Abstract
Objective
There are some anthraquinones, anthraquinones and flavonones in Sennae Folium which exhibited significant acidity, such as sennoside A/B and sennoside C/D. The current strategies used in separating these components are mainly based on conventional column chromatography which is time consuming, laborious and costly. This study is aimed at exploring a method of precipitation extraction of acid components in Sennae Folium. Using alkaloid as a “hook”, it is reasonable to use the principle of “acid-alkali complexation” to "fish" the acidic components in Sennae Folium.
Methods
Isothermal titration calorimeter (ITC) was used to measure the extraction efficiency of different alkaloids. Then, alkaloid determined by ITC was mixed with extracting solution of Sennae Folium to form complex. High performance liquid chromatography coupled with mass spectrometry (HPLC-MS2) was used to investigate the ingredients “fished” by berberine (Ber). The mechanism of “fishing” process was explained by ITC, optical activity, fluorescence spectrometry and scanning electron microscope.
Results
The ITC results proved that the choice of “hook” was particularly important in the process of “fishing”. Among the hooks, the fishing efficiency of the isoquinoline alkaloids (Ber) was the highest, reaching 10.3%. Nine ingredients were detected and determined by HPLC-MS2, and the main components were sennoside A/B and sennoside C/D. Based on ITC test of Ber and sennoside A, the combination mechanism of the two ingredients was a chemical reaction with a nearly binding ratio (2:1). Fluorescence and optical properties of the active ingredients were changed after complexation. By scanning electron microscope, we found that two types of components had obviously self-assembled behavior during the formation process.
Conclusion
Ber successfully “fished” the main acidic components, sennoside A/B and sennoside C/D, from Sennae Folium. Combined with different characterizations, the “fishing” process was determined as a chemical association reaction induced by electrostatic interaction or π-π stacking. Therefore, with special identification ability, the “fishing” process had the potential of practical application.
Keywords: acid-alkali complexation, fishing, isothermal titration calorimeter method, Sennae Folium, weak ponds
1. Introduction
Direct self-assembly of molecules induced by weak bonds, such as hydrogen bonds, van der Waals forces, π-π stacking, etc., has been widely reported (Wang et al., 2018; Zheng et al., 2019). Inspired by these studies, we found that different molecules with acidity and alkalinity were easy to self-assemble under the effect of coulomb gravity to form different scale units (Zhang et al., 2016). This finding was summarized as acid-alkali complexation developed by our team to clarify the self-assembly phenomena in traditional Chinese medicine (TCM) decoction (Li, Wang, et al. 2017a; Li, Zhang, et al. 2017b; Tian et al., 2017; Wang et al., 2017). It could be expressed that obvious self-precipitation happened in the solution which consisted of one component containing the carboxyl group and others containing quaternary ammonium groups. This discovery had many applications, and we had reported its utility in the quality evaluation of TCM. For example, berberine (Ber) as a single component could form complex with baicalin, wogonoside, etc., which showed acidic in Scutellaria baicalensis decoction. The amount of the complex exhibited the level of the acidic components, which was used to evaluate the quality of the S. baicalensis (Li, Wang, et al. 2017a; Li, Zhang, et al. 2017b). This study was another application of acid-alkali complexation to explore its potential in the extraction of TCM components.
Sennae Folium, the dried leaflet of Cassia angustifolia Vahl or Cassia acutifolia Delile, locates mainly in India and Egypt (Bi et al., 2017). The acidic elements in Sennae Folium include anthraquinone derivatives, such as sennoside, aloeemodin glucoside, emodin glucoside and so on, as well as flavonoids, such as kaempferol glucoside, quercetin and so on (Wu et al., 2007). The methods used in separating these components were mainly based on conventional column chromatography which was time consuming, laborious and costly (Cui & Liu, 2007; Gu et al., 2010; Zhang et al., 2006). It was also difficult to implement mass production. Based on acid-alkali complexation, we compared an alkaloid to “hook”, compared the anthraquinone glycosides and flavonoid glycosides in Sennae Folium to “fish”, the “acid-alkali complexation” theory was the “bait”, then we would “fish” the acidic compositions from Sennae Folium directly (Fig. 1.).
Fig. 1.
"Fishing" process using Ber as “hook”.
