Abstract
Objective
To evaluate antihypertensive and antioxidant activities of Allium hookeri root (AHR) fermented with Lactobacillus plantarum, Leuconostoc mesenteroides, and Weissella cibaria.
Methods
The novel fermented AHR products using L. plantarum, L. mesenteroides, and W. cibaria were developed and ACE inhibitory activity, DPPH radical scavenging activity, hydroxyl radical scavenging activity, superoxide anion radical scavenging activity, total phenolic content, and total thiosulfinate content were determined. The antihypertensive and antioxidant effects of fermented AHR were further investigated in spontaneously hypertensive rats (SHRs).
Results
Administration of fermented AHR to SHRs had an attenuating effect on both diastolic and systolic blood pressure. The SHRs treated with fermented AHR showed lower plasma ACE activity and higher plasma NO levels. Furthermore, fermented AHR administration led to parallel improvements in plasma oxidative stress biomarkers in SHRs.
Conclusion
Our results highlight the potential usefulness of fermented AHR for the prevention of hypertension.
Keywords: antihypertensive activity, antioxidant activity, hypertension, microbiological fermentation, roots of Allium hookeri
1. Introduction
Hypertension is a common chronic disease in adults characterized by an increased systemic arterial blood pressure, and it may occur as the combined consequences of the demographic, genetic, and environmental stimuli (Montezano & Touyz, 2012). It is estimated that the number of adults suffering from hypertension will be up to 1.56 billion by 2025 (Kearney et al., 2005, Mills et al., 2016). If the hypertension condition is not properly controlled, it may induce cardiovascular disease, impairing quality of life. The increased prevalence of hypertension would confer an enormous public healthy and economic burden pertaining morbidity and mortality. Despite tremendous social, economic, and scientific efforts are done to understand and curb this disease over the past years, limited success is obtained. About 90% of current hypertension individuals are classified as primary hypertension, and the underlying mechanism remain elusive (Kearney, et al., 2005).
The increased reactive oxygen species or oxidative stress is well acknowledged to be an important contributing factor for the pathogenesis of hypertension and some other related diseases (Touyz & Briones, 2011). Hypertension itself is able to induce oxidative stress in the vascular system (Touyz & Briones, 2011), and thus can create a vicious cycle of oxidative stress and further hypertension. The search of safe and efficacious approaches to combat continuously expanding hypertension and oxidative stress from natural products emerges as a crucial strategy for hypertension management because they contain diverse biologically active compounds. The Allium vegetable genus, a promising herbal resource, have been exploited and used traditionally as foods and flavoring ingredients and for various medicinal purposes in some parts of the world such as Korea, China, and Japan (Nicastro, Ross, & Milner, 2015). Several experimental and clinical evidence demonstrates the beneficial effects of the Allium vegetables on hypertension (Bahadoran, Mirmiran, Momenan, & Azizi, 2017).
Allium hookeri, a member of Allium genus in Alliaceae family, like garlic, onion, scallion, leek and chive, is mainly cultivated and consumed as foods and flavoring ingredients for thousands of years in South Asia and Southeast Asia countries. A. hookeri is characterized by a high content of carbohydrates, proteins, lipids, organic acids, minerals as well as other nutrients (Park & Yoon, 2014). It is reported to have several pharmacological properties, including antiobesity, antidiabetic, anticancer, and antiasthmatic effects (Kim et al., 2019, Yang et al., 2016, Yang et al., 2017). For instance, supplementation of mice fed high-fat diet with A. hookeri root (AHR) extracts reduces body weight gain and adipose tissue weight, decreases adipocyte cell size, and modulates the expression of genes related to lipolysis, lipogenesis, adipogenesis in the white adipose tissues (Kim, et al., 2019). Treatment of 3 T3-L1 adipocytes with AHR water extracts increase glucose transporter 4 expression, inhibit adipogenesis, and enhance glucose uptake into adipocytes (Yang, et al., 2016). AHR extracts significantly ameliorate asthmatic changes, as demonstrated by reduced white blood cell count, eosinophil count as well as IgE levels in an ovalbumin‑induced asthma mouse model (Bok, et al., 2019). AHR extracts inhibit the proliferation of two human cancer cell lines, MDA-MB-231 and HT-1080, in a dose-dependent manner (Myint, et al., 2020).
Due to their health-promoting properties, lactic acid bacteria are widely used in the production of fermented foods. Here, our aim is to develop novel fermented AHR products using Lactobacillus plantarum, Leuconostoc mesenteroides, and Weissella cibaria. Angiotensin converting enzyme (ACE), a pivotal component of the renin-angiotensin system and plays a critical role in circulatory homeostasis by causing blood vessels to constrict, the in vitro antihypertensive activities of fermented AHR were investigated by the inhibitory capacity against ACE. The inhibitory activity, antioxidant potential, total phenolic and thiosulfinate contents of fermented AHR products were evaluated. Then, using spontaneously hypertensive rats (SHRs) as animal model of essential hypertension, we further determine the potential of fermented AHR products to attenuate hypertension and oxidative stress.
