ABSTRACT
Nitrate metabolism is an adaptation mechanism used by many bacteria for survival in anaerobic environments. As a by-product of inflammation, nitrate is used by the intestinal bacterial pathogens to enable gut infection. However, the responses of bacterial respiratory pathogens to nitrate are less well understood. Actinobacillus pleuropneumoniae is an important bacterial respiratory pathogen of swine. Previous studies have suggested that adaptation of A. pleuropneumoniae to anaerobiosis is important for infection. In this work, A. pleuropneumoniae growth and pathogenesis in response to the nitrate were investigated. Nitrate significantly promoted A. pleuropneumoniae growth under anaerobic conditions in vitro and lethality in mice. By using narQ and narP deletion mutants and single-residue-mutated complementary strains of ΔnarQ, the two-component system NarQ/P was confirmed to be critical for nitrate-induced growth, with Arg50 in NarQ as an essential functional residue. Transcriptome analysis showed that nitrate upregulated multiple energy-generating pathways, including nitrate metabolism, mannose and pentose metabolism, and glycerolipid metabolism via the regulation of NarQ/P. Furthermore, narQ, narP, and its target gene encoding the nitrate reductase Nap contributed to the pathogenicity of A. pleuropneumoniae. The Nap inhibitor tungstate significantly reduced the survival of A. pleuropneumoniae in vivo, suggesting that Nap is a potential drug target. These results give new insights into how the respiratory pathogen A. pleuropneumoniae utilizes the alternative electron acceptor nitrate to overcome the hypoxia microenvironment, which can occur in the inflammatory or necrotic infected tissues.
KEYWORDS: nitrate, Actinobacillus pleuropneumoniae, growth, pathogenicity, regulation, NarP, NarQ
INTRODUCTION
Bacteria have to rapidly adjust their metabolic behaviors according to the external environment and/or the microenvironment of the infected tissue inside their hosts. Utilizing alternative electron acceptors is an essential adaptive mechanism for bacteria to cope with anaerobic environments, which can be significant to overcome oxygen limitation during pathogen invasion and colonization in damaged tissues. Due to its high redox potential, nitrate is preferentially utilized in anaerobic respiration as an alternative electron acceptor (1). Nitrate accumulation occurs mainly during disease and inflammation in the host. Inflammation upregulates the expression of inducible nitric oxide synthase (iNOS), which catalyzes the production and sustained release of NO and reactive nitrogen species from l-arginine (2). The bactericidal activity of reactive nitrogen species quickly wanes through conversion to harmless and stable NO3− in the presence of carbon dioxide (CO2) (3). Members of the Enterobacteriaceae—for example, Escherichia coli (4) and Salmonella enterica serovar Typhimurium (5–8)—can take advantage of the accumulated inflammatory by-product NO3− to upregulate nitrate respiration, enabling proliferation and invasion at the inflamed site. By this metabolic adaptation, pathogenic bacteria with the ability to use inflammatory by-products can gain a fitness advantage in the inflamed gut over the resident microflora. In E. coli, the two-component systems (TCSs) NarX/L and NarQ/P are responsible for sensing nitrate and regulating intracellular processes (9). Three nitrate reductases have been reported as functional in E. coli: the periplasmic dissimilatory nitrate reductase (Nap), membrane-bound respiratory nitrate reductase (Nar), and cytoplasmic assimilatory nitrate reductase (Nas) (10, 11). narGHJI and napFDAGHBC transcription are both tightly regulated by the nitrate regulator Fnr (fumarate and nitrate reductase regulation protein), and the nitrate response regulator NarL/P, depending on oxygen and nitrate availability (11). The process of nitrate reduction can create a flow of protons across the cell membrane, and the resulting electron gradient drives massive ATP synthesis (12), allowing bacteria to survive under oxygen limitation.
