Abstract
Hepatocellular carcinoma (HCC) is the sixth most common cancer. The main risk factors associated with HCC development include hepatitis B virus, hepatitis C virus, alcohol consumption, aflatoxin B1, and nonalcoholic fatty liver disease. However, hepatocarcinogenesis is a complex multistep process. Various factors lead to hepatocyte malignant transformation and HCC development. Diagnosis and surveillance of HCC can be made with the use of liver ultrasound (US) every 6 mo. However, the sensitivity of this imaging method to detect HCC in a cirrhotic liver is limited, due to the abnormal liver parenchyma. Computed tomography (CT) and magnetic resonance imaging (MRI) are considered to be most useful tools for at-risk patients or patients with inadequate US. Liver biopsy is still used for diagnosis and prognosis of HCC in specific nodules that cannot be definitely characterized as HCC by imaging. Recently the American College of Radiology designed the Liver Imaging Reporting and Data System (LI-RADS), which is a comprehensive system for standardized interpretation of CT and MRI liver examinations that was first proposed in 2011. In 2018, it was integrated into the American Association for the Study of Liver Diseases guidance statement for HCC. LI-RADS is designed to ensure high sensitivity, precise categorization, and high positive predictive value for the diagnosis of HCC and is applied to “high-risk populations” according to specific criteria. Most importantly LI-RADS criteria achieved international collaboration and consensus among liver experts around the world on the best practices for caring for patients with or at risk for HCC.
Keywords: Hepatocellular carcinoma, Hepatocarcinogenesis, Computed tomography, Magnetic resonance imaging, Liver Imaging Reporting and Data System
Core Tip: Worldwide, hepatocellular carcinoma (HCC) is the sixth most common malignancy and fourth cause of cancer-related death. Progress on the diagnosis and treatment possibilities of HCC has been made over the past decades. We herein review the available knowledge on the carcinogenesis, surveillance of HCC, and relevant data with the Liver Imaging Reporting and Data System (LI-RADS) algorithm that was recently integrated into the American Association for the Study of Liver Diseases clinical practice guidelines. LI-RADS aims to avoid false-positive examinations and ensure high sensitivity and positive predictive value in high-risk populations with simultaneous achievement of probabilistic categorization of the tumor.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death worldwide[1]. Up to 90% of HCC cases arise on the background of established liver cirrhosis or advanced fibrosis, which has been developed due to a known etiology[2]. The most predominant risk factors for HCC worldwide are hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol consumption, and nonalcoholic fatty liver disease[3].
The prognosis of HCC is significantly influenced by the disease stage at the time of diagnosis. Patients with HCC detection at an early stage can be treated with potentially curative therapies[4]. Imaging plays an important role in the early diagnosis of HCC, both in the context of surveillance strategies of at-risk populations and in the phase of confirmatory studies for a new liver nodule. Most importantly, imaging studies for the diagnosis of HCC, usually performed with computed tomography (CT) or magnetic resonance imaging (MRI), have approximately 100% specificity. Therefore, HCC is among the few malignancies for which management can be established on the basis of noninvasive imaging, without histologic confirmation[5].
CT and MRI are extensively used in clinical practice for the diagnosis of HCC. However, their diagnostic accuracy is decreased when strong criteria are not applied. In addition, the variable algorithms and recommendations from different scientific societies have created some degree of confusion in the interpretation of the radiological findings. To standardize the performance of liver imaging in patients with risk factors for developing HCC, the American College of Radiology (ACR) introduced in 2011 the first version of the Liver Imaging Reporting and Data System (LI-RADS)[6]. The LI-RADS criteria were created to standardize the terminology used for HCC imaging, allow probabilistic categorization, and provide personalized medical care[7]. It has been updated in 2013, 2014, 2017, and 2018 and is considered a dynamic document, which can be expanded and refined in response to new knowledge and user feedback[8].
In this pictorial review, we analyze the rational for the radiological diagnosis of HCC, present the LI-RADS criteria and associate them with characteristic images per category in order to enhance the familiarization of clinicians with the use of this system in clinical practice.
HEPATOCARCINOGENESIS AND IMAGING CORRELATES
Hepatocarcinogenesis is a complex multistep process characterized by alterations at several levels (molecular, cellular, and histologic levels). Chronic liver inflammation can lead to continuous cycles of persistent cell injury, death, and regeneration, causing subsequent genetic damage and epigenetic alterations of hepatocytes[9]. Various factors lead to hepatocyte malignant transformation and HCC development, such as genetic susceptibility, mutual interplays between viral and non-viral risk factors, cellular microenvironment, immune cells, and the severity of the underlying chronic liver disease. An altered microenvironment plays a significant role in all stages of neoplastic growth, from the early mutation stage, through to invasion, and eventually, to metastatic stage[10,11].
The molecular pathogenesis of HCC varies according to the distinct etiologies[10]. HBV and HCV are the two main viruses, which can be implicated in the development of HCC[12]. HBV genome inte-gration can target cancer-relevant genes, and protein x can alter the expression of growth-control genes and inactivate the tumor suppressor p53 leading to HCC development[12,13]. On the other hand, HCV infection is not characterized by oncogenic effects; however mutations can result from oxidative stress caused by chronic inflammation[12]. Alcohol-induced hepatocarcinogenesis is another risk factor for developing HCC. Chronic alcohol intake is associated with proinflammatory cytokine production and oxidative stress, which can lead to the development of fibrosis, cirrhosis, and ultimately HCC[14]. Aflatoxin-B1 also plays a role in hepatocarcinogenesis. This fungal toxin is associated with a specific p53 mutation and activation of oncogenes such as the Human Harvey-ras proto-oncogene[15,16].
Irrespective of etiology, cirrhosis is the main underlying cause for most HCC cases. During cirrhosis, regenerating nodules are developed, which provide an appropriate microenvironment for the development of normal hepatocytes to dysplastic hepatocytes to neoplastic lesions and culminating in HCC through the subsequent agglomeration of multiple DNA alterations[17]. Histologically, abnormal precursor hepatic lesions including cirrhotic nodules, low-grade dysplastic nodules, and high-grade dysplastic nodules dedifferentiate and progressively develop to form early and eventually advanced HCCs[5]. This continuum biologic procedure may happen simultaneously at various rates in different parts of the liver.
During hepatocarcinogenesis, the vascular supply of the nodules modifies together with malignant progression and the number of intranodular portal tracts diminishes progressively, while the number of unpaired arteries increases. Eventually, HCC is supplied mainly by the abnormal hepatic artery system via neoangiogenic unpaired arteries[18]. It is important for both clinicians and radiologists to understand these hemodynamic changes during hepatocarcinogenesis as these are correlated with the enhancing pattern precursor hepatic lesions and finally of HCCs on imaging studies. Arterial phase hyperenhancement and portal venous and/or delayed phases wash-out relative to the background liver on contrast enhanced multiphasic CT and MRI are the most important imaging features of HCC and are correlated with the blood supply of the nodules with malignant transformation.
Features during hepatocarcinogenesis also include tumor capsule and fibrous septa formation and fat accumulation within hepatocytes during the early phases (dysplasia and early HCC), while fat usually regresses with the progression to overt HCC.
