Abstract
Objectives
To conduct a systematic review and meta-analysis of available studies regarding the effects of the traditional herb Rosa damascena (as topical application and oral intake) on the severity of acute pain in adults.
Methods
A systematic search was performed on the following databases: Cochrane Central Register of Controlled Trials, PubMed, Scopus, Web of Science Core Collection, Embase, Cumulative Index to Nursing and Allied Health Literature, Scientific Information Database, and Magiran from inception to 20 March 2021. We included parallel-group and cross-over randomized controlled trials that compared the effects of any products containing R damascena in oral and topical administration forms to placebo, non-treatment, or conventional treatment. Two researchers independently performed the document screening and selection, data extraction, and risk of bias assessment. A random-effect model was used to pool the data.
Results
From a total of 11 studies that met the inclusion criteria, four studies administered R damascena through topical application and seven by oral intake. Nine studies recruited only females. Ten studies had parallel-group design, while one adopted cross-over design. The oral intake of Rdamascena reduced pain severity non-significantly (standardized mean difference (SMD) = -0.55, 95% CI: -1.27–0.17; p =0.132). However, the topical application of this treatment had no pain-alleviating effect (SMD = 0.10, 95% CI: -0.75–0.96; p =0.814). One study reported mild allergic rhinitis as an adverse effect of the treatment. Risk of bias assessment revealed that three of the eleven studies had good methodological quality, six had fair quality, and two were of poor quality.
Conclusions
This systematic review and meta-analysis suggests that the oral intake of R damascena may have a non-significant alleviating effect on acute pain severity in adults. However, its topical application has not shown pain-alleviating effect. More robust randomized controlled trials are needed for accurate estimation of the effects of oral and topical use of R damascena on the severity of different types of acute pain in adults.
Keywords: Acute pain, Adult, Analgesics, Rosa, Review, Iran
Introduction
Rosa damascena, popularly known as damask rose, is a medicinal herb belonging to the Rosaceae family.1 This herb is cultivated in Iran, Bulgaria, Pakistan, Turkey, Morocco, and India.2 R damascena is traditionally considered as the king of flowers and is often associated with purity, inspiration, love, happiness, and beauty.3 R damascena is also called Gole-Mohammadi by Iranian people because its fragrance reminds them of Prophet Muhammad.4
R damascena is currently used worldwide in food, perfume, cosmetic, and pharmacological industries.3,5 The pharmacological properties of this herb are attributed to a high percentage of glycosides, terpenes, flavonoids, and anthocyanins.2,6 Traditionally, different products of R damascena have been used for managing conditions as varied as erectile dysfunction, arthritis, hepatitis, cardiovascular disorders, respiratory tract infections, and digestive disorders.3,6,7 R damascena preparations are also suggested to alleviate pain by traditional physicians in Iran and elsewhere and some modern medical practitioners.2,5,6,8-10 In recent years, several in vivo and in vitro studies have assessed the pain-alleviating properties of R damascena in aromatherapy, oral intake, and topical application.11-40
Recent reviews have suggested positive effects of R damascena in aromatherapy form on reducing pain severity.5,6,8,41,42 However, the potential pain-alleviating effects of this herb in oral and topical forms have not yet been addressed in a comprehensive review. Based on the recent randomized controlled trials (RCTs), either topical application or oral intake of R damascena could alleviate different types of pain (e.g., pregnancy-related low back pain, menstrual-related pain, postoperative pain, and aphthous stomatitis-induced pain).24,30,31,35,36,43 On the contrary, two RCTs found no significant difference in sexual-related pain among women who received R damascena capsule compared to those who received placebo capsule.27,28 Similarly, no significant difference was reported between students’ menstrual-related abdominal pain when they received R damascena and mefenamic acid capsules in a cross-over design.25 In addition, oral intake of R damascena had a non-significant alleviating effect on menstrual-related abdominal pain and headache among females with primary dysmenorrhea (PD) and premenstrual syndrome (PMS).26,29 Moreover, topical application of this herbal medicine had no significant effect on pain induced by migraine headaches and aphthous ulcers.32,34
Although the results of recent RCTs are inconclusive on the pain-alleviating effects of R damascena in topical and oral administration forms, to the best of our knowledge, no review has analyzed the conflicting findings of these RCTs. Therefore, we aimed to systematically identify and summarize the results of recent RCTs regarding the effects of topical application and oral intake of R damascena on acute pain severity in adults and also to pool the obtained findings in a meta-analysis.
Methods
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020205071). The review was also reported based on the statements presented by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).44
Search strategy
A systematic search was performed on the Cochrane Central Register of Controlled Trials, PubMed, Scopus, Web of Science Core Collection, Embase, Cumulative Index to Nursing and Allied Health Literature, Scientific Information Database (http://www.sid.ir/), and Magiran (http://www.magiran.com). All the searches were conducted by two researchers independently. The searches were conducted in respect of two time periods: (a) from the inception date of the database till 30 October 2020; and (b) from 30 October 2020 to 20 March 2021. Additionally, the Iranian Registry of Clinical Trials and the World Health Organization International Clinical Trials Registry Platform were searched for any records of clinical trials involving R damascena. The corresponding authors of the retrieved trials were contacted via email to get additional information on their trials. Likewise, the reference lists of the eligible trials were checked to avoid missing related studies.
A combination of the following keywords was used in the systematic search: (‘Rosa’ OR ‘Rose’ OR ‘Rosaceae’ OR ‘Rosewater’ OR ‘Rose water’ OR ‘Rose oil’ OR ‘R damascena’ OR ‘R damascena’ OR ‘R X damascena’ OR ‘Damask rose’ OR ‘Rose damask’ OR ‘Damascus rose’ OR ‘Gole Mohammadi’ OR ‘Gol-E-Muhammadi’ OR ‘Gol-E-Mohammadi’) AND (Oral OR Supplement* OR Syrup OR Suspension* OR Emulsion OR Linctus OR Drop* OR Solution OR Extract* OR Oil* OR Capsule* OR Tablet OR Spray OR Ointment* OR Gel* Or Cream OR Lotion OR Massage OR Topical) AND (Pain OR Analgesi*OR Antinocicepti*).
To ensure that none of the related studies were missed, no restrictions were applied with regard to studies’ participants, clinical conditions, language, or publication date in the literature search.
Eligibility criteria and studies selection
The studies were included based on the elements of the participants, intervention, comparison, outcomes, and study design (PICOS), as per the criteria in Table 1.
Table 1. Inclusion criteria for considering studies on the effects of topical application and oral intake of R damascena on adults’ acute pain.
| Items | Criteria |
|---|---|
| Participants | Individuals within the age range of 18-60 years who experienced any types of moderate to severe acute pain. |
| Intervention | Administration of any products of Rosa damascena (e.g., essential oil, extract, absolute or concrete, syrup or juice, Jollab, petal jam, Gulkand, rose water, tea, drop, capsule, mouthwash) in the form of topical application or oral intake for a treatment group. |
| Comparison | Placebo treatment, non-treatment, and conventional treatment. |
| Outcomes | Pain severity, analgesics use, and adverse effects of the treatment. |
| Study design | Parallel-group and cross-over randomized controlled trials. |
Studies were excluded if they: (a) did not have English abstract; (b) were conference papers, theses, letters, comments, short communications, reviews, meta-analyses, and animal studies; (c) administered R damascena in aromatherapy form; (d) administered R damascena in combination with other herbal products; (e) administered other species of Rosa; (f) recruited individuals who experienced chronic pain; and (g) recruited individuals > 60 years of age. Redundant studies with limited data were also excluded.
