamine ethyl ester (TMRE) was purchased from Biolite Biotech (Tianjin, China). Annexin V-FITC/PI apoptosis detection kit was purchased from Nanjing jiancheng (Nanjing, China). Hoechst-PI staining assay kit, GSH assay kit and ATP assay kit were provided by Beyotime (Shanghai, China). All antibodies were from Affinity Biosciences (OH, USA). Mito-Tracker deep red (MTDR) was obtained from Thermo Fisher (Shanghai, China).
2.2. Cell culture
HepG2 cells were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science. The cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. They were maintained at 37 °C in a humidified incubator with 5% CO2.
2.3. Cell viability assay
HepG2 cells (1 × 104 cells/well) were incubated with Chry (12.5, 25, 50, 100, and 120 μmol/L) in triplicate in 96-well plates for 48 h at 37 °C in a final volume of 100 μL. Additionally, to determine the combination effects of Chry with VP-16 and Cis-Pt, cells were incubated with Chry (25 μmol/L) and VP-16 (10, 20, 30, and 60 μmol/L) or Cis-Pt (5, 10, 20, and 40 μmol/L) for 48 h. At the end of the treatment, MTT assay was performed to assess the cell viability using a microplate reader (Thermo Scientific Multiskan GO, Finland).
2.4. Mitochondrial uptake assay of Chry
HepG2 cells (5 × 104 cells/dish) were cultured on coverslips and treated with Chry (50 µmol/L) for 15 min followed by staining with 100 nmol/L Mito-Tracker deep red for another 15 min. Then the cells were washed three times with phosphate-buffered saline (PBS, pH = 7.4) and photographed using a confocal microscopy (FV 1000MPE, Olympus, Japan) by argon laser at 488 nm and helium–neon laser at 633 nm. The colocalization coefficient is analyzed by the software along with the confocal microscope.
2.5. Detections of ΔΨm, ATP, superoxide anion O2–· and GSH/GSSG
After incubation of HepG2 cells (5 × 104 cells/well) with Chry (0, 50, 100 µmol/L) for 6, 12, 24, 48 h, the cells were washed with PBS. For ΔΨm assay, 100 µL of fresh medium containing 200 nmol/L tetramethylrhodamine ethyl ester (TMRE) was added to each well and incubated for another 30 min at 37 °C. Cells were then washed with PBS three times. For ATP assay, it was detected by ATP Assay Kit following the manufacturer’s instruction. For superoxide anion O2–· assay, 50 µL of 0.2 µmol/L MitoSOX probe working solution was added and incubation continued for another 45 min. Cells were then rinsed with PBS and the fluorescence intensity was determined by a microplate reader (Varioskan Flash, Thermo scientific, Finland) at 510 nm excitation and 580 nm emission. For GSH/GSSG assay, the assay was performed using GSH and GSSG Assay Kit according to the manufacturer's instructions.
2.6. Determination of apoptosis and/or necrosis
After the cells were pretreated with CsA (1 µmol/L) for 1 h, Chry (0, 50, 100 µmol/L) was added and incubation continued for another 48 h. The percentages of apoptotic cells were determined by the AnnexinV-FITC/PI kit using FACS Canto flow cytometer (Canto, Becton Dickinson, USA). For Hoechst-PI double staining assay, cells were stained with 5 µL Hoechst 33,342 and 5 µL propidium iodide (PI) for 25 min at 4 °C. After staining, cells were washed by PBS and examined by fluorescence microscopy (Germany Leica).
2.7. Western blots
After treatment, mitochondrial fractions were isolated. Equal amounts of denatured proteins (20–40 mg) were separated by 12% SDS PAGE followed by electroblotting onto polyvinylidene difluoride membranes. Targeted proteins were detected with specific primary antibodies. Corresponding secondary antibodies were then utilized and immunoreactive bands were visualized by fully automatic chemiluminescence analysis system (Tanon, Shanghai, China).
