ABSTRACT
Dysbiosis of the vaginal microbiome as a result of overgrowth of anaerobic bacteria, such as Gardnerella vaginalis, and low levels of “healthy” lactobacilli leads to bacterial vaginosis (BV), usually associated with a low-grade inflammatory process. Despite appropriate antibiotic treatment, G. vaginalis-associated BV is characterized by significant recurrence. The use of probiotics could be an interesting alternative therapy due to their ability to rebalance vaginal microbiota. In this study, we investigated the effects of a well-characterized probiotic strain, Lacticaseibacillus rhamnosus Lcr35, on epithelial vaginal and dendritic cell (DC) immune responses after G. vaginalis infection. In an in vitro coculture model with human monocyte-derived dendritic cells and a vaginal epithelial cell (VEC) monolayer, the Lcr35 strain induced DC activation, as evidenced by the induction of maturation and synthesis of interleukin-8 (IL-8) and CCL-20 chemokines upon apical challenge of the VECs by G. vaginalis. Analysis of the vaginal epithelial response showed that the presence of Lcr35 significantly increased the production of the proinflammatory cytokines IL-8 and IL-1β and human β-defensin 2 (HBD-2), whereas the concentration of secretory leukocyte protease inhibitor (SLPI) was decreased in G. vaginalis-infected vaginal epithelial cells. Treatment with recombinant SLPI was associated with upregulation of Lcr35-stimulated IL-8 and HBD-2 production. These results suggest that inhibition of SLPI by Lcr35 in vaginal epithelial cells contributes to the host defense response against G. vaginalis infection.
KEYWORDS: Gardnerella vaginalis, Lactobacillus, antimicrobial peptides, cytokines, dendritic cells, epithelial cells, vaginosis
INTRODUCTION
The human vaginal microbiome is a complex bacterial community that interacts closely with vaginal epithelial cells impacting the mucosal immune response and its responses to pathogenic bacteria. A healthy vaginal microbiome is dominated by Lactobacillus, including Lactobacillus crispatus, Lactobacillus jensenii, Lactobacillus gasseri, and Lactobacillus vaginalis, resulting in a dynamic balance that inhibits overgrowth of potential anaerobic pathogens, such as Gardnerella vaginalis, Atopobium spp., and Prevotella spp. (1–3).
The female reproductive tract (FRT) can be divided into two immunological regions, the upper (endocervix, uterus, and oviduct) and lower (vagina and ectocervix) FRT. In the lower FRT, interactions between vaginal cells, lactobacilli, and their metabolic products create a physical and immunological barrier against pathogens that contributes to the prevention of invasion by exogenous microbes and regulation of the inflammatory response (4–6).
Despite these local host defense mechanisms, as many as 75% of women may experience an occasional vaginal infection, and some 5% to 10% suffer from recurrent bacterial vaginosis (BV) (7, 8).
BV is characterized by a polymicrobial imbalance, or dysbiosis, of the natural microflora of the cervicovaginal (CV) space. G. vaginalis is found in up to 94% of all cases of BV and has recently been suggested as the main “early colonizer species” that displaces lactobacilli and forms a biofilm, thereby creating a favorable environment for colonization by BV-associated bacteria, such as Prevotella bivia, Atopobium vaginae, and Megasphaera type 1 (9). BV can also produce a state of local immunosuppression that increases susceptibility to HIV and other sexually transmitted diseases (8, 10). Unlike vulvovaginal candidiasis and vaginitis, BV is typically distinguished by a lack of inflammation: IL-8 concentrations and leukocyte counts are not significantly greater in BV-positive women than in healthy control subjects (11–13). A deficiency in antimicrobial peptides, such as human β-defensin-1 and -2, has also been reported in vaginal fluid from women with BV (14). In contrast, vaginal interleukin-1β (IL-1β) levels are largely increased (13, 15), suggesting that BV-associated G. vaginalis interacts with the host vaginal immunity specifically to dampen IL-8 production. BV has also been associated with a significant reduction in vaginal levels of the secretory leukocyte protease inhibitor (SLPI). SLPI is produced by both epithelial and immune cells (16) and is bactericidal for pathogens such as Neisseria gonorrhoeae (17); it has also been shown to participate in the mucosal defense by reducing inflammation (18).
The currently recommended treatment for BV is antibiotics (metronidazole and clindamycin), but the rates of recurrence following antibiotic treatment are extremely high and >50% of women have recurrent episodes within 6 to 12 months (19). In this context, probiotics appear as an interesting alternative strategy due to their role in maintaining a healthy vaginal flora (3, 20). Their beneficial action could be related to the competitive exclusion of pathogenic bacteria, competition for nutrients, production of antimicrobial substances, and/or activation of the immune system (21, 22).
Lactobacillus rhamnosus Lcr35, recently renamed Lacticaseibacillus rhamnosus Lcr35, is a well-characterized probiotic strain that has been used in clinical practice for more than 50 years, in particular to restore vaginal health (GynOphilus). The antimicrobial effects of this probiotic strain have been largely documented over the past decade (23, 24), but little is known about its interactions with the vaginal immune system. In the present study, we assessed the immunomodulatory effect of Lcr35 in innate vaginal cells infected by G. vaginalis. We show that Lcr35 has an immunostimulatory effect on vaginal cells infected by G. vaginalis by activating both epithelial and dendritic cell (DC) responses. These effects are associated with modulation of SLPI production, offering a novel mechanism for the regulation of innate response by lactobacilli during G. vaginalis infection.
RESULTS
Lacticaseibacillus rhamnosus Lcr35 stimulates the cross talk between epithelial and dendritic cells when coincubated with Gardnerella vaginalis.