2. Materials and methods
2.1. Chemicals and strains
Cysteine, folin-Ciocalteu’s phenol reagent, 2,2′-dithiobis (5-nitropyridine), 2,2-diphenyl-1-picryl-hydrazyl (DPPH), ferric sulfate, gallic acid, H2O2, hippuryl-histidyl-leucine (Hip-His-Leu), lung acetone powder from rabbit, N-1-napthylethylenediamine dihydrochloride, NADH, potassium ferricynide, nitrotetrazolium blue chloride, phenazine methosulfate, potassium ferricynide, sulfanilamide, superoxide dismutase (SOD) assay kit (19160-1KT-F), thiobarbituric acid, trichloroacetic acid, vanadium (III) chloride, and zinc sulfate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293, Lactobacillus plantarum subsp. plantarum ATCC 14917, and Weissella cibaria KCTC 3746 were obtained from the Korean collection for Type Cultures (KCTC, Daejeon, Korea). All chemicals and reagents used in the present study were of analytical grade.
2.2. Preparation of fermented AHR
Allium hookeri roots (AHR) were purchased from the local market in Jeonnam, South Korea. The roots were carefully washed with fresh water to remove contaminants and then shredded into slice (1–2 cm) and autoclaved for 10 min (Autoclave, JSAC-80, Js Research, Gongju, Korea). Steamed AHR was mixed with sterilized water (1:1, mass to volume ratio), oligosaccharides (10:1, mass ratio), and sucrose (10:1, mass ratio) in a fermentation bucket. The final fermentation medium (1 kg) was comprised of 45 g of oligosaccharides, 45 g of sucrose, 455 g of water, and 455 g of AHR. Starter solutions of L. plantarum, L. mesenteroides, and W. cibaria were inoculated to the fermentation mixture separately (6–7 log CFU/mL). Afterwards, the fermentation buckets were tightly covered and placed at 35 °C. After 3 d, the fermentation mixture was centrifuged at 250 g for 10 min to collect supernatants, which were stored at − 20 °C for the further analyses. In this paper that nonfermented AHR is used as control.
2.3. Determination of ACE inhibitory activity
The in vitro antihypertensive activities of fermented AHR were investigated by the inhibitory capacity against ACE according to the method developed by Cushman & Cheung (Cushman & Cheung, 1971). Briefly, ACE solution was extracted from the lung acetone powder of rabbit using 0.1 mol/L borate buffer (containing 0.3 mol/L NaCl, pH 8.3) at 4 °C for 24 h. Then 50 μL of diluted sample (2 folds) was pre-incubated with 100 μL of 5 mmol/L Hip-His-Leu and 100 μL of borate buffer at 37 °C. After 10 min, 100 μL of ACE solution was added and the final mixture was incubated at 37 °C for 1 h. To stop the reaction, 200 μL of 1 mol/L HCl was added to the mixture. The formed hippuric acid was subsequently extracted by 2 mL of ethyl acetate, followed by centrifuge for 5 min at 380 g. An aliquot of supernatant was collected and heated at 100 °C to evaporate ethyl acetate. Then, hippuric acid was dissolved in distilled water (3 mL) and the absorbance of the solution was recorded at 228 nm using a spectrophotometer (Ultrospec 5300, Biochrom Ltd., CB, UK). The inhibitory activities of examined samples were calculated according to formula (1):
Where As represents the absorbance of mixture in the presence of fermented AHR, Ab represents the absorbance of blank that ACE was inactivated before the addition of fermented AHR, and Ac represents the absorbance of control (without fermented AHR).
2.4. Determination of DPPH radical scavenging activity
The DPPH radical scavenging ability of samples was investigated according to the description of Yang et al (Yang, et al., 2009). Briefly, 2 mL of 0.1 mmol/L DPPH dissolved in ethanol was mixed with 2 mL of sample. The mixture was then vortexed vigorously and left to incubate in the dark for 0.5 h. The absorbance at 517 nm was determined using a spectrophotometer (Ultrospec 5300, Biochrom Ltd.) and the DPPH radical scavenging capacity of sample was calculated from formula (2):
Where As represents the absorbance of sample and Ac represents the absorbance of control without sample.
2.5. Determination of hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of fermented AHR was determined as described by Smirnoff & Cumbes (Smirnoff & Cumbes, 1989). Briefly, the reaction mixture containing 0.7 mL of 6 mmol/L H2O2, 1 mL of diluted (5 folds) sample, 1 mL of 1.5 mmol/L ferric sulfate, and 0.3 mL of 20 mmol/L sodium salicylate was incubated for 1 h at 37 °C. The absorbance at 562 nm was recorded by a spectrophotometer (Ultrospec 5300, Biochrom Ltd.) and the hydroxyl radical scavenging capacity was calculated according to equation (2).
2.6. Determination of superoxide anion radical scavenging activity
The superoxide radical scavenging activity of fermented AHR was determined according to the method described by Nishikimi et al with some modification (Nishikimi, Appaji, & Yagi, 1972). Briefly, 1.5 mL of diluted (20 folds) sample, 0.5 mL of 468 μmol/L NADH, 0.5 mL of 300 μmol/L nitrotetrazolium blue chloride, and 0.5 mL of 60 μmol/L phenazine methosulfate were added to a test tube orderly. The mixture was then left to incubate 5 min. The absorbance at 560 nm was measured by a spectrophotometer (Ultrospec 5300, Biochrom Ltd.) and the superoxide anion radical scavenging capacity was calculated based on equation (2).