2.8. Molecule docking between chrysophanol and CypD
The 3D structure of chrysophanol was drawn in SYBYL 6.9 software (SYBYL, Version 6.9, Tripos Inc., St. Louis, MO). Partial atomic charges were calculated using the Tripos force field with a convergence criterion of 0.01 kcal/mol. Then, the CDOCKER module of Discovery Studio 2.5 software was used to dock chrysophanol with CypD (PDB code: 2BIT) (Discovery Studio version 2.5, Accelrys Inc., CA, 2009). We defined the binding site sphere through receptor cavities, and set the radius of the sphere to 7 Å (Liu et al., 2015).
2.9. Statistical analysis
The data are expressed as the means ± SD of at least three independent experiments. Statistical differences between two groups were measured by one-way factorial analysis of variance (ANOVA), using Duncan’s post-hoc test (SPSS 19.0). P < 0.05 versus control was used as the criterion for statistical significance.
3. Results
3.1. Uptake of Chry in mitochondria
Having a planar polycyclic aromatic system, Chry displays the inherent fluorescence with λex = 445 nm, and λem = 525 nm (Fig. 1A). Thus, we mapped its cellular localization in HepG2 cells by laser confocal microscopy (Fig. 1B). Upon treatment with Chry, the cells exhibited bright green fluorescence in discrete subcellular distribution (A), likely to be mitochondria around the nucleus. In order to test this assumption, a recognized method for mitochondrial imaging by MTDR-staining was performed (B). The merging of A and B presented a clear yellow colocalization signal (C) with a colocalization coefficient of 0.591, confirming that Chry could permeate into mitochondria and localize in and around mitochondria.
Fig. 1.
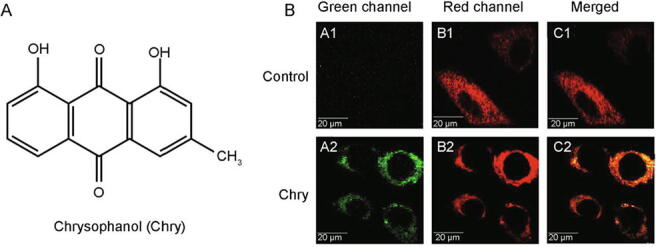
Detection of Chry in mitochondria of HepG2 cells by laser confocal microscopy. (A) The chemical structure of Chry. (B) Fluorescent imaging of mitochondria. The cells were incubated with 50 μmol/L of Chry for 15 min, and then stained with MTDR (an exclusive dye for mitochondria), for another 15 min.
3.2. Induction of mitochondrial disfunction and cell death
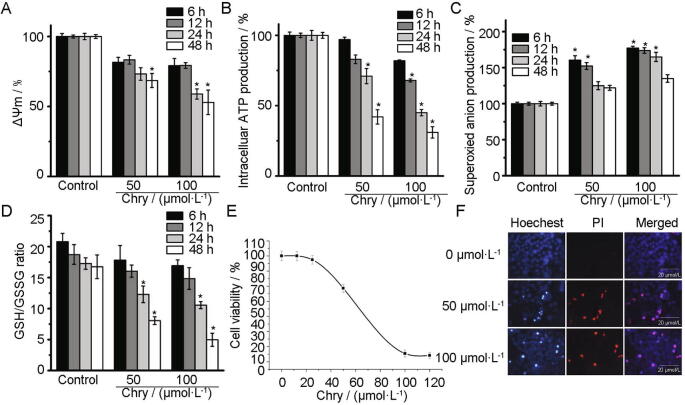
As expected, Chry decreased ΔΨm and ATP levels in dose- and time-dependent manner (Fig. 2A and B). Simultaneously, Chry-promoted O2–· generation in mitochondria remained significantly higher than the controls. The maximum O2–· production was triggered after exposure of the cells to 100 μmol/L Chry for 6 h (Fig. 2C). Due to the overproduction of reactive oxygen species (ROS), cellular GSH was oxidized to GSSG immediately. Hence, the ratio of GSH/GSSG was decreased in a dose- and time-dependent manner (Fig. 2D), showing an occurrence of redox imbalance. Next, it has reported that Chry triggered necrotic signaling in human hepatoma cells, such as J5 cells (Lu et al., 2010) and H3B cells (Ni et al., 2012). Herein, we also found that Chry killed HepG2 cells predominantly through the induction of necrosis. The IC50 value from the curve of cell viability was around (74.5 ± 4.65) μmol/L (Fig. 2E). However, Chry did not show significant cytotoxicity toward normal liver cells L02 at 100 μmol/L after 48 h. The images of Hoechst/PI staining showed that Chry primarily induced cellular necrosis, appearing red and purple cells (Fig. 2F).