The lung environment is different from that inside the gut (13). Oxygen partial pressure gradients occur along the respiratory tract (14), and during disease and inflammation, serum leakage, increased mucus production, and tissue necrosis create hypoxia zones in the respiratory tract (15). In inflamed lungs, activated macrophages can continuously release NO for several weeks (16), resulting in elevated nitrate and nitrite concentrations (17). As a representative of respiratory pathogens, Pseudomonas aeruginosa was found to use nitrates abundant in sputum derived from cystic fibrosis patients for microaerobic growth (17, 18). But unlike Enterobacteriaceae, P. aeruginosa has an intact functional multistep denitrification pathway, reducing NO3− to the final product N2 (13). However, the role of nitrate utilization and the regulatory mechanisms in respiratory pathogens having an incomplete denitrification pathway are still unknown.
Actinobacillus pleuropneumoniae is an economically important respiratory pathogen of pigs, causing highly contagious pleuropneumonia with high mortality (19). In subclinically infected herds, long-term colonizers of A. pleuropneumoniae in the tonsils and lungs may cause disease outbreaks when the pigs encounter “trigger” stressors (20, 21). Infection and persistence of A. pleuropneumoniae are mediated by multiple virulence factors (22, 23), including RTX exotoxins (24, 25), lipopolysaccharides, outer membrane proteins (26–29), global regulators related to environmental adaptation (30–32), and potentially the genes involved in adapting to the environments mimicking that inside the host (33–36). The incomplete tricarboxylic acid (TCA) cycle in the genome of A. pleuropneumoniae suggests that the anaerobic metabolism is critical for the survival of this pathogen (37, 38). Comparing gene expression profiles under anaerobic and aerobic conditions, A. pleuropneumoniae remodels the central carbon metabolism pathway under anaerobic conditions (39). Anaerobic metabolism-related factors, such as the regulators HlyX (FNR) and ArcA, the enzymes FrdABCD (fumarate reductase), DmsA (dimethyl sulfoxide reductase), and AspA (aspartate ammonia-lyase), involved in anaerobic respiration have been shown to be essential determinants of A. pleuropneumoniae virulence (30, 31, 40, 41).
In our previous work, we found that A. pleuropneumoniae may respond to nitrate through its TCS NarQ/P and established a high-throughput method to successfully identify the binding sequence and target genes of NarP (42). In this study, we further detect the roles of nitrate in A. pleuropneumoniae growth and pathogenicity. The genome analysis suggests that A. pleuropneumoniae has an incomplete denitrification pathway, with only one nitrate reductase, Nap. Nitrate was found to promote A. pleuropneumoniae growth and lethality of mice. The metabolic changes of A. pleuropneumoniae in response to nitrate were studied, and the regulons under the control of NarP and the nitrate sensor NarQ in the presence of additional nitrate were also identified. The function of nitrate reductase Nap in A. pleuropneumoniae was further confirmed to contribute to nitrate-induced growth. The genes encoding NarQ/P and Nap were found to contribute to A. pleuropneumoniae survival in vivo. Our results also suggest that Nap and other enzymes containing molybdenum as a cofactor involved in anaerobic respiration are potential drug targets for this pathogen.
RESULTS
Nitrate promotes A. pleuropneumoniae growth and pathogenicity.