2.7. Determination of total phenolic content
The total phenolic content of fermented AHR was measured based on method described by Singleton and Rossi (Singleton & Rossi, 1965). Briefly, 100 μL of sample was diluted with distilled water (2 mL) and mixed with Folin-Ciocalteau phenol reagent (0.2 mL). After 5 min of reaction, 1 mL of 15% sodium carbonate was added and the mixture was left to incubate in the dark for 120 min. The absorbance at 765 nm was measured using a spectrophotometer (Ultrospec 5300, Biochrom Ltd.). Total phenolic content was calculated and expressed as mg gallic acid equivalents (GAE)/100 mL of samples (y = 3.6286x + 0.0601, R2 = 0.9969), where gallic acid was used as a standard compound.
2.8. Determination of total thiosulfinate content
The total thiosulfinate content of fermented AHR was determined based on the previous method with slight modification (Han, Lawson, Han, & Han, 1995). Briefly, sample (0.5 mL) was pre-incubated with cysteine (0.5 mL, 2 mmol/L) and HEPES buffer (4 mL, 50 mmol/L, pH 7.5) at 27 °C for 15 min. The pre-incubated solution (1 mL) was then mixed with 2,2′-dithiobis(5-nitropyridine) buffer (1 mL, 0.4 mmol/L). The mixture was incubated at 27 °C for 10 min and the absorbance at 412 nm was measured using a spectrophotometer (Ultrospec 5300, Biochrom Ltd.). The total thiosulfinate content was calculated according to the following equation:
In this equation, A0 represents the absorbance of cysteine where sample was replaced by distilled water, A1 represents the absorbance in the presence of sample, and B represents the dilution factor of cysteine.
2.9. Animal studies
Four weeks old spontaneously hypertensive rats (SHRs) were purchased from Japan SLC Inc. (Shizuoka, Japan). They were maintained in a humidity- (45 ± 5%) and temperature-controlled (23 ± 1 °C) room with a 12-hour light/dark cycle. All the rats were allowed free access to food and water. After 2 weeks of acclimatization, the rats were randomly divided into four groups (n = 8) to assess the effect of chronic fermented AHR exposure (8 weeks) on blood pressure: SHRs administrated with water (control group), AHR fermented with L. plantarum (ALP group), AHR fermented with L. mesenteroides (ALM group), or AHR fermented with W. cibaria (AWC group). The fermented AHR was added to the drinking water of SHRs with 30% final concentration (the average dose volume for control group, ALP group, ALM group, and AWC group were 9.50 mL, 9.93 mL, 9.87 mL, and 9.83 mL, respectively). Weekly body weight and daily water intakes were recorded. At the end of experiment, the animals were sacrificed by exsanguination under diethyl ether anesthesia. The blood was collected in tubes (BD Vacutainer, NJ, USA) with lithium heparin as anticoagulant. To obtain the plasma, the blood was centrifuged at 860 g for 15 min at 4 °C. Collected plasma was kept at − 80 °C for further study. The animal experimental procedures used in the present study were approved by the Ethics Committee of Mokpo National University (IACUC, 2015–034).
2.10. Measurement of blood pressure and heart rates
Animals were heated at 37 °C for 10–15 min to raise their body temperature, after which, diastolic blood pressures (DBP), systolic blood pressure (SBP) as well as heart rates were recorded by tail cuff method using a sphygmomanometer (Panlab, Spain). Blood pressure and heart rates of SHRs were measured biweekly performed in the morning, between 8:00 a.m. and 11:00 a.m., approximately 30 min after drugs administration.
2.11. Determination of plasma ACE activity
The plasma ACE activity was determined by previously reported method (Cushman & Cheung, 1971). Briefly, plasma (50 μL) was pre-incubated with borate buffer (100 μL, 0.1 M, pH 8.3) at 37 °C for 10 min. After which, Hip-His-Leu (100 μL, 5 mM) were added to the mixture. The enzyme reaction was kept at 37 °C for 1 h. To stop the reaction, HCl (200 μL of 1 mol/L) was added to the reaction system. Ethyl acetate (2 mL) was used to extract the formed hippuric acid. After being centrifuged at 380 g for 5 min, the supernatant (1.5 mL) was collected in a glass tube and evaporated at 100 °C using a heating block. Afterward, the ethyl acetate was removed and hippuric acid was dissolved in distilled water (3 mL). The absorbance at 228 nm was determined.
2.12. Determination of plasma nitric oxide (NO) level
Plasma NO concentration was determined based on the method previously reported (Moshage, Kok, Huizenga, & Jansen, 1995). Appropriate diluted plasma (100 μL) was placed in an Eppendorf tube, to which zinc sulfate solution (50 μL, 75 mM) was added. The mixture was centrifuged at 5000 g for 10 min to collect supernatants. Thereafter, supernatant (100 μL) was placed in 96-well plate, followed by the addition of 100 μL of vanadium (III) chloride and 100 μL of Griess reagent (2% sulfanilamide in 3 mol/L HCl and 0.1% N-1-napthylethylenediamine dihydrochloride in distilled water). The plate was incubated at 37 °C for 20 min and the absorbance at 540 nm was measured using a microplate reader (μQuant MQX200, BioTek, VT, USA).
2.13. Determination of plasma SOD activity
Plasma SOD activity was determined by a commercial SOD assay kit (19160-1KT-F, Sigma). Plasma (20 μL) was well mixed with assay solution (200 μL) and enzyme (20 μL). After twenty minutes later, the absorbance at 450 nm was measured using a microplate reader (μQuant MQX200, BioTek).
