Abstract
Syringa oblata is a traditional Mongolian medicine mainly distributed in the Helan Mountains (the boundaries of Inner Mongolia and Ningxia, China) and the north of Yan Mountains (Aohan Qi, Inner Mongolia, China). It is clinically used to treat diseases caused by Heyi, such as heartache and heat pathogen in the heart. Phytochemical studies on S. oblata revealed the presence of iridoids, lignans, triterpenes, phenylpropanoids, phenylethanoids, and volatile components. Pharmacological investigations revealed a broad spectrum of bioactivities, such as antimicrobial, antioxidant, antiproliferative, and hepatoprotective effects. This article summarized the chemical components and pharmacological activities of S. oblata, providing a scientific rationale for its bioactive constituents, quality control, and utilization as an important medicine.
Keywords: chemical constituent, heartwood, Mongolian medicine, pharmacological activity, Syringa oblata Lindl.
1. Introduction
Syringa oblata Lindl. is a species belonging to the Oleaceae family and its heartwood is used as a traditional Mongolian medicine named A-La-Ge-A-Ga-Ru (Inner Mongolia Mongolian Medicine Standard, 2015). S. oblata has several synonyms in China, such as Zidingxiang, Dingxiang, and Huabei Dingxiang in Chinese and Gao-Li-De-Bao-Ri in Mongolian. S. oblata and S. oblata Lindl. var. alba Rehderis are recorded as medicines in Herbal Materials of Inner Mongolia, with the spicy and bitter flavor and the nature of cold in Chinese medicine. Due to their pharmacological effects, such as clearing heat and inhibiting Heyi, pain, and cough, they are clinically used to treat heat pathogens in the heart, heartache, head dizziness, insomnia, palpitation, asthma, and other diseases caused by Heyi (Food and Drug Administration of Inner Mongolia Autonomous Region, 2015, Wu et al., 2014, Zhao et al., 2020). S. oblata is distributed in East Asia, such as China and Korea (Wu et al., 2014, Liu, 1996). In China, it is widely distributed from the Northeast to the Southwest, including Shandong, Shanxi, and Sichuan provinces. Natural resources are rich in the Helan Mountains and the north of Yan Mountains (Dahei Mountain in Aohan Qi, Inner Mongolia). Usually, their habits are shaded foothills or valleys with an altitude of 2000 m.
There are over 400 species belonging to 28 genera in Oleaceae family. Among them, about 20 are from Syringa genus. To our best known, at least 16 species, including 10 endemic species, are distributed in Southwest and North China (Jilin Provincial Health Bureau, 1997, Jilin Institute of Traditional Chinese Medicine, 1982). In Inner Mongolia, there are six Syringa species including two cultivators.
S. oblata is a medicinal, edible, and ornamental plant with multiple values. As a well-known ornamental plant, it is usually cultivated in parks, roadsides, and homegardens. Due to its potent ability to clear SO2, it is also widely planted as an eco-friendly plant in urban areas (Jiangsu Institute of Botany, 1990). In addition, for edible purposes, the tender leaves are made into herbal teas and the flowers are raw materials for producing essential oils. Different plant parts including leaves, barks, flower buds, seeds, roots, and heartwoods of S. oblata have been recorded as traditional medicines in Chinese and Mongolian medicinal documents as well. In the Drug Standard of Jilin Province, the leaf is used as a hepatoprotective and anti-dysentery medicine with the flavor of bitter and nature of cold, for treating bacterial dysenteries and infectious hepatitis (Xing, 2006). The leaves and barks are both recorded as folk medicines for treating diarrhea and hepatitis in Herbal Materials in Changbai Mountain (Gao, 2010). In Xinhua Bencao Gangyao, S. oblata leaves are recorded as Chinese medicines with a spicy flavor and a warm nature, which are used for detoxification, clearing heat, and inhibiting inflammation. Clinical uses with an external application include treating acute icteric hepatitis, microbial infections, conjunctivitis, sores ulceration, and inflamed disorders (Ma, 1980). In Chinese Medicines of Ningxia, similar medicinal uses are documented, and the leaves and barks are both recorded as medicinal materials (Cui, Gao, & Liu, 2009). In Medicinal Plants of Chifeng, the roots and heartwoods, possessing spicy and bitter flavors and the nature of cold, are used as Mongolian medicines for treating diseases such as heat pathogens in the heart, heartache, dizziness, insomnia, palpitation, and asthma (Alashan Medicinal Plants Color Atlas, 2016). The flower buds, leaves, and seeds are recorded as medicines in Chinese Medicines of Liaoning. With a bitter flavor and being cold in nature, they have pharmacological effects including clearing heat, detoxification, and anti-diarrhea activities and are used for treating dysentery, enteritis, upper respiratory tract infection, tonsillitis, and hepatitis (Mengke, 2018). Records describing the medicinal uses of S. oblata are similar in other books including Color Atlas of Medicinal Plant in Alashan and Illustrated Book of Medicines in Wushenqi (Su et al., 2015, Zhang and Zhang, 2007).
S. oblata is often used as a substitute for agarwood in Alashan, Inner Mongolia. In Mongolian medicine, it is also used as a substitute for agarwood or S. pinnatifolia in A-Ga-Ru prescriptions, which are used for treating heartache and disorders caused by Heyi. For example, when used as an ingredient in the Mongolian prescription Chenxiang Anshen San as a substitute for S. pinnatifolia, it showed potent pharmacological effects in clinic. Recent phytochemical studies on the leaves, barks, seed coats, stems, twigs, flowers, and flower buds of S. oblata have been conducted. These raw plant materials were usually extracted by methanol, ethanol, or aqueous. Leaves and flowers were also made into essential oils. Chemical profiling of these extracts was conducted by various chromatographic methods including silica gel, Sephadex LH-20, macroporous resin, HPLC, and GC coupled with multiple spectroscopic analyses, such as ultraviolet, nuclear magnetic resonance, and mass spectra. Results revealed the presence of iridoids, iridoid glycosides, lignans, triterpenoids, phenylpropanoids, phenylethanoids, flavonoids, and others (Jilin Provincial Health Bureau, 1997, Yu et al., 2016, Ma et al., 2020, Li et al., 2018b, Li et al., 2018a, Gao et al., 2020). Pharmacological investigations revealed the antimicrobial, antioxidant, antiproliferative, and hepatoprotective activities of extracts from S. oblata leaves and flower buds. In the current paper, the phytochemical and pharmacological research of S. oblata were summarized, providing an up-to-date review, a scientific rationale for the medicinal uses of S. oblata, and a beneficial reference for future studies on the quality control, clinical uses, and utilization of Mongolian medicinal products.
2. Chemical constituents of S. oblata
Previous studies showed that phytochemicals present in the leaves, barks, seed coats, stems, twigs, flowers, and flower buds of S. oblata are iridoids, lignans, triterpenoids, phenylpropanoids, phenylethanoids, phenolic acids, flavonoids, and others. Those compounds reported from S. oblata include 22 iridoids, 14 lignans, 19 triterpenoids, 25 phenols, seven flavonoids, and 10 others.
2.1. Iridoids
Iridoids are monoterpenoids derived from iridodial, in the general form of cyclopentanopyranl. They are a group of medicinally valuable metabolites that are found in a wide variety of plants, especially in dicotyledons (Wu et al., 2002, Wu, 2005). Since 1958 when the basic core of iridoids was firstly identified, numerous researchers had focused on it. Up to now, over 1000 iridoids have been reported and the number is still increasing. Notably, many of them were found to have potent bioactivities, such as protecting the cardiovascular system, regulating the immune system, and antitumor, antivirus, hepatoprotective, choleretic, antidiabetic, antihyperlipidemic, anti-inflammatory, anticoagulant, antioxidant, antispasmodic, laxative, and neuroprotective effects (Ma, Tian, Zhang, & Xu, 2008; Xing et al., 2009, Wang et al., 2019, Kong et al., 2021).