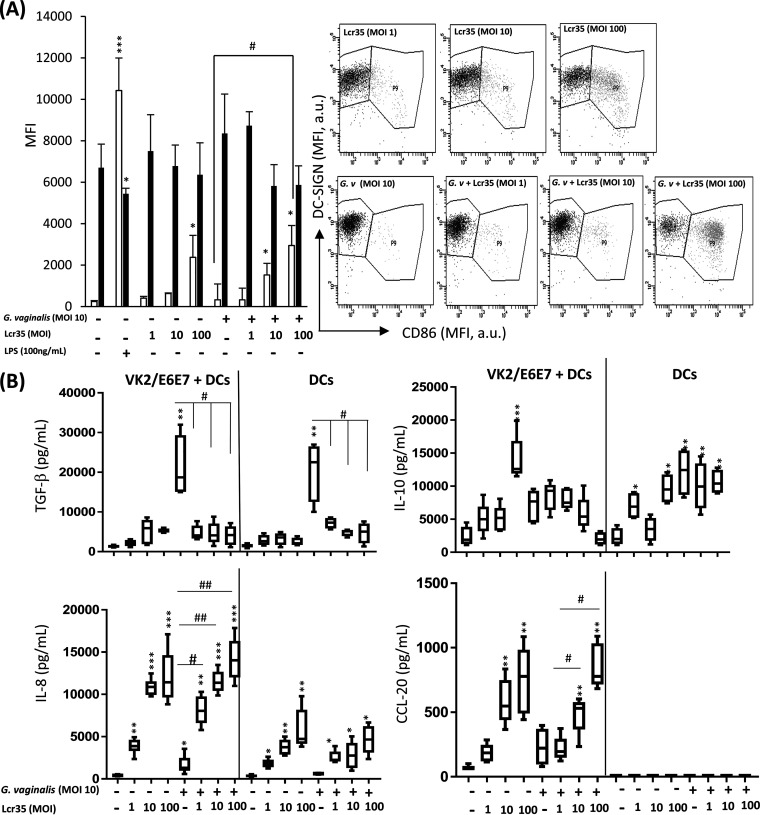
In a coculture model composed of human monocyte-derived dendritic cells in the lower part and vaginal epithelial cell monolayer (VK2/E6E7) in the upper part (see Fig. S1 in the supplemental material), the addition of G. vaginalis in the upper part did not induce any change in the DC surface phenotype compared to that of immature DCs as measured by determination of CD86 and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) expression (Fig. 1A). In contrast, the addition of Lcr35 in the upper part of the same coculture system induced a dose-dependent higher surface expression of CD86 and a lower surface expression of DC-SIGN than noninfected cells (Fig. 1A). Analysis of other activation markers such as CD40, CD206, CD83, and HLA-DR confirmed that incubation with Lcr35 in the presence or absence of G. vaginalis induced maturation of DCs (see Fig. S2B and C in the supplemental material). In addition, increased expression of CD86 and decreased expression of DC-SIGN were also observed when both Lcr35 and G. vaginalis were added directly onto DCs (see Fig. S3 in the supplemental material). For controls, an immature DC profile was observed with low levels of CD86 and high levels of DC-SIGN expression, whereas addition of lipopolysaccharide (LPS) at the apical surface of the epithelial monolayer induced a phenotype characteristic of fully mature DCs with increased levels of CD86 combined with decreased levels of DC-SIGN (Fig. 1A). Determination of the concentrations of immune attractive chemokines (CCL-20 and IL-8) and immunosuppressive cytokines (transforming growth factor β [TGF-β] and IL-10) in the lower compartment of the cell coculture model (corresponding to the basolateral surface of the epithelial monolayer) showed an increase in both TGF-β and IL-8 production when the cells were incubated with G. vaginalis alone (multiplicity of infection [MOI], 10). Incubation of the same cells with Lcr35 induced dose-dependent increased secretion of IL-8, IL-10, and CCL-20 but not TGF-β. Infection of DCs with both bacteria, G. vaginalis and Lcr35, resulted in phenotypes similar to those observed when cells were incubated with Lcr35 alone (Fig. 1B). Direct contact of Lcr35 with DCs induced an increase of IL-8 secretion and a decrease of TGF-β and IL-10 levels compared to those of noninfected cells in the presence or absence of G. vaginalis (Fig. 1B). No production of CCL-20 was detected when DCs were directly incubated with bacteria (Fig. 1B).
FIG 1.
Effect of Lcr35 on G. vaginalis-infected dendritic cells in a coculture model. Human monocyte-derived dendritic cells were exposed indirectly through the vaginal epithelial cell monolayer to UV-inactivated G. vaginalis (MOI, 10) alone or UV-inactivated Lcr35 (MOI, 1 to 100) for 48 h. (A) The effects of DC functional maturation were determined by measuring the DC-surface expression of DC-SIGN (black bars) and CD86 (white bars) by flow cytometry. The dot plots and histograms show MFI values on gated DCs. DC-SIGN/CD86 dot plots gated on human DCs. (B) The secretions of cytokines TGF-β, IL-10, IL-8, and CCL-20 were measured in the supernatant of coculture VK2/E6E7 + DC (lower part) or DC alone by ELISA. Values are the means ± SEM; n = 4 to 5; *, P < 0.05; **, P < 0.01 compared with noninfected DCs. #, P < 0.05; #, P < 0.01 compared with DCs infected with G. vaginalis.
Lcr35 with and without Gardnerella vaginalis induces human epithelial vaginal cell response in a NF-κB-dependent manner.
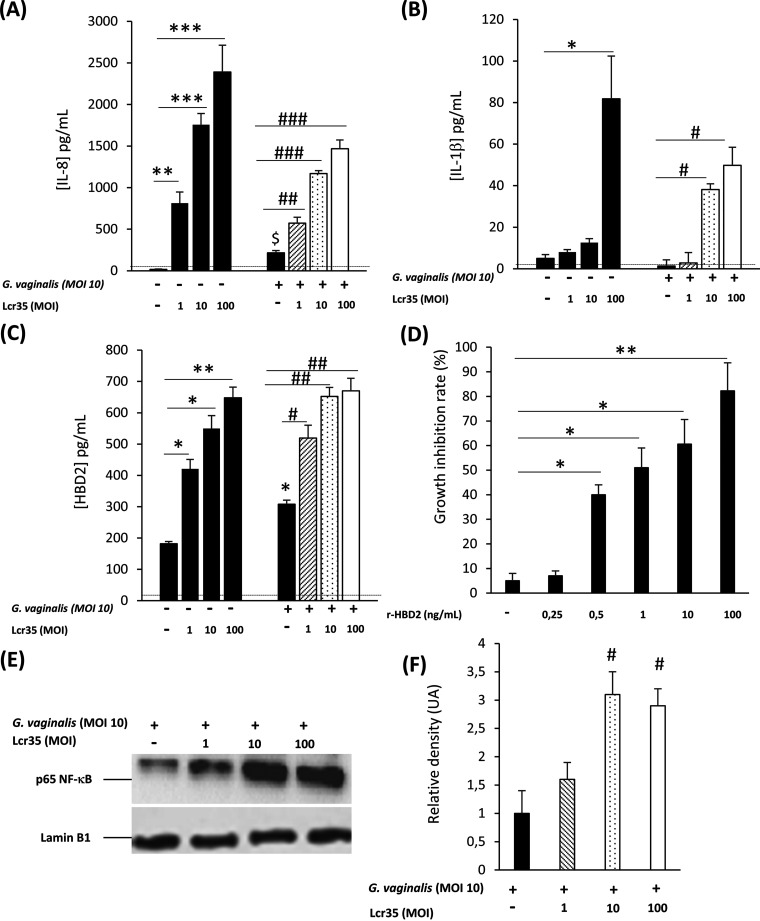
To determine whether Lcr35 influences the immune response of VK2/E6E7 cells to G. vaginalis, the production of inflammatory cytokines by cells incubated separately with Lcr35 or G. vaginalis was first assessed. After incubation with Lcr35, the cells specifically induced the production of proinflammatory cytokine IL-8 and IL-1β and antimicrobial human β-defensin 2 (HBD-2) in a dose-dependent manner, whereas no significant change in the production of IL-6 and IL-10 was observed (Table 1). Incubation with different doses of G. vaginalis (MOI from 1 to 100) induced a low vaginal epithelial response, as shown by the absence of significant change in all innate cell markers except IL-8 (Table 1).