2.14. Measurement of plasma malondialdehyde (MDA)
MDA level in plasma was measured according to the method described by Tong et al. (Tong, Ko, Kim, Ham, & Kang, 2015). Briefly, plasma (100 μL) was mixed with 20% trichloroacetic acid (500 μL) and 0.6 % thiobarbituric acid (1 mL). The mixture was then heated for 20 min in boiling water. Thereafter, n-butanol (800 μL) was added and the mixture was centrifuged at 6000 g for 10 min. The supernatant (100 μL) was transferred into a new 96-well plate and the absorbance was recorded at 532 nm.
2.15. Statistical analysis
All data are expressed as mean ± SD (standard deviation). Statistical analysis was performed using the SPSS 23.0 statistical program (SPSS Inc., Chicago, IL, USA). Differences between groups were analyzed using One-way ANOVA, followed by post-hoc Duncan’s multiple range tests.
3. Results and discussion
3.1. ACE inhibitory activity of fermented AHR
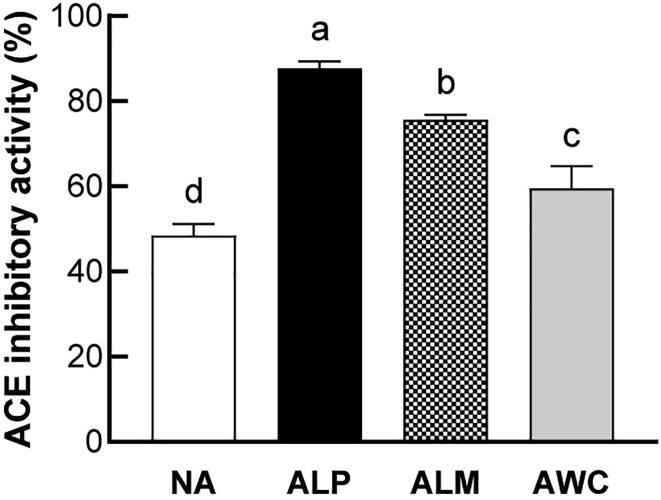
ACE, a well-known zinc-metallopeptidase, plays important roles in blood pressure control and is considered as a therapeutic target for hypertensive diseases. It acts mainly through converting inactive angiotensin-I (Ang-I) to angiotensin-II (Ang-II), a powerful vasoconstrictor and aldosterone-stimulating peptide. Moreover, ACE degrades vasodilator such as bradykinin and inactives enkephalin, neurotensin as well as tachykinins including substance P (Lopez-Sublet, et al., 2018). ACE inhibitor is currently recommended as first-choice therapy for the management of patients with cardiovascular disease by most guidelines. In the present study, all fermented AHR showed superior ACE inhibitory activities compared with nonfermented AHR. ALP exhibited the strongest inhibitory activity against ACE, followed by ALM and AWC (Fig. 1). Fermentation is demonstrated to be an effective way to enhance the ACE inhibitory activity of extract from plant-based foods, such as vegetables, fruits, and cereals. For example, Fujita et al. reported that fermentation with L. plantarum enhances the ACE enzyme inhibitory activity of camu–camu and soymilk combination (Fujita, Sarkar, Genovese, & Shetty, 2017). Fermentation of milk with L. mesenteroides strain produces intense ACE inhibitory activity (Pihlanto, Virtanen, & Korhonen, 2010).
Fig. 1.
ACE inhibition activity of fermented AHR. NA, nonfermented AHR; ALP, AHR fermented with L. plantarum; ALM, AHR fermented with L. mesenteroides; AWC, AHR fermented with W. cibaria. Different letters (a, b, c or d) above the bars mean statistically different at P < 0.05.
3.2. In vitro antioxidant activities of fermented AHR
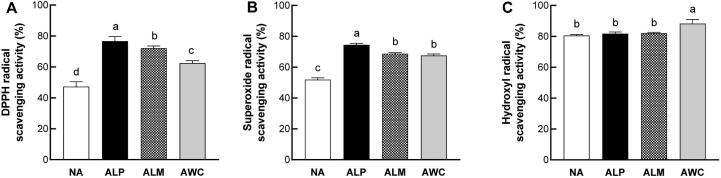
It is well established that fermentation can improve the antioxidant capacity of the plant materials and thus is believed to be a useful method to develop functional foods (Hur, Lee, Kim, Choi, & Kim, 2014). For example, L. plantarum fermentations significantly increases the antioxidant capacity of water-soluble lentil extracts (Torino, et al., 2013). Here, the in vitro antioxidant activities of fermented AHR were determined using several well-known antioxidant models. We found that all fermented AHR showed stronger DPPH and superoxide radical scavenging capacities compared to nonfermented AHR. And ALP displayed the most excellent DPPH (Fig. 2A) and superoxide radical scavenging capacity (Fig. 2B), followed by ALM and AWC. The hydroxyl radical scavenging capacities of ALP and ALM were similar to that of nonfermented AHR. AWC exhibited stronger scavenging capacity against hydroxyl radical, as compared with nonfermented AHR (Fig. 2C). These results support the notion that the antioxidative activities of fermented plant-based foods can be influenced by the microbial species used during the fermentation (Hur, et al., 2014).
Fig. 2.
