Abstract
Objective
Fufang Biejia Ruangan Tablet (FBRT) is widely used for the treatment of liver fibrosis. However, Hominis Placenta (HP), as an important adjuvant of FBRT, has been restricted for medicinal using due to the limited availability, ethical controversy and safety issues. The present study aimed to investigate the therapeutic effects of novel FBRT (N-FBRT) with sheep placenta (SP) as substitute for HP on liver fibrosis and explore its possible mechanisms. Different dosages of SP in N-FBRT were also evaluated.
Methods
Rats were subcutaneously injected with CCl4 to induce liver fibrosis and then treated with N-FBRT and FBRT. The anti-hepatic fibrosis effect was determined based on biomarkers analysis of liver function and hepatic fibrosis, and the liver pathology was visualized by H&E staining and Masson staining. The oxidative stress and inflammatory cytokines were also detected. Immunohistochemical staining of α-SMA, real time PCR and Western blotting were performed to evaluate hepatic stellate cells (HSCs) activation and TGF-β1/Smad signaling pathway.
Results
N-FBRT and FBRT could ameliorate CCl4-induced liver fibrosis and improve liver function, as evidenced by lowering serum biomarkers levels of liver function and hepatic fibrosis, and decreasing hepatic Hyp content and collagen deposition, and improving the hepatic morphology and architecture changes. Moreover, the anti-liver fibrosis effect was better when the dosage of SP used in N-FBRT was 1/2 of HP in FBRT. Administration of N-FBRT markedly alleviated oxidative stress and inflammatory cytokines, and inhibited α-SMA expression. Furthermore, the mRNA expression of Col I, Col III, α-SMA and TGF-β1, and proteins expression of α-SMA, TGF-β1, Smad2/3 and p-Smad2/3 were significantly down-regulated by N-FBRT treatment.
Conclusion
SP can be used as substitute for HP to prepare N-FBRT for the treatment of liver fibrosis and the anti-liver fibrosis effect of N-FBRT is achieved by eliminating oxidative stress and inflammation, and inhibiting HSCs activation and ECM production by blocking TGF-β1/Smad signaling pathway.
Keywords: Fufang Biejia Ruangan Tablet, Hominis Placenta, liver fibrosis, sheep placenta, substitute, TGF-β1/Smad signaling pathway
1. Introduction
Currently, traditional Chinese medicine (TCM) has shown its unique advantages in inhibiting the progression of liver fibrosis and promoting the reversion of liver fibrosis (Zhang & Schuppan, 2014). Fufang Biejia Ruangan Tablet (FBRT) is the first Chinese medicine formula approved by the China Food and Drug Administration for treating liver fibrosis (Dong et al., 2016, Yang et al., 2013). It is prepared from the following 11 TCMs: Trionycis Carapax, Curcuma phaeocaulis Valeton, Paeonia lactiflora Pall., Angelica sinensis (Oliv.) Diels, Panax notoginseng (Burkill) F.H.Chen, Codonopsis pilosula (Franch.) Nannf., Astragalus propinquus Schischkin, Hominis Placenta, Cordyceps, Isatis tinctoria L., and Forsythia suspensa (Thunb.) Vahl (Huang et al., 2019, Dong et al., 2016). With the TCM effects of “softening and resolving hard masses, dissolving blood stasis and detoxication, replenishing qi and blood”, FBRT is widely used for the treatment of liver fibrosis in China (Huang et al., 2019, Yang et al., 2016, Wu et al., 2014, Chen et al., 2007). It was reported that FBRT could ameliorate liver fibrosis via restraining the proliferation and activation of hepatic stellate cells (HSCs) and down-regulating fibrogenic signal transduction of the TGF-β/Smad pathway (Yang et al., 2013, Guo et al., 2004).
As an important adjuvant in FBRT prescription, Hominis Placenta (HP), known as Ziheche in Chinese, has TCM effects of “warming kidney and replenishing vital essence, qi and blood”. It is a dried placenta isolated from healthy pregnant women after delivery (Park et al., 2014, Lee et al., 2013) and was reported to have various bioactive substances, such as proteins, peptides, hormones, growth factors, cytokines, amino acids, enzymes, minerals, and trace elements (Donnelly and Campling, 2016, Park et al., 2014, Lee et al., 2013, Kawakatsu et al., 2013). However, the application of HP was restricted due to the limited availability, ethical controversy and safety issues. Moreover, HP and all the TCM preparations containing HP documented in the 2010 edition of Chinese Pharmacopoeia have been removed in the 2015 edition of Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2015). These issues may affect the production and application of TCM preparations containing HP, such as FBRT.
To overcome the aforementioned problems, we have modified the formula of FBRT and evaluated the effect of the modified FBRT (MF-FBRT, where HP was removed) on liver fibrosis in rats induced by CCl4 (Deng et al., 2018). We found that the MF-FBRT could still effectively inhibit CCl4-induced liver fibrosis, as conferred by reducing oxidative stress, inhibiting collagen accumulation, reversing hepatic stellate cell activation, and inhibiting the expression of pro-fibrogenic cytokines (Deng et al., 2018). But, the anti-fibrotic effects of the MF-FBRT were slightly weaker than the original FBRT, indicating that the function of HP in the FBRT prescription cannot be ignored. Since HP has been limited for medical using, there is urgent need to find new substitutes of this traditional Chinese medicine.
Exploring new substitutes for TCMs that are in shortage or endangered state has been studied for many years. Some reports have demonstrated that placenta from domestic animals, such as sheep and pigs, exerted similar functions as HP, such as hepatoprotection, anti-oxidation, wound healing, and immunomodulatory effects (Wu et al., 2003, Park et al., 2011, Teng et al., 2011, Liu et al., 2019, Xu et al., 2018). Sheep placenta (SP) and HP are all listed in Compendium of Materia Medica and possess similar TCM effects. Therefore, we supposed that SP can be used as substitute for HP to prepare novel FBRT (N-FBRT) for the treatment of liver fibrosis. The present study was designed to investigate the therapeutic effects of N-FBRT with SP as substitute for HP on liver fibrosis induced by CCl4 in rats and explore its possible mechanisms. Different dosages of SP in N-FBRT were also evaluated.