The screening and selection of the studies were performed by two researchers independently.
A total of 1429 records were found from the electronic search. Based on the screening of title and abstract of 953 records, 938 were removed and full-texts of the 15 remaining studies were assessed for compliance with the inclusion criteria. Of these, four were excluded: two were redundant publications,43,45 one had a single-group study,39 and one study recruited individuals > 60 years of age.34 Finally, 11 studies were considered eligible for this review [Figure 1].24-32,34,36
Figure 1.

Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for identification of the studies and selection process.
Data extraction
The following data were extracted for each study by two independent researchers: (a) study details, (b) participants’ characteristics, (c) intervention details, and (d) mean or mean changes and SD, as well as number and percentage for the measured outcomes. In four studies with multiple intervention groups, data were extracted from the R damascena and control groups.26,29,35,36 Of these, one considered both placebo and non-treated groups; hence, data were extracted from the placebo group for comparison.36 If the studies contained unclear or insufficient information, the corresponding authors were contacted via email or phone call to get additional information on their studies. Any disagreement in data extraction between the researchers was resolved by discussion.
Assessment of risk of bias
The risk of bias for each included study was assessed by two independent researchers using the Cochrane Risk of Bias Assessment Tool, which consists of seven items: (a) random sequence generation (selection bias), (b) allocation concealment (selection bias), (c) blinding of participants and personnel (performance bias), (d) blinding of outcome assessment (detection bias), (e) incomplete outcome data (attrition bias), (f) selective reporting (reporting bias), and (g) other biases.46 Disagreements between the researchers were resolved by discussing with a third researcher and reaching a consensus.
Data analysis
Various studies recorded pain at different post-treatment times; hence, we calculated the changes of mean and SD in each study group by comparing the baseline and the end-of-trial values, using standard methods.42,47 In a cross-over trial, the effect sizes of the first and second phases were pooled.25 Also, the effect sizes of one study over the same participants were pooled before conducting the meta-analysis.26
All effect sizes were reported as standardized mean difference (SMD) of outcomes with their 95% CI, using a random-effect model to take between-study heterogeneity into account. To assess heterogeneity, the I2 statistic value of ≥ 50% and Cochran’s Q test value of < 0.05 were considered as significant heterogeneity. Subgroup analysis was performed to determine probable sources of heterogeneity and investigate any possible differences between studies about clinical condition, R damascena total administration dosage and duration as well as administration form, study tool, and study quality. To conduct a subgroup analysis based on total administration dosage, we converted R damascena products to a similar product or unit if possible. According to the administration dosage reported in the majority of included studies, 10 drops of R damascena was estimated as 1 mL. Also, we considered 1 mg of R damascena as equal to 0.001 mL. To find the dependency of the overall estimate on the effect size from a single study, a sensitivity analysis was conducted. The Begg’s and Egger’s tests and also a visual inspection of funnel plots were used to assess potential publication bias. All statistical analyses were performed using Stata, version 11.2 (Stata Corp, College Station, TX). P-values were considered significant at the level of < 0.05.
Results
General characteristics
Out of the 1429 records found from our electronic search, only 11 studies were eligible to be included in this study. All the included studies were conducted in Iran, and three were published in Farsi.24,29,32 These studies were conducted on individuals in the age range 18–40 years who experienced different types of acute pain. All studies recorded only pain severity, except one which recorded frequency and dosage of administrated analgesics in addition to pain severity.30 One study suggested an association of mild allergic rhinitis with R damascena therapy [Table 2].35
Table 2. Summary of included studies for the effects of topical application and oral intake of R damascena on adults’ acute pain.
| Authors | Study design | Participants | Sample size/ age (mean ± SD) | Intervention | Outcome/ study tool (measurement times) | Adverse events/ findings* | ||
|---|---|---|---|---|---|---|---|---|
| Study arms | Administration route | Administration dosage and duration; total dosage and duration† | ||||||
| Shirazi et al,35 | Triple-blind, placebo-controlled, 3-arm, parallel-group | Pregnant women with low back pain | I: 37/27.7 ± 0.8 C: 38/27.9 ± 0.7 |
I: R.D drop (essential oil in carrier of almond oil) + standard care C: Placebo drop (almond oil) + standard care |
Topical application* | 7 drops of each product (estimated as 0.7 mL), 2 times daily for 4 consecutive weeks; total dosage: 39 mL; total duration: 28 days | Pregnancy-related low back pain/VAS (baseline, 2nd week of intervention, 2 weeks after the end of intervention) | Mild allergic rhinitis/Sig. |
| Khatibi et al,32 | Double-blind, placebo-controlled, 2-arm, parallel-group | Females and males with minor aphthous ulcers | I: 50/30 ± 13.81 C: 50/24.5 ± 8.34 |
I: R.D drop + standard care C: Placebo drop (Diphenhydramine syrup) + standard care |
Topical application** | 10 drops of each product (estimated as 1 mL), 4 times daily for 1 week; total dosage: 28 mL; total duration: 7 days | Aphthous ulcer pain/VAS (baseline, 2nd, 4th, and 7th days of intervention) | Nrep./NS |
| Sadeghi Aval Shahr et al,36 | Single-blind, placebo-controlled, 3-arm, parallel-group | Female college students with PD | I: 25/26 ± 3.6 C: 25/24.6 ± 3.1 |
I: R.D drop (essential oil in carrier of almond oil) C: Placebo drop (almond oil) |
Topical application*** | 5 drops of each product (estimated as 0.5 mL) at the 1st day of menstruation for 2 subsequent MC; total dosage: 1 mL; total duration: 2 days | Menstrual-related abdominal pain/VAS (before and after intervention in 1st and 2nd MC) | Nrep./Sig. only at the 2nd MC |
| Hoseinpour et al,31 | Double-blind, placebo-controlled, 2-arm, parallel-group | Females and males with minor aphthous ulcers | I: 25/34.4 ± 9.6 C: 25/33.6 ± 14.4 |
I: R.D mouthwash C: Placebo mouthwash |
Topical application**** | 5 mL of each product, 4 times daily for 2 weeks; total dosage: 280 mL; total duration: 14 days | Aphthous ulcer pain/ perceived pain rating scale (baseline and 4th, 7th, 11th, and 14th days of intervention) | Nrep./Sig. only at 4th and 7th days |
| Farnia et al,28 | Double-blind, placebo-controlled, 2-arm, parallel-group | Opioid-dependent females with methadone-related sexual dysfunction | I: 25/38.92 ± 8.31 C: 25/38.72 ± 7.24 |
I: R.D soft gelatin capsule (filled with 2 mL essential oil) + standard care C: Placebo soft gelatin capsule (filled with 2 mL oil-water solution) + standard care |