First, ITC was used to compare the “fishing” efficiency of different types of alkaloids; and Ber was proved to be an economical and reasonable solution for “fishing” hook. Then, HPLC-MS2 was used to certify the accuracy of the “fishing”. Meanwhile, the content of Ber in the complex was measured by HPLC. Fluorescence spectroscopy characterized the change of fluorescence properties after complexation. Scanning electron microscope (SEM) was used to explore the scientific evidence of the interaction between the two types of ingredients from a microscopic point of view. Finally, we found that acid-alkali complexation was feasible in the extraction of the active ingredients of TCM.
2. Materials and methods
2.1. ITC analysis to conduct the selective “hook” and formation mechanism test
The ITC experiments were performed with an Auto-ITC isothermal titration calorimeter (TA Instruments, Shanghai, China). The ITC experiments were performed at 303.00 K. First of all, ITC was used to evaluate the binding capacity of different types of alkaloids to the extraction of Sennae Folium (Sen). The injector was sequentially filled with Ber, palmatine, higenamine and matrine (superfluous) and the working cell with Sen. The ITC titrations were carried out at a stirring speed of 250 r/min. The titration was sustained for six injections with injections of 8 µL, and an interval of 200 s between injections to ensure complete equilibration. In background blank titration, the injector was also filled with Ber solution with the same concentration, but the sample cell was just filled with the deionized water. Then, the formation mechanism experiments of sennoside A and Ber were performed by ITC. The injector was sequentially filled with Ber and the working cell with sennoside A. In order to reduce the energy of the system, the binding would generally be exothermic, otherwise it would not combine. Therefore, we could see clearly that the reaction between Ber solution and Sen was exothermic. After the titration, the Nano Analyze software was used to process the data of ITC. Through a series of fitting processes, the thermodynamic parameters including Ka, Kd, n, ΔH, ΔS were calculated.
2.2. HPLC-MS2 analysis of the constituents of complex of sen and ber (Sen-Ber)
HPLC-MS2 system was consists of several units: Thermo Accela Ultra-High Performance Liquid Chromatograph (Accela 600 Pump with Accela Open Autosampler and Quaternary Solvent Controller), Thermo LQT Orbitrap XL Mass Spectrometer, Xcalibur Workstation, ultra-pure helium as collision gas and high purity nitrogen for atomization. A TC—C18 (4.6 mm × 50 mm, 5 µm, Agilent) column was used for analysis. The column temperature was kept at 30 °C. The mobile phases consisted of water containing 0.1% formic acid water (A) and acetonitrile (B). The gradient elution was done as follows: 10%−80% B (0 − 30 min); the sample injection volume was 10 µL. The eluent flow rate was 0.2 mL/min. A full scan was run in the negative mode with a mass range from m/z 100 to 1000 amu. The capillary pressure with the ESI ion source was +3.4 kV. The tapered voltage was +110 V and the ion source temperature and capillary temperature were 300 °C and 350 °C, respectively. The collision gas was helium, the collision energy was 35 V and the sheath air flow and auxiliary air flow were 35 L/min and 10 L/min, respectively.
2.3. Optical properties of sennoside a
Sennoside A was dissolved in 0.1% of NaHCO3 and Ber (Non-optical properties) was dissolved in deionized water. Automatic polarimeter (Rudolph research analytical) was used to measure the optical properties of sennoside A before and after complexation with Ber.
2.4. Fluorescence properties of Ber and Sen-Ber
The Ber and Sen-Ber dissolved in methanol were measured by Fluorescence Spectrometer (LS 45, PerkinElmer). After preliminary experiments, we found that Ber had the maximum absorbance at 350 nm. And the absorption peak of Sen at 350 nm was significantly lower than Ber. So, excitation wavelength was set to 350 nm and the scan range to 300−600 nm. Then we gained the fluorescence spectrums.
2.5. Scanning electron microscope analysis of Sen-Ber
First of all, 10 µL of 0.25 mg/mL Ber solution and 0.75 mg/mL Sen solution was added to the conductive plastic, mix it evenly and react completely. Then, it was dried to remove moisture. We used FESEM (ZEISS-SUPRA55) to analyze the micromorphology of self-assemblies. Before the observation, it was necessary to electroplate Au coating using LEICA-EM-ACE600, because of the low conductivity of samples.