Fig. 2.
Effects of Chry on mitochondria function and cell viability toward HepG2 cells. (A) Determination of ΔΨm by TMRE probe. (B) Determination of ATP by ATP Assay Kit. (C) Determination of O2–·production by MitoSOX probe. (D) Determination of GSH/GSSG by GSH/GSSG Assay Kit. The concentrations and the action time were indicated on the figures. (E) Cell viability for 48 h was determined by MTT assay. (F) Analysis of cell death by nuclear condensation using Hoechst/PI staining. Scale bars of fluorescence images: 20 μmol/L. The values are expressed as the means ± SD; n = 3, *P < 0.05 vs control.
3.3. Involvement of CypD in cell death
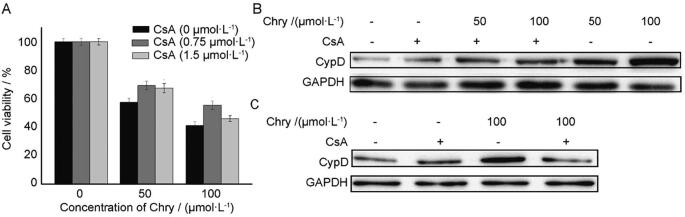
Further, in order to determine whether CypD was involved in Chry-evoked cell death, CsA as CypD inhibitor was employed. Inclusion of CsA moderately attenuated cell death (Fig. 3A). On the other hand, Chry increased the expression of CypD in a dose-dependent manner (Fig. 3B), while CsA obviously attenuated the Chry-evoked protein expression of CypD (Fig. 3B). Besides, CypD is sensitive to redox conditions and oxidized CypD induces the opening of mPTP and leads to cell death (Linard et al., 2009). Then, we explored the function of antioxidant N-acetyl-L-cysteine (NAC) on CypD expression. Owing to exposure of the cells to 100 μmol/L Chry for 6 h led to a maximum production of ROS, we performed this condition for following assay. Pretreatment of the cells with NAC remarkably suppressed the CypD level (Fig. 3C), indicating that ROS participated in the relevant role of CypD. It was also found that NAC significantly inhibited Chry-induced cell death (data not shown). Finally, the molecular docking was used to evaluate whether Chry could bind to the active sites of CypD. Chry formed two hydrogen bonds with Gln-63 and Arg-82 respectively, including the binding affinity value of −11.94 kal/mol (Fig. 4). Because of these hydrogen bonds, the effective interaction between Chry and CypD is relatively strong.
Fig. 3.
Effects of CsA on Chry-induced cell death and CypD expression, including the effects of NAC on CypD expression. A) Analysis of cell viability after 48 h. (B&C) Analysis of CypD level in the presence of CsA (1 μmol/L) or NAC (5 mmol/L) by Western blots. GAPDH was used as internal control. The values are expressed as the means ± SD; n = 3, *P<0.05 vs control.
Fig. 4.
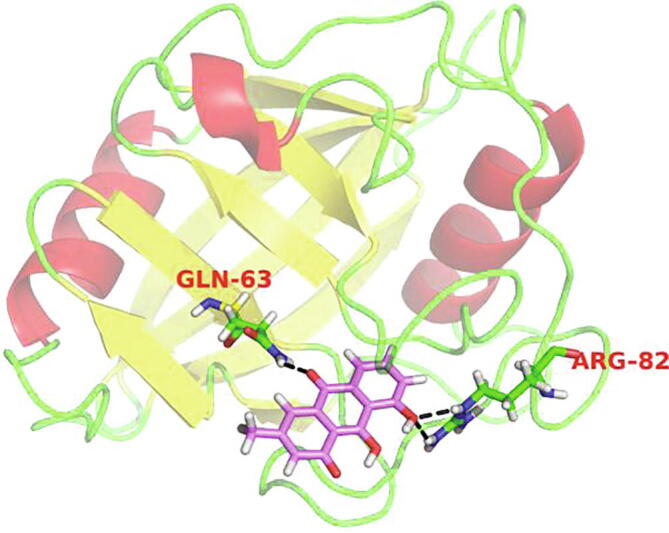
Docking of Chry in binding site of CypD in 3D style. For above configuration magenta sticks represent the ligand (Chry and CypD), and black dashed lines represent the hydrogen bonds.
