Abstract
Chinese herbal medicines (CHMs) are one of the important bioresources of medicine, which works by unlocking nature's ability to prevent diseases and recover from illnesses. Recently, it has ascended to the world stage and become a global icon. Nowadays, a considerable of researches have focused on the quality evaluation of CHMs. However, it is difficult to meet the reasonable needs of human beings for safe drug use to evaluate the quality of a huge number of inferior goods for the CHMs contaminated by pesticides and heavy metals. Hence to explore an eligible medicinal plant cultivation pattern, which can provide high quality CHMs sustainably, is most promising. This review analyzed the situation and characteristics of medicinal plant resources in different periods, including wild-harvested and cultivated resources during different stages, putting forward that ecological cultivation must be the way to develop medicinal plant cultivation and to obtain high quality CHMs.
Keywords: Chinese herbal medicines, drug safety, ecological cultivation, pesticide residues, soil quality
1. Introduction
Chinese herbal medicines (CHMs) have served for the prevention, treatment and health care of human beings for thousands of years, and been extremely favored in both developing and developed countries (WHO, 2013). With the improvement of international demand and recognition, CHMs are getting more and more attention (Chen et al., 2016). Firstly, CHMs are the material basis and prerequisite for the healthy development of traditional Chinese medicine (TCM) and other related industries. Secondly, CHMs are also one of China's export advantages (Cheng, Yang, Li, & Huang, 2020). According to relevant data from the National Bureau of Statistics of China, the export amount of Chinese patent medicines in 2018 was $ 262.34 million, increased by 33.71% compared with 2010. At the same time, the “Chinese medicine diplomacy” in “the belt and road” clearly recognizes the position and role of Chinese medicines in international affairs. Therefore, the demand of CHMs resources is soaring worldwide.
In order to meet the market demand, some wild medicinal plants have been overexploited, such as Astragalus membranaceus (Fisch.) Bge. (Astragalus, Astragali Radix), Rhodiola crenulate (Hook. f. et Thoms.) H. Ohba (Rhodiola, Rhodiolae Crenulatae Radix et Rhizoma) and Glycyrrhiza uralensis Fisch. (Licorice, Glycyrrhizae Radix et Rhizoma), resulting in the deterioration of the ecological environment. Additionally, a considerable of medicinal plants, such as Panax ginseng C. A. Mey. (Ginseng, Ginseng Radix et Rhizoma), Gastrodia elata Bl. (Gastrodiae Rhizoma) and Lonicera japonica Thunb. (Lonicerae, Lonicerae Japonicae Flos) are cultivated artificially to make up for the market gap. In 2018, the planting area of Chinese herbal medicines in China reached 2,392.43 thousand hectares, increased by 89.53% compared with 2010 (excluding forest land and wild herbs). However, as a negative impact, some new problems gradually emerged: (i) The ecological environment has been artificially destroyed which led to the decrease of wild CHMs resources constantly (Guo, Fu, & Chen, 2019). (ii) The abuse of fertilizers and pesticides resulted in excessive pesticide residues and heavy metals in CHMs, threatening the drug safety (Kang et al., 2016). (iii) Serious farmland soil pollution affects sustainable cultivation of CHMs (Ye et al., 2010). (iv) Continuous cropping obstacle affects the quality of medicinal materials (Guo et al., 2015).
As we all known, a stable ecosystem is the prerequisite for plants survival. In the wild ecosystem, the sustainable resource of CHMs depends on the dynamic and balanced ecosystem. However, in the farmland ecosystem, medicinal plants were cultivated as same as cropping common plants, which used massive chemicals for chasing yield. This may extremely affect the quality and safety of CHMs. Unfortunately, many studies have paid attention to the quality evaluation of CHMs, rather than the cultivation of high-quality medicinal plants. Hence we overviewed the characters of CHMs resources in different periods at great length, to look for the way to obtain high quality CHMs.
2. Characteristics of CHMs resources in different periods
2.1. Wild-harvested resources of medicinal plants
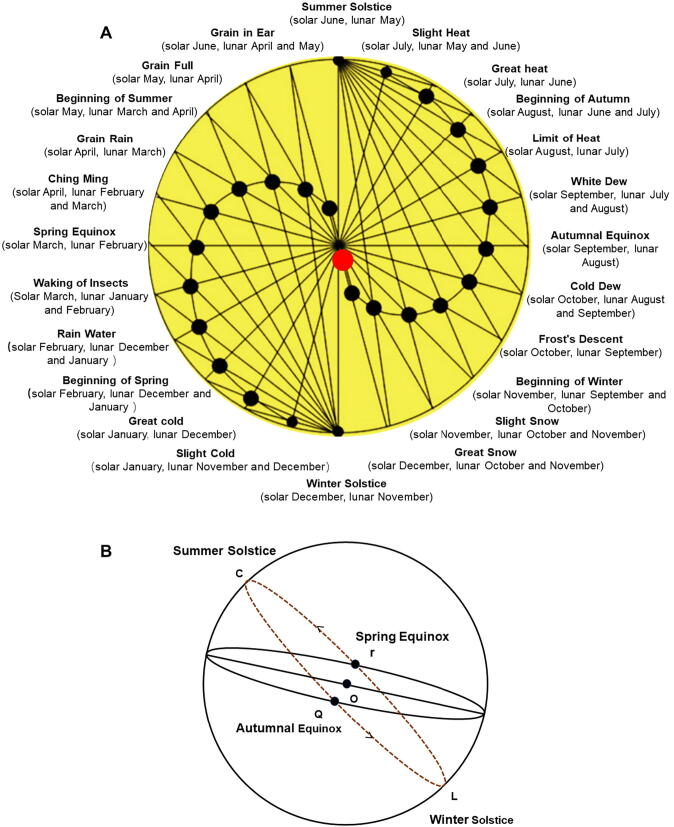
In ancient times, people obtained CHMs mainly depending on harvesting wild plants. The earliest literature about wild-harvested resources of medicinal plants in China is the Book of Rites in the Shang Dynasty (11th to 6th century B.C). It recorded the first month of summer (solar April, lunar May) was the time for gathering all kinds of herb medicines. Moreover, the Book of Songs also contains poems and songs about collection works on Ge (Kudzu, Pueraiae Lobatae Radix, Pueraria lobata (Willd.) Ohwi) and Ling (Licorice, Glycyrrhizae Radix et Rhizoma, Glycyrrhiza uralensis Fisch.) (Confucius, 2015). Shennong Herbal Classic, one of the classic works of TCM and completed in the Han Dynasty (202 BCE−220 CE), described 252 medicinal herbs in morphology, nature and flavor, usage, distribution, habitat, harvest time and storage (Shen, 2007). The Yongyao Faxiang pointed out: the efficacy of CHMs would be better only if the genuine herbal, woody and animal medicinal materials from the orgin place, and the root, leaf, flower and fruit herbs harvested at proper harvest time. Once missed geographical and phenological basis, it would have a negative impact on clinical efficacy (Xie, 2001). Those works indicated that Chinese ancient people had already known phenology was very important to agricultural activities and geography was very fatal to find proper herbs. For example, they picked Pinellia ternata (Thunb.) Breit. (Pinelliae Rhizoma) and Prunella vulgaris L. (Prunellae Spica) at the summer solstice, harvested radish (Raphanus sativus L.) in the first frost year by year.
Huainanzi Tianwenxun summarized the twenty-four solar terms completely for the first time, that were completed by An Liu (179–122 BCE) and listed in the list of UNESCO's representative works of human intangible cultural heritage on November 30, 2016. The 24 solar terms is closely related with variation of climate and living creatures on the earth. It is one of the basic theories that guides agriculture and medical activities, especially TCM, in China until now (Fig. 1). In Tang Dynasty (618–907 CE), Qianjin Yifang by Si-miao Sun recorded that the herbs had no clinical effect when harvested at inappropriate time and dried by wrong methods in initial processing (Sun, 2011). Therefore, phenology had been applied to guide the haveresting of CHMs in ancient times.
Fig. 1.
Twenty-four solar terms (A) established by fixing qi method of fixing qi and ecliptic of sun (B). Note: Method of “fixing qi” is a method to determine solar terms according to the position of the sun on ecliptic (Fig. 1B, the ecliptic of the sun relative to the earth. When the observer is at the center of the celestial body, LrCQ is the ecliptic, the sun's trajectory in a year). It is divided into 24 equal parts in a circle (the sun's apparent path on the celestial sphere in a year). Spring equinox is 0 degrees (0 degrees of ecliptic) as the starting point. Every time 15 degrees forward is a solar term, it will return spring equinox again after a round (twenty-four solar terms). The degrees of each solar term are equal, and the time is not.
Additionally, geographical characteristics are also the fatal reference factor to obtain CHMs with high quality. First of all, the geographical indications of CHMs names have confirmed it. For example, the Shennong Herbal Classic recorded Bajitian (Morindae Officinalis Radix, Morinda officinalis How), Qinpi (Fraxini Cortex, Fraxinus chinensis Roxb.), Badou (Crotonis Fructus, Croton tiglium L.), Shujiao (Zanthoxyli Pericarpium, Zanthoxylum bungeanum Maxim.), and Shuzao (Shanzhuyu, Corni Fructus, Cornus officinalis Sieb. et Zucc.) with obvious regional characteristics, (Ba, Shu and Qin namely refers to Chongqing City, Sichuan Province and Shaanxi Province, China) (Shen, 2007). Secondly, medical experts believed that CHMs from different regions might have diverse clinical effects. China's first official pharmacopoeia, Xinxiu Bencao in Tang Dynasty, described that TCM in genuine and non-genuine areas would produce different clinical effects. It was recorded that Asari Radix et Rhizoma (Asarum, Asarum sieboldii Miq.) produced in Huayin City (Huai'an City, Jiangsu Province) should be discarded because of its little clinical effect (Su, 2005), which certified that all kinds of herbs have specific living environment. Modern pharmacological researches have also proved this point. For example, Fritillariae Cirrhosae Bulbus (Chuanbeimu, Fritillaria cirrhosa D. Don) is good at “moistening the lung, eliminating phlegm, stopping cough and asthma”, while Fritillariae thunbergii Bulbus (Zhebeimu, Fritillaria thunbergii Miq.) is better at “relieving carbuncle and toxin, breaking the crux, eliminating excess phlegm and enriching malignant sores” (Pharmacopoeia Committee of P. R. China, 2015).