Iron may also accumulate within hepatocytes during the dysplastic phases of hepatocarcinogenesis and usually also regresses with progression to HCC. Finally organic anionic transporting polypeptides expression declines during hepatocarcinogenesis (organic anion transporting polypeptide 8 expression levels decrease during hepatocarcinogenesis prior to reduction in portal tracts and increases in unpaired arteries). It is worth noting that abnormal venous drainage of HCCs occurs during hepatocarcinogenesis from hepatic veins to sinusoids and then to portal veins, which may explain why HCC has a tendency to spread through the portal venous system rather than hepatic veins[5].
HCC DIAGNOSIS
Early diagnosis of HCC is enhanced by the use of surveillance strategies in certain populations that are at risk of developing HCC. Typically these patients include cirrhotic patients with Child-Pugh stage A and B as well as cirrhotic patients with Child-Pugh stage C awaiting liver transplantation[19,20]. Regarding patients with chronic hepatitis B, the use of risk scores (especially PAGE-B) is recommended to prioritize patients for surveillance[21]. Finally, it is recommended that patients with severe fibrosis enter surveillance programs on an individual basis after careful assessment of their risk[19,20]. Regarding tools that should be used for surveillance, most international societies recommend only the use of abdominal ultrasound (US) every 6 mo, as the use of alpha-fetoprotein is still controversial[19,20]. CT or MRI may be utilized for surveillance in selected patients with inadequate US visualization or at high risk for inadequate US[20].
Once a new nodule is discovered in the liver of a cirrhotic patient, further imaging work-up with either CT or MRI is recommended[19,20]. These modalities are also the gold standard for HCC diagnosis outside the use of surveillance programs. The peculiar vascular derangement that occurs during hepatic carcinogenesis, as previously described, correlate with imaging phases and have led to the implementation of accurate non-invasive criteria for HCC diagnosis. Integration of LI-RADS version 2018 into the American Association for the Study of Liver Diseases (AASLD) HCC clinical practice guidelines, was a major step. As a result, the AASLD management suggestions are directly associated with the LI-RADS categories[20].
The routine use of liver biopsy is not suggested for the diagnosis of HCC[20]. Biopsy shortcomings include risks associated with the procedure. Another important issue is that small lesions < 2 cm are often difficult to target in a cirrhotic liver leading to high false negatives. However, the role of tumor tissue analysis in HCC is essential in cases of a cirrhotic patient that lacks typical imaging characteristics or in cases where molecular profiling is needed to determine systemic therapy options[22]. It should also be noted that the diagnosis should be confirmed with pathology in non-cirrhotic patients[19]. In all of these cases, the pathological diagnosis of HCC should be based on International Consensus recommendations using appropriate histopathological analyses and immunohistochemistry[23,24].
DESCRIPTION OF LIRADS
LI-RADS has provided a standardized guidance, which contributes to a universal language for liver imaging findings. All LI-RADS diagnostic tables can be found for free at the ACR website (https://www.acr.org/ClinicalResources/Reporting-and-Data-Systems/LI-RADS). By philosophy, LI-RADS diagnostic algorithm is developed to ensure high specificity and positive predictive value (PPV) for HCC diagnosis to avoid false-positive examinations. To obtain a high PPV, LI-RADS should be used only to populations at high risk for developing HCC[25]. This “high-risk population” includes adult patients with liver cirrhosis (except for vascular causes of cirrhosis), chronic HBV infection with or without cirrhosis, and patients diagnosed with current or prior HCC (Figures 1 and 2)[26].
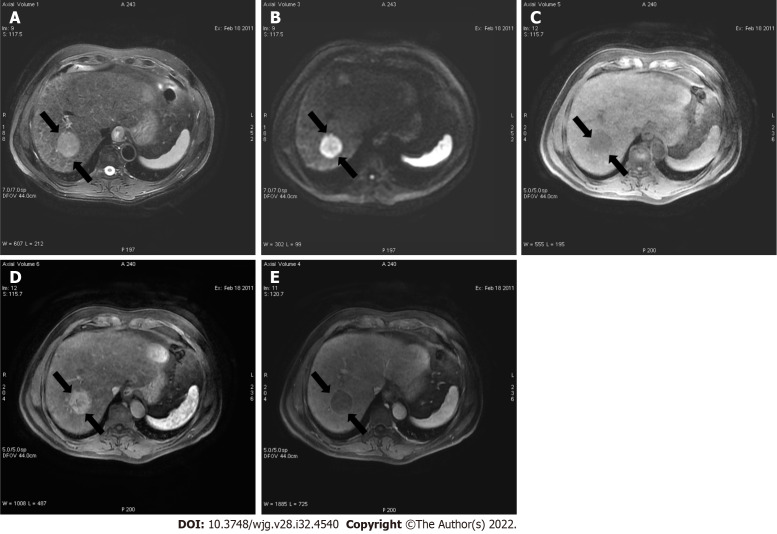
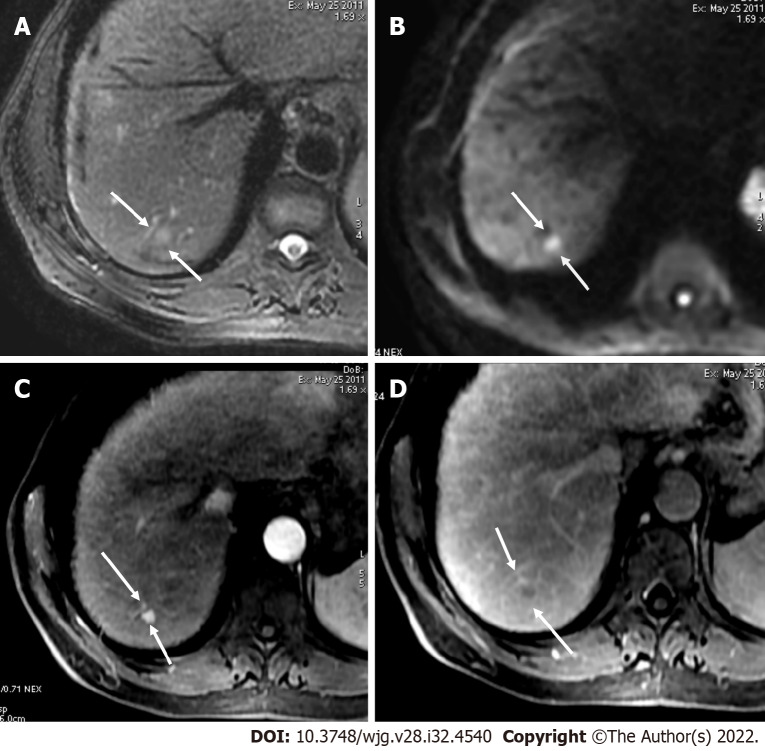
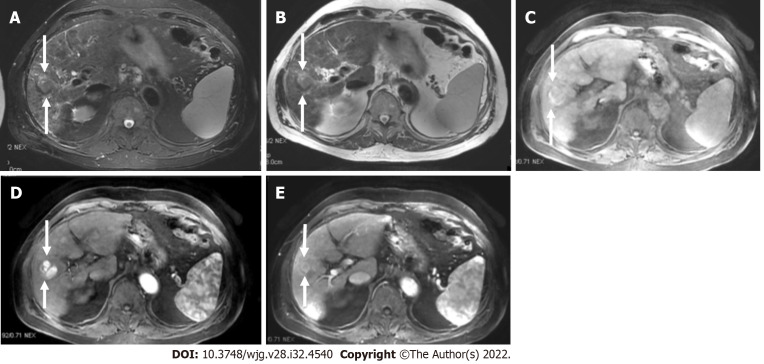
Figure 1.