3. Results and discussion
3.1. ITC analysis to conduct the selective “hook”
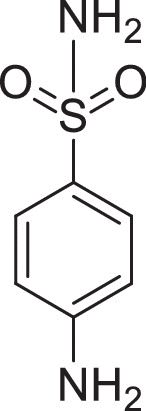
ITC was used to conduct the selective experiments on “hook”. Firstly, there was no visible precipitation during reacting with inorganic bases, such as ammonium thiosulfate, ammonium hydroxide. Then, four different structures of alkaloids, including Ber, palmatine, matrine, higenamine and sulfanilamide, were compared. As expected, the complexation of alkaloids with Sen showed obvious precipitation except sulfonamide (Table 1). This suggested that only satisfying constraint alkaline substance could be used as a “hook” for “fishing”. From the phenomenon of complexation, the reaction sequence was palmatine ≥ Ber > matrine > higenamine. Moreover, we compared the energy changes of four alkaloids in the process of complexation with Sen. As shown in Table 2, ITC results were accordance with the precipitation phenomenon. Energy span (ΔQ) showed that palmatine had the highest calorific value during the reaction, followed by Ber, matrine, and higenamine in sequent. It was indicated that isoquinoline alkaloids were more efficient in the process of “fishing” for acidic components. At the same time, due to economic reasons, we finally chose Ber as a “hook” for “fishing”.
Table 1.
Comparisons of complexations between alkaloids and inorganic base.
| Items | Berberine | Palmatine | Matrine | Higenamine | Sulfanila-mide | Ammonium thiosulfate |
|---|---|---|---|---|---|---|
| Structure | 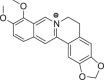 |
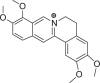 |
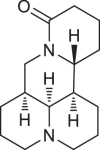 |
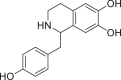 |
 |
 |
| Reaction phenomenon |  |
 |
 |
 |
 |
 |
| Intensity* | (+++) | (+++) | (++) | (+) | (-) | (-) |
“+++” means high intensity, “++” means medium intensity and “+” means low intensity of the reactions. “-” means no reaction.
Table 2.
Binding heat of all titrations of four alkaloids.
| No. | Berberine into Sen (µJ) | Palmatine into Sen (µJ) | Matrine into Sen (µJ) | Higenamine into Sen (µJ) |
|---|---|---|---|---|
| 1 | −1393 | −1716 | −227.0 | −67.02 |
| 2 | −1130 | −1117 | −290.5 | −94.56 |
| 3 | −802.5 | −677.2 | −126.5 | −64.00 |
| 4 | −555.1 | −437.3 | −54.67 | −32.81 |
| 5 | −390.5 | −297.3 | −18.84 | −10.04 |
| 6 | −198.4 | −229.0 | −7.880 | −1.297 |
| ΔQa of ITC | −1095 | −1487 | −219.1 | −65.72 |
The energy span between the first drop and the sixth drop.
3.2. Preparation of complex
Sennae Folium (6 g) was extracted with 100 mL 0.1% NaHCO3 solution under ultrasound for 30 min. Adding excess Ber (2 mg/mL, 5 mL) to 1 mL filtered 0.1% NaHCO3 solution of Sennae Folium, then a large amount of precipitate (Table 1) would appear. After filtrating, washing and drying, Sen-Ber was obtained.
3.3. HPLC analysis of Sen-Ber
3.3.1. Quantitative analysis of Ber in Sen-Ber
In order to explore the content of Ber and Sennae Folium extract in the complex and determine the extraction efficiency of the “fishing” process, HPLC experiment was designed. After Sen-Ber was prepared, the content determination experiment of Ber was carried out. HPLC was used to make a standard curve of Ber. The equation was y = 1.2208x + 40.452 (10.00 < x < 50.00 µg/mL), whose corresponding correlation coefficients (R2) was 0.9994. The quantitative result of Ber was 25.0% (Quality percentage). Therefore, we could further determine the content of Sennae Folium extract through the proportion of Ber in the complex, so as to obtain the extraction efficiency. The quantitative data were shown in Table 3.
Table 3.