In vitro antioxidant activities of fermented AHR. (A) DPPH radicals scavenging activity of fermented AHR. (B) Superoxide radicals scavenging activity of fermented AHR. (C) Hydroxyl radicals scavenging activity of fermented AHR. NA, nonfermented AHR; ALP, AHR fermented with L. plantarum; ALM, AHR fermented with L. mesenteroides; AWC, AHR fermented with W. cibaria. Different letters (a, b, c or d) above the bars mean statistically different at P < 0.05.
3.3. Total phenolic and total thiosulfinate contents of fermented AHR
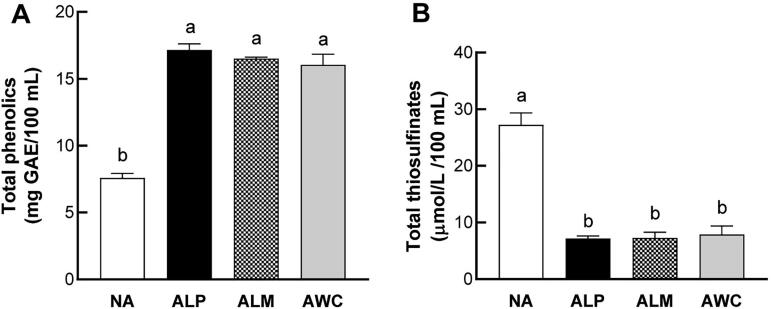
The ability of fermentation to enhance ACE inhibitory and antioxidant activities is mainly attribute to the microbial hydrolysis reaction during fermentation, which lead to the structural breakdown of plant cell walls and subsequent synthesis or liberation of various bioactive compounds including phenolic compounds (Hur, et al., 2014). For example, fermentation of cowpea flours by L. plantarum results in significant improvement in phenolic compound concentration (Duenas, Fernandez, Hernandez, Estrella, & Munoz, 2005). We next determined the total phenolic content of fermented AHR and found that, in general, fermented AHR had higher level of total phenolic contents compared with nonfermented AHR. No significant difference in total phenolic content values among the three fermented AHR samples was found (Fig. 3A).
Fig. 3.
Total phenolic (A) and total thiosulfinate (B) contents of fermented AHR. NA, nonfermented AHR; ALP, AHR fermented with L. plantarum; ALM, AHR fermented with L. mesenteroides; AWC, AHR fermented with W. cibaria. Different letters (a, b, c or d) above the bars mean statistically different at P < 0.05.
Thiosulfinate compounds are known to be responsible for the typical pungent flavor of the Allium genus and are formed under enzyme reaction. In the present study, we found that fermentation with L. plantarum, L. mesenteroides, and W. cibaria led to a significant decrease in total thiosulfinate contents of AHR (Fig. 3B). This may be due to the volatile nature of thiosulfinate compounds and the utilization by the Lactobacilli involved in the fermentation. In agreement with our results, Yang et al. found that in Korean leeks fermented with W. confusa and L. plantarum, the content of allicin (diallyl thiosulfinate), one of the main thiosulfinate compounds responsible for the special strong odor of leeks, decreased during fermentation (J. Yang, et al., 2014). Our result also indicated that fermentation with L. plantarum, L. mesenteroides, and W. cibaria can improve the flavor of AHR by reducing the strong pungent odor related to thiosulfinate compounds.
3.4. Effects of fermented AHR on blood pressure and heart rates of SHRs
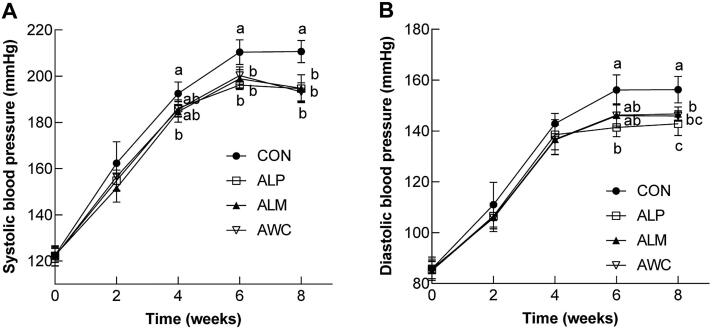
Based on the positive results obtained from a series of in vitro experiments, we further evaluate the ability of fermented AHR to attenuate hypertension and oxidative stress in spontaneously hypertensive rats, an animal model of essential hypertension that have been widely used for the past 50 years (Wexler, Iams, & Judd, 1977). As expected, the development of hypertension in SHRs is age-dependent: at 4th week (the real age of SHR was 10 weeks old), SBP of control rats has exceeded 180 mmHg, and DBP has risen to around 140 mmHg; at the end of experiments, the SBP and DBP of control rats were 210 and 156 mmHg, respectively. Concurrent administration of ALM reduced SBP of mice in a time-dependent manner and this beneficial effect of ALM was evident from 4th week. Similarly, the SBP in ALP- and AWC-treated rats was significantly lower than that of control rats at 6th and 8th week (Fig. 4A). Parallel improvements in DBP were observed after fermented AHR treatment. Significantly lower DBP were observed in ALP-treated rats from 6th week (Fig. 4B). The DBP in ALM- and AWC-treated rats was significantly lower than that of control rats after 8 weeks of treatment. Treatments of ALP, ALM, or AWC showed no significant effects on the heart rates of SHRs (data not shown).
Fig. 4.