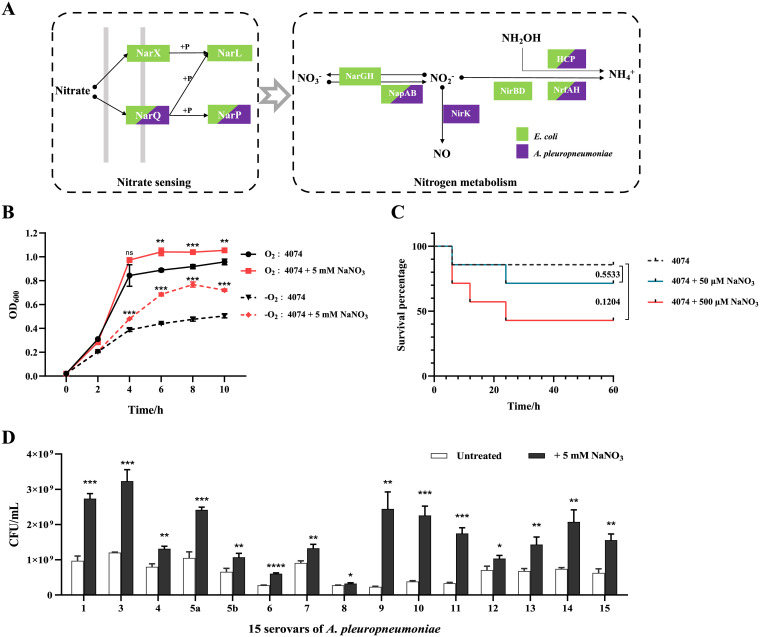
In our previous study, nitrate was found to promote the growth of A. pleuropneumoniae 4074 under both aerobic and anaerobic conditions (42). Thus, here, the A. pleuropneumoniae genes involved in nitrate sensing and metabolism were analyzed. Compared with the genome sequences of E. coli (Fig. 1A), the A. pleuropneumoniae genome contains homologous genes encoding the nitrate sensing protein NarQ and its cognate regulator, NarP. For the nitrate reduction pathway, A. pleuropneumoniae contains the nitrate reductase Nap (encoded by the napFDAGHBC operon), the nitrite reductase (cytochrome c552) Nrf (encoded by the nrfABCD operon), and the nitrite reductase NirK (encoded by the gene nirK) generating NO from nitrite, indicating that A. pleuropneumoniae possesses an incomplete denitrification pathway and a complete dissimilatory reduction pathway (37). To further explore the effect of nitrate on A. pleuropneumoniae growth, different concentrations of nitrate were added to the bacterial medium. Under the growth conditions used, 5 mM NaNO3 showed the most optimal effect on growth promotion in vitro, with optical density at 600 nm (OD600) values showing significant difference between nitrate-treated and untreated bacteria from 5 h after subculture (see Fig. S1A and B in the supplemental material). As a control, 5 mM NaCl did not show any effect on A. pleuropneumoniae growth (Fig. S1C). In addition, compared with the untreated group, 5 mM NaNO3 promoted accelerated growth under anaerobic compared to aerobic conditions, with OD600 values showing a significant difference between nitrate-treated and untreated bacteria from 4 h after subculture under anaerobic conditions and from 6 h after subculture under aerobic conditions (Fig. 1B). Further validation was carried out by viable counts of bacteria in the selected growth phase, and similar growth promotion effects of nitrate were observed in strains of various serotypes of A. pleuropneumoniae (Fig. 1D). Subsequently, the effect of nitrate on bacterial virulence was determined in the mouse infection model. The mice were intranasally infected with log-phase bacteria together with gradient doses of NaNO3. Although the mortality of the infected mice did not show significant difference between the wild-type (WT)-infected group and the groups infected with the WT and treated with nitrate, the mortality increased with an increased concentration of nitrate. (Fig. 1C). Based on these results, with a nitrate sensing two-component system and an incomplete denitrification pathway in its genome, A. pleuropneumoniae can use nitrate to enhance its growth ability in vitro and animal lethality in vivo.
FIG 1.