Oral intake | One capsule of each product (estimated as 2 mL), daily for 8 consecutive weeks; total dosage: 112 mL; total duration: 56 days | Sexual-related pain/FSFI (baseline, 4th, and 8th weeks of intervention) | Nrep./NS |
| Davaneghi et al,26 | Double-blind, placebo-controlled, 4-arm, parallel-group | Females with PD | I: 27/22.63 ± 0.47 C: 25/22.08 ± 0.39 |
I: R.D hard gelatin capsule (filled with 800 mg R.D extract) + fish oil soft gelatin capsule (placebo) C: R.D hard gelatin capsule (filled with placebo) + fish oil soft gelatin capsule (placebo) |
Oral intake | One capsule of each product (estimated as 0.8 mL), daily from the first day of menstruation until 60 consecutive days; total dosage: 48 mL; total duration: 60 days | Menstrual-related headache and abdominal pain/VAS (baseline, 30th, and 60th days of intervention) | Nrec./NS |
| Ataollahi et al,24 | Double-blind, placebo-controlled, 2-arm, parallel-group | Female college students with PD | I: 55/21.41 ± 1.49 C: 55/21.38 ± 1.72 |
I: R.D oral drop C: Placebo drop (water and sugar) |
Oral intake | 10 drops of each product (estimated as 1 mL), 2 times daily during first 3 days of menstruation for 2 subsequent MC; total dosage: 12 mL; total duration: 6 days | Menstrual-related abdominal pain/McGill (baseline, end of 2nd MC) | Nrec./Sig. |
| Farnia et al,27 | Double-blind, placebo-controlled, 2-arm, parallel-group | Females with SSRI-induced sexual dysfunction | I: 25/32.45 ± 5.68 C: 25/34.02 ± 6.45 |
I: R.D soft gelatin capsule (filled with 2 mL essential oil) + standard care C: Placebo soft gelatin capsule (filled with 2 mL oil-water solution) + standard care |
Oral intake | One capsule of each product (estimated as 2 mL), daily for 8 consecutive weeks; total dosage: 112 mL; total duration: 56 days | Sexual-related pain/FSFI (baseline, 4th, and 8th weeks of intervention) | Nrep./NS |
| Bani et al,25 | Double-blind, placebo-controlled, 2-arm, cross-over groups | Female college students with PD | I: 46/22.20 ± 2.11 C: 46/22.13 ± 2.06 |
I: R.D hard gelatin capsule (filled with 200 mg R.D extract) C: Mefenamic acid capsule (250 mg) |
Oral intake | One capsule of each product (estimated as 0.2 mL), 4 times daily during first 3 days of menstruation for 2 subsequent MC; total dosage: 4.8 mL; total duration: 6 days | Menstrual-related abdominal pain/VAS (baseline and 1, 2, 3, 6, 12, 24, 48, and 72 hours after taking the first drug during 1st and the 2nd MC) | Nrep./NS |
| Jamilian et al,29 | Double-blind, placebo-controlled, 3-arm, parallel-group | Females with PMS | I: 40/25.93 ± 4.68 C: 40/26.56 ± 3.53 |
I: R.D oral drop C: Placebo drop (distilled water) |
Oral intake | 15 drops of each product (estimated as 1.5 mL), 2 times daily from 14 days before menstruation until end of menstruation for 3 subsequent MC; total dosage: 180 mL; total duration: 60 days | Menstrual-related headache/ DSRS (baseline, end of 3rd MC) | Nrec./NS |
| Mostafa-Gharabaghi et al,30 | Double-blind, placebo-controlled, 2-arm, parallel-group | Females undergoing C/S | I: 46/27.78 ± 4.04 C: 46/22.28 ± 5.04 |
I: R.D hard gelatin capsule (filled with 400 mg R.D extract) + standard care C: Placebo hard gelatin capsule (filled with 400 mg starch) + standard care |
Oral intake | 2 capsules of each product (each estimated as 0.4 mL); during 15 min before anesthesia; total dosage: 0.8 mL | Post-operative pain/VAS (baseline and 3, 6, 12, and 24 hours after surgery) | Nrep./Sig. |
| Frequency and dosage of administrated analgesics (baseline, end of intervention) | ||||||||
C: control; I: intervention; C/S: cesarean section; DSRS: daily symptom rating scale; DW: distilled water; FSFI: female sexual function index; MC: menstrual cycle; McGill: McGill pain questionnaire; min: minutes; nrep.: not reported; nrec.: not recorded; NS: not significant; PD: primary dysmenorrhea; PMS: premenstrual syndrome; R.D: rosa damascena; Sig.: significantly; SSRI: selective serotonin-reuptake inhibitors; VAS: visual analog scale.
† Ten drops and 1 mg of Rosa damascena was estimated as 1 mL and 0.001 mL, respectively.
*Products were self-administered topically for 100 cm2 of the painful part of the skin (without massage).
**Products were self-administered topically on the lesions using a sterile swab (without massage and after meals, and before sleep).
***Products were self-administered topically on the abdomen and then the abdomen was massaged by clockwise circular movements for 15 min.
****Products were swished around the mouth for 30 seconds and then were expelled (preferably after oral-hygiene procedures).
*Significantly lower in the intervention group compared to the comparison group after the intervention.
Topical application of R damascena
Of the four studies that administered R damascena in topical form, three used 0.5–1 mL topical drop,32,35,36 and the other one administered 5 mL mouthwash.31 The total administration duration of R damascena products varied from 2 to 28 days. The products were self-administered without massage in all studies, except one which applied abdomen massage with R damascena essential oil.36 All studies had a parallel-group design and two recruited only females.35,36 The total sample size of the placebo group (i.e., Diphenhydramine syrup and almond oil) and the R damascena group were 137 and 138, respectively [Table 2].
Based on the combined effect sizes of the four RCTs, topical application of R damascena had no pain-alleviating effect (SMD = 0.10, 95% CI: -0.75–0.96; p = 0.814). Heterogeneity was significant between studies in the overall analysis (I2 = 91.3%, p < 0.001) [Figure 2]. After excluding one study which applied R damascena using massage,36 a non-significant reducing effect of treatment was observed (SMD = -0.06, 95% CI: -1.13–1.00; p = 0.906).
Figure 2.

Forest plot for the effect of topical application of Rosa damascena on adults’ acute pain.
Oral intake of R damascena
Of the seven studies that investigated R damascena in oral form, five administered soft or hard gelatin capsule containing either 200–800 mg extract25,26,30 or 2 mL essential oil.27,28 Two remaining studies used 1 mL or 1.5 mL oral drop.24,29 The total administration duration of R damascena products varied from one day to 60 days. All oral intake studies recruited only females. Six studies had parallel-group design, while one adopted cross-over design.25 The total sample sizes of the placebo/conventional treatment groups (i.e., mefenamic acid capsule) was 262 and that of the R damascena groups was 264 [Table 2].
Based on the combined effect sizes of seven RCTs, oral intake of R damascena reduced pain severity non-significantly compared to the placebo or conventional treatment (SMD = -0.55, 95% CI: -1.27–0.17; p = 0.132). Significant heterogeneity (I2) was found among the included studies for main analysis (I2 = 94.3, p < 0.001) [Figure 3]. After excluding one cross-over study,25 the results of primary meta-analysis did not change (SMD = -0.68, 95% CI: -1.55–0.20; p = 0.129). Based on subgroup analysis, pain severity was significantly reduced when R damascena was administered using oral drop (p = 0.024) [Table 3].
Figure 3.
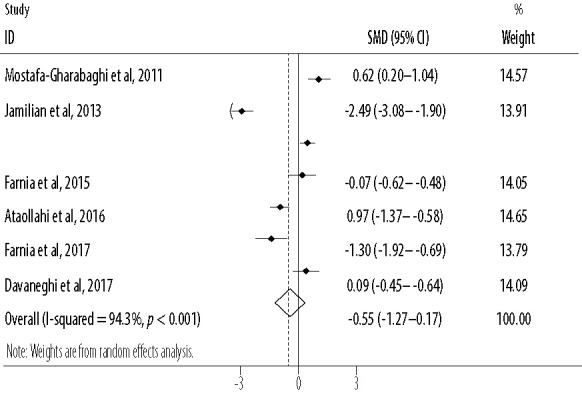
Forest plot for the effect of oral intake of Rosa damascena on adults’ acute pain.