In a word, Chinese ancient people believed that both phenology and geography contributed to the growth and quality of herbs. However, it was not easy for people in this period to collect medicinal plants due to scattered distribution of herbs, especially in remote mountains and forests, obstructing the development of medical activities.
2.2. Ancient agriculture
With the development of production practice, in addition to phenology and geographical characteristics, intelligent ancients have explored mysteries related to the growth of medicinal plants gradually. In ancient agricultural period, there was a wider and deeper understanding of the cultivation theory of medicinal plants.
Qimin Yaoshu, which provided a theoretical basis for the introduction and cultivation of ancient Chinese medicinal plants, described 20 kinds of medicinal plant cultivation recipes, including Rehmannia glutinosa Libosch. (Rehmannia, Rehmanniae Radix), Carthamus tinctorius L. (Safflower, Carthami Flos) and Evodia rutaecarpa (Juss.) Benth. (Evodiae Fructus), and summed Chinese agriculture from the primitive period to the Northern Wei Dynasty periods (533–544 CE). The author thought crops cultivation should follow seasonal changes: sowing and germination in spring, growing in summer, harvesting in autumn and storing in winter. Moreover, many advanced thoughts were also permeated in Qimin Yaoshu: (i) Soil fertility can be improved through green manure. (ii) Adopting ecological principles controls pest, such as the use of cattle and sheep bones to attract ants, lime to control pests and animal phototaxis to kill pests. (iii) Crop yield can be improved by increasing multiple cropping index and land use rate. (iv) The relationship between environment and heredity was well understood. For example, the author thought environmental factors may cause morphological variation of the same species (Jia & Shi, 2015).
In addition to the development of important agricultural theory, the medicinal plant cultivation practice also got national's attention. In the Sui Dynasty (581–618 CE), the royal family set up positions of “main medicine” and “medicine gardener” in the imperial doctor's office, responsible for medicinal plants cultivating and medicinal plant cultivators training (Guo, 2009). Xinxiu Bencao of Tang Dynasty (618–907 CE) recorded the planting activity of Aconitum carmichaelii Debx. (Aconm Lateralis Radix Praeparaia) in Mianzhou (the east of Mianyang, Sichuan Province) for the first time (Huang et al., 2011). During the Ming Dynasty (1368–1644 CE), more than 200 kinds of medicinal plants were cultivated. The Compendium of Materia Medica, described the cultivation methods of about 180 kinds of medicinal plants, especially the part of “Caobu” (a section specifically referring to herbal medicinal plants), which described the artificial cultivation of 62 kinds of medicinal plants such as Schizonepeta tenuifolia Briq. (Schizonepetae Herba) and Ophiopogon japonicus (L.f) Ker-GawL (Ophiopogonis Radix), providing extremely valuable scientific reference of medicinal plant cultivation (Li, 2004).
Although these successful introduction and cultivation experiences partially expanded CHMs source, it is undeniable the main method of obtaining CHMs was still relying on harvesting wild herbs (Guo, 2009).
2.3. Chemical agriculture
The cultivation of medicinal plants in China has developed rapidly since 1949 for the expansion of planting scale and improvement of technology. Up to now, there are more than 200 kinds of medicinal plants cultivated successfully in China, such as Chrysanthemum morifolium Ramat. (Chrysanthemi Flos), Fritillaria cirrhosa (Fritillariae Cirrhosae Bulbus) and Panax notoginseng (Burk.) F.H. Chen (Notoginseng Radix et Rhizoma). Through the efforts of Chinese medicine workers, more than 20 kinds of foreign rare medicinal plants have been successfully cultivated in China, such as Panax quinquefolium L. (American ginseng, Panacis Quinquefolia Radix), Sterculia lychnophora Hance (Sterculiae Lychnophorae Semen) and Eugenia caryophyllata Thunb. (Caryophylli Flos) (Cai, Lu, Li, Guo, & Dai, 2015, Dao, Meng, & Li, 2000, Gu, Yu, Luan, & Wang, 2006).
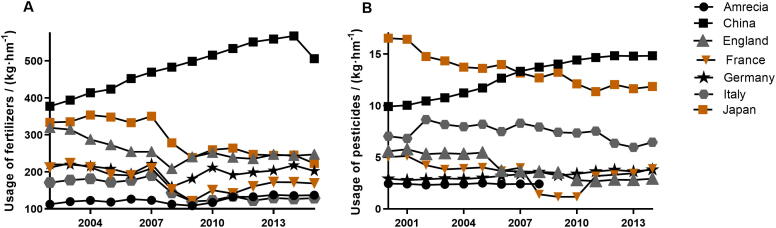
One thing that must be recognized is that chemical pesticides and fertilizers played an important role at this time (Li et al., 2014). Since 1882, bordeaux mixture was used to control grape downy mildew, the world has gradually entered the era of chemical agriculture. Applying chemical fertilizers can increase the output of crops by 250 million tons per year, and spraying pesticides can greatly reduce labor intensity and obtain high yield (Xie et al., 2019). In 2015, the amount of China's fertilizer use (506.12 kg/hm) far exceeded the average use of developed countries in the world (Fig. 2A). Moreover, pesticides used in China have been growing steadily since 2000 (Fig. 2B).
Fig. 2.
Use of fertilizers (A) and pesticides (B) in several countries.
However, the application of chemical fertilizers and pesticides has negative effects on medication safety, soil reuse and organism in the ecology. It was found that there were 38 pesticides tested totally and at least one pesticide residue in each sample was detected in 60 samples of Lonicera japonica, including organophosphorus, organochlorines, pyrethroids, carbamates, triazole and amides (Li et al., 2017a). Forty-eight of 65 CHMs tested pesticide residues positively with 51 different pesticide residues. Among them, six pesticides, including phorate, carbofuran, fipronil, methamidophos, aldicarb, and profenofos, have been prohibited in China. In Sanqi (Panax notoginseng (Burk.) F. H. Chen) flower, the residue of thiophanate-methyl was 500 times over the maximum European residue limit. In honeysuckle (Lonicerae, Lonicera japonica Thunb.), it exceeded the limit by 100 times (Greenpeace, 2013). Excessive application of nitrogen and phosphorus fertilizer can also lead to soil acidification and even reduced productivity (Monica, 2018, Savci, 2012). Among the pesticides applied, only 10%−30% are effective for crops, and 50%−60% remaining in the soil affect the reuse ability of soil and endanger life safety (Hua & Jiang, 2000). Furthermore, the excessive application of chemical substances is harmful to organisms on the earth (Hill & Hausbeck, 2008).
This is contrary to our willingness to produce a non-harmful, non-contaminated, and quality-stable CHMs. Actually, planting medicinal plants under chemical agriculture pattern fails to deliver health to people. Therefore, some attempts have been made to ensure drug safety and sufficient medicinal resources.
“Good Agriculture Practice (GAP)” was put forward under the background of non-standardized production and planting technology, and the inferior CHMs (Guo, Zhang, Zhu, Wang, & Huang, 2014). It worked through effective quality control of the whole process of CHMs (ecological environment, germplasm and breeding materials, cultivation and breeding management, etc.) to ensure the safe and stable source of CHMs (Yang, Guo, Zhou, & Huang, 2016). As of January 2016, about 194 Chinese herbal medicine planting bases have obtained GAP certification. Thankfully, the concept of standardized planting during the implementation period has been reached among pharmaceutical planting enterprises and planting personnel. However, the incomplete GAP medicinal certification system failed to distinguish the quality gap of the GAP medicinal materials from the general, let alone the price. As a result, planting enterprises and individuals have lost their enthusiasm for producing GAP CHMs. It was reported that only 2% of the planting area of ginseng in Jilin Province passed the national GAP certification, and it was difficult to find the ginseng medicinal materials with GAP certification in the medicinal material market (Yang, Guo, Zhou, & Huang, 2016). Therefore, the Practice of Quality Management of Traditional Chinese Medicine (Trial) has been implemented for 12 years since 2002 and was canceled on February 3, 2016.
Further, the wild tending of Chinese medicinal materials emerged, which is the organic combination of the collection and cultivation of medicinal plants. It is carried out by providing suitable growth and increasing the number of population artificially or naturally in the original or similar environment, to meet the drug demand and ensure medicinal plant reproduction (Chen et al., 2004). At present, the research on the wild tending is mainly aimed at the Chinese medicinal materials with harsh growth conditions, relatively expensive selling price, or significant quality difference between the cultivated and the wild one, such as Heterosmilax yunnanensis Gagneb. (Wei et al., 2018), Notopterygium incisum ex H,T-Chang (Notopterygii Rhizoma et Radix) (Yang et al., 2020), Dysosina versipellis (Hance) M. Cheng (Liu et al., 2010), etc. However, due to the multiple medicinal plants and the complex medicinal plant habitats, wild tending has not been popularized in the cultivation of all medicinal plants.
Still, it was gradually realized the importance of suitable ecological environment and appropriate planting technology in medicinal plants cultivation. Therefore, a new cultivation mode of medicinal plants has arisen.
2.4. Ecological cultivation
With the improvement of people's awareness of drug safety and sustainable utilization of soil, ecological cultivation, as a promising planting pattern, has grown boomingly. It applies the principles of unity, harmony, circulation and recycling of the ecosystem to achieve the simultaneous improvement of ecological, economic and social benefits in crop planting. It seeks to harmonize the relationship between organism and nature by comprehensively managing agricultural environments to get maximized economic and environmental benefits in a limited space. It emphasizes the interaction between the populations within the ecosystem, such as plants and plants, plants and microorganisms, with plant health as an indicator, aiming at providing a healthy and excellent ecological environment for plant growth (Wang, Qin, Huang, & Zhang, 2007, Xu, 2002). Some mature and feasible ecological planting patterns in Chinese herbal medicine cultivation have been formed, such as understory planting of Paris polyphylla Smith var. yunnanensis (Franch.) Hand. Mazz. mode (Lin et al., 2018) and Gastrodia elata Bl. Phallus impudicus sequential planting pattern (Zhang et al., 2020). However, the cultivation of some medicinal herbs has been proved difficult because of specific ecological requirements (Canter, Howard, & Edzard, 2005).