2. Materials and methods
2.1. Experimental drugs and reagents
The raw material powders of FBRT (no excipients, batch number: X20161205) and N-FBRT were prepared and provided by Inner Mongolia Furi Medical Science Co., Ltd. (Inner Mongolia, China). The dosage of SP used in N-FBRT were 0.5, 1, 2 and 4 times of HP in FBRT, respectively, referring to N-FBRT1 (batch number: X20161201), N-FBRT2 (batch number: X20161202), N-FBRT3 (batch number: X20161203) and N-FBRT4 (batch number: X20161204). Colchicine Tablet (batch number: 160604) was purchased from Beijing Jialin Pharmaceutical Co., Ltd. (Beijing, China). CCl4 was purchased from Beijing Chemical Reagent Company (Beijing, China). All other chemicals were of analytical grade and commercially available.
2.2. Animals and treatment
Male Sprague Dawley rats, weighing (180–220) g, were purchased from the SPF (Beijing) Biotechnology Co., Ltd. (Beijing, China). All animals were housed with free access to food and water in a temperature [(25 ± 2) °C] and humidity [(60 ± 10)%] controlled room with a 12 h light/dark cycle (lights on from 6:00 am to 6:00 pm). Animal experiments were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Institution Ethical Committee of 302 Military Hospital.
The rats were randomly divided into control (n = 10) and CCl4 model group (n = 70). The rats in CCl4 model group were injected subcutaneously with CCl4 (mixed 2:3 in soybean oil) at a dose of 5 mL/kg for the first time and subsequently 3 mL/kg twice a week for 6 weeks to induce liver fibrosis (Deng et al., 2018). Rats in normal control group were received equal volume of soybean oil. After 6 weeks of CCl4 treatment, 12 rats died during modeling, two rats from each group were selected to verify the success of the modeling and the left rats in CCl4 model group were further divided into seven groups with eight rats per group as follows: CCl4 model group, N-FBRT1 group (0.58 g/kg), N-FBRT2 group (0.60 g/kg), N-FBRT3 group (0.66 g/kg), N-FBRT4 group (0.77 g/kg), FBRT group (0.60 g/kg) and colchicine group (0.1 mg/kg) as a positive control. The dosages of FBRT and N-FBRT were calculated according to the human dosage of FBRT (one 6 g pill per day) and the dosage of SP contained in N-FBRT. The raw material powders of FBRT and N-FBRT were suspended in 0.5% CMC-Na solution for administration. All rats in the FBRT, N-FBRT and colchicine groups were respectively administrated drugs by gavage once a day for another 6 weeks, while rats in normal control and CCl4 model groups were administrated equivalent volume of 0.5% CMC-Na solution.
At the end of the 12th week, all rats were sacrificed after overnight fast. Blood samples were collected, centrifuged at 8000 rpm for 15 min at 4 °C and serum were kept at −80 °C for biochemical analysis. Some liver tissues were fixed in 10% formaldehyde for histological and immunohistochemical analysis and the others were snap frozen in liquid nitrogen and stored at −80 °C for biochemical analysis.
2.3. Serum biochemical analysis
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), alkaline phosphatase (ALP) were measured by an automatic biochemistry analyzer using commercial kits (Mindray Medical, Shenzhen, China) according to the manufacturer’s instructions.
2.4. Liver fibrosis biomarkers and hydroxyproline content analysis
The levels of hyaluronic acid (HA), laminin (LN), collagen type IV (Col-IV) and procollagen III (PC-III) in serum were detected using enzyme-linked immunosorbent assay (ELISA) kits (CUSABIO, Wuhan, China) according to the manufacturer’s instructions. Liver hydroxyproline (Hyp) content was examined by alkaline hydrolysis method using commercial kits (CUSABIO, Wuhan, China).
2.5. Histological analysis and immunohistochemistry staining
The fixed liver tissues were dehydrated by graded ethanol, embedded in paraffin and sectioned. Liver sections were stained with hematoxylin and eosin (H&E) for observing the pathological changes of the liver tissues and with Masson’s trichrome stains to evaluate the collagen deposition in liver (Hong et al., 2016, Zhang et al., 2018). Five different fields (×200 magnification) of the tissue slide from each rat was randomly selected and analyzed by Image J (www.imagej.net). The area of Masson staining was divided by the net field area and multiplied by 100% to evaluate the relative fibrosis area (Cheng et al., 2013).
The immunohistochemistry staining was performed for the assessment of hepatic α smooth muscle actin (α-SMA) (Li et al., 2016, Anuja et al., 2018, Yuan et al., 2018, Tian et al., 2019). The liver sections were deparaffinized, hydrated and incubated in 3% hydrogen peroxide. Sections were then treated with EDTA (pH 8.0) antigen retrieval solution and non-specific binding sites were blocked with 5% bovine serum albumin. The samples were incubated with α-SMA antibody (1:500 dilution; mouse IgG2a α-SMA; Abcam, Cambridge, UK) overnight at 4 °C, followed by incubation with a species-specific secondary antibody (1:500 dilution, horseradish peroxidase-linked goat antimouse IgG; DAKO, Glostrup, Denmark) for 1 h at 37 °C. The α-SMA positive staining was done by incubation with diaminobenzidine (DAB) and the nuclei was counterstained using hematoxylin. The α-SMA positive staining area was analyzed by Image J.
2.6. Oxidative stress and inflammatory cytokines analysis
The serum levels of superoxide dismutase (SOD), reduced glutathione (GSH) and malondialdehyde (MDA) were estimated by colorimetric method as described in commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The concentrations of interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in liver tissue were determined by ELISA kits (MultiSciences Biotech Co., Ltd., Hangzhou, China).