Table 3. Subgroup analysis for the effects of topical application and oral intake of Rosa damascena on adults’ acute pain.
| Variables | Effect sizes (n) | I2 (% of heterogeneity) | Cochran’s Q test | SMD (95%CI) | p-value |
|---|---|---|---|---|---|
| Topical application | |||||
| Clinical condition | |||||
| Menstrual-related pain | 1 | - | - | 0.63 (0.03–1.22) | 0.039 |
| Pregnancy-related low back pain | 1 | - | - | -1.10 (-1.59–-0.61) | < 0.001 |
| Aphthous ulcer pain | 2 | 87.0 | 0.006 | 0.45 (-0.53–1.42) | 0.372 |
| Total administration dosage | |||||
| ≤ 39 mL | 3 | 90.6 | < 0.001 | -0.18 (-1.09–0.74) | 0.702 |
| 280 mL | 1 | - | - | 0.97 (0.38–1.56) | 0.001 |
| Total administration duration | |||||
| ≤ 14 days | 3 | 77.1 | 0.013 | 0.49 (-0.14–1.12) | 0.125 |
| 28 days | 1 | - | - | -1.10 (-1.59––0.61) | < 0.001 |
| Administration form | |||||
| Drop | 3 | 90.6 | < 0.001 | -0.18 (-1.09–0.74) | 0.702 |
| Mouthwash | 1 | - | - | 0.97 (0.38–1.56) | 0.001 |
| Study tool | |||||
| VAS | 3 | 90.6 | < 0.001 | -0.18 (-1.09–0.74) | 0.702 |
| Perceived pain rating scale | 1 | - | - | 0.97 (0.38–1.56) | 0.001 |
| Study quality | |||||
| Poor1 | 1 | - | - | -0.03 (-0.42–0.36) | 0.818 |
| Fair2 | 3 | 94.2 | < 0.001 | 0.16 (-1.17–1.48) | 0.883 |
| Oral intake | |||||
| Clinical condition | |||||
| Sexual-related pain | 2 | 88.3 | 0.003 | -0.68 (-1.89–0.53) | 0.270 |
| Menstrual-related pain | 4 | 95.9 | < 0.001 | -0.79 (-1.85–0.27) | 0.143 |
| Post-operative pain | 1 | - | - | 0.62 (0.20–1.04) | 0.004 |
| Total administration dosage | |||||
| ≤ 12 mL | 3 | 93.8 | < 0.001 | -0.07 (-0.92–0.78) | 0.874 |
| ≥ 48 mL | 4 | 94.1 | < 0.001 | -0.94 (-2.12–0.25) | 0.121 |
| Total administration duration | |||||
| ≤ 6 days | 3 | 93.8 | < 0.001 | -0.07 (-0.92–0.78) | 0.874 |
| ≥ 56 days | 4 | 94.1 | < 0.001 | -0.94 (-2.12–0.25) | 0.121 |
| Administration form | |||||
| Soft or hard gelatin capsule | 5 | 84.9 | < 0.001 | -0.07 (-0.60–0.46) | 0.793 |
| Oral drop | 2 | 94.3 | < 0.001 | -1.71 (-3.20–-0.23) | 0.024 |
| Study tool | |||||
| VAS, McGill (0–10 scales) | 4 | 90.8 | < 0.001 | -0.03 (-0.69–0.63) | 0.927 |
| Other | 4 | 94.2 | < 0.001 | -1.29 (-2.69–0.12) | 0.072 |
| Study quality | |||||
| Poor1 | 1 | - | - | 0.62 (0.20–1.04) | 0.004 |
| Fair2 | 3 | 95.0 | < 0.001 | -1.12 (-2.42–0.19) | 0.094 |
| Good3 | 3 | 88.6 | < 0.001 | -0.38 (-1.19–0.44) | 0.363 |
I2: statistic value; McGill: McGill pain questionnaire; n: number; SMD: standardized mean difference; VAS: visual analog scale.
1Cochrane risk of bias assessment tool: High risk of bias in one item and unclear risk of bias in more than two items.
2Cochrane risk of bias assessment tool: High risk of bias in one item or unclear risk of bias in one item or two items.
3Cochrane risk of bias assessment tool: Low risk of bias in all items.
Assessment of the risk of bias
From the perspective of risk of bias, three out of 11 studies had good quality (low risk of bias for all items),25,27,28 while two had poor quality (high risk of bias in one item and unclear risk of bias in more than two items).30,32 The remaining six studies had fair quality (high risk of bias in one item or unclear risk of bias in one item or two items) [Figures 4 and 5, Table 4].24,26,29,31,35,36
Figure 4.

Risk of bias graph for studies on the effects of topical application and oral intake of Rosa damascena on adults’ acute pain.
Figure 5.
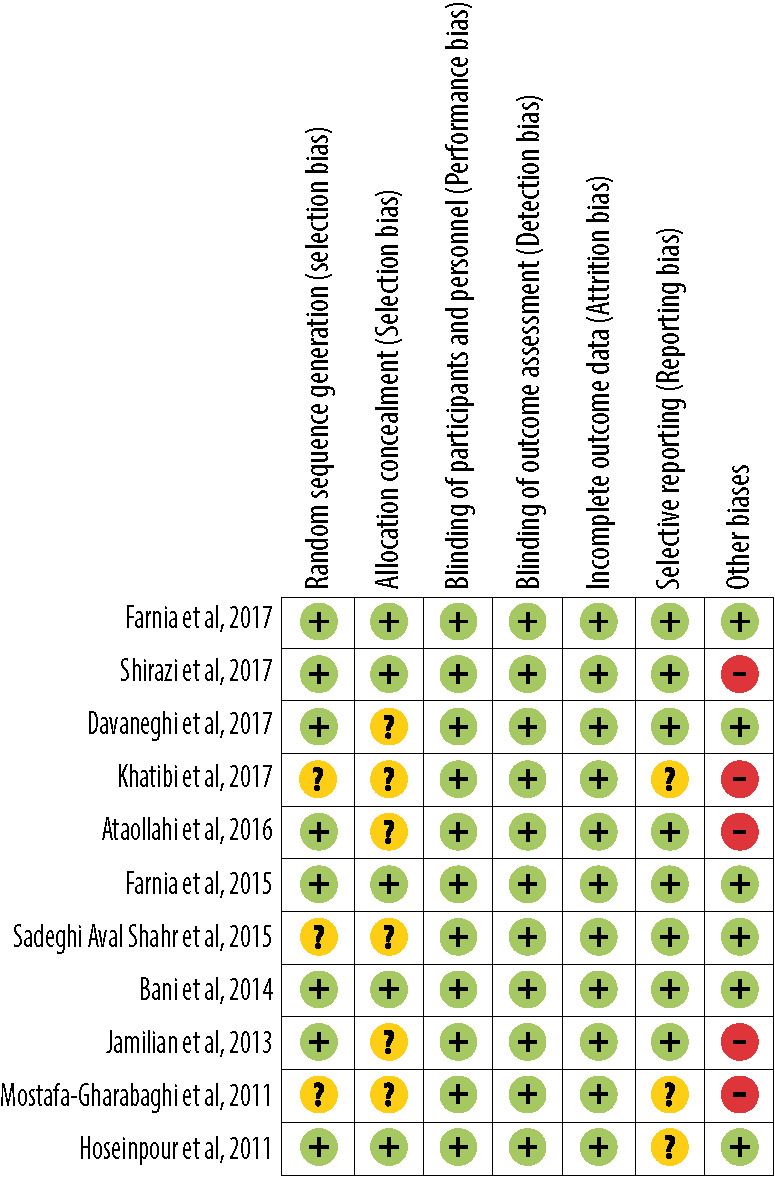
Summary of risk of bias within studies on the effects of topical application and oral intake of Rosa damascena on adults’ acute pain.