Analysis of nitrate sensing and utilization pathways in A. pleuropneumoniae genome and the effects of nitrate on growth and pathogenicity. (A) Nitrate sensing and metabolism pathways in E. coli and A. pleuropneumoniae based on genome analysis. Each rectangle is colored according to the protein present in E. coli (green) and A. pleuropneumoniae (purple). Arrows indicate the direction of the pathways, and solid dots indicate products of the process. (B) Growth curves of A. pleuropneumoniae cultured under aerobic conditions with shaking (O2) and anaerobic conditions in a static anaerobic chamber (−O2) with or without nitrate in NAD-supplemented TSB. The OD600 of bacterial cultures was determined every 2 h. (C) Survival rate of mice intranasally infected with A. pleuropneumoniae with different doses of nitrate (7 mice per group); (D) viable counts of different serovars of A. pleuropneumoniae at mid-log phase (4 h) cultured under anaerobic conditions in NAD-supplemented TSB in a static anaerobic chamber with additional nitrate. For growth curves and viable counts of bacteria, data are shown as means ± SD from three independent replicates. The two-tailed t test was used for statistical analysis. For comparisons of mortalities of mice, a log rank (Mantel-Cox) test was performed for statistical analysis (**, P < 0.01; ***, P < 0.001; ns, not significant).
Nitrate modulates multiple metabolic pathways in A. pleuropneumoniae.
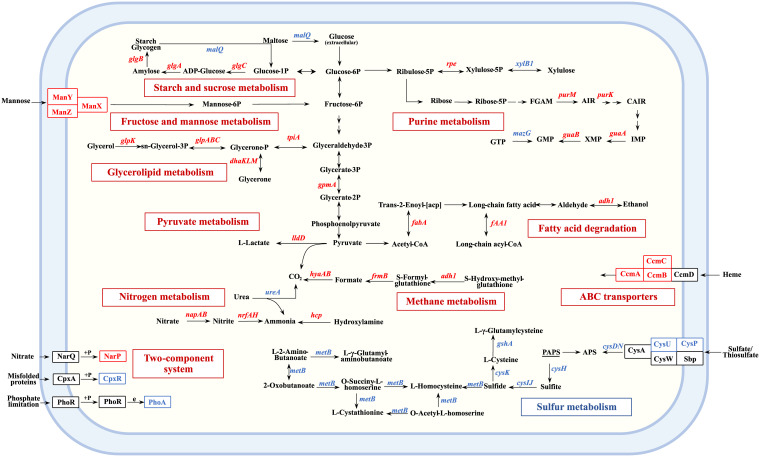
To explore the mechanism of nitrate-induced growth and pathogenicity of A. pleuropneumoniae, the differentially expressed genes after nitrate treatment under the anaerobic conditions were identified by transcriptomic analysis. Among the differentially expressed genes, 112 were upregulated and 189 were downregulated (Table S1). Pathway enrichment analysis was performed on the differentially expressed genes. Genes involved in metabolic pathways (75.9% of the upregulated genes, 56.8% of the downregulated genes) and environmental information processing pathways (14.5% of the upregulated genes, 13.6% of the downregulated genes) accounted for 80.1% of all the differentially expressed genes. Notably, the expression of genes involved in nitrogen metabolism (nap and nrf operon), mannose metabolism (manXYZ), glycerolipid, and glycerophospholipid metabolism (dhlKLM and glp operon), starch and sucrose metabolism (glgABC), purine metabolism (guaAB and purKM), and their associated transport systems (heme exporter encoded by ccmABC and mannose transporter ManXYZ) were significantly upregulated (Fig. 2). Activation of these metabolic pathways suggests that A. pleuropneumoniae in this nitrate-rich context alters its core metabolic network to obtain energy from multiple substrates (43). In contrast, sulfur metabolism, the sulfur relay system, and biofilm formation pathways were significantly downregulated by nitrate (Fig. 2). These results indicated that nitrate modulated multiple metabolic pathways of A. pleuropneumoniae, including upregulation of those with roles in carbohydrate, lipid, and energy metabolism, to promote A. pleuropneumoniae growth.
FIG 2.
Schematic representation of A. pleuropneumoniae metabolic pathways differentially regulated by nitrate. +p, transfer of phosphate groups. Red letters indicate upregulated genes, and blue letters indicate downregulated genes.
