Abstract
BACKGROUND
The use of antidepressant therapy alone has a limited efficacy in patients with childhood trauma-associated major depressive disorder (MDD). However, the effectiveness of antidepressant treatment combined with psychodrama in these patients is unclear.
AIM
To evaluate the effectiveness of antidepressant treatment combined with psychodrama.
METHODS
Patients with childhood trauma-associated MDD treated with antidepressants were randomly assigned to either the psychodrama intervention (observation group) or the general health education intervention (control group) and received combination treatment for 6 mo. The observation group received general health education given by the investigator together with the “semi-structured group intervention model” of Yi Shu psychodrama. A total of 46 patients were recruited, including 29 cases in the observation group and 17 cases in the control group. Symptoms of depression and anxiety as well as coping style and resting-state functional magnetic resonance imaging were assessed before and after the intervention.
RESULTS
Symptoms of depression and anxiety, measured by the Hamilton Depression Scale, Beck Depression Inventory, and Beck Anxiety Inventory, were reduced after the intervention in both groups of patients. The coping style of the observation group improved significantly in contrast to the control group, which did not. In addition, an interaction between treatment and time in the right superior parietal gyrus node was found. Furthermore, functional connectivity between the right superior parietal gyrus and left inferior frontal gyrus in the observation group increased after the intervention, while in the control group the connectivity decreased.
CONCLUSION
This study supports the use of combined treatment with antidepressants and psychodrama to improve the coping style of patients with childhood trauma-associated MDD. Functional connectivity between the superior parietal gyrus and inferior frontal gyrus was increased after this combined treatment. We speculate that psychodrama enhances the internal connectivity of the cognitive control network and corrects the negative attention bias of patients with childhood trauma-associated MDD. Elucidating the neurobiological features of patients with childhood trauma-associated MDD is important for the development of methods that can assist in early diagnosis and intervention.
Keywords: Major depressive disorder, Childhood trauma, Yi Shu psychodrama, Cognitive control network, Coping style
Core Tip: Antidepressant therapy alone has limited efficacy in patients with childhood trauma-associated major depressive disorder. In our study, we treated patients with childhood trauma-associated major depressive disorder with antidepressants combined with psychodrama. After treatment, the internal connectivity of the cognitive control network increased in patients with childhood trauma-associated depression. Antidepressants combined with psychodrama were more effective in improving patients’ coping styles and cognitive control network than combined with a general health education intervention.
INTRODUCTION
Major depressive disorder (MDD) is a common psychiatric condition and leads to significant physical, psychological, and economic distress in individuals, families, and society[1,2]. Traumatic experiences during childhood, as shown by a meta-analysis[3], are significant psychosocial risk factors for MDD, and their presence represents a major reason for the refractory and recurrent nature of depression[1,4,5]. Childhood trauma, also known as early life stress, early life adverse life events, childhood adversity, and early negative events, generally refers to a variety of adverse life events that occurred in childhood or adolescence that the child or adolescent was unable to cope with; these include experiences such as abuse, neglect, parental divorce, and parental death. In China, the depression associated with childhood trauma is estimated to be as high as 55.5%[6].
The psychologist A. T. Beck proposed a cognitive model of depression in which it was proposed that early negative events can lead to the formation of a negative cognitive schema and can thus have a significant impact on cognitive functions such as information processing, interpretation, attention, and memory[7]. Cognitive function plays an important role in coping with environmental changes and in guiding problem-solving, decision-making, and behavioral responses in new situations[8]. Therefore, the coping style can reflect the cognitive function of individuals to some extent. As a continuing stressor for the individual, childhood trauma may affect the coping style. Some studies have pointed out that depressed patients with childhood trauma have inappropriate coping styles[9]. Patients with depression were also found to pay more attention to negative stimuli when presented with external environmental stimuli such as visual space than patients without depression[10]. More attention to negative information may hinder the regulation of emotion and the use of positive coping strategies in patients with depression[11]. Furthermore, depressed patients with a history of childhood trauma were more likely to pay attention to negative information (such as facial expression) than those without childhood trauma[12].
Resting-state functional magnetic resonance imaging (MRI) is helpful for researchers to understand the activity and neural functions of brain neurons. Functional connectivity (FC) is defined as the correlation between spatially nonadjacent brain regions in neurophysiological activities and is often used to evaluate information transmission by different brain regions[13]. Childhood is a critical period in human brain development[14], and the experience of childhood trauma may be sufficiently stressful to cause changes in both brain structure and function. Several studies have found that connectivity changes in the cognitive control network (CCN) may be the basis of cognitive impairment in patients with depression[15]. The CCN is located in the frontal and parietal lobes, primarily in the dorsolateral prefrontal cortex, dorsal anterior cingulate cortex, posterior parietal lobe, and posterior cingulate cortex[16]. It has been observed that compared with healthy controls, there was reduced internal connectivity in the CCN in patients with depression[17-19]. A study of multiple brain networks in patients with childhood trauma-associated MDD also found similar changes[20].
Antidepressants alone appear to have limited effectiveness in treating patients with depression resulting from childhood trauma. It has been found that psychotherapy is more effective in these patients compared with those without childhood trauma[21]. An intervention study on patients with chronic childhood traumatic depression found that the remission rate of clinical symptoms after treatment with antidepressants combined with psychotherapy was higher than that with antidepressants alone[22]. Brain imaging studies have pointed out that the internal connectivity of the CCN in patients with depression after receiving antidepressant medication is still lower than that in healthy controls[18,23]. However, the inferior frontal gyrus (IFG) connection in the CCN in depression patients increased after psychotherapy[24], which suggests that antidepressant therapy and psychotherapy may have different effects on the CCN in patients with depression. However, research on the effects of psychotherapy on CCN connectivity in patients with childhood trauma-associated MDD is limited.
At present, cognitive behavioral therapy (CBT) is the most effective form of psychotherapy for treating depression[25]. However, researchers have pointed out that because CBT is a psychotherapeutic model developed by A. T. Beck, an American psychologist, patients suffering from symptoms of depression from other cultures and non-English speaking countries may not be as responsive to CBT intervention[26].
Psychodrama is a type of group psychotherapy founded by J. L. Moreno, a psychiatrist and psychotherapist. Studies have shown that the symptoms of depression in patients were significantly improved after psychotherapy and that the levels of cortisol, a marker related to stress, were also significantly decreased. These findings suggest that psychodrama may significantly improve depression and effectively reduce the physical and mental distress caused by stressors[27].