TABLE 1.
Production of innate vaginal components by VK2/E6E7 vaginal epithelial cells after incubation with Lcr35a
| Mediator | Production of innate vaginal components (pg/mL) |
||||||
|---|---|---|---|---|---|---|---|
| NI | Lcr35 |
G. vaginalis
|
|||||
| MOI, 1 | MOI, 10 | MOI, 100 | MOI, 1 | MOI, 10 | MOI, 100 | ||
| Cytokine | |||||||
| IL-8 | 16.8 ± 6.1 | 814.5 ± 133.3** | 1,757.9 ± 135.9*** | 2,391.2 ± 322.7*** | 192.2 ± 8.6 | 216.1 ± 28.1 | 317.3 ± 34.5 |
| IL-1β | 5.2 ± 1.8 | 7.9 ± 1.2 | 12.6 ± 1.9 | 81.8 ± 20.6* | 3.1 ± 1.3 | 4.7 ± 2.1 | 18.2 ± 3.6 |
| IL-6 | ND | ND | ND | ND | ND | ND | ND |
| IL-10 | ND | ND | ND | ND | ND | ND | ND |
| Antimicrobial peptide | |||||||
| HBD-2 | 182.0 ± 7.2 | 421.6 ± 30.2* | 550.3 ± 41.8* | 648.9 ± 34.4** | 350.3 ± 20.8* | 335.4 ± 11.2* | 318.9 ± 10.4* |
VK2/E6E7 cells were incubated with Lcr35 or G. vaginalis (MOI, 1 to 100) for 6 h. Subsequently, cytokines or host defense peptides concentration were measured in the culture supernatants by ELISA. Each value is the mean ± SEM of n = 3 to 8 independent experiments. ND, not detected. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with noninfected cells (NI).
The cells were then treated with both Lcr35 and G. vaginalis. Increased production of IL-8 and IL-1β in an Lcr35 MOI-dependent manner was observed compared to that of cells infected with G. vaginalis alone (Fig. 2A and B). In all cases, no cytotoxic effect was observed (see Fig. S4 in the supplemental material), and no significant change in the production of IL-6 and IL-10 was observed (data not shown). Similar assays performed with other Lactobacillus species (L. vaginalis, L. gasseri, Lactobacillus plantarum, and L. crispatus) gave rise to similar results (see Fig. S5 in the supplemental material).
FIG 2.
Effect of Lcr35 on innate immune response in G. vaginalis-infected vaginal epithelial cells. VK2/E6E7 cells were infected with G. vaginalis (MOI, 10) alone or with Lcr35 at different MOIs (MOI, 1 to 100). IL-8 (A), IL-1β (B), and HBD-2 (C) concentrations were analyzed 6 h postinfection by ELISA. The detection limits of IL-8 and IL-1β were 312 and 39 pg/mL, respectively. (D) Growth inhibition rates of G. vaginalis after treatment with various concentrations of HBD-2 for 4 h. (E) The presence of the p65 NF-κB and Lamin B1 proteins were detected by Western blotting in cellular extracts of VK2/E6E7 cells stimulated for 3 h with G. vaginalis alone or with Lcr35. (F) Densitometric analysis of the data in Fig. 2E by using Image Lab 2.0 software (n = 3). Representative data of 3 to 8 independent experiments. Values are the means ± SEM; *, P < 0.05; **, P < 0.01 compared with uninfected cells. #, P < 0.05; ##, P < 0.01 compared with G. vaginalis-infected cells.
Likewise, the production of antimicrobial peptide HBD-2 increased significantly in vaginal epithelial cells treated by both Lcr35 and G. vaginalis compared to that of cells infected with G. vaginalis alone (Fig. 2C). Incubation of purified HBD-2 in the range of 0.5 to 100 ng/mL impaired G. vaginalis growth (Fig. 2D) but had no impact on Lcr35 growth (data not shown). Moreover, SLPI at the range of 0.2, 0.5, 1, 5 and 10 ng/mL did not show antimicrobial effect with Lcr35 or G. vaginalis (data not shown).
The level of p65 NF-κB in VK2/E6E7-infected cells was then measured to determine if the NF-κB pathway was involved in response to the presence of G. vaginalis and Lcr35. Infection with both G. vaginalis and Lcr35 resulted in larger amounts of p65 NF-κB protein than cells infected with the pathogen alone, as shown by Western blotting assays (Fig. 2E).
Modulation of innate immune factor production by Lcr35 under the control of SLPI production in G. vaginalis-infected cells.