Table 4. Assessment of risk of bias within studies with support for judgment.
| Risk of bias items | Authors’ judgment | Support for judgment |
|---|---|---|
| Shirazi et al, 201735 | ||
| Random sequence generation | Low risk | It was done using shuffling envelopes. |
| Allocation concealment | Low risk | It was done using sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | Blinding of outcome assessment has been ensured. |
| Incomplete outcome data | Low risk | Missing outcome data balanced in numbers across groups. |
| Selective reporting | Low risk | The protocol is available (IRCT2014091419150N1) and all outcomes have been reported. |
| Other biases | High risk | Measurement time is not well specified and is not based on the protocol. |
| Khatibi et al, 201732 | ||
| Random sequence generation | Unclear risk | No specific information. |
| Allocation concealment | Unclear risk | No specific information. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | Blinding of outcome assessment has been ensured. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Unclear risk | The protocol is not available. |
| Other biases | High risk | The registered protocol does not exist, ethical approval does not exist, and no specified funding source. |
| Sadeghi Aval Shahr et al, 201536 | ||
| Random sequence generation | Unclear risk | No specific information. |
| Allocation concealment | Unclear risk | No specific information. |
| Blinding of participants and personnel | Low risk | Blinding of participants has been ensured. |
| Blinding of outcome assessment | Low risk | No blinding of outcome assessment, but the outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Low risk | The protocol is available (IRCT2012081310182N2) and all outcomes have been reported. |
| Other bias | Low risk | No other sources of bias. |
| Hoseinpour et al, 201131 | ||
| Random sequence generation | Low risk | It was done using a computer random number generator. |
| Allocation concealment | Low risk | It was done using sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | Blinding of outcome assessment has been ensured. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Unclear risk | The protocol is not available. |
| Other biases | Low risk | No other sources of bias. |
| Farnia et al, 201728 | ||
| Random sequence generation | Low risk | It was done using the drawing of lots. |
| Allocation concealment | Low risk | It was done using sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | Blinding of outcome assessment has been ensured. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Low risk | The protocol is available (IRCT2015091523705N2) and all outcomes have been reported. |
| Other biases | Low risk | No other sources of bias. |
| Davaneghi et al, 201726 | ||
| Random sequence generation | Low risk | It was done using a random number table. |
| Allocation concealment | Unclear risk | No specific information. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | Blinding of outcome assessment has been ensured. |
| Incomplete outcome data | Low risk | Missing outcome data balanced in numbers across groups. |
| Selective reporting | Low risk | The protocol is available (IRCT201403105670N8) and all outcomes have been reported. |
| Other biases | Low risk | No other sources of bias. |
| Ataollahi et al, 201624 | ||
| Random sequence generation | Low risk | It was done using block randomization. |
| Allocation concealment | Unclear risk | No specific information. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | No blinding of outcome assessment, but unlikely to have influenced the outcome measurement. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Low risk | The protocol is available (IRCT201311216807N10) and all outcomes have been reported. |
| Other biases | High risk | Outcome measurements have not been reported based on the protocol. |
| Farnia et al, 201527 | ||
| Random sequence generation | Low risk | It was done using the drawing of lots. |
| Allocation concealment | Low risk | It was done using sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | Blinding of outcome assessment has been ensured. |
| Incomplete outcome data | Low risk | Missing outcome data balanced in numbers across groups. |
| Selective reporting | Low risk | The protocol is available (IRCT2013100114333N9) and all outcomes have been reported. |
| Other biases | Low risk | No other sources of bias. |
| Bani et al, 201425 | ||
| Random sequence generation | Low risk | It was done using block randomization. |
| Allocation concealment | Low risk | It was done using sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | No blinding of outcome assessment, but not likely to have influenced the outcome measurement. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Low risk | The protocol is available (IRCT201207267618N2) and all outcomes have been reported. |
| Other biases | Low risk | No other sources of bias. |
| Jamilian et al, 201329 | ||
| Random sequence generation | Low risk | It was done using digital random number generator. |
| Allocation concealment | Unclear risk | No specific information. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | No blinding of outcome assessment, but may not have influenced the outcome measurement. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Low risk | The protocol is available (IRCT201108237405N1) and all outcomes have been reported. |
| Other biases | High risk | Outcome measurements have not been reported based on the protocol. |
| Mostafa-Gharabaghi et al, 201130 | ||
| Random sequence generation | Unclear risk | No specific information. |
| Allocation concealment | Unclear risk | No specific information. |
| Blinding of participants and personnel | Low risk | Blinding of participants and key study personnel have been ensured. |
| Blinding of outcome assessment | Low risk | No blinding of outcome assessment, but the outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data | Low risk | No missing outcome data. |
| Selective reporting | Unclear risk | The protocol is not available. |
| Other biases | High risk | The registered protocol does not exist, ethical approval does not exist, and no specified funding source. |
Publication bias and sensitivity analysis
With regard to topical application, no evidence of publication bias was found based on the visual inspection of the funnel plot as well as the Begg’s test (p = 0.734) and Egger’s test (p = 0.527). Such findings were also obtained for oral intake based on the funnel plot and also the Begg’s test (p = 0.230) and Egger’s test (p = 0.236) [Figure 6].
Figure 6.
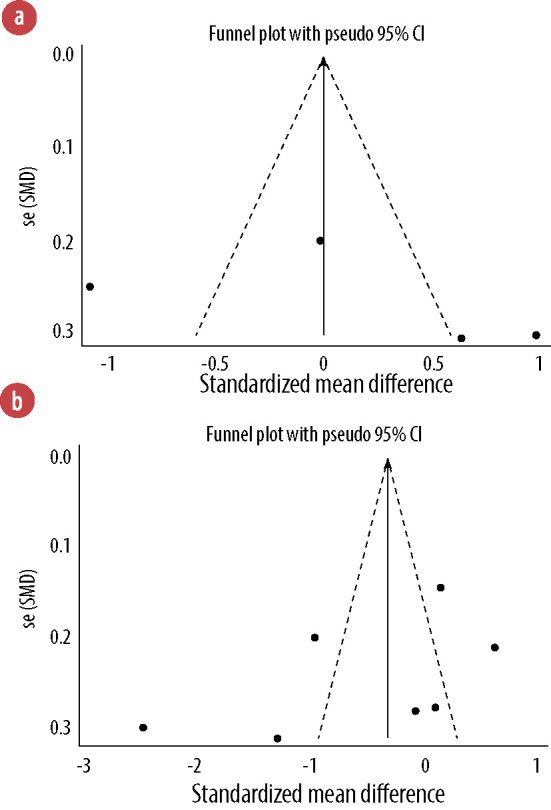
Funnel plots for the effects of topical application (a) and oral intake (b) of Rosa damascena on adults’ acute pain.
