Abstract
Objective
To isolate and identify the major bioactive components from the leaves of Lysiphyllum strychnifolium, an indigenous herb used in traditional Thai medicine for detoxification, longevity, and some other health related issues.
Methods
Comparative HPLC analyses of the crude extracts from three provenances were carried out for an overview of characteristic compound profiles. Isolation of the major compounds was undertaken with chromatographic methods. Chemical structures were elucidated by NMR spectroscopic techniques and mass spectrometry. DPPH scavenging assay was carried out to determine the free radical scavenging activity of isolated compounds.
Results
Yanangdaengin (3), a dihydrochalcone glucoside galloyl ester, has been isolated together with its corresponding dihydrochalcone glucoside trilobatin (2) as major compounds from the leaves of L. strychnifolium. Additionally, gallic acid (1) was co-chromatographically identified. Free radical scavenging activity of isolated compounds were determined. Compound 3 exhibited higher free radical scavenging activities in comparison to Trolox and quercetin.
Conclusion
The isolated compounds could be used as chemical markers for quality assessment. The present work could promote the quality control and herbal medicinal product development of this plant.
Keywords: antioxidative properties, Bauhinia strychnifolia Craib, dihydrochalcone, Lysiphyllum strychnifolium (Craib) A. Schmitz, Ya-Nang-Daeng
1. Introduction
Lysiphyllum strychnifolium (Craib) A. Schmitz, is an endemic herb commonly known as “Kha-Yan” or “Ya-Nang-Daeng” in Thailand (Larsen and Larsen, 1984, Pooma and Suddee, 2014). This plant was previously listed as Bauhinia strychnifolia Craib and re-classified based on results of a phylogenetic study of nuclear ribosomal DNA (Hao et al., 2003). This species occurs mainly in dry deciduous dipterocarp forests in northern, central and eastern parts of Thailand (Tangnak et al., 2018). In traditional Thai medicine (TTM), aqueous extracts from leaves and/or stems of L. strychnifolium are used for detoxification, longevity and some other health-related issues (Luengthong et al., 2016, Maitree et al., 2018, Sutiyaporn et al., 2018, Wutthithammawet, 1997). From extracts of this plant, various activities such as cytotoxic effects against human cancer cell lines (Kaewpiboon et al., 2012, Yuenyongsawad et al., 2013), anti-HIV-1 and anti-allergic (Bunluepuech et al., 2013), and antihyperuricemic effects (Sutiyaporn et al., 2018) have been reported. Although this plant is commonly used by Thai traditional practitioners, there are no reports on the economic importance of this plant. Mostly, Thai traditional practitioners cultivate this plant in their gardens for medical purposes. Scientific research in depth of its phytochemical composition and pharmacological mode of actions may promote this plant as a potential economic crop for developing herbal medicinal products.
To date, several flavonoids such as quercetin, 3,5,7,3′,5′-pentahydroxy-flavanonol-3-O-α-l-rhamnopyranoside, 3,5,7-trihydroxychromone-3-O-α-l-rhamnopyranoside and the triterpenoids β-sitosterol, and stigmasterol are known from stems (Bunluepuech et al., 2013, Yuenyongsawad et al., 2013). Gallic acid was detected in leaves of L. strychnifolium (syn. B. strychnifolia) (Maitree et al., 2018, Sutiyaporn et al., 2018). However, despite the long traditional use of this plant, the quality control and pharmacological research have been hindered because the major bioactive compounds were unknown. Herein, we are able to report the isolation and structure elucidation of yanangdaengin (3), a dihydrochalcone glucoside galloyl ester of trilobation. Furthermore, the dihydrochalcone trilobatin (2) and gallic acid (1) could be identified. Compounds 2 and 3 represent the major active constituents of this plant. From all three compounds we assessed the free-radical scavenging activity, especially compounds 1 and 3 showed impressive activities in comparison to Trolox.
2. Materials and methods
2.1. Reagents
HPLC grade methanol and glacial acid were purchased from Labscan (Thailand). Deionized water was purified by Ultra Clear system (Siemen Water Technologies Corp., USA). Gallic acid was purchased from Tokyo Chemical Industry Co., Ltd. (Japan). 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) was purchased from Sigma (St. Louis, MO).
2.2. Plant materials
The samples were collected in the provinces Ratchaburi, Udonthani and Uttaradit (Thailand) and also purchased from Charoensuk Pharma Supply Co., Ltd., Nakhon Pathom, Thailand. The samples were identified by Ms. Pajaree Inthachub. Voucher specimens were deposited at Drug Discovery and Development Center, Office of Advanced Science and Technology, Thammasat University, Thailand. The samples were dried at 50 °C for 72 h.