Traditional Chinese medical agriculture (TCMA), a derivative of ecological cultivation, applies the principles “harmony between man and nature” in traditional Chinese medicine to agriculture, realizes integration between traditional Chinese medicine and ecological cultivation. In TCMA, it was considered reasonable, that the biological elements and natural mineral elements can be made into nutrients or biogenic pesticides to promote plant growth and control plant diseases, which can effectively realize organic production and reduce drug residues (Yu and Hao, 2018, Zhang et al., 2018a, Zhang et al., 2017). At present, the principle of TCMA has been applied to the culture of rice and tea trees (Zhang et al., 2018a, Zhu, 2017). It was reported that the planting products produced by the principle of TCMA have excellent taste and yield (Zhang & Zhang, 2018, Zhu, 2017). Moreover, some researchers found that fertilizers made up of herbal medicines can increase crop yield and increase the number of soil bacteria, fungi and actinomycetes (Bi, Xia, Zhu, & Shi, 2008, Shi, Bi, Xia, Zhu, & Sun, 2010).
However, it is undeniable that the ecological cultivation of CHMs is still in the exploratory stage because of weak research foundation and little experience. There are little specific measures applied in ecological cultivation. Therefore, the following part looked forward to the key factors in ecological cultivation, which can be highly valued in practice, so as to serve the medical and health undertakings of mankind.
3. Key factors of ecological cultivation
There is no doubt that the bioactive components of medicinal plants are the most indispensable and worthy of attention. Obviously, the climatic and soil factors related to plant growth are the guiding points in this cultivation process. Additionally, in order to improve the quality and the yield of CHMs and facilitate the reuse of soil, botanical pesticides and appropriate cultivation measures are also recommended methods for ecological cultivation.
3.1. Climate factors determine the distribution of medicinal plant, as well as accumulation of secondary metabolites
Climate is an imperative factor affecting plant distribution. In recent years, the relationship between the distribution of medicinal plants and climate has been widely concerned. By analyzing the climate characteristics and geographical distribution of medicinal plants in Sichuan Province, Zhang et al. (2018c) found that the Sichuan Basin with high precipitation and short sunshine had the richest species diversity, while the western Sichuan Plateau with little precipitation and abundant light and heat had the least species diversity. Zhang et al. (2018b) reported that 12 species of Curcuma plants were distributed in 17 provinces in China, however, they were mainly distributed in subtropical regions such as Guangxi Zhuang Autonomous Region and Guangdong Province. In recent years, Global Geographic Information System (GIS) for medicinal plants has been used to identify the climate and soil regions with high ecological similarity and suitable for planting medicinal plants (Wu et al., 2019b). Dong et al. (2017) reported that the suitable cultivation area of Swertia mussotii Franch based on GIS technology was basically consistent with its distribution in practice.
The yield and quality of medicinal plants are closely related to climate change, just like the famous phenomenon of Nanju Beizhi (orange changes in morphological performances with their environment). Temperature, water, light and other factors are the basis of quality formation of medicinal plants. Lu et al. (2018) found that artificial high temperature can promote the biosynthesis of artemisinin in Artemisia annua L. by promoting the expression of synthetase genes in artemisinin synthesis pathway and inhibiting the expression of synthetase genes in artemisinin-competition pathway. Alhaithloul, Soliman, Ameta, El-Esawi, & Elkelish, 2019 reported that drought and heat stress triggered the accumulation of osmolytes proline, sugars, glycine betaine, and sugar alcohols including inositol and mannitol tannins, terpenoids, and alkaloids in Mentha piperita L. and Catharanthus roseus (L.) G. Don plants. Li et al. (2019a) found that the red/blue (2:1) light can promote the chlorophyll content, protein and total polysaccharides accumulation in Paris polyphylla significantly, and the blue light was beneficial for the accumulation of total saponins.
3.2. Healthy soil related to accumulation of secondary metabolites of medicinal plants
Medicinal plants-microorganism-soil forms a complicated ecosystem, in which microorganism, organic carbon and mineral elements in soil are closely associated with the quality formation of CHMs.
3.2.1. Beneficial microorganisms are the promoters of soil material and energy
3.2.1.1. Soil and microorganism in soil
In ancient China, pharmacists believed that the soil environment is the key to the formation of the CHMs quality (daodi nature). Healthy soil is a good foundation for medicinal plants growth. It was affected by complex soil factors, such as soil composition, structure and texture, soil fertility, and soil pH. Among them, the soil fertility is mainly affected by the soil humus, which is closely related to the activities of some microorganisms in the soil. More than 80% nitrogen in plants comes from biological nitrogen fixation. The nitrogen fixing bacteria in soil mainly include Paenibacillus, Rhizobium and Bradyrhizobium (Pastor-Bueis, Sanchez-Canizares, James, & Gonzalez-Andres, 2019, Zimmer et al., 2016). These bacteria can convert the nitrogen element in the air into the ionic nitrogen element for plant absorption. Phosphate solubilizing bacteria mainly include Trichoderma, Pseudomonas and Talaromyces (Bononi, Chiaramonte, Pansa, Moitinho, & Melo, 2020, Li et al., 2017b, Wu et al., 2019a). Inoculation of nitrogen-fixing bacteria Enterpriser Cloacae AKS7 into soil can significantly improve soil nitrogen content and nitrogen-fixing microorganism diversity (Chakraborty et al., 2019). P-solubilizing Pseudomonas sp. inoculated into P. ginseng rhizosphere can improve the activities of urease, acid phosphatase and peroxidase in ginseng soil, as well as promote the root biomass and ginsenosides (Li et al., 2017b). To sum up, these microorganisms can improve soil fertility, and provide a healthy environment for the growth of medicinal plants.
3.2.1.2. Endophyte and medicinal plants
In recent years, endophytes, especially endophytic fungi, as a part of plant symbiosis system, involved in the growth, metabolism and quality formation of medicinal plants, gained a lot of attention. Some endophytes can secrete indole acetic acid (IAA) to promote its host growth. Rehmannia glutinosa endophytic fungus Ceratobasidium sp. can secrete IAA to promote the increase of chlorophyll, root shoot ratio and biomass of R. glutinosa (Chen et al., 2011a). Bidens pilosa Libosch. endophytes Bacillus sp., Pseudomonas sp. and Burkholderia sp. can produce IAA with a yield of 57.48–312.22 μg/mL (Hu, Liu, Hu, & Li, 2019). Moreover, some endophytes are involved in plant resistance system regulation. B. subtilis 50–1 can decrease the death rate of ginseng seedlings (Dong et al., 2018b). Diisobutyl formate (DiBP) can inhibit the germination of P. ginseng seeds and the growth of ginseng buds, Sphingobacterium sp. PG-1 can decrease the content of DiBP and the mortality of ginseng after the inoculation (Dong et al., 2018a). And some endophytes are also related to medicinal plant quality formation. Ligusticum chuanxiong Hort. endophytic fungi were widely distributed and stable, while the structure of endophytic fungi of Xixiong in Gansu Province and Yunxiong in Yunnan Province was different from Chuanxiong in Sichuan (Wang & Yan, 2009). Hyphae polysaccharides secreted by the endophytic fungi Fusarium oxysporum dzf17 of Dioscorea zingiberensis C. H. Wright can induce the production of diosgenin (Zhang, Li, Xu, & Zhou, 2011).
3.2.1.3. Rhizosphere microorganisms and medicinal plants
Rhizosphere microorganism is known as the “second genome” of plant. It is the material and energy converter between soil and plant, which can influence the growth, quality, and the health of medicinal plants through microbial metabolisms and host interactions (Köberl, Schmidt, Ramadan, Bauer, & Berg, 2013). At present, researches on rhizosphere microorganisms and medicinal plants, mainly focus on the effects of rhizosphere microorganisms on the yield, quality and growth status of medicinal plants. Azotobacter chroococcum, Bacillus subtilis, Pseudomonas aeruginosa and B. pumilis, isolated from the rhizosphere of Cicer arietinum, were used as compound microbial inoculants, which can improve the germinating index, plant height, stem diameter and chlorophyll content of Cicer arietinum (Pandey, Gupta, & Ramawat, 2019). Stenotrophomonas maltophilia and P. fluorescens, isolated from the rhizosphere of Nicotiana glauca, can promote the growth of Hypericum perforatum and the secretion of hypericin and pseudohypericin (Mañero, Algar, Martín Gómez, Saco Sierra, & Solano, 2012). Wei et al. (2020b) found that the pathogenic fungi Cylindrocarpon, Alternaria and Fusarium were mainly concentrated in the rhizosphere of ginseng with rust root disease, which indicated that the microbial community structure of rhizosphere was closely related to the health status of plants.