Parameters during “fishing” process.
| No. | Weight of Sennae Folium/g | Selective “hook” complex/mg | Weight of extract from Sennae Folium/mg | Extraction efficiency/% |
|---|---|---|---|---|
| 1 | 6.01 | 93.13 | 69.80 | 9.50 |
| 2 | 6.02 | 111.75 | 83.83 | 10.88 |
| 3 | 5.99 | 97.10 | 72.82 | 10.53 |
| Average | 6.00 ± 0.01 | 100.66 ± 4.85 | 75.48 ± 6.03 | 10.30 ± 0.59 |
3.3.2. HPLC-MS2 coupled with mass spectrometry analysis of the constituents of Sen-Ber
HPLC-MS2 was used to analyze the main constituents of Sen-Ber. The total ion current of Sen-Ber was shown in Fig. 2A. Compared with the total ion current of Sen (Fig. 2B), the “fishing” process also showed the strong specificity. For instance, the peak at retention time of 5.7 min contained sennoside C/D, as well as numerous impurities (Fig. 2B). After complexation, sennoside C/D as the basic ingredients in Sennae Folium were extracted. At the same time, nine compounds were characterized and determined based on their retention behaviors and MS data (Table 4), mainly including sennoside A/B and sennoside C/D. In the first-order full-scan mass spectrometry, the quasi-molecular ion of peak c was at m/z 847.2010 [M-H]−, and the cleavage fragment was at m/z 609.1445 (Fig. 2C) which exhibited a [M + H2O-glucoside-COOH—CH3O—H]− product. Hence, we could easily determine that it was sennoside C/D according to the literatures (Terreaux et al., 2002). The quasi-molecular ion of peak d was at m/z 861.1920 (Fig. 2D), and the cleavage fragment was at m/z 699.1370 which exhibited [M-glucoside-H]−. Hence the peak d was sennoside A/B (Duan et al., 2017). As shown in Table 4, the results showed that the main ingredients were anthraquinone derivatives and flavonoid derivatives which were consistent with the literature (Terreaux et al., 2002). What's more, the major monomer compositions were sennoside A/B and sennoside C/D. Thus, the method based on the “acid-alkali complexation” theory was reasonable and feasible. As shown in Fig. 3, this result also fully demonstrated the feasibility of using Ber as “bait” to capture the acid components.
Fig. 2.
Total ion current of Sen-Ber (A) and Sen (B); Secondary cleavage of peak c (C) and peak d (D).
Table 4.
| Peaks | tR/ min |
m/z, [M-H]− |
Formula | Major fragments (negative ion mode) | Identification | |
|---|---|---|---|---|---|---|
| Measured | Mass error (× 10−6) | |||||
| a | 1.36 | 593.1522 | 1.69 | C27H30O15 | 353.0630, 285.0402, 227.4849 | Kaempferol-3-rutinoside |
| b | 4.25 | 609.1452 | 1.48 | C27H30O16 | 285.0402 | Kaempferol diglucoside |
| c | 6.18 | 847.2110 | 2.24 | C42H40O19 | 586.1556, 609.1445, 386.0946, 284.9497 | Sennoside C/Da |
| d | 7.66 | 861.1920 | 4.88 | C42H38O20 | 699.1370, 549.6667, 430.0839, 386.1036 | Sennoside A/Ba |
| e | 7.92 | 447.0889 | 4.65 | C21H20O11 | 402.8314, 285.0309, 241.0406 | Kaempferol glucoside |
| f | 8.41 | 407.1347 | 0.25 | C20H24O9 | 345.0595, 287.0880, 245.0837 | Tinnevellin glucoside |
| g | 12.25 | 301.0362 | 0.67 | C16H12O6 | 255.0665, 178.9966 | Quercetin |
| h | 16.25 | 285.0414 | 3.16 | C15H10O6 | 239.0361, 167.0516 | Kaempferol |
| i | 23.45 | 283.0261 | 4.59 | C15H8O6 | 239.0345, 211.0365 | Rhein |
The isomerism of sennoside A, B and sennoside C, D were not determined in this study.
Fig. 3.
Calorimetric titration of Ber solution into sennoside A and deionized water (A); Heating curve of sennoside A titrated by Ber solution (B); Structures of Ber (C) and sennoside A (D).