Sensitivity analysis showed that the pooled effect sizes obtained for topical application (lower CI limit: -1.17–-0.06; upper CI limit: 0.73–1.48) and oral intake (lower CI limit: -1.55–-0.79; upper CI limit: 0.01–0.34) did not depend on a particular study or group of studies [Figure 7].
Figure 7.
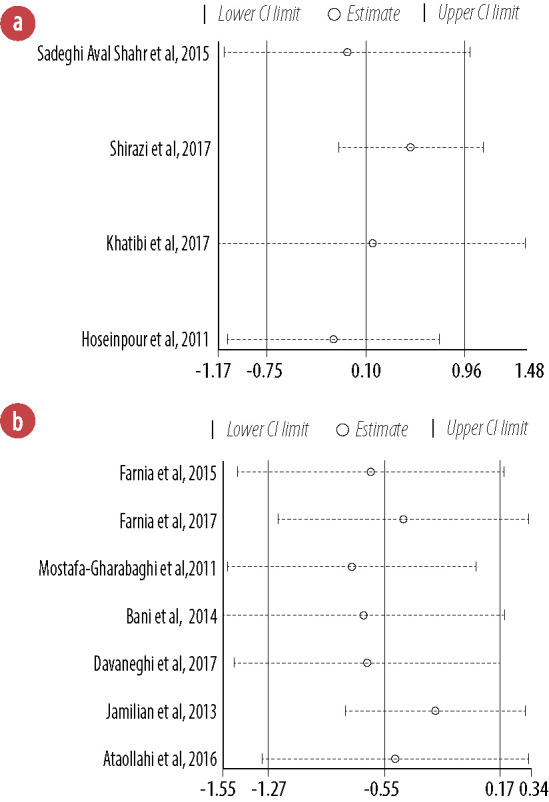
Sensitivity analysis for the effects of topical application (a) and oral intake (b) of Rosa damascena on adults’ acute pain.
Discussion
In Asian countries, herbs have been important in traditional and complementary medicine for alleviating various painful conditions.48,49 Currently, different products of R damascena are being used in Asian countries for their pain-alleviating properties; however, there is a paucity of comprehensive evidence to support their applications.3,7,42 Accordingly, we performed this review to summarize the effects of topical application and oral intake of this herbal medicine on treating acute pain in adults.
Based on the meta-analysis findings, administration of oral intake of R damascena reduced pain severity non-significantly. However, the topical application of this herbal medicine had no pain-alleviating effect, which might be due to the limited number of included studies and also their suboptimal methodological quality. It also might be due to the participants' gender as all the oral medications were administered to females mostly, and that too, for gender-specific pains; only two topical-application studies recruited both sexes. Also, it seems that our findings obtained for topical application studies were affected by one study which applied R damascena by massaging it.36 After excluding that study, pain severity reduction of topical application method was non-significant.
The findings of this review update the available reviews regarding the analgesic effect of R damascena. In a recent meta-analysis by Koohpayeh et al,47 the pooled analysis of five RCTs on the effects of oral intake and aromatherapy of R damascena reduced the menstruation-related pain non-significantly (weighted mean difference (WMD) = –1.39; 95% CI: -3.21–0.43; p = 0.133). Also, in a systematic review of herbal medications for postoperative pain, Arruda et al,50 found no significant reduction in the need for analgesics after oral intake of R damascena in combination with gingeRosa. However, Nayebi et al,2 reported the analgesic effects and safety of R damascena in the forms of inhalation aromatherapy, topical treatment, or massage application on pain induced by surgery, PD, pregnancy, and aphthous ulcer. In another systematic review, Mohebitabar et al,8 found promising evidence for the effectiveness of inhalation use of R damascena on pain of menstruation, renal colic, and surgery. Moreover, Mahboubi et al,5 and Boskabady et al,6 reported the analgesic activities of R damascena based on the results of both in vivo and in vitro studies.
Differences in study objectives might be the main reason for the differences observed in the findings of previously mentioned reviews and in the current review. In this meta-analysis, we included only RCTs that addressed pain-alleviating effects of R damascena using oral or topical administration routes, while the above-mentioned systematic or narrative reviews neither focused specifically on the analgesic properties of R damascena nor they stratified the administration routes of this treatment. However, Nasiri et al,42 pooled 15 RCTs on the effect of aromatherapy with R damascena on adults’ acute pain severity and found a promising pain-alleviating effect of treatment (WMD = -2.12; 95% CI: -2.85–1.40; p < 0.001). Also, they included studies that evaluated the effect of R damascena in form of aromatherapy; whereas, we considered oral intake or topical application of R damascena which can justify the differences in the obtained findings.
The analgesic effects of R damascena induced by oral intake or topical application have been attributed to chemical components in this herbal medicine. Hongratanaworakit has reported the analgesic effects of R damascena oil without olfactory stimulation, and she presumed that molecules of R damascena could enter the bloodstream by dermal absorption.51 In a recent animal study, the non-water soluble ingredients of R damascena oil such as quercetin and kaempferol were reported as responsible for its analgesic effect.33 Likewise, 2-phenylethanol found in R damascena might be a pain signal inhibitor that could block pain receptors.17 Moreover, the topical effects of R damascena on reducing pain might be explained by the high tannin content of the extract of this herbal medicine.31 Further studies are recommended to determine the biochemical mechanisms responsible for analgesic activities of oral intake and topical application of R damascena.
Implications of findings
The findings of the present review can increase our understanding of the value of R damascena as a holistic care approach and non-pharmacological agent. We found that oral intake of R damascena led to a 0.55 unit reduction in pain severity. Also, the administration of R damascena was reported to be free of side effects in most included RCTs. However, we confirmed a paucity of well-designed trials in this area as most included studies had a fair or poor methodological quality. Considering the low-cost and simple application of R damascena, future studies with improved methodological quality are suggested to evaluate the pain-alleviating potencies of this herbal medicine to reach an evidence-based conclusion.
Although we used subgroup analysis, we could not find a source of between-study heterogeneity or a significant difference within subgroups in most cases, which might be due to the limited number of included studies. Based on studies that evaluated the oral intake of R damascena, it seems that pain severity reduced more when the treatment was administrated in the form of oral drops, at higher dosages, and for longer duration. On the contrary, for topical applications of R damascena, it seems that lower dosage and shorter administration duration may have been more beneficial. Further studies are suggested to compare the effects of oral intake and topical application of R damascena on pain severity in different groups of participants with different administration durations, dosages, and forms.
Limitations
Initially, we did not receive any response or feedback from corresponding authors of the published studies in some cases when we requested further information via email. In the following, contact was made with the authors via phone call and the required details were obtained. Regarding one study,30 where the contact number of the author was not available, estimations were made based on our internal discussion and consensus. Secondly, according to pool data using meta-analysis, we compared the changes in baseline and end-of-trial values due to a minor variation in assessment time of pain severity after treatment. Hence, different choices of an endpoint might have led to different effect sizes or heterogeneities. Thirdly, the results obtained by subgroup analysis might be affected by the limited number of studies in each subgroup. Fourthly, we could not perform a dose-response analysis due to the limited number of included studies and the low variations of R damascena administration dosages and durations. Finally, all studies were conducted in Iran and nearly all (except two studies on topical application of the herb) recruited female participants only; hence, the findings may not be generalizable to men, or those outside Iran.