2.7. Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from liver tissue using Trizol reagent (Life Technologies, CA, USA) and then was reverse-transcribed to complementary DNA (cDNA) using First-Strand cDNA Synthesis kits (Thermo Fisher Scientific, MA, USA) according to the manufacturer's instructions. qRT-PCR was then performed to measure the mRNA expression levels of collagen type I (Col-I), collagen type III (Col-III), α-SMA and transforming growth factor-beta1 (TGF-β1) using SYBR Green PCR Master Mix kit and a Stepone plus PCR System (Applied Biosystems, CA, USA). The primers sequences used in the present study were as follows: α-SMA: forward, 5′-AGCTGCTCCAGCTATGTGTG-3′/ reverse, 5′-TCCCAGTTGGTGATGATGCC-3′, TGF-β1: forward, 5′-GGCGGTGCTCGCTTTGTA-3′/ reverse, 5′-TCCCGAATGTC-TGACGTATTGA-3′; Col-I: forward, 5′- AGAGGCATAAAGGGTCATCGTG-3′/ reverse, 5′- AGACCGTTGAGTCCATCTTTGC-3′; Col-III: forward, 5′- AGAGGCT-TTGATGGACGCAA-3′/ reverse, 5′- GGTCCAACCTCACCCTTAGC-3′; GAPDH: forward, 5′-TTCCTACCCCCAATGTATCCG-3′/reverse, 5′-CATGAGGTCCA-CCACCCTGTT-3′. Relative expression level of genes was calculated using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control. Data was analyzed by 2−ΔΔCT method.
2.8. Western blot analysis
A total of 100 mg of liver tissue samples were lysed for total protein extraction in radio immunoprecipitation assay (RIPA) lysis buffer (Servicebio, Wuhan, China) and the protein concentration was quantified by the bicinchoninic acid (BCA) protein assay kit (Servicebio, Wuhan, China). The proteins were mixed with loading buffer and then heated at 100 °C for 10 min for protein denaturation. The denatured protein samples were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA). The membranes were blocked with 5% fat-free milk in Tris-buffered saline (TBST, 100 mmol/L NaCl, 50 mmol/L Tris, 0.1% Tween-20, pH 7.5) at room temperature for 1 h and then incubated overnight at 4 °C with primary antibodies, including α-SMA, TGF-β1, Smad2/3, p-Smad2/3 (Abcam) and β-actin (Servicebio) as an internal control, followed by incubating with appropriate secondary antibodies for 1 h at room temperature. The protein bands were detected by the BeyoECL plus kit (Beyotime, Shanghai, China) and β-actin was used as internal control.
2.9. Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS 13.0 statistical analysis software and one-way analysis of variance (ANOVA) followed by a LSD-t test was used for multigroup comparisons. Differences were considered significant at P < 0.05.
3. Results
3.1. Effects of N-FBRT and FBRT on liver injury
Serum biochemical analysis and histological changes visualized by H&E staining were performed to assess the protective effects of N-FBRT and FBRT against long-term CCl4-induced liver injury in rats (Tian et al., 2019). As shown in Table 1, compared with control group, the liver weight, liver index and the levels of serum ALT, AST, ALP and TBIL were significantly increased in CCl4 model group (P < 0.01), while animals treated with N-FBRT, FBRT or colchicine represented obviously reduced liver weight, liver index and serum levels of ALT, AST, ALP and TBIL (P < 0.05 or P < 0.01). But administration of FBRT or N-FBRT4 didn’t significantly decrease the serum TBIL level compared to CCl4 model group (P > 0.05). Furthermore, results of H&E stain showed that the liver sections of control group rats exhibited normal lobular architecture with radiating hepatic cords and central veins, no appreciable morphological alterations was observed; while CCl4 induced obvious hepatic damage including disordered lobular structure, vacuolar degeneration, hepatocytes swelling and necrosis, inflammatory cell infiltration and formation of fibrous septum. Treatment with N-FBRT, FBRT or colchicine significantly reversed the hepatic morphology and architecture changes (Fig. 1A).
Table 1.
Effects of N-FBRT and FBRT on liver weight, liver index and serum biochemical markers of rats with liver fibrosis (mean ± SD, n = 8).
| Groups | Liver weight/g | Liver index/% | ALT/(U/L) | AST/(U/L) | ALP/(U/L) | TBIL/(μmol/L) |
|---|---|---|---|---|---|---|
| Control | 9.06 ± 2.38 | 2.47 ± 0.20 | 47.40 ± 5.22 | 100.80 ± 11.71 | 133.40 ± 48.04 | 0.74 ± 0.24 |
| CCl4 | 15.09 ± 3.57** | 3.82 ± 0.47** | 571.17 ± 145.00** | 578.50 ± 134.23** | 211.67 ± 115.44* | 1.85 ± 0.78** |
| N-FBRT1 | 10.39 ± 1.50## | 2.66 ± 0.32## | 37.50 ± 8.70## | 78.80 ± 17.86## | 106.40 ± 37.41## | 1.05 ± 0.52## |
| N-FBRT2 | 10.49 ± 2.10## | 2.55 ± 0.30## | 50.90 ± 16.93## | 117.90 ± 40.05## | 130.80 ± 69.12# | 0.72 ± 0.39## |
| N-FBRT3 | 9.29 ± 1.77## | 2.39 ± 0.18## | 40.10 ± 6.44## | 147.20 ± 69.90## | 111.30 ± 42.25## | 1.30 ± 0.60# |
| N-FBRT4 | 9.89 ± 1.81## | 2.45 ± 0.12## | 37.20 ± 9.53## | 105.20 ± 21.66## | 123.30 ± 74.50## | 1.31 ± 0.34 |
| FBRT | 9.71 ± 1.88## | 2.50 ± 0.25## | 37.20 ± 12.32## | 89.10 ± 24.32## | 105.70 ± 26.06## | 1.34 ± 0.83 |
| Colchicine | 10.11 ± 1.94## | 2.48 ± 0.29## | 43.10 ± 15.47## | 82.40 ± 21.17## | 114.60 ± 43.77## | 0.82 ± 0.31## |
Liver index = (liver weight/body weight) × 100%. *P < 0.05, **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs CCl4 model group.