The winner of the American Group Psychotherapy and Psychodrama Society’s Lifetime Achievement Award, and trainer, educator, and practitioner certified by The American Board of Examiners in Psychodrama, Sociometry and Group Psychotherapy, Chinese-American Dr. Gong Shu integrated the five elements of Eastern philosophy, the psychological theory of traditional Chinese medicine, and the balance of Yin and Yang in Taoist culture with classic psychodrama and explored and developed Yi Shu psychodrama in line with Chinese culture. Patients have reported significant improvement and the relief of physical and emotional distress following the use of Yi Shu psychodrama, which healed both emotions and the body together[28].
We hypothesized that the combination of first-line antidepressants and psychodrama therapy, or general health education, would improve the clinical symptoms and coping styles of patients with childhood trauma-associated MDD. We also hypothesized that the internal connectivity of the CCN would be altered after the combination therapy. Therefore, MDD patients with childhood trauma were selected after taking first-line antidepressants in the acute phase (8 wk) treatment and randomly divided into two groups, namely the observation group in which antidepressants were combined with Yi Shu psychodrama and the control group in which antidepressants were combined with general health education. The effects on clinical symptoms, coping style, and the CCN were then observed. It is hoped that these findings will enrich empirical research on the clinical treatment of childhood traumatic depression and will provide scientific data for the specific application of psychodrama in clinical practice.
MATERIALS AND METHODS
Participants and grouping
Participants were recruited from the Department of Psychiatry outpatients in the First Affiliated Hospital of Chongqing Medical University from July 2017 to July 2019. Inclusion criteria: all participants were between the ages of 18 and 50, with a minimum of 9 years of education, right-handed, and had received only first-line antidepressants (selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors). The prospective participants received structured clinical interviews with ICD-10 (International Classification of Diseases-10) conducted by two different licensed clinical psychologists who did not participate in the study. All the participants were required to meet the ICD-10 criteria for a current episode of MDD. According to the questionnaire survey of childhood trauma experience and standardized interview of childhood experience, the MDD patients should have had at least one experience of childhood trauma. Exclusion criteria: (1) MDD accompanied by severe physical diseases; (2) MDD accompanied by mental retardation or dementia, obvious psychotic symptoms, bipolar disorder, post-traumatic stress-related disorders, or severe personality disorders; (3) Patients with serious suicide risk and self-injury behavior within the previous 3 mo; (4) Patients addicted to alcohol or other substances; (5) Patients who had undergone major surgery, received electric shock, or transcranial magnetic therapy within the previous 3 mo; (6) Patients receiving other systematic psychotherapy at the same time; (7) Patients being treated with hormonal drugs; (8) Pregnant or lactating women; and (9) Patients with MRI taboos or claustrophobia.
The patients were divided into 2 groups using computer-generated random numbers: an observation group and a control group. Imaging data that could not be analyzed or the data of patients who were unwilling to participate in the intervention study or who had dropped out during the observation period were excluded. All patients provided written informed consent, and the study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Intervention process
General health education: During the 6-mo observation period, the investigator provided general health education to the control group through the distribution of the health manual for depression and providing and explaining information about depression either in the outpatient setting or on the phone.
Psychodrama: Yi Shu psychodrama intervention was conducted in small, closed groups (6-10 patients in each batch) in batches by Er-Dong Wang, who is a Clinical Practitioner certified by The American Board of Examiners in Psychodrama, Sociometry and Group Psychotherapy and was supervised by Dr. Gong Shu. The intervention frequency for each group was 4 d for each intervention, once every 2 mo for a total of three times lasting for 6 mo. There were several psychiatric medical staff who had been trained in psychodrama as professional auxiliary egos and could deal with possible clinical crises.
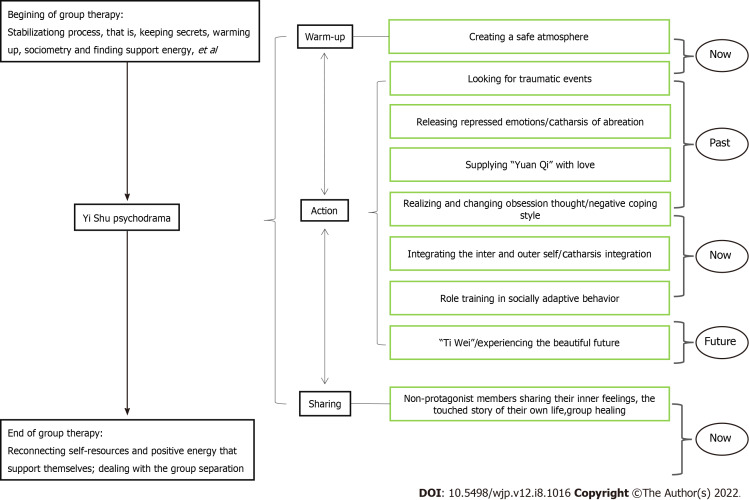
In this study, we applied the “semi-structured group intervention model” of Yi Shu psychodrama for depression (Figure 1). This included the three classic “structure” stages of psychodrama: the warm-up phase, the enactment/action phase, and the sharing/integration phase. The protagonist is allowed to go from the “now” back to the “past” to explore the influence of past experiences, then to return to the present to “integrate self” and experience the possibility of the future in surplus reality, and finally return to anchoring in the present.
Figure 1.
Model of Yi Shu psychodrama for patients with major depressive disorder.
Since the enrolled depression patients had all experienced childhood trauma, we added a stabilization process. The structural stabilization work was carried out during the half day at the beginning and the half day at the end, running through the whole process. In the warm-up phase, the use of music, dancing, painting, body feelings, and dreams, amongst others, assisted patients to become aware of implicit or body memories often associated with traumatic events. In the enactment or action phase, the impacts of traumatic events were explored, and the patients’ negative cognition was corrected through typical psychodrama techniques such as role-playing, role reversal, double, mirroring, and soliloquy, amongst others. In addition, energy blockages in both the body and emotions were released simultaneously. In the sharing or integration phase, patients shared their own stories related to the protagonist during the psychodrama enactment.
General information and assessment indicators
All subjects completed the Hamilton Depression Scale (HAMD-17), 13-item Beck Depression Inventory (BDI-13), 21-item Beck Anxiety Inventory (BAI-21), and Trait Coping Style Questionnaire (TCSQ) twice, at the beginning and at the end of the 6-mo observation period. In addition, the Childhood Trauma Questionnaire-Short Form was used to quantitatively assess the type and degree of childhood trauma. The sociodemographic information form was designed to acquire the patient’s general information before the experiment. All the observation indicators are described below.