2.3. Extraction and isolation
The dried and powdered leaves (overall 200 g) were macerated with methanol for 3 × 72 h with occasional shaking. After HPLC profiling, the extracts were pooled, filtered, and the solvent was evaporated using a rotary evaporator. This yielded 16 g of crude methanolic extract. This extract was roughly separated by column chromatography (CC) (Merck silica gel 60, 70–230 mesh) eluted with ethyl acetate and methanol (95:5, volume percent). Fractions were analyzed by TLC (silica gel 60 F254) and HPLC. Further purification was made using CC (Merck LiChroprep RP-18, 40–63 µm) with methanol and water (50:50, volume percent). Purification by CC over Sephadex LH-20 eluted isocratic with methanol yielded 34 mg of trilobatin (2) and 8 mg of yanangdaengin (3).
2.4. NMR and mass spectrometry
All NMR spectra were recorded at room temperature either on a Bruker Avance II 400 (resonance frequencies 400.13 MHz for 1H and 100.63 MHz for 13C) or a Bruker Avance III 600 (resonance frequencies 600.25 MHz for 1H and 150.95 MHz for 13C) with standard Bruker pulse programs. The samples were dissolved in 0.6 mL of MeO-d4 (99.9% D). Chemical shifts are given in ppm, referenced to residual solvent signals (δH 3.31, δC 49.0)
Mass spectra were recorded on a high-resolution time-of-flight (HR-TOF) mass spectrometer (maXis, Bruker Daltonics) by direct infusion electrospray ionization (ESI) in positive ionization mode (mass accuracy ± 5 × 10−6) as well as in negative mode (mass accuracy ± 10 × 10−6). HR-TOF MS measurements have been performed within the selected mass range of m/z 100–2500. ESI was made by the capillary voltage of 4 kV to maintain a (capillary) current between 30 and 50nA. Nitrogen temperature was maintained at 180 °C using a flow rate of 4.0 L/min and the N2 nebulizer gas pressure at 0.3 bar.
2.5. HPLC analysis
HPLC analyses were performed on an Agilent 1260 Series (Agilent Technologies) equipped with a 1260 Quat pump VL quaternary pump, 1260 ALS autosampler, 1260 TCC column thermostat, 1260 DAD VL diode array detector and a Hypersil BDS C18 column (4.6 mm × 100 mm; 3.5 µm particle size); injection volume was 10 µL and the wavelength of detection was set at 254 nm. The mobile phases were (A) 0.5% acetic acid in water and (B) methanol. Gradient elution was used from 0% to 100% B for 40 min, 100% B for 10 min. The column was equilibrated with 100% A for 10 min prior to each analysis. The flow rate was set at 1.0 mL/min at 25 °C.
2.6. Determination of free radical scavenging activity
The free radical scavenging activity of isolated compounds was determined using the DPPH radical scavenging assay (Sithisarn et al., 2015, Vongsak et al., 2015). The stock solution of the plant extract (1 mg/mL) was serially diluted to concentrations of 1.56 to 100 µg/mL in a microplate (100 µL sample in each well) and 100 µL DPPH• solution (152 µmol/L in methanol) were added into each well. After incubation at 25 °C for 30 min, the absorbance at 517 nm was measured using a microplate reader. The corresponding blank was also determined and percent inhibition was calculated as follows: Scavenging activity (%) = [1 – (A1 – A2) / A0] × 100%, where A0 was the absorbance of control (DPPH• solution without sample), A1 = absorbance of DPPH• solution in the presence of the sample; A2 = was the absorbance without DPPH• solution.
3. Results and discussion
Dried and ground leaves of L. strychnifolium samples were extracted with 50% methanol in water. In order to get an overview of characteristic compound profiles, comparative HPLC analyses of the crude extracts from different provenances were carried out (Fig. 1). With regard to previous studies of this plant (Bunluepuech et al., 2013, Yuenyongsawad et al., 2013), none of the reported constituents could be detected in major peaks. Therefore isolation of the major compounds was undertaken. This led to yanangdaengin (3), a galloyl ester of dihydrochalcone glucoside, together with its known derivative trilobatin (2). Their structures were elucidated by NMR and MS analyses and the spectroscopic data were compared with published data. Although, the structure of yanangdaengin (3) has been mentioned in Tao et al. (2012) as 4,2′,6′-trihydroxy-dihydrochalcone-4′-O-(6′′-galloyl)-β-d-glucopyranose without supporting data and was referenced to Tanaka et al. (2005), the structure of yanangdaengin (3) could not be found in this reference. Therefore, in the present work we now provide the NMR as well as HR-ESI-MS data of compound 3. Identification of gallic acid (1) in L. strychnifolium leaves was done by HPLC coupled with UV diode array detection and TLC comparison with an authentic standard. These results are well in line with previous studies (Maitree et al., 2018, Sutiyaporn et al., 2018).