Effects of fermented AHR on systolic blood pressure (A) and diastolic blood pressure (B) (mean ± SD, n = 8). SHRs were treated with water (CON), AHR fermented with L. plantarum (ALP), AHR fermented with L. mesenteroides (ALM), or AHR fermented with W. cibaria (AWC). The fermented AHR was added to the drinking water of SHRs with 30% final concentration. Different letters (a, b, c or d) mean statistically different at P < 0.05.
It has been estimated that a 5 mmHg reduction of SBP in the population can lead to significant decreases in mortality caused by coronary heart disease (9%) or stroke (14%) (Whelton, et al., 2002). Recently, development of functional foods possessing hypotensive effects as a possible option to reduce the increased risk for cardiovascular disease has attracted much attention. Here, we demonstrated that fermented AHR significantly reduced blood pressure of SHRs, highlighting the potential usefulness of fermented AHR for the management of cardiovascular disease.
3.5. Effects of fermented AHR on body and organ weights of SHRs
Water intakes were comparable among four groups. After 8 weeks of experiment, no significant change in body weight was found in SHRs for all groups (data not shown). Meanwhile, the weights of abdominal and brown adipose tissues, heart, lung, liver, and kidney showed no significant differences among the groups (data not shown).
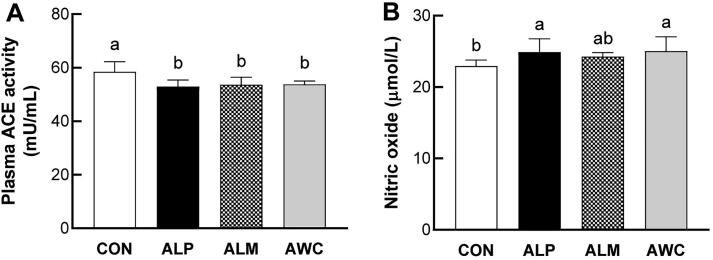
3.6. Effects of fermented AHR on plasma ACE activity
In the present study, the SHR rats treated with ALP, ALM, or AWC had lower plasma ACE activity compared with control rats, and no significant difference in plasma ACE activity was found among the ALP, ALM, or AWC groups (Fig. 5A). Similar to our results, garlic is reported to exert a significant effect in reducing blood pressure in 2K1C hypertensive rats and its antihypertensive effects of garlic is thought to be partly due to inhibition of serum ACE activity (Sharifi, Darabi, & Akbarloo, 2003).
Fig. 5.
Effects of fermented AHR on plasma ACE activity (A) and NO concentration (B) (mean ± SD, n = 8). SHRs were treated with water (CON), AHR fermented with L. plantarum (ALP), AHR fermented with L. mesenteroides (ALM), or AHR fermented with W. cibaria (AWC). The fermented AHR was added to the drinking water of SHRs with 30% final concentration. Different letters above the bars mean statistically different at P < 0.05.
3.7. Effects of fermented AHR on plasma NO concentration
NO is one of the most potent vasodilators synthesized in vascular endothelium and the loss of NO bioavailability is a hallmark of hypertension. It is dominantly synthesized from amino acid L-arginine by endothelial NO synthase (NOS). When diffused into vascular smooth muscle cells, NO triggers the generation of cyclic guanosine monophosphate, which then induces the membrane hyperpolarization of vascular smooth muscle cells and increase in vascular contraction or leads to vasodilatation (Furchgott & Vanhoutte, 1989). Therefore, the increased NO production in plasma may be benefit for NO-dependent vasodilation and hypertension treatment. For example, it was reported that the blood-pressure lowering effect of fresh garlic in two-kidney, one-clip model of hypertension is mediated, at least partially, via increasing NO generation (Al-Qattan, et al., 2006). In the present study, AWC- and ALP-treated SHR rats exhibited higher plasma NO concentration than that of control rats. There was a tendency for higher plasma NO level in ALM-treated rats compared to control rats, although no statistical difference was observed (Fig. 5B).
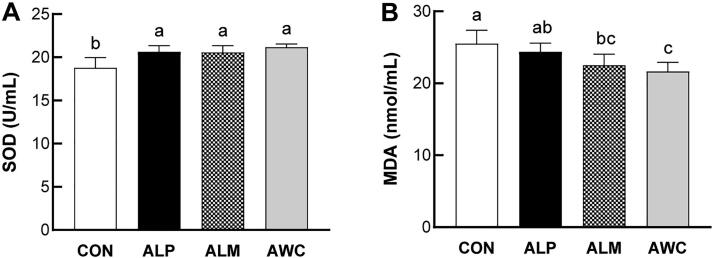
3.8. Effects of fermented AHR on plasma SOD and MDA levels
Over production of reactive oxygen species causes the unbalance between reactive oxygen species and internal antioxidant systems, resulting in oxidative stress and lipid oxidation. Increased superoxide levels and oxidative stress have been reported in SHRs and other hypertensive animal models (Shouk, Abdou, Shetty, Sarkar, & Eid, 2014). Treatment of SHRs with antioxidant-fortified diet is demonstrated to lower the blood pressure levels of SHRs (Zhan, Sindhu, & Vaziri, 2004). Along these lines, many antihypertensive drugs currently adopted in clinical practice, including ACE inhibitors, angiotensin (AT)1 receptor blockers, besides lowering blood pressure, show anti-oxidant effects, and improve oxidative markers (Sorriento, De Luca, Trimarco, & Iaccarino, 2018).