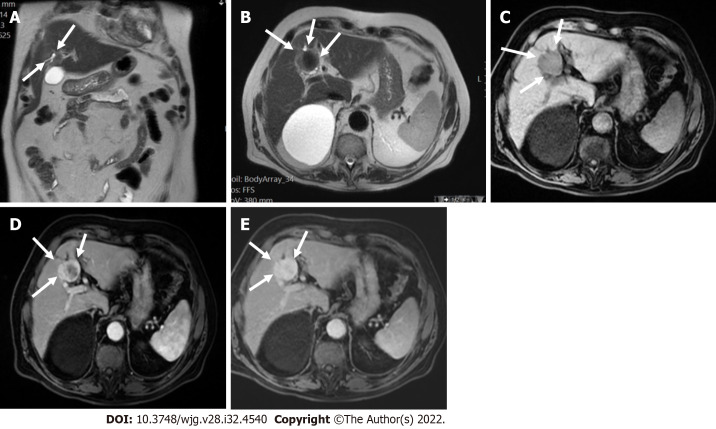
A 52-year-old male with hepatocellular carcinoma and chronic hepatitis B. A: A segment VII mass showed a high signal on the short tau inversion recovery image; B: High on diffusion-weighted imaging; C: Low on unenhanced volumetric interpolated breath-hold examination image; D: Non-rim arterial phase hyperenhancement on arterial phase; E: Washout and enhancing capsule on portal phase. Lesion showed typical imaging findings of hepatocellular carcinoma categorized as LR-5 according to Liver Imaging Reporting and Data System criteria.
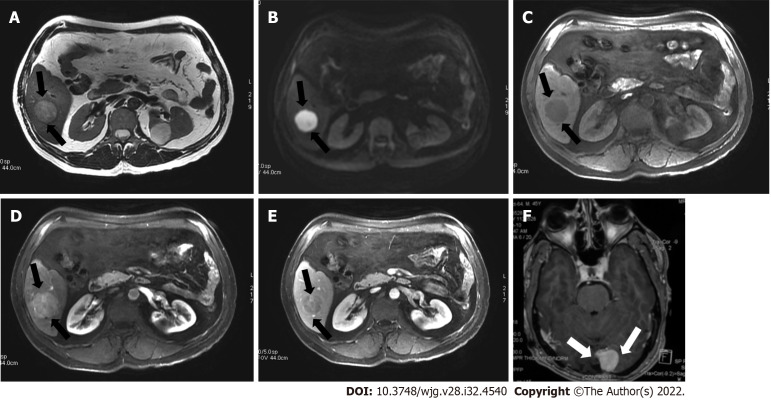
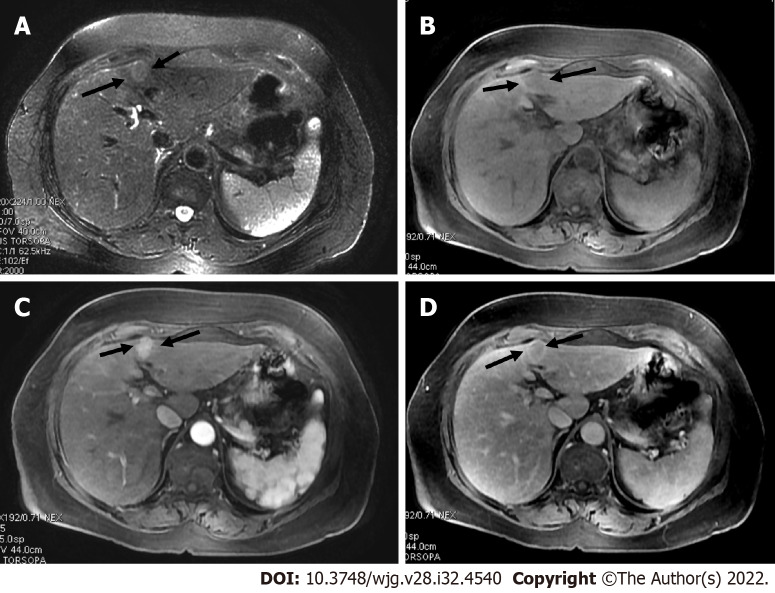
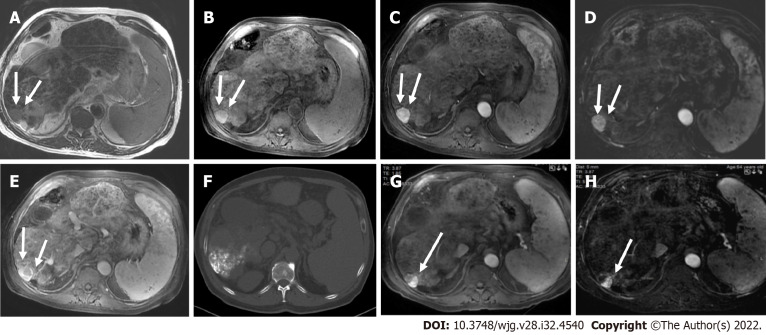
Figure 2.
A 45-year-old man with a segment VI lesion. A: The lesion demonstrated a high signal on T2 weighted image; B: High on diffusion-weighted imaging; C: Low on volumetric interpolated breath-hold examination; D: Non-rim arterial-phase hyper-enhancement on arterial-phase image; E: Washout on portal phase. If we applied Liver Imaging Reporting and Data System (LI-RADS) criteria, the erroneous diagnosis of hepatocellular carcinoma could be made. However, the patient did not meet the at-risk criteria to apply LI-RADS. Histologic examination of the lesion demonstrated metastatic hemangiopericytoma; F: Magnetic resonance imaging of the brain, axial post-contrast T1-weighted image showed an extra-axial lobulated, occipital enhancing mass corresponding to recurrence of hemangiopericytoma 12 mo after total surgical excision.
Determining patients who meet the at-risk criteria is not always easy. Cirrhotic patients are usually diagnosed based only on clinical signs, symptoms, and diagnostic markers with or without histological confirmation.
Measurement of liver stiffness using FibroScan could be a useful predictor of HCC appearance in patients with chronic hepatitis B and/or C[26]. If the patient is considered with a high pre-test probability of HCC, LI-RADS may be used with a recommendation that the referring physician confirms the patient’s risk status (Figure 3).
