Abstract
Plants of genus Cichorium are famous due to their therapeutic and medicinal properties. They are used as traditional medicine and edible food. To date, several scholars concentrated on compounds belonging to coumarins, flavonoids, sesquiterpenoids, triterpenoids, steroids, organic acids and other chemical constituents. Pharmacological effects such as photo-protective, hepatoprotective, anti-diabetic and lipid lowering, antioxidant, anti-inflammation, antifungal, antimalarial, increased bone mineral density, as well as vasorelaxant and antitumour activity were wildly reported. In this study, botanical resources, ethnopharmacological application, chemical constituents and bioactivities, as well as safety and toxicity of clinical applications of genus Cichorium were reviewed, which may provide a reliable basis for further development and utilization of Cichorium genetic resources.
Keywords: chemical constituents, Cichorium endivia L., Cichorum glandulosum Boiss. et Huet, Cichorium intybus L., ethanopharmacological application, pharmacological activities
1. Introduction
Cichorium genus (Asteraceae) includes 10–12 species in this genus, four to six of which are wild species. They are perennial herb, as a typical Mediterranean plant indigenous to Europe, Western Asia and North America (Fernald, 1950). Plants of genus Cichorium are of great importance due to their outstanding therapeutic and medicinal properties. They are used as traditional medicine and edible food. To our knowledge, Cichorium, known as Ctchorium, was first found in Egypt about 2500 years ago. As a kind of folk medicine, seeds, roots, flowers, leaves, and whole weeds are all used as medicinal parts, and are widely used in several countries (e.g., Armenia, Turkey, Georgia, Italy, etc.) for their function of improving the gastrointestinal tract, diabetes, jaundice, malaria, gallstones, hemorrhoids, and anemia. In the Chinese Pharmacopoeia, the medical parts originate from the aerial parts or roots of Cichorum glandulosum Boiss. et Huet and Cichorium intybus L. This plant has also influences on clearing away heat and damp elimination, promoting digestion, protecting liver, etc. (Pharmacopoeia Commission of PRC, 2015). As an edible food, young leaves are mainly used in salad, and roasted root is an appropriate alternative of coffee. The fresh roots are also used in milk soup. Several scholars have concentrated on the chemical composition and bioactivities of genus Cichorium. We herein attempted to review bioactivities, as well as safety and toxicity evaluation clinical applications of genus Cichorium, which provided the basis for the further development and utilization of Cichorium resources.
2. Botanical resources
Numerous reports have focused on C. intybus, C. endivia, C. glandulosum and C. spinosum and their botanical resources are as follows: C. intybus. is also known as chicory, blue sailors, succory, coffee weed and cornflower. The leaves or roots are baked and used as coffee or additive. Chicory has the height of about 30–100 cm with a tough, grooved, less hairy stem. The leaves are stalked, lanceolate and unlobed. The flowers are light blue, sometimes white, or pink, and heads of flowers have the width of 2–4 cm wide. It has two rows of involucral bracts: in which the inner is the long and erect one, and the outer is short and spread one. Blooms first appear in late spring and continue into mid fall. The achenes have no pappus, but do have toothed scales on top, and the weight of 1000 Wedge-shaped seeds is 1.2–1.5 g (https://en.wikipedia.org/wiki/Chicory).
The analysis of the nutritional composition of the air-dried root piece per 100 g includes water 9.22 g, ash 2.67 g, total carbohydrate 71.32 g, fat 0.56 g, total protein 8.55 g, crude fiber 6.61 g, crude saponin 1.09 g, Ca 16.02 mg, Mg 4.72 mg, P 17.13 mg, Fe 4.01 mg, Zn 0.19 mg, Si 17.8 mg, and Cu 0.035 mg (Zheng, Hu, Xu, Chang, & Li, 2003).
Cichorium endivia (C. endivia) is a species of flowering plant belonging to the genus Cichorium, and is widely cultivated as one of the species of similar bitter-leafed vegetables known as endive and escarole. Leaves alternate, simple or pinnatifid, sessile, broad, up to 45 cm × 18 cm, slightly crumpled, margin entire or dentate (escarole type) or very narrow, deeply pinnatifid and strongly curled (curly-leaved type), progressively smaller upwards on stem, slightly pubescent or glabrous, pale to dark green or yellowish, sometimes reddish along midrib. Inflorescence a head, 1–6 together, sessile or on up to 20 cm long, apically thickened peduncle; involucre with outer row of five bracts (7–10 mm × 2–5 mm), and inner row of eight bracts (8–12 mm × 1–3 mm). Flowers 15–20 per head, all ligulate; corolla up to 2 cm long, blue, sometimes white, 5-lobed at apex; stamens 5 with anthers fused into a tube; ovary inferior, 1-celled, style slender, hairy, with 2 slender stigmatic lobes. Fruit, an obovoid to cylindrical achene (2–3 mm × 1–1.5 mm), brown, with pappus of 1–3 rows of small, persistent membranous scales (Fig. 1).
Fig. 1.
Different parts (A, whole weed; B, flowers; C, seeds; D, roots) of C. glandulosum.
The nutritional composition of escarole per 100 g edible portion includes water 94 g, protein 1.3 g, fat 0.2 g, carbohydrate 3.4 g, fibre 3.1 g, Ca 52 mg, Mg 15 mg, P 28 mg, Fe 0.8 mg, Zn 0.8 mg, vitamin A 2167 IU, thiamin 0.08 mg, riboflavin 0.08 mg, niacin 0.4 mg, folate 142 μg, and ascorbic acid 6.5 mg (USDA, 2002). The nutritional composition of endive is about the same, except for the ascorbic acid content, which is 13 mg per 100 g (Grubben and Denton, 2004).
C. glandulosum is a traditional medicine, mainly distributed in Xinjiang and Caucasus region (Editorial Committee of Xinjiang Plant Flora, 1999). It is also called kasina and keku qiqi in Uyghur and Mongolian. In morphology, there is something different with C. intybus, and the stems and leaves of C. glandulosum are stronger, comoser and bigger than those of C. intybus (Jiangsu New Medical School, 1977). The contents of eight metal elements are as follows: Ca 65.84 mg/g, Fe 24.38 mg/g, Mg 278.17 mg/g and K 18.50 mg/g, Ni 0.004 38 mg/g, Mn 0.52 mg/g, Cu 0.0165 mg/g and Zn 0.18 mg/g (Pei et al., 2009).
C. spinosum also known as “stamnagathi” in Greek language. It is deciduous, the perennials reach heights of 10–20 cm (Erhardt, Götz, & Bödeker, 2008). It is a native plant of the Mediterranean basin and can be also found in different regions (e.g., Balearic Islands, Cyprus, Greece, Italy and Spain), as well as typically in coastal areas and plateaus of the mainland, and constitutes a very common ingredient of the so-called Mediterranean diet (Klados & Tzortzakis, 2014). It is dark-green with alternated simple leaves. It produces solitary light purple many-stellate flowers from June to August, as well as achenes every year (Christopher, 2003).
The nutritional composition is as follows: water 887–937 g/kg, ash 6.0–25.9 g/kg, proteins 4.2–22 g/kg, fats 1.98–4.1 g/kg, carbohydrates 47.44–76.2 g/kg, carbohydrates, energy 999–1519 kJ/kg and total sugars 4.48–17.01 g/kg (Spyridon et al., 2016).
3. Ethnopharmacological application
C. intybus could be used as a folk medicine in many countries, and it is also admitted in the books by Horace, Virgil, Ovid and Pliny, respectively (https://www.orleanscoffee.com/how_to/what-is-chicory/).
Many conciry the alcoholic extraction or decoction of roots is typically applied to treat wounds and tumors due to bites of poisonous snakes and scorpions. In addition, it can be used for lumbar pain, gout, rheumatism and skin diseases (Street, Sidana, & Prinsloo, 2013). The decoction derived from the aerial parts is also employed for hemorrhoids and eczema, the ash obtained from roots is used for wound healing (Yesilada et al., 1999), the latex at the tip of leaf stalks are used for treating warts (Tuzlaci, Alparslan Is-bilen, & Bulut, 2010).
The roots of the plant can also be used as a substitute of coffee or used in a kind of milk soup (Bussmann et al., 2017), and young leaves are highly appropriate to be used in salad. In Italy, flowers, leaves and roots of Cichorum intybus L. are helpful for protecting and cleaning liver, preventing diabetes, decreasing glucose level, regulating bladder function, as well as purifying human body (Dei Cas, Pugni, & Fico, 2015). In several countries, different parts of C. intybus were previously used almost in the same manner (Street et al., 2013).
In China, C. glandulosum and C. intybus were admitted in Chinese Pharmacopoeia. The roots or whole herbs could be used to clear away heat, eliminate dampness, and promote digestion (National Institute for the Control of Pharmaceutical and Biological Products, 1990).
The earliest note about C. glandulosum was presented in Xinjiang Herbal Handbook; and can be used for the treatment of dampness-heat, jaundice, stomachache, anorexia, oedema, and oliguria diseases (Board of health of Xinjiang Uygur Autonomous Region, 1976, Xinjiang Institute of Biology, Pedology and Desert Research, 1984).
In pharmaceutical criterion-Uyghur part, four folk recipes are collected. Granules of kasina is made from C. glandulosum alone, and are highly appropriate for protecting liver. The seeds and roots of C. glandulosum are used in the other folk recipes (e.g. Syrup of Yanxiao Dinaer, Granules of Hugan Buzure, Granules of Fufang muniziqi) (Pharmacopoeia Commission of PRC, 1999).
4. Phytochemistry of Cichorium genus
The genus Cichorium (Asteraceae) is made up of six species with major geographical presence in Europe and Asia. C. intybus, commonly known as chicory, is well known as a coffee substitute, while it is also widely used medicinally to treat various ailments ranging from wounds to diabetes. Cichorium is a genus of plants in the dandelion tribe within the sunflower family. The genus includes two cultivated species commonly known as chicory or endive, plus several wild species. To date, coumarin, flavonoid, sesquiterpenoid, triterpenoid, steroid, organic acid, and other components have been found in genus Cichorium.
4.1. Phenylpropenoids
A total of 12 phenylpropenoids such as esculetin (1), esculin (2), cichoriin (3), umbelliferone (4) and scopoletin (5) have been extracted from flowers of C. intybus. or seeds of C. glandulosum; cichoriin-6-p-hydryopheyl acetate (6) from leaves of C. intybus, syringin (7), from roots of C. pumilum and rearrangements, syringaresinol monoglucoside (8), 4α-4″-O-hydroxysyring-aresinol (9), 4α-hydroxy-syringaresinol-4″-O-β-glucopyranoside (10), 4β-hydroxysyringaresinol-4″-O-β-glucopyranosides (11), (7S,8R)-3″-Demethyl dehydro diconiferyl alcohol-3″-O-β-glucopyranoside (12). Their structures were given in previous paper (Fan et al., 2017, Fan et al., 2016, Long et al., 2014, Malarz et al., 2013, Wu et al., 2007).