To elucidate the effect of fermented AHR administration on in vivo antioxidant capacity of SHRs, we sought to determine the plasma levels of oxidative stress biomarkers such as SOD and MDA. SOD plays an essential role in removing of superoxide anion radicals. It protects the cell matrix and maintains the balance between antioxidation and oxidation by catalyzing the cellular oxidative metabolism of superoxide anion radicals into hydrogen peroxide (Tiwari, Jena, & Mishra, 2018). Treatment of SOD mimetic tempol to salt-loaded stroke-prone spontaneously hypertensive rats has been reported to significantly attenuate the progression of hypertension, decrease the superoxide anion generation, and improve the plasma antioxidant status (Park, Touyz, Chen, & Schiffrin, 2002). MDA level is widely used to evaluate oxidative stress as a marker of lipid oxidation and oxidative stress and increased MDA level has been reported in hypertensive patients (Redón et al., 2003). In the present study, a significant increase in plasma SOD activity was found in all fermented AHR-treated rats compared with control rats (Fig. 6A). Administration of ALM and AWC significantly decreased the plasma MDA levels. Slight but non-significant decrease was found in the plasma MDA level of ALP-treated rats compared to control rats (Fig. 6B).
Fig. 6.
Effects of fermented AHR on plasma SOD (A) and MDA levels (B) (mean ± SD, n = 8). SHRs were treated with water (CON), AHR fermented with L. plantarum (ALP), AHR fermented with L. mesenteroides (ALM), or AHR fermented with W. cibaria (AWC). The fermented AHR was added to the drinking water of SHRs with 30% final concentration.. Different letters above the bars mean statistically different at P < 0.05.
4. Conclusion
We found that fermented AHR prepared with L. plantarum, L. mesenteroides, or W. cibaria exhibits strong inhibitory activity against ACE and concurrent antioxidant activity in vitro. Administration of fermented AHR to SHRs significantly attenuate the elevation of both systolic and diastolic blood pressure, decease plasma ACE activities, and increase plasma NO production. Moreover, fermented AHR significantly improve the in vivo antioxidant status of SHRs, as evidenced by the increased SOD level and decreased MDA level. Although the effects of fermented AHR in humans have yet to be explored, the present study highlights the possible importance of fermented AHR for the management of hypertension and its related complications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Leaders in Industry-university Cooperation, Mokpo National University, Mokpo, Korea and 2115 Talent Development Program of China Agricultural University.
Contributor Information
Cheng-Mei Zhang, Email: zcm152@126.com.
Seong-Gook Kang, Email: sgkang@mokpo.ac.kr.
References
- Al-Qattan, K. K., Thomson, M., Al-Mutawa'a, S., Al-Hajeri, D., Drobiova, H., & Ali, M. (2006). Nitric oxide mediates the blood-pressure lowering effect of garlic in the rat two-kidney, one-clip model of hypertension. The Journal of Nutrition, 136, 774S-776S. [DOI] [PubMed]
- Bahadoran Z., Mirmiran P., Momenan A.A., Azizi F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: A longitudinal follow-up study. Journal of Hypertension. 2017;35:1909–1916. doi: 10.1097/HJH.0000000000001356. [DOI] [PubMed] [Google Scholar]
- Bok S.H., Seo J.H., Bae C.S., Kang B., Cho S.S., Park D.H. Allium hookeri root extract regulates asthmatic changes through immunological modulation of Th1/Th2related factors in an ovalbumininduced asthma mouse model. Molecular Medicine Reports. 2019;20:3215–3223. doi: 10.3892/mmr.2019.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman D.W., Cheung H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochemical Pharmacology. 1971;20(7):1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Duenas M., Fernandez D., Hernandez T., Estrella I., Munoz R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. Journal of the Science of Food and Agriculture. 2005;85:297–304. [Google Scholar]
- Fujita A., Sarkar D., Genovese M.I., Shetty K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochemistry. 2017;59:133–140. [Google Scholar]
- Furchgott R.F., Vanhoutte P.M. Endothelium-derived relaxing and contracting factors. The FASEB Journal. 1989;3(9):2007–2018. [PubMed] [Google Scholar]
- Han J., Lawson L., Han G., Han P. Spectrophotometric method for quantitative-determination of allicin and total garlic thiosulfinates. Analytical Biochemistry. 1995;225(1):157–160. doi: 10.1006/abio.1995.1124. [DOI] [PubMed] [Google Scholar]
- Hur S.J., Lee S.Y., Kim Y.C., Choi I., Kim G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chemistry. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee M.J., Jang J.Y., Lee S.H. Allium hookeri root extract inhibits adipogenesis by promoting lipolysis in high fat diet-induced obese mice. Nutrients. 2019;11:2262. doi: 10.3390/nu11102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sublet M., Caratti di Lanzacco L., Danser A.H.J., Lambert M., Elourimi G., Persu A. Focus on increased serum angiotensin-converting enzyme level: From granulomatous diseases to genetic mutations. Clinical Biochemistry. 2018;59:1–8. doi: 10.1016/j.clinbiochem.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Mills K.T., Bundy J.D., Kelly T.N., Reed J.E., Kearney P.M., Reynolds K.…He J. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezano A.C., Touyz R.M. Molecular mechanisms of hypertension–reactive oxygen species and antioxidants: A basic science update for the clinician. Canadian Journal of Cardiology. 2012;28(3):288–295. doi: 10.1016/j.cjca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Moshage H., Kok B., Huizenga J.R., Jansen P.L. Nitrite and nitrate determinations in plasma: A critical evaluation. Clinical Chemistry. 1995;41:892–896. [PubMed] [Google Scholar]