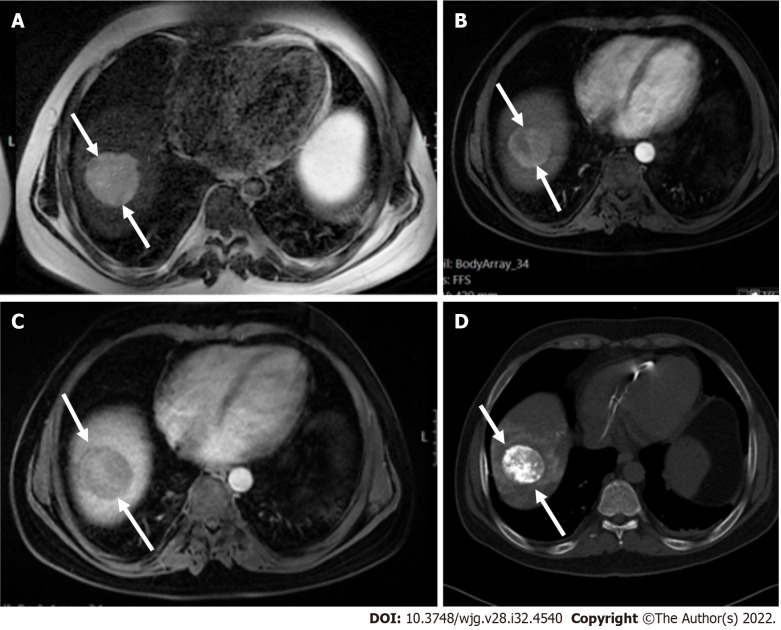
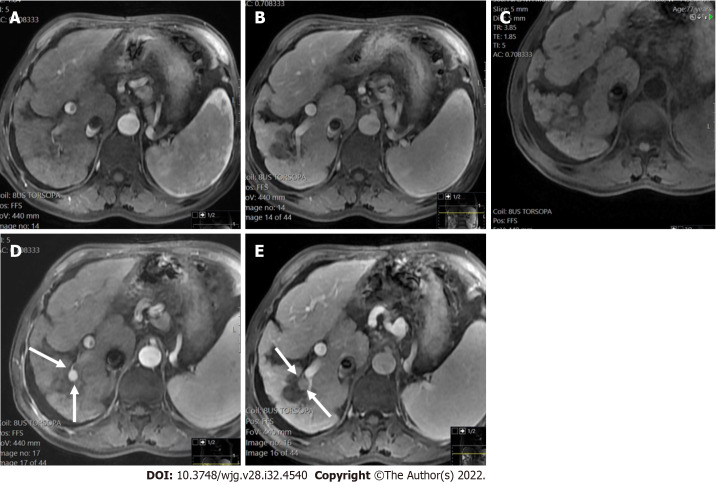
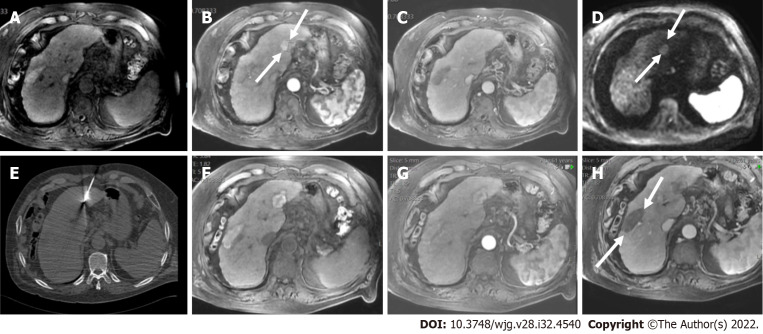
Figure 3.
A 47 mm liver mass was incidentally found, in a 40-year-old man with beta thalassemia, during cardiac magnetic resonance investigation for myocardial iron overload. A-C: On abdominal magnetic resonance imaging, the lesion demonstrated high signal intensity on T2 weighted image (A), arterial phase hyperenhancement on post-contrast arterial phase (B), and washout on portal phase (C). Although there are no current evidence-based guidelines for patients with beta thalassemia without cirrhosis, the hepatologist confirmed the patient’s risk status and proposed that Liver Imaging Reporting and Data System (LI-RADS) should be applied. According to the LI-RADS criteria, the lesion was categorized as LR-5 (definite hepatocellular carcinoma); D: Unenhanced computed tomography scan after transarterial chemoembolization showed accumulation of iodized oil (arrow) within the tumor.
Although HCC may develop in patients with non-alcoholic fatty liver disease/steatohepatitis without cirrhosis, sufficient data are lacking to support that LI-RADS would provide an adequately high PPV for HCC in this sub-cirrhotic population group[27]. LI-RADS does not apply to patients < 18-years-old and patients with liver cirrhosis due to vascular diseases (e.g., cardiac hepatopathy, Budd-Chiari syndrome), because the above-mentioned diseases are characterized by hypervascular benign liver lesions in liver imaging, which raise the probability of false positive results and reduce the PPV for HCC diagnosis[17].
LI-RADS CATEGORIES
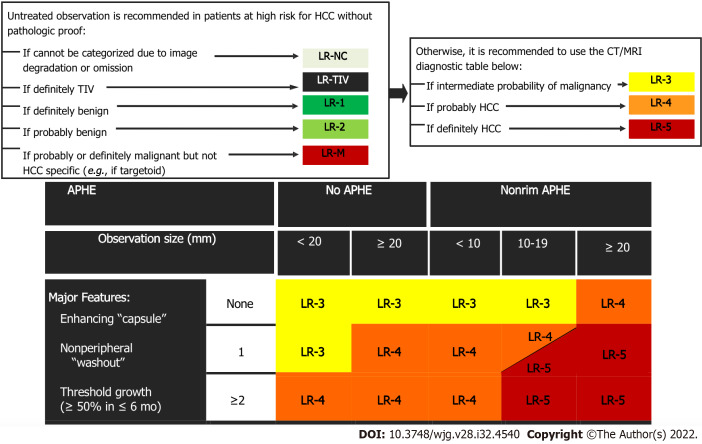
LI-RADS CT/MRI Diagnostic Algorithm version 2018 (v2018) is characterized by eight diagnostic categories, which are used for individual liver lesions with increasing probability of HCC and malignancy with higher categories. The several LI-RADS categories do not have an exact association to the histologic categories but instead reflect the probability of individual liver lesions being benign, HCC, or non-HCC malignancy. The LI-RADS CT/MRI algorithm is used in a stepwise manner starting with LR-not categorizable (NC). It is followed by LR-tumor in vein (TIV), LR-1, LR-2, and LR-M. Once the above categories are excluded, the CT/MRI diagnostic table is used to determine an observation LR-3, LR-4, or LR-5 category (Figure 4). LI-RADS criteria uses the term observation to generically refer to an area with an imaging appearance that is distinctive from the rest of the liver. Such an area may be a true lesion or a pseudolesion.
Figure 4.
Diagnostic algorithm of liver lesions in computed tomography/magnetic resonance imaging (Liver Imaging Reporting and Data System v2018 CORE). Observations in the cell LR-4 and LR-5 are categorized based on one additional major feature: LR-4 if enhancing “capsule” and LR-5 if nonperipheral “washout” or threshold growth. APHE: Arterial phase hyperenhancement; CT: Computed tomography; HCC: Hepatocellular carcinoma; MRI: Magnetic resonance imaging; TIV: Tumor in vein.
LR-NC
CT and MRI need to meet minimal technical requirements to yield the desired diagnostic accuracy. For CT, multidetector CT (≥ 8 detectors) is requisite, whereas for MRI, 1.5 or 3 T magnets are required according to LI-RADS guidance. Administration of intravenous contrast and acquisition of a multiphase liver protocol including images before contrast, late hepatic arterial phase, portal venous phase, and delayed phase images is required to allow for diagnosis of HCC[20]. The most important characteristic is the acquisition of a technically adequate and well-timed arterial phase.
LI-RADS guidance strongly suggests that imaging should be performed during the late arterial phase, i.e. when the hepatic artery and portal vein are both enhanced, as this correlates with the time when arterialized tumor tissue is maximally enhanced relative to background liver (Figure 5). If an observation cannot be categorized due to image degradation or omission, then radiologists may choose to assign an LR-NC category (Figure 6). LI-RADS currently does not provide guidance on scoring the severity of artifact. In these cases, radiologists should use their judgment in suggesting either repeat or alternative imaging within 3 mo to better characterize the liver lesion.
Figure 5.