3.4. Involvement of mtDNA in cell death
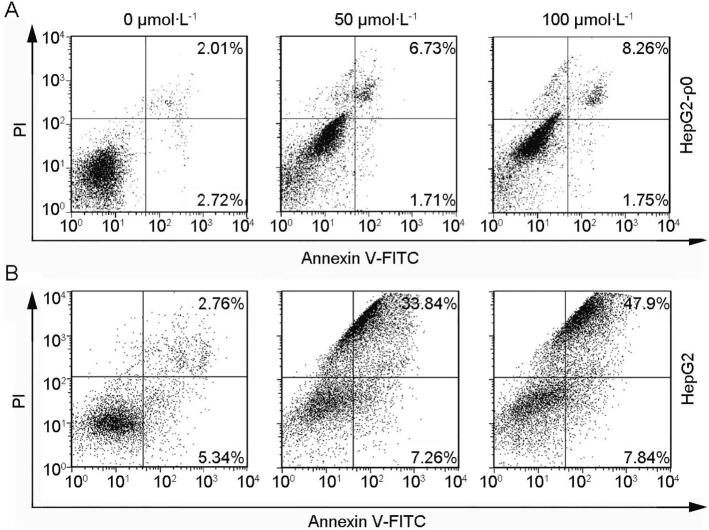
Due to uptake of Chry by mitochondria, we determined whether mtDNA was also involved in cell death. The mtDNA-deficient HepG2 cells (termed ρ0 cells) was used. Obviously, HepG2-ρ0 cells were much resistant to the growth suppressive induction after exposure to Chry, suggesting that mtDNA damage was at least in part involved in the necrotic mechanism of action of Chry (Fig. 5).
Fig. 5.
Efects of Chry on apoptosis in HepG2-ρ0 cells. The cells were incubated with Chry for 48 h. After that, the percentages of apoptosis were determined by flow cytometry according to the instructions of AnnexinV-FITC/PI kit.
3.5. Combination effects of Chry with VP-16- or Cis-Pt
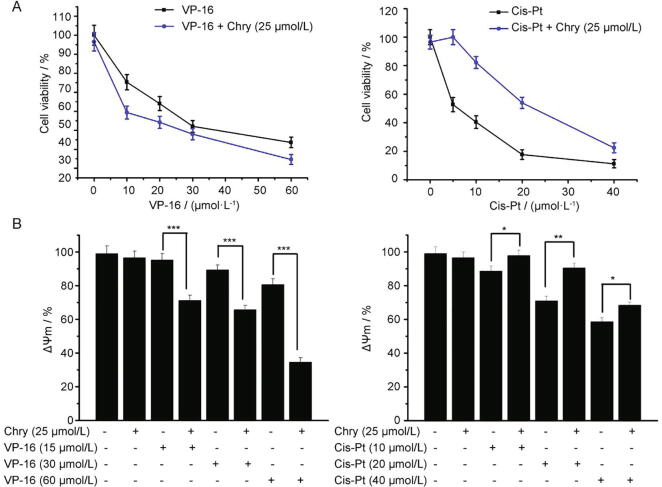
Due to weak antiproliferative activity of Chry, we sought to investigate whether Chry could sensitize HepG2 cells to the anti-cancer effect of VP-16 or Cis-Pt. VP-16 and Cis-Pt are two of the first-line chemotherapeutic drugs. First, the combination effects of VP-16 or Cis-Pt with Chry on cell viability were evaluated using MTT assay. As shown in Fig. 6A, Chry at 25 μmol/L alone showed no inhibitory activity; Chry at the above dose in combination with VP-16 decreased the cell viability more intensely relative to cell viability only affected by VP-16 alone, showing the occurrence of synergistic effect. To our surprise, concomitant treatment of cells with Cis-Pt and Chry led to the higher cell viability than treatment with Cis-Pt alone, suggesting an antagonism effect existing between Chry and Cis-Pt. In order to confirm the combination effects, their effects on ΔΨm were evaluated. From Fig. 6B, VP-16 combined with Chry markedly collapsed ΔΨm relative to VP-16 alone; however, the decrease of ΔΨm was much less for Cis-Pt combined with Chry than that for Cis-Pt alone. Thus, the same conclusion was obtained from ΔΨm assay.