Fig. 1.

HPLC at 254 nm overview of crude extracts of L. strychnifolium leaves demonstrating same pattern of major compounds (gallic acid (1). trilobatin (2) and yanangdaengin (3)) from Uttaradit (A), Udonthani (B) and Ratchaburi (C).
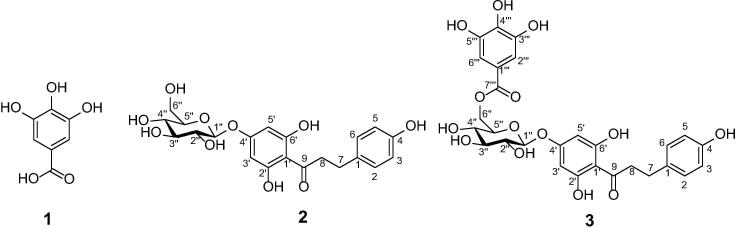
Yanangdaengin (phloretin 4′-O-(6′′-O-galloyl)-β-D-glucoside, 3) was isolated as a white amorphous powder (m.p.165–166 °C). The 1H NMR spectrum was closely related to that of trilobatin (2) (Qin et al., 2015), with the signals of the phloroglucinol ring at δ 6.08 (s, 2H, H-3′ and H-5′), the para-hydroxybenzene moiety at δ 7.04 (d, 2H, H-3, H-5), 6.69 (d, 2H, H-2, H-6), two mutually coupled methylene groups at δ 3.30 (m, H-8) and 2.85 (m, H-7), and the resonances of a glucose group. The 13C NMR data also confirmed the presence of the phloretin-4′-O-β-glucoside subunit. Additional 13C resonances at δ 168.3, 146.5 (2C), 139.8, 121.2, and 110.2 (2C) and a two-proton singlet in the 1H NMR at δ 7.08 were indicative for a galloyl group. The connecting position of the galloyl group was determined as CH2-O of the glucoside (C-6) moiety on the basis of characteristic low field shifts of H-6′′[δ4.54 (dd, J = 12.1, 2.2 Hz) and 4.45 (dd, J = 12.1, 4.8 Hz)] compared to those of trilobatin (2) and furthermore by long-range cross-peaks of these protons to the galloyl carboxyl carbon C-7′′′. Accordingly, the structure of compound 3 was elucidated as phloretin 4′-O-(6′′-O-galloyl)-β-D-glucoside (Fig. 2), which was also confirmed by high-resolution mass spectral data revealing a [M + Na]+ peak at m/z 611.1360 (calcd for C28H28O14Na: 611.1371) in positive mode and in the negative mode a [M−H]− peak at m/z 587.1414 (calcd for C28H27O14: 587.1406), respectively. The assignment of all the 1H and 13C NMR data was accomplished by 1H–1H COSY, 1H–13C HSQC, and 1H–13C HMBC NMR data are listed in Table 1.
Fig. 2.
Chemical structures of gallic acid (1), trilobatin (2) and yanangdaengin (3).
Table 1.
1H and 13C NMR spectroscopic data for compounds 2 and 3.