Sociodemographic information form: This part of the questionnaire contained general information on the participant’s age, sex, years of education, and the types of antidepressants taken.
Childhood Trauma Questionnaire-Short Form: The Childhood Trauma Questionnaire-Short Form, with modifications by Bernstein et al[29] in 2003 was used; this has validity in diverse clinical and nonreferred populations. This questionnaire has a total of 28 items (25 items plus the 3-item validity scale) and divides childhood trauma into five dimensions: emotional neglect, physical neglect, sexual abuse, emotional abuse, and physical abuse. The internal consistency coefficient of the questionnaire was 0.73.
HAMD-17: The HAMD-17 was used to evaluate the severity of depressive symptoms. Two psychiatrists or postgraduates who had received consistent training were given HAMD joint examinations, and the prescribed guidelines were used at the same time. After the examination, the scores were determined by two independent examiners who were unaware of the grouping of the patients to avoid subjective scoring. This questionnaire has passed the reliability and validity tests in China, and its internal consistency coefficient was 0.714. The total score for no depression was 0-7, and the total score for mild depression was 8-17. Patients with moderate depression scored between 18 and 24, and patients with severe depression scored over 25. Reductions in the HAMD-17 score of ≥ 75% or a total score of ≤ 7 points after the intervention indicated significant effectiveness. A HAMD-17 score reduction rate ≥ 50% was defined as effective, a 25% ≤ score reduction rate < 50% was defined as improvement, and a score reduction rate < 25% was defined as invalid.
BDI-13: The degree of depression of the patients was assessed at the same time by the BDI-13, which was translated into Chinese. The questionnaire had passed the Chinese test of reliability and validity, and its internal consistency coefficient was 0.86. Each item of the BDI-13 was rated as 0-3, with a total score of 0-4 for no depression, 5-7 for mild depression, 8-15 for moderate depression, and more than 16 for severe depression.
BAI-21: The degree of anxiety was assessed by the BAI-21. Each item was scored by 1-4 grades. The higher the total score, the more serious the anxiety level of the patients. The internal consistency coefficient of the questionnaire was 0.95.
TCSQ: The TCSQ for Chinese was used for direct measurement of coping style and indirect assessment of cognitive schema. This questionnaire includes two dimensions of positive and negative coping. Each dimension comprised 10 items, with the score of each item ranging from 1 (absolutely no) to 5 (absolutely yes). The higher the score on a given subscale, the more an individual tends to adopt the respective coping style. The validity and reliability of the TCSQ have been established, and the Cronbach’s alpha coefficients for positive coping and negative coping were 0.790 and 0.776, respectively[30,31].
Data collection and analysis
Questionnaire data acquisition and analysis: The subjects completed the questionnaires online through the QuestionStar Internet platform (https://www.wjx.cn/) by scanning a two-dimensional code before and after the intervention. The researchers confirmed the submissions immediately and evaluated the questionnaire results in the background on the same day. SPSS 25.0 was used to process and analyze the questionnaire data. The t test was used for normally distributed measurement data, and the results were expressed as mean ± standard deviation. Nonparametric tests were used to compare measurement data that did not conform to the normal distribution, and the results were expressed by M (Q). The count data were compared by the χ2 test, and the results were expressed as percentages.
MRI data acquisition: All imaging data (baseline and after intervention) were acquired using a Signa 3.0 Tesla MRI system (GE Medical Systems, Waukesha, WI, United States) at the First Affiliated Hospital of Chongqing Medical University. At the baseline scan, T1-weighted and BOLD data were collected. In addition, T2-Flair image data of all the participants were also collected at the baseline scan because if any brain illnesses were found the participant would be removed from the study and examined by the Neurology Department. Both the T1-weighted and BOLD scan sequences were described in our previous article[32]. Participants were instructed to keep their eyes closed but be awake during the scan, and head motion during scanning was restricted by restraining the head using foam pads inserted on each side.
Resting-state functional MRI data preprocessing: The resting-state functional MRI data preprocessing were carried out using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and the GRETNA toolbox[33] (http://www.nitrc.org/projects/gretna/), which are based on MATLAB. The first 10 volumes were excluded, and the remaining 230 volumes were corrected for head motion. In this step, the middle slice was used as the reference slice. If the participant’s head motion exceeded 3 mm in distance or a 3° angle during scanning, whether at baseline or after the intervention, all the patient’s data were excluded. Individual 4D volumes were then spatially normalized to the Montreal Neurological Institute space, retaining a voxel of size 3 mm× 3 mm× 3 mm (originally acquired at 3.75 mm× 3.75 mm× 3.75 mm), using diffeomorphic anatomical registration through exponentiated lie algebra[34] and were then spatially smoothed with a 6-mm full width at half-maximum Gaussian kernel. It is worth mentioning that a smooth step only exists in the preprocessing step of voxel-wise functional connection analysis based on the node efficiency result. Next, linear trends were removed to account for scanner drift, and temporal band-pass filtering (0.01–0.1 Hz) was performed. Finally, multiple linear regression was performed on the Friston-24 parameters of head motion[35] and the signals of the white matter and cerebrospinal fluid.
Functional brain network construction and node efficiency analysis: All networks are composed of nodes and connected edges. In the functional brain network, nodes refer to the brain regions with internal consistency and external independence, and the edge connection between nodes can be regarded as the temporal behavioral consistency between the two spatially independent nodes. From a statistical point of view, the meaning of the edge is statistically dependent on the time series of two brain regions.
In this study, we constructed a functional brain network for each subject according to the automated anatomical labeling template[36] that divides the brain into 90 anatomical regions, with each region defined as a node. Then, positive Pearson’s correlation coefficients between the time series of two nodes (xi, xj) were computed as the edges to produce a 90 × 90 correlation matrix for each subject.
Then, the correlation matrix was transformed into a binary matrix according to the preset threshold value, that is, when Rij is greater than the threshold value, the corresponding element of the binary matrix is 1; otherwise it is 0. In this study, sparsity was used to set a series of continuous thresholds to construct a brain network in a threshold space. Sparsity is defined as the ratio of the number of edges in the network to the maximum number of edges that may exist in the network. The sparsity range in this study was S∈(0.01, 0.5). Within this range, binary brain networks for all subjects were constructed under all sparsity degrees with a step size of 0.1.
When the brain network is constructed, the node efficiency of each node in each sample under all selected thresholds is calculated. In this case, a graph of node efficiency can be constructed for each node, and the area under the curve can be calculated to characterize the overall characteristics of node efficiency within the selected threshold. The area under the curve was used in the subsequent statistical analysis.