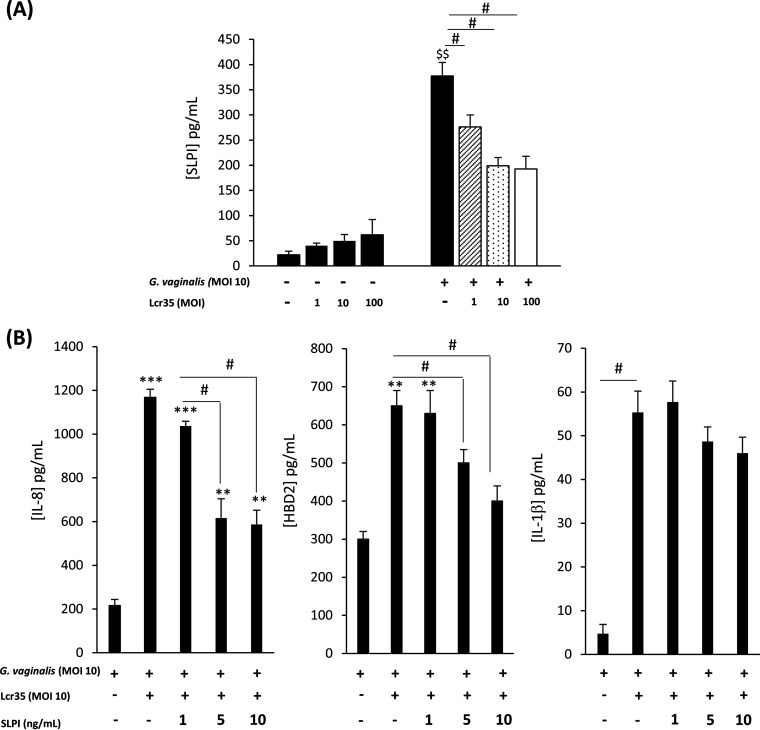
To further characterize the vaginal epithelial innate immune response to Lcr35 and/or G. vaginalis, we monitored SLPI host defense peptide production in vaginal cells. Incubation with Lcr35 did not elicit secretion of SLPI whatever the bacterial concentration tested, whereas a strong induction was observed in G. vaginalis-infected vaginal cells (Fig. 3A). Coincubation of the two bacterial strains, Lcr35 and G. vaginalis, with VK2/E6E7 cells resulted in lower SLPI levels in the cell supernatants than in cells infected with G. vaginalis alone (Fig. 3A).
FIG 3.
Modulation of innate immune factor production by Lcr35 under the control of SLPI production in G. vaginalis-infected cells. VK2/E6E7 cells were infected with G. vaginalis (MOI, 10) alone or with Lcr35 at different MOIs (MOI, 1 to 100). (A) SLPI concentration was analyzed 6 h postinfection by ELISA. Recombinant SLPI protein was added to VK2/E6E7 cells incubated with G. vaginalis (MOI, 10) and Lcr35 (MOI, 10). (B) IL-8, HBD-2, and IL-1β concentrations were detected by ELISA in supernatants after 6 h of incubation. Values are the means ± SEM; n = 3 to 4; $$, P < 0.05 compared with uninfected cells; *, P < 0.05; **, P < 0.01 compared with G. vaginalis-infected cells. #, P < 0.05; #, P < 0.01 compared with vaginal cells infected with G. vaginalis and Lcr35.
Because SLPI has immunomodulatory properties via inhibition of the NF-κB pathway (25), we examined the effect of purified peptide on G. vaginalis growth and proinflammatory cytokine production in G. vaginalis-infected cells. The addition of recombinant SLPI significantly decreased IL-8 and HBD-2 production compared to that observed with G. vaginalis and Lcr35 (MOI, 10) in vaginal epithelial cells (Fig. 3B). No significant decrease in IL-1β concentration was observed (Fig. 3B).
DISCUSSION
Several studies have previously demonstrated the efficacy of the probiotic strain Lcr35 in the prevention of recurrent vulvovaginal candidiasis and bacterial vaginosis (23, 24, 26, 27). Clinical studies performed with GynOphilus, the commercial vaginal form of Lcr35, reported a positive effect on the recurrence of infections and patient quality of life (26, 28). In the present study, we showed that Lcr35 modified the host defense response of innate immune cells exposed to the pathogen G. vaginalis, the key player in the pathogenesis of BV.
Using a coculture model mimicking the vaginal epithelium with both vaginal epithelial cells (VK2/E6E7 cell line) and adjacent DCs, we showed that apical challenge of the epithelial cells with Lcr35 induces a dose-dependent maturation of DCs, whereas incubation with G. vaginalis had no effect. When the two bacteria were coincubated on the cells, the immunostimulatory effect of Lcr35 was not modified. Analysis of cytokine and chemokine concentrations in the basolateral compartment demonstrated an induction of chemokine secretion (IL-8 and CCL20) and a decrease in the TGF-β immunosuppressive cytokine when challenged with Lcr35 regardless of the presence of G. vaginalis. Altogether these data suggest that (i) Lcr35 is able to activate DCs across the epithelial barrier and (ii) vaginal epithelial cells have an active role during G. vaginalis infection and are able to “sense” bacteria. Similar results were obtained in a murine coculture model (intestinal epithelial cells and bone marrow-derived DCs) mimicking the intestinal barrier by Grangette et al., who observed differential responses in intestinal epithelial activation and DC maturation between Escherichia coli and lactobacilli strains (29). These findings suggest that vaginal epithelial cells, like intestinal epithelial cells, have an “immunological filtering” role that predicts DC activation and consequently modulates adaptive response.
To characterize this signal, we first assessed the effect of Lcr35 on VK2/E6E7 cell response by measuring the release of several cytokines. We showed that Lcr35 stimulates production by VK2/E6E7 cells of the proinflammatory cytokines IL-8 and IL-1β and the antimicrobial peptide HBD-2 via the NF-κB pathway. Although the immunomodulatory properties of lactobacilli are strain-specific, we observed that this specific pathway was also induced by four other lactobacilli species: L. vaginalis, L. gasseri, L. crispatus, and L. plantarum. In the vagina, lactobacilli have been described as noninducers of cytokine production (5, 30, 31). Nevertheless, vaginal epithelial cells possess Toll-like receptor (TLR), and some in vitro studies propose that they respond to both commensal bacteria and invading pathogens by producing cytokines and immune mediators (32, 33). In addition, incubation with Lactobacillus iners has been associated with higher production of IL-6 and IL-8 by VK2 cells (34), colonization of vaginal epithelial cells by L. jensenii led to increased IL-6 and tumor necrosis factor alpha (TNF-α) synthesis (35), and L. rhamnosus GG, L. rhamnosus LC705, and L. rhamnosus GR-1 were able to induce IL-8, IL-6, and TNF-α production in VK2 cells (36). The effect of lactobacilli on vaginal epithelial cells, in the absence of another immune stimulus, is therefore still a controversial issue. However, the Lcr35-induced inflammatory effect observed in this study is moderate compared with that induced by the pathogen Candida albicans with the level of IL-8 reaching 2,635 ± 213 μg/mL (our unpublished data). Tolerance of Lcr35 (GynOphilus) was previously evaluated in clinical studies, and no inflammatory signs (tingling, dryness, burning, itching, and pelvic pain) were reported (37). The same clinical study reported high basal levels of some cytokines in healthy women (12, 13, 38) in accordance with our in vitro results. All of these data suggest that a “basal moderate inflammatory state” is naturally induced in the vaginal environment to activate vaginal innate and adaptive immunity against pathogens.