Fig. 6.
Determinations of cell viability and ΔΨm in HepG2 cells when treated with Chry + VP-16 or Chry + Cis-Pt. For Chry, a fixed concentration of 25 μmol/L was used; for VP-16 and Cis-Pt, the gradient concentration was applied marked in the figures. (A) The cell viability was examined using MTT assay after the treatment for 48 h. (B) The level of ΔΨm was examined using the TMRE probe by a flow cytometry after the treatment for 30 h. The data of ΔΨm (%) were presented, in order to get the differences clearly. The values are expressed as the means ± SD; n = 3, *P<0.05 vs control.
4. Discussion
Rhubarb is a well-known folk herbal medicine and has been widely used for treating gastrointestinal disease, acute hepatitis, blood diseases, chronic renal failure, diabetes, and especially, constipation due to its effective laxative activity (Cao et al., 2017, Zheng et al., 2013). In rhubarb, anthraquinones, including rhein, aloe-emodin, Chry, emodin, physcion and their glycosides are though to be the major active components. These five anthraquinones may applied as lead compounds for the development of future drugs. Here, we observed that Chry mainly distributed in mitochondria of HepG2cells analyzed by its inherent fluorescence (Fig. 1B). Next, anticancer drugs with anthraquinones skeleton, such as doxorubicin, mitoxantrone and daunorubicin act partly through induction of ROS, because quinones could be activated to the semiquinone radical intermediate, which in turn could react with oxygen to produce ROS. Once the level of cellular ROS over threshold, it will attack DNA strand, destroy unsaturated lipids, proteins and other macromolecules resulting in cell damage. On the other hand, semiquinone radicals were also found to equallly contribute to cytotoxicity (Malik & Müller, 2016). We found that Chry enhanced O2–· accumulation within mitochondria, consequently causing intracellular redox imbalance as determined by the level of GSH and GSSG (Fig. 2 C and D). Moreover, induction of diminished level of ATP, collapsion of ΔΨm together with necrosis (Fig. 2) were also observed. Hence, we concluded that mitochondria could act as a primary cellular site for enrichment of Chry which induced mitochondria-mediated cell death.
Being an integral constituent of mPTP, CypD plays a decisive role in opening of mPTP which induce execution of cell death. The immunosuppressant CsA is the CypD ligand that has been available for studying the role of CypD in mitochondria-mediated cell death (Li et al., 2004, Gutiérrez-Aguilar and Baines, 2015). Here, we proposed CypD as a target of Chry in mitochondria and demonstrated Chry-induced necrosis through a previously uncharacterized mechanism by regulating this protein. First, CsA effectively attenuated both necrosis percentages and CypD expression triggered by Chry in dose-dependent manner, suggesting that the process of necrosis was closely associated with enhancement of CypD expression. Second, it has reported that oxidation of CypD has an impact on its conformation and activity, facilitating cell death (Linard et al., 2009). We found that antioxidant NAC almost completely inhibited CypD expression, suggesting that ROS production caused by Chry might participate in upregulation of CypD expression. Third, in CypD-Chry complex: Residues of two amino acids (Gln-63, Arg-82) are involved in H-bond interactions based on molecule docking procedure, showing an effective combination between Chry with CypD. On the other hand, promotion of DNA strand scission and alkylation, intercalation into DNA, inhibition on DNA topoisomerase I and II are recognized as the prominent mechanisms underlying anticancer activity of anthraquinones (Malik & Müller, 2016). Here, we found that involvement of mtDNA damage to the cytotoxicity of Chry was demonstrated by mtDNA-deficient HepG2-ρ0 cells. HepG2-ρ0 cells were more resistant than HepG2 cells to the growth suppressive induction after exposure to Chry (Fig. 5). Taken together, our data suggested that Chry might target CypD and mtDNA in HepG2 cells to induce cell death.