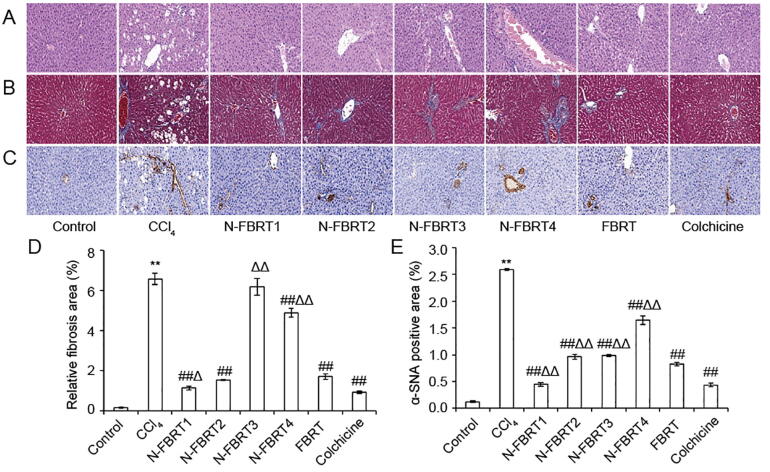
Fig. 1.
Effects of N-FBRT and FBRT on hepatic histopathological changes of rats with liver fibrosis (magnification, 200×). (A) H&E staining; (B) Masson staining; (C) α-SMA immunohistochemical staining; (D) Relative fibrosis area; (E) α-SMA positive area. Data are expressed as mean ± SD (n = 8). **P < 0.01 vs control group; ##P < 0.01 vs CCl4 model group; ΔP < 0.05, ΔΔP < 0.01 vs FBRT group.
3.2. Effects of N-FBRT and FBRT on liver fibrosis
Serum levels of HA, LN, PC-III and Col-IV in CCl4 model group were aberrantly increased compared to control group, whereas these collagen biomarkers in N-FBRT, FBRT and colchicine groups were distinctly decreased than CCl4 model group (Table 2, P < 0.05 or P < 0.01). But the serum HA level in N-FBRT3 group, the serum LN level in N-FBRT3 and N-FBRT4 groups and the serum PC-III level in N-FBRT2, N-FBRT3 and N-FBRT4 groups were not significantly decreased compared to CCl4 model group (P > 0.05). Compared with control group, hepatic Hyp content increased obviously in CCl4 model group and decreased obviously in N-FBRT and FBRT groups except N-FBRT4 group (P < 0.01).
Table 2.
Effects of N-FBRT and FBRT on serum fibrosis biomarkers and hepatic hydroxyproline content of rats with liver fibrosis (mean ± SD, n = 8).
| Groups | HA/(ng/mL) | LN/(ng/mL) | PC-III/(ng/mL) | C-IV/(ng/mL) | Hyp/(ng/g) |
|---|---|---|---|---|---|
| Control | 78.35 ± 14.93 | 45.31 ± 2.98 | 23.00 ± 4.66 | 94.07 ± 12.23 | 36.06 ± 6.36 |
| CCl4 | 183.00 ± 29.05** | 75.67 ± 8.28** | 51.00 ± 9.14** | 256.16 ± 26.90** | 132.54 ± 11.47** |
| N-FBRT1 | 108.75 ± 17.02## | 52.04 ± 4.07## | 31.25 ± 6.29## | 123.95 ± 21.84## | 39.40 ± 12.07## |
| N-FBRT2 | 123.00 ± 27.08# | 45.95 ± 2.95## | 38.00 ± 13.88 | 132.84 ± 22.71## | 66.84 ± 26.04## |
| N-FBRT3 | 137.50 ± 28.79 | 55.56 ± 6.10# | 40.85 ± 7.56 | 144.50 ± 10.69## | 70.94 ± 15.60## |
| N-FBRT4 | 147.00 ± 17.39 | 61.59 ± 5.91 | 43.00 ± 8.6 | 143.92 ± 19.77## | 97.30 ± 39.80 |
| FBRT | 113.35 ± 22.35## | 47.60 ± 4.54## | 34.85 ± 4.91# | 122.87 ± 22.21## | 66.87 ± 14.21## |
| Colchicine | 107.50 ± 18.44## | 44.59 ± 7.37## | 32.15 ± 5.15## | 115.37 ± 27.57## | 47.93 ± 23.71## |
P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs CCl4 model group.
Collagen deposition in liver tissue was observed by Masson's trichrome staining. As shown in Fig. 1B, an extensive collagen deposition appeared and intersected at multiple portal areas in liver of CCl4 model group rats, bridging fibrosis and pseudo lobule formation were also discovered. With treatment of N-FBRT, FBRT or colchicine, the collagen deposition were significantly reduced. The relative fibrosis area of liver sections in CCl4 model group was much larger than that in control group, but significantly decreased in N-FBRT and FBRT groups except N-FBRT3 group (Fig. 1D, P < 0.01). Moreover, the N-FBRT1 group showed more efficient to decrease the relative fibrosis area than FBRT group (P < 0.05).
Hepatic fibrosis is also characterized by an excessive α-SMA expression (Xia et al., 2018). The α-SMA expression in liver tissue was analyzed by immunohistochemical staining and brown staining in the cytoplasm or nucleus was regarded as positive for α-SMA expression (Cai et al., 2010). As shown in Fig. 1C and E, only very little α-SMA expression was observed in vascular smooth muscle cells of liver in control group. CCl4 model group showed the α-SMA expression was markedly increased as compared to control group (P < 0.01) and mainly distributed in the portal area and fibrous septa. In contrast, the α-SMA expression was remarkable reduced by treatment with N-FBRT, FBRT or colchicine (P < 0.01). Furthermore, the α-SMA positive staining area in N-FBRT1 group was significantly lower than that in FBRP group (P < 0.01).