In vitro, infection by G. vaginalis has been associated with divergent effects on immune cell populations and the cytokine network in the vaginal mucosa. Clinically, BV is mainly differentiated from vaginitis by the absence of an inflammatory response and is characterized by the absence of the chemotactic effect of IL-8 correlated the lack of leukocyte recruitment and increased vaginal antigen-specific immune levels (39–41). Our results show that G. vaginalis induces a low inflammatory response in vaginal epithelial cells characterized by low levels of IL-8, IL-6, and IL-1β. When Lcr35 was added together with G. vaginalis on the upper part of the cell monolayer, the response was exacerbated compared to that observed with G. vaginalis alone in terms of proinflammatory and antimicrobial signals involving the NF-κB pathway. Most studies describe an anti-inflammatory effect of Lactobacillus strains when they are associated with proinflammatory vaginal pathogens like C. albicans or Atopobium vaginae or used in pretreatment before inflammatory stimuli (5, 30, 42, 43). In our study, the effects of Lcr35 were assessed on epithelial cells infected with G. vaginalis, a noninflammatory pathogen. In this tripartite relation, Lcr35—and other Lactobacilli species—induced an immunostimulatory effect. We thus hypothesized that lactobacilli are able to permit immune homeostasis through an interaction with epithelial cells. In a low inflammatory environment, such as the one encountered in BV, Lactobacillus could induce an “alert” immunostimulatory signal. However, when the vaginal cavity is contaminated by highly proinflammatory pathogens, lactobacilli could decrease the production of cytokines to control the inflammation and therefore avoid deleterious hyperinflammation. Although the precise mechanism underlying the Lcr35 immunomodulatory action remains to be elucidated, exogenous addition of Lcr35 is also likely to restore the balance of microbiota and thus contribute to an effective vaginal immune defense against pathogens. Specific induction of HBD-2 by Lcr35 could also be an effective mechanism to increase antimicrobial defense against both G. vaginalis and bacterial vaginosis-associated bacteria (44, 45). In our study, G. vaginalis alone strongly induced SLPI production by vaginal epithelial cells, whereas decreased levels of SLPI were observed in vivo in vaginal fluids of BV-positive women (46). This multifunctional antimicrobial protein expressed at mucosal surfaces plays a key role in infectious and inflammatory processes and suppresses inflammatory responses and proinflammatory cytokines (e.g., IL-8 and HBD-2) by controlling the activity of NF-κB in various immune cells (25, 47–49). Our findings showed that the induction of IL-8 and HBD-2 by Lcr35 in G. vaginalis-infected cells involves the inhibition of SLPI, which suggests a novel role of lactobacilli in the modulation of the epithelial immune response. The direct or indirect action of Lcr35 on the SLPI response still remains to be elucidated in the immune escape mechanism during G. vaginalis infection. The bactericidal activity of Lcr35 against G. vaginalis (27) could in part explain the anti-SLPI mechanisms. However, Lcr35 could also directly interact with epithelial cells as demonstrated by its highly dynamic adhesion abilities to vaginal ectocervical and endocervical cell lines (27). The mechanism(s) underlying the process by which SLPI is regulated by Lcr35 is likely multifaceted.
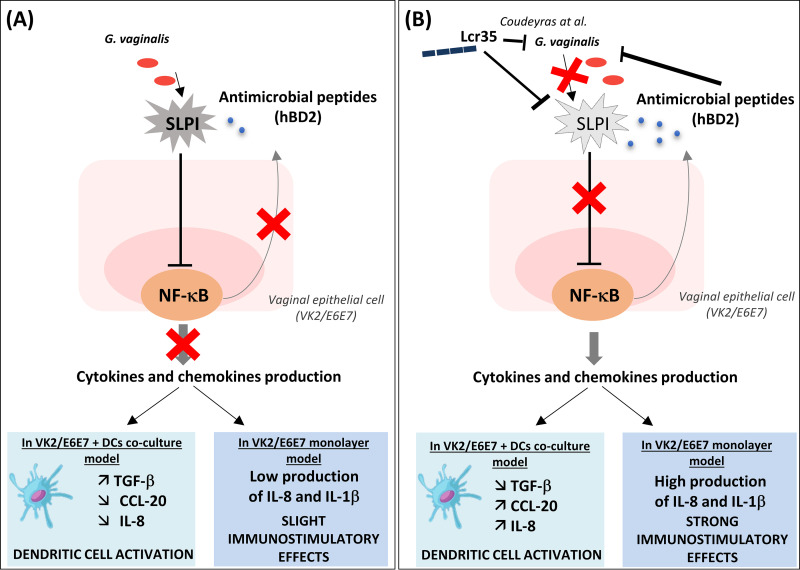
In summary, this study shows that the probiotic Lcr35 induces host responses against G. vaginalis by modulating the synthesis of both proinflammatory cytokines and host defense peptides in vaginal cells and also by enhancing the dialogue between epithelial and dendritic cells (Fig. 4). These immunostimulatory properties could restore a beneficial local immune response during G. vaginalis infections in line with the positive effects observed in clinical studies.
FIG 4.
Model for immunostimulatory effect of Lcr35 on G. vaginalis-infected vaginal epithelial cells. G. vaginalis induces the secretion of SLPI, which interacts with the NF-κB pathway and can inhibit immune cell recruitment (A). Lcr35 counteracts these immunosuppressive effects by SLPI inhibition, which leads to secretion of immunostimulatory cytokines and defensins and an increase in immune cell (e.g., dendritic cells) recruitment (B).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Lacticaseibacillus rhamnosus 35 (Lcr35), Lactobacillus crispatus BLL2005, Lactobacillus vaginalis CIP 105932, Lactobacillus gasseri CIP 102991-43, and Lactobacillus plantarum CIRM653 (24, 50) were grown without agitation in De Man, Rogosa, Sharpe (MRS) medium (Becton, Dickinson, Franklin Lakes, NJ, USA) at 37°C overnight. G. vaginalis ATCC 14018 was grown in brain heart infusion (BHI) medium (Becton, Dickinson) supplemented with maltose (0.1%), glucose (0.1%), yeast extract (1%), and horse serum (10%) in 5% CO2 at 37°C for 72 h.