3.4. Mechanism of fishing process
3.4.1. ITC analysis of Ber and sennoside A
To study the mechanism of the fishing work, we chose the main component of sennoside A to simulate the molecular thermodynamics of the complexation process. The thermodynamic progress of titrating Ber solution into sennoside A was measured by ITC. The heat flow for each injection (µJ/s) as a function of time (seconds) was shown in Fig. 3A. The upper panel (blue peaks) showed heat rates of the titration of Ber solution into sennoside A; The lower panel (red peaks) showed the heat rates of the titration of Ber solution into deionized water. As shown in Fig. 3B, the binding thermodynamic parameters (ΔH, ΔS, ΔG, n, Ka, Kd) of Ber and sennoside A were shown after fitting process, summarized in Table 5. The panels showed different trends in thermodynamics. The upward peaks in Fig. 3A indicated exothermic reaction and as the sennoside A became progressively consumed during titration, the exothermicity of the peaks decreased and eventually saturated. As shown in Table 5, the binding constant (n) of Ber and sennoside A was 1.955, which proved that one molecule of sennoside A would bind to two molecules of Ber. This information was related to the molecular structures of Ber and sennoside A (Fig. 3C and D). Ka = 2.548 × 103 1/M, Kd = 3.924 × 10−4 (Ka/Kd = 6.5 × 106), indicating the tendency of Ber and sennoside A to react positively was large. Moreover, ΔH = −1437 kJ/mol, -TΔS = −1417 kJ/mol, ΔG = −19.44 kJ/mol, which indicated that it was very likely to be a chemical reaction by electrostatic interaction (Li et al., 2019), meaning that the separation of active constituents from Sennae Folium using Ber solution by acid-alkali complexation theory was feasible.
Table 5.
Binding thermodynamics of Ber into sennoside A.
| No. | ΔH /(kJ•mol−1) | −TΔS /(kJ•mol−1) | ΔG /(kJ•mol−1) | n | Ka (1/M) | Kd /(mol•L − 1) |
|---|---|---|---|---|---|---|
| Ber to sennoside A | −20.09 | 0.6508 | −19.44 | 1.955 | 2.548 × 103 | 3.924 × 10−4 |
In addition, after Ber was complexed with sennoside A, the optical rotation of sennoside A changed from to (c = 0.43 mg/mL). This was also a strong evidence of their complexation.
3.4.2. Spectral properties of Ber and Sen-Ber
The fluorescent properties of Ber and Sen-Ber were measured in methanol solvents at room temperature (25 °C). In the UV–vis spectrum, Ber exhibited a strong absorbance at 350 nm, which was significantly higher than Sen (Fig. 4A). However, when the excitation wavelength given to Sen was 350 nm, substantially no fluorescent property of Sen was exhibited (Fig. 4B). This indicated that when studying the change of fluorescence properties of Ber after complexation, Sen would not affect the results. Therefore, the excitation wavelength of Ber was set at 350 nm. The concentration of Ber was 10 µg/mL and the concentration of Sen-Ber was 40 µg/mL. As shown in Fig. 4B, the fluorescence emission spectrum of Ber had two peaks which were at 430 nm and 523 nm, when excited at 350 nm. The maximum fluorescence wavelength (λem (max)) of Ber was at 523 nm. However, the fluorescence emission spectrum of Sen-Ber only had one peak which was at 520 nm when excited at 350 nm. Obviously, the λem (max) of Sen-Ber shifted to the left compared to the Ber, and the fluorescence intensity of the fluorescence emission spectrum was reduced. The fact was that the conjugated system of Ber was affected after the quaternary ammonium group of Ber complexed with ingredients containing electron withdrawing groups such as a carboxyl group (Xiao, He, Huang & Li, 2012). The electron-withdrawing effect weakened the conjugate system and (π-π*) of Ber, causing λem (max) blue shift. Since (π-π*) affected the vibrational relaxation of Ber, the peak of Ber at 430 nm disappeared. At the same time, these were also the main factors that reduced the fluorescence intensity of the fluorescence emission spectrum. In a word, the change in the fluorescence properties of Ber and Sen-Ber was also a strong evidence of the complexation reaction between the two compositions.
Fig. 4.
UV-visible spectrum of Ber and Sen (A); Fluorescence emission spectra of Ber, Sen and Sen-Ber (B).