In principle, the biological significances of targeting mitochondria and its subunits, such as CypD and mtDNA in cancer cells are at least as following. First, mitochondria is considered as a drug target which has manifested the clinical effectiveness in combating relapsed or refractory cancer (Xia et al., 2019). More importantly, as mitochondria is a control center of cell death and an energy factory for ATP production, the drugs targeting mitochondria can directly perturb its function and therefore bypass multidrug resistance (Wang et al., 2018, Wang et al., 2020). Second, CypD regulates the formation of mPTP, leading to overproduction of ROS and alteration in cellular redox states. Meanwhile, CypD is also oxidized by ROS and oxidized CypD in turn promotes the opening of mPTP. Bigi et al. found that oncogenic Ras might enhance CypD protein through Raf-1/MEK/ERK pathway which has a deterministic role in tumor progression (Bigi et al., 2016). Third, the damage to mtDNA may elicit dysfunction of electron transport chain which will lead to an increase in mitochondria-generated ROS (Fliss, 2000). ROS are involved in cell death by imposing an oxidative stress. We believe that all these factors contribute to the cellular function of Chry.
However, Chry displayed the weak antiproliferative activity against HepG2 cells with the IC50 around 74.5 μmol/L. Then, we assumed whether Chry could be used as an ancillary drug for cancer treatment. Here, we found that Chry combined with VP-16 showed the synergistic effects on inhibition of cell growth and deplolarization of ΔΨm, while Chry with Cis-Pt showing the antagonism effects (Fig. 6). As we known, it has reported that Chry could be inserted into the base pair of double-stranded DNA and damage DNA (Yang et al., 2012). Cis-Pt as a DNA crosslinking agent, its DNA binding mode is sensitive to DNA conformation (Bubley et al., 1996). We hypothesized that Chry could influence DNA topology which partly prevented the DNA crosslinks by Cis-Pt. For example, Daunorubicin (Dauno) is also an anthraquinone (9,10-dioxoanthracenes), binding to DNA and inhibiting topoisomerase II (Gewirtz, 1999). Recently, it has reported that Dauno at low concentration elicited the antagonism with Cis-Pt through the decrease of interstrand crosslinks of Cis-Pt and the diminished Dauno uptake in the presence of Cis-Pt (Erfaneh, Thibaut, László, Péter, Szabolcs, Zsolt, Frank, & Gábor, 2020). Hopefully, the antagonism mechanism of Dauno combined with Cis-Pt might help us to speculate antagonism of Chy with Cis-Pt.
5. Conclusion
In summary, we have discovered mitochondria as a main location of Chry in HepG2 cells and reported that Chry induced necrotic cell death at least partly through CypD upregulation and mtDNA damage. The observations of the combination effects of Chry with VP-16 or Cis-Pt provided a deep insight into the understanding the anticancer action of Chry in vivo.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (Grant No. lzujbky-2018-131), the National Natural Sciences Foundation of China (Grant No. 21302079).