Bacterial cells were harvested by centrifugation (11,000 × g for 10 min), and the pellet was resuspended in appropriate cell culture medium. Optical density (OD) was measured at 620 nm to adjust the final concentration of the bacterial suspension, and the exact number of CFU was determined by plating serial dilutions of the inoculum on MRS or BHI plates (Becton, Dickinson).
For experiments with DCs, the bacterial cells were inactivated by exposure to UV for 1 h. Successful inactivation of bacteria was assessed by plating the final suspension on agar plates.
Cultures of epithelial cells and infections.
Human vaginal (VK2/E6E7, ATCC CRL-2616) epithelial cells were grown to confluence in keratinocyte serum-free medium (Thermo Fisher Scientific) supplemented with 0.1 ng/mL human recombinant epidermal growth factor (EGF), 0.05 mg/mL bovine pituitary extract, and additional calcium chloride at 44.1 mg/L (final concentration, 0.4 mM) (all from Sigma-Aldrich). For bacterial infection, cells were seeded in 24- or 6-well tissue culture plates (Thermo Fisher Scientific) and incubated with G. vaginalis and/or Lcr35 at a multiplicity of infection of 1 to 100 in complete medium in the presence or absence of 10 to 100 ng/mL recombinant human SLPI (R&D Systems, Minneapolis, MN). Following infection, cells were washed, and (i) proteins were extracted from lysed cells (3 h postinfection), and (ii) supernatants were collected (6 h postinfection) to determine cytokine secretion in the supernatants.
In vitro differentiation of monocyte-derived dendritic cells.
DCs were generated from peripheral blood mononuclear cells (PBMC) obtained from the local French blood agency (Etablissement Français du Sang [EFS], Saint-Etienne). Blood donation requires the systematic information of the volunteers (article R.1221-5 of the Public Health Code, 01/12/2009 and 06/11/2006 decrees) and written informed consents were obtained by EFS from all donors involved in our study.
Briefly, PBMCs were isolated from the buffy coat of healthy volunteers by Ficoll-Histopaque (Sigma, Saint-Quentin Fallavier, France) density gradient centrifugation. For phenotypic assays, the PBMCs were washed twice with RPMI 1640 (Cambrex Bio Science, Verviers, Belgium), and the monocytes were then isolated by adherence (2 h). The surface of the plates (75 cm2 flasks; BD Falcon, Le Pont de Claix, France) was precoated with 1 μg/mL poly-l-lysine (PLL) (Sigma) for 2 h at 4°C. Conversely, for transcriptional assays, the PBMCs were resuspended in PBS supplemented with 2% fetal calf serum (FCS) and 1 mM EDTA at a final concentration of 5.107 cells/mL, and the monocytes were purified by negative selection using the EasySep human monocyte enrichment kit as recommended by the manufacturer (StemCell Technologies, Grenoble, France). In both cases, the monocytes were then cultured for 5 days in RPMI 1640 supplemented with 1% l-glutamine (Sigma), 10% FCS (Biowest-Abcys, Paris, France), and 0.5% penicillin-streptomycin (Sigma) and containing 500 U/mL IL-4 (R&D systems, Lille, France) and 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (from R&D systems). After 3 days of incubation, one-half volume of fresh medium containing 2× doses of IL-4 and GM-CSF was added to each well. The purity of the DCs was assessed by flow cytometry analysis using a marker highly specific of the DC lineage, the DC-SIGN, and was always above 90% (see Fig. S2A in the supplemental material).
Vaginal epithelial and dendritic cell coculture.
VK2/E6E7 cells were seeded (2.5 × 105 cells/well) on a 6-well format cell culture Transwell insert (3-μM nucleopore size; Costar, Corning Inc., USA). The medium was changed every 2 days, and cells were cultured until confluence. For coculture experiments, inserts containing confluent vaginal epithelial cell monolayers were transferred to six-well plates containing DCs (2 × 106 cells/well in 3 mL complete RPMI) (see Fig. S1 in the supplemental material). The apical surface of VK2/E6E7 monolayers was challenged by addition of UV-treated bacteria in the upper chamber of each well. For direct interaction, DCs (2 × 106 cells/well in 3 mL complete RPMI) were directly challenged by addition of the bacteria at 1 × 107 CFU/well or LPS (10 ng/mL). All plates were incubated at 37°C in a 5% CO2/95% air atmosphere for 48 h. After incubation, cells were collected, centrifuged, and resuspended in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) (Sigma). Cell surfaces were stained with the appropriate fluorescence-labeled murine antibodies as follows: APC-Cy7-conjugated anti-CD14 (specific molecule of the monocytes, coreceptor of the LPS), PE-conjugated anti-CD86 (costimulatory molecule, activation marker), fluorescein isothiocyanate (FITC)-conjugated anti-CD83 (specific marker of mature DCs whose biological functions are not yet clear), PE-conjugated anti-HLA-DR, PerCP-Cy5.5-conjugated anti-DC-SIGN (CD209, member of the CLR family, specific marker of immature DCs), Alexa488-conjugated anti-MR (Mannose Receptor or CD206, member of the CLR family, specific marker of immature DCs and macrophages), and streptavidin APC-conjugated anti-TLR4 (biotin antibody, a LPS receptor with activation functions). Antibodies were obtained from BD Biosciences except anti-MR from BioLegend and anti-HLA-DR from Beckman Coulter. The cells were analyzed using BD-LSRII with FACSDiva Software (BD Biosciences) from the Centre d'Imagerie Cellulaire Santé (CICS), Université Clermont Auvergne. Gates were set on living DCs based on forward/side scatter properties (Fig. S2A). The analysis was based on a count of 3,000 DCs. The level of staining was expressed as the mean fluorescence intensity (MFI). The culture supernatant was collected and stored at −20°C until cytokine analysis.
ELISA.
The human IL-1β, IL-8, HBD-2, SLPI, TGF-β, IL-10, CCL20, and IL-6 cytokines were assayed in culture supernatants with enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, San Diego, CA, USA) according to the manufacturers’ instructions.
Protein extraction and Western blotting.
Cells were lysed with the NE-PER nuclear protein extraction kit (Thermo Fisher Scientific) containing a protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined by the BCA protein assay (Thermo Fisher Scientific). Western blotting was performed with 20 μg of protein per lane. Membranes were probed with a rabbit anti-p65NF-κB polyclonal antibody (BioLegend; 1:1,000), or a rabbit anti-lamin B1 polyclonal antibody (BioLegend; 1:5,000) overnight at 4°C. After washes, the membranes were incubated for 1 h at room temperature with anti-rabbit horseradish peroxidase-conjugated IgG (Sigma-Aldrich). Signals were visualized with Clarity Western enhanced chemiluminescence (ECL) substrate (Bio-Rad). Quantification was performed with Image Lab 2.0 software (Bio-Rad). Most representative full, uncropped blots used for Fig. 1E are available in Fig. S6 in the supplemental material.