Rational and effective use of the interaction between medicinal plants and beneficial microorganisms is of great practical significance, to promote the growth of medicinal plants, enhance their ability of resistance to abiotic stress and biotic stress. In ecological cultivation, microbial inoculants, composed of plant growth promoting rhizobacteria (PGPR) and other nutrient compounds, are realistic alternatives to chemical pesticides and fertilizers. They are commonly used to regulate the stability of soil microecology and improve the herb quality by promoting the absorption of plant nutrients, regulating the dynamic changes of plant hormones, and inhibiting the growth of pathogenic microorganisms (Compant, Clément, & Sessitsch, 2010, Naik, Mishra, Srichandan, Singh, & Sarangi, 2019). Gradually, microbial inoculants showed increasing application prospects in medicinal plant cultivation. Firstly, microbial inoculants can promote the accumulation of effective ingredients in medicinal materials. Tianxiadiyijun microbial inoculant applied to Salvia miltiorrhiza Bge. reduced the Cd content in by 37.90% under Cd stress, and promoted the increase of total tanshinone content by 40.45% (Wei et al., 2020a). The application of Glomus intraradices microbial inoculant increased ginsenosides Re, Rg1, Rb1, Rb2, Rc, and Rd in P. ginseng roots, improved the activities of polyphenol oxidase and catalase, and increased the relative abundance of rhizosphere beneficial bacteria, such as Bacillus sp., Flavobacterium sp., and Rhizobium sp. (Tian et al., 2019). Secondly, microbial inoculant can be used to control plant diseases. The application of different doses of microbial inoculant significantly reduced the incidence of P. ginseng root rot by 40.3%−47.3%, and increased the yield and ginsenoside content. Moreover, it significantly increased the relative abundance of potential beneficial microorganisms, Bacillus, Burkholderia, Rhizobium, Streptomyces, and Mycobacterium (Dong et al., 2019). Ning shield microbial inoculant can reduce 51.08% of the nematode disease of medicinal plant Trichosanthes meloidogyne and increase 36.26% of its yield, as well as improve the soil (Jiang et al., 2018). Thirdly, microbial inoculant can also be used for degradating pesticide residues. Paenibacillus polymyxa microbial inoculant can significantly reduce the contents of fluazinam, benzenehexachloride (BHC), pentachloronitrobenzene (PCNB), chlorpyrifos and dichlorodiphenyltrichloroethane (DDT) in ginseng roots by 66.07% 46.24%, 21.05%, 72.40% and 54.21%, respectively, which proved that P. polymyxa is an effective degrading bacterium for these pesticides (Zhang et al., 2019).
3.2.2. Carbon element is material basis of plant growth
Medicinal plants, as the botanical source of herbal medicine, serve the health of human beings and animals. They can get carbon source from a large number of straws and animal's feces besides photosynthesis. In the farmland system, the carbon elements in the soil flow from plants to animals, and finally return to the soil, which is the most common carbon cycle in the ecosystem. This is very important for soil quality and plant development. Some studies showed that returning straws to the field can not only change the taxonomic composition of arbuscular mycorrhizal (AM) fungi, but also increase the biomass of AM fungi (Ma, Song, Wang, Ma, & An, 2019). Moreover, straws can enrich microorganisms variety in salinized soil, affect the composition of Enterobacteriaceae, Sphingomonadaceae and Xanthomonidaceae, improve the aggregate structure of salinized soil, increase the content of soil organic matter, accelerate the release of mineral elements in soil, and finally promote the growth of plants (He et al., 2019, Li et al., 2019c). Furthermore, the mixture of animal manure and plant waste can significantly promote the growth of crops. For example, the mixture of fermented chicken manure, tea residue and greenhouse soil can significantly improve the plant height, stem diameter, fresh and dry quality, chlorophyll content, invertase activity and humus content in the soil (Chen, Wu, Fan, Zhu, & Li, 2019).
However, the utilization of animal and plant waste returned to the field is not optimistic up to now. In 2016, the amount of straw returned to the field in Ningxia Hui Autonomous Region, China, accounted for only 2.9% of the total straw resources (Ma, Song, Wang, Ma, & An, 2019). With the popularization of chemical agriculture and the increase of labor cost in recent years, most plant straws and animal feces are discharged into rivers or burned on the spot. This is not only to a large extent reduction of input of soil carbon sources, but also detrimental to the aquatic and atmospheric environment. Therefore, the decrease of soil carbon source input and the increase of chemical fertilizer wouldn’t change the “hunger” of soil carbon, but will lead to soil hardening and acidification (Shen, Wang, Wang, & Lv, 2004).
Biochar, as another supplemental soil carbon source, is a kind of highly aromatic and refractory solid-state high polymer which is produced by pyrolysis and carbonization of animal and plant residues or other creatures at relatively low temperature (<700 degreesC) under complete or partial anoxia (Oliveira et al., 2017). Firstly, it can not only improve soil pH, enhance soil water holding capacity, increase soil organic matter, etc., but also affects the structure of soil microorganisms, changes the abundance of soil bacteria and fungi, and promotes the improvement of soil microbial ecosystem (Jin et al., 2019, Li et al., 2019d, Yan, Niu, Yan, Zhang, & Liu, 2020). For example, with the increase of planting years, the rhizosphere soil microbial diversity of Andrographis paniculata (Burm.f.) Nees decreased, which may be one of the main reasons for the continuous cropping obstacles. However, biochar increased the number of bacteria, actinomycetes, as well as the Shannon and McIntosh index. Consquencely, the continuous cropping obstacle was relieved slightly (Li et al., 2019d). Secondly, biochar can be treated as a new type of soil amendment which can be used in remediation of heavy metal contaminated soil (Liu et al., 2020). Thirdly, the appropriate application of biochar may be beneficial to CHMs yield. Song, Xue, Chen, He, & Dai, 2014 reported that the mass ratios of biochar to soil is 1:1, 1:2, 1:3, 1:4 and 1:5 were better for accumulation of garlic biomass than that of control, and the mass ratio 1:4 was the most favorable one. In conclusion, there are sufficient evidences that biochar will contribute to medicinal plant cultivation.
3.2.3. Mineral elements in soil closely related to life activities
In chemical agriculture, we have fully realized the importance of nitrogen (N), phosphorus (P) and potassium (K) for plant growth and development. N is the main element of protein, and proper supplement of N can promote plant growth. P element is closely related to energy metabolism, storage and transmission of genetic information in plant cells. K element is associated with photosynthesis and stress resistance of plants. At the same time, we also realize that the scientific ratio of NPK can promote the accumulation of medicinal plant yield (Niu et al., 2020, Wang et al., 2020). However, there are fewer researches on mineral fertilizer for the growth of plants. According to the report issued by the World Health Organization in 2015, nearly 5 billion people in the world have different forms of micronutrient deficiency. About 2 billion people lack iron and 2 billion people lack iodine. Modern medicine shows that 70% of chronic diseases, such as diabetes, cardiovascular disease, cancer and so on, are related to the imbalance of human nutrition intake. “Hidden hunger” is becoming an invisible killer affecting people's health. Generally speaking, mineral elements are directly or indirectly derived from soil. They make all the difference for the composition of soil aggregates, plant disease resistance and insect defense. In the continuous cropping system of medicinal plants, the nutrient preference absorption leads to some elements decrease along with the cultivation years increasing. If the elements are not supplemented in time, the soil would become poorer, and the plant growth will be more and more unbalanced, thus indirectly affecting human health (Lv, 2006). The contents of nitrogen and phosphorus in the three-year-old P. notoginseng soil decreased significantly, while iron, boron and aluminum was increased, suggesting that soil acidification and the imbalance of mineral element may be one of the reasons of continuous cropping obstacles (Sun et al., 2015).
Essential mineral elements involved life activities are mainly including potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), molybdenum (Mo), etc. They are not only important components of cell substance, but also participate in cell metabolism. Iron is an important raw material for photosynthesis and heme biosynthesis (Georg et al., 2017). Zinc is an important component of carbonic anhydrase (CA), which can catalyze the hydration of carbon dioxide. Other elements are also related to plant life activities and the formation of medicinal plant quality.
In cultivation practice, a small amount of accurate mineral elements is beneficial to improve the quality of medicinal plants. When cultivating balangu (Lallemantia iberica (M.B.) Fischer & Meyer) in winter, nano-NPK + nano-Fe fertilizers are the most appropriate treatment to acquire the highest qualitative and quantitative yield (Mohammad Ghasemi, Siavash Moghaddam, Rahimi, Pourakbar, & Popovic-Djordjevic, 2020). After the addition of exogenous 300 mol/L Cu, the vinblastine content in Catharanthus roseus (L.) G. Don was increased by 1.7 times, and at the same time, the plant biomass was enhanced (Lin, Gao, Yu, & Chen, 2016). Adding 0.2% nano-sized Zn compounded with 5-aminolevulinic acid can increase the content of (E)-anisole, β-bisphenol butene, germanium acridine d, methyl chavicol and α-turmeric in Pimpinella anisum L. (Tavallali, Rahmati, & Rowshan, 2017). In summary, mineral elements can improve the quality of medicinal plants and precisely light up the future of ecological cultivation.
3.3. Secondary metabolites — A guardian of medicinal plants and human health
3.3.1. Primary functions of secondary metabolites of medicinal plants
The secondary metabolites are the main active components of herbal medicines. When encountering special environment, medicinal plants synthesize phenols, terpenes and nitrogen compounds. These substances in plants are significant in their ecological adaptability, serving as natural defense weapons. Here we call those functions as the primary functions of secondary metabolites. Phenols, (Chen et al., 2011b, Pei, Du, Nie, Zhai, & Zhang, 2014), terpenes, (Wang & Isman, 2014, Wu, 2015), and compounds containing nitrogen (Gao, Zhang, Hu, Qi, & Tan, 2006), are the main secondary metabolites in plants. These secondary metabolites are the fatal biological weapons, which can help plants to resist adversity.
Further, the secondary metabolites can be used as a source of biocide for bio-control of insect pests. On the one hand, the secondary metabolites of medicinal plants or their derivatives can be directly used as botanical pesticides to participate in the management of diseases and insect pests. The essential oil blend (1:1) form Melaleuca leucadendra (L.) L. and Schinus terebinthifolius Raddi showed toxicity to Tetranychus urticae, and can work with Neoseiulus californicus for the integrated management of T. urticae (De Araujo, Da Camara, Born, & De Moraes, 2020). Itol A, an isoryanodane diterpene derived from Itoa orientalis Hemsl. (Flacourtiaceae), can prolong the growth period, weaken the quality of pupae and cause various abnormalities of Spodoptera litura (Ling, He, Zeng, & Tang, 2020). Honokiol, isolated from the bark and leaf of Magnolia officinalis and M. obovata, its derivatives can be used to control the larvae of Mythimna separata Walker and Plutella xylostella Linnaeus, whose control effect is better than that of toosendanin, a well-known botanical insecticide (Zhi et al., 2020). On the other hand, the secondary metabolites of medicinal plants can indirectly participate in the biological control of insect pests and phytopathogen. The new materials manufactured by the secondary metabolites of medicinal plants and nano materials have the potential to control important agricultural phytopathogen in vitro and planta. The silver nanoparticles (P-AgNPs) reduced by aqueous fruit peel extract of Momordica charantia L. showed 85% mortality in Aedes albopictus at 20 ug/mL concentration (Shelar et al., 2019). Importantly, some biocompatible iron oxide nanoparticles (Fe2O3-NPs) made from the leaf extractions of Skimmia laureola (DC.) Sieb. et Zucc. ex Walp. have the potential to control phytopathogen Ralstonia solanacearum and enhance the growth of plant shoots, root length and fresh biomass (Alam et al., 2019). Anyway, the secondary metabolites of medicinal plants can be acted as botanical substances utilized in the field management, which is in line with the development of ecological cultivation.