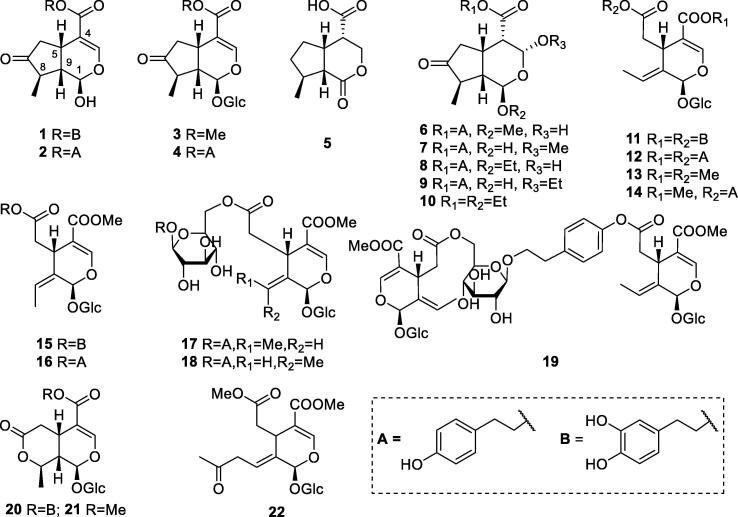
The presence of iridoids in S. oblata was firstly reported in 1982, isolating and identifying syringopicroside (4) (Wang et al., 1982). It was reported that there were two dominant conformations, and the coupling constant of H-1 and H-9 (J < 3 Hz or J = 7–10 Hz) was corresponding to the dihedral angle of H-1 and H-9 (60° or 180°, respectively). (8E)-gstroside (16), which was previously reported from S. vulgaris L. and S. pubescens subsp. patula (Palib.) M. C. Chang & X. L. Chen, was isolated from the EtOAc fraction of EtOH extract of S.oblata barks for the first time (Zhang, Zhang, & Wang, 2006). Wang et al. (2010) conducted a phytochemical study on the EtOAc extract of S.oblata seed coats. Syringopicrogenin A (6), along with syringopicrogenin B (1), syringopicroside (4), oleuropein (15), and (8E)-ligstroside (14) were isolated and identified, indicating that compounds 4 and 15 also existed in seed coats. Syringopicrogenin B (1) was also found in the EtOAc extract of S.oblata flower buds (Dong, Wang, Zhao, & Zhang, 2011). In continuous phytochemical studies on the EtOH extract of S. oblata leaves, syringopicrogenin C (7) and 7β-d-glucopyranosyl-11-methyloleoside (22) were reported from the n-BuOH portion (Zhang, Chen, Li, & Yang, 2011). A new iridoid syringopicrogenin F (10) was reported from the EtOAc portion (Zhang, Li, Li, Wang, & Zhao, 2014). Then, another four iridoids from S.oblata leaves were found for the first time in 2018, including syringopicroside B (2), (8E)-ligstroside B (11), (8E)-ligstroside A (12), and fliederoside B (3) (Zhang et al., 2018). Other iridoids reported from S. oblata leaves are 5 (Li, Xu, Hao, Yang, & Li, 2009), 8 and 9 (Zhao et al., 2016), 13 (Zhang et al., 2018), 20 (Wang, 2008), and 21 (Tian, Li, Lv, Zhang, & Liu, 2013). Compound 5 has a chiral center at C-4 and does not possess the carbonyl group at C-7, which is different from other typical iridoids in S. oblata. Detailed information on these isolates was listed in Table 1 and Fig. 1.
Table 1.
Iridoids isolated from S. oblata (1–22).
| No. | Compounds | Plant parts | References |
|---|---|---|---|
| 1 | Syringopicrogenin B | Seed coats, flower buds, leaves | Wang et al., 2010, Dong et al., 2011, Zhang et al., 2018 |
| 2 | Syringopicroside B | Leaves | Zhang et al., 2018 |
| 3 | Fliederoside B | Leaves | Zhang et al., 2018 |
| 4 | Syringopicroside A | Leaves | Wang, 2008 |
| 5 | 7-Methyl-1-oxo-octahydro-cyclopenta[c]pyran-4-carboxylic acid | Leaves | Li, Xu, Hao, Yang, & Li, 2009 |
| 6 | Syringopicrogenin A | Seed coats, leaves |
Wang et al., 2010, Han, 2012, Zhang et al., 2018 |
| 7 | Syringopicrogenin C | Leaves | Han, 2012, Zhang et al., 2018 |
| 8 | Syringopicrogenin D | Leaves | Han, 2012, Zhao et al., 2016, Zhang et al., 2018 |
| 9 | Syringopicrogenin E | Leaves | Han, 2012, Zhao et al., 2016, Zhang et al., 2018 |
| 10 | Syringopicrogenin F | Leaves | Zhang et al., 2018 |
| 11 | (8E)-Ligstroside B | Leaves | Zhang et al., 2018 |
| 12 | (8E)-Ligstroside A | Leaves | Zhang et al., 2018 |
| 13 | 7-Dehydrologanin | Leaves | Zhang et al., 2018 |
| 14 | (8E)-Ligstroside | Seed coats, leaves, twigs |
Wang et al., 2010, Zhao et al., 2012, Zhang et al., 2018 |
| 15 | Oleuropein | Barks, twigs, leaves |
Zhang, Zhang, & Wang, 2006; Zhao et al., 2012, Zhang et al., 2018 |
| 16 | (8E)-Gstroside | Barks | Zhang, Zhang, & Wang, 2006 |
| 17 | 8E-Nüzhenide | Seeds | Zhang, Guo, Han, Zhao, & Wang, 2011 |
| 18 | 2-(p-Hydroxyphenyl)-ethyl-2,6-bis(2S,3E,4S)-3-ethylidene-2-(β-d-glucopyranosyloxy)-3,4-dihydro-5-(methoxycarbonyl)-2H-pyran-4-acetate | Seeds | Zhang, Guo, Han, Zhao, & Wang, 2011 |
| 19 | 4-O-11-Methyloleoside-p-hydroxyphenyl-(6–11-methyloleoside)-β-d-glucopyranoside | Seeds | Zhang, Guo, Han, Zhao, & Wang, 2011 |
| 20 | Syringalactone B | Leaves | Wang, 2008 |
| 21 | 2-(3,4-Dihydroxyphenyl)ethyl(1R,4aS,8R,8aS)-8-methyl-6-oxo-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethy)oxan-2-yloxy-4a,5,8,8a-tetrahydro-1H-pyrano[3,4-c]pyran-4-carboxylate | Leaves | Tian, Li, Lv, Zhang, & Liu, 2013 |
| 22 | 7β-d-Glucopyranosyl-11-methyloleoside | Seeds | Zhang, Guo, Han, Zhao, & Wang, 2011 |
Fig. 1.
Structures of iridoids in S. oblata (1–22).
Iridoids are a group of major components of S. oblata leaves. Among them, three iridoids syringopicroside (32.63 mg/g), oleuropein (28.43 mg/g), and ligstroside (15.83 mg/g) from the 75% ethanol extract of S. oblata leaves were characterized as the major compounds by an HPLC-MS-based quantitative analysis, and the structures of these major compounds were further confirmed by NMR data (Zhu et al., 2021). An HPLC-MS-based chemical profiling of S. oblata var. alba leaves also revealed the major compounds including syringopicroside, oleuropein, and ligstroside (Nenadis, Vervoort, Boeren, & Tsimidou, 2007).
2.2. Lignans
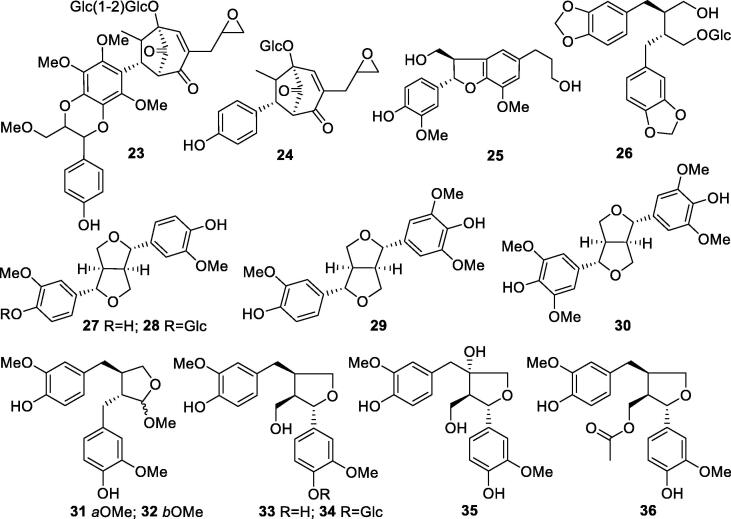
Lignans are another major group of chemical constituents from S. oblata. Lignans are derived from two monolignols, which possess a C6-C3 core. And the coupling of the monolignols usually occurs at C-8 (Wu, 2010). The name derives from the Latin word for “wood”. They are widely found in roots, rhizomes, stems, leaves, flowers, fruits, heartwoods, and resins of plants, especially rich in heartwood and resins (Feng, 2007). Due to the high structural diversity of lignans, they showed multiple biological activities including antivirus, antitumor, antioxidant, anticoagulant, antidiabetic, and anti-inflammatory effects (Zhang et al., 2007, Wu et al., 2021).
Syringa oblata ligninosides A and B (23, 24) are two macrophyllin-type bicyclo[3.2.1]octanoid neolignans reported from aqueous extract of S. oblata leaves, which belong to a group of bioactive compounds showing platelet-activating factor antagonistic activities (Wei, 2004). A dihydrobenzofuran lignan named (7R,8S)-4,9,9′-trihydroxyl-3,3′-dimethoxyl-7,8-dihydrobenzofuran-1′-propylneolignan (25), a lignan glycoside 3,4:3′,4′-bis(methylene-dioxy)-9-hydroxyl-lignane-9-methyl-O-β-d-glucopyranoside (26), and lariciresinol-4-O-β-d-glucopyranoside (34) were also isolated from S. oblata leaves (Zhao et al., 2016, Zhou, 2005, Tian et al., 2013). The EtOAc extracts of S. oblata seed coats and twigs were studied in 2010, leading to the isolation of (+)-syringaresinol (30) (Wang et al., 2010, Zhao et al., 2012). Another four typical C-8–C-8′ connected lignans, (9R)-9-O-methylcubebin (31), (9S)-9-O-methylcubebin (32), 4,4′,8,9-tatrahydroxyl-3,3′-dimethyoxyl-7,9′-monoepoxylignin (35), and lariciresinol acetate (36), and a neolignan 4,4′-dihydroxyl-3,3′,5-trimethyoxyl bisepoxylignan (29) were found from the EtOAc and n-BuOH fractions of EtOH extract of S. oblata twigs (Zhao, Han, Lv, & Zhang, 2012). Detailed information about lignans in S. oblata was described in Table 2 and Fig. 2.