Statistical analysis using the MATLAB statistical toolkit, NBS statistical method[37], and repeated measurement analysis of variance was carried out on the node efficiency area under the curves of 90 nodes in the two groups of patients. The results were not corrected by multiple comparisons, and the significance level was set as 0.001.
Functional connection analysis based on node efficiency result: Based on the results of node efficiency, the brain regions of the two groups with node efficiency interacting with treatment and time were selected as seed points for voxel-wise FC analysis of the whole brain. SPM was used for statistical analysis and flexible design was used for treatment time interaction analysis. SPSS was used for t tests, covariate regression was used for sex and age, and multiple comparison correction was performed by Gaussian random field correction with a voxel level of 0.001 and a mass level of 0.05.
RESULTS
Comparative results of demographic information
Both questionnaire and MRI data, before and after the intervention, were collected from 46 subjects between July 2017 and July 2019. There were 29 cases in the observation group and 17 cases in the control group (complete questionnaire and MRI data were collected from 33 cases in the observation group, with 4 cases dropping out, and from 27 cases in the control group, with 10 cases dropping out). There were no statistically significant differences between the two groups of patients in terms of demographics and medication information (P > 0.05) (Table 1).
Table 1.
Baseline demographic comparison between the two groups
|
Item
|
Observation group, n = 29
|
Control group, n = 17
|
t
/χ2
|
P
value
|
| Age, yr | 25.970 ± 7.189 | 28.120 ± 6.214 | -1.029 | 0.309 |
| Sex, F/M | 0.405 | 0.525 | ||
| Female | 22 (76) | 15 (88) | ||
| Male | 7 (24) | 2 (12) | ||
| Education, yr | 15.030 ± 2.179 | 13.710 ± 2.443 | 1.909 | 0.063 |
| Med, SSRIs/SNRI | 0.423 | 0.515 | ||
| SSRIs | 24 (83) | 16 (94) | ||
| SNRI | 5 (17) | 1 (6) | ||
| CTQ | 50.210 ± 9.715 | 48.880 ± 8.908 | 0.460 | 0.648 |
Data are mean ± SD or n (%). Due to rounding, the total % might be more than 100%. SSRIs: Selective serotonin reuptake inhibitors; SNRI: Serotonin noradrenaline reuptake inhibitors; CTQ: Childhood Trauma Questionnaire; F: Female; M: Male.
Changes in the clinical and psychological questionnaire information after intervention
Comparison of the clinical efficacy of two intervention methods: The χ2 test was used to analyze the clinical efficacy of HAMD-17 between the two groups. In the observation group, the number of significantly effective scores was 23 (79.31%), the number of effective scores was 1 (3.45%), and there were 2 improvements (6.90%). In the control group, the number of significantly effective scores was 12 (70.59%), with 2 effective (11.76%) and 3 improvements (5.89%). No significant differences in clinical efficacy were observed between the two groups (P > 0.05) (Table 2).
Table 2.
Comparison of clinical efficacy between the two groups
|
Item
|
Total cases
|
General improvement
|
Invalid
|
| Observation group | 29 | 26 (89.66) | 3 (10.34) |
| Control group | 17 | 15 (88.24) | 2 (11.76) |
| χ2 | 0.022 | ||
| P value | 0.881 |
Data are n (%). Due to rounding, the total % might be more than 100%.
Comparison of HAMD, BDI, BAI, and coping style scores before and after interventions: The HAMD, BDI, BAI, positive coping style, and negative coping style scores were analyzed by the generalized estimation equation. There were statistically significant differences in the time effect and interaction effect on HAMD, BDI, and BAI between the two groups (P < 0.01). There were also statistically significant differences in the between-group effects, time effect, and interaction effect between the two groups of patients in the positive coping style and negative coping style (P < 0.05) (Table 3).
Table 3.
Comparison of the scores of each scale between the two groups before and after the intervention
|
Item
|
Group
|
Pre-intervention
|
Post-intervention
|
Wald χ2
|
||
|
Between-group effect
|
Time effect
|
Interaction effect
|
||||
| HAMD | Observation group | 19.690 ± 6.887 | 6.240 ± 7.342 | 0.000 | 125.683b | 137.316b |
| Control group | 18.410 ± 9.625 | 7.590 ± 7.246 | ||||
| BDI | Observation group | 14.000 ± 5.898 | 4.480 ± 5.096 | 0.004 | 97.162b | 105.231b |
| Control group | 13.120 ± 8.455 | 5.590 ± 5.269 | ||||
| BAI | Observation group | 38.380 ± 10.584 | 31.100 ± 9.828 | 0.142 | 19.415b | 20.096b |
| Control group | 36.350 ± 8.536 | 31.290 ± 9.225 | ||||
| P-coping style | Observation group | 22.000 ± 5.988 | 26.790 ± 7.379 | 3.898a | 8.635b | 12.891b |
| Control group | 20.760 ± 5.663 | 21.650 ± 6.800 | ||||
| N-coping style | Observation group | 32.030 ± 7.580 | 22.140 ± 4.875 | 4.017a | 18.020b | 60.931b |
| Control group | 30.350 ± 8.775 | 31.240 ± 7.164 | ||||
P < 0.05.
P < 0.01.
Data are mean ± SD or n (%). Due to rounding, total % might be more than 100%. HAMD: Hamilton Depression Scale; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; P-coping style: Positive coping style; N-coping style: Negative coping style.
Simple effect analysis of HAMD, BDI, BAI, and coping style scores before and after interventions: We conducted a further analysis based on the results shown in Table 3. The HAMD, BDI, BAI, positive coping style, and negative coping style scores between and within the two groups were tested by independent-sample t tests or Mann Whitney U tests with two independent samples and paired-sample t tests. There were no significant differences in the baseline scores of each scale between the two groups before the intervention (P > 0.05). After the intervention, while there were no significant differences in the HAMD, BDI, and BAI scores between the two groups (P > 0.05), the score for positive coping style in the observation group was significantly higher than that in the control group (P < 0.05), and the score for negative coping style in the observation group was significantly lower than that in the control group (P < 0.01). The HAMD, BDI, BAI, and negative coping style scores in the observation group were significantly lower than those before the intervention (P < 0.01), while the scores for positive coping style were significantly increased (P < 0.01). The HAMD, BDI, and BAI scores in the control group after intervention were lower than those before intervention (P < 0.05), while the scores for positive coping style and negative coping style were not statistically significant (P > 0.05) (Table 4).
Table 4.