| Carbons |
δH |
δC |
||
|---|---|---|---|---|
| 2a | 3b | 2c | 3d | |
| 1 | – | – | 133.8 (C) | 133.9 (C) |
| 2, 6 | 7.04 (2H, d, J = 8.5 Hz) | 7.04 (2H, d, J = 8.6 Hz) | 130.3 (CH) | 130.3 (CH) |
| 3, 5 | 6.69 (2H, d, J = 8.5 Hz) | 6.69 (2H, d, J = 8.6 Hz) | 116.1 (CH) | 116.1 (CH) |
| 4 | – | – | 156.5 (C-OH) | 156.5 (C-OH) |
| 7 | 2.86 (2H, m) | 2.85 (2H, m) | 31.2 (CH2) | 31.2 (CH2) |
| 8 | 3.30 (2H, m) | 3.30 (2H, m) | 47.6 (CH2) | 47.5 (CH2) |
| 9 | – | – | 207.0 (C = O) | 207.0 (C = O) |
| 1′ | – | – | 106.8 (C) | 107.0 (C) |
| 2′, 6′ | – | – | 165.4 (C-OH) | 165.4 (C-OH) |
| 3′, 5′ | 6.09 (2H, s) | 6.08 (2H, s) | 96.4 (CH) | 96.4 (CH) |
| 4′ | – | – | 165.0 (C) | 164.9 (C) |
| 1′′ | 4.93 (1H, d, J = 7.4 Hz) | 4.98 (1H, d, J = 7.5 Hz) | 101.1 (CH) | 101.1 (CH) |
| 2′′ | 3.43 (1H, m) | 3.47 (1H, m) | 74.6 (CH) | 74.6 (CH) |
| 3′′ | 3.39 (1H, m) | 3.50 (1H, m) | 77.9 (CH) | 77.7 (CH) |
| 4′′ | 3.45 (1H, m) | 3.53 (1H, m) | 71.1 (CH) | 71.1 (CH) |
| 5′′ | 3.45 (1H, m) | 3.74 (1H, ddd, J = 9.3, 4.8, 2.2 Hz) | 78.3 (CH) | 75.7 (CH) |
| 6′′ | 3.90 (1H, dd, J = 12.1, 2.1 Hz) 3.71 (1H, dd, J = 12.1, 5.5 Hz) |
4.55 (1H, dd, J = 12.1, 2.2 Hz) 4.46 (1H, dd, J = 12.1, 4.8 Hz) |
62.3 (CH2) | 64.3 (CH2) |
| 1′′′ | – | – | – | 121.2 (C) |
| 2′′′, 6′′′ | – | 7.08 (2H, s) | – | 110.2 (CH) |
| 3′′′, 5′′′ | – | – | – | 146.5 (C-OH) |
| 4′′′ | – | – | – | 139.8 (C-OH) |
| 7′′′ | – | – | – | 168.3 (C = O) |
1H NMR spectrum (600 MHz, methanol‑d4).
1H NMR spectrum (400 MHz, methanol‑d4).
13C NMR spectrum (150 MHz, methanol‑d4).
13C NMR spectrum (100 MHz, methanol‑d4).
The free-radical scavenging activities of the isolated compounds were determined by the DPPH• free radical scavenging assay and the IC50 values are reported in Table 2. The obtained results clearly showed the high potentials of compounds 1 and 3, whereas 2 only showed moderate anti-oxidative properties. We assume that the gallic acid moiety of 3 and free gallic acid (1) content in the leaves are responsible for this positive effect. The higher amount of 3 in the leaves justify the usage as an anti-oxidative drug. This proposed active principle of the herbal drug now may promote the quality control and herbal medicinal product development.
Table 2.
Content of compounds 1–3 in leaves of three examined samples and their free radical scavenging activity.
| Compounds | Content/% |
IC50/(mmol·L−1)a | ||
|---|---|---|---|---|
| Uttaradit | Udonthani | Ratchaburi | ||
| Gallic acid (1) | 0.15 (±0.01) | 0.10 (±0.01) | 0.17 (±0.01) | 5.99 ± 0.29 |
| Trilobatin (2) | 3.42 (±0.06) | 5.40 (±0.19) | 3.94 (±0.23) | 51.59 ± 1.67 |
| Yanangdaengin (3) | 1.34 (±0.24) | 0.97 (±0.13) | 0.67 (±0.23) | 5.03 ± 0.37 |
| Quercetin | 8.52 ± 0.25 | |||
| Trolox | 12.25 ± 0.39 | |||
aData expressed as mean ± SD for triplicate analysis.
4. Conclusion
Yanangdaengin (3) has been isolated together with its corresponding dihydrochalcone glucoside trilobatin (2) as major compounds from the leaves of L. strychnifolium. Additionally, gallic acid (1) was also identified. Free radical scavenging activities were determined, revealing high potentials of compounds 1 and 3, whereas 2 only showed moderate anti-oxidative properties. These compounds could also be used as active chemical markers for quality assessment. The present study provided a useful basis for quality control and further development of this plant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors greatly acknowledge the financial support provided by Thammasat University Research Fund under the TU Research Scholar, Contract No. 2/29/2561. We thank Ms. Pajaree Inthachub for plant collection, discussion, and identification. We thank Drug Discovery and Development Center and Office of Advanced Science and Technology for laboratory facilities.