3.3. Effects of N-FBRT and FBRT on oxidative stress and inflammatory cytokines
The effect of N-FBRT and FBRT on oxidative stress and inflammatory cytokines of rats with liver fibrosis are shown in Table 3. Compared to control group, the serum levels of SOD and GSH were significantly decreased in rats of CCl4 model group, while the serum MDA content and hepatic concentrations of inflammatory cytokines including IL-1β, IL-6 and TNF-α were markedly increased (P < 0.01). Inversely, treatment with N-FBRT, FBRT or colchicine obviously increased the levels of SOD and GSH, and decreased MDA content and inflammatory cytokines levels (P < 0.05 or P < 0.01). However, the SOD level in N-FBRT3 and N-FBRT4 groups and the GSH level in N-FBRT4 group were not significantly increased compared to CCl4 model group (P < 0.01).
Table 3.
Effects of N-FBRT and FBRT on serum levels of SOD, MDA, GSH and concentration of IL-1β, IL-6, TNF-α in liver tissue of rats (mean ± SD, n = 8).
| Groups | SOD/(U/mL) | GSH/(μg/mL) | MDA/(nmol/mL) | IL-6/(pg/mg) | IL-1β/(pg/mg) | TNF-α/(pg/mg) |
|---|---|---|---|---|---|---|
| Control | 169.27 ± 23.11 | 15.10 ± 3.33 | 3.22 ± 0.68 | 33.91 ± 24.19 | 30.72 ± 21.91 | 1.21 ± 0.95 |
| CCl4 | 130.43 ± 4.57** | 6.35 ± 1.08** | 5.97 ± 0.61** | 149.97 ± 47.99** | 841.56 ± 178.76** | 21.90 ± 0.71** |
| N-FBRT1 | 164.13 ± 21.23## | 11.87 ± 3.09## | 3.57 ± 0.79## | 45.24 ± 43.34## | 74.67 ± 37.15## | 1.67 ± 1.14## |
| N-FBRT2 | 157.32 ± 5.66# | 9.72 ± 2.04# | 4.10 ± 0.85## | 77.64 ± 59.13# | 117.18 ± 35.43## | 3.45 ± 3.12## |
| N-FBRT3 | 143.00 ± 4.81 | 10.13 ± 1.58# | 3.77 ± 0.21## | 38.19 ± 37.47## | 260.10 ± 74.63##Δ | 3.03 ± 1.43## |
| N-FBRT4 | 136.27 ± 2.26 | 9.21 ± 1.72 | 3.62 ± 0.45## | 80.53 ± 76.26# | 132.69 ± 52.24## | 2.42 ± 2.08## |
| FBRT | 160.50 ± 4.75## | 10.03 ± 2.42# | 3.47 ± 0.33## | 41.05 ± 8.42## | 80.22 ± 36.85## | 1.51 ± 0.37## |
| Colchicine | 156.00 ± 13.12## | 9.74 ± 1.37# | 4.00 ± 0.53## | 55.24 ± 20.69## | 51.15 ± 28.39## | 2.73 ± 0.27## |
P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs CCl4 model group; ΔP < 0.05 vs FBRT group.
3.4. Effects of N-FBRT and FBRT on fibrosis-associated mRNA expression
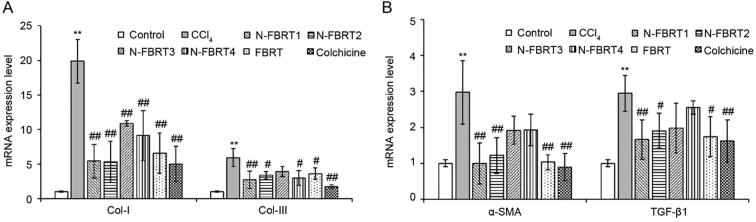
As shown in Fig. 2, compared with control group, the expression levels of Col-I, Col-III, α-SMA and TGF-β1 were up-regulated significantly in CCl4 model group and down-regulated remarkably by N-FBRT, FBRT or colchicine treatment (P < 0.05 or P < 0.01). However, the levels of Col-III, α-SMA and TGF-β1 mRNA expression in N-FBRT3 group and α-SMA, TGF-β1 mRNA expression in N-FBRT4 group showed no statistical significance as compared to CCl4 model group (P > 0.05).
Fig. 2.
Effects of N-FBRT and FBRT on mRNA expression levels of Col-I and Col-III (A), α-SMA and TGF-β1 (B) in liver of rats with liver fibrosis. Data are expressed as mean ± SD (n = 4). **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs CCl4 model group.
3.5. Effects of N-FBRT and FBRT on fibrosis-associated protein expression
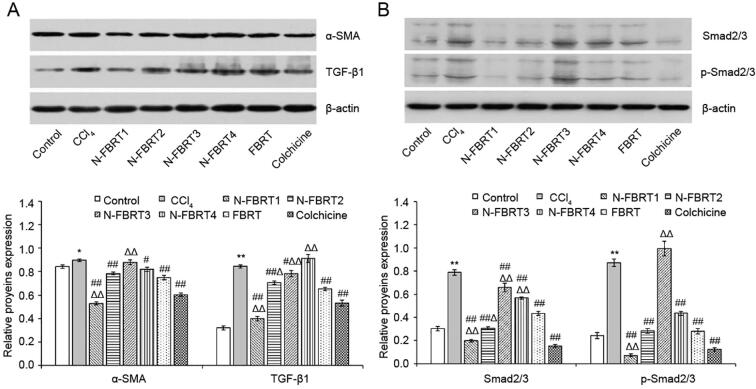
The expression of α-SMA, TGF-β1, Smad2/3 and p-Smad2/3 proteins in liver was determined by Western blot. As shown in Fig. 3, the α-SMA, TGF-β1, Smad2/3 and p-Smad2/3 proteins expressions in liver were excessively higher in CCl4 model group than that in control group (P < 0.05 or P < 0.01); Whereas N-FBRT, FBRT or colchicine significantly reversed these proteins expression (P < 0.05 or P < 0.01). Moreover, the down-regulation of these proteins expression was more remarkable in N-FBRT1 than that in FBRT. But N-FBRT3 exerted no effect on the up-regulated expression of α-SMA and p-Smad2/3 induced by CCl4 and N-FBRT4 exhibited no effect on the up-regulated of TGF-β1 protein expression induced by CCl4 (P > 0.05).