Hepatocellular carcinoma in a 60-year-old man with hepatitis C. A: Computed tomography scan, late arterial phase were the hepatic artery and portal vein are both enhanced, showed a segment VIII lesion measuring 27 mm with non-rim arterial phase hyper-enhancement; B: In the portal phase, washout was seen.
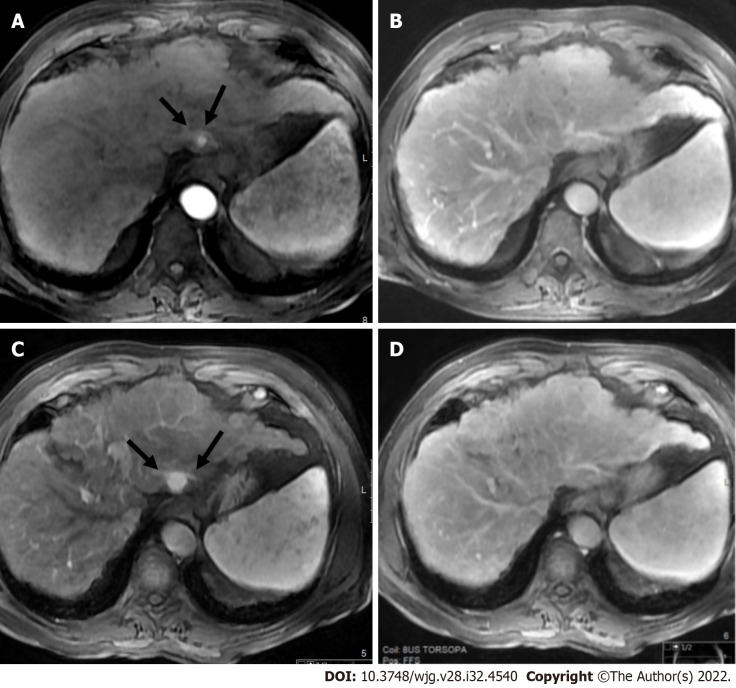
Figure 6.
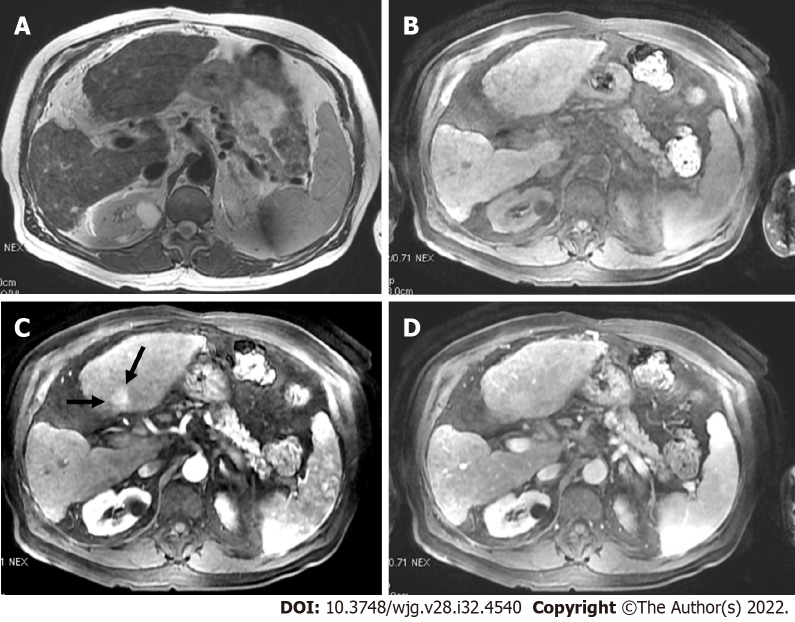
LR-non-categorizable category. Magnetic resonance imaging in a 74-year-old man with hepatitis C. A: Axial short tau inversion recovery shows an observation in segment VIII with high signal intensity (arrow) within a nodular liver consistent with cirrhosis; On arterial post-contrast image (Β) and on portal phase (C) (arrows) the observation shows low signal intensity. However the arterial phase is early to characterize a possible arterialized lesion (LR-NC category).
LR-TIV
The presence of TIV should be ruled out. LR-TIV category is defined by definite enhancing tissue with a vein, indicating the presence of TIV. Whenever is possible the most likely etiology of TIV should be assigned (due to HCC or non HCC malignancy) (Figures 7 and 8).
Figure 7.
LR-tumor in vein (definite tumor in vein) in a 70-year-old man with alcoholic liver cirrhosis. Magnetic resonance imaging arterial phase and portal venous phase demonstrated a large inhomogeneous enhancing mass with definite enhancing soft tissue extending to the right portal vein (arrows). A: Magnetic resonance imaging arterial phase; B: Portal venous phase.
Figure 8.
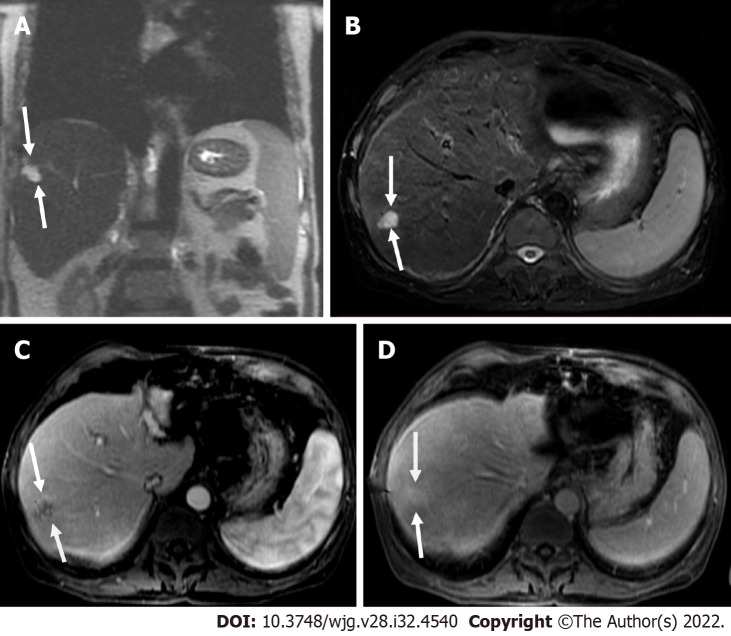
Two-dimensional transthoracic echocardiogram revealed a mobile right atrial mass protruding from the inferior vena cava, in a patient with chronic hepatitis B and cirrhosis. A-C: Axial T1 weighted image (T1WI) (A), arterial post-contrast (B) and portal phase (C) showed direct right atrial extension of hepatic segment VIII-IVA tumor through the inferior vena cava, categorized as LR-tumor in vein (arrows); D and E: Coronal T2 weighted image (D) and coronal post-contrast T1WI (E) showed better craniocaudal tumor extension (arrows).
LR-1 definitely benign
LR-1 is applied to observations that are benign. Management should be returned to routine surveillance at 6 mo intervals.
LR-2 probably benign
LR-2 is applied to observations that are probably benign. Management should be returned to routine surveillance at 6 mo intervals (Figure 9).
Figure 9.