4.2. Flavonoids
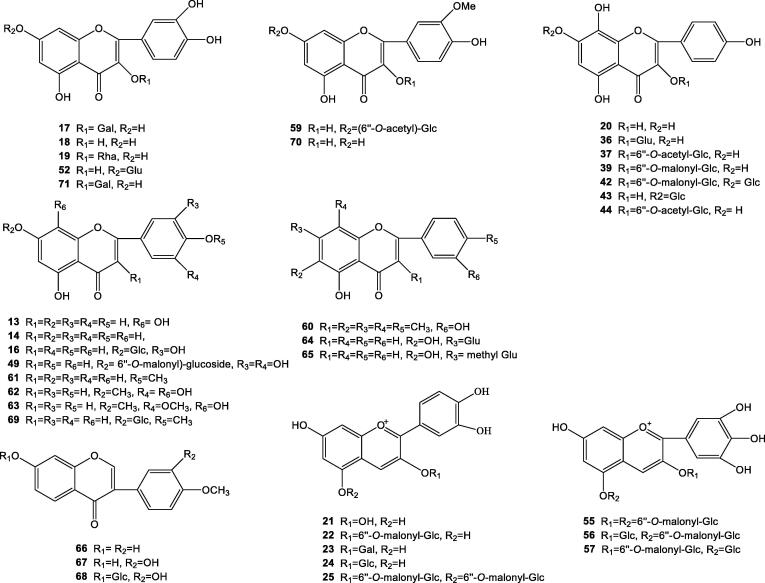
Flavonoids are the most common and widely distributed group of plant phenolic compounds, occurring virtually in all plant parts, particularly in the photosynthesising plant cells. They are an important class of natural products; particularly, they belong to a class of plant secondary metabolites having a polyphenolic structure, widely found in fruits, vegetables and certain beverages. They have miscellaneous favorable biochemical and antioxidant effects associated with various diseases such as cancer, Alzheimer's disease, atherosclerosis, etc. 59 flavnoids had been identified from different parts of C. intybus, frozen red chicory and C. glandulosum. Isoscutellarein (13), apigenin (14), apigenin-7-O-L-arabinoside (15), luteolin-7-O-β-D-glucopyranoside (16), hyperin (17), quercetin (18), quercitrin (19) and kaempferol (20) were isolated from aerial parts of C. intybus; besides, cyanidin (21) cyanidin-3-O-(6″-O-malonyl)-glucoside (22), cyanidin-3-O-galactoside (23), cyanidin-3-O-glucoside (24), cyanidin-3,5-di-O-(6″-O-malonyl)-glucoside (25), isorhamnetin-7-O-neohesperidoside (26), isorhamnetin-7-O-glycoside (27), isorhamnetin-7-O-glucuronide (28), isorhamnetin-7-O-(6″-O-malonyl)-glucoside (29), quercetin-7-O-galactoside (30), quercetin-3-O-glucuronide-7-O-(6″-O-malonyl)-glucoside (31), quercetin-3-O-(6″-O-malonyl)-glucoside (32), quercetin-7-O-(6″-O-acetyl)-glucoside (33), quercetin-7-O-p-coumaroylglucoside (34) kaempferol-3-O-glucoside (35), kaempferol-3-O-glucuronide (36), kaempferol-3-O-(6″-O-acetyl)-glucoside (37), kaempferol-3-O-glucosyl-7-O-(6″-O-malonyl)-glucoside (38), kaempferide-3-O-(6″-malonyl)-glucoside (39), kaempferol-3-O–sophoroside (40), kaempferol-3-O-glucuronide-7-O-glucoside (41), kaempferol-7-O-glucosyl-3-O-(6″-malonyl)-glucoside (42), kaempferol-7-O-glucoside (43), kaempferol-3-O-(6″-O-acetyl)-glucoside (44), kaempferol-7-O-(6″-O-malonyl)-glucoside (45), kaempferol-7-O-(6″-O-acetyl)-glucoside (46), kaempferol-7-O-rutinnoside (47), kaempferol-7-O-neohesperidoside (48), myricetin-7-O-(6″-O-malonyl)-glucoside (49), and petunidin-3-O-(6″-O-malonyl)-glucoside (50) were isolated from fresh leaves of C. intybus. Additionally, quercetin-7-O-glucoside (51), quercetin-7-O-glucuronide (52), pelargonidin-3-O-monoglucuronide (53) and kaempferol-7-O-glucuronide (54) were isolated from whole weed of C. intybus. Moreover, delphinidin-3,5-di-O-(6″-O-malonyl-β-D-glucoside) (55), delphinidin-3-O-β-D-glucoside-5-O-(6″-O-malonyl-β-D-glucoside) (56), delphinidin-3-O-(6″-O-malonyl-β-D-glucoside)-5-O-β-D-glucoside (57) from flowers of C. intybus (Bergantin et al., 2017, Carazzone et al., 2013, Dem'yanenko and Dranik, 1973, EI-Lakany et al., 2004, Liu, 2005, Norbaek, Nielsen, & Kondo, 2002, Wu, 2008).
Compounds of Nos. 23, 24, 26, 29, 32, 33, 34, 46, 50, apigenin-7-O-glycoside (58), isorhamnetin-7-O-(6″-O-acetyl)-glucoside (59) were isolated from frozen red chicory (Bergantin et al., 2017, Carazzone et al., 2013, Dem'yanenko and Dranik, 1973, EI-Lakany et al., 2004, Norbaek, Nielsen, & Kondo, 2002).
Furthermore, 4′,5-Dihydroxy-3,3′,6,7-tetramethoxy flavone (60), 2-(4-methoxy)-phenoxy-5,7-dihydrixy-chromone (61) were isolated from whole herbs of C. glandulosum (Yili, 2003), besides, 5,8,3″,4″-tetrahydroxy-7-methoxy flavone (62), 5,8,4″-trihydroxy-7,3′-dimethoxy flavone (63), baicalin (64), methyl baicalin (65), formononetin (66), calycosin (67), calycosin-7-O-β-D-glycoside (68) were isolated from roots of C. glandulosum (Liu, 2005), compound 35, tilianin (69), isorhamnetin (70), quercetin-3-O-β-D-galactoside (71) isolated from seeds of C. glandulosum (Wu, 2008).
The structures of compounds of Nos. 15, 26–35, 38, 40–41, 45–48, 50–51, 53–54 and 58 were shown in a previous paper (Long et al., 2014) and others were shown in Fig. 2.
Fig. 2.
Chemical structures of flavonoids in Cichorium genus. Note: Glc = Glucose, Gal = Galactose, Rha = Rhamnose, Glu = Glucuronide.
4.3. Triterpenoids and steroids
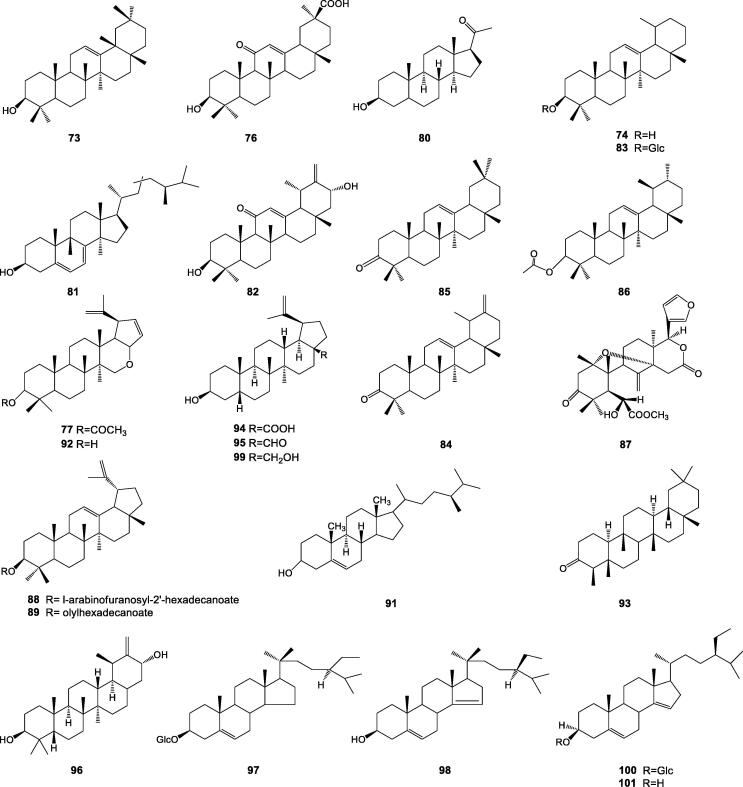
To date, more than 30 triterpenoids and steroids have been found in Cichorium genus, α-amyrin (72), β-amyrin (73), taraxasterol (74), Ф-taraxasterol (75), 3β-hydroxy-11-oxo-18β-olean-12-en-30-oic acid (76), lupeol acetate (77), β-sitosterol (78), daucosterol (79), pregnenolone (80), ergosterol (81) were isolated from seeds of C. glandulosum. Compounds 78, 79, and stigmasterol 3-O-β-D-glucoside (82) were also isolated from roots of C. glandulosum (Liu, 2005, Wu, 2008). Taraxasterol-3-O-β-D-glucoside (83), 20(30)-taraxasten-3β,21α–diol (84) from stems of C. glandulosum (Wu, Su, Xin, & Aisa, 2010).
Compounds 72, 74, 75, 78, 79, taraxerone (85), bauerenyl acetate (86), intybusoloid (87), lup-12,20(29)-dien-3β-ol-3β-l-arabinofuranosyl-2′-hexadecanoate (88), lup-12,20(29)-dien-3β-olyl hexadecanoate (89) were isolated from roots of C. intybus (He et al., 2002, Kumari et al., 2010). Compounds 78, 79, 87, stigmasterol (90), and campesterol (91) were isolated from leaves of C. intybus (Krebsky et al., 1999, Atta-ur-Rahman et al., 2008). Lupeol (92), friedelin (93), betulinic acid (94), betulin aldehyde (95), cichoridiol (96), stigma-5(6)-ene-3-α-O-D-glucopyranoside (97), cichosterol (98) betulin (99), bacosterol-3-O-β-D-glucopyranoside (100), 13,14-seco-stigma-5(6), 14(15)-diene-3-α-ol (101) were only isolated from seeds of C. intybus (Ahmed, Bawa, Siddiqui, & Alam, 2002; Sheng, 2006, Atta-ur-Rahman et al., 2008, Zou, 2013). The chemical structures of compounds 72, 75, 78, 79 and 90 are well known, and others are listed in Fig. 3.
Fig. 3.
Chemical structures of triterpenoids and steroids from Cichorium genus.