Fig. 3.
Effects of N-FBRT and FBRT on proteins expression of α-SMA and TGF-β1(A), Smad2/3 and p-Smad2/3 (B) in liver of rats with liver fibrosis. Bands 1–8 of western-blot images represent the control, CCl4 model, N-FBRT1, N-FBRT2, N-FBRT3, N-FBRT4, FBRT and colchicine groups, respectively. Data expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs CCl4 model group; ΔP < 0.05, ΔΔP < 0.01 vs FBRT group.
4. Discussion
In the present study, the rats’ model of CCl4-induced liver fibrosis was established to investigate the protective effect of N-FBRT with SP as substitute for HP against CCl4-induced liver fibrosis. The results showed that N-FBRT with different dosages of SP as substitute for HP could attenuate CCl4-induced liver fibrosis and improve liver function, as evidenced by reducing liver weight and liver index, lowering serum biochemical markers (ALT, AST, ALP and TBIL), decreasing liver fibrosis biomarkers (HA, LN, PC-III and Col-IV), hepatic Hyp content and collagen deposition, and improving the hepatic morphology and architecture changes. Moreover, N-FBRT1 exerted superior effect against CCl4-induced liver fibrosis based on the analysis of the relative fibrosis area as compared to FBRT. These results indicated that SP could be used as substitute for HP in FBRT for the treatment of liver fibrosis and the anti-liver fibrosis effect was better when the dosage of SP used in N-FBRT was 1/2 of HP in FBRT.
Oxidative stress is caused by an imbalance between oxidative and antioxidant systems, which plays an important role in the formation and development of liver fibrosis (Sun et al., 2019). MDA is the final product of lipid peroxidation and its content reflects the degree of lipid peroxidation, which indirectly represents the level of oxidative injury (Yuan et al., 2018). As important cellular antioxidant enzyme and antioxidant, SOD and GSH can scavenge the harmful free oxygen radicals (Shen et al., 2015). In this study, administration of N-FBRT1 or FBRT significantly increased SOD activity and GSH level, and decreased MDA content. These data suggested that N-FBRT1 or FBRT could eliminate oxidative stress and alleviate oxidative injury by inhibiting lipid peroxidation and restoring the anti-oxidative defense system.
Sustained liver inflammation and its corresponding regenerative wound-healing response is one of the main pathogenesis of liver fibrosis (Zhang et al., 2018). It can drive the maladaptive wound-healing response in the pathogenesis of CCl4-induced liver fibrosis via the activation of Kupffer cells and release of inflammatory cytokines like TNF-α, IL-1β and IL-6 (Zheng et al., 2019). The inflammatory cytokines TNF-α, IL-1β and IL-6 are important inflammatory mediators in the progression of liver injury, liver fibrosis and even cirrhosis, and their over-secretion can activate HSCs and directly induce hepatocyte damage (Kawaratani et al., 2017). Our study showed that administration of N-FBRT1 or FBRT significantly decreased the IL-1β, IL-6 and TNF-α levels in liver of rats with liver fibrosis, which was beneficial for alleviating CCl4-induced liver inflammation.
HSCs activation is the core event in the pathogenesis of liver fibrosis, leading to increased HSCs proliferation as well as overproduction of extracellular matrix proteins (ECMs), particularly collagens (Tian et al., 2019). The protein expression of α-SMA is a sensitive and specific marker for the HSCs activation (Zhang et al., 2018). In the present study, N-FBRT1 or FBRT treatment significantly reduced the collagen deposition and overexpression of α-SMA in the liver. Additionally, treatment with N-FBRT1 or FBRT also markedly down-regulated the mRNA expressions of Col I, Col III, α-SMA, and the protein expression of α-SMA. These results suggested that N-FBRT1 or FBRT can alleviate collagen accumulation accompanied with the inhibition of HSC activation.
TGF-β1/Smad signaling pathway plays a vital role in HSCs activation and fibrosis regulation (Xia et al., 2018). TGF-β1 is the main cytokine activating HSCs and promoting ECMs production (Gong et al., 2020) and HSCs autocrine after the initiation activation is the major source of TGF-β1 production (Tian et al., 2019). Smad2/3 are two crucial intracellular mediators downstream of TGF-β signaling (Wu et al., 2019). TGF-β1 leads to phosphorylation of Smad2/3 and the phosphorylated Smad2/3 (p-Smad2/3) then form a heterotrimeric complex with Smad4 that migrates to the nucleus and modulates transcription of multiple target genes related to liver fibrosis, resulting in excessive production of ECM proteins (Xie et al., 2020, Sun et al., 2019, Xia et al., 2018, Zhou et al., 2016). In our study, the results showed that N-FBRT1 or FBRT not only inhibited TGF-β1 and Smad2/3 proteins expression, but also down-regulated the phosphorylation of Smad2/3 and the mRNA expressions of TGF-β1 in liver of rats with liver fibrosis, suggesting that the protective effect of N-FBRT1 and FBRT against CCl4-induced liver fibrosis is associated with blocking TGF-β1/Smad2/3 signaling pathway.
5. Conclusion
The present study demonstrated that SP could be used as substitute for HP to prepare N-FBRT for the treatment of liver fibrosis and the anti-liver fibrosis effect of N-FBRT was likely to be better when the dosage of SP used in N-FBRT was 1/2 of HP in FBRT. Moreover, the anti-liver fibrosis mechanism of N-FBRT is the same as that of FBRT, which is achieved by eliminating oxidative stress, alleviating inflammation, and inhibiting HSCs activation and ECM production by blocking TGF-β1/Smad signaling pathway.