References
- Bigi A., Beltrami E., Trinei M., Stendardo M., Pelicci P.G., Giorgio M. Cyclophilin D counteracts P53-mediated growth arrest and promotes ras tumorigenesis. Oncogene. 2016;35(39):5132–5143. doi: 10.1038/onc.2016.42. [DOI] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A.…Molkentin J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bubley G.J., Xu J., Kupiec N., Sanders D., Foss F., O'Brien M.…Patierno S.R. Effect of DNA conformation on cisplatin adduct formation. Biochemical Pharmacology. 1996;51(5):717–721. doi: 10.1016/s0006-2952(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Cao Y.-J., Pu Z.-J., Tang Y.-P., Shen J., Chen Y.-Y., Kang A.n.…Duan J.-A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chinese Medical Journal. 2017;12(1) doi: 10.1186/s13020-017-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.-S. Chrysophanic acid induces necrosis but not necroptosis in human renal cell carcinoma Caki-2 cells. Journal of Cancer Prevention. 2016;21(2):81–87. doi: 10.15430/JCP.2016.21.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-H., Li D.-L., Yang F., Wu Z., Zhao Y.-Y., Jiang Y. Gemcitabine-induced pancreatic cancer cell death is associated with MST1/Cyclophilin D mitochondrial complexation. Biochimie. 2014;103:71–79. doi: 10.1016/j.biochi.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Chen B., Xu M., Zhang H., Wang J.-x., Zheng P., Gong L.…Dai T.u. Cisplatin-induced non-apoptotic death of pancreatic cancer cells requires mitochondrial cyclophilin-D-p53 signaling. Biochemical and Biophysical Research Communications. 2013;437(4):526–531. doi: 10.1016/j.bbrc.2013.06.103. [DOI] [PubMed] [Google Scholar]
- Erfaneh, F. N., Thibaut, V. A., László. I., Péter, N. J., Szabolcs, T., Zsolt, B., Frank, V., & Gábor, S. (2020). Interactions of Cisplatin and Daunorubicin at the chromatin level. Scientific Reports, 10, 1107-1118. [DOI] [PMC free article] [PubMed]
- Fliss M.S. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Aguilar M., Baines C.P. Structural mechanisms of cyclophilin D-dependent control of the mitochondrial permeability transition pore. Biochimica et Biophysica Acta General Subjects. 2015;1850(10):2041–2047. doi: 10.1016/j.bbagen.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishko V., Rachek L., Musiyenko S., LeDoux S.P., Wilson G.L. Involvement of mtDNA damage in free fatty acid-induced apoptosis. Free Radical Biology and Medicine. 2005;38(6):755–762. doi: 10.1016/j.freeradbiomed.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology. 1999;57(7):727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Lim W., An Y., Yang C., Bazer F.W., Song G. Chrysophanol induces cell death and inhibits invasiveness via mitochondrial calcium overload in ovarian cancer cells. Journal of Cellular Biochemistry. 2018;119(12):10216–10227. doi: 10.1002/jcb.27363. [DOI] [PubMed] [Google Scholar]
- Lim W., Yang C., Bazer F.W., Song G. Chrysophanol induces apoptosis of choriocarcinoma through regulation of ROS and the AKT and ERK1/2 pathways. Journal of Cellular Physiology. 2017;232(2):331–339. doi: 10.1002/jcp.25423. [DOI] [PubMed] [Google Scholar]
- Liu H., An X., Li S., Wang Y., Li J., Liu H. Interaction mechanism exploration of R-bicalutamide/S-1 with WT/W741L AR using molecular dynamics simulations. Molecular BioSystems. 2015;11(12):3347–3354. doi: 10.1039/c5mb00499c. [DOI] [PubMed] [Google Scholar]
- Lu J.-H., Shi Z.-F., Xu H. The mitochondrial cyclophilin D/p53 complexation mediates doxorubicin-induced non-apoptotic death of A549 lung cancer cells. Molecular and Cellular Biochemistry. 2014;389(1-2):17–24. doi: 10.1007/s11010-013-1922-1. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Cha E.Y., Sul J.Y., Song I.S., Kim J.Y. Chrysophanic acid blocks proliferation of colon cancer cells by inhibiting EGFR/mTOR pathway. Phytotherapy Research. 2011;25(6):833–837. doi: 10.1002/ptr.3323. [DOI] [PubMed] [Google Scholar]