4.4. Sesquiterpenoids
There are several sesquiterpenoids in plants of Asteraceae family. The majority of sesquiterpenoids have the skeleton of guaiane sesquiterpenes, and few of them are eudesmane sesquiterpene. Lactucopicin could be found in the whole herbs, and it is the source of bitterness. Besides, 54 sesquiterpenoids have been isolated: in addition, 3,4-dihydrolactucin (102), 11α,13-dihydro-8-deoxylactucin (103), 11β,13-dihydro-8-deoxylactucin (104), intybulide A (105), 11β,13-dihydrolactupicrin-15-al (106) from aerial part of C. intybus, 11β, 13-dihydrolactucin (107), cichoriolide A (108), crepidiaside A (109), crepidiaside B (110), sonchuside A (111), picriside B (112), sonchuside C (113), cichoriosides A (114), cichoriosides B (115), cichoriosides C (116), cichorioside D (117), 8-deoxylactucin (118), Ierisoside D (119), lactucopicrin (120), 11-epiartesin (121), santamarine (122), intybulide (123), crepidiaside B (124), jacquinelin (125), 10α-hydroxycichopumilide (126), 8α-angeloyloxycichoralexin (127), artesin (128), magnolialide (129) from roots of C. intybus, moreover, compounds 107, 119, as well as cichoralexin (130), cichoralexin (isomer) (131), 3,4β-dihydro-15-dehydrolactucopicrin (132) from leaves of C. intybus. Cichotyboside (133) was extracted from seeds of C. intybus. Besides, iIxerisoside D (134) was originated from hairy root culture of C. intybus. Lactupicrin methyl ester (135), lactucopicrin-15-oxalate (136), chicoralexin (137), 1α,5α-epoxy-4α-hydroxyl-4β,10β-dimethyl-7αH,10αH-guaia-l1(13)-en-12-oic acid (138), 3α-R-santamarine (139) were derived from whole plant of C. intybus. Compounds 107, 119, as well as epi-8α-angeloxycichoralexin (140), 8-O-methyl senecioylaustricin (141) were derived from stems of C. glandulosum, while compound 120 was also be extracted from roots of C. glandulosum.
It is noteworthy that10β-Hydroxyguala-4,13-dlen-6 (142) was obtained from roots of C. pumilum, and 6S,7E-6-hydroxy-4,7-megastigmadien-3,9-dione(s(+)-dehydrovomifoliol (143), roseoside (6S,7E,9R)-6,9-dihydroxy-4,7-megastigmadien-3-one (144), 9-O-β-glucopyranoside (145), and 6S,7E,9S-diastereomer (146) were isolated from aerial parts of C. pumilum. Additionally, iacquinelin 15-O-α-L-rhamnopyranosyl-(1–6)-β-D-glucopyranoside (147), cichorioside L (148), cichorioside M (149), cichorioside N (150), 11β,13-dihydro-13-prolyllactucopicrin (151), cichorioside J (152), and cichorioside K (153) were derived from roots of C. endivia, leucodin (154) and tanacetin (155) were originated from the aerial parts of C. spinosum. The structures of the above-mentioned compounds were depicted previously (Long et al., 2014, Aisa and Xin, 2015).
4.5. Nitrogen-contained compounds
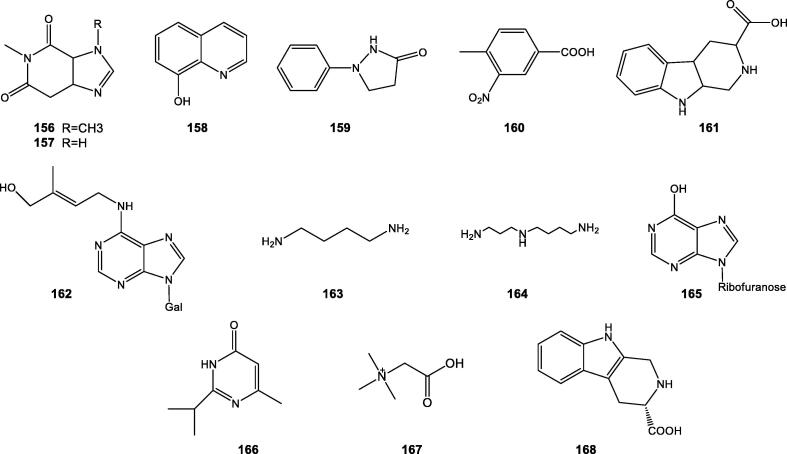
Only a limited number of nitrogen compounds such as caffeine (156), theophylline (157), 8-hydroxyquinoline (158), 1-phenyl-3-pyrazolidone (159), 4-methyl-3-nitrobenzoic acid (160), 2,3,4,9-tetrahydro-1H-pyrido-(3,4-b)indole-3-carboxylic acid (161), zeatin and riboside (162) (Fig. 4), were isolated from roots of C. intybus (Bui-Dang-Ha and Nitsch, 1970, He et al., 2002, Liu, 2005, Atta-ur-Rahman et al., 2008), and putrescine (163) and spermidine (164) were found in leaves of C. intybus (Mao, 2010).
Fig. 4.
Chemical structures of nitrogen-contained compounds from Cichorium genus.
Inosine (165) and 2-isopropyl-6-methylpyrimidin-4(3H)-one (166) isolated from seeds and stems of C. glandulosum (Wu, 2008).
Betaine (167) and (3S)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (168) were found in whole herbs and leaves of C. endivia (Wang et al., 2013).
4.6. Organic acids
Organic acids and organic ester compounds, which were found in genus Cichorium were listed as follows: tartaric acid (169), 4-hydroxycinnamic acid (170), oxalic acid (171), quinic acid (172), ascorbic acid (173), citric acid (174), malic acid (175), 4-O-feruloylquinic acid (176), 5-O-feruloylquinic acid (177), 3-caffeoylquinic acid (178), 4-caffeoylquinic acid (179), 5-caffeoylquinic acid (180), cis-5-caffeoylquinic acid (181), cis-caftaric acid (182), trans-caftaric acid (183), 5-caffeoylshikimic acid (184), 5-p-coumaroylquinic acid (185), 1,3-di-O-caffeoylquinic acid (186), 1,3-di-O-caffeoylquinic acid (187), 3,4-di-O-caffeoylquinic acid (188), 3,5-di-O-caffeoylquinic acid (189), and cichoric acid (190). The above-mentioned compounds were obtained from leaves of C. intybus (Rücker and Noldenn, 1991, Papetti et al., 2008, Carazzone et al., 2013, Papetti et al., 2013, Bergantin et al., 2017, Petropoulos et al., 2018). Compounds 171, 172, 173, 177, 178, 180, 185, succinic acid (191), and shikimic acid (192) were also obtained from frozen red chicory (Papetti et al., 2013). Additionally, tetracosanoic acid (193), 4,4′-dimethy-1,7-heptanedioic acid (194), phthalic acid bis-(2-ethyl-hexyl) ester (195), dibutylphthalate (196) and ethyl caffeate (197) were isolated from seeds of C. glandulosum (Wu, 2008). Compounds 177 and 188 were also found in C. endivia (Papetti et al., 2008).
4.7. Others
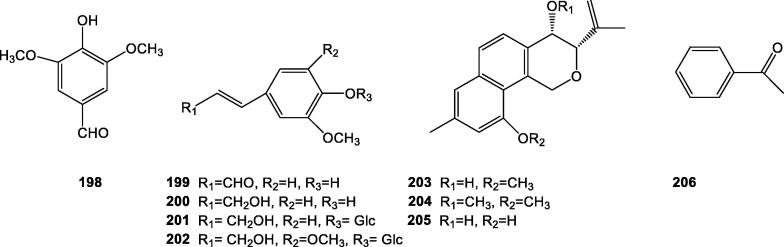
In addition to the above mentioned compounds, there are a number of other compounds such as syringaldehyde (198), coniferyl aldehyde (199), coniferyl alcohol (200), coniferin (201), syringing (202), cichorins A, B, C (203, 204, and 205) were isolated from roots and whole herbs of C. intybus (Kisiel et al., 2004, Hussain et al., 2011) and acetophenone (206) from C. endivia (Götz-Schmidt & Schreier, 1986), and their chemical structures were showed in Fig. 5.
Fig. 5.
Other chemical structures of Cichorium genus.
4.8. Essential oils
In recent years, the essential oil of C. intybus. has been thoroughly investigated, and the volatile compounds were obtained by hydrodistillation of dried material and analyzed by gas chromatography-mass spectrometry (GC/MS). Octane, n-nonadecane, pentadecanone, hexadecane and a tentatively identified compound (pentenyl salicilate) were found as principal components among all volatile constituents. The amounts of octane and the tentatively identified compound were significantly higher in roots (34.3–69.8% and 4.8–22.7%, respectively) than in the aerial parts (8.0–25.6% and 0–0.9%, respectively). An opposite relation was observed for nonadecane; and its content was found to be higher in the aerial parts (5.1–46.9%) than that in roots (0.3–3.9%). Aliphatic compounds and their derivatives comprised the main fraction (63.2–76.9% and 64.1–81.3%, respectively in the aerial parts and roots), while terpenoids were minor constituents. Additionally, 28 identified compounds comprised 75.2–84.7% and 83.4–95.1% of the total content in the aerial parts and roots, respectively (Judzentiene & Udien, 2008). In C. glandulosum, the same way was used for analyzing the essential oil of seeds, in which 24 compounds were identified. Ethylene glycol diethyl ether (56.47%) was the mainly consistent, and 6,10,14-trimethyl-2-pentadecanone (9.38%) was the flavor components. Several kinds of long chain alkanes, alcohols, lipids, and ketone were found in the essential oil as well (Wu, Fan, Ba, Liao, & Aisa, 2005).
4.9. Polysaccharides
Augustín reported optimization of isolation of inulin which is a major polysaccharudes in C. intybus. The obtained experimental results are compared and evaluated with quality (whiteness) and the yield of the obtained final product. They also studied different applications of inulin in pharmaceutical, medical, cosmetological, food industrial, and fodder production fields (Augustín, 2005).
Wu et al. reported the results about carbohydrates from C. glandulosum seeds. Water-soluble polysaccharides (WSPS-1 and 2) were obtained in yield of 1.06% and 1.39%, in which they were light-brown powders that can dissolved in water. WSPS-1 is a mixture of acidic and neutral polysaccharides. The dominant monosaccharides of WSPS-1 are rhamnose, xylose, and galactose; and those of WSPS-2, mainly include mannose and galactose. After removing WSPS, pectinic substances (PS) predominantly contained rhamnose, mannose, and galactose and Hemicellulose (HC-A, B) which are mainly consist of mannose (HC-A), arabinose and xylose (HC-B) and their yield are 2.28%, 0.92% and 4.28% respectively(Wu, Aisa, Rakhmanberdyeva, Zhauynbaeva, & Xin, 2008).
5. Pharmacological activities of Cichorium genus
As an edible plant, C. intybus can be used in salads, as well as being a replacement of coffee in Europe, and C. intybus and C. glandulosum have shown several bio-activities in traditional and modern medicine. The majority of pharmacological activities of C. intybus published before 2012 had been reviewed (Street et al., 2013). Herein, only some activities or pharmacological activities of C. intybus published after 2012 were presented, while other plants in Cichorium were reviewed.
5.1. Photo protective and anti-radical effects
The ethanolic extracts of roots, stalks, and inflorescences of populations of wild Cichorum endivia in terms of protection against sunburn, and in prevention of UVB-induced pyrimidine dimer formation and interleukin-6 (IL-6) mRNA expression were tested in the human keratinocyte cell line (HaCaT). The results showed that the ethanolic extract of C. endivia roots absorbed radiation in the UVB spectrum and partially prevented induction of pyrimidine dimers and IL-6 expression. Application of the root extract on the skin prior to UVB irradiation totally prevented erythema (Enk et al., 2004). Dried seeds of C. intybus were found that it irradiated with 30 kR from a 60Co source, and was grown side by side with controls. (Haque & Godward, 1985) Heimler et al. compared the anti-radical action of C. intybus in which they considered two growing periods: in the first, the plants were subjected to severe water stress, and in the second, the stress was absent. They found that polyphenol content was higher in samples obtained from the former than in the latter (Heimler, Isolani, Vignolini, & Romani, 2009).