- Myint A.A., Aregay M.G., Kang M., Kim B.S., Lee Y.W., Kim J. Comprehensive study on the formation mechanism of highly bioactive compounds from Allium hookeri root using subcritical water and their antioxidant and anticancer effects. Journal of Supercritical Fluids. 2020;157:104709. [Google Scholar]
- Nicastro H.L., Ross S.A., Milner J.A. Garlic and onions: Their cancer prevention properties. Cancer Prevention Research. 2015;8(3):181–189. doi: 10.1158/1940-6207.CAPR-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi M., Appaji Rao N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Park J.-Y., Yoon K.-Y. Comparison of the nutrient composition and quality of the root of Allium hookeri grown in Korea and Myanmar. Korean Journal of Food Science and Technology. 2014;46(5):544–548. [Google Scholar]
- Park J.B., Touyz R.M., Chen X., Schiffrin E.L. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. American Journal of Hypertension. 2002;15(1):78–84. doi: 10.1016/s0895-7061(01)02233-6. [DOI] [PubMed] [Google Scholar]
- Pihlanto A., Virtanen T., Korhonen H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. International Dairy Journal. 2010;20(1):3–10. [Google Scholar]
- Redón J., Oliva M.R., Tormos C., Giner V., Chaves J., Iradi A., Sáez G.T. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41(5):1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- Sharifi A.M., Darabi R., Akbarloo N. Investigation of antihypertensive mechanism of garlic in 2K1C hypertensive rat. Journal of Ethnopharmacology. 2003;86(2-3):219–224. doi: 10.1016/s0378-8741(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Shouk R., Abdou A., Shetty K., Sarkar D., Eid A.H. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutrition Research. 2014;34(2):106–115. doi: 10.1016/j.nutres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal Ecology Viticulture. 1965:144–158. [Google Scholar]
- Smirnoff N., Cumbes Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28(4):1057–1060. [Google Scholar]
- Sorriento D., De Luca N., Trimarco B., Iaccarino G. The antioxidant therapy: New insights in the treatment of hypertension. Frontiers in Physiology. 2018;9:258. doi: 10.3389/fphys.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M.K., Jena N.R., Mishra P.C. Mechanisms of scavenging superoxide, hydroxyl, nitrogen dioxide and methoxy radicals by allicin: Catalytic role of superoxide dismutase in scavenging superoxide radical. Journal of Chemical Sciences. 2018;130:150. [Google Scholar]
- Tong T., Ko D.O., Kim B.S., Ham K.S., Kang S.G. Beneficial effect of seaweed on high-fat diet-induced oxidative stress and insulin resistance in rats. Food Science and Biotechnology. 2015;24(6):2185–2191. [Google Scholar]
- Torino M.I., Limón R.I., Martínez-Villaluenga C., Mäkinen S., Pihlanto A., Vidal-Valverde C., Frias J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chemistry. 2013;136(2):1030–1037. doi: 10.1016/j.foodchem.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertension Research. 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- Wexler B.C., Iams S.G., Judd J.T. Comparative effects of adrenal regeneration hypertension on non-arteriosclerotic and arteriosclerotic Sprague-Dawley vs spontaneously hypertensive rats. Atherosclerosis. 1977;26(1):1–15. doi: 10.1016/0021-9150(77)90134-4. [DOI] [PubMed] [Google Scholar]
- Whelton P.K., He J., Appel L.J., Cutler J.A., Havas S., Kotchen T.A.…Karimbakas J. Primary prevention of hypertension: Clinical and public health advisory from The National High Blood Pressure Education Program. The Journal of the American Medical Association. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- Yang H.S., Choi Y.J., Jin H.Y., Lee S.C., Huh C.K. Effects of Allium hookeri root water extracts on inhibition of adipogenesis and GLUT-4 expression in 3T3-L1 adipocytes. Food Science and Biotechnology. 2016;25(2):615–621. doi: 10.1007/s10068-016-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ji Y., Park H., Lee J., Park S., Yeo S.…Holzapfel W.H. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel.) International Journal of Food Microbiology. 2014;191:164–171. doi: 10.1016/j.ijfoodmicro.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Yang M.H., Kim N.H., Heo J.D., Rho J.R., Ock K.J., Shin E.C., Jeong E.J. Comparative evaluation of sulfur compounds contents and antiobesity properties of Allium hookeri prepared by different drying methods. Evidence-Based Complementary and Alternative Medicine. 2017;2017:1–10. doi: 10.1155/2017/2436927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.M., Yu W., Ou Z.P., Ma H.l., Liu W.M., Ji X.L. Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Foods for Human Nutrition. 2009;64(2):167–173. doi: 10.1007/s11130-009-0120-5. [DOI] [PubMed] [Google Scholar]
- Zhan C.D., Sindhu R.K., Vaziri N.D. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: Reversal by lifelong antioxidant supplementation. Kidney International. 2004;65(1):219–227. doi: 10.1111/j.1523-1755.2004.00372.x. [DOI] [PubMed] [Google Scholar]