Conclusion
Although the growing trend of recent RCTs about pain-alleviating effects of topical application and oral intake of R damascena provides a scientific rationale for its clinical properties, the present meta-analysis indicated that oral intake of this herbal medicine had a non-significant alleviating effect on adults’ acute pain severity. Also, the topical application of R damascena had no pain-alleviating effect. Therefore, further robust RCTs are needed to elicit reliable conclusions in this regard.
Disclosures
The authors declared no conflicts of interest. No funding was received for this study.
References
- 1.Nunes H, Miguel MG. R damascena essential oils: A brief review about chemical composition and biological properties. Trends Phytochem Res. 2017;1(3):111-128. [Google Scholar]
- 2.Nayebi N, Khalili N, Kamalinejad M, Emtiazy M. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complement Ther Med 2017. Oct;34:129-140. 10.1016/j.ctim.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 3.Akram M, Riaz M, Munir N, Akhter N, Zafar S, Jabeen F, et al. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: a literature review. J Pharm Pharmacol 2020. Feb;72(2):161-174. 10.1111/jphp.13185 [DOI] [PubMed] [Google Scholar]
- 4.Nikbakht A, Kafi M. A study on the relationships between Iranian people and Damask Rose (R damascena) and its therapeutic and healing properties. Acta Hortic 2008;(790):251-254 . 10.17660/ActaHortic.2008.790.36 [DOI] [Google Scholar]
- 5.Mahboubi M. Rosa damascena as holy ancient herb with novel applications. J Tradit Complement Med 2015. Oct;6(1):10-16. 10.1016/j.jtcme.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boskabady MH, Shafei MN, Saberi Z, Amini S. Pharmacological effects of rosa damascena. Iran J Basic Med Sci 2011. Jul;14(4):295-307. [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari S, Zeenat F, Ahmad W, Ahmad I. Therapeutics and pharmacology of Gul-e-Surkh (R damascena Mill): an important Unani drug. Int J Adv Pharm Med Bioallied Sci. 2017;5(3):195-205. [Google Scholar]
- 8.Mohebitabar S, Shirazi M, Bioos S, Rahimi R, Malekshahi F, Nejatbakhsh F. Therapeutic efficacy of rose oil: A comprehensive review of clinical evidence. Avicenna J Phytomed 2017. May-Jun;7(3):206-213. [PMC free article] [PubMed] [Google Scholar]
- 9.Heydarirad G, Keyhanmehr AS, Mofid B, Nikfarjad H, Mosavat SH. Efficacy of aromatherapy with Rosa damascena in the improvement of sleep quality of cancer patients: A randomized controlled clinical trial. Complement Ther Clin Pract 2019. May;35:57-61. 10.1016/j.ctcp.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 10.Hamedi A, Zarshenas MM, Sohrabpour M, Zargaran A. Herbal medicinal oils in traditional Persian medicine. Pharm Biol 2013. Sep;51(9):1208-1218. 10.3109/13880209.2013.777462 [DOI] [PubMed] [Google Scholar]
- 11.Abbasijahromi A, Hojati H, Nikooei S, Jahromi HK, Dowlatkhah HR, Zarean V, et al. Compare the effect of aromatherapy using lavender and Damask rose essential oils on the level of anxiety and severity of pain following C-section: A double-blinded randomized clinical trial. J Complement Integr Med 2020. Sep;17(3). 10.1515/jcim-2019-0141 [DOI] [PubMed] [Google Scholar]
- 12.Amini R, Alizadeh F. Investigating musical effects and aromatherapy on anxiety and pain in patients undergoing surgery. Indian J Forensic Med Toxicol. 2018;12(4):170-176 . 10.5958/0973-9130.2018.00219.0 [DOI] [Google Scholar]
- 13.Ayan M, Tas U, Sogut E, Suren M, Gurbuzler L, Koyuncu F. Investigating the effect of aromatherapy in patients with renal colic. J Altern Complement Med 2013. Apr;19(4):329-333. 10.1089/acm.2011.0941 [DOI] [PubMed] [Google Scholar]
- 14.Babatabar Darzi H, Vahedian-Azimi A, Ghasemi S, Ebadi A, Sathyapalan T, Sahebkar A. The effect of aromatherapy with rose and lavender on anxiety, surgical site pain, and extubation time after open-heart surgery: A double-center randomized controlled trial. Phytother Res 2020. Oct;34(10):2675-2684. 10.1002/ptr.6698 [DOI] [PubMed] [Google Scholar]
- 15.Bikmoradi A, Harorani M, Roshanaei G, Moradkhani S, Falahinia GH. The effect of inhalation aromatherapy with damask rose (Rosa damascena) essence on the pain intensity after dressing in patients with burns: A clinical randomized trial. Iran J Nurs Midwifery Res 2016. May-Jun;21(3):247-254. 10.4103/1735-9066.180380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chughtai A, Navaee M, Alijanvand MH, Yaghoubinia F. Comparing the effect of aromatherapy with essential oils of R damascena and lavender alone and in combination on severity of pain in the first phase of labor in primiparous women. Crescent J Med Biol Sci 2018;5(4):312-319. [Google Scholar]
- 17.Hamdamian S, Nazarpour S, Simbar M, Hajian S, Mojab F, Talebi A. Effects of aromatherapy with Rosa damascena on nulliparous women’s pain and anxiety of labor during first stage of labor. J Integr Med 2018. Mar;16(2):120-125. 10.1016/j.joim.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 18.Nehbandani S, Galeh MR, Bordbari M, Koochakzai M. Comparison the effects of aromatherapy with rose extract and lavender on the pain of the active phase of labor in primipara women. Majallah-i Ilmi-i Danishgah-i Ulum-i Pizishki-i Kurdistan 2018;23(5):45-54. [Google Scholar]
- 19.Roozbahani N, Attarha M, Akbari Torkestani N, Amiri Farahani L, Heidari T. The effect of rose water aromatherapy on reducing labor pain in primiparous women. Complement Med J. 2015;5(1):1042-1053. [Google Scholar]
- 20.Seyyed-Rasooli E, Amiri M-R, Zamanzadeh V, Peron K, Aghakeshizadeh M. Effect of aromatherapy on anxiety and pain in patients undergoing cholecystectomy. Adv Herb Med. 2014;1(1):1-7. [Google Scholar]
- 21.Uysal M, Doğru HY, Sapmaz E, Tas U, Çakmak B, Ozsoy AZ, et al. Investigating the effect of rose essential oil in patients with primary dysmenorrhea. Complement Ther Clin Pract 2016. Aug;24:45-49. 10.1016/j.ctcp.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Vahaby S, Abedi P, Afshari P, Haghighizadeh MH, Zargani A. Effect of aromatherapy with rose water on pain severity of labor in nulliparous women: a random clinical trial study. Majallah-i Ilmi-i Danishgah-i Ulum-i Pizishki-i Rafsanjan 2016;14(12):1049-1060. [Google Scholar]
- 23.Heydari N, Abootalebi M, Tayebi N, Hassanzadeh F, Kasraeian M, Emamghoreishi M, et al. The effect of aromatherapy on mental, physical symptoms, and social functions of females with premenstrual syndrome: A randomized clinical trial. J Family Med Prim Care 2019. Sep;8(9):2990-2996. 10.4103/jfmpc.jfmpc_452_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ataollahi M, Amir Ali Akbari S, Mojab F, Roshanaie G. Effects of aromatherapy by Rceous on the severity and systemic symptoms of primary dysmenorrhea. Adv Nurs Midwifery. 2016;25(89):59-67. [Google Scholar]