Sultana et al. demonstrated that the extracts of C. intybus in the reaction mixture containing calf thymus DNA and free radical generating system can protect DNA against oxidative damage to its deoxyribose sugar moiety. The effect was dose-dependent manner on the concentration of plant extracts, which might be due toits ability to suppress the oxidative degradation of DNA in the tissue debris (Sultana, Perwaiz, Iqbal, & Athar, 1995).
5.2. Liver protection
Traditional medicine unveiled that C. intybus and C. glandulosum have protect effects on liver, which was confirmed by modern experiments of pharmacology as well. Zheng et al. studied the therapeutic effect of chicory polysaccharides (CPS) on non-alcoholic fatty-liver disease (NAFLD), and also investigated its pharmacodynamics on high-fat diet and white wine induced rat model of NAFLD. Dongbao Gantai was used as positive control, and rats with NAFLD were treated by CPS for 4 weeks and levels of total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), superoxide dismutase (SOD), and malondialdehyde (MDA) were detected as well (Zheng, Chang, Cao, & Qi, 2008).
Hassan et al. investigated the modulating effect of chicory-supplemented diet against nitrosamnine-induced oxidative stress and hepatotoxicity in male rats. Their results revealed that rats that received nitrosamine precursors showed a significant increase in liver TBARS, total lipids, total cholesterol, bilirubin and enzyme activities (AST, ALT, ALP and GGT) in both serum and liver. However a significant decrease in the levels of GSH, GSH-Rx, SOD, catalase, total protein and albumin was detected. On the other hand, chicory-supplemented diet successfully modulate these observed abnormalities resulting from nitrosamine compounds as indicated by the reduction of TBARS and the pronounced improvement of the investigated biochemical and antioxidant parameters. Thus, they concluded that chicory has a promising role and can be considered as a natural substance for ameliorating the oxidative stress and hepatic injury induced by nitrosamine compounds (Hassan & Yousef, 2010).
Nallamilli et al. obtained the similar result. They investigated possible hepatoprotective activity of C. intybus against carbon tetrachloride induced hepatotoxicity in albino Wistar rats. They showed that both alcoholic and aqueous extracts of the C. intybus roots were found to have significant hepatoprotective activity, reduces the elevated levels of SGOT, SGPT, SALP and Total bilirubin. But aqueous extract found to have hepatoprotective activity almost compared to silymarin (Nallamilli et al., 2013).
Saggu et al. elucidated the modulating effect of chicory (C. intybus) fruit extracts (CFR) against 4-tert-OP-induced oxidative stress and hepatotoxicity in male rats. They indicated that CFR extract succeeded to improve the biochemical and antioxidant parameters destroyed by 4-tert-OP. Histopathological evidence and observed PCNA and DNA fragmentation supported the effect of CFR extract on improving liver detoxicity (Saggu et al., 2014).
C. intybus was reported to protect against CCl4-induced hepatic fibrosis in rats, which was found as a promising anti-fibrotic therapeutic agent (Li et al., 2014). The flavonoids from seeds of C. glandulosum had hepatoprotective activity on carbon tetrachloride-induced hepatotoxicity in vitro and in vivo. Moreover, the total flavonoids exhibited significant suppression of LPO and pancreatic lipase capacity, which may be the mechanisms of hepatoprotective effects against CCl4 (Tong et al., 2015). Similar studies have demonstrated the hepatoprotective effect of esculetin, a phenolic compound, and cichotyboside, a guaianolide sesquiterpene glycoside reported from C. intybus (Ahmed, Khan, Masood, & Siddique, 2008). The diabetic rats possessing the characteristic of hepatic steatosis showed a significant decrease in fat accumulation and fibrosis after the treatment of C. glandulosum seeds extract (Ziamajidi et al., 2013). The similar effects of C. glandulosum root extraction (CGRE) was found by Upur et al, which supported the traditional usage of C. glandulosum for hepatoprotection due to its antioxidant propriety (Upur, Armt, Blažeković, & Talip, 2009).
Yang et al. investigated the hepatoprotective effects of a sesquiterpene-rich fraction (SRF) from the aerial part of C. glandulosum on carbon tetrachloride (CCl4)-induced acute hepatotoxicity in mice, and on priming with Bacillus Calmette-Guerin (BCG) followed by lipopolysaccharide (LPS)-induced immunological liver injury in mice. They found that SRF is hepatoprotective in animal models of chemical and immunological acute liver injury. Xin et al. studied the antioxidant effects of plant extraction (CGE60) in vitro and in vivo, and find the mechanism of liver protection in Bacille Calmette-Guerin vaccine (BCG) +Lipopolysaccharides (LPS) induced liver injury in mice. They demonstrated that CGE60 possesses antioxidant activity and this activity associates with hepatoprotective effect in the mice of BCG +LPS model, and the mechanisms underlying these effects may involve antioxidant actions and anti-inflammation activities (Yang et al., 2012, Xin et al., 2014).
5.3. Anti-diabetic and hypolipidemic effects
Cha et al. assessed effect of chicory root extract (CRE) on the triglyceride metabolism in orotic acid (OA)-fed rats. They demonstrated that CRE reduces the liver TG accumulation by reduced diacylglycerol acyltransferase (DGAT) and microsomal triglyceride transfer protein (MTP) activities without diminishing MTP mRNA expression by OA administration (Cha, Park, & Cho, 2010).
Caffeoylquinic acid-rich extract from chicory seeds have shown influences on improving glycemia, atherogenic index, and antioxidant status in rats, which could be used as a diet supplement for carrying out clinical trial (Jurgoński, Juśkiewicz, Zduńczyk, & Król, 2012).
Azay-Milhau et al. compared the antihyperglycemic effects of caffeic acid, ferulic acid and natural chicoric acid extract (NCRAE, 50 and 100 mg/mL) on the three major tissues implicated in glycemic regulation (pancreas, muscle and liver) in vitro and in vivo. It was uncovered that caffeic acid mainly promotes a decrease in hepatic glycogenolysis; besides, ferulic acid elicits a clear increase of insulin release and a reduction of hepatic glycogenolysis. They also found that NCRAE provokes an increase of insulin release and glucose uptake without any effect on hepatic glycogenolysis (Azay-Milhau et al., 2013).
The flavonoids from C. glandulosum seeds have antioxidant activity both in vivo and in vitro. They reported that in treatment groups with TFs (100, 200, 400 mg/kg), a significant decrease in the MDA level and an increase in the levels of SOD and glutathione (Yao, Zhu, Chen, Tian, & Wang, 2013).
Lin et al. (2015) studied the protective and anti-obesity effects of inulin from C. intybus on serum lipid concentration and abdominal fat pad mass in quail model induced by protein and purine rich diet. They found that inulin of C. intybus significantly improved lipid metabolism of diet that induced abdominal obesity in quails. The possible mechanism of anti-obesity activity appeared to be either associated with decreasing acetyl-Coa carboxlyase (ACC) protein expression and fatty acid synthase (FAS) activity, or decreasing serum TG level (Lin, Zhang, Li, Wang, & Zhu, 2015).
In a randomized double-blind placebo-controlled study, Nishimura et al. examined the effects of chicory root extract by some biomarkers for diabetes in 47 healthy adult participants. The extract from roasted chicory roots could delay or prevent the early onset of diabetes mellitus and improve bowel movements, including improvement of blood glucose and lipid metabolism. This result indicated the extract could suspend occurrence and development of diabetes mellitus (Nishimura et al., 2015).
A number of scholars demonstrated that anti-inflammatory action of chicory seed extract (CSE) is due to a direct modulation of cytokine expression. The dependency of chicory action on the presence of insulin indicates its usefulness in the early stages of diabetes and for the purpose of preventing and delaying diabetes onset (Rezagholizadeh et al., 2016). Recently, that research team further tested the usefulness of CSE in preventing diabetes-induced kidney damage. CSE can repair the kidney damage and a1-microglobulin, which is sensitive enough to allow monitoring of the improvement caused by the treatment (Pourfarjam et al., 2017).
Polysaccharides from C. glandulosum (CGP) significantly decreased TC, TG, the mRNA expression of SREBP-1 and Fas, while increased the expression of PPARα/β. Facilitation of lipolysis (mainly beta-oxidation) or inhibition of lipogenesis was found as the main pathway for hypolipidemic ability of CGP. Furthermore, CGP could prevent and cause the regression of steatosis in NAFLD via its regulatory effects on lipid metabolism (Li et al., 2018).
5.4. Antihyperuricemia
From 1998 to 2004, C. intybus extracts were reported by Zhang et al. to reduce whole blood viscosity, plasma specific viscosity, blood glucose, serum uric acid and blood lipid in quail models which were establishedon the basis of high fat diet (Zhang et al., 1998). C. intybus extract also led to the decrease of blood glucose in diabetic rabbit models induced by alloxan. Additionally, Zhang et al. further explained the mechanism of Cichorium extracts on a quail model of hyperuricemia (Zhang et al., 1998; Gao et al., 1999; Hyunm et al., 1999, Zheng et al., 2000, Kong et al., 2003, Liu et al., 2003, Sa et al., 2004). Chicory remarkably decreased the serum uric acid level, whereas noticeably increased the intestinal uric acid excretion in rats with hyperuricemia induced by 10% fructose via down-regulating the mRNA and protein expressions of ABCG2 (Wang, Lin, Zhang, Nie, & Bian, 2017). Chicory also markedly attenuated serum levels of urate and creatinine, while promoted the clearance of creatinine and urate, as well as improving renal pathologic changes resulted from hyperuricemia. Expressions of URAT1 and GLUT9 were remarkably inhibited by chicory in a dose-dependent manner, while no effect was found on expression of OAT1 or OAT3. The mechanism of its uricosuric effect is related to promoting renal excretion of urate by inhibiting urate reabsorption, which might be associated to down-regulation of mRNA and protein expression of URAT1 and GLUT9 (Wang, Lin, Zhang, Wang, & Chu, 2019).
5.5. Antioxidant and anti-inflammation
The fraction rich of sesquiterpene from C. glandulosum and chicory seed extract showed anti-inflammation effect in mice with liver-injuried by Bacille Calmette-Guerin vaccine (BCG)+Lipopolysaccharides (LPS) (Xin et al., 2014) and in addition to diabetic rats induced by STZ+niacinamide (Rezagholizadeh et al., 2016).
Organic acids such as hydroxycinnamic acids, isomers of chlorogenic acid, caftaric acid, cichoric acid flavonoids are the main compounds in leaves of C. intybus which exhibited superior antioxidant and enzyme inhibitory activities on α-glucosidase (Dalar & Konczak, 2014). The half maximal inhibitory concentration (IC50) value of chicory leaves extract was found to be (67.2 ± 2.6) μg/mL in 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay (Abbas et al., 2015). Recently, Zeb et al. evaluated the effects of microwave cooking (MWC) on antioxidant activity of C. intybus leaves for the first time. They found that cooking of chicory leaves or its pre-treatment with microwave in food industries enhanced the important bioactive substances for consumer health (Zeb, Haq, & Murkovic, 2019).