3.5. Scanning electron microscope study of Sen-Ber
To analyze the "acid-alkali complexation" theory and explore the scientific evidence of the interaction from a microscopic point of view, SEM was used to investigate the morphological characteristics of the Sen-Ber. As shown in Fig. 5, the morphology of Sen-Ber showed a strong regularity, which was quite different from Sen and Ber. As shown in Fig. 5c, many clusters were formed by a lot of bifurcated spikes arranged radially at low power. However, the fact was that each cluster was composed of many nanoflakes, as the magnification increased (Fig. 5d). This was a regular molecular arrangement. It was easy to understand self-assembly occurred between two types of molecules during the process of the “acid-alkali complexation”. Meanwhile, the self-assembly phenomena proved that the "acid-alkali complexation" theory was feasible from the microscopic point of view.
Fig. 5.
SEM images of Sen (a), Ber (b), and Sen-Ber (c, d).
In recent years, the self-assembly behavior of molecules was more and more widely used in the field of materials. Different types of weak interactions exist in acid-alkali complexation, such as π-π interactions, van der Waals’ force, hydrophobic force, hydrogen-bonding interactions, etc. (Gao, Liang, Hu & Ju, 2018) Under the action of these forces, there were many self-assembly forms, such as bowl, ball, bundle, flower and so on (Cal, Jaremko, Jaremko & Stefanowicz, 2013; Goskulwad et al., 2016; Ning, Xie, Pan, Li & Yu, 2010; Pachauri, Kern & Balasubramanian, 2010). Changes in microscopic morphology often pointed to changes in molecular arrangement, and the regular arrangement of the molecules indicated that self-assembly occurred. Therefore, it was proved that Ber and the acidic components of Sen could be self-assembled induced by weak bonds.
4. Conclusions
In this study, ITC was used to perform selective experiments on “hooks”. And it proved that the reaction of Ber and sennoside A was spontaneous chemical reactions induced by electrostatic interaction and π-π stacking. It demonstrated that the complexation reaction of Ber and Sen was scientific from molecular thermodynamics whose binding ratio was 2. HPLC-MS2 detection method was established to analyze the ingredients of Sen-Ber. Nine compounds were characterized based on their retention behaviors and MS data. Spectral and SEM tests indicated that the properties of Ber changed significantly after complex of Ber and Sen. Various characterization methods indicated that the process of extracting acidic components from Sennae Folium by Ber was a chemical reaction and had good application potential.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This research was funded by Beijing Natural Science Foundation (No. 7202116), National Natural Science Foundation of China (No. 81603256), Project of China Association of Chinese Medicine (CACM-2018-QNRC2B08), the Fundamental Research Funds for the Central Universities (BUCM-2019-JCRC002, 2019-JYB-TD005, and BUCM-2018−2021), Beijing Key Laboratory for Basic and Development Research on Chinese Medicine (Beijing, 100102).
Contributor Information
Peng-long Wang, Email: wpl581@126.com.
Hai-min Lei, Email: hm_lei@126.com.
References
- Bi H.L., Xiao Y.Z., Hu J., Song X.H., Yuan Q.F., Pan Y. Study on the research of folium sennae. Chinese Journal of Veterinary Medicine. 2017;53(4):108–111. [Google Scholar]
- Cal M., Jaremko Ł., Jaremko M., Stefanowicz P. The metallacrowns as templates for spontaneous self-assembly of polypeptides into a tetrahelical bundle. New Journal of Chemistry. 2013;37(11):3770. [Google Scholar]
- Cui X.Q., Liu Y.G. Study on separation and purification of active components from rhubarb by macroporous resin. Yunnan Journal of Traditional Chinese Medicine. 2007;(8):52–53. [Google Scholar]
- Duan W.J., Wu Q.X., Li Y., Yang G.H., Shi X.G., Sun W.H. Separation and purification of glycosidic compounds from folium sennae by high-speed counter-current chromatography. Shandong Science. 2017;30(1):15–19. [Google Scholar]