Coronal T2 weighted image and axial short tau inversion recovery image. A and B: Coronal T2 weighted image (T2WI) (A) and axial short tau inversion recovery image (B) showed a 2 cm lesion in segment VII with high signal intensity (bright) within a nodular liver consistent with cirrhosis; C: Post-contrast image showed nodular peripheral enhancement; D: On delay image, the lesion showed progressive filling. In progressive cirrhosis, hemangiomas are likely to decrease in size, become more fibrotic, and are difficult to diagnose radiologically. However, the bright signal on T2WI and the enhancement pattern was pathognomonic for hemangioma of the liver consistent with LR-2.
LR-M
LR-M for HCC observations display imaging characteristics of malignancy but not features that are specific. The LR-M category was created to maintain the specificity of the LI-RADS guidance for diagnosis of HCC while not losing sensitivity for liver malignancies. LR-M features include a targetoid or non-targetoid mass with infiltrative appearance, marked diffusion restriction, necrosis, or other characteristics suggestive of non-HCC malignancy[28]. Targetoid mass is defined by rim enhancement in the arterial phase, peripheral washout, and progressive central enhancement on delayed phases. Although any liver malignancy can appear with characteristics of LR-M, the most common entities in patients at increased risk for developing HCC are intrahepatic cholangiocarcinoma (CCA), combined tumors (CCA-HCC), and atypical HCC. LR-M observations are commonly biopsied to propose the appropriate treatment (Figure 10).
Figure 10.
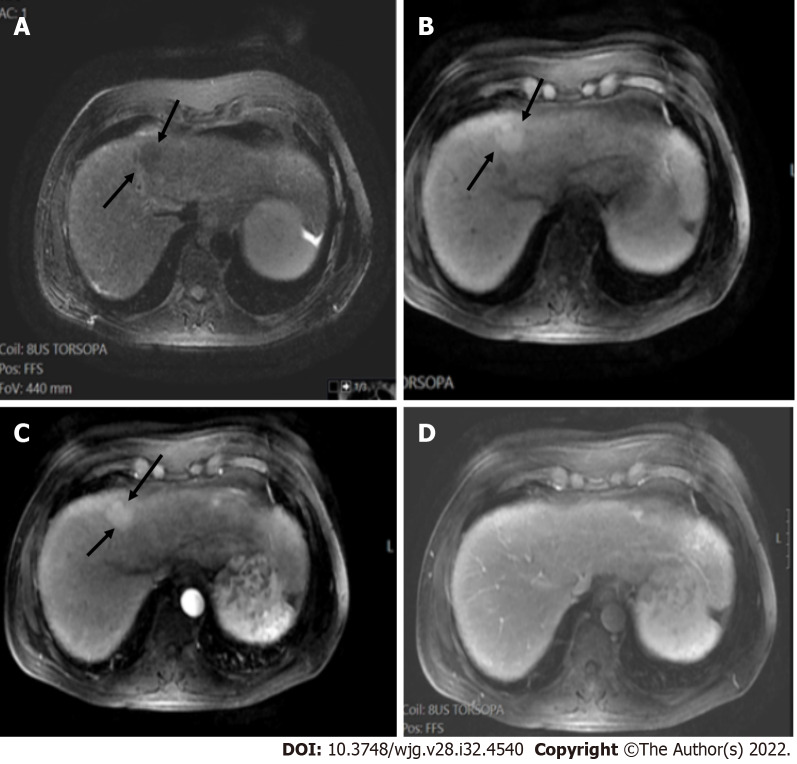
T1 weighted image and T2 weighted image. A and B: Coronal (A) and axial (B) T2 weighted images showed a low signal segment V mass in a patient with chronic hepatitis B (arrows); C: The mass showed low signal intensity (arrows) on T1 weighted image; D and E: In the arterial phase (D), there was peripheral enhancement (arrows) and progressive filling (arrows) in the portal phase (E) categorized as LR-M. Histologic examination confirmed the diagnosis of cholangiocarcinoma and not hepatocellular carcinoma.
LI-RADS diagnostic table
If the above categories (LR-TIV, LR-M, LR-1, and LR-2) are considered and excluded, the radiologist should use the LI-RADS diagnostic criteria based on combinations of major imaging features. The diagnostic table includes the predominant imaging characteristics of HCC in order to generate categories ranging from intermediate probability to definite HCC (LR-3, LR-4, LR-5). For CT and MRI, the LI-RADS predominant characteristics consist of the following: (1) Size; (2) The presence of non-rim arterial phase hyperenhancement (APHE), which is defined by the appearance of enhancement that is unequivocally significantly than that of the background liver[29]. On MRI the enhancing portion must have higher intensity and on CT higher attenuation than background liver in the arterial phase; (3) Non-peripheral washout, which is defined as a visually assessed temporal decrease in enhancement in whole or at least in part relative to composite liver tissue from earlier to later phase leading to hypoenhancement; and (4) Enhancing capsule, which is defined as a smooth, uniform, and sharply outlined area of enhancement around a liver lesion which is either thicker or more conspicuous than fibrotic tissue detected in chronic liver disease.
It is worth mentioning that no single major imaging characteristic in the algorithm is specific enough to categorize a liver lesion as LR-5, and that LI-RADS liver lesions less than 10 mm in diameter cannot be categorized as LR-5.
LR-3 intermediate probability of malignancy
Liver lesions categorized as LR-3 have intermediate probability of being considered HCC, and the recommendation is to undergo short follow-up imaging in 3 to 6 mo (Figures 11 and 12).
Figure 11.
T2 weighted image and volumetric interpolated breath-hold examination. A and B: Axial T2 weighted image (A) and volumetric interpolated breath-hold examination (VIBE) (B) showed a shrunken and nodular liver that was consistent with cirrhosis; C and D: On post-contrast VIBE arterial-phase image (C), a 1.6 cm lesion in segment IVb demonstrated hyperenhancement (arrows) without washout on portal venous phase image (D). According to Liver Imaging Reporting and Data System criteria, the lesion was categorized as LR-3.
Figure 12.
Axial short tau inversion recovery image. A and B: A IVa segment observation with low signal intensity (arrows) measuring > 20 mm (A) and high signal intensity on pre-contrast image (arrows on B); C and D: On arterial (C) and portal (D) phase there was no arterial phase hyperenhancement or washout and the lesion was categorized as LR-3.
LR-4 probably HCC
Observations categorized as LR-4 have a high probability of being considered definite HCC, and the suggestion is for an individualized multidisciplinary approach that may comprise biopsy, probable treatment, or follow-up (Figure 13).
Figure 13.
Axial short tau inversion recovery and diffusion-weighted imaging. A and B: Axial short tau inversion recovery (A) and diffusion-weighted imaging (DWI) (B) images showed a < 10 mm segment VII observation with high signal intensity; C and D: In the arterial phase (C), arterial phase hyperenhancement was seen with washout in the portal phase (D) categorized as LR-4. Restriction on DWI does not allow to upgrade from LR-4 to LR-5, because ancillary features lack sufficient specificity for hepatocellular carcinoma to allow for an LR-5 upgrade.
LR-5 definitely HCC
It is worth noting that for LR-5 categorization, non-rim APHE must be present. The criteria for LR-5 category has a PPV approaching 100% in patients at high risk for developing HCC. Liver lesions categorized as LR-5 are considered definite HCC and therefore staging and treatment is suggested without biopsy (Figures 14-17).
Figure 14.