NarQ and NarP play key roles in growth promotion of A. pleuropneumoniae in response to nitrate.
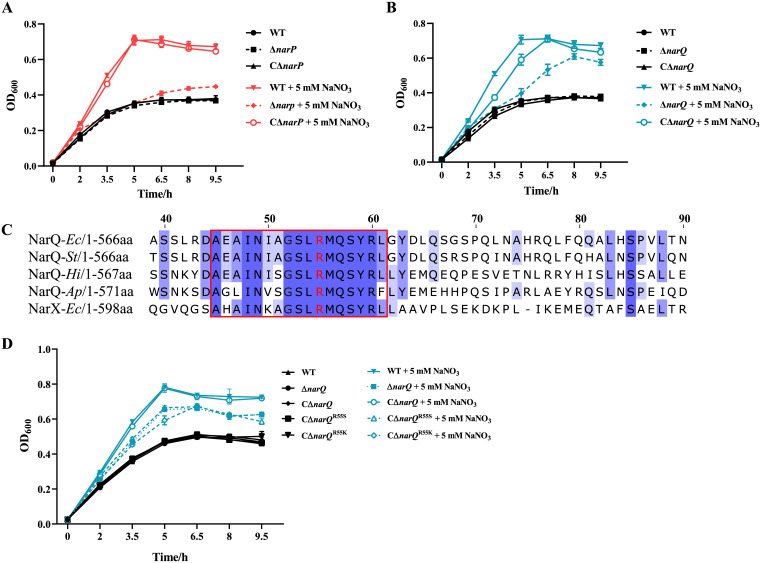
The deleted mutants and the complementary strains of the homologous genes of the E. coli nitrate sensing TCS narP and narQ in A. pleuropneumoniae were constructed in our previous study (42) and in this work (Fig. S2). When cultured anaerobically, ΔnarP and ΔnarQ mutants failed to display the same growth advantage after the addition of 5 mM NaNO3 as that which occurred in the wild-type (WT) strain, while their corresponding complementary strains, CΔnarP and CΔnarQ, reverted to the WT strain characteristics (Fig. 3A and B). Subsequently, multiple-sequence alignments of A. pleuropneumoniae NarQ with NarX and NarQ of E. coli and other organisms were carried out (Fig. 3C). Among the 17 conserved amino acid residues of the P-box region of the sensor domain of NarQ/X (44), 12 residues of A. pleuropneumoniae NarQ were found to be identical to those in the aligned proteins. The Arg residue in the P-box of NarQ/X, which has been identified to form hydrogen bonds with nitrate ions (45–47), was also conserved in A. pleuropneumoniae NarQ (Arg50). Thereafter, a single-residue mutation of Arg50 was constructed by changing it into Ser50 (R50S) or Lys50 (R50K) in the complementary vector carrying the NarQ coding sequence. The mutated complementary vectors were transformed into the ΔnarQ mutant to obtain the single-residue-mutated complementary strains CΔnarQR50K and CΔnarQR50S. These two mutated complementary strains showed similar growth characteristics to the ΔnarQ mutant (Fig. 3D), confirming that Arg50 was a key residue in A. pleuropneumoniae NarQ. Taken together, A. pleuropneumoniae NarQ and NarP were confirmed to be essential in the enhanced growth of this bacterium in response to nitrate, with Arg50 as an essential residue for function locating in the P-box of NarQ.
FIG 3.
Roles of NarP, NarQ and its nitrate sensing residue in the growth enhancement of A. pleuropneumoniae in response to nitrate. (A and B) Growth curves of WT, ΔnarP, ΔnarQ, and complementary strains supplemented with or without nitrate; (C) sequence alignment of NarQ and NarX from different species. The aligned partial sensor domain sequences of NarQ are shown. The P-box is indicated by a red rectangle. The numbers ahead of sequences are amino acid positions of A. pleuropneumoniae NarQ. Highly conserved sites are highlighted in blue. The Arg residues reported to bind with nitrate are shown in red. Genome names are shown as italicized abbreviations (Ec for E. coli, St for Salmonella Typhimurium, Hi for Haemophilus influenzae, Ap for A. pleuropneumoniae). (D) Growth curves of the WT, ΔnarQ, complementary, and mutated complementary strains with single-residue mutations at Arg50 supplemented with or without nitrate. R50K indicates mutation of Arg50 into Lys, while R50S indicates mutation of Arg50 into Ser. The bacterial strains were cultured anaerobically in NAD-supplemented TSB in a static anaerobic chamber. Data are shown as means ± SD from three independent replicates.
Analysis of nitrate-activated and -repressed genes under the regulation of NarQ/P.
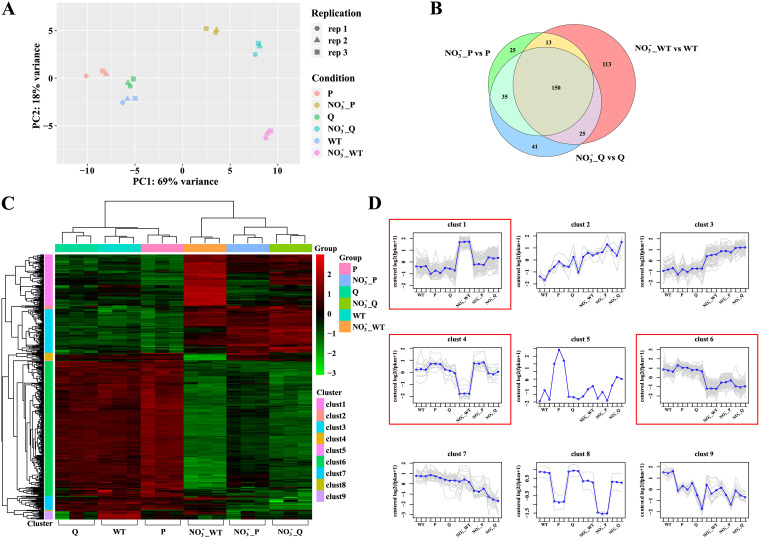
Subsequently, to identify the genes directly regulated by NarQ/P in response to nitrate, differentially expressed genes of the ΔnarQ and ΔnarP mutants after treatment with NaNO3 were identified by transcriptomic analysis. Differentially expressed genes in response to nitrate of the WT strain and the mutants were also compared. Principal-component analysis (PCA) was performed on all the transcriptome sequencing (RNA-Seq) data. As expected, the gene expression patterns of the samples from each bacterial strain under the same condition clustered tightly on the PCA plot (Fig. 4A). The nitrate-supplemented groups were clearly differentiated on the x axis (PC1) and y axis (PC2) compared with nonsupplemented groups, showing significant transcriptional changes in A. pleuropneumoniae in response to nitrate (Fig. 4A). In addition, greater separation was observed between WT strain and the ΔnarQ or ΔnarP mutant the under nitrate-treated condition compared with that under the untreated condition (Fig. 4A). This indicated that the gene expression pattern affected by narQ or narP was much more significant in the presence of nitrate. In other words, the two-component system NarQ/P could be critical for A. pleuropneumoniae response to nitrate. Venn diagrams were used to represent the comparison of differentially expressed genes from different treatment groups. Few genes were found to be differentially expressed between the mutants and the WT strain under anaerobic culture without nitrate treatment, but after treatment with nitrate, a significant fraction of the differentially expressed genes in the WT strain (113 genes, as shown in the red zone in Fig. 4B) were identified as no longer significantly changed in response to nitrate in the two mutants. These genes contained the regulons that were coregulated by NarQ and NarP in response to nitrate (Fig. 4B; Table S2). Furthermore, two-way cluster analysis was performed on the differentially expressed genes of all the comparison groups (Fig. 4C). Differentially expressed genes were divided into nine clusters according to their expression patterns (Fig. 4D; Table S2). Genes in cluster 1 (80 genes) and cluster 4 (12 genes) were significantly upregulated or downregulated only in the nitrate-treated WT groups compared with the nontreated controls, but not changed in the nitrate-treated mutant groups, which indicated the genes were coregulated by NarQ/P in response to nitrate. In cluster 6 (211 genes), genes were downregulated by nitrate in WT groups with increased expression levels in the nitrate-treated mutant groups, but not restored to the level of the nontreated WT groups. These genes may be under the regulation of NarQ/P as well as other regulators in response to nitrate. Genes in cluster 2 (6 genes) and cluster 3 (68 genes) showed a consistent upregulated trend in all the nitrate-treated groups compared with their untreated controls, while the genes in cluster 7 (22 genes) and cluster 9 (13 genes) displayed a downregulated pattern in all of the nitrate-treated groups. The genes in these four clusters indicated the genes regulated by nitrate independent of NarQ/P.
FIG 4.
Clustering analysis revealing divergent data sets of the differential expression patterns of WT and mutant strains (Q, ΔnarQ; P, ΔnarP) with or without nitrate treatment. (A and B) Principal-component analysis (A) and Venn (B) diagram of differentially expressed genes of WT and mutant strains treated with nitrate under anaerobic conditions. Different symbols represent three repetitions (rep) within a group. (C) Heat map of differentially expressed genes from all comparison groups and bidirectional cluster analysis of the samples. The horizontal line represents genes; each column is a sample. Red indicates genes with high expression levels, and green indicates genes with low expression levels. (D) Clusters of differentially expressed genes according to their expression patterns. Each gray line indicates the expression of each gene in a cluster, and each blue line indicates the average expression of all of the genes in a cluster.
Verification of expression levels of target genes by qRT-PCR.
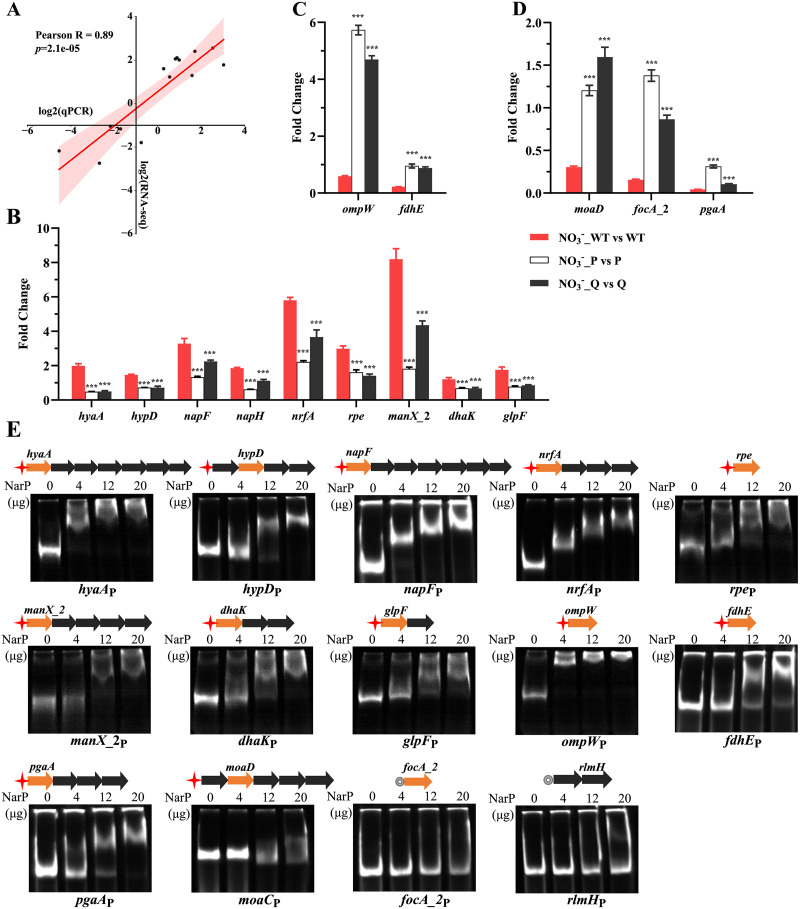
According to the above gene clustering analysis, 14 target genes in different clusters were selected to determine their transcription levels by quantitative reverse transcription-PCR (qRT-PCR) under the same culture conditions as that set up for RNA-Seq (Fig. 5A to D). The results from qRT-PCR analysis correlated well with the transcriptomic data (Pearson R = 0.89) (Fig. 5A). The qRT-PCR results verified that the genes from cluster 1, including those encoding glycerol uptake protein GlpF, proteins involved in carbohydrate metabolism (DhaK, ManX_2, and Rpe), enzymes involved in nitrate metabolism (NapF, NapH, and NrfA), and hydrogenases (HypD and HyaA), were significantly upregulated by NarQ/P in response to nitrate (Fig. 5B). The genes encoding outer membrane protein OmpW and formate dehydrogenase accessory protein FdhE in cluster 4 were selected and verified by qRT-PCR to be downregulated by NarQ/P in response to nitrate (Fig. 5C). The genes encoding molybdopterin subunit MoaD, biofilm extracellular matrix poly-β-1,6-N-acetyl-d-glucosamine synthesis protein PgaA, and formate transporter FocA_2 in cluster 6 were confirmed to be downregulated by NarQ/P after nitrate stimulation by qRT-PCR (Fig. 5D).
FIG 5.
Verification of differentially expressed genes by qRT-PCR and EMSA. (A) Pearson coefficient correlation analysis. x axis, log2 value from qRT-PCR; y axis, log2 value from RNA-Seq. (B to D) qRT-PCR showed the transcript levels of 14 candidate genes from different clusters in the WT, ΔnarQ, and ΔnarP strains after nitrate treatment compared to the corresponding untreated groups. The A. pleuropneumoniae 16S rRNA gene was used as an endogenous control. The threshold cycle (2−ΔΔCT) was calculated to determine the relative transcript levels of each gene. Experiments were performed independently in triplicate. Data are shown as means ± SD from four independent replicates. The two-tailed t test was used for statistical analysis (**, P < 0.01; ***, P < 0.001). (E) Assessment of the binding between NarP and the promoter regions of the candidate genes by EMSA. Schematic structures depicting the organization of the candidate genes in each operon are shown above the corresponding EMSA image. Arrows indicate the transcription direction. Colored arrows in orange are differentially expressed genes validated by qRT-PCR. Red asterisks indicate the presence of NarP binding motif. Gray circles indicate the absence of the NarP binding motif.
Binding activity of NarP to the upstream region of the candidate genes.
To validate the direct role of NarP in the regulation of the differential expressed genes, the electrophoretic mobility shift assay (EMSA) was used to assess the interaction between NarP and the promoter regions of the candidate genes identified from transcriptomic analysis and qRT-PCR. The NarP binding motif identified in our previous study (42) was used to search the putative promoter region of the selected genes in gene clusters 1, 4, and 6 (Table S2). These genes contain nine genes upregulated and five downregulated by nitrate. As shown in Fig. 5E, each promoter region containing the NarP binding motif displayed a dose-dependent mobility shift with the NarP protein. The promoter region of focA_2 and the gene unregulated by nitrate, rlmH, which did not contain the NarP binding motif served as negative controls. The promoter regions showed no band migration with the NarP protein (Fig. 5E). Therefore, the selected genes with the NarP binding motif in their promoter regions were confirmed to be directly regulated by NarP in response to nitrate.
Functional analysis of nitrate reductase Nap and its