Simple effect analysis of each scale in two groups before and after intervention
|
Item
|
Comparison between the two groups before intervention
|
Comparison between the two groups after intervention
|
Comparison of before and after intervention in the observation group
|
Comparison of before and after intervention in the control group
|
| HAMD | 0.523 | -0.481 | -8.985b | -6.614a |
| BDI | 0.416 | -0.586 | -8.453b | -5.035b |
| BAI | 0.671 | -0.114 | -3.517b | -2.619a |
| P-coping style | 0.689 | -2.211a | 3.003b | 0.642 |
| N-coping style | 0.685 | -5.124b | -6.744b | 0.436 |
P < 0.05.
P < 0.01.
The value in the table is the statistical value t/Z (where t is the t-value of the test two independent samples or paired-samples t test and Z is the statistical value of the Mann-Whitney U test). HAMD: Hamilton Depression Scale; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; P-coping style: Positive coping style; N-coping style: Negative coping style.
The results of node efficiency and FC
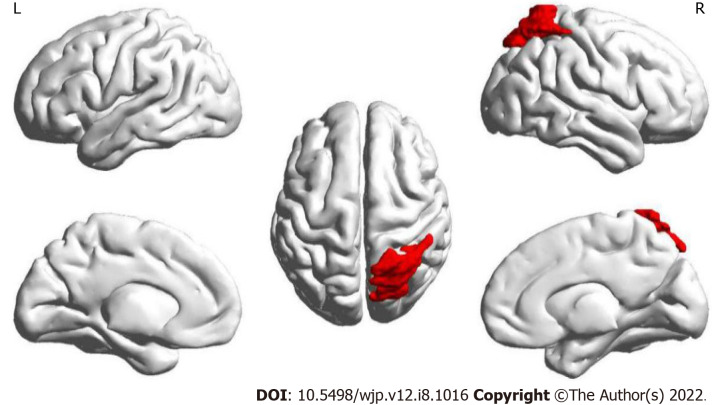
The results of this part of the study found that only the node efficiency of the right superior parietal gyrus (SPG) in brain area 60 showed an interaction between treatment and time (Figure 2). It was found that the node efficiency of brain area No. 60 increased after intervention in the observation group and decreased in the control group.
Figure 2.
Location of brain regions with interactions of node efficiency. The red brain area marked in the figure is the right superior parietal gyrus, the brain region where the node efficiency interacts after intervention in the two groups.
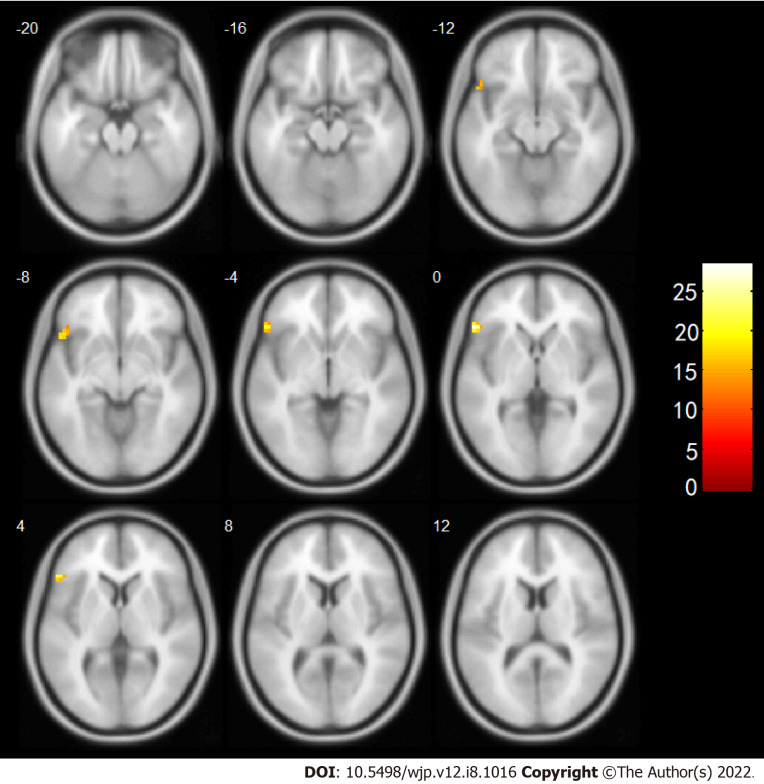
Based on these results, brain area No. 60 was subsequently used as a seed point to conduct a whole-brain voxel-wise FC connection analysis. The results showed that after the intervention, the change in the FC strength of a mass in the right SPG and the left IFG was associated with a significant interaction between treatment and time. Further post-examination analysis found that compared with before the intervention the connection between the right SPG and the left IFG of the observation group was enhanced after the intervention, while the connection in the control group was weakened (Table 5, Figure 3).
Table 5.
Connections to the brain area interacting with the right superior parietal gyrus after intervention
|
Brain region
|
Voxels
|
MNI Coordinate (X, Y, Z) (mm)
|
Peak intensity
|
t
A
(pA)
|
t
B
(pB)
|
| Inferior frontal gyrus | 39 | (-54, 27, 0) | 28.3857 | 2.492 (0.019) | -2.156 (0.047) |
X, Y, Z: Coordinates of primary peak locations in the Montreal Neurological Institute space. MNI: Montreal Neurological Institute. Peak intensity: The statistical value of the interactive brain region that passes the Gauss random field corrected P < 0.05. tA (pA): Comparison of after intervention and before intervention in the observation group; tB (pB): Comparison of after intervention and before intervention in the control group. A positive value for t indicates a stronger connection after treatment, and a negative value for t indicates a weaker connection after the intervention.
Figure 3.
Increased connectivity with the right superior gyrus after intervention. The numbers in the figure represent the axial coordinates of the brain profile in Montreal Neurological Institute space, and the brightness of the color represents the significance level of the interaction, with brighter color indicating higher significance.
DISCUSSION
Emotional and physical neglect account for a high proportion of childhood traumatic experiences in MDD patients[6]. Chinese parents have paid a great deal of attention to education over the past 40 years, with many Chinese parents pushing their children to study hard and succeed to the possible detriment of the children’s emotional and physical well-being. Both emotional and physical neglect can play significant roles in the development of depression. Depressive patients who have experienced childhood trauma often have negative coping styles[9], an aspect that should receive more attention in psychological intervention.