After treated with seed extract of C. intybus (CIE 250 mg and 500 mg/kg) for 3 weeks, CIE exerted the cardio-protective effect via inhibition of oxidative stress and pro-inflammatory cytokines (Ahmed, 2018).
Ghaffari et al. assessed the effects of chicory seeds (CHI) and Curcuma longa L (TUR) + chicory seeds consumption on antioxidant status and inflammatory biomarkers in patients with NAFLD. They found that chicory seed and combination of chicory seed and turmeric significantly reduced serum levels of MDA in comparison with placebo. Combination of turmeric and chicory seed marginally reduced serum level of IL-6. They concluded that turmeric and chicory seed may be applicable for reducing risk factors for NAFLD (Ghaffari, Rafraf, Navekar, & Asghari-Jafarabadi, 2018).
5.6. Antimicrobial effect
The antibacterial and antifungal activities of C. intybus crude extract and its different solvent soluble fractions were carried out by using standard methods on Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus epidermidis, methicillin-resistant Staphylococcus aureus, and Bacillus subtilis. The water and ethyl acetate fractions showed promising activity against Fusariun solani and Aspergillus niger. Furthermore, chloroform fraction was also found to be highly active against Fusariun solani (Rehman, Ullah, Ullah, & Ahmad, 2014). All the seed extracts of C. intybus showed antimicrobial activity against tested microorganisms whereas S. aureus was found to be the most sensitive against aqueous extract and had the widest zone of inhibition. Ethyl acetate and ethanol extract were found to be significant against P. aeruginosa and S. aureus (Shaikh, Rub, & Sasikumar, 2016). Leaves of red chicory also had antimicrobial properties on Saccharomyces yeast and four lactic acid bacteria (Kagkli et al., 2016).
5.7. Antimalarial activity
Bischoff et al. reported that the aqueous root extracts of C. intybus could be served as a light-sensitive plant remedy for malaria. Lactucin and lactucopicrin could be regarded as antimalarial compounds (Bischoff et al., 2004). Pan et al. reviewed the antimalarial activity of plant metabolites included C. intybus (Pan, Xu, Shi, Tsang, & Zhang, 2018).
5.8. Antiosteoporotic effect
Roberfroid et al. investigated the antiosteoporotic effect of inulin at the dosages of 0, 5 and 10 g/100 g and 0.2, 0.5 or 1 g Ca/100 g in diet by comparing whole-body bone mineral content (WBBMC), whole-body bone area (WBBA) and whole-body bone mineral density (WBBMD) in liver of adult male rats. Although inulin significantly increased WBBMC (P < 0.05) and WBBMD (P < 0.001) at all ages and dietary calcium concentrations, it had no effect on WBBA (Roberfroi, Cumps, & Devogelaer, 2002).
C. intybus extract had shown positive effects on rats with osteoporosis induced by glucocorticoid. Fourty female rats were injected with 0.1 mg/kg b.wt. dexamethasone to induce the model. After treated with aqueous parsley extract (2 g/kg b.wt.), aqueous basil extract (400 mg/kg b.wt.) and aqueous chicory extract (100 mg/kg b.wt), bone protective effects were observed. It was noted that therapeutic effectiveness was more remarkable with Chicory in comparison with parsley and basil based on flavonoids and inulin (Hozayen, El-Desouky, Soliman, Ahmed, & Khalief, 2016).
5.9. Tumour inhibitory activity
Chen et al. discussed about the anti-tumor effect and mechanism of chicory polysaccharide (CPS). A number of mice received tumor cell after CPS oral administration for 10 days. It was unveiled that CPS inhibited the growth of sarcoma S180, mouse forestomach carcinoma (MFC) cell line, especially in inhibiting the growth of S180 and prolonging the survival time at a lower dose. Meanwhile, the phagocyte index A, expuragation index K, thymus coefficient and the transform function of the lymphocyte were increased in vitro (Chen, Chang, Zheng, Ma, & Chang, 2004). Another research demonstrated that (3S)-1, 2, 3, 4-Tetrahydro-β-carboline-3-carboxylic acid from C. endivia can induce apoptosis of HCT-8 cells (Wang et al., 2013).
Al-Akhras et al.also found that chicory extract significantly increased the levels of P. carbonyl (PC) and malondialdehyde (MDA) while decreased the hepatic levels of total antioxidant capacity (TAC) andSOD in benign breast tumors-induced group compared with control. It also significantly reduced the number of estrogen receptors in tumor masses. These results suggested that chicory extracts could be used as herbal photosensitizing agent against protecting against benign breast tumor in rats (Al-Akhras, Aljarrah, & Al-Khateeb, 2012).
5.10. Immunomodulatory effect
Amirghofran et al. evaluated the immunoregulative effect of extract from C. intybus. No direct mutagenic effect was found on human lymphocytes or thymocytes, while it inhibited the proliferation of lymphocytes effectively (Amirghofran, Azadbakht, & Karimi, 2000).
Ni et al. (2005) tested mice that received CPS in different concentrations by orally for 20 d. As a result, the number of antibody-forming cells, the function of mice macrophage, weight indices of thymus gland and spleen increased significantly (Ni, Zheng, & Chang, 2005).
In 2008, the effect of CPS on human immunity was observed in 58 volunteers (18 men and 40 women). They were randomly divided into two group, and received health food via the bi-blind method. The volunteers consumed CPS 1.5 g/d orally without changing the life habits during two consecutive months. Various biochemical indexes and the content of urea acid in blood out of veins were measured before and after the experiment, and CPS has greater enhancement effects on human immunity (Ren, Chang, Zheng, Cao, & Qi, 2008).
5.11. Neuritogenesis and neurotrophic effects
Two sesquiterpene lactones extracted from chicory roots, 8-deoxylactucin and lactucopicrin showed a significant potent AChE inhibitory activity with IC50 of 308.1 μmol/L (Dem’yanenko & Dranik, 1971) and 150.3 μmol/L (Vardanyan, 1979) respectively in a dose-dependent manner. The dichloromethane extract of chicory roots had inhibitory activity with 70% inhibition at 1 mg/mL on AChE feature-based pharmacophore model and a docking procedure, sesquiterpenoids with low molecular weight exhibited distinct interactions in pharmacophore model (Rollinger et al., 2005).
Lactucopicrin, as an inhibitor of acetylcholine esterase (AChE) ameliorated oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway, increase of intracellular Ca2+ and the expression of muscarinic acetylcholine receptor M1 (CHRM1) in N2a cells. Lactucopicrin also had effect on neurite outgrowth by CaMKII and further activated ATF1-TrkA-ERK1/2-AKT- and synaptophysin 1 in N2a cells. Additionally, it also increased the levels of neurotrophins including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT3) in C6 cells, while the effects of lactucopicrin on NGF secretion and neuritogenesis could not be prevented by PI3K inhibitor LY294002 (Venkatesan et al., 2016, Venkatesan et al., 2017).
Supplementation with inulin during pregnancy attenuates acrylamide-induced maternal and fetal brain oxidative dysfunctions, anxiety and neurotoxicity in rat. After supplement with inulin, ACR treatment decreased fetal/placental weights which were marginally restored, levels of hydroperoxides and nitric oxide in maternal brain regions of rats was significantly attenuated, levels of protein carbonyls in fetal brain were completely normalized, dopamine levels and acetylcholinesterase activity were noticeably diminished (Gokul & Muralidhara, 2015).
5.12. Anthelmintic activities
Mansour et al. reported that saponification of petroleum ether extract results in saponifiable and unsaponifiable fractions, in which the latter is highly toxic than the former. They also demonstrated that fractionation of the chicory plant (C. intybus) is in favor of toxicity, increasing towards the insect pests (Mansour, Ibrahim, & EL-Gengaihic, 2014). Sesquiterpene lactones-containing extracts from forage chicory showed to inhibitory effects on feeding of free-living larvae and exerted direct effects against parasitic stages of O. ostertagi (Peña-Espinoza et al., 2015).
C. oncophora eggs were inhibited by chicory extract with 95% inhibition at 2500 μg extract/mL (EC50 = 619 μg extract/mL) in dose-dependent manner. Adult worms showed a total paralysis after 12 h of incubation with chicory extract at ≥500 μg extract/mL. A complete inhibition of worm motility was observed after 48 h of incubation at ≥250 μg extract/mL (Peña-Espinoza, Williams, Thamsborgb, Simonsenc, & Enemarkd, 2017). Chicory also be reported for its antiparasitic activity against GI parasites in livestock, and the bioactive compounds were focused on sesquiterpene lactones components (Peña-Espinoza et al., 2018). Extracts from roots or aerial parts of Chicory (C. intybus) exhibited strong larvicidal activity against mosquito vectors of malaria, dengue fever, and filariasis. Methanol extract of roots of C. intybus showed the highest larvicidal activity with LC50 of 18.88, 40.15, 64.56 and LC90 of 107.16, 231.28, 247.54 μg/mL for An. Stephensi, Ae. Aegypti and Cx. quinquefasciatus, respectively (Ali, Gopalakrishnan, & Venkatesalu, 2018).
5.13. Gastroprotective effect
The methanol extracted from C. intybus roots improved the gastric ulcer in a rat model, suggesting that it had gastroprotective effect when given orally (Gürbüz, Ustün, Yeşilada, Sezik, & Akyürek, 2002). Another research showed that the red part of Treviso red chicory leaf has a high content of anthocyanins, which could be used as a potential food supplement to improve intestinal diseases (D’evoli et al., 2013). C. intybus root superfine powder could regulate the disturbance of intestinal microflora in immunosuppressive mice induced by cyclophosphamide (Wu, 2016).
5.14. Other bioactivies
In addition to the pharmacological activities mentioned above, plants in Cichorium genus also have anti-rheumatic (Sharma, 1991), antiallergic (Hyunm et al., 1999), cardioprotective (Nayeemunnisa & Kumuda, 2003), vasorelaxant (Sakurai et al., 2003), anti-aging (Qi, Cao, Zheng, & Chang, 2008), antifatigue (Zheng, Hu, Wu, Chang, & Ma, 2004) and anti-HIV-1 effects (Lee, Shin, Lee, & Lee, 2007).
6. Safety and toxicity evaluation
A number of researches about the evaluation on safety and toxicity of inulin and chicory root extract were presented (Aisa & Xin, 2015). Microtox acute toxicity test of C. intybus extracts has also finished by Vibrio fscheri bioluminescence inhibition test. Less than 20% inhibition of bioluminescence was obtained by this way, suggesting C. intybus extracts were considered to be safe for people (Conforti et al., 2008). Yang et al. detected the acute toxicity of C. glandulosum aqueous extracts (MS). All mice were killed and grossly anatomized after 14 days. There was no acute toxicity in mice treated with MS at the maximum dose of 150 g/kg (Yang et al., 2017).
7. Clinical application
The clinical application of C. glandulosum had been previously prescribed (Aisa & Xin, 2015). In this study, other clinical applications about C. intybus were collected. The safety and tolerability of chicory root extracts were tested in patients with osteoarthritis. The results suggested that it has a potential effect in treatment of osteoarthritis, while other merits need to be further investigation (Schumacher et al., 2011). After a week of chicory and coffee intake, depending on the aggregation test, the viscosity in both plasma and whole blood were significantly reduced, in addition to decrease of MIF levels in serum. The efficacy of herbal medicine Liv-52 which content about 24% C. intybus had been used in 36 cirrhotic patients for 6 month, the protective effect was identified and this effect may be attributed to the anti-oxidative, diuretic, anti-inflammatory and immunoregulatory properties (Ahmed et al., 2003, Huseini, Alavian, Heshmat, Heydari, & Abolmaali, 2005).