Author’s Contributions
Hailong Yuan conceived the experiments; Baode Shen and Li Deng designed the experiments; Li Deng, Baode Shen, Yuan Liu, Ruisheng Li, Chengying Shen and Xiao Liu performed the experiments; Li Deng and Baode Shen analyzed and interpreted the data; Yinchao Li performed the histological analysis; Baode Shen drafted the manuscript; All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by Inner Mongolia Science and Technology Key Project of China (2015ZY0024), the Chinese Foundation for Hepatitis Prevention and Control Project (WBE20170066).
References
- Anuja G.I., Shine V.J., Latha P.G., Suja S.R. Protective effect of ethyl acetate fraction of Drynaria quercifolia against CCl4 induced rat liver fibrosis via Nrf2/ARE and NF kappa B signaling pathway. Journal of Ethnopharmacology. 2018;216:79–88. doi: 10.1016/j.jep.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Cai H.B., Sun X.G., Liu Z.F., Liu Y.W., Tang J., Liu Q.…Lv Z.P. Effects of dahuangzhechong pills on cytokines and mitogen activated protein kinase activation in rats with hepatic fibrosis. Journal of Ethnopharmacology. 2010;132(1):157–164. doi: 10.1016/j.jep.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Chen J.M., Yang Y.P., Chen D.Y., Han J., Jin X.Y., Huang Z.X.…Shen Y.M. Efficacy and safety of Fufang Biejia Ruangan tablet in patients with chronic hepatitis B complicated with hepatic fibrosis. Chinese Journal of Experimental and Clinical Virology. 2007;21(4):358–360. [PubMed] [Google Scholar]
- Cheng Q., Li N., Chen M.Q., Zheng J.M., Qian Z.P., Wang X.Y.…Shi G.F. Fuzheng Huayu inhibits carbon tetrachloride-induced liver fibrosis in mice through activating hepatic NK cells. Journal of Ethnopharmacology. 2013;145(1):175–181. doi: 10.1016/j.jep.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2015. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- Deng L., Shen B.D., Liu Y., Liu X., Lian W.Q., Li Y.C., Yuan H.L. Effect of modified formula Compound Biejia Ruangan Pills on hepatic fibrosis in rats induced by CCl4 and its mechanism. Chinese Traditional and Herbal Drugs. 2018;49(6):1371–1378. [Google Scholar]
- Dong Q., Qiu L.L., Zhang C.E., Chen L.H., Feng W.W., Ma L.N.…Xiao X.H. Identification of compounds in an anti-fibrosis Chinese medicine (Fufang Biejia Ruangan Pill) and its absorbed components in rat biofluids and liver by UPLC-MS. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2016;1026:145–151. doi: 10.1016/j.jchromb.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Donnelly L., Campling G. Functions of the placenta. Anaesthesia and Intensive Care Medicine. 2016;17(7):349–353. [Google Scholar]
- Gong Z., Lin J., Zheng J., Wei L., Liu L., Peng Y.…Hu G. Dahuang Zhechong pill attenuates CCl4-induced rat liver fibrosis via the PI3K-Akt signaling pathway. Journal of Cellular Biochemistry. 2020;121(2):1431–1440. doi: 10.1002/jcb.29378. [DOI] [PubMed] [Google Scholar]
- Guo S.G., Zhang W., Jiang T., Dai M., Zhang L.F., Meng Y.C.…Niu J.Z. Influence of serum collected from rat perfused with compound Biejiaruangan drug on hepatic stellate cells. World Journal of Gastroenterology. 2004;10(10):1487–1494. doi: 10.3748/wjg.v10.i10.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Han Y.Q., Wang Y.Z., Gao J.R., Li Y.X., Liu Q., Xia L.Z. Paridis Rhizoma sapoinins attenuates liver fibrosis in rats by regulating the expression of RASAL1/ERK1/2 signal pathway. Journal of Ethnopharmacology. 2016;192:114–122. doi: 10.1016/j.jep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Huang C., Shen D., Sun S., Huang Y., Xin Y., Luo H.…Chen X. Effect of Fufang Biejia Ruangan Tablet on lowering biochemical and virological parameters of hepatic fibrosis in patients with chronic hepatitis B: Protocol for a systematic review and meta-analysis of randomized controlled trials and cohort studies. Medicine (Baltimore) 2019;98(17) doi: 10.1097/MD.0000000000015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu M., Urata Y., Goto S., Ono Y., Li T.S. Placental extract protects bone marrow-derived stem/progenitor cells against radiation injury through anti-inflammatory activity. Journal of Radiation Research. 2013;54(2):268–276. doi: 10.1093/jrr/rrs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaratani H., Moriya K., Namisaki T., Uejima M., Kitade M., Takeda K.…Yoshiji H. Therapeutic strategies for alcoholic liver disease: Focusing on inflammation and fibrosis (Review) International Journal of Molecular Medicine. 2017;40(2):263–270. doi: 10.3892/ijmm.2017.3015. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Ji H.M., Kim D.W., Choi S.M., Kim S., Yang E.J. Effects of Hominis placenta on LPS-induced cell toxicity in BV2 microglial cells. Journal of Ethnopharmacology. 2013;147(2):286–292. doi: 10.1016/j.jep.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Li X.M., Peng J.H., Sun Z.L., Tian H.J., Duan X.H., Liu L.…Hu Y.Y. Chinese medicine CGA formula ameliorates DMN-induced liver fibrosis in rats via inhibiting MMP2/9, TIMP1/2 and the TGF-beta/Smad signaling pathways. Acta Pharmacologica Sinica. 2016;37(6):783–793. doi: 10.1038/aps.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.W., Luo S.T., Yang J., Ren F.Z., Zhao Y., Luo H.L.…Zhang H. The protective effect of sheep placental extract on concanavalin A-induced liver injury in mice. Molecules. 2019;24(1):28. doi: 10.3390/molecules24010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J., Suh H.G., Kim J.H., Jang A., Jung H.J., Lee S.D.…Song H. Immune modulation effect of pig placenta extracts in a mouse model: Putative use as a functional food supplement. Korean Journal for Food Science of Animal Resources. 2011;31(5):701–709. [Google Scholar]