Table 2.
Lignans isolated from S. oblata (23–36).
| No. | Compounds | Plant parts | References |
|---|---|---|---|
| 23 | Syringa oblata ligninoside B | Leaves | Wei, 2004 |
| 24 | Syringa oblata ligninoside A | Leaves | Wei, 2004 |
| 25 | 7R,8S-4,9,9′-Trihydroxyl-3,3′-dimethoxyl-7,8-dihydrobenzofuran-1′-propylneolignan | Leaves | Zhao et al., 2016 |
| 26 | 3,4:3′,4′-bis(Methylene-dioxy)-9′-hydroxyl-lignane-9-methyl-O-β-d-glucopyranoside | Leaves | Zhou, 2005 |
| 27 | (+)-Pinoresinol | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 28 | (+)-Pinoresinol-4″-O-β-d-glucopyranoside | Leaves | Tian, Li, Lv, Zhang, & Liu, 2013 |
| 29 | 4,4′-Dihydroxyl-3,3′,5-trimethyoxyl bisepoxy lignan | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 30 | (+)-Syringaresinol | Twigs, seed coats | Wang et al., 2010, Zhao et al., 2012 |
| 31 | (9R)-9-O-Methylcubebin | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 32 | (9S)-9-O-Methylcubebin | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 33 | Lariciresinol | Twigs, leaves | Zhao et al., 2012, Zhang et al., 2018 |
| 34 | Lariciresinol-4-O-β-d-glucopyranoside | Leaves | Tian, Li, Lv, Zhang, & Liu, 2013 |
| 35 | 4,4′,8,9-Tatrahydroxyl-3,3′-dimethyoxyl-7,9′-monoepoxylignin | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 36 | Lariciresinol acetate | Leaves | Zhang, Li, Li, Wang, & Zhao, 2014 |
Fig. 2.
Structures of lignans in S. oblata (23–36).
According to the previous phytochemical studies on S. oblata and other Syringa species, lignans are the major group of compounds from Syringa plants, especially the leaves, roots, and stems (Zhu et al., 2021). However, the stems and roots of S. oblata, which are used as medicinal parts in Mongolian medicine, have rarely been studied. Phytochemical research on lignans from this Mongolian medicine is necessary for revealing its therapeutic constituents.
2.3. Triterpenoids
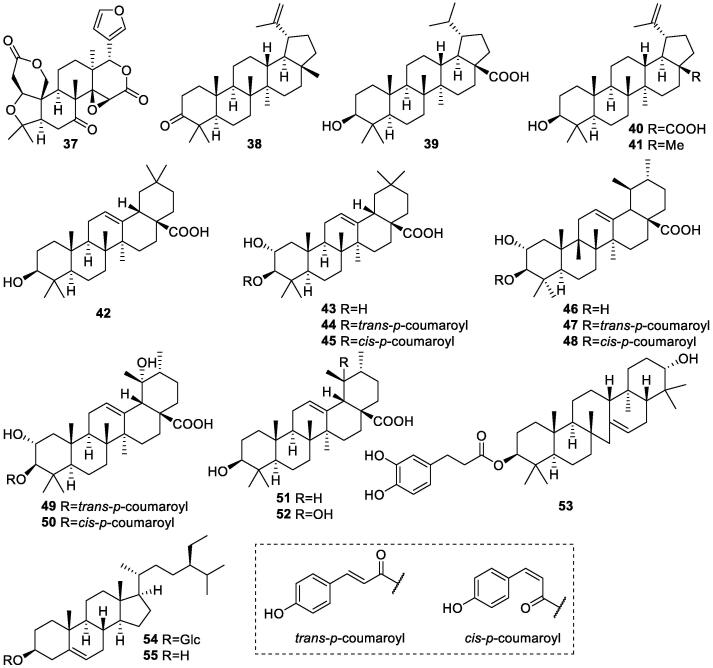
Obaculactone (37), also known as limonoic acid di-delta-lactone, is the first triterpenoid found in the aqueous extract of S. oblata leaves (Lu, Li, & Li, 2003). It is a member of furanolactones with a bitter flavor and a broad spectrum of bioactivities. Then, a series of triterpenoids were found from different plant parts of S. oblata. Three compounds, lupanic acid (39), oleandic acid (42), and daucosterol (54), were firstly reported from the EtOH extract of S. oblata barks in 2006 (Zhang, Zhang, & Wang, 2006). From the EtOAc extract of S. oblata flower buds, four triterpenoids including oleandic acid (42), ursolic acid (51), lupanic acid (39), and luprol (41) were isolated and identified (Dong, Wang, Zhao, & Zhang, 2011). In continuous phytochemical studies on S. oblata leaves, from the dichloromethane and EtOAc fractions of EtOH extract, 19α-hydroxyl ursolic acid (52) was found for the first time in 2009 (Li, Xu, Hao, Yang, & Li, 2009); from the EtOAc extract, 21α-hydroxy-serrat-14-en-3β-yl-dihydrocaffeate (53) was found in the n-BuOH fraction (Zhang, Chen, Li, & Yang, 2011). These above-mentioned compounds were shown in Table 3 and Fig. 3.
Table 3.
Triterpenoids isolated from S. oblata (37–55).
| No. | Compounds | Plant parts | References |
|---|---|---|---|
| 37 | Obaculactone | Leaves | Lu, Li, & Li, 2003 |
| 38 | Lup-20(29)-en-3-one | Leaves | Wei, 2004 |
| 39 | Lupanic acid | Barks, flower buds | Zhang, Zhang, & Wang, 2006; Zhang, Chen, Li, & Yang, 2011 |
| 40 | Betulinic acid | Leaves | Li et al., 2009, Zhang et al., 2018 |
| 41 | Luprol | Flower buds | Dong, Wang, Zhao, & Zhang, 2011 |
| 42 | Oleandic acid | Barks, leaves, flower buds | Zhang, Zhang, & Wang, 2006; Dong et al., 2011, Cao, 2017, Zhang et al., 2018, Li et al., 2019a |
| 43 | Maslinic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 44 | 3-O-trans-p-Coumaroyl maslinic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 45 | 3β-O-cis-p-Coumaroyl maslinic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 46 | 2α-Hydroxy ursolic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 47 | 3β-O-trans-p-Coumaroyloxy-2α-hydroxyurs-12-en-28-oic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 48 | 3β-O-cis-p-Coumaroyloxy-2α-hydroxyurs-12-en-28-oic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 49 | 3β-O-trans-p-Coumaroyl tormentic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 50 | 3β-O-cis-p-Coumaroyl tormentic acid | Leaves | Zhang, Chen, Li, & Yang, 2011 |
| 51 | Ursolic acid | Leaves, flower buds | Li et al., 2009, Zhang et al., 2011, Han, 2012, Zhang et al., 2018, Li et al., 2019a |
| 52 | 19α-Hydroxy ursolic acid | Leaves | Li, Xu, Hao, Yang, & Li, 2009 |
| 53 | 21α-Hydroxy-serrat-14-en-3β-yl-dihydrocaffeate | Seeds | Zhang, Guo, Han, Zhao, & Wang, 2011 |
| 54 | Daucosterol | Barks, leaves, Twigs | Zhang, Zhang, & Wang, 2006; Li et al., 2009, Zhao et al., 2012 |
| 55 | β-Sitosterol | Leaves, flower buds | Zhou, 2005; Wang, 2008; Dong, Wang, Zhao, & Zhang, 2011 |
Fig. 3.
Structures of triterpenoids in S. oblata (37–55).
Most of the triterpenoids found in S. oblata are pentacyclic triterpenoids. Many of them including compounds 40, 51, and 54 were reported to have antitumor, antiviral, and anti-inflammatory activities. However, they may not be the unique bioactive compounds in S. oblata, because they are widely distributed in the barks of many plant species. Those triterpenoids substituted by coumaroyls may need to be deeply studied for explaining if they contribute to the pharmacological effects of S. oblata.