8. Conclusion
The plants in genus Cichorium has been used globally since several decades ago as a coffee substitute, vegetable crop, and medicinal plant, and were occasionally used as animal forage as well. Toxicological data on plants related to this genus are currently limited; the clinical applications of this genus could be supported by investigation of phytochemical and biological activities.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgment
This study was supported by National Natural Science Foundation of China (NSFC), Grant No. U1703235.
References
- Ahmed B., Al-Howiriny T.A., Siddiqui A.B. Antihepatotoxic activity of seeds of Cichorium intybus. Journal of Ethnopharmacology. 2003;87(2–3):237–240. doi: 10.1016/s0378-8741(03)00145-4. [DOI] [PubMed] [Google Scholar]
- Abbas Z.K., Saggu S., Sakeran M.I., Zidan N., Rehman H., Ansari A.A. Phytochemical, antioxidant and minera composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi Journal of Biological Sciences. 2015;22:322–326. doi: 10.1016/j.sjbs.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed B., Khan S., Masood M.H., Siddique A.H. Antihepatotoxic activity of cichotyboside, a sesquiterpene glycoside from the seeds of Cichorium intybus. Journal of Asian Natural Products Research. 2008;10:223–231. doi: 10.1080/10286020701590764. [DOI] [PubMed] [Google Scholar]
- Ahmed B., Bawa S., Siddiqui A.B., Alam S.A. Components fromseeds of Cichorium intybus Linn. Indian Chem. J. 2002;41B:2701–2705. [Google Scholar]
- Ahmed D. Cichorium intybus attenuates streptozotocin induced diabetic cardiomyopathy via inhibition of oxidative stress and inflammatory response in rats. Free Radical Biology and Medicine. 2018;120:S45–S166. doi: 10.2478/intox-2019-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Akhras M.A.H., Aljarrah K., Al-Khateeb H. Introducing Cichorium pumilum as a potential therapeutical agent against drug-induced benign breast tumor in rats. Electromagnetic Biology and Medicine. 2012;31(4):299–309. doi: 10.3109/15368378.2012.662193. [DOI] [PubMed] [Google Scholar]
- Aisa H.A., Xin X.L. Springer; 2015. Dietary Chinese herbs chemistry, pharmacology and clinical evidence. chapter 80. [Google Scholar]
- Ali S.I., Gopalakrishnan B., Venkatesalu V. Chicory (Cichorium intybus) and wormwood (Artemisia absinthium) extracts exhibit strong larvicidal activity against mosquito vectors of malaria, dengue fever, and filariasis. Parasitology International. 2018;67:781–786. doi: 10.1016/j.parint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Amirghofran Z., Azadbakht M., Karimi M.H. Evaluation the immunomodulatory effects of five herbal plants. Journal of Ethnopharmacology. 2000;72:167–172. doi: 10.1016/s0378-8741(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Atta-ur-Rahman Z.S., Choudhary M.I., Akhtar M.N., Khan S.N. α-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus. Journal of Natural Products. 2008;71(5):910–913. doi: 10.1021/np800001v. [DOI] [PubMed] [Google Scholar]
- Augustín J. Optimization of isolation of inulin from Cichorium intybus L. and some of its uses in social practice. Ceská a Slovenská farmacie. 2005;54(3):145–150. [PubMed] [Google Scholar]
- Azay-Milhau J., Ferrare K., Leroy J., Aubaterre J., Tournier M., Lajoix A.D., Tousch D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): A comparative in vitro study with the effects of caffeic and ferulic acids. Journal of Ethnopharmacology. 2013;150:755–760. doi: 10.1016/j.jep.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Bergantin C., Annalisa M., Cavazzini A., Pasti L., Tedesci P., Brandolini V., Marchetti N. Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in Red Chicory (Cichorium intybus) Journal of Functional Foods. 2017;33:94–102. [Google Scholar]
- Bischoff T.A., Kelley C.J., Karchesy Y., Laurantos M., Nguyen-Dinh P., Arefi A.G. Antimalarial activity of lactucin and lactucopicrin: Sesquiterpene lactones isolated from Cichorium intybus L. Journal of Ethnopharmacology. 2004;95(2–3):455–457. doi: 10.1016/j.jep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Board of health of Xinjiang Uygur Autonomous Region, Xinjiang Institute of Biology, Pedology and Desert Research, Xinjiang Rear Service Department and health department of The Chinese People's Liberation Army, Ed. 1976. Chinese herb in Xinjiang. Urumqi Xinjiang People’s publishing House.
- Bui-Dang-Ha D., Nitsch J.P. Isolation of zeatin. riboside from chicory root. Planta. 1970;95:119–126. doi: 10.1007/BF00387244. [DOI] [PubMed] [Google Scholar]
- Bussmann R.W., Paniagua Zambrana N.Y., Sikharulidze S., Kikvidze Z., Kikodze D., Tchelidze D.…Hart R.E. Plant and fungal use in Tusheti, Khevsureti, and Pshavi, Sakartvelo (Republic of Georgia) Caucasus. Acta Societatis Botanicorum Poloniae. 2017;86(2):3517. [Google Scholar]
- Carazzone C., Mascherpa D., Gazzani G., Papetti A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass pectrometry. Food Chemistry. 2013;138:1062–1071. doi: 10.1016/j.foodchem.2012.11.060. [DOI] [PubMed] [Google Scholar]
- Cha J.Y., Park C.K., Cho Y.S. Hepatoprotective effect of chicory (Chicorium intybus) root extract against orotic acid-induced fatty liver in rats. Food Science & Biotechnology. 2010;19(4):865–871. [Google Scholar]
- Chen L.G., Chang Y.Q., Zheng H.Y., Ma J.R., Chang X.T. The experimental study of CPS antitumor. Food Science. 2004;25(11):276–280. [Google Scholar]
- Christopher B. Third edition. Dorling Kindersley; London: 2003. RHS A-Z encyclopedia of garden plants. [Google Scholar]
- Conforti F., Ioele G., Statti G.A., Marrelli M., Ragno G., Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food and Chem. Toxicol. 2008;46(10):3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Dalar A., Konczak I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Industrial Crops & Products. 2014;60:79–85. [Google Scholar]
- Dem’yanenko V.G., Dranik L.I. Coumarins of the racemes of Cichorium in tybus. Chemistry of Natural Compounds. 1971;7(1):104. [Google Scholar]
- Dem’yanenko V.G., Dranik L.I. Flavonoids of Cichorium intybus. Chemistry of Natural Compounds. 1973;9(1):115. [Google Scholar]
- Dei Cas L., Pugni F., Fico G. Tradition of use on medicinal species in Valfurva (Sondrio, Italy) Journal of Ethnopharmacology. 2015;163:113–134. doi: 10.1016/j.jep.2014.12.054. [DOI] [PubMed] [Google Scholar]
- Devoli L., Morroni F., Lombardi-Boccia G., Lucarini M., Hrelia P., Cantelli-Forti G., Tarozzi A. Red Chicory (Cichorium intybus L. cultivar) as a potential source of antioxidant anthocyanins for intestinal health. Oxidative Medicine and Cellular Longevity. 2013 doi: 10.1155/2013/704310. 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial committee of xinjiang Plant flora, Ed. (1999) Flora of Xinjiang. Urumqi Xinjiang Sci-Tech and Public Health Press.
- EI-Lakany A.M., Aboul-Ela M.A., Abdul-Ghani M.M., Mekky H. Chemical constituents and biological activities of Cichorium intybus L. Journal of Natural Products. 2004;10(2):69–73. [Google Scholar]
- Enk C.D., Hochberg M., Torres A., Lev O., Dor I., Srebnik M., Dembitsky V.M. Photoprotection by Cichorum endivia extracts: Prevention of UVB-induced erythema, pyrimidine dimer formation and IL-6 expression. Skin Pharmacology and Physiology. 2004;17(1):42–48. doi: 10.1159/000074062. [DOI] [PubMed] [Google Scholar]
- Erhardt W., Götz E., Bödeker N. Eugen Ulmer KG; Stuttgart: 2008. Siegmund Seybold: Der große Zander. [Google Scholar]
- Fan H., Chen J., Liang C.Y., Ren B.R., Li W.L. Advance in studies on chemical constituents of Cichorii Herba and their pharmacological effects. Chinese Traditional and Herbal Drugs. 2016;47(4):680–688. [Google Scholar]
- Fan H., Chen J., Lv H., Ao X.C., Wu Y.X., Ren B.R., Li W.L. Isolation and identifcation of terpenoids from chicory roots and their inhibitory activities against yeast α-glucosidase. European Food Research and Technology. 2017;243:1009–1017. [Google Scholar]
- Fernald, M. L. Ed. (1950). Gray’s Manual of Botany American Book
- Götz-Schmidt E.M., Schreier P. Neutral volatiles from blended endive(Cichorium endivia, L.) J. Agric. Food Chem. 1986;34(2):212–215. [Google Scholar]
- Gürbüz I., Ustün O., Yeşilada E., Sezik E., Akyürek N. In vivo gastroprotective effects of five Turkish folk remedies against ethanol-induced lesions. Journal of Ethnopharmacology. 2002;83(3):241–244. doi: 10.1016/s0378-8741(02)00248-9. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y, Zhang B., Jiang P.F., Zheng H.M., He T., Jiao Y.F. The study of pharmacological activity of ethanol extraction and water extraction. Journal of Bejing university of TMC. 1999;22(3):43–44. [Google Scholar]
- Ghaffari A., Rafraf M., Navekar R., Asghari-Jafarabadi M. Effects of turmeric and chicory seed supplementation on antioxidant and inflammatory biomarkers in patients with non-alcoholic fatty liver disease (NAFLD) Advances in Integrative Medicine. 2018;5:89–95. [Google Scholar]
- Gokul K., Muralidhara Inulin (a nondigestible oligosaccharide) supplements during pregnancy attenuates acrylamide-induced maternal and fetal brain oxidative dysfunctions, anxiety and neurotoxicity in rats. International Journal of Developmental Neuroscience. 2015;47:77. [Google Scholar]
- Grubben, G. J. H., Denton, O. A. Ed. (2004). Plant resources of Tropical Africa, vegetables. PROTA Foundation, Wageningen, Netherlands/, Earthprint Limited.