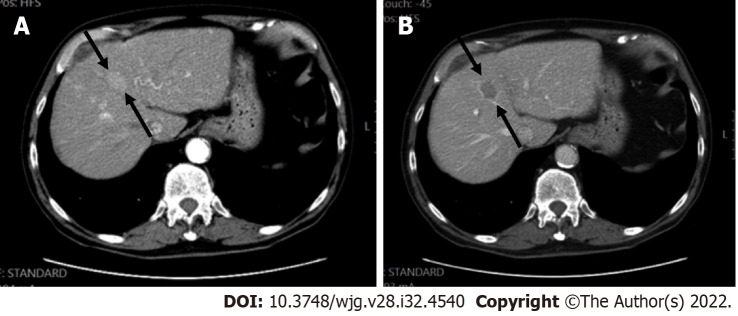
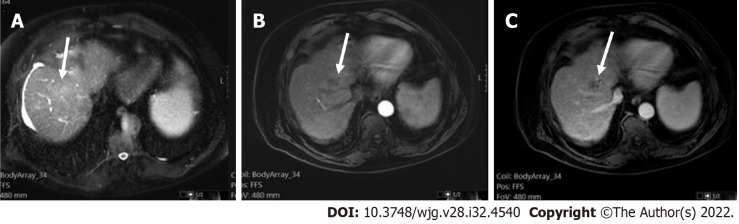
A 62-year-old female with chronic hepatitis C. A: On axial short tau inversion recovery image, a 21 mm IVb segment observation with relative high signal intensity is seen (arrows); B: On axial volumetric interpolated breath-hold examination image the observation shows low signal intensity (arrows); C and D: On post-contrast images, major features of hepatocellular carcinoma (HCC), arterial phase hyperenhancement (arrows) on arterial phase (C), washout and enhancing capsule (arrows) on portal (D) were seen, indicating LR-5 (definitely HCC).
Figure 17.
LR-5 observation. A and B: Axial post-contrast images arterial (A) and portal phase (B) showed an ablation defect in segment VI without residual areas of arterial enhancement or washout. This was classified as LR-treatment response non-viable; C-E: Later pre-contrast (C) arterial (D) and portal (E) phase showed a new nodule (arrows) measuring 12 mm with strong arterial enhancement and washout categorized as LR-5. If a new tumor arises within or adjacent to a treated lesion, this observation should be assigned a new non-treated LI-RADS category.
Figure 15.
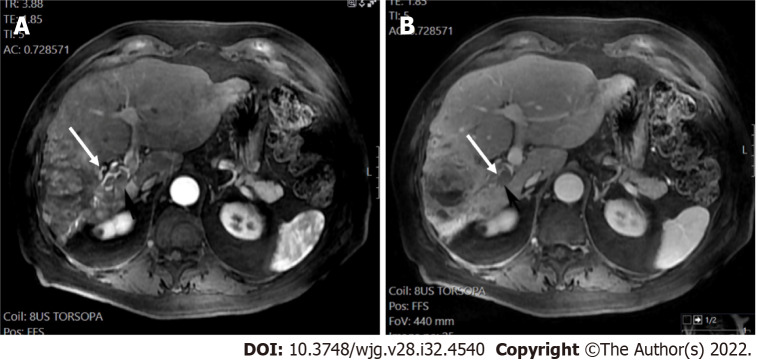
A 60-year-old male with hepatitis B and cirrhosis. A and B: A < 10 mm segment II observation demonstrated arterial phase hyperenhancement (APHE) (arrows) on arterial post-contrast images (A) without washout (B), categorized as LR-3 observation; C and D: 1-year later, the observation measured 18 mm, showed APHE (arrows) in the arterial phase (C) without definite washout in the portal phase (D). However due to threshold growth, it was categorized as LR-5.
Figure 16.
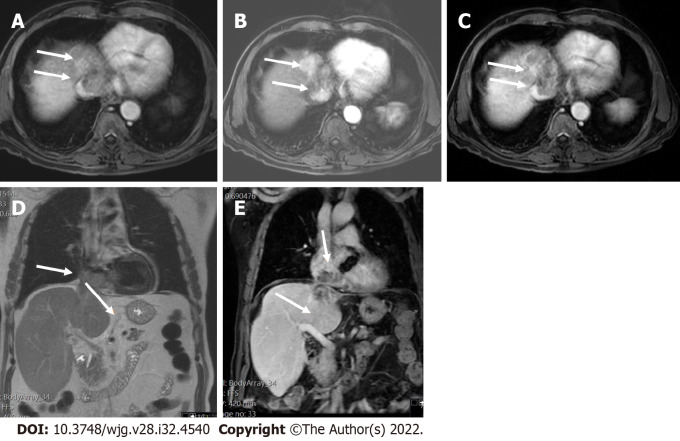
LR-5 observation. A and B: Axial short tau inversion recovery (A) and T2 weighted (B) showed a segment VI observation with relative high signal intensity (arrows); C: On pre-contrast volumetric interpolated breath-hold examination image, peripherally high signal was seen; D: In the arterial phase, multiple hyperenhanced nodules within nodule were seen (arrows); E: In the portal phase, segmental washout was seen (arrows) categorized as LR-5.
Ancillary features
Ancillary features (AFs) can be used at the radiologist’s discretion to adapt the category and to enhance the ability for reaching the final diagnosis. Although AFs are an important component of LI-RADS, their impact may be small (Figure 13). Various AFs likely can be taken out from LI-RADS criteria without compromising diagnostic performance[30].
Treatment response algorithm (LI-RADS)
LI-RADS treatment response guidance (LR-TR) offers imaging criteria for evaluation of the success rate of locoregional therapy (Figures 17, 18, and 19). However, the guidance is not created for evaluating the response to systemic therapy, which is often at the patient-level rather than a lesion-level evaluation.
Figure 18.
LR-5 observation. A and B: Axial T2 weighted (A) and volumetric interpolated breath-hold examination (B) show a segment VI observation with high signal intensity (arrows); C and D: On arterial phase (C) strong enhancement is seen, better depicted on subtracted image (D); E: On portal phase washout is seen categorized as LR-5; F: Unenhanced computed tomography scan after TACE shows accumulation of iodized oil (arrow) within the tumor; G and H: However on post-contrast follow up magnetic resonance imaging on arterial phase (G) there is residual nodular enhancement within a lesion clearly depicted on subtracted image (H) categorized as LR-treatment response viable.
Figure 19.
Axial volumetric interpolated breath-hold examination, arterial phase. A-C: Axial volumetric interpolated breath-hold examination (A), arterial phase (B) showed an observation measuring almost 20 mm with arterial phase hyperenhancement (arrows) without washout on portal image (C) categorized as LR-4; D and E: On diffusion-weighted imaging (D), the observation showed high signal intensity (arrows) but the ancillary features lacked sufficient specificity for hepatocellular carcinoma to allow for an LR-5 upgrade. After multidisciplinary discussion, ablation was performed (E); F-H: On follow-up, an ablation defect was seen without residual areas of arterial enhancement or washout. This was classified as LR-treatment response non-viable. Note a previous ablation defect in the right lobe (arrows).
LI-RADS IN CLINICAL PRACTICE
The LI-RADS “lexicon” provide strict diagnostic criteria and reporting guidelines in order to enhance the consistency and clarity of radiologist interpretation and reporting. Subsequently, the communication between radiologists and clinicians has been improved. The adoption of a universal liver imaging lexicon is of great importance. This lexicon was designed to standardize the terminology used to describe liver imaging findings and is suggested for use in various patient settings including patients with cirrhosis, chronic HBV infection, current or prior HCC and following loco-regional therapy to assess tumor response[31,32]. In this sense, the integration of LI-RADS into the AASLD HCC clinical practice guidelines was a major achievement. However, global unification of diagnostic algorithms of HCC is still an unmet need.