2.4. Simple phenols
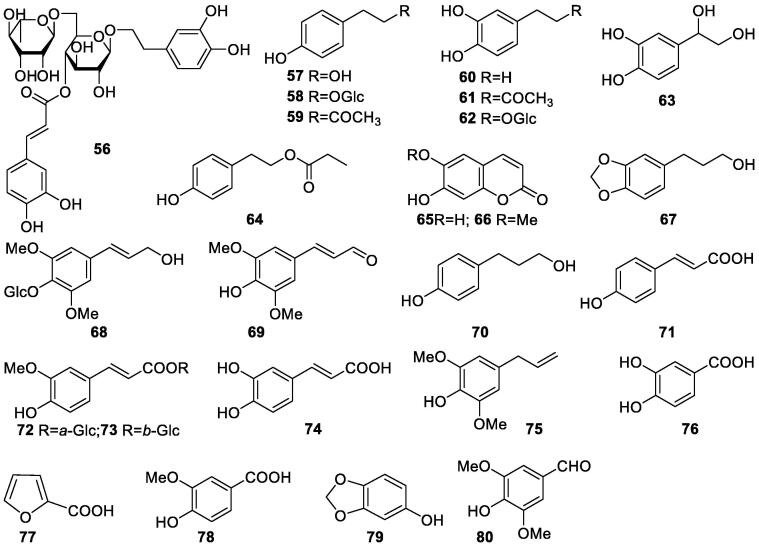
Simple phenolic compounds include phenylpropanoids, phenylethanoids, coumarins, and other phenolics with only one benzene ring. They are a group of precursors contributing to the structural diversity of iridoids, lignans, and triterpenoids in S. oblata. Five compounds including three phenylpropanoids (61, 68, 69) and two simple phenolic derivates (79 and 80) were found in twigs of S. oblata (Zhao, Han, Lv, & Zhang, 2012). A phytochemical study on the EtOH extract of S. oblata barks resulted in the isolation and identification of two phenylethanoids, 3,4-dihydroxy phenylethano1 (60) and 2-(3,4-dihydroxy)phenyl ethyl acetate (62) (Zhang, Zhang, & Wang, 2006). In another study on the EtOAc extract of S. oblata barks, compounds 60, 3,4-dihydroxylphenylethanolglucoside (82), and hydroxylphenylethanolglucoside (58) were found (Zhang, Jiao, Wang, & Zhang, 2007). From the EtOAc extract of S. oblata seed coats, compounds 58, 60, and p-hydroxyphenyl ethyl acetate (59) were reported (Wang, Zhang, Dong, Zhao, & Zhang, 2010). From the aqueous extract of S. oblata leaves, 59 and 3,4-dihydroxybenzene-styrene glycol (63) were isolated and identified (Han, 2012, Tian et al., 2013). Verbascoside (56) is a caffeoyl phenylethanoid glycoside, in which the phenylpropanoid caffeic acid and the phenylethanoid hydroxytyrosol form an ester and an ether bond respectively, to the rhamnose part of a disaccharide (Andary, Wylde, Laffite, Privat, & Winternitz, 1982). Several studies on the S. oblata leaves revealed the presence of 56 and other 12 phenolic constituents (Zhang et al., 2018, Wang et al., 1982, Zhou, 2005, Li et al., 2009). Simple phenolics reported from S. oblata were described in Table 4 and Fig. 4.
Table 4.
Phenolic constituents isolated from S. oblata (56–80).
| No. | Compounds | Plant parts | References |
|---|---|---|---|
| 56 | Verbascoside | Leaves | Zhang et al., 2018 |
| 57 | p-Hydroxyphenylethyl alcohol | Leaves | Wang et al., 1982, Zhou, 2005 |
| 58 | p-Hydroxylphenylethanolglucoside | Seed coats, barks | Wang et al., 2010, Zhang et al., 2007 |
| 59 | p-Hydroxyphenyl ethyl acetate | Seed coats, leaves | Wang et al., 2010, Han, 2012 |
| 60 | 3,4-Dihydroxyl benzene ethyl alcohol | Leaves, barks, seed coats, flowers | Wang et al., 1982; Zhang, Zhang, & Wang, 2006; Wang et al., 2010, Zhang et al., 2018, Zhang et al., 2007 |
| 61 | 2-(3,4-Dihydroxy)phenyl ethyl acetate | Barks, leaves, twigs | Zhang, Zhang, & Wang, 2006; Han, 2012, Zhao et al., 2012 |
| 62 | 3,4-Dihydroxylphenylethanolglucoside | Barks | Zhang, Jiao, Wang, & Zhang, 2007 |
| 63 | 3,4-Dihydroxybenzene-styrene glycol | Leaves | Tian, Li, Lv, Zhang, & Liu, 2013 |
| 64 | p-Hydroxyphenylethyl propyl ester | Seeds | Zhang, Guo, Han, Zhao, & Wang, 2011 |
| 65 | Esculetin | Barks | Zhang, Zhang, & Wang, 2006 |
| 66 | 7-Hydroxy-6-methoxy-2H-chromen-2-one | Leaves | Li, Xu, Hao, Yang, & Li, 2009 |
| 67 | 1,3-Benzodioxole-5-propanol | Leaves | Zhang et al., 2018 |
| 68 | Syringin | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 69 | 3,5-Dimethyoxyl-4-hydroxyl cinnamaldehyde | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 70 | p-Hydroxy benzene propyl alcohol | Flower buds, leaves | Dong et al., 2011, Zhang et al., 2018 |
| 71 | trans-p-Hydroxycinnamic acid | Leaves | Wang et al., 1982 |
| 72 | 6-O-(E)-feruloyl-(α)–glucopyranoside | Leaves | Tian, Li, Lv, Zhang, & Liu, 2013 |
| 73 | 6-O-(E)-feruloyl-(β)-glucopyranoside | Leaves | Tian, Li, Lv, Zhang, & Liu, 2013 |
| 74 | Caffeic acid | Flowers | Cao, 2017 |
| 75 | Dictamnoside A | Flowers | Cao, 2017 |
| 76 | 3,4-Dihyroxybenzoic acid | Leaves | Wang et al., 1982, Zhou, 2005 |
| 77 | 2-Furancarboxylic acid | Leaves | Zhou, 2005, Lu et al., 2003 |
| 78 | 4-Hydroxy-3-methoxybenzoic acid | Leaves | Li, Xu, Hao, Yang, & Li, 2009 |
| 79 | Sesamol | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
| 80 | 3,5-Dimethoxy-4-hydroxybenzaldehyde | Twigs | Zhao, Han, Lv, & Zhang, 2012 |
Fig. 4.
Structures of phenols in S. oblata (56–80).
2.5. Flavonoids
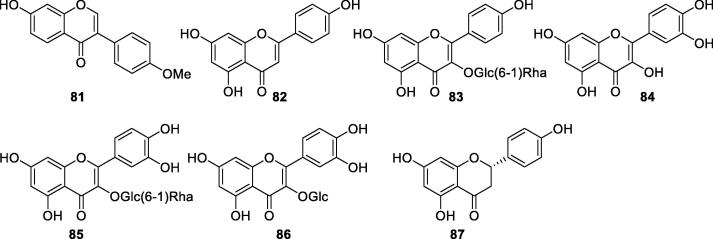
Phytochemicals in the aqueous extract of S. oblata leaves were studied by (Lu, Li, & Li, 2003), leading to the isolation of a flavonoid named formonometin (81). Another study on the dichloromethane and EtOAc fractions from EtOH extract of S. oblata leaves reported the presence of 5,7,4′-trihydroxyl flavanone (82), which was also found in S. oblata twigs (Li et al., 2009, Zhao et al., 2012). Five flavonoids, kaempferol-3-O-α-l-rhamnosyl-(1 → 6)-β-d-glucoside (83), quercetin (84), rutin (85), quercetin-3-O-β-d-glucoside (86), and naringenin (87) were isolated and identified from the S. oblata leaves (Bai et al., 2016, Cao, 2017). Detailed information on the flavonoids was shown in Table 5 and Fig. 5.
Table 5.
Flavonoids isolated from S. oblata (81–87).
| No. | Compounds | Plant parts | References |
|---|---|---|---|
| 81 | Formonometin | Leaves | Zhou, 2005, Lu et al., 2003 |
| 82 | 5,7,4′-Trihydroxyl flavanone | Leaves, twigs | Li et al., 2009, Zhao et al., 2012 |
| 83 | Kaempferol-3-O-α-l-rhamnosyl-(1 → 6)-β-d-glucoside | Flowers | Bai et al., 2016, Cao, 2017, Cui et al., 2019 |
| 84 | Quercetin | Flowers | Cao, 2017, Cui et al., 2019 |
| 85 | Rutin | Flowers | Cao, 2017, Cui et al., 2019 |
| 86 | Quercetin-3-O-β-d-glucoside | Flowers | Cao, 2017, Cui et al., 2019 |
| 87 | Naringenin | Flowers | Cao, 2017, Cui et al., 2019 |
Fig. 5.
Flavonoids from S. oblata (81–87).
Different from the iridoids, triterpenoids, and lignans which were mainly found in S. oblata leaves, these flavonoids were mostly isolated from flowers. However, rutin was characterized as a bioactive compound with antimicrobial effects in S. oblata leaves, with a concentration ranging from 0.175 to 0.216 mg/mL (Liu et al., 2018). It deserves a further phytochemical study on the flavonoids from S. oblata leaves, barks, and other medicinal parts.