References
- Bunluepuech K., Wattanapiromsakul C., Madaka F., Tewtrakul S. Anti-HIV-1 integrase and anti-allergic activities of Bauhinia strychnifolia. Songklanakarin Journal of Science and Technology. 2013;35:659–664. [Google Scholar]
- Hao G., Zhang D.X., Zhang M.Y., Guo L.X., Li S.J. Phylogenetics of Bauhinia subgenus Phanera (Leguminosae: Caesalpinioideae) based on ITS sequences of nuclear ribosomal DNA. Botanical Bulletin of Academia Sinica (Taipei) 2003;44:223–228. [Google Scholar]
- Kaewpiboon C., Lirdprapamongkol K., Srisomsap C., Winayanuwattikun P., Yongvanich T., Puwaprisirisan P.…Assawalapsakul W. Studies of the in vitro cytotoxic, antioxidant, lipase inhibitory and antimicrobial activities of selected Thai medicinal plants. BMC Complementary and Alternative Medicine. 2012;12:217. doi: 10.1186/1472-6882-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen K., Larsen S.S. Leguminosae-Caesalpinioideae. Flora of Thailand. 1984;4:24. [Google Scholar]
- Luengthong N., Lin C., Muangman V., Wongyai S. Preliminary study of the effect of Bauhinia strychnifolia Craib on blood alcohol levels in healthy volunteers. Journal of Thai Traditional and Alternative Medicine. 2016;14:177–187. [Google Scholar]
- Maitree M., Sato V.H., Sithisarn P., Chewchinda S. Evaluation of antioxidant activities and HPTLC analysis of Lysiphyllum strychnifolium (Craib) A. Schmitz leaf extract. Thai Journal of Pharmaceutical Sciences. 2018;42:S22–S26. [Google Scholar]
- Pooma, R., & Suddee, S. (2014). Thai plant names Tem Smitinand revised edition 2014. Office of the Forest Herbarium, Department of National Park, Wildlife and Plant Conservation: Bangkok.
- Qin X., Xing Y.F., Zhou Z., Yao Y. Dihydrochalcone compounds isolated from crabapple leaves showed anticancer effects on human cancer cell lines. Molecules. 2015;20:21193–21203. doi: 10.3390/molecules201219754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithisarn P., Rojsanga P., Sithisarn P., Kongkiatpaiboon S. Antioxidant activity and antibacterial effects on clinical isolated Streptococcus suis and Staphylococcus intermedius of extracts from several parts of Cladogynos orientalis and their phytochemical screening. Evidence Based Complementary and Alternative Medicine. 2015;2015 doi: 10.1155/2015/908242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutiyaporn A., Sato V.H., Parichatikanond W., Chewchinda S. The study of antihyperuricemic effect of Lysiphyllum strychnifolium (Craib) A. Schmitz leaf extract. Thai Journal of Pharmaceutical Sciences. 2018;42:S111–S114. [Google Scholar]
- Tanaka T., Uehara R., Nishida K., Kuono I. Galloyl, caffeoyl and hexahydroxydiphenoyl esters of dihydrochalcone glucosides from Balanophora tobiracola. Phytochemistry. 2005;66:675–681. doi: 10.1016/j.phytochem.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Tangnak N., Hongtrakul V., Keeratinijakal V. Analysis of genetic diversity evaluation of Lysiphyllum strychnifolium (Craib) A. Schmitz in Thailand using amplified fragment length polymorphism markers. Agriculture and Natural Resources. 2018;52:341–346. [Google Scholar]
- Tao J., Zhao J., Zhao Y., Cui Y., Fang W. BACE inhibitory flavanones from Balanophora involucrata Hook. f. Fitoterapia. 2012;83:1386–1390. doi: 10.1016/j.fitote.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Vongsak B., Kongkiatpaiboon S., Jaisamut S., Machana S., Pattarapanich C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Revista Brasileira de Farmacognosia. 2015;25:445–450. [Google Scholar]
- Wutthithammawet W. Odeanstore; Bangkok: 1997. Herbal Encyclopaedia. [Google Scholar]
- Yuenyongsawad S., Bunluepuech K., Wattanapiromsakul C., Tewtrakul S. Anti-cancer activity of compounds from Bauhinia strychnifolia stem. Journal of Ethnopharmacology. 2013;150:765–769. doi: 10.1016/j.jep.2013.09.025. [DOI] [PubMed] [Google Scholar]