We found that while both interventions produced similar clinical effects in decreasing the levels of depression and anxiety among patients diagnosed with MDD with childhood trauma, the combination of first-line antidepressants and psychodrama was found to be more effective than that of the combination of first-line antidepressants and general health education in reducing the passive coping styles and enhancing the positive coping styles of patients, which is similar to the conclusion of Stanisławski’s study[38]. Other studies have also found that positive support can reduce the impact of childhood traumatic experiences on depressive symptoms[39]. Perceived social support has been identified as a classic coping strategy[9]; however, it has been observed that individuals with childhood trauma have difficulty seeking support[40]. Furthermore, depressed patients’ disproportionate preferences for negative information has been found to affect their coping strategies[11].
The Yi Shu psychodrama group provided emotional support for its members. For example, “action performance” and “love hugs” during the Yi Shu psychodrama sessions could nourish the body and mind. With Yi Shu psychodrama “the trauma treatment and self-integration intervention structure” allows patients to receive corrective emotional experiences for their childhood trauma by altering their negative cognition, reconnecting internal and external resources, and integrating themselves, leading to improved coping style and the ability to adapt to environmental change. Therefore, we speculate that Yi Shu psychodrama is more effective than general health education in influencing the coping style. This may be because psychodrama can correct the patient’s perception of distress by altering the disproportionate attention to negative information in depression patients with childhood trauma, and the psychodrama groups can provide individual physical and mental support.
We found that after 6 mo of intervention, the node efficiency of the right SPG increased and the connection with the left IFG increased in the group receiving first-line antidepressants combined with psychodrama, while the node efficiency in the other group that received first-line antidepressants combined with general health education decreased and the connection with the left IFG decreased. Node efficiency is a measure of the ability of a node to transmit information to other nodes. The higher the node efficiency, the greater the importance of the node in the network, and the easier it spreads information to other nodes, resulting in greater integration in the brain[41]. SPG, as an important brain region integrating multi-channel information of visual, auditory, and sensory movements, participates in the processes of attention control and target selection[42].
In our study, Yi Shu psychodrama aimed to reverse the negative effects of childhood trauma on the individual through various channels such as vision, hearing, and kinesthetic sense. Therefore, we suggest the use of the SPG as the functional MRI target when using psychodrama as a treatment. Some studies have observed significantly lower activation of the bilateral frontal lobe and right SPG than in healthy controls[43], which may be the reason depression patients tend to pay more attention to negative information[44]. It has been pointed out that CCN abnormalities in depression patients are usually manifested as an inability to effectively transmit information between the parietal lobe and the frontal lobe. As a result, depression patients cannot adjust the parietal lobe attentional bias in a way that is beneficial to individual development. This may be the general mechanism underlying impairments in cognitive performance in patients with depression[45]. Our study found that the two intervention methods had different effects on the right SPG.
The IFG participates in response inhibition[46], that is to say, it inhibits the individual’s spontaneous response to a specific environmental stimulus[47]. Some studies have also pointed out that the IFG may be involved in individual monitoring of the external environment to establish or maintain attention to a certain objective of the current external environment[48,49]. The activation of the IFG in individuals with childhood trauma may be related to their high vigilance against the external environment[50]. Our research found that the SPG, which is responsible for integrating visual information in the CCN, and the IFG, which has the function of reflecting inhibition or monitoring the external environment, showed increased connectivity after the intervention in the observation group, while such connections appeared reduced in the control group after intervention. These results are similar to those of previous studies that found a decrease in the internal connectivity of the CCN after antidepressant treatment, while there was increased internal connectivity of the CCN after psychotherapy[23,24,51].
Other studies have found that the frontal lobe controls the area of attention of the parietal lobe through top-down regulation[52]. The impairment of CCN function in patients with depression leads to reduced control over the hyperactivation of the limbic system (i.e. the higher cognitive level areas cannot effectively regulate the activities of lower cognitive level areas), and its top-down regulation of attention and emotion is reduced[53-56]. Our study suggests that the enhanced internal connectivity of the CCN after the intervention of first-line antidepressants combined with psychodrama may be due to an enhanced top-down attention control from the IFG to the SPG. The cognitive control capability of the whole network was restored, and the negative attention bias was corrected. However, the treatment of first-line antidepressants combined with general health education did not restore the cognitive control capability of the network, and the negative attention bias of the patients was not corrected. This is similar to the finding that even if patients with depression recover from a depressive episode, their attention is still negatively biased[57]. We further speculate that psychodrama can enhance the internal connectivity of the CCN and correct the patient’s negative attentional bias better than general health education.
It has been pointed out that psychotherapy works through a top-down mechanism[58]. Top-down cognitive control by the CCN has been found to overcome hyperactivity of the limbic system[59]. The ability of the individual to regulate the response to negative stimuli depends on the attention to negative stimuli when facing the visual spatial environment[57]. Based on this indirect evidence and our own research evidence, we speculate that psychodrama may restore the cognitive control capability of the CCN in depressive patients from the top-down, inhibiting overactivity of the limbic system and thus reducing the patient’s negative attentional bias. Then, like CBT, it could weaken the patient’s perception of negative cognitive schemas[60] and improve their coping styles.
CONCLUSION
This study provides initial support for the use of antidepressants combined with psychodrama to improve the coping style of MDD patients with childhood trauma, which was found to increase the functional connectivity between the SPF and IFG. However, antidepressants combined with general health education did not produce these effects. We speculate that psychodrama can enhance the internal connectivity of the CCN and can thus correct the negative attention bias of patients.
In conclusion, we preliminarily found that antidepressant drugs combined with Yi Shu psychodrama therapy have better short-term effects in improving the coping style of these patients than antidepressant drugs combined with general health education, which provides a new option for clinical intervention with childhood traumatic depression. This study shows that psychodramas enhanced characteristics of cognitive network connectivity will be beneficial for the development of methods for early diagnosis and treatment of such patients. In the future, we will combine more abundant clinical psychological indicators and neurobiological indicators to conduct joint exploration to lay a foundation for the early diagnosis of depression with childhood trauma and the exploration of effective intervention targets.
ARTICLE HIGHLIGHTS
Research background
The use of antidepressant therapy alone has a limited efficacy in patients with childhood trauma-associated major depressive disorder. However, the effectiveness of antidepressant treatment combined with psychodrama in these patients is unclear.
Research motivation
To evaluate the effectiveness of antidepressant treatment combined with psychodrama.
Research objectives
Patients with childhood trauma-associated major depressive disorder treated with antidepressants.