2.6. Other compounds
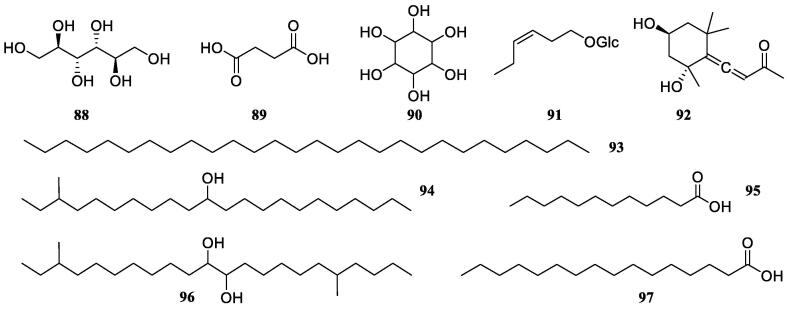
Except for the abovementioned five groups of phytochemicals, several other types of compounds were reported from S. oblata, including fatty acids and polyhydric alcohols. A phytochemical study on the EtOAc and n-BuOH fractions from the aqueous extract of S. oblata leaves resulted in the isolation and identification of d-mannitol (88) (Wang et al., 1982). In another study on the aqueous extract of S. oblata leaves, (Lu, Li, & Li, 2003) found succinic acid (89). Also, from the aqueous extract of S. oblata leaves, cyclohexanehexol (90) was reported in the genus Syringa (Zhou, 2005). Another two compounds, 3(Z)-enol glucoside (91) and grasshopper ketone (92) were first isolated from S. oblata leaves (Zhang et al., 2018). From the EtOAc and n-BuOH fractions of EtOH extract of S. oblata barks, palmitic acid (97) and lauric acid (95) were isolated, resulting in the first report of 97 in the genus Syringa (Cao, 2017). Other classes of compounds from S. oblata were described in Table 6 and Fig. 6.
Table 6.
Other classes of compounds reported from S. oblata (88–97).
| No. | Compounds | Plant parts | References |
|---|---|---|---|
| 88 | d-Mannitol | Leaves | Wang et al., 1982, Zhou, 2005, Lu et al., 2003 |
| 89 | Succinic acid | Leaves | Zhou, 2005, Lu et al., 2003 |
| 90 | Cyclohexanehexol | Leaves | Zhou, 2005 |
| 91 | 3(Z)-Enol glucoside | Leaves | Zhang et al., 2018 |
| 92 | Grasshopper ketone | Leaves | Zhang et al., 2018 |
| 93 | Nonacosane | Leaves | Wei, 2004 |
| 94 | 4-Methyl-12-tricosanol | Leaves | Wei, 2004 |
| 95 | Lauric acid | Flowers | Cao, 2017, Cui et al., 2019 |
| 96 | 4,19-Dimethyl-12,13-dihydroxy-docosane | Leaves | Wei, 2004 |
| 97 | Palmitic acid | Flowers | Cao, 2017, Cui et al., 2019 |
Fig. 6.
Structures of other compounds in S. oblata (88–97).
Essential oils from the leaves, flowers, and stems of S. oblata were chemically profiled by several researchers, using GC–MS-based metabolomics. In the essential oil of S. oblata leaves, eugenol (40.43%) was found to be the dominant component. Eugenol acetate (28.78%) was the second major component, followed by β-caryophyllene (21.99%) and α-caryophyllene (3.46%) (Jing et al., 2018). Terpenes and oxygenated terpenes, aromatic compounds, a series of alkanes, and heterocyclic compounds were characterized as the main components of S. oblata flowers and buds (Zhao, Liang, Fang, & Li, 2005). In another GC–MS-based study, the variation of volatile compounds emitted from S. oblata var. alba flowers in different florescence stages was investigated (Li, Lee, & Shen, 2006). Results revealed that lilac aldehydes A–D, lilac alcohols A–D, α-pinene, sabinene, β-pinene, myrcene, d-limonene, eucalyptol, cis-ocimene, benzaldehyde, terpinolene, linalool, benzene acetaldehyde, α-terpineol, p-methoxyanisole, p-anisaldehyde, (Z,E)-α-farnesene and (E,E)-α-farnesene were the most abundant volatiles released from fresh flowers and may contribute to the scent of fresh flowers. In our previous study on essential oils from S. oblata stems, 46 chemical components were identified by GC–MS (Gegen et al., 2022). α-Cadinol (15.65%), T-cadinol (11. 68%), and isocalamenediol (7.11%) were the top three major compounds. The major compounds from essential oils of leaves, flowers, and stems of S. oblata are significantly different. It may help to explain the different medicinal uses of these plant parts. Moreover, S. pinnatifolia, a famous Mongolian medicine and a botanical relative of S. oblata, has been well studied on its stems. Sesquiterpenoids are a specific group of compounds in S. pinnatifolia that were confirmed to be bioactive compounds contributing to its cardiac protective effects (Li et al., 2022). However, these compounds have not been studied in S. oblata. A further study on the volatile components and sesquiterpenoids from S. oblata stems may help to reveal scientific evidence of its traditional use in Mongolian medicine.
3. Pharmacological effects of S. oblata
S. oblata has shown potent medicinal properties including antimicrobial, antioxidant, antitumor, hepatoprotective, and choleretic activities, drawing the interest of many researchers.
3.1. Antimicrobial activities
The antibacterial activities of dichloromethane, EtOAc, and n-BuOH fractions of the EtOH-aqueous extract of S. oblata leaves were evaluated. Results showed that the EtOAc fraction had the strongest activity. A further bioactivity-guided fractionated afforded five compounds possessing inhibitory effects against Staphylococcus aureus, Shigella flexneri, Escherichia coli, and Pseudomonas aeruginosa. Among them, 3,4-dihydroxy phenylethano1 was significantly active, with a minimum bacteriostatic concentration of 6.25 μg/mL (Wang et al., 1982). Another antibacterial assay conducted by (Hu et al., 1993) showed that S. oblata could inhibit Staphylococcus aureus, S. epidermidis, and Proteus. Eugenol, a major volatile metabolite in the flower buds of S. oblata, showed potential antimicrobial activity against Alternaria alternata, Phytophthora parasitica var. nicotianae, and Ralstonia solanacearum. It exhibited the best antimicrobial effect on A. alternata, with a minimum bacteriostatic concentration of 150 μg/mL and a minimum bactericidal concentration of 250 μg/mL (Jing et al., 2017, Jing et al., 2018). Another study showed that eugenol dose-dependently inhibited catalase and succinate dehydrogenase in Pseudomonas solanacearum at concentrations ranging from 0.1 to 0.3 mg/mL (Bai, Kong, Lin, & Zhang, 2016). The aqueous extract of S. oblata showed an antibiofilm effect against Streptococcus suis by inhibiting the synthetase in the bacteria (Liu et al., 2018). A further study revealed that rutin was the bioactive compound, showing an antibiofilm activity by targeting the chloramphenicol acetyltransferase. The total flavonoids extracted from S. oblata leaves showed a potential inhibitory effect against E. coli K 87 and K99, indicating that the extract could be a promising antidiarrhea agent for pigs (Wang, 2013). The antibacterial effects of essential oils from S. oblata flower buds and extract of S. oblata leaves were evaluated by in vivo experiments on mice infected by S. aureus, results showed that both the essential oil and leaves extract showed inhibitory effects against E. coli (Wang, 2008; Zhang et al., 2020, Yang, 2016).
3.2. Antioxidant activities
A phenolics extract from S. oblata leaves was evaluated for its inhibitory effect against the oxidization of low-density lipoprotein (LDL) by detecting the concentration of malondialdehyde and lipofuscin in the oxidized LDL. At concentrations ranging from 50 μg/mL to 400 μg/mL, the extract of S. oblata leaves showed inhibitory rates of 10.0%–71.9% against malondialdehyde and 29.2%–56.2% against lipofuscin. At the concentration of 100, 200, and 400 μg/mL, the extract inhibited the relative electrophoretic mobility of oxidized LDL, with initiatory rates of 17.5%, 22.4%, and 31.2%, respectively. Besides, the extract of S. oblata leaves also showed potent antioxidant activity in inhibiting the oxidization of cooking oil (Wang and Zhao, 2006, Wang and Zhao, 2007, Tóth et al., 2016). A bioactivity-guided fractionation of phenolics from S. oblata showed that nonpolar constituents had the strongest antioxidant activity (Zhao, Lv, Zhu, Zhang, & Guo, 2015). At the concentration of 70 μg/mL, the DPPH free radical scavenging capacity was 79.44%. Furthermore, the nonpolar constituents were found to exhibit a stronger antioxidant activity when they were treated with simulated gastric fluid, but to show a lower antioxidant activity when treated with simulated intestinal fluids. Wu (2015) found that a higher relative content of phenolic acids in the extract of S. oblata leaves resulted in a stronger antioxidant effect within the concentration from 8 to 16 mg/mL in DPPH assays, indicating that the antioxidant activity may be associated with phenolic acids in S. oblata.
3.3. Antiproliferative activities
The antiproliferative activities of syringin against HepG2 human liver cancer cells (IC50 = 40.13 μg/mL) and PC-3 human prostate cancer cells (IC50 = 88.08 μg/mL) were evaluated (Qin & Zhu, 2018). Results showed that syringin inhibited the viabilities of these two tumor cell lines by accelerating their apoptosis in a dose-dependent manner at 10–160 μg/mL. Hu, Li, Tie, and Jin (2019) found that the essential oil of S. oblata flowers could dose-dependently accelerate the apoptosis of several human gastric cancer cell lines using the TUNEL method. The strongest antiproliferative activity was found in HGC-27 cells, indicating that the essential oil of S. oblata flowers may be a potential antitumor agent against HGC-27 cells. A phenylethanoid hydroxytyrosol and a glycosylated seco-iridoid oleuropein both showed inhibitory activity against the H2O2-induced oxidative stress in LLC-PK1 (Liu, 2013). And hydroxytyrosol exhibited a stronger inhibition than oleuropein.