3.3.2. Secondary function of secondary metabolites
Secondary metabolites of medicinal plants also participate in human health management. Here we call those functions as the secondary function of secondary metabolites. With the further research on modern pharmacology and phytochemistry, more and more secondary metabolites in CHMs have been verified. In the 20th century, facing the global malaria epidemic, You-you Tu, got inspiration from the traditional Chinese Medicine Classics, Handbook of Precriptions for Emergency (Zhouhou Beiji Fang), which recorded that the decoction of Artemisiae Hnnuae Herba (Qinghao in Chinese) can treat malaria. Firstly, she found and purified artemisinin. This discovery provided valuable support for malaria drug treatment worldwide (Li et al., 2019b). Dioscoreae Rhizoma (Chinese yam) was first recorded in Shennong Herbal Classic, which was used to tonify the middle and replenish qi. In recent years, it has been found that the diosgenin in Dioscotea opposite Thunb not only has good anti-tumor, anti-inflammatory, hypolipidemic, but also has a certain effect on neuroimmunity and behavior improvement (Leng et al., 2020). Coptids Rhizoma has effect on clearing heat and dampness, purging fire and detoxification. Chemical analysis revealed that berberine, coptisine and epiberberine were the main active components of Coptis chinensis Fransh. Among them, berberine has the effect of clearing away heat and detoxification, and is widely used as a traditional anti-inflammatory and anti-bacterial drug because of its small side effects (Zhou, Xiang, Zhang, & Yang, 2020). In brief, the secondary metabolites are also functional substances of CHMs, which are closely related to the prevention and treatment of diseases.
3.3.3. Secondary metabolites production and TCM quality
The accumulation of secondary metabolites is closely related to medicinal plant’s living environment. First of all, the genuine nature of CHMs suggests that the production of medicinal plants has regional characteristics. Therefore, representative authentic medicinal materials have been formed in various places, such as Huaishanyao in Henan, Hangbaiju in Hangzhou, Chuanbeimu in Sichuan, etc. Secondly, special stress would directly or indirectly affect the accumulation of secondary metabolites. “Adversity effect” considers that “sub-optimal” conditions would promote the accumulation of the secondary metabolites (Yuan, Zhou, & Huang, 2016). For some medicinal plants, the content of secondary metabolites of some medicinal plants is higher under adversity. For example, the amount of volatile oil in Angelica sinensis (Oliv.) Diels and Mentha haplocalyx Briq, and rographolide in Andrographis paniculata (Burm.f.) Nees, belladonna alkaloid in Atropa belladonna L. leaves and digitalis glycoside C in Digitalis purpurea L. leaves under high temperature are higher than those of in humid environment. When the external environment changes, plants will produce some signal molecules, and react at morphological structure, tissue cell and molecular level to adapt to environment (Liu et al., 2013). Many scholars have studied plant resistance gene expression under stress to explore the relationship between secondary metabolites and stress. Coronatine insensitive 1 (COI1) has the function of regulating plant resistance and quality formation (Stotz et al., 2011, Ye et al., 2012). This gene of Salvia miltiorrhiza is involved in regulating the insect resistance (Chen et al., 2018). Dehydration responsive element binding protein (DREB) is a unique transcription factor related to environmental stress. It can be combined with DRE cis-acting elements to regulate the expression of stress response genes (Song et al., 2019). When plants are suffering from abiotic stress such as drought, salinity, high temperature and low temperature, they can grow up by regulating the synthesis of osmoregulation substances, stress-resistant proteins and hormones and the regulation of antioxidant system (Liang et al., 2016, Lu et al., 2018).
Generally, some specific cultivation measures will trigger the improvement of medicinal plants quality and yield. Gao et al. (2016) compared the contents of lobetyolin in Codonopsis Radix (Codonopsis pilosula (Franch.) Nannf.) cultivated in different cultivation measures, and found that the appropriate cultivation measure was not using Zhuanggenling foliar fertilizer, not pinching and shelving. In addition, the intercropping patterns of some medicinal plants are beneficial to the accumulation of secondary metabolites. For example, the intercropping of Mentha piperita L. and Vicia Faba L. can increase the content of menthone (Machiani, Javanmard, Morshedloo, & Maggi, 2018), and the intercropping of Dracocephalum moldavica L. (dragonhead) and Glycine max increased the content of geranyl acetate (Fallah, Rostaei, Lorigooini, & Abbasi Surki, 2018).
4. Future prospects
In ecological cultivation, besides suitable climatic factors, proper soil conditions should also be provided for the growth of plants, because healthy soil brings laudable life. Soil not only provides food and fiber for people to live on, but also is an imperative material source of biomedicine, which serves as fatal role in the medicinal plant growth’s medium of water and nutrient reserves. However, over the past few decades, too much attention was paid to the quality evaluation of CHMs, neglected the role of soil in the cultivation process. Therefore, to develop ecological cultivation of medicinal plants, we should focus on soil quality urgently right now.
4.1. Soil quality assessment and cultivation of medicinal plants
Soil quality refers to the capacity of soil working within ecosystem boundaries to sustain biological productivity, preserve environmental quality, and promote the health of plant and animal (Bünemann et al., 2018). The function of soil biological productivity means the ability of soil to provide plant nutrients and produce biomass. Generally, soil fertility index (i.e., soil organic matter, pH, available phosphorus, available nitrogen and available potassium) and soil enzyme (i.e., catalase, polyphenol oxidase, transferase, urease and phosphatase) activity can be measured to guide the cultivation of medicinal plants. The capacity of soil environmental quality preservation refers to the function of soil to contain, absorb and degrade various environmental pollutants. Through the dynamic monitoring of heavy metals or pesticides can tell us whether the soil is suitable for further cultivation. Promoting plant and animal health refers to soil affecting survival of human and animals producing safe by providing high-quality plants. Soil organisms related to soil function closely, and can be an excellent indicator of soil health function. The next generation sequencing (NGS) technology based on 16S rRNA (Fu et al., 2017) and internal transcribed spacer (ITS) rRNA (Wei et al., 2020b) gene sequences can truly reflect the composition of soil microbial community structure reliably, as well as soil nutrient cycling, which is one of the important research directions in the ecological cultivation.
4.2. Simulation model of soil quality evolution to guide cultivation activities
Soil quality fluctuation is a complex geochemical process, which is affected by many factors such as climate, plant growth, soil properties and human activities. Modern statistical analysis technology, such as R program language, can reveal the role and contribution of multiple factors to soil quality (Wu & Liu, 2014). For the prediction of soil organic carbon evolution trend, CENTURY (Kwon et al., 2017), RothC (Yang et al., 2003), DNDC (Cui & Wang, 2019) and other models can be applied based on a series of long-term experimental data. These models are indispensable tools to develop and construct soil quality prediction models suitably for complex climate types and diversified planting conditions, and to guide the cultivation of medicinal plants.
4.3. Soil quality and ecological cultivation of medicinal plants
Medicinal plants are important parts of farmland ecosystem. Therefore, the development of ecological planting of medicinal plants is bound to focus on the important soil factors for the growth of medicinal plants. On the basis of soil quality assessment, it is possible to realize the ecological cultivation of medicinal plants by introducing beneficial microorganisms and important elements of plant growth to regulate soil quality. Previous studies have shown that beneficial microorganisms can promote the yield and quality of medicinal plants and reduce the incidence rate of plant diseases (Dong et al., 2019, Wei et al., 2020a). At the same time, as an integral part of cell structure, additional carbon supplement can promote the growth of plants (Meng et al., 2019). In addition to NPK fertilizers, the supplement of mineral elements required by the growth of medicinal plants can promote the growth and development of plants, and alleviate the lack of some mineral elements in soil (Mohammad Ghasemi, Siavash Moghaddam, Rahimi, Pourakbar, & Popovic-Djordjevic, 2020). Furthermore, targeted agronomic measures and botanical pesticides can be involved in the field management of ecological cultivation to reduce the adverse effects of chemical products on soil quality (Machiani, Javanmard, Morshedloo, & Maggi, 2018, Zhi et al., 2020).
In a word, soil quality is not only related to the ecological cultivation of medicinal plants, but also the environmental management and agricultural production. Importantly, they are in line with the ecological development concept of “Lucid waters and lush mountains are invaluable assets” in China. At present, the transmission of high-quality Chinese herbal medicine laid on healthy soil must be contained in the development of ecological cultivation. Ecological cultivation based on the healthy soil not only ensures healthy botanical source of herb medicine for today and future, but also protects ecosystem for sustainable cultivation of medicinal plants. Although there is still a lot of specific work to be overcomed, from the perspective of practical application effect, it will lighten the future of Chinese medicine.
5. Conclusion
With the increasing demand for global herbal medicine, the safety and quality of CHMs is hard to ignore. Domestic cultivated medicinal plants are the main source of herbal medicine. Ecological cultivation focusing on the climate and soil factors of the growth of medicinal plants, may light up the future of high-quality herbal medicine production.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by grants from the National Key Research and Development Program of China (2019YFC1604701).