- 25.Bani S, Hasanpour S, Mousavi Z, Mostafa Garehbaghi P, Gojazadeh M. The effect of R damascena extract on primary dysmenorrhea: a double-blind cross-over clinical trial. Iran Red Crescent Med J 2014. Jan;16(1):e14643. 10.5812/ircmj.14643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davaneghi S, Tarighat Esfanjani A, Safaiyan A, Fardiazar Z. Effective reduction of primary dysmenorrheal symptoms through concurrent use of n-3 fatty acids and R damascena extract (RDE). Prevent Care Nurs Midwif J. 2017;7(2):33-40. [Google Scholar]
- 27.Farnia V, Hojatitabar S, Shakeri J, Rezaei M, Yazdchi K, Bajoghli H, et al. Adjuvant R damascena has a small effect on SSRI-induced sexual dysfunction in female patients suffering from MDD. Pharmacopsychiatry 2015. Jul;48(4-5):156-163. 10.1055/s-0035-1554712 [DOI] [PubMed] [Google Scholar]
- 28.Farnia V, Tatari F, Alikhani M, Yazdchi K, Taghizadeh M, Sadeghi Bahmani D, et al. Rosa Damascena oil improved methadone-related sexual dysfunction in females with opioid use disorder under methadone maintenance therapy - results from a double-blind, randomized, and placebo-controlled trial. J Psychiatr Res 2017. Dec;95:260-268. 10.1016/j.jpsychires.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 29.Jamilian M, Jamilian H, Mirzaie S. Rose damascena vs. Omega-3 in the treatment of premenstrual syndrome: a randomized, and placebo-controlled clinical trial. Complement Med J. 2013;3(3):541-551. [Google Scholar]
- 30.Mostafa-Gharabaghi P, Tabatabei F, Abdollahi Fard S, Sayyah-Melli M. Evaluation of the effect of preemptive administration of R damascena extract on post-operative pain in elective cesarean sections. Afr J Pharm Pharmacol 2011;5(16):1950-1955. [Google Scholar]
- 31.Hoseinpour H, Peel SA, Rakhshandeh H, Forouzanfar A, Taheri M, Rajabi O, et al. Evaluation of Rosa damascena mouthwash in the treatment of recurrent aphthous stomatitis: a randomized, double-blinded, placebo-controlled clinical trial. Quintessence Int 2011. Jun;42(6):483-491. [PubMed] [Google Scholar]
- 32.Khatibi M, Mohammadian S, Arezoobakhsh M. The comparison of the effectiveness of Rose extract and diphenhydramine on aphthus ulcers in oral mucosa. J Res Dent Sci. 2017;14(2):70-76. [Google Scholar]
- 33.Mahabob N, Mohan J. Preparation of mouthwash and gel from R damascena mill and evaluating its effectiveness-An in vivo analysis. J Pharm Bioallied Sci 2019. May;11(Suppl 2):S198-S202. 10.4103/JPBS.JPBS_294_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niazi M, Hashempur MH, Taghizadeh M, Heydari M, Shariat A. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med 2017. Oct;34:35-41. 10.1016/j.ctim.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 35.Shirazi M, Mohebitabar S, Bioos S, Yekaninejad MS, Rahimi R, Shahpiri Z, et al. The effect of topical R damascena (Rose) oil on pregnancy-related low back pain: a randomized controlled clinical trial. J Evid Based Complementary Altern Med 2017. Jan;22(1):120-126. 10.1177/2156587216654601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadeghi Aval Shahr H, Saadat M, Kheirkhah M, Saadat E. The effect of self-aromatherapy massage of the abdomen on the primary dysmenorrhoea. J Obstet Gynaecol 2015. May;35(4):382-385. 10.3109/01443615.2014.958449 [DOI] [PubMed] [Google Scholar]
- 37.Anbari S, Estaji Z, Rastaqhi S. Assessment effect of R damascena juice aromatherapy on elderly chronic musculoskeletal pain in Sabzevar retirement clubs. Salmand Iran J Ageing 2018;13(2):250-261 . 10.32598/sija.13.2.250 [DOI] [Google Scholar]
- 38.Bastani F, Samady Kia P, Haghani H. The effect of inhalation aromatherapy with damask rose (R damascena) on the pain of elderly after knee arthroplasty. J Client-Cent Nurs Care. 2017;3(2):153-160 . 10.32598/jccnc.3.2.153 [DOI] [Google Scholar]
- 39.Mahabob N, Mohan J, Gunasekaran S. Clinical effectiveness of R damascena mill in the management of oral mucosal lesions. World J Pharm Med Res. 2018;4(9):155-160. [Google Scholar]
- 40.Mahabob N, Mohan J, Gunasekaran S. Anti-inflammatory and analgesic properties of R damascena mill - an in vitro analysis. World J Pharm Med Res. 2018;4(3):321-325. [Google Scholar]
- 41.Lakhan SE, Sheafer H, Tepper D. The effectiveness of aromatherapy in reducing pain: a systematic review and meta-analysis. Pain Res Treat 2016;2016:8158693. 10.1155/2016/8158693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasiri M, Torkaman M, Feizi S, Bigdeli Shamloo MB. Effect of aromatherapy with Damask rose on alleviating adults’ acute pain severity: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med 2021. Jan;56:102596. 10.1016/j.ctim.2020.102596 [DOI] [PubMed] [Google Scholar]
- 43.Sadeghi Aval Shahr H, Saadat M. khairkhah M, Saadat E. The effect of aromatherapy with rose oil on primary dysmenorrhea. Complement Med J. 2014;4(2):787-797. [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009. Jul;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mostafa-Gharabaghi P, Delazar A, Mostafa Gharabaghi M, Jafari Shobeiri M, Khaki A. The view of cesarean pain after preemptive use of R damascena extract in women with elective cesarean section. World Sci J. 2013;4:226-235. [Google Scholar]
- 46.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). 2011 [cited 2019 August 3]. Available from: https://handbook-5-1.cochrane.org/index.htm#front_page.htm.
- 47.Koohpayeh SA, Hosseini M, Nasiri M, Rezaei M. Effects of Rosa damascena (Damask rose) on menstruation-related pain, headache, fatigue, anxiety, and bloating: A systematic review and meta-analysis of randomized controlled trials. J Educ Health Promot 2021. Jul;10:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nik Shafii NA, Yaacob LH, Ishak A, Kadir AA. Traditional and complementary medicine use in knee osteoarthritis and its associated factors among patients in Northeast Peninsular Malaysia. Oman Med J 2018. Mar;33(2):148-153. 10.5001/omj.2018.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Divakar MC, Al-Siyabi A, Varghese SS, Rubaie MA. The practice of ethnomedicine in the Northern and Southern provinces of Oman. Oman Med J 2016. Jul;31(4):245-252. 10.5001/omj.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arruda AP, Zhang Y, Gomaa H, Bergamaschi CC, Guimaraes CC, Righesso LA, et al. Herbal medications for anxiety, depression, pain, nausea and vomiting related to preoperative surgical patients: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019. May;9(5):e023729. 10.1136/bmjopen-2018-023729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hongratanaworakit T. Relaxing effect of rose oil on humans. Nat Prod Commun 2009. Feb;4(2):291-296. 10.1177/1934578X0900400226 [DOI] [PubMed] [Google Scholar]