- Gao Y.X., Liang Y., Hu J., Ju Y. Supramolecular chiral self-Assembly based on small molecular natural products. Progress in Chemistry. 2018;30(6):737–752. [Google Scholar]
- Goskulwad S.P., La D.D., Bhosale R.S., AlKobaisi M., Bhosale S.V., Bhosale S.V. Golf ball-like architecture fabricated by supramolecular self-assembly of naphthalene diimide. RSC Advances. 2016;6(45):39392–39395. [Google Scholar]
- Gu Y., Xue M., Liu M., Zhang S.Y., Yan J., Zhang C.B. Study on purification process of total glucosides of senna. Chinese Traditional Patent Medicine. 2010;32(11):1986–1988. [Google Scholar]
- Li T., Wang H., Zhang H., Tian X.H., Chen Q.H., Fang K. Formation mechanism of precipitation in huanglian jiedu decoction based on molecular thermodynamic characteristics. Chinese Traditional and Herbal Drugs. 2017;48(17):3505–3510. [Google Scholar]
- Li T., Wang P.L., Guo W.B., Huang X.M., Tian X.H., Wu G.R. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano. 2019;13:6770–6781. doi: 10.1021/acsnano.9b01346. [DOI] [PubMed] [Google Scholar]
- Li T., Zhang H., Tian X.H., Wang H., Ren G.X., Jing W.G. New qualities control strategy of scutellaria baicalensis by isothermal titration calorimetry. China Journal of Chinese Materia Medica. 2017;42(20):3969–3973. doi: 10.19540/j.cnki.cjcmm.20170807.008. [DOI] [PubMed] [Google Scholar]
- Ning G.H., Xie T.Z., Pan Y.J., Li Y.Z., Yu S.Y. Self-assembly of bowl-like trinuclear metallo-macrocycles. Dalton Transactions. 2010;39(13):3203–3211. doi: 10.1039/b926138a. [DOI] [PubMed] [Google Scholar]
- Pachauri V., Kern K., Balasubramanian K. Template-free self-assembly of hierarchical ZnO structures from nanoscale building blocks. Chemical Physics Letters. 2010;498(4):317–322. [Google Scholar]
- Terreaux C., Wang Q., Ioset J.R., Ndjoko K., Grimminger W., Hostettmann K. Complete LC/MS analysis of a tinnevelli senna pod extract and subsequent isolation and identification of two new benzophenone glucosides. Planta Medica. 2002;68(4):349–354. doi: 10.1055/s-2002-26738. [DOI] [PubMed] [Google Scholar]
- Tian X.H., Zhang H., Li T., Wang H., Li J.Y., Wang P.L. New strategy on scientific connotation of Chinese materia medica compatibility enlightened by precipitation from Chinese materia medica formula decoction. Chinese Traditional and Herbal Drugs. 2017;48(22):4778–4783. [Google Scholar]
- Wang D.L., Yu C.Y., Xu L., Shi L.L., Tong G.S., Wu J.L. Nucleoside analogue-based supramolecular nanodrugs driven by molecular recognition for synergistic cancer therapy. Journal of the American Chemical Society. 2018;140(28):8797–8806. doi: 10.1021/jacs.8b04556. [DOI] [PubMed] [Google Scholar]
- Wang H., Li T., Xiang H.J., Zhang X.Y., Fang K., Wu G.R. Origin and formation mechanism investigation of compound precipitation from the traditional Chinese prescription huanglian jiedu decoction by isothermal titration calorimetry. Molecules (Basel, Switzerland) 2017;22:1456. doi: 10.3390/molecules22091456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.P., Wang Z.J., Fu M.H., Tang L.Y., He Y., Fang W. Chemical constituents from the leaves of cassia angustifolia. Journal of Chinese Medicinal Materials. 2007;(10):1250–1252. [PubMed] [Google Scholar]
- Xiao Y., He J., Huang H.M., Li S. Berberine detection based on a fluorescent conjugated polyelectrolyte. Chemical Sensors. 2012;32(1):59–63. [Google Scholar]
- Zhang C.Z., Zhao R., Yan W.Q., Wang H., Jia M.L., Zhu N.L. Compositions, formation mechanism, and neuroprotective effect of compound precipitation from the traditional Chinese prescription huanglian jiedu decoction. Molecules (Basel, Switzerland) 2016;21:1094. doi: 10.3390/molecules21081094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Tianjin University; 2006. The research on the separation of dianthrone in folium senna with the macroporous resin; pp. 27–46. [Google Scholar]
- Zheng J., Fan R., Wu H.Q., Yao H.H., Yan Y.J., Liu J.M. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nature Communications. 2019;10(1):160. doi: 10.1038/s41467-019-09601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]