References
- Alam T., Khan R.A.A., Ali A., Sher H., Ullah Z., Ali M. Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Materials Science and Engineering: C. 2019;98:101–108. doi: 10.1016/j.msec.2018.12.117. [DOI] [PubMed] [Google Scholar]
- Alhaithloul H., Soliman M., Ameta K., El-Esawi M., Elkelish A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules. 2019;10(1):43. doi: 10.3390/biom10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J., Xia G., Zhu G., Shi G. Effects of plant-sourced officinal-fertilizer on earthnut growth, anti-pest efficiency and soil activity. Chinese Journal of Soil Science. 2008;39(5):1097–1101. [Google Scholar]
- Bononi L., Chiaramonte J.B., Pansa C.C., Moitinho M.A., Melo I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Scientific Reports. 2020;10(1):2858. doi: 10.1038/s41598-020-59793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann E.K., Bongiorno G., Bai Z., Creamer R.E., De Deyn G., de Goede R., et al. Soil quality – a critical review. Soil Biology and Biochemistry. 2018;120:105–125. [Google Scholar]
- Cai J., Lu J., Li Q., Guo S., Dai Y. Analysis on volatile components of Caryophylli Flos from different habitats. Plant Science Journal. 2015;33(2):251–258. [Google Scholar]
- Canter P.H., Howard T., Edzard E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends in Biotechnology. 2005;23(4):180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Chakraborty P., Sarker R.K., Roy R., Ghosh A., Maiti D., Tribedi P. Bioaugmentation of soil with Enterobacter cloacae AKS7 enhances soil nitrogen content and boosts soil microbial functional-diversity. 3 Biotech. 2019;9(7):253. doi: 10.1007/s13205-019-1791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Wang M., Hu Y., Lin Z., Yu R., Huang L. Preliminary study on promoting effects of endophytic fungi to growth of Rehmannia glutinosa. Journal of Chinese Medicinal Materials. 2011;36(9):1137–1140. [PubMed] [Google Scholar]
- Chen C., Cao X., Hua W., Huang Y., Lv T., Zhang Y., et al. Roles of SmCOI1 in pest resistance and secondary metabolism regulation based on Salvia miltiorrhiza Bunge genome. Science in China (Series C) 2018;48(4):399–411. [Google Scholar]
- Chen J., Hong J., Du Q., Li M. Prevention of flavonoids from Oxytropis falcate Bunge against UVB-induced damage to HaCaT keratinocytes. Pharmaceutical Journal of Chinese People's Liberation Army. 2011;27(4):289–292. [Google Scholar]
- Chen S., Wei J., Huang L., Guo B., Xiao P. Probing into the theory and practice of wild medicinal materials tending. Journal of Chinese Medicinal Materials. 2004;29(12):1123–1126. [Google Scholar]
- Chen S., Yu H., Luo H., Wu Q., Li C., Steinmetz5 A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chinese Medicine. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wu J., Fan W., Zhu W., Li X. Effects of different organic materials on the morphology and composition of soil humus binding in primary saline and alkaline land. Journal of Soil and Water Conservation. 2019;33(1):200–205. [Google Scholar]
- Cheng M., Yang G., Li Y., Huang L. A brief introduction to the report on the development of traditional Chinese medicine resources. China Food & Drug Administration Magazine. 2020;01:4–11. [Google Scholar]
- Compant S., Clément C., Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biology and Biochemistry. 2010;42(5):669–678. [Google Scholar]
- Confucius . Zhonghua Book Company; Beijing: 2015. The Book of Songs. [Google Scholar]
- Cui G., Wang J. Improving the DNDC biogeochemistry model to simulate soil temperature and emissions of nitrous oxide and carbon dioxide in cold regions. Science of the Total Environment. 2019;687:61–70. doi: 10.1016/j.scitotenv.2019.06.054. [DOI] [PubMed] [Google Scholar]
- Dao X., Meng Q., Li X. Comparative analysis of the quality of Sterculia lychnophora. Chinese Traditional and Herbal Drugs. 2000;31(7):556–558. [Google Scholar]
- De Araujo M.J.C., Da Camara C.A.G., Born F.S., De Moraes M.M. Acaricidal activity of binary blends of essential oils and selected constituents against Tetranychus urticae in laboratory/greenhouse experiments and the impact on Neoseiulus californicus. Experimental & Applied Acarology. 2020;80(3):423–444. doi: 10.1007/s10493-020-00464-8. [DOI] [PubMed] [Google Scholar]
- Dong L., Li Y., Xu J., Yang J., Wei G., Shen L., et al. Biofertilizers regulate the soil microbial community and enhance Panax ginseng yields. Chinese Medcine. 2019;14(1) doi: 10.1186/s13020-019-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Xu J., Li Y., Fang H., Niu W., Li X., et al. Manipulation of microbial community in the rhizosphere alleviates the replanting issues in Panax ginseng. Soil Biology and Biochemistry. 2018;125:64–74. [Google Scholar]
- Dong L., Xu J., Zhang L., Cheng R., Wei G., Su H., et al. Rhizospheric microbial communities are driven by Panax ginseng at different growth stages and biocontrol bacteria alleviates replanting mortality. Acta Pharmaceutica Sinica B. 2018;8(2):272–282. doi: 10.1016/j.apsb.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.B., Luo Y., Zhu C., Peng W.F., Xu X.L., Fang Q.M. Application of remote sensing and GIS in study of suitability distribution of Swertia mussotii, a Tibetan medicine in Sichuan province. Journal of Chinese Medicinal Materials. 2017;42(22):4387–4394. doi: 10.19540/j.cnki.cjcmm.2017.0189. [DOI] [PubMed] [Google Scholar]
- Fallah S., Rostaei M., Lorigooini Z., Abbasi Surki A. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead- soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Industrial Crops and Products. 2018;115:158–165. [Google Scholar]
- Fu L., Penton C.R., Ruan Y., Shen Z., Xue C., Li R., et al. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biology and Biochemistry. 2017;104:39–48. [Google Scholar]
- Gao S.M., Liu J.S., Sun T., Liu F.S., Jing H., Qi Y.D., et al. Influence of different cultivation measures on chemical quality of Codonopsis Radix. China Journal of Chinese Materia Medica. 2016;41(20):3753–3760. doi: 10.4268/cjcmm20162009. [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhang X., Hu Y., Qi J., Tan C. The new prosess in studies of L-Canavanine. Chemical Intermediate. 2006;3:5–7. [Google Scholar]
- Georg J., Kostova G., Vuorijoki L., Schön V., Kadowaki T., Huokko T., et al. Acclimation of oxygenic photosynthesis to iron starvation Is controlled by the sRNA isaR1. Current Biology. 2017;27(10):1425–1436.e7. doi: 10.1016/j.cub.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Greenpeace. (2013). Chinese Herbs: elixir of health or pesticide cocktail Retrieved from http://www.greenpeace.org/eastasia/campaigns/food-agriculture/Chinese-Herbs-Elixir-ofHealth/
- Gu X., Yu W., Luan C., Wang X. The ecological environment and distribution of Panax ginseng C.A.Meyer and P. qiunqueflins Linn germplasm resources. Renshen Yanjiu. 2006;2:4–7. [Google Scholar]
- Guo L., Zhou L., Mo G., Wang S., Huang L. Ecological agriculture: Future of good agriculture practice of Chinese materia medica. Journal of Chinese Medicinal Materials. 2015;40(17):3360–3366. [PubMed] [Google Scholar]
- Guo L.P., Zhang Y., Zhu S.D., Wang G.H., Huang L.-Q. Good agricultural practice (GAP) of Chinese materia medica (CMM) for ten years: Achievements, problems and proposals. Journal of Chinese Medicinal Materials. 2014;39(7):1143–1151. [PubMed] [Google Scholar]
- Guo M., Fu C., Chen Y. The current situation and sustainable exploitation and utilization of traditional Chinese medicine resources. Journal of Pharmaceutical Research. 2019;38(5):295–298. [Google Scholar]
- Guo Q. Beijing China Higher Education Press; 2009. Medicinal plant cultivation. [Google Scholar]
- He Z., Dong H., Lou C., Wang X., Han Y. Effects of corn stalks on microenvironment of facility secondary salinized soil and tomato growth. China. 2019;Vegetables(6):39–44. [Google Scholar]
- Hill S.N., Hausbeck M.K. Virulence and fungicide sensitivity of Phytophthora cactorum isolated from American ginseng gardens in Wisconsin and Michigan. Plant Disease. 2008;92(8):1183–1189. doi: 10.1094/PDIS-92-8-1183. [DOI] [PubMed] [Google Scholar]
- Hu Z., Liu Y., Hu C., Li S. Diversity, heavy-metal tolerance and indoleacetic acid production of bacterial endophytes in Bidens pilosa. Microbiology. 2019;46(1):29–41. [Google Scholar]
- Hua X., Jiang X. Characteristics and control countermeasures of pesticide pollution and its damage on environmental in China. Research of environmental science. 2000;13(3):40–43. [Google Scholar]
- Huang Q., Zhou Z., Wang J., Liu R. Investigation and collection development pattern for genuineness of Aconitum carmichali. Journal of Chinese Medicinal Materials. 2011;36(18):2599–2601. [PubMed] [Google Scholar]
- Jia S., Shi S. Zhonghua Book Company; 2015. Qimin Yaoshu. [Google Scholar]