- Park J.Y., Lee J., Jeong M., Min S., Kim S.Y., Lee H.…Park H.J. Effect of Hominis Placenta on cutaneous wound healing in normal and diabetic mice. Nutrition Research and Practice. 2014;8(4):404–409. doi: 10.4162/nrp.2014.8.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Chen H., Shen C., Xu P., Li J., Shen G.…Han J. Hepatoprotective effects of lignans extract from Herpetospermum caudigerum against CCl(4)-induced acute liver injury in mice. Journal of Ethnopharmacology. 2015;164:46–52. doi: 10.1016/j.jep.2015.01.044. [DOI] [PubMed] [Google Scholar]
- Sun X., Huang X., Zhu X., Liu L., Mo S., Wang H.…Lin J. HBOA ameliorates CCl4-incuded liver fibrosis through inhibiting TGF-beta1/Smads, NF-kappaB and ERK signaling pathways. Biomedicine & Pharmacotherapy. 2019;115 doi: 10.1016/j.biopha.2019.108901. [DOI] [PubMed] [Google Scholar]
- Teng D.K., Fang Y., Song X.Y., Gao Y.X. Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food and Bioproducts Processing. 2011;89(C3):202–208. [Google Scholar]
- Tian H., Liu L., Li Z., Liu W., Sun Z., Xu Y.…Peng J. Chinese medicine CGA formula ameliorates liver fibrosis induced by carbon tetrachloride involving inhibition of hepatic apoptosis in rats. Journal of Ethnopharmacology. 2019;232:227–235. doi: 10.1016/j.jep.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen W., Ding H., Li D., Wen G., Zhang C.…Yang Y. Salvianolic acid B exerts anti-liver fibrosis effects via inhibition of MAPK-mediated phospho-Smad2/3at linker regions in vivo and in vitro. Life Sciences. 2019;239 doi: 10.1016/j.lfs.2019.116881. [DOI] [PubMed] [Google Scholar]
- Wu C.H., Chang G.Y., Chang W.C., Hsu C.T., Chen R.S. Wound healing effects of porcine placental extracts on rats with thermal injury. British Journal of Dermatology. 2003;148(2):236–245. doi: 10.1046/j.1365-2133.2003.05164.x. [DOI] [PubMed] [Google Scholar]
- Wu G., He H., Li H., Chen W. Clinical effect of combination therapy with Fufang Biejia Ruangan tablet and entecavir in patients with hepatitis B virus-related cirrhosis. Chinese Journal of Hepatology. 2014;22(8):604–608. doi: 10.3760/cma.j.issn.1007-3418.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Xia Y., Yu B., Ma C., Tu Y., Zhai L., Yang Y.…You P. Yu Gan Long reduces rat liver fibrosis by blocking TGF-beta1/Smad pathway and modulating the immunity. Biomedicine & Pharmacotherapy. 2018;106:1332–1338. doi: 10.1016/j.biopha.2018.07.081. [DOI] [PubMed] [Google Scholar]
- Xie, H., Su, D., Zhang, J., Ji, Mao, J., Hao, M., Wang, Q., Yu, M., Mao, C., & Lu, T. (2020). Raw and vinegar processed Curcuma wenyujin regulates hepatic fibrosis via bloking TGF-beta/Smad signaling pathways and up-regulation of MMP-2/TIMP-1 ratio. Journal of Ethnopharmacology, 246, 111768. [DOI] [PubMed]
- Xu L., Nagata N., Nagashimada M., Zhuge F., Ni Y., Chen G.…Ota T. A porcine placental extract prevents steatohepatitis by suppressing activation of macrophages and stellate cells in mice. Oncotarget. 2018;9(19):15047–15060. doi: 10.18632/oncotarget.24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.R., Fang B.W., Lou J.S. Effects of Fufang Biejia Ruangan pills on hepatic fibrosis in vivo and in vitro. World Journal of Gastroenterology. 2013;19(32):5326–5333. doi: 10.3748/wjg.v19.i32.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N.H., Yuan G.S., Zhou Y.C., Liu J.W., Huang H.P., Hu C.G.…Zhou Y.P. Entecavir combined with Fufang Biejia Ruangan tablet in treatment of chronic hepatitis B patients with liver fibrosis: 96-week efficacy analyses. Journal of Sourthern Medical University. 2016;36(6):775–779. [PubMed] [Google Scholar]
- Yuan X.X., Gong Z.Q., Wang B.Y., Guo X.Y., Yang L., Li D.D., Zhang Y.L. Astragaloside inhibits hepatic fibrosis by modulation of TGF-beta 1/Smad signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2018;2018:3231647. doi: 10.1155/2018/3231647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Schuppan D. Traditional Chinese medicine (TCM) for fibrotic liver disease: Hope and hype. Journal of Hepatology. 2014;61(1):166–168. doi: 10.1016/j.jhep.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Miao H., Yan H.Y., Sheng Y.C., Ji L.L. Hepatoprotective effect of Forsythiae Fructus water extract against carbon tetrachloride-induced liver fibrosis in mice. Journal of Ethnopharmacology. 2018;218:27–34. doi: 10.1016/j.jep.2018.02.033. [DOI] [PubMed] [Google Scholar]
- Zheng H., Wang X., Zhang Y., Chen L., Hua L., Xu W. Pien-Tze-Huang ameliorates hepatic fibrosis via suppressing NF-kappaB pathway and promoting HSC apoptosis. Journal of Ethnopharmacology. 2019;244 doi: 10.1016/j.jep.2019.111856. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Tong X., Ren S., Wang X., Chen J., Mu Y.…Liu P. Synergistic anti-liver fibrosis actions of total astragalus saponins and glycyrrhizic acid via TGF-beta1/Smads signaling pathway modulation. Journal of Ethnopharmacology. 2016;190:83–90. doi: 10.1016/j.jep.2016.06.011. [DOI] [PubMed] [Google Scholar]