- Haque M.Z., Godward M.B.E. Effects of seed irradiation on M1 achenes of Lactuca and Cichorium. Environmental and Experimental Botany. 1985;2(1):53–65. [Google Scholar]
- Hassan H.A., Yousef M.I. Ameliorating effect of chicory (Cichorium intybus L)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food and Chemical Toxicology. 2010;48(8–9):2163–2169. doi: 10.1016/j.fct.2010.05.023. [DOI] [PubMed] [Google Scholar]
- He T., Guo Y.J., Gao Y.N. The study of Chemical constituents on the root of Cichorium intybus L. China Journal of Chinese Materia Medica. 2002;27(3):209–210. [PubMed] [Google Scholar]
- Heimler D., Isolani L., Vignolini P., Romani A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chemistry. 2009;114:765–770. [Google Scholar]
- Hozayen W.G., El-Desouky M.A., Soliman H.A., Ahmed R.R., Khalief A.K. Antiosteoporotic effect of Petroselinum crispum, Ocimum basilicum and Cichorium intybus L. in glucocorticoid-induced osteoporosis in rats. Bmc Complementary and Alternative Medicine. 2016;16:165–175. doi: 10.1186/s12906-016-1140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseini H.F., Alavian S.M., Heshmat R., Heydari M.R., Abolmaali K. The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo- controlled first approach. Phytomedicine. 2005;12(9):619–624. doi: 10.1016/j.phymed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Hussain H., Hussain J., Saleem M., Miana G.A., Riaz M., Krohn K., Anwar S. Cichorin A: A new benzo-isochromene from Cichorium intybus. Journal of Asian Natural Products Research. 2011;13(6):566–569. doi: 10.1080/10286020.2011.573789. [DOI] [PubMed] [Google Scholar]
- Hyunm M.K., Hyw W.K., Yeoung S.L., Jin H.W., Dae K.K., Young M.L.…Nyeon H.A. Inhibitory effect of mask cell-mediated immediated-type allergic reactions by cichorium intybus. Pharmacological Research. 1999;40(1):61–65. doi: 10.1006/phrs.1999.0474. [DOI] [PubMed] [Google Scholar]
- Jiangsu New Medical School. 1977. “ZHHONYIYAODACIDIAN.” 2008.
- Judzentiene A., Udien J.B. Volatile constituents from aerial parts and roots of Cichorium intybus L. (chicory) grown in Lithuania. Chemija. 2008;19(2):25–28. [Google Scholar]
- Jurgoński A., Juśkiewicz J., Zduńczyk Z., Król B. Caffeoylquinic acid-rich extract from chicory seeds improves glycemia, atherogenic index, and antioxidant status in rats. Nutrition. 2012;28:300–306. doi: 10.1016/j.nut.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Kagkli D.M., Corich V., Bovo B., Lante A., Giacomini A. Antiradical and antimicrobial properties of fermented red chicory (Cichorium intybus L.) by-products. Annals of Microbiology. 2016;66:1377–1386. [Google Scholar]
- Kisiel W., Michalska K., Szneler E. Norisoprenoids from aerial parts of Cichorium pumilum. Biochemical Systematics and Ecology. 2004;32:343–346. [Google Scholar]
- Klados E., Tzortzakis N. Effects of substrate and salinity in hydroponically grown Cichorium spinosum. Journal of Soil Science and Plant Nutrution. 2014;14(1):211–222. [Google Scholar]
- Kong Y., Zhang B., Liu X.Q., Sa Y., Ye G.H. Effect and mechanism study of Cichorii extracts hyperuricemic model quail. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2003;12(11):1138–1139. [Google Scholar]
- Krebsky E.O., Geunsb J.M.C., Proft M.D. Polyamines and sterols in Cichorium heads. Phytochemistry. 1999;550(50):549–553. [Google Scholar]
- Kumari R., Ali M., Aeri V. Two new triterpenoids from Cichorium intybus L. roots. Journal of Asian Natural Products Research. 2010;14(1):7–13. doi: 10.1080/10286020.2011.619181. [DOI] [PubMed] [Google Scholar]
- Lee S.U., Shin C.G., Lee C.K., Lee Y.S. Caffeoylglycolic and caffeoylamino acid derivatives, halfmers of L-chicoric acid, as new HIV-1 integrase inhibitors. European Journal of Medicinal Chemistry. 2007;42(10):1309–1315. doi: 10.1016/j.ejmech.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Li G.Y., Gao H., Huang J., Lu J., Gu J.K., Wang J.H. Hepatoprotective effect of Cichorium intybus L., a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fbrosis in rats. World Journal of Gastroenterology. 2014;20(16):4753–4760. doi: 10.3748/wjg.v20.i16.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.R., Ma J., Ahmad O., Cao Y., Wang B., He Q.Q.…Shang J. Lipid-modulate activity of Cichorium glandulosum Boiss. et Huet polysaccharide in nonalcoholic fatty liver disease larval zebrafish model. Journal of Pharmacological Sciences. 2018;138:257–262. doi: 10.1016/j.jphs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Lin Z.J., Zhang B., Li L.Y., Wang H.P., Zhu C.S. Experimental study on protective and anti-obesity effects of inulin from chicory (Cichorium intybus L.) on quail model. Integrative Medicine Research. 2015;1–41:2. [Google Scholar]
- Liu X.Q., Zhang B., Hu J., Liu C.M., Hong Q.T., Zhang H.J., Kong Y. Study on establishing hyperuricemia quail model and effect of cichorii extracts. Chinese Journal of Integrated Traditional and Western Medicine. 2003;23(1):45–47. [Google Scholar]
- Liu W.B. Beijing University Master degree paper; 2005. Studies on the chemical constituents of traditional Uygur medicine-Cichorum glandulosum Boiss.et Hue. [Google Scholar]
- Long T., Gao Y., Niu Y.J., Wang H. Research progress in chemical constituents and pharmacological activities of plants in Cichorium genus. Strait Pharmaceutical Journal. 2014;26(6):1–6. [Google Scholar]
- Mao Q.L. Xinjiag University; 2010. Preliminary studies on the chemical composition of Cichorium glandulosum (D) [Google Scholar]
- Malarz J., Stojakowska A., Szneler E., Kisiel W. A new neolignan glucoside from hairy roots of Cichorium intybus. Phytochemistry Letters. 2013;6:59–61. [Google Scholar]
- Mansour S.A., Ibrahim R.M., El-Gengaihic S.E. Insecticidal activity of chicory (Cichorium intybus L.) extracts against two dipterous insect-disease vectors: Mosquito and housefly. Industrial Crops and Products. 2014;54:192–202. [Google Scholar]
- Nallamilli B.R., Kumar C.S.P., Reddy K.V., Prasannac M.L., Maruthic V., Sucharita P. Hepatoprotective activity of Cichorium intybus (Linn.) root extract against carbon tetrachloride induced hepatotoxicity in albino Wistar rats. Drug Inovention. 2013;5:311–314. [Google Scholar]
- National Agricultural Statistics Service Information USDA, 2002.
- National Institute for the Control of Pharmaceutical and Biological Products, Ed. (1990). Chinese traditional medicine. Beijing, People's Hygiene Publishing House
- Nayeemunnisa M., Kumuda R. Cardioprotective effects of Cichorium intybus in ageing myocardium of albino rats. Current Science. 2003;84(7):10. [Google Scholar]
- Ni X.Z., Zheng H.Y., Chang Y.Q. Immunological regulation of chicory polysaccharides. Food Science. 2005;26(9):534–536. [Google Scholar]
- Nishimura M., Ohkawara T., Kanayama T., Kitagawa K., Nishimura H., Nishihira J. Effects of the extract from roasted chicory (Cichorium intybus L.) root containing inulin-type fructans on blood glucose, lipid metabolism, and fecal properties. Jouranl of Traditional and Complementary Medicine. 2015;5:161–167. doi: 10.1016/j.jtcme.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti A., Daglia M., Aceti C., Sordelli B., Spini V., Carazzone C., Gazzani G. Hydroxycinnamic acid derivatives occurring in Cichorium endivia vegetables. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(2):472–476. doi: 10.1016/j.jpba.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Norbaek R., Nielsen K., Kondo T. Anthocyanins from flowers of Cichorium intybus. Phytochemistry. 2002;60:357–359. doi: 10.1016/s0031-9422(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Pan W.H., Xu X.Y., Shi N., Tsang S., Zhang H.J. Antimalarial activity of plant metabolites. International Journal of Molecular Sciences. 2018;19(5):1382. doi: 10.3390/ijms19051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti A., Mascherpa D., Carazzone C., Stauder M., Spratt D.A., Wilson M.…Gazzani G. Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria. Food Chemistry. 2013;138:1706–1712. doi: 10.1016/j.foodchem.2012.10.148. [DOI] [PubMed] [Google Scholar]
- Pei L.P., Zhou X.Y., Cui J., Pan Z.R., Liu H.B., Ge L. Determination of eight metal elements in Cichorium glandulosum Boiss et Huet by microwave digestion-FAASL. Spectroscopy and Spectral Analysis. 2009;29(12):3412–3415. [PubMed] [Google Scholar]
- Peña-Espinoza M., Boas U., Williams A.R., Thamsborg S.M., Simonsen H.T., Enemark H.L. Sesquiterpene lactone containing extracts from two cultivars of forage chicory (Cichorium intybus) show distinctive chemical profiles and in vitro activity against Ostertagia ostertagi. International Journal for Parasitology Drugs & Drug Resistance. 2015;5:191–200. doi: 10.1016/j.ijpddr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Espinoza M., Williams A.R., Thamsborgb S.M., Simonsenc H.T., Enemarkd H.L. Anthelmintic effects of forage chicory (Cichorium intybus) against free-living and parasitic stages of Cooperia oncophora. Veterinary Parasitology. 2017;243:204–220. doi: 10.1016/j.vetpar.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Peña-Espinoza M., Valente A.H., Thamsborg S.M., Simonsen H.T., Boas U., Enemark H.L.…Williams A.R. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: A review Parasites. Vectors. 2018;11:475–488. doi: 10.1186/s13071-018-3012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos S.A., Fernandes Â., Vasileios A., Ntatsi G., Barros L., Ferreira I.C.F.R. Chemical composition and antioxidant activity of Cichorium spinosum L. leaves in relation to developmental stage. Food Chemistry. 2018;239:946–952. doi: 10.1016/j.foodchem.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Pharmacopoeia Commission of PRC, Ed. 1999. Pharmacopoeia of the People's Republic of China-Uygur medicine part. Urumqi, Xinjiang Sci-Tech and Public Health Press.