3.4. Hepatoprotective and choleretic activities
S. oblata has been traditionally used to treat hepatitis in folk, and this usage is recorded in Medicinal Plants of Changbai Mountain. Some modern pharmacological studies also validated the hepatoprotective effect of S. oblata (Wang et al., 2000, Gao et al., 2003a). The extract of S. oblata leaves was made into clinical medicines to treat acute icteric hepatitis, resulting in that 93.7% of patients being cured (Department of Infectious Diseases, 1978). An in vitro study showed that the extract of S. oblata leaves inhibited hepatitis B viral proteins HBeAg and HBsAg, indicating the inhibitory activity against the hepatitis B virus (Bai et al., 2016, Gao et al., 2003a). As previously reported, S. oblata leaves extract could also relieve the liver damage induced by chemical drugs (Hao, 2008), and the hepatoprotection may be associated with triterpenoid glycosides (Bai et al., 2016, Gao et al., 2003b). Another in vivo study showed that the extract of S. oblata leaves protected mice from the CCl4-induced liver injury at an effective concentration of 200 mg/kg (Li et al., 2018b, Li et al., 2018a). The extract (100 and 200 mg/kg) significantly reversed CCl4-induced changes in serum and liver biochemical parameters and showed antioxidant activities both in vitro and in vivo, significantly depressing the supernatant and serum levels of ALT, AST, and GSTA1 as well as the cell and tissue level of MDA. Besides S. oblata leaves were reported to possess a choleretic activity. Wang et al. (1982) conducted a phytopharmacological study on S. oblata leaves, revealing that syringopicroside was the bioactive compound contributing to the choleretic activity.
3.5. Other pharmacological activities
As previously reported, the extract of S. oblata leaves sometimes was used to treat epilepsy and acute icteric hepatitis (Department of Microbiology and Pharmacy and College, 1978, Department of Infectious Diseases, Bethune Medical University, 1978). Due to the folk use of S. oblata leaves for treating Pink Eye, some researchers conducted pharmacological studies to evaluate the efficiency of treating keratitis and conjunctivitis (Yang and Xing, 1990, Xing, 1992, Xing and Li, 1996). The medicine made from S. oblata leaves could treat herpes simplex virus keratitis by changing the pH of tears and regulating the level of IgA, IgG, and IgM. It showed a potential efficiency in treating epidemic hemorrhagic conjunctivitis as well. Besides, iridoids from S. oblata inhibited ulcerative colitis in rats by inhibiting the oxidization of immunoglobulins (Liu & Wang, 2011). Oleuropein was reported to protect from kidney damage by inhibiting the H2O2-induced oxidative stress on LLC-PK1 (Liu, 2013). Terpenoids from S. oblata could inhibit the production of NO, TNF-α, and IL-6 in LPS-induced BV-2 murine microglial cells, indicating the potential anti-inflammatory effect of S. oblata (Jing et al., 2018, Liu et al., 2018, Yang, 2016). Moreover, the aqueous extract of S. oblata flowers and lauric acid showed in vitro coagulation activities by shortening the activated partial thromboplastin time, prothrombin time, and thrombin time in the plasma of rabbits (Cui et al., 2019).
4. Conclusion and discussion
S. oblata has been traditionally used in Chinese medicine. And its leaves, barks, seed coats, twigs, flowers, and flower buds have been systematically investigated in phytochemistry and pharmacology. Phytochemicals from S. oblata, including iridoids, phenylpropanoids, triterpenoids, phenylethanoids, and flavonoids, have been well studied in their biological activities. Iridoid glycosides showed potent anti-inflammatory activity. The antimicrobial activities of syringopicroside and flavonoids were examined. Antiproliferative effects of syringin were found in several cancer cell lines. And the total saponins extract of S. oblata exhibited a hepatoprotective effect. Unique chemical constituents and pharmacological effects have been found in S. oblata. It merits further studies on the chemical composition as well as systematic evaluation and mechanisms of the pharmacological effects.
The medicinal parts and applications of S. oblata in Mongolian medicine are different from those in traditional Chinese medicine. S. oblata and its botanical relative S. pinnatifolia are similarly used as Mongolian medicines in Alashan, Inner Mongolia. The medicinal part of S. oblata in Mongolian medicine is the heartwood, and the traditional use is to treat heart diseases caused by Heyi. The traditional medicinal uses of S. oblata documented in the legal drug standards of Mongolian medicines are also similar to S. pinnatifolia. It is documented in Mongolian Medicine Standards of Inner Mongolia (supplemental edition in 2015). Recently, the anti-myocardial ischemia, antimicrobial, antitumor, antidiabetic, hepatoprotective, pain-relieving, and sedative effects of S. pinnatifolia have been well studied by many researchers (Ma et al., 2020, Su et al., 2015). Although there are several studies on S. oblata in Chinese medicine, it lacks a systematic study on the heartwood of S. oblata in Mongolian medicine. Especially, modern research in the ethnopharmacology of S. oblata in Mongolian medicine has rarely been reported.
Moreover, in Newly Revised Jingzhu Bencao, it is recorded that the stems of S. oblata and its botanical relatives in the genus Syringa could be used as substitutes for sandalwood in Tibet medicine, the substitutes were called Huang-Tan-Xiang (Luo, 2004). The abovementioned traditional uses and modern pythopharmacological studies both supported that S. oblata is a medicinally important plant. However, there is a gap in the systematic study of S. oblata heartwoods. Are there any sesquiterpenoids that are characteristic compounds in the genus Syringa (Ma et al., 2020)? Are there lignans which may have synergy with sesquiterpenoids (Li et al., 2019b, Su et al., 2015)? It deserves further phytochemical and pharmacological studies in this Mongolian medicine. An integrated study on the system biology and phytopharmacology of S. oblata may help to uncover the multiple targets and signaling pathways involved in its protection of the cardiovascular system, to provide scientific evidence for clinic uses, and to give references to the utilization of Mongolian medicinal products made from S. oblata.
Last but not least, all ethnomedicines containing S. oblata deserve a deep thought about their pharmacologically active components, botanical source verification, and resource survey.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors thank for the support of the science foundation projects: The Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (No. 2060302), Natural Science Foundation of China (No. 82104342), the Open Project of NMPA Key Laboratory for Quality Control of Traditional Chinese Medicine, Mongolian Medicine (No. MDK2021069), and Inner Mongolia Natural Science Foundation(2022QN08028).
References
- Andary C., Wylde R., Laffite C., Privat G., Winternitz F. Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry. 1982;21(5):1123–1127. [Google Scholar]
- Bai W., Kong F., Lin Y., Zhang C. Extract of Syringa oblata: A new biocontrol agent against tobacco bacterial wilt caused by Ralstonia solanacearum. Pesticide Biochemistry and Physiology. 2016;134:79–83. doi: 10.1016/j.pestbp.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Cao P.R. Henan University; Kaifeng: 2017. Study on bioactive components of agastaches and lilacs. [Google Scholar]
- Cui G.D., Gao M.W., Liu G.R. Science and Technology Press; Chifeng: 2009. Medicinal plants of Chifeng; p. 366. [Google Scholar]
- Cui L., Hu M., Cao P., Niu Y., Li C., Liu Z., Kang W. Chemical constituents and coagulation activity of Syringa oblata Lindl flowers. BMC Chemistry. 2019;13(1):1–8. doi: 10.1186/s13065-019-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Microbiology and Pharmacy, Heilongjiang College of Traditional Chinese Medicine (1978). Study on antibacterial active components of Syringa oblata leaves. Selected Works of Popular Science, (36–46).