Research methods
Patients with childhood trauma-associated major depressive disorder treated with antidepressants were randomly assigned to either the psychodrama intervention (observation group) or the general health education intervention (control group) and received combination treatment for 6 mo. The observation group received general health education given by the investigator together with the “semi-structured group intervention model” of Yi Shu psychodrama. A total of 46 patients were recruited, including 29 cases in the observation group and 17 cases in the control group. Symptoms of depression and anxiety as well as coping style and resting-state functional magnetic resonance imaging were assessed before and after the intervention.
Research results
Symptoms of depression and anxiety, measured by the Hamilton Depression Scale, Beck Depression Inventory, and Beck Anxiety Inventory, were reduced after the intervention in both two groups of patients. The coping style of the observation group improved significantly in contrast to the control group, which did not. In addition, an interaction between treatment and time in the right superior parietal gyrus node was found. Furthermore, functional connectivity between the right superior parietal gyrus and left inferior frontal gyrus in the observation group increased after the intervention, while in the control group the connectivity decreased.
Research conclusions
This study supports the use of combined treatment with antidepressants and psychodrama to improve the coping style of patients with childhood trauma-associated major depressive disorder. Functional connectivity between the superior parietal gyrus and inferior frontal gyrus was increased after this combined treatment. We speculate that psychodrama enhances the internal connectivity of the cognitive control network and corrects the negative attention bias of patients with childhood trauma-associated major depressive disorder.
Research perspectives
Elucidating the neurobiological features of patients with childhood trauma-associated major depressive disorder is important for the development of methods that can assist in early diagnosis and intervention.
Footnotes
Institutional review board statement: All patients provided written informed consent, and the study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 22, 2022
First decision: June 11, 2022
Article in press: July 27, 2022
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ignácio ZM, Brazil; Khosravi M, Iran S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
Contributor Information
Ren-Qiang Yu, Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Huan Tan, Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Er-Dong Wang, College of Art, Soochow University, Suzhou 215006, Jiangsu Province, China.
Jie Huang, Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing 40016, China.
Pei-Jia Wang, Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing 40016, China.
Xiao-Mei Li, Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing 40016, China.
Han-Han Zheng, Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing 40016, China.
Fa-Jin Lv, Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Hua Hu, Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing 40016, China. huhuateam@126.com.
References
- 1.Aghamohammadi-Sereshki A, Coupland NJ, Silverstone PH, Huang Y, Hegadoren KM, Carter R, Seres P, Malykhin NV. Effects of childhood adversity on the volumes of the amygdala subnuclei and hippocampal subfields in individuals with major depressive disorder. J Psychiatry Neurosci. 2021;46:E186–E195. doi: 10.1503/jpn.200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu R, Tan H, Peng G, Du L, Wang P, Zhang Z, Lyu F. Anomalous functional connectivity within the default-mode network in treatment-naive patients possessing first-episode major depressive disorder. Medicine (Baltimore) 2021;100:e26281. doi: 10.1097/MD.0000000000026281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson J, Klumparendt A, Doebler P, Ehring T. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br J Psychiatry. 2017;210:96–104. doi: 10.1192/bjp.bp.115.180752. [DOI] [PubMed] [Google Scholar]
- 4.Alnefeesi Y, Chen-Li D, Krane E, Jawad MY, Rodrigues NB, Ceban F, Di Vincenzo JD, Meshkat S, Ho RCM, Gill H, Teopiz KM, Cao B, Lee Y, McIntyre RS, Rosenblat JD. Real-world effectiveness of ketamine in treatment-resistant depression: A systematic review & meta-analysis. J Psychiatr Res. 2022;151:693–709. doi: 10.1016/j.jpsychires.2022.04.037. [DOI] [PubMed] [Google Scholar]
- 5.Nikkheslat N, McLaughlin AP, Hastings C, Zajkowska Z, Nettis MA, Mariani N, Enache D, Lombardo G, Pointon L, Cowen PJ, Cavanagh J, Harrison NA, Bullmore ET NIMA Consortium, Pariante CM, Mondelli V. Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav Immun. 2020;87:229–237. doi: 10.1016/j.bbi.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie P, Wu K, Zheng Y, Guo Y, Yang Y, He J, Ding Y, Peng H. Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. J Affect Disord. 2018;228:41–48. doi: 10.1016/j.jad.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 8.Fasina OB, Wang J, Mo J, Osada H, Ohno H, Pan W, Xiang L, Qi J. Gastrodin From Gastrodia elata Enhances Cognitive Function and Neuroprotection of AD Mice via the Regulation of Gut Microbiota Composition and Inhibition of Neuron Inflammation. Front Pharmacol. 2022;13:814271. doi: 10.3389/fphar.2022.814271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Feng L, Hu C, Pao C, Xiao L, Wang G. Associations Among Depressive Symptoms, Childhood Abuse, Neuroticism, Social Support, and Coping Style in the Population Covering General Adults, Depressed Patients, Bipolar Disorder Patients, and High Risk Population for Depression. Front Psychol. 2019;10:1321. doi: 10.3389/fpsyg.2019.01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duque A, Vázquez C. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. J Behav Ther Exp Psychiatry. 2015;46:107–114. doi: 10.1016/j.jbtep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Günther V, Dannlowski U, Kersting A, Suslow T. Associations between childhood maltreatment and emotion processing biases in major depression: results from a dot-probe task. BMC Psychiatry. 2015;15:123. doi: 10.1186/s12888-015-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, Nichols TE, Robinson EC, Salimi-Khorshidi G, Woolrich MW, Barch DM, Uğurbil K, Van Essen DC. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 15.Jiao K, Xu H, Teng C, Song X, Xiao C, Fox PT, Zhang N, Wang C, Zhong Y. Connectivity patterns of cognitive control network in first episode medication-naive depression and remitted depression. Behav Brain Res. 2020;379:112381. doi: 10.1016/j.bbr.2019.112381. [DOI] [PubMed] [Google Scholar]