- Jiang C.H., Xie P., Li K., Xie Y.S., Chen L.J., Wang J.S., et al. Evaluation of root-knot nematode disease control and plant growth promotion potential of biofertilizer Ning shield on Trichosanthes kirilowii in the field. Brazilian Journal of Microbiology. 2018;49(2):232–239. doi: 10.1016/j.bjm.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Chen C., Chen X., Hopkins I., Zhang X., Han Z., et al. The crucial factors of soil fertility and rapeseed yield - A five year field trial with biochar addition in upland red soil, China. Science of the Total Environment. 2019;649:1467–1480. doi: 10.1016/j.scitotenv.2018.08.412. [DOI] [PubMed] [Google Scholar]
- Kang C., Guo L., Zhou T., Zhao D., Kang L., He Y., et al. Discussion on present situation of study on pesticide residues in Chinese herbal medicines. Journal of Chinese Medicinal Materials. 2016;41(2):155–159. doi: 10.4268/cjcmm20160201. [DOI] [PubMed] [Google Scholar]
- Köberl M., Schmidt R., Ramadan E.M., Bauer R., Berg G. The microbiome of medicinal plants: Diversity and importance for plant growth, quality, and health. Frontiers in Microbiology. 2013;4:400. doi: 10.3389/fmicb.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Ugarte C.M., Ogle S.M., Williams S.A., Wander M.M., Silva L.C.R. Use of inverse modeling to evaluate CENTURY-predictions for soil carbon sequestration in US rain-fed corn production systems. PLoS One. 2017;12(2):e0172861. doi: 10.1371/journal.pone.0172861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng J., Li X., Tian H., Liu C., Guo Y., Zhang S., et al. Neuroprotective effect of diosgenin in a mouse model of diabetic peripheral neuropathy involves the Nrf2/HO-1 pathway. BMC Complementary Medicine and Therapies. 2020;20(1) doi: 10.1186/s12906-020-02930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Tang Z., Wang N., Sun X., Huang W., Song Z., et al. Effects of different LED light quality on physiological characteristics and component accumulation of Paris polyphylla in Shaanxi province. Modern Chinese Medcine. 2019;21(10):1386–1391. [Google Scholar]
- Li H., Qin D., Ge W., Wang Z., Cao L., Xiao W., et al. Research progress on chemical constituents of Artemisia annua and its pharmacological activities. Chinese Traditional and Herbal Drugs. 2019;50(14):3461–3470. [Google Scholar]
- Li J., Gong L., Ji X., Zhang J., Miao P. Development paths of China's agricultural pharmaceutical industry under eco-agriculture background. Pakistan Journal of Pharmaceutical Sciences. 2014;27(4 Suppl):1049–1055. [PubMed] [Google Scholar]
- Li J., Gu Y., Xue J., Jin H., Ma S. Analysis and risk assessment of pesticide residues in a Chinese herbal medicine Lonicera japonica Thunb. Chromatographia. 2017;80(3):503–512. [Google Scholar]
- Li L., Sun H., Liu Z., Zhang C., Yayu Z. Screening and identification of phosphate solubilizing bacteria from ginseng root zone and its effect on ginseng growth. Soil and Fertilizer Sciences in China. 2017;6:163–170. [Google Scholar]
- Li S. Jiangsu People's Publishing. LTD; Nanjing: 2004. Compendium of Materia Medica. [Google Scholar]
- Li Y., Li Y., Liu Z., Meng X., Hu Y., Jin L., et al. Effects of straw incorporation on aggregate stability and organic carbon content of black soil in continuous cropping maize. Soil and Crop. 2019;8(2):129–138. [Google Scholar]
- Li Z., Chen Y., Chen R., Yang C., Chen N., Lu W. Effects of biochar on rhizospheric soil microbial community of continuous cropping Andrographis Paniculata. Journal of Shangrao Normal University. 2019;39(6):50–55. [Google Scholar]
- Liang J., Jia X., Liu Y., Wu Y., Zhou R., Feng Q. Effects of drought stress on seedling growth and accumulation of secondary metabolites in the roots of Astragalus membranaceus var.mongholicus. Acta Ecologica Sinica. 2016;36(14):4415–4422. [Google Scholar]
- Lin J., Zhang M., Chen T., Zhou X., Guo J., Wang T., et al. Research status and analysis on understory planting of Paris polyphylla. Modern Chinese Medcine. 2018;20(10):30–34. [Google Scholar]
- Lin S., Gao J., Yu B., Chen H. Effects of copper stress on the alkaoids of Catharanthus roseus. Bulletin of Botanical Research. 2016;36(3):374–379. [Google Scholar]
- Ling S.Q., He B., Zeng D.Q., Tang W.W. Effects of botanical pesticide itol against the tobacco cutworm, Spodoptera litura (Fab.) Environmental Science And Pollution Research International. 2020;27(11):12181–12191. doi: 10.1007/s11356-020-07824-2. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhao Z., Chen L., Wang L., Ji L., Xiao Y. Influences of arbuscular mycorrhizae, phosphorus fertiliser and biochar on alfalfa growth, nutrient status and cadmium uptake. Ecotoxicology and Environment Safety. 2020;196:110537. doi: 10.1016/j.ecoenv.2020.110537. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen H., Yang Y., Zhang Z., Wei J., Meng H., et al. Hole-tree agarwood-inducing technique: An efficient novel. Molecules. 2013;18(3):3086–3106. doi: 10.3390/molecules18033086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu Z., Xiao B., Hu K., Li J., Feng X. Wildlife tending of endangered herbal plant Dysosina versipellis. Chinese Agricultural Science Bulletin. 2010;26(05):276–278. [Google Scholar]
- Lu J., Zhang D., Ding D., Gao H., Han Z., Liu X., et al. Mechanism of high temperature promoting artemisinin biosynthesis in Artemisia annua. China Journal of Chinese Materia Medica. 2018;43(20):4169–4176. doi: 10.19540/j.cnki.cjcmm.20180726.011. [DOI] [PubMed] [Google Scholar]
- Lv W. Effects of cucumber continuous cropping on the soil physi-chemical characters and biological activities. Chinese Journal of Eco-agriculture. 2006;14(2):119–121. [Google Scholar]
- Ma K., Song L., Wang M., Ma Z., An Y. Effects of maize straw returning on arbuscular mycorrhizal fungal community structure in soil. Chinese Journal of Applied Ecology. 2019;30(8):2746–2756. doi: 10.13287/j.1001-9332.201908.034. [DOI] [PubMed] [Google Scholar]
- Machiani M.A., Javanmard A., Morshedloo M.R., Maggi F. Evaluation of yield, essential oil content and compositions of peppermint (Mentha piperita L.) intercropped with faba bean (Vicia faba L.) Journal of Cleaner Production. 2018;171:529–537. [Google Scholar]
- Mañero F.J., Algar E., Martín Gómez M.S., Saco Sierra M.D., Solano B.R. Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharmaceutical Biology. 2012;50(10):1201–1209. doi: 10.3109/13880209.2012.664150. [DOI] [PubMed] [Google Scholar]
- Meng L., Sun T., Li M., Saleem M., Zhang Q., Wang C. Soil-applied biochar increases microbial diversity and wheat plant performance under herbicide fomesafen stress. Ecotoxicology and Environment Safety. 2019;171:75–83. doi: 10.1016/j.ecoenv.2018.12.065. [DOI] [PubMed] [Google Scholar]
- Mohammad Ghasemi V., Siavash Moghaddam S., Rahimi A., Pourakbar L., Popovic-Djordjevic J. Winter cultivation and nano fertilizers improve yield components and antioxidant traits of dragon’s head (Lallemantia iberica (M.B.) Fischer & Meyer) Plants. 2020;9:252. doi: 10.3390/plants9020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monica B. The nitrogen cycle in the soil, impact on the environment. Environmental Analysis & Ecology Studies. 2018;4(4) [Google Scholar]
- Naik K., Mishra S., Srichandan H., Singh P.K., Sarangi P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatalysis and Agricultural Biotechnology. 2019;21:101326. [Google Scholar]
- Niu S., Xu H., Sun Z., Wang D., Zhao W., Ma W. Effect of NPK application rates and basal/dressing ratios on yield and nutrient utilization of yam. Journal of Plant Nutrition and Fertilizers. 2020;26(09):1702–1713. [Google Scholar]
- Oliveira F.R., Patel A.K., Jaisi D.P., Adhikari S., Lu H., Khanal S.K. Environmental application of biochar: Current status and perspectives. Bioresource Technology. 2017;246:110–122. doi: 10.1016/j.biortech.2017.08.122. [DOI] [PubMed] [Google Scholar]
- Pandey S., Gupta S., Ramawat N. Unravelling the potential of microbes isolated from rhizospheric soil of chickpea (Cicer arietinum) as plant growth promoter. 3 Biotech. 2019;9(7):277. doi: 10.1007/s13205-019-1809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Bueis R., Sanchez-Canizares C., James E.K., Gonzalez-Andres F. Formulation of a highly effective Inoculant for common bean based on an autochthonous elite strain of Rhizobium leguminosarum bv. phaseoli, and genomic-based insights into its agronomic performance. Frontiers in Microbiology. 2019;10(2724) doi: 10.3389/fmicb.2019.02724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Du X., Nie J., Zhai H., Zhang B. Allelopathy of gallic acid on Microcystis aeruginosa. Bulletin of Botanical Research. 2014;34(6):840–844. [Google Scholar]
- Pharmacopoeia Committee of P. R. China. (2015). Chinese pharmacopoeia. Beijing: China Medical Science Press.