- Department of Infectious Diseases, First Hospital of Bethune Medical University of Jilin Province Preparation of clove tablets and their clinical effect on yellow gangrene hepatitis. Journal of Chinese Herbal Medicine. 1978;1:18. [Google Scholar]
- Department of Infectious Diseases, Bethune Medical University Preparation of clove leaf agent and its clinical effect on 434 cases of acute icteric hepatitis. Journal of Chinese Herbal Medicine. 1978;1:18–19. [Google Scholar]
- Dong L.W., Wang J.L., Zhao M., Zhang S.J. Chemical constituents of the Alabastrum of Syringa oblata Lindl. Natural Product Research & Development. 2011;23(4):658–660. [Google Scholar]
- Editorial Board of Alashan Medicinal Plants Color Atlas . Inner Mongolia People’s Publishing House; Huhehaote: 2016. Alashan medicinal plants color atlas; p. 208. [Google Scholar]
- Feng R.H. Northwest A&F University; Yangling: 2007. Isolation and insecticidal activity of lignans from Sabina vulgaris. [Google Scholar]
- Food and Drug Administration of Inner Mongolia Autonomous Region . Inner Mongolia People’s Publishing House; Huhehaote: 2015. Inner Mongolia Mongolian medicine standard; p. 61. [Google Scholar]
- Gao J.Q., Jiao S.G., Ma J.Y., Liu J., Chai X.Y. Advances on terpenoids from genus Syringa. China Journal of Chinese Materia Medica. 2020;45(10):2343–2352. doi: 10.19540/j.cnki.cjcmm.20200220.203. [DOI] [PubMed] [Google Scholar]
- Gao S. Liaoning Science and Technology Press; Shenyang: 2010. Traditional Chinese medicine records of Liaoning (Plants) p. 750. [Google Scholar]
- Gao S.Q., Niu J.Q., Wang F., Liu X.D., Jin Z.X., Wang Y.L. Comparative studies on the resistance of hepatitis B by Syringa extract, IFN and ganyanling in HepG2. 2.15 cells. Chinese Journal of Cellular and Molecular Immunology. 2003;19(4):385–386. [PubMed] [Google Scholar]
- Gao S.Q., Chi B.L., Wang F., Liu X.D., Jin Z.X., He H. Effect of the extract of Syringa on the secretion of HBsAg and HBeAg in HepG2.2.15 cells. Journal of Jilin University (Medical Edition) 2003;29(4):468–470. [Google Scholar]
- Gegen Z.L., Gao Y., Site G.L., Alatan C.M., Tai B.L., Tu Y. Mechanism of Syringa oblata in treating angina pectoris based on GC-MS and network pharmacology. China Journal of Chinese Materia Medica. 2022;47(3):836–845. doi: 10.19540/j.cnki.cjcmm.20210927.201. [DOI] [PubMed] [Google Scholar]
- Han J. Qiqihar University; Qiqihar: 2012. Study on the digestive components of Syringa oblata and Angelica sinensis. [Google Scholar]
- Hao T.T. Jilin Agricultural University; Changchun: 2008. Study on Syringa oblata, a new drug for hepatitis. [Google Scholar]
- Hu G.Q., Li J.Z., Zhang C.Y., Wang X.R., Pan S.H., Lang J. Experimental study on antibacterial effect of traditional Chinese medicine in Heilongjiang. Heilongjiang Journal of Traditional Chinese Medicine. 1993;5:47–48. [Google Scholar]
- Hu J.R., Li P., Tie J., Jin S. Study on antioxidant and antitumor activity of essential oil from flowers of Syringa oblata. Biotechnology Bulletin. 2019;35(12):16–23. [Google Scholar]
- Jiangsu Institute of Botany. (1990). Xinghua Bencao Gangyao, Volume III (pp. 252). Shanghai: Shanghai Science and Technology Press.
- Jilin Provincial Health Bureau. (1997). Jilin provincial drug standards (pp.249).
- Jilin Institute of Traditional Chinese Medicine . Jilin People’s Publishing House; Changchun: 1982. Flora of Changbai Mountain; p. 897. [Google Scholar]
- Jing C., Gou J., Han X., Wu Q., Zhang C. In vitro and in vivo activities of eugenol against tobacco black shank caused by Phytophthora nicotianae. Pesticide Biochemistry and Physiology. 2017;142:148–154. doi: 10.1016/j.pestbp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Jing C., Zhao J., Han X., Huang R., Cai D., Zhang C. Essential oil of Syringa oblata Lindl. as a potential biocontrol agent against tobacco brown spot caused by Alternaria alternata. Crop Protection. 2018;104:41–46. [Google Scholar]
- Kong Y.F., Vencent Y., Hu Y.L., Dong C.H. Research advance on structural modification and structure-activity relationship of iridoids. Natural Product Research and Development. 2021;33(7):1236–1250. [Google Scholar]
- Li G., Yan H., Liu X. Simultaneous purification and separation of syringoside and oleuropein from Syringa oblata Lindl. extract using macroporous resin. Journal of Chemistry. 2019;2019:1–9. [Google Scholar]
- Li J., Ge F., Wuken S., Jiao S., Chen P., Huang M.…Huang L. Zerumbone, a humulane sesquiterpene from Syringa pinnatifolia, attenuates cardiac fibrosis by inhibiting of the TGF-β1/Smad signaling pathway after myocardial infarction in mice. Phytomedicine. 2022;100:154078. doi: 10.1016/j.phymed.2022.154078. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang R., Wuken S., Du X., Jiao S., Su G.…Chai X. Phytochemical and chemotaxonomic study of Syringa pinnatifolia Hemsl. (Oleaceae) Biochemical Systematics and Ecology. 2018;81:58–61. [Google Scholar]
- Li J.J., Ge F.X., Jiao S.G., Wuken S.N., Chen S.Y., Tu P.F., Chai X.Y. Pharmacological evaluation of Mongolian medicine Syringa pinnatifolia fraction I against acute myocardial ischemia in mice. China Journal of Chinese Materia Medica. 2019;44(23):5240–5247. doi: 10.19540/j.cnki.cjcmm.20190923.401. [DOI] [PubMed] [Google Scholar]
- Li Q., Xu Q.M., Hao L.L., Yang S.L., Li X.R. Studies on chemical constituents of Syringa oblata leaves. Chinese Traditional and Herbal Drugs. 2009;40(3):369–371. [Google Scholar]
- Li Y., Li Z., Li C., Ma X., Chang Y., Shi C.…Liu F. Evaluation of hepatoprotective activity of Syringa oblata leaves ethanol extract with the indicator of glutathione S-transferase A1. Revista Brasileira de Farmacognosia. 2018;28:489–494. [Google Scholar]
- Li Z.G., Lee M.R., Shen D.L. Analysis of volatile compounds emitted from fresh Syringa oblata flowers in different florescence by headspace solid-phase microextraction–gas chromatography–mass spectrometry. Analytica Chimica Acta. 2006;576(1):43–49. doi: 10.1016/j.aca.2006.01.074. [DOI] [PubMed] [Google Scholar]
- Liu S.W. Flora of Qinghai. 1996;Volume III:44. [Google Scholar]
- Liu X., Wang J. Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. Journal of Ethnopharmacology. 2011;133(2):780–787. doi: 10.1016/j.jep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Liu X.P. Northeast Forestry University; Haerbin: 2013. Extraction technology of total flavonoids from clove leaves and evaluation of antibacterial effect. [Google Scholar]
- Liu Y.Y., Chen X.R., Gao L.F., Chen M., Cui W.Q., Ding W.Y., Onaghise God’s power B., Li Y.H. Spectrum-effect relationships between the bioactive ingredient of Syringa oblata Lindl. leaves and its role in inhibiting the biofilm formation of Streptococcus suis. Frontiers in Pharmacology. 2018;9:570. doi: 10.3389/fphar.2018.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Li Y.P., Li J.H. Studies on chemical constituents of Syringa oblata leaves. Chinese Traditional and Herbal Drugs. 2003;34(8):687–689. [Google Scholar]
- Luo D.S. Sichuan Publishing Group Sichuan Science and Technology Press; Chengdu: 2004. Newly revised Jingzhu Bencao; p. 197. [Google Scholar]
- Ma L.N., Tian C.W., Zhang T.J., Zhang L.J., Xu X.H. Advances in study on iridoids in plants of Swertia L. and their pharmacological activity. Chinese Traditional and Herbal Drugs. 2008;39(5):790–795. [Google Scholar]
- Ma J.Y., Liu S.H., Jiao S.G., Xing W.W., Sun J.J., Luo Y.G.…Chai X.Y. Phytochemical and pharmacological progress on genus Syringa. China Journal of Chinese Materia Medica. 2020;45(8):1833–1843. doi: 10.19540/j.cnki.cjcmm.20200224.202. [DOI] [PubMed] [Google Scholar]
- Ma Y.Q. Flora of Inner Mongolia. 1980;Vol. 5:65. [Google Scholar]
- Mengke N. Inner Mongolia People’s Publishing House; Huhehaote: 2018. Wushenqi drug illustrated book; p. 65. [Google Scholar]
- Nenadis N., Vervoort J., Boeren S., Tsimidou M.Z. Syringa oblata Lindl var. alba as a source of oleuropein and related compounds. Journal of the Science of Food and Agriculture. 2007;87(1):160–166. [Google Scholar]
- Qin L.D., Zhu A.H. Study on the screening and mechanism of syringin anticancer activity. Journal of Anhui Agricultural Sciences. 2018;46(14):107–108. [Google Scholar]