Antimicrobial activity.
The antibacterial activity of HBD-2 (PeproTech) and SLPI (R&D systems) was evaluated by incubating G. vaginalis (1.108 CFU/mL) or Lcr35 inoculum (1.107 CFU/mL) with different concentrations of HBD-2 (0.25 to 100 ng/mL) or SLPI (0.2 to 10 ng/mL) for 4 h. After incubation, serial decimal dilutions of cultures were prepared in PBS and plated onto BHI or MRS agar and incubated for 48 h to 72 h. The number of CFU was assessed after incubation. The results of antibacterial activity were expressed as the percentage of growth inhibition.
Cytotoxicity assays.
The impact of bacteria on the viability of the cultured VK2 cells was determined with the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. After the 6 h exposure, 5 μg/mL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT reagent, Life Technologies) was added to each well, and the plates were incubated at 37°C for 2 h in the dark. Subsequently, the media was thoroughly removed, about 400 μL of dimethyl sulfoxide (DMSO) was added to each well and the plate was incubated with shaking (150 rpm) at room temperature for 15 min to allow color development. The OD was measured at 540 nm.
Statistical analysis.
One-way analysis of variance (ANOVA) (Kruskal-Wallis with Dunn’s multiple comparison test) tests were performed with GraphPad Prism 6 software. A P value of less than 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars depict mean ± standard error of the mean (SEM).
ACKNOWLEDGMENTS
We thank the CICS (Centre d'Imagerie Cellulaire Santé) of the Université Clermont Auvergne and, in particular, Christelle Blavignac.
We declare that no conflict of interest exists.
Footnotes
Supplemental material is available online only.
Contributor Information
Christiane Forestier, Email: christiane.forestier@uca.fr.
Manuela Raffatellu, University of California San Diego School of Medicine.
REFERENCES
- 1.Chen X, Lu Y, Chen T, Li R. 2021. The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol 11: 631972. 10.3389/fcimb.2021.631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma B, Forney LJ, Ravel J. 2012. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 66:371–389. 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chee WJY, Chew SY, Than LTL. 2020. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact 19:203. 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarbrough VL, Winkle S, Herbst-Kralovetz MM. 2015. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 21:353–377. 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 5.Manhanzva MT, Abrahams AG, Gamieldien H, Froissart R, Jaspan H, Jaumdally SZ, Barnabas SL, Dabee S, Bekker LG, Gray G, Passmore J-AS, Masson L. 2020. Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota. Sci Rep 10:6196. 10.1038/s41598-020-62184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, Dobozy A, Kemeny L. 2005. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human β-defensin-2 in vaginal epithelial cells. Microbes Infect 7:1117–1127. 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 193:1478–1486. 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 8.Allsworth JE, Peipert JF. 2007. Prevalence of bacterial vaginosis: 2001–2004 national health and nutrition examination survey data. Obstet Gynecol 109:114–120. 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 9.Jung H, Ehlers MM, Peters RPH, Lombaard H, Redelinghuys MJ, Bezuidenhoudt JE, Kock MM. 2020. Growth forms of Gardnerella spp. and Lactobacillus spp. on vaginal cells. Front Cell Infect Microbiol 10:71. 10.3389/fcimb.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirmonsef P, Krass L, Landay A, Spear G. 2012. The role of bacterial vaginosis and Trichomonas in HIV transmission across the female genital tract. Curr HIV Res 10:202–210. 10.2174/157016212800618165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauci S, Guaschino S, Driussi S, De Santo D, Lanzafame P, Quadrifoglio F. 2002. Correlation of local interleukin-8 with immunoglobulin A against Gardnerella vaginalis hemolysin and with prolidase and sialidase levels in women with bacterial vaginosis. J Infect Dis 185:1614–1620. 10.1086/340417. [DOI] [PubMed] [Google Scholar]
- 12.Eschenbach DA. 1993. History and review of bacterial vaginosis. Am J Obstet Gynecol 169:441–445. 10.1016/0002-9378(93)90337-I. [DOI] [PubMed] [Google Scholar]
- 13.Cauci S. 2003. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9:53–58. 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 14.Valore EV, Wiley DJ, Ganz T. 2006. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 74:5693–5702. 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturm Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ. 2000. High levels of tumor necrosis factor-α and interleukin-1β in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J Infect Dis 182:467–473. 10.1086/315713. [DOI] [PubMed] [Google Scholar]
- 16.Majchrzak-Gorecka M, Majewski P, Grygier B, Murzyn K, Cichy J. 2016. Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev 28:79–93. 10.1016/j.cytogfr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MD, Roberts MH, Barauskas OL, Jarvis GA. 2012. Secretory leukocyte protease inhibitor binds to Neisseria gonorrhoeae outer membrane opacity protein and is bactericidal. Am J Reprod Immunol 68:116–127. 10.1111/j.1600-0897.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doumas S, Kolokotronis A, Stefanopoulos P. 2005. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun 73:1271–1274. 10.1128/IAI.73.3.1271-1274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradshaw CS, Brotman RM. 2015. Making inroads into improving treatment of bacterial vaginosis – striving for long-term cure. BMC Infect Dis 15:292. 10.1186/s12879-015-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senok AC, Verstraelen H, Temmerman M, Botta GA. 2009. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev (4):CD006289. 10.1002/14651858.CD006289.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Boirivant M, Strober W. 2007. The mechanism of action of probiotics. Curr Opin Gastroenterol 23:679–692. 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 22.Forsythe P, Bienenstock J. 2010. Immunomodulation by commensal and probiotic bacteria. Immunol Invest 39:429–448. 10.3109/08820131003667978. [DOI] [PubMed] [Google Scholar]
- 23.Forestier C, De Champs C, Vatoux C, Joly B. 2001. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res Microbiol 152:167–173. 10.1016/s0923-2508(01)01188-3. [DOI] [PubMed] [Google Scholar]