- 16.Gudayol-Ferré E, Peró-Cebollero M, González-Garrido AA, Guàrdia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front Hum Neurosci. 2015;9:582. doi: 10.3389/fnhum.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stange JP, Bessette KL, Jenkins LM, Peters AT, Feldhaus C, Crane NA, Ajilore O, Jacobs RH, Watkins ER, Langenecker SA. Attenuated intrinsic connectivity within cognitive control network among individuals with remitted depression: Temporal stability and association with negative cognitive styles. Hum Brain Mapp. 2017;38:2939–2954. doi: 10.1002/hbm.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Linn KA, Shinohara RT, Oathes DJ, Cook PA, Duprat R, Moore TM, Oquendo MA, Phillips ML, McInnis M, Fava M, Trivedi MH, McGrath P, Parsey R, Weissman MM, Sheline YI. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc Natl Acad Sci U S A. 2019;116:8582–8590. doi: 10.1073/pnas.1900801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serbanescu I, Walter H, Schnell K, Kessler H, Weber B, Drost S, Groß M, Neudeck P, Klein JP, Assmann N, Zobel I, Backenstrass M, Hautzinger M, Meister R, Härter M, Schramm E, Schoepf D. Combining baseline characteristics to disentangle response differences to disorder-specific versus supportive psychotherapy in patients with persistent depressive disorder. Behav Res Ther. 2020;124:103512. doi: 10.1016/j.brat.2019.103512. [DOI] [PubMed] [Google Scholar]
- 22.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shou H, Yang Z, Satterthwaite TD, Cook PA, Bruce SE, Shinohara RT, Rosenberg B, Sheline YI. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464–470. doi: 10.1016/j.nicl.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Bhui K. Culture and complex interventions: lessons for evidence, policy and practice. Br J Psychiatry. 2010;197:172–173. doi: 10.1192/bjp.bp.110.082719. [DOI] [PubMed] [Google Scholar]
- 27.Erbay LG, Reyhani İ, Ünal S, Özcan C, Özgöçer T, Uçar C, Yıldız S. Does Psychodrama Affect Perceived Stress, Anxiety-Depression Scores and Saliva Cortisol in Patients with Depression? Psychiatry Investig. 2018;15:970–975. doi: 10.30773/pi.2018.08.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang ZQ, Huang HM, Benko A, Wu Y. The Spread and Development of Psychodrama in Mainland China. Front Psychol. 2018;9:1368. doi: 10.3389/fpsyg.2018.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y, Yang Y, Yang X, Zhang T, Qiu X, He X, Wang W, Wang L, Sui H. The Mediating Role of Coping Style in the Relationship between Psychological Capital and Burnout among Chinese Nurses. PLoS One. 2015;10:e0122128. doi: 10.1371/journal.pone.0122128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao Z, Chen L, Chen M, Guan X, Wang L, Jiao Y, Yang J, Tang Q, Yang X, Qiu X, Han D, Ma J, Yang Y, Zhai X. Prevalence and factors associated with occupational burnout among HIV/AIDS healthcare workers in China: a cross-sectional study. BMC Public Health. 2016;16:335. doi: 10.1186/s12889-016-2890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du L, Wang J, Meng B, Yong N, Yang X, Huang Q, Zhang Y, Yang L, Qu Y, Chen Z, Li Y, Lv F, Hu H. Early life stress affects limited regional brain activity in depression. Sci Rep. 2016;6:25338. doi: 10.1038/srep25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386. doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 36.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 37.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 38.Stanisławski K. The Coping Circumplex Model: An Integrative Model of the Structure of Coping With Stress. Front Psychol. 2019;10:694. doi: 10.3389/fpsyg.2019.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzik M, Umarji R, Sexton MB, Davis MT. Family Social Support Modifies the Relationships Between Childhood Maltreatment Severity, Economic Adversity and Postpartum Depressive Symptoms. Matern Child Health J. 2017;21:1018–1025. doi: 10.1007/s10995-016-2197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagdon S, Ross J, Robinson M, Contractor AA, Charak R, Armour C. Assessing the Mediating Role of Social Support in Childhood Maltreatment and Psychopathology Among College Students in Northern Ireland. J Interpers Violence. 2021;36:NP2112–2136NP. doi: 10.1177/0886260518755489. [DOI] [PubMed] [Google Scholar]
- 41.Onoda K, Yamaguchi S. Dissociative contributions of the anterior cingulate cortex to apathy and depression: Topological evidence from resting-state functional MRI. Neuropsychologia. 2015;77:10–18. doi: 10.1016/j.neuropsychologia.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. Neuroscience. 2010;167:97–103. doi: 10.1016/j.neuroscience.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foland-Ross LC, Hamilton JP, Joormann J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychol Sci. 2013;24:334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brzezicka A. Integrative deficits in depression and in negative mood states as a result of fronto-parietal network dysfunctions. Acta Neurobiol Exp (Wars) 2013;73:313–325. doi: 10.55782/ane-2013-1939. [DOI] [PubMed] [Google Scholar]
- 46.Yi K, Kim C. Dissociable neural correlates of spatial attention and response inhibition in spatially driven interference. Neurosci Lett. 2020;731:135111. doi: 10.1016/j.neulet.2020.135111. [DOI] [PubMed] [Google Scholar]
- 47.Head J, Tenan MS, Tweedell AJ, Wilson KM, Helton WS. Response Complexity Reduces Errors on a Response Inhibition Task. Hum Factors. 2020;62:787–799. doi: 10.1177/0018720819852801. [DOI] [PubMed] [Google Scholar]
- 48.Depue BE, Orr JM, Smolker HR, Naaz F, Banich MT. The Organization of Right Prefrontal Networks Reveals Common Mechanisms of Inhibitory Regulation Across Cognitive, Emotional, and Motor Processes. Cereb Cortex. 2016;26:1634–1646. doi: 10.1093/cercor/bhu324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swick D, Chatham CH. Ten years of inhibition revisited. Front Hum Neurosci. 2014;8:329. doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackiewicz Seghete KL, Kaiser RH, DePrince AP, Banich MT. General and emotion-specific alterations to cognitive control in women with a history of childhood abuse. Neuroimage Clin. 2017;16:151–164. doi: 10.1016/j.nicl.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, Satchell EK, Shou H, Sheline YI. Cognitive Behavioral Therapy Is Associated With Enhanced Cognitive Control Network Activity in Major Depression and Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:311–319. doi: 10.1016/j.bpsc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szczepanski SM, Pinsk MA, Douglas MM, Kastner S, Saalmann YB. Functional and structural architecture of the human dorsal frontoparietal attention network. Proc Natl Acad Sci U S A. 2013;110:15806–15811. doi: 10.1073/pnas.1313903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, Huang X, Kemp GJ, Mechelli A, Gong Q. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 54.Song Z, Zhang M, Huang P. Aberrant emotion networks in early major depressive disorder patients: an eigenvector centrality mapping study. Transl Psychiatry. 2016;6:e819. doi: 10.1038/tp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li BJ, Friston K, Mody M, Wang HN, Lu HB, Hu DW. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci Ther. 2018;24:1004–1019. doi: 10.1111/cns.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 58.Weingarten CP, Strauman TJ. Neuroimaging for psychotherapy research: current trends. Psychother Res. 2015;25:185–213. doi: 10.1080/10503307.2014.883088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 60.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]