- Su G., Cao Y., Li C., Yu X., Gao X., Tu P., Chai X. Phytochemical and pharmacological progress on the genus Syringa. Chemistry Central Journal. 2015;9(1):1–12. doi: 10.1186/s13065-015-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G.Z., Chen J., Cao Y., Bai R.F., Chen S.Y., Tu P.F., Chai X.Y. Phytochemical and pharmacological progress on peeled stem of Syringa pinnatifolia, a Mongolian folk medicine. China Journal of Chinese Materia Medica. 2015;40(22):4333–4338. [PubMed] [Google Scholar]
- Tian L., Li Y.J., Lv S.W., Zhang L., Liu T. Chemical constituents of Syringa oblata leaves. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(1):144–147. [Google Scholar]
- Tóth G., Barabás C., Tóth A., Kéry Á., Béni S., Boldizsár I.…Noszál B. Characterization of antioxidant phenolics in Syringa vulgaris L. flowers and fruits by HPLC-DAD-ESI-MS. Biomedical Chromatography. 2016;30(6):923–932. doi: 10.1002/bmc.3630. [DOI] [PubMed] [Google Scholar]
- Wang D.D., Liu S.Q., Chen Y.J., Wu L.J., Sun J.Y., Zhu T.R. Study on the active constituents of Syringa oblata Lindl. Acta Pharmaceutica Sinica. 1982;17(12):951–954. [PubMed] [Google Scholar]
- Wang F., Wen Y.J., Niu J.Q., Jiang W.C., Xu C., Cai Z.C. Experimental studies of the phamacologic and toxical effects of the dingxiangye tablet. Journal of Clinical Hepatology. 2000;16(2):94–96. [Google Scholar]
- Wang Y.X. Northeast Agricultural University; Haerbin: 2013. Optimization of extraction process and evaluation of bacteriostatic effect of total flavonoids in Syringa leaves. [Google Scholar]
- Wang F.F., Zhang Y.M., Zheng X.W., Dai Z., Liu B., Ma S.C. Research progress of the structure and biological activities of iridoids compounds. Chinese Pharmaceutical Affairs. 2019;33:91–98. [Google Scholar]
- Wang J., Zhang G., Dong L., Zhao M., Zhang S. Chemical constituents in seed crust of Syringa oblata. Chinese Traditional and Herbal Drugs. 2010;41(10):1598–1601. [Google Scholar]
- Wang J.L. Shenyang Pharmaceutical University; Shenyang: 2008. Preliminary pharmacognostical investigation of Folium Syringae and the research on its active fractions. [Google Scholar]
- Wang L.L., Zhao X.H. The antioxidation effect of extract from clove leaf in two edible fats. Science and Technology of Cereals, Oils and Foods. 2006;14(6):39–40. [Google Scholar]
- Wang L.L., Zhao X.H. Inhibition of the extract from clove leaf (Syringa oblata) on oxidative modification of low-density lipoprotein. Journal of Northeast Agricultural University. 2007;38(5):641–644. [Google Scholar]
- Wei Z.J. Jilin University; Changchun: 2004. Studies on the chemical constituents of Syringa oblata L. from the leaf and its pharmacological activities. [Google Scholar]
- Wu L.J. People’s Health Publishing House; Beijing: 2005. Natural medicine chemistry; p. 228. [Google Scholar]
- Wu L.J. 5th ed. People’s Health Publishing House; Beijing: 2010. Natural medicinal chemistry; p. 124. [Google Scholar]
- Wu Q., Wang S.S., Sheng W.B., Yao M., Yuan H.W., Wang W.…Peng C.Y. Advances on natural sources norlignan compounds and their biological activities. Chinese Traditional and Herbal Drugs. 2021;52(5):1522–1535. [Google Scholar]
- Wu S.J., Zhao T., Qin Y.Q. China Medical Science and Technology Press; Beijing: 2002. Composition chemistry of modern Chinese herbal medicine; pp. 718–732. [Google Scholar]
- Wu X.C., Wang Y.P., Sui Y.H. Shandong University Press; Jinan: 2014. Common Garden Plants in Northern China; p. 291. [Google Scholar]
- Wu Y. Northeast Agricultural University; Haerbin: 2015. Optimization of extraction process of total phenolic acids from Folium Syringae leaves and its antioxidant activity research. [Google Scholar]
- Xing J., Xu W.R., Liu P., Liu B.N., Fu H.X., Liu W.…Tang L.D. Virtual evaluation on anti-inflammatory activity of iridoids from cape jasmine fruit and adhesive rehmannia root. Chinese Traditional and Herbal Drugs. 2009;40(6):930–935. [Google Scholar]
- Xing M.Y. Changes of tear pH before and after treatment of herpes simplex keratitis with Syringa oblata preparation. Journal of Ophthalmology of Integrated Traditional Chinese and Western Medicine. 1992;10(3):131–132. [Google Scholar]
- Xing M.Y., Li B. Syringa oblata leaf preparation for the treatment of epidemic hemorrhagic conjunctivitis. Journal of Ophthalmology of Integrated Chinese and Western Medicine. 1996;14(1):30. [Google Scholar]
- Xing S.R. Chronicles of traditional Chinese medicine in Ningxia. 2006;Vol. II:966. [Google Scholar]
- Yang S. Inner Mongolia Agricultural University; Huhehaote: 2016. Extraction and composition analysis of three kinds of cloves essential oil, and their bacteriostatic effect assay. [Google Scholar]
- Yang W.W., Xing M.Y. Immunoassay of Syringa oblata before and after treatment of herpes simplex keratitis. Journal of Practical Ophthalmology. 1990;8(4):225–226. [Google Scholar]
- Yu T.J., Wang L.B., Wu L.J. Research progress of the chemical constituents and pharmacological action of Syringa Linn. Journal of Anhui Agricultural Sciences. 2016;44(2):168–170. [Google Scholar]
- Zhang D.X., Chen Z., Li X.R., Yang S.L. Triterpenoids from Syringa oblata leaves. Guide of China Medicine. 2011;9(11):45–46. [Google Scholar]
- Zhang G.L., Li N., Lin L.L., Wang M.W. Recent progresses in studies on bioactive lignans from plants. China Journal of Chinese Material Medica. 2007;32(20):2089–2094. [PubMed] [Google Scholar]
- Zhang S.J., Zhang J.F, Wang J.L. Chemical constituents in stem bark of Syringa oblata. Chinese Traditional and Herbal Drugs. 2006;37(11):1624–1626. [Google Scholar]
- Zhang J.F., Zhang S.J. The research progress in chemical composition and pharmacological action of Syringa. Natural Science Journal of Hainan University. 2007;25(2):201–205. [Google Scholar]
- Zhang J.F., Jiao H., Wang J.L., Zhang S.J. Chemical constituents in barks of Syringa oblata Lindl. (II) Natural Product Research & Development. 2007;19(4):617–619. [Google Scholar]
- Zhang S.J., Guo H.Q., Han J., Zhao M., Wang J.L. Chemical constituents from seeds of Syringa oblata. Chinese Traditional and Herbal Drugs. 2011;42(10):1894–1899. [Google Scholar]
- Zhang S.J., Li Y.F., Li J., Wang J.L., Zhao M. A new iridoid from leaves of Syringa oblata. Chinese Traditional and Herbal Drugs. 2014;45(5):608–610. [Google Scholar]
- Zhang S.J., Shi Z.C., Wang D., Wang J.L., Zhao M., Li J. Chemical constituents from leaf of Syringa oblata. Chinese Traditional and Herbal Drugs. 2018;49(16):3747–3757. [Google Scholar]
- Zhang, Y., Han, D., Yu, S., An, C., Liu, X., Zhong, H., Xu, Y., Jiang, L., & Wang, Z. (2020). Protective effect of iridoid glycosides of the leaves of Syringa oblata Lindl. on dextran sulfate sodium-induced ulcerative colitis by inhibition of the TLR2/4/MyD88/NF-κB signaling pathway. BioMed Research International, 2020, 7650123. [DOI] [PMC free article] [PubMed]
- Zhao C.X., Liang Y.Z., Fang H.Z., Li X.N. Temperature-programmed retention indices for gas chromatography–mass spectroscopy analysis of plant essential oils. Journal of Chromatography A. 2005;1096(1–2):76–85. doi: 10.1016/j.chroma.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Lv X.F., Zhu X.R., Zhang N., Guo Q.Q. Optimization of extraction technology for lilac polyphenols and their antioxidant activity. Journal of Beijing Forestry University. 2015;37(10):125–129. [Google Scholar]
- Zhao Y.Z., Zhao L.Q., Cao R. 3rd ed. Inner Mongolia People’s Publishing House; Huhehaote: 2020. Flora of Inner Mongolia; p. 62. [Google Scholar]
- Zhao M., Han J., Lv S.Y., Zhang S.J. Study on chemical constituents in twigs of Syringa oblata. Chinese Traditional and Herbal Drugs. 2012;43(2):251–254. [Google Scholar]
- Zhao M., Tang W.X., Li J., Bai L.M., Wang J.L., Zhang W.Z., Zhang S.J. Two new monoterpenoids from the fresh leaves of Syringa oblata. Chemistry of Natural Compounds. 2016;52(6):1023–1025. [Google Scholar]
- Zhou Y.M. Shenyang Pharmaceutical University; Shenyang: 2005. Studies on the active constituents from leaves of Syringa oblata Lindl. [Google Scholar]
- Zhu, W.B., Su, F.Z., Sun, Y.P., Yang, B.Y., Wang, Q.H., & Kuang, H.X. (2021). Antipharyngitis effects of Syringa oblata L. ethanolic extract in acute pharyngitis rat model and anti-Inflammatory effect of iridoids in LPS-induced RAW 264.7 Cells. Evidence-Based Complementary and Alternative Medicine, 2021, 5111752. [DOI] [PMC free article] [PubMed]
- Zhu W., Wang Z., Sun Y., Yang B., Wang Q., Kuang H. Traditional uses, phytochemistry and pharmacology of genus Syringa: A comprehensive review. Journal of Ethnopharmacology. 2021;266 doi: 10.1016/j.jep.2020.113465. [DOI] [PubMed] [Google Scholar]