- 24.Dausset C, Bornes S, Miquel S, Kondjoyan N, Angenieux M, Nakusi L, Veisseire P, Alaterre E, Bermúdez-Humarán LG, Langella P, Engel E, Forestier C, Nivoliez A. 2020. Identification of sulfur components enhancing the anti-Candida effect of Lactobacillus rhamnosus Lcr35. Sci Rep 10:17074. 10.1038/s41598-020-74027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taggart CC, Cryan S-A, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O'neill SJ, McElvaney NG. 2005. Secretory leucoprotease inhibitor binds to NF-κB binding sites in monocytes and inhibits p65 binding. J Exp Med 202:1659–1668. 10.1084/jem.20050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petricevic L, Witt A. 2008. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG Int J Obstet Gynaecol 115:1369–1374. 10.1111/j.1471-0528.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- 27.Coudeyras S, Jugie G, Vermerie M, Forestier C. 2008. Adhesion of human probiotic Lactobacillus rhamnosus to cervical and vaginal cells and interaction with vaginosis-associated pathogens. Infect Dis Obstet Gynecol 2008:549640. 10.1155/2008/549640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohbot JM, Cardot JM. 2012. Vaginal impact of the oral administration of total freeze-dried culture of Lcr 35 in healthy women. Infect Dis Obstet Gynecol 2012:503648. 10.1155/2012/503648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoumpopoulou G, Tsakalidou E, Dewulf J, Pot B, Grangette C. 2009. Differential crosstalk between epithelial cells, dendritic cells and bacteria in a co-culture model. Int J Food Microbiol 131:40–51. 10.1016/j.ijfoodmicro.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP. 2008. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 10:439–446. 10.1016/j.micinf.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Abramov V, Khlebnikov V, Kosarev I, Bairamova G, Vasilenko R, Suzina N, Machulin A, Sakulin V, Kulikova N, Vasilenko N, Karlyshev A, Uversky V, Chikindas ML, Melnikov V. 2014. Probiotic properties of Lactobacillus crispatus 2,029: homeostatic interaction with cervicovaginal epithelial cells and antagonistic activity to genitourinary pathogens. Probiotics Antimicrob Proteins 6:165–176. 10.1007/s12602-014-9164-4. [DOI] [PubMed] [Google Scholar]
- 32.Benjelloun F, Quillay H, Cannou C, Marlin R, Madec Y, Fernandez H. 2020. Activation of Toll-like receptors differentially modulates inflammation in the human reproductive tract: preliminary findings. Front Immunol 11:1655. 10.3389/fimmu.2020.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fazeli A, Bruce C, Anumba DO. 2005. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod 20:1372–1378. 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 34.Hesham H, Mitchell AJ, Bergerat A, Hung K, Mitchell CM. 2021. Impact of vaginal douching products on vaginal Lactobacillus, Escherichia coli and epithelial immune responses. Sci Rep 11:23069. 10.1038/s41598-021-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto HS, Xu Q, Fichorova RN. 2013. Homeostatic properties of Lactobacillus jensenii engineered as a live vaginal anti-HIV microbicide. BMC Microbiol 13:4. 10.1186/1471-2180-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrova MI, Macklaim JM, Wuyts S, Verhoeven T, Vanderleyden J, Gloor GB, Lebeer S, Reid G. 2018. Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR-1. Front Microbiol 9:1278. 10.3389/fmicb.2018.01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dausset C, Patrier S, Gajer P, Thoral C, Lenglet Y, Cardot J-M, Judlin P, Ravel J, Nivoliez A. 2018. Comparative phase I randomized open-label pilot clinical trial of Gynophilus (Lcr regenerans) immediate release capsules versus slow release muco-adhesive tablets. Eur J Clin Microbiol Infect Dis 37:1869–1880. 10.1007/s10096-018-3321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fidel PL, Ginsburg KA, Cutright JL, Wolf NA, Leaman D, Dunlap K, Sobel JD. 1997. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Thl-type responses following intravaginal challenge with “Candida” antigen. J Infect Dis 176:728–739. 10.1086/514097. [DOI] [PubMed] [Google Scholar]
- 39.Weissenbacher T, Walter C, Mylonas I, Scholz C, Gingelmaier A, Friese K. 2010. Interleukin-6, interleukin-10 and interleukin-12 in vaginal fluid from women with bacterial vaginosis. Arch Gynecol Obstet 281:77. 10.1007/s00404-009-1072-6. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert NM, Lewis WG, Lewis AL. 2013. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One 8:e59539. 10.1371/journal.pone.0059539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cauci S. 2004. Vaginal immunity in bacterial vaginosis. Curr Infect Dis Rep 6:450–456. 10.1007/s11908-004-0064-8. [DOI] [PubMed] [Google Scholar]
- 42.Wagner R, Johnson SJ. 2012. Correction: probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J Biomed Sci 19:84. 10.1186/1423-0127-19-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzo A, Losacco A, Carratelli CR. 2013. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunol Lett 156:102–109. 10.1016/j.imlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Wiesner J, Vilcinskas A. 2010. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1:440–464. 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 45.Kotani H, Koshizuka T, Matsubara K, Nishiyama K, Sugiyama T, Suzutani T. 2020. Relationship between human β-defensin 2 and the vaginal environment. Jpn J Infect Dis 73:214–220. 10.7883/yoken.JJID.2019.190. [DOI] [PubMed] [Google Scholar]
- 46.Novak RM, Donoval BA, Graham PJ, Boksa LA, Spear G, Hershow RC, Chen HY, Landay A. 2007. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol 14:1102–1107. 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nugteren S, Samsom JN. 2021. Secretory leukocyte protease inhibitor (SLPI) in mucosal tissues: protects against inflammation, but promotes cancer. Cytokine Growth Factor Rev 59:22–35. 10.1016/j.cytogfr.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Taggart CC, Greene CM, McElvaney NG, O'Neill S. 2002. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IκBα degradation without affecting phosphorylation or ubiquitination. J Biol Chem 277:33648–33653. 10.1074/jbc.M203710200. [DOI] [PubMed] [Google Scholar]
- 49.Greene CM, McElvaney NG, O'Neill SJ, Taggart CC. 2004. Secretory leucoprotease inhibitor impairs Toll-like receptor 2- and 4-mediated responses in monocytic cells. Infect Immun 72:3684–3687. 10.1128/IAI.72.6.3684-3687.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagrafeuille R, Miquel S, Balestrino D, Vareille-Delarbre M, Chain F, Langella P, Forestier C. 2018. Opposing effect of Lactobacillus on in vitro Klebsiella pneumoniae in biofilm and in an in vivo intestinal colonisation model. Benef Microbes 9:87–100. 10.3920/BM2017.0002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6. Download iai.00309-22-s0001.pdf, PDF file, 1.5 MB (1.6MB, pdf)