- Savci S. An agricultural pollutant: Chemical fertilizer. Environmental Science and Development. 2012;3(1):73–80. [Google Scholar]
- Shelar A., Sangshetti J., Chakraborti S., Singh A.V., Patil R., Gosavi S. Helminthicidal and larvicidal potentials of biogenic Silver nanoparticles synthesized from medicinal plant Momordica charantia. Journal of Medicinal Chemistry. 2019;15(7):781–789. doi: 10.2174/1573406415666190430142637. [DOI] [PubMed] [Google Scholar]
- Shen A., Wang Y., Wang S., Lv A. Current situation of research and development of microorganism fertilizer and its application prospects. Journal of Henan Agricultural Sciences. 2004;33(4):34–36. [Google Scholar]
- Shen N. Xueyuan Press of China; Beijing: 2007. Shennong herbal classic. [Google Scholar]
- Shi G., Bi J., Xia G., Zhu G., Sun G. Effects of plant-sourced medical fertilizer on potato and physical and chemical properties of soil. Chinese Agricultural Science Bulletin. 2010;26(1):115–120. [Google Scholar]
- Song T., Li K., Wu T., Wang Y., Zhang X., Xu X., et al. Identification of new regulators through transcriptome analysis that regulate anthocyanin biosynthesis in apple leaves at low temperatures. PLoS One. 2019;14(1):e0210672. doi: 10.1371/journal.pone.0210672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.D., Xue X.Y., Chen D.Z., He P.J., Dai X.H. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere. 2014;109:213–220. doi: 10.1016/j.chemosphere.2014.01.070. [DOI] [PubMed] [Google Scholar]
- Stotz H.U., Jikumaru Y., Shimada Y., Sasaki E., Stingl N., Mueller M.J., et al. Jasmonate-dependent and COI1-independent defense responses against Sclerotinia sclerotiorum in Arabidopsis thaliana: Auxin is part of COI1-independent defense signaling. Plant and Cell Physiology. 2011;52(11):1941–1956. doi: 10.1093/pcp/pcr127. [DOI] [PubMed] [Google Scholar]
- Su J. Vol. 1. Anhui Science and Technology Press; Hefei: 2005. (Xinxiu Bencao). [Google Scholar]
- Sun S. Second Military Medical University Press; Shanghai: 2011. Qianjin Yifang. [Google Scholar]
- Sun X., Long G., Zhang G., Meng Z., Chen Z., Yang S., et al. Properties of soil physical-chemistry and activities of soil enzymes in context of continuous cropping obstacles for Panax notoginseng. Ecology and Environment Sciences. 2015;24(3):409–417. [Google Scholar]
- Tavallali V., Rahmati S., Rowshan V. Characterization and influence of green synthesis of nano-sized zinc complex with 5-Aminolevulinic acid on bioactive compounds of aniseed. Chemistry & Biodiversity. 2017;14(11) doi: 10.1002/cbdv.201700197. [DOI] [PubMed] [Google Scholar]
- Tian L., Shi S., Ma L., Zhou X., Luo S., Zhang J., et al. The effect of Glomus intraradices on the physiological properties of Panax ginseng and on rhizospheric microbial diversity. Journal of Ginseng Research. 2019;43(1):77–85. doi: 10.1016/j.jgr.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Qin I., Huang L., Zhang L. Ecological agriculture in China: Principles and applications. Advances in Agronomy. 2007;94(06):181–208. [Google Scholar]
- Wang Q., Isman M.B. Plant essential oils for pest and disease managentent. China Plant Protection. 2014;34(3):77–79. [Google Scholar]
- Wang Y., Wang Z., Yan X., Wang Q., Yang Q., Tang H., et al. Effects of different NPK ratios on growth indexs and yield of Alpinia oxyphylla. Journal of Chinese Medicinal Materials. 2020;43(10):2345–2349. [Google Scholar]
- Wang Y., Yan Z. Population stability of endophytic fungi in Ligusticum chuanxiong Hort. LIishizhen Medicine and Materia Reseach. 2009;20(12):2948–2949. [Google Scholar]
- Wei H.G., Lei Z.H., Guan Z.G., Wang Y.L., Li J., Wu S.Y., et al. Tending of wild Heterosmilax yunnanensis. Journal of Chinese Medicinal Materials. 2018;43(22):4427–4432. doi: 10.19540/j.cnki.cjcmm.20180807.006. [DOI] [PubMed] [Google Scholar]
- Wei X., Cao P., Wang G., Han J. Microbial inoculant and garbage enzyme reduced cadmium (Cd) uptake in Salvia miltiorrhiza (Bge.) under Cd stress. Ecotoxicology and Environment Safety. 2020;192:110311. doi: 10.1016/j.ecoenv.2020.110311. [DOI] [PubMed] [Google Scholar]
- Wei X., Wang X., Cao P., Gao Z., Chen A.J., Han J. Microbial community changes in the rhizosphere soil of healthy and rusty Panax ginseng and discovery of pivotal fungal genera associated with rusty roots. Biomed Research International. 2020;2020:1–13. doi: 10.1155/2020/8018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2013). WHO traditional medicine strategy: 2014-2023.
- Wu A., Zhang Y., Wan S., Fang X., Liu R., Hu T., et al. Phosphate solubilizing characteristics of Talaromyces aurantiacus and its growth-promoting effect on Phyllostachys edulis seedlings. Chinese Journal of Applied Ecology. 2019;30(1):176–182. doi: 10.13287/j.1001-9332.201901.011. [DOI] [PubMed] [Google Scholar]
- Wu C. A brief discussion on the poisoning of Rehmannia glutinosa drugs. World Latest Medicine Information. 2015;55:133–134. [Google Scholar]
- Wu J., Li X., Huang L., Meng X., Hu H., Luo L., et al. A new GIS model for ecologically suitable distributions of medicinal plants. Chinese Medcine. 2019;14(1) doi: 10.1186/s13020-019-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Liu S. Improvement of the R-SWAT-FME framework to support multiple variables and multi-objective functions. Science of the Total Environment. 2014;466–467:455–466. doi: 10.1016/j.scitotenv.2013.07.048. [DOI] [PubMed] [Google Scholar]
- Xie S., Yang F., Feng H., Wei C. General characteristics of the chemical fertilizers and pesticides use and the analysis of use reduction effect in China. Environmental Pollution and Control. 2019;41(4):490–495. [Google Scholar]
- Xie Z. Chinese medicinal materials should be harvested timely and appropriately, with high quality, high yield and sustainable utilization as the criterion. Journal of Chinese Medicinal Materials. 2001;26(03):8–11. [Google Scholar]
- Xu C. Recent development of sustainable agriculture and its relation to Chinese ecological agriculture. Chinese Journal of Eco-agriculture. 2002;10(4):1–5. [Google Scholar]
- Yan S., Niu Z., Yan H., Zhang A., Liu G. Influence of soil organic carbon on the aroma of tobacco leaves and the structure of microbial communities. Current Microbiology. 2020;77(6):931–942. doi: 10.1007/s00284-020-01895-7. [DOI] [PubMed] [Google Scholar]
- Yang G., Guo L.P., Zhou X.T., Huang L.Q. Analysis of several key problems of good agricultural practice (GAP) of Chinese materia medica. Journal of Chinese Medicinal Materials. 2016;41(7):1173–1177. doi: 10.4268/cjcmm20160702. [DOI] [PubMed] [Google Scholar]
- Yang P., Wang H.L., Sun H., Zhu W.T., Qiu T., Du J.Z., et al. Correlation between growth characteristics and quality of rhizomes of Notopterygium incisum under wild tending. Journal of Chinese Medicinal Materials. 2020;45(4):739–745. doi: 10.19540/j.cnki.cjcmm.20191223.103. [DOI] [PubMed] [Google Scholar]
- Yang X.M., Zhang X.P., Fang H.J., Zhu P., Ren J., Wang L.C. Long-term effects of fertilization on soil organic carbon changes in continuous corn of northeast China: RothC model simulations. Environmental Management. 2003;32(4):459–465. doi: 10.1007/s00267-003-0082-6. [DOI] [PubMed] [Google Scholar]
- Ye B., Tang H., Tang H., Tang X., Xu Y. Status and prevention measurements of farmland pollution in China. Chinese Agricultural Science Bulletin. 2010;26(7):295–298. [Google Scholar]
- Ye M., Luo S.M., Xie J.F., Li Y.F., Xu T., Liu Y., et al. Silencing COI1 in rice increases susceptibility to chewing insects and impairs inducible defense. PLoS One. 2012;7(4):e36214. doi: 10.1371/journal.pone.0036214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Hao X. Research on characteristics of essential factors, development predicament and countermeasures of Chinese medical eco-agriculture. Ecological Economy. 2018;34(11):14–20. [Google Scholar]
- Yuan Y., Zhou J., Huang L. Experimental verification and prospect on stress effect of Dao-di herbs Scutellaria baicalensis. Journal of Chinese Medicinal Materials. 2016;41(1):139–143. doi: 10.4268/cjcmm20160127. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J., Li B. Thinking about using the “traditional Chinese medicine agriculture” to improve the production level of the bee industry. China Animal Husbandry and Veterinary abstracts. 2018;34(6):1–2. [Google Scholar]
- Zhang L., Wang D., Liu R. “Traditional Chinese medicine agriculture” promote the construction of the human destiny community. China Animal Husbandry and Veterinary abstracts. 2017;33(12):1–3. [Google Scholar]
- Zhang L., Wei J., Yang Z., Chen F., Xian Q., Su P., et al. Distribution and diversity of twelve Curcuma species in China. Natural Product Research. 2018;32(3):327–330. doi: 10.1080/14786419.2017.1350667. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang L. Development strategy and prospect of “TCM agriculture”. Agricultural Outlook. 2018;14(11):72–76. [Google Scholar]
- Zhang Q., Zhang D., Sun C., Xie C. Numerical analysis of climatic characteristics and geographical distribution of medicinal plants-Sichuan province as an example. Modern Chinese Medcine. 2018;20(2):145–151. [Google Scholar]
- Zhang R., Li P., Xu L., Zhou L. Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules. 2011;16(12):10631–10644. doi: 10.3390/molecules161210631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Gao Y., Zang P., Zhao Y., He Z., Zhu H., et al. Study on the simultaneous degradation of five pesticides by Paenibacillus polymyxa from Panax ginseng and the characteristics of their products. Ecotoxicology and Environment Safety. 2019;168:415–422. doi: 10.1016/j.ecoenv.2018.10.093. [DOI] [PubMed] [Google Scholar]
- Zhang X., Xie Z., Lang D., Chu Y., Cui G., Jia X. Bacillus pumilus improved drought tolerance in Glycyrrhiza uralensis G5 seedlings through enhancing primary and secondary metabolisms. Physiologia Plantarum. 2020;171(3):388–399. doi: 10.1111/ppl.13236. [DOI] [PubMed] [Google Scholar]
- Zhi X.Y., Jiang L.Y., Li T., Song L.L., Wang Y., Cao H., et al. Semisynthesis and insecticidal bioactivities of benzoxazole and benzoxazolone derivatives of honokiol, a naturally occurring neolignan derived from Magnolia officinalis. Bioorganic & Medicinal Chemistry Letters. 2020;30(9):127086. doi: 10.1016/j.bmcl.2020.127086. [DOI] [PubMed] [Google Scholar]
- Zhou R., Xiang C., Zhang J., Yang H. Research progress on chemical compositions of Coptidis Rhizoma and pharmacological effects of berber. Journal of Chinese Medicinal Materials. 2020;20(2):1–14. doi: 10.19540/j.cnki.cjcmm.20200527.202. [DOI] [PubMed] [Google Scholar]
- Zhu L. The role of the “traditional Chinese medicine agriculture” model in the ecological planting of rice. Grain Issues Research. 2017;4:9–11. [Google Scholar]
- Zimmer S., Messmer M., Haase T., Piepho H.P., Mindermann A., Schulz H., et al. Effects of soybean variety and Bradyrhizobium strains on yield, protein content and biological nitrogen fixation under cool growing conditions in Germany. European Journal of Agronomy. 2016;72:38–46. [Google Scholar]