Variability in terminology and definitions creates confusion between clinicians and challenges the significance of the published literature. For example, multiple terms have been used to describe nodules without APHE that are hypointense in the hepatobiliary phase of a gadoxetate-enhanced MRI examination[33]. The LI-RADS Hepatobiliary Agent Working Group suggests standardized terminology for these nodules: “hepatobiliary phase hypointense nodules without APHE.” A single term with a uniform definition enhances the ability of scientific investigators to define and pool data associated with these nodules and assists radiologists and referring clinicians in interpreting their clinical significance[31]. However, there are still areas of uncertainty. Non-cirrhotic patients at increased risk for developing HCC, such as those with chronic HCV infection or nonalcoholic steatohepatitis with advanced fibrosis, do not meet criteria for diagnosis of HCC with the use of LI-RADS. This is an example of an important gap in the generalized implementation of the LI-RADS criteria which necessitates further research.
CONCLUSION
The diagnosis of HCC in clinical practice is based on imaging, using mainly CT and MRI. As the early diagnosis of HCC is associated with the overall prognosis, accurate identification of potential malignant liver lesions using these modalities is essential. LI-RADS is a comprehensive guidance that provides standardized interpretation and reporting of CT and MR liver imaging. LI-RADS is suggested to be used only to patients at high risk for developing HCC in a step-wise fashion that analyzes all the relevant radiologic characteristics according to a pre-defined manner. Clinicians that care for patients with liver disease must be familiar with the characterization of liver lesions using the LI-RADS criteria as this offers correct assessment of liver lesions and truthful communication between specialties.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 31, 2022
First decision: February 24, 2022
Article in press: July 27, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishikawa T, Japan; Kumar SKY, India; Tamori A, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Chen YX
Contributor Information
Christina Liava, 4th Department of Internal Medicine, Hippokratio Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Emmanouil Sinakos, 4th Department of Internal Medicine, Hippokratio Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece. em_sinakos@yahoo.com.
Elissavet Papadopoulou, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Lamprini Giannakopoulou, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Stamatia Potsi, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Anestis Moumtzouoglou, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Anthi Chatziioannou, 4th Department of Internal Medicine, Hippokratio Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Loukas Stergioulas, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Lydia Kalogeropoulou, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Ioannis Dedes, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
Evangelos Akriviadis, 4th Department of Internal Medicine, Hippokratio Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Danai Chourmouzi, Department of Radiology, Interbalkan Medical Center, Thessaloniki 57001, Greece.
References
- 1.World Health organization. International Agency for Research on Cancer, Global Cancer Observatory Cancer Today 2020–IARC. [cited 10 January 2022]. Available from: https://gco.iarc.fr/today/fact-sheets-cancers .
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282–5294. doi: 10.3748/wjg.v23.i29.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635–654. doi: 10.1148/radiol.14132361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha GM, Sirlin CB, Fowler KJ. Imaging diagnosis of hepatocellular carcinoma: LI-RADS. Chin Clin Oncol. 2021;10:3. doi: 10.21037/cco-20-107. [DOI] [PubMed] [Google Scholar]
- 7.Santillan CS, Tang A, Cruite I, Shah A, Sirlin CB. Understanding LI-RADS: a primer for practical use. Magn Reson Imaging Clin N Am. 2014;22:337–352. doi: 10.1016/j.mric.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Moura Cunha G, Chernyak V, Fowler KJ, Sirlin CB. Up-to-Date Role of CT/MRI LI-RADS in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:513–527. doi: 10.2147/JHC.S268288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina (Kaunas) 2019;55 doi: 10.3390/medicina55090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 11.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 12.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 13.Tokino T, Tamura H, Hori N, Matsubara K. Chromosome deletions associated with hepatitis B virus integration. Virology. 1991;185:879–882. doi: 10.1016/0042-6822(91)90564-r. [DOI] [PubMed] [Google Scholar]
- 14.McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 15.Ozturk M. p53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet. 1991;338:1356–1359. doi: 10.1016/0140-6736(91)92236-u. [DOI] [PubMed] [Google Scholar]
- 16.Riley J, Mandel HG, Sinha S, Judah DJ, Neal GE. In vitro activation of the human Harvey-ras proto-oncogene by aflatoxin B1. Carcinogenesis. 1997;18:905–910. doi: 10.1093/carcin/18.5.905. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2:367–383. doi: 10.1159/000343852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang HY, Chen J, Xia CC, Cao LK, Duan T, Song B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J Gastroenterol. 2018;24:2348–2362. doi: 10.3748/wjg.v24.i22.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 21.Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000–4013. doi: 10.3748/wjg.v24.i35.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 24.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. IARC press, 2010. [Google Scholar]
- 25.Franette C. Hepatocellular Carcinoma: Moving into the 21st Cenury, An Issue of Clinics in Liver Disease. 1st ed. Elsevier press, 2020. [Google Scholar]
- 26.Tatsumi A, Maekawa S, Sato M, Komatsu N, Miura M, Amemiya F, Nakayama Y, Inoue T, Sakamoto M, Enomoto N. Liver stiffness measurement for risk assessment of hepatocellular carcinoma. Hepatol Res. 2015;45:523–532. doi: 10.1111/hepr.12377. [DOI] [PubMed] [Google Scholar]
- 27.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 28.Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816–830. doi: 10.1148/radiol.2018181494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kielar AZ, Chernyak V, Bashir MR, Do RK, Fowler KJ, Mitchell DG, Cerny M, Elsayes KM, Santillan C, Kamaya A, Kono Y, Sirlin CB, Tang A. LI-RADS 2017: An update. J Magn Reson Imaging. 2018;47:1459–1474. doi: 10.1002/jmri.26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Pol CB, Dhindsa K, Shergill R, Zha N, Ferri M, Kagoma YK, Lee SY, Satkunasingham J, Wat J, Tsai S. MRI LI-RADS Version 2018: Impact of and Reduction in Ancillary Features. AJR Am J Roentgenol. 2021;216:935–942. doi: 10.2214/AJR.20.23031. [DOI] [PubMed] [Google Scholar]
- 31.Marks RM, Masch WR, Chernyak V. LI-RADS: Past, Present, and Future, From the AJR Special Series on Radiology Reporting and Data Systems. AJR Am J Roentgenol. 2021;216:295–304. doi: 10.2214/AJR.20.24272. [DOI] [PubMed] [Google Scholar]
- 32.Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR, Marks RM, Kamaya A, Do RKG, Kono Y, Fowler KJ, Tang A, Bashir MR, Hecht EM, Jambhekar K, Lyshchik A, Rodgers SK, Heiken JP, Kohli M, Fetzer DT, Wilson SR, Kassam Z, Mendiratta-Lala M, Singal AG, Lim CS, Cruite I, Lee J, Ash R, Mitchell DG, McInnes MDF, Sirlin CB. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma. 2019;6:49–69. doi: 10.2147/JHC.S186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motosugi U, Murakami T, Lee JM, Fowler KJ, Heiken JP, Sirlin CB LI-RADS HBA Working Group. Recommendation for terminology: Nodules without arterial phase hyperenhancement and with hepatobiliary phase hypointensity in chronic liver disease. J Magn Reson Imaging. 2018;48:1169–1171. doi: 10.1002/jmri.26515. [DOI] [PubMed] [Google Scholar]