- Lu C.C., Yang J.S., Huang A.C., Hsia T.C., Chou S.T., Kuo C.L.…Chung J.G. Chrysophanol induces necrosis through the prodcution of ROS and alteration of ATP levels in J5 human liver cancer cells. Molecular Nutrition & Food Research. 2010;54:967–976. doi: 10.1002/mnfr.200900265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linard D., Kandlbinder A., Degand H., Morsomme P., Dietz K.-J., Knoops B. Redox characterization of human cyclophilin D: Identification of a new mammalian mitochondrial redox sensor. Archives of Biochemistry and Biophysics. 2009;491(1-2):39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Li Y., Johnson N., Capano M., Edwards M., Crompton M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochemical Journal. 2004;383:101–109. doi: 10.1042/BJ20040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik E.M., Müller C.E. Anthraquinones as pharmacological tools and drugs. Medicinal Research Reviews. 2016;36(4):705–748. doi: 10.1002/med.21391. [DOI] [PubMed] [Google Scholar]
- Ni C.-H., Chen P.-Y., Lu H.-F., Yang J.-S., Huang H.-Y., Wu S.-H.…Chung J.-G. Chrysophanol-induced necrotic-like cell death through an impaired mitochondrial ATP synthesis in Hep3B human liver cancer cells. Archives of Pharmacal Research. 2012;35(5):887–895. doi: 10.1007/s12272-012-0514-z. [DOI] [PubMed] [Google Scholar]
- Prateeksha, Yusuf M.A., Singh B.N., Sudheer S., Kharwar R.N., Siddiqui S.…Gupta V.K. Chrysophanol: A natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 2019;9(2):68. doi: 10.3390/biom9020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lim W., Song G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathway. Toxicology and Applied Pharmacology. 2018;360:201–211. doi: 10.1016/j.taap.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Qin L.S., Jia P.F., Zhang Z.Q., Zhang S.M. ROS-p53-cyclophilin-D signaling mediates salinomycin-induced glioma cell necrosis. Journal of Experimental & Clinical Cancer Research. 2015;34:1–12. doi: 10.1186/s13046-015-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Li Z., Dai C., Zhao D., Wang Y., Ma C., Liu C. Chrysophanol inhibits proliferation and induces apoptosis through NF-B/cyclin D1 and NF-B/Bcl-2 signaling cascade in breast cancer cell lines. Molecular Medicine Reports. 2018;17:4376–4382. doi: 10.3892/mmr.2018.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.K., Russell J., Sigala B., Zhang Y., Williams J., Keshav K.F. Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene. 1999;18(48):6641–6646. doi: 10.1038/sj.onc.1203056. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang F., Wen H., Shi W., Huang Q., Huang Y.…Zhou Y.i. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. Journal of Nanobiotechnology. 2020;18(1) doi: 10.1186/s12951-019-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Gao Z., Liu X., Agarwal P., Zhao S., Conroy D.W.…He X. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nature Communications. 2018;9(1) doi: 10.1038/s41467-018-02915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.-Y., Yao W.-X., Ji W.-Y., Wei J.-q., Peng S.-q. Qualitative and quantitative analysis of anthraquinones in rhubarbs by high performance liquid chromatography with diode array detector and mass spectrometry. Food Chemistry. 2013;141(3):1710–1715. doi: 10.1016/j.foodchem.2013.04.074. [DOI] [PubMed] [Google Scholar]
- Xia M., Zhang Y., Jin K., Lu Z., Zeng Z., Xiong W. Communication between mitochondria and other organelles: A brand-new perspective on mitochondria in cancer. Cell & Bioscience. 2019;9:27–45. doi: 10.1186/s13578-019-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, L., Tang, H., Song, J., Long, J., Zhang, L., & Li, X. (2019). Chrysophanol: A review of its pharmacology, toxicity and pharmacokinetics. Journal of Pharmacy & Pharmacology, 71, 1475-1487. [DOI] [PubMed]
- Yang X.M., Li J.S., Huang G.X., Li Q.Q., Yan L.J. Study on potential toxic mechanism of chrysophanol binding DNA by saturation value binding DNA. Asian Journal of Chemistry. 2012;24:551–557. [Google Scholar]
- Zheng Q.X., Wu H.F., Guo J., Nan H.J., Chen S.L., Yang J.S., Xu X.D. Review of rhubarbs: Chemistry and pharmacology. Chinese Herbal Medicines. 2013;5:9–32. [Google Scholar]