- Pharmacopoeia Commission of PRC, Ed. 2015 Pharmacopoeia of the People's Republic of China China Medical Science and Technology Publishing House Press. 310
- Pourfarjam Y., Rezagholizadeh L., Nowrouzi A., Meysamie A., Ghaseminejad S., Ziamajidi N., Norouzi D. Effect of Cichorium intybus L. seed extract on renal parameters in experimentally induced early and late diabetes type 2 in rats. Renal Failure. 2017;39(1):211–221. doi: 10.1080/0886022X.2016.1256317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y.X., Cao B.Y., Zheng H.Y., Chang Y.Q. Experimental study on anti-aging effect of chicory polysaccharides. Food Science. 2008;29(9):564–566. [Google Scholar]
- Rücker G., Noldenn U. Polyacetylenes from the underground parts of Cichorium intybus. Planta Medica. 1991;57(1):97–98. doi: 10.1055/s-2006-960038. [DOI] [PubMed] [Google Scholar]
- Rehman A., Ullah N., Ullah H., Ahmad I. Antibacterial and antifungal study of Cichorium intybus. Asian Pacific Journal of Tropical Disease. 2014;4(Suppl 2):S943–S945. [Google Scholar]
- Ren B.G., Chang Y.Q., Zheng H.Y., Cao B.Y., Qi Y.X. Study on Effect of Chicory Polysaccharides on Human. Food Science. 2008;29(11):579–581. [Google Scholar]
- Rezagholizadeh L., Pourfarjam Y., Nowrouzi A., Nakhjavani M., Meysamie A., Ziamajidi N., Nowrouzi P.S. Effect of Cichorium intybus L. on the expression of hepatic NF-κB and IKKβ and serum TNF-α in STZ− and STZ+ niacinamide-induced diabetes in rats. Diabetology & Metabolic Syndrome. 2016;8:11–20. doi: 10.1186/s13098-016-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroi M.B., Cumps C.J., Devogelaer J.P. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. The Journal of Nutrition. 2002;132(12):3599–3602. doi: 10.1093/jn/132.12.3599. [DOI] [PubMed] [Google Scholar]
- Rollinger J.M., Mocka P., Zidorn C., Ellmerer E.P., Langer T., Stuppner H. Application of the in combo screening approach for the discovery of non-alkaloid acetylcholinesterase inhibitors from Cichorium intybus. Current Drug Discovery Technologies. 2005;2(3):185–193. doi: 10.2174/1570163054866855. [DOI] [PubMed] [Google Scholar]
- Sa Y., Zhang B., Liu X.Q., Ye G.H. Effect of herba Cichorii extracts in reducing serum uric acid and blood lipid. Traditional Chinese Drug Research & Clinical Pharmacology. 2004;15(4):227–229. [Google Scholar]
- Saggu S., Sakeran M.I., Zidan N., Tousson E., Mohan A., Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food and Chemical Toxicology. 2014;72:138–146. doi: 10.1016/j.fct.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Sakurai N., Iizuka T., Nakayama S., Funayama H., Noguchi M., Nagai M. Vasorelaxant activity of caffeic acid derivatives from Cichorium intybus and Equisetum arvense. Journal of the Pharmaceutical Society of Japan. 2003;123(7):593–598. doi: 10.1248/yakushi.123.593. [DOI] [PubMed] [Google Scholar]
- Schumacher E., Vigh E., Molnár V., Kenyeres P., Fehér G., Késmárky G.…Garai J. Trombosis preventive potential of chicory coffee consumption: A clinical study. Phytotherapy Research. 2011;25(5):744–748. doi: 10.1002/ptr.3481. [DOI] [PubMed] [Google Scholar]
- Shaikh T., Rub R.A., Sasikumar S. Antimicrobial screening of Cichorium intybus seed extracts. Arabian Journal of Chemistry. 2016;9:S1569–S1573. [Google Scholar]
- Sharma P. Herbal remedies for treating rheumatic pains in Jammu and Kashmir. Indian Journal of Forestry. 1991;14:206–210. [Google Scholar]
- Sheng L. Sichuan University Master degree’s paper; 2006. Studies On Cichorum intybus in Xinjiang GAP BAS. [Google Scholar]
- Spyridon A.P., Angela F., Georgia N., Efi L., Lillian B., Isabel C.F.R.F. Nutritional profile and chemical composition of Cichorium spinosum ecotypes. LWT-Food Science Technology. 2016;73:95–101. [Google Scholar]
- Street R.A., Sidana J., Prinsloo G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evidence-based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/579319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S., Perwaiz S., Iqbal M., Athar M. Crude extracts of hepatoprotective plants, Solanum nigrum and Cichorium intybus inhibit free radical-mediated DNA damage. Journal of Ethnopharmacology. 1995;45(3):189–192. doi: 10.1016/0378-8741(94)01214-k. [DOI] [PubMed] [Google Scholar]
- Tong J., Yao X.C., Zeng H., Zhou G., Chen Y.X., Ma B.X., Wang Y.W. Hepatoprotective activity of flavonoids from Cichorium glandulosum seeds in vitro and in vivo carbon tetrachloride-induced hepatotoxicity. Journal of Ethnopharmacology. 2015;174:355–363. doi: 10.1016/j.jep.2015.08.045. [DOI] [PubMed] [Google Scholar]
- Tuzlaci E., Alparslan Is-bilen D.P., Bulut G. Turkish folk medicinal plants. VIII: Lalapasa (Edirne) Marmara Pharmaceutical Journal. 2010;14:47–52. [Google Scholar]
- Upur H., Armt N., Blažeković B., Talip A. Protective effect of Cichorium glandulosum root extract on carbon tetrachloride-induced and galactosamine-induced hepatotoxicity in mice. Food and Chemical Toxicology. 2009;47(8):2022–2030. doi: 10.1016/j.fct.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Venkatesan R., Subedi L., Yeo E.J., Kim S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamineinduced neurotoxicity through activation of the NRF2 pathway. Neurochemistry International. 2016;99:133–146. doi: 10.1016/j.neuint.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Vardanyan S. Pharmacology in ancient Armenia. Hist Philol J. 1979;2:179–194. [Google Scholar]
- Venkatesan R., Shim W.S., Yeo E.J., Kim S.Y. Lactucopicrin potentiates neuritogenesis and neurotrophic effects by regulating Ca2+/CaMKII/ATF1 signaling pathway. Journal of Ethnopharmacology. 2017;198:174–183. doi: 10.1016/j.jep.2016.12.035. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin Z.J., Zhang B., Wang X., Chu M.Z. Chicory (Cichorium intybus L.) inhibits renal reabsorption by regulating expression of urate transporters in fructose-induced hyperuricemia. Chinese Medical Sciences Journal. 2019 [Google Scholar]
- Wang F.X., Deng A.J., Li M., Wei J.F., Qin H.L., Wang A.P. (3S)-1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid from Cichorium endivia L induces apoptosis of human colorectal cancer HCT-8 cells. Molecules. 2013;18:418–429. doi: 10.3390/molecules18010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lin Z.J., Zhang B., Nie A.Z., Bian M. “Cichorium intybus L. promotes intestinal uric acid excretion by modulating ABCG2 in experimental hyperuricemia. Nutrition & Metabolism. 2017;14:38–48. doi: 10.1186/s12986-017-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.K., Fan Y., Ba H., Liao L.X., Aisa H.A. Analysis of essential oil from seeds of Cichorium gladulosum Boiss et Hout by GS-MS. Chinese Journal of Spectroscopy Laboratory. 2005;12(4):694–696. [Google Scholar]
- Wu H.K., Su Z., Yili A., Xiao Z.P., Ba H., Aisa H.A. Isolation of esculetin from Cichorium glandulosum by high-speed countercurrent chromatography. Chemistry of Natural Compounds. 2007;43(1):109. [Google Scholar]
- Wu H.K., Aisa H.A., Rakhmanberdyeva R.K., Zhauynbaeva K.S., Xin X.L. Polysaccharides from Cichorium glandulosum seeds. Chemistry of Natural Compounds. 2008;44(1):79–80. [Google Scholar]
- Wu, H. K. (2008). Studies on chemical constituents and bioactivities of Cichorium glandulosum Xinjiang Technical Institute of Physics and Chemistry Chinese Academy of Sciences Doctor degree’s paper.
- Wu H.K., Su Z., Xin X.L., Aisa H.A. Two new sesquiterpene lactones and triterpen glycoside from Cichorium glandulosum. Helvetica Chimica Acta. 2010;93(3):414–421. [Google Scholar]
- Wu W. Effect of superfine powder of Cichorium intybus L. root on intestinal microflora in immunosuppressive mice. Natural Product Research and Development. 2016;28:429–432. [Google Scholar]
- Xin X.L., Yang W.J., Yasen M., Zhao H.Q., Aisa H.A. The mechanism of hepatoprotective effect of sesquiterpene rich fraction from Cichorum glandulosum Boiss. et Huet on immune reaction-induced liver injury in mice. Journal of Ethnopharmacology. 2014;155 doi: 10.1016/j.jep.2014.06.014. 1068-1061-1075. [DOI] [PubMed] [Google Scholar]
- Xinjiang Institute of Biology, Pedology and Desert Research (1984). Flora of xinjiang. Urumqi, Xinjiang People’s publishing House, III: 172-173.
- Yao X., Zhu L., Chen Y., Tian J., Wang Y.W. In vivo and in vitro antioxidant activity and α-glucosidase, α-amylase inhibitory effects of flavonoids from Cichorium glandulosum seeds. Food Chemistry. 2013;139(1–4):59–66. doi: 10.1016/j.foodchem.2012.12.045. [DOI] [PubMed] [Google Scholar]
- Yang W.J., Luo Y.Q., Aisa H.A., Xin X.L., Totahon Z., Mao Y.…Zhang R.P. Hepatoprotective activities of a sesquiterpene-rich fraction from the aerial part of Cichorium glandulosum. Chin Med-UK. 2012;7:21–28. doi: 10.1186/1749-8546-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.L., Liu Y., Shi Y.Z., Wang X., Si L.J., Huang H. Acute toxicity research of Cichorium glandulosum aqueous extracts and it’s protective effect on acute liver injury mice. Chinese Journal of Modern Applied Pharmacy. 2017;34(7):957–963. [Google Scholar]
- Yesilada E, Sezik E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey IX. Folk medicine in north-west Anatolia. Journal of Ethnopharmacology. 1999;64:195–210. doi: 10.1016/s0378-8741(98)00133-0. [DOI] [PubMed] [Google Scholar]
- Yili A. Xinjiang medical university Master's degree paper; 2003. The study of effective part of traditional Uygur medicine–Cichorum glandulosum Boiss. et Hue. [Google Scholar]
- Zeb A., Haq A., Murkovic M. Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (chicory) leaves. European Food Research and Technology. 2019;245:365–374. [Google Scholar]
- Zhang B., Liu X.Q., Hu J.H., Jiao Y.F., Jia X.D., He T., Yang X. The influence of Cichorium capsule of blood glucose, serum uric acid and blood lipid on alloxan induced diabetic rabbit models. Traditional Chinese Drugs Research and Clinical Pharmacology. 1998;9(4):221–223. [Google Scholar]
- Zheng H.Y., Chang Y.Q., Cao B.Y., Qi Y.X. Study on therapeutic effect of chicory polysaccharides on fatty liver function of rat. Food Science. 2008;29(9):575–579. [Google Scholar]
- Zheng H.Y., Hu Y.H., Xu Y., Chang Y.Q., Li Z. Analysis of the ingredients of chicory nutrition. Journal of Jilin Agricultural University. 2003;25:462–465. [Google Scholar]
- Zheng H.Y., Hu Y.H., Wu R.J., Chang X.T., Ma J.R. Study on the extracting technique of fresh chicory. Journal of Jilin Agricultural University. 2004;26(1):116–118. [Google Scholar]
- Zheng H.M., Wang D.G., Gao Y.Y., Zhang B. The influence of n-hexane extraction of Cichorium on blood glucose and lipid. Chinese Journal of Information on Traditional Chinese Medicine. 2000;7(4):38–39. [Google Scholar]
- Ziamajidi N., Khaghani S., Hassanzadeh G., Vardasbi S., Ahmadian S., Nowrouzi A.…Abdirad A. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPAR-alpha and SREBP-1. Food and Chemical Toxicology. 2013;58:198–209. doi: 10.1016/j.fct.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Zou L. Zhejiang University; 2013. Studies on chemical constituents of Cichorium intybus L. and Kleinhovia hospita L [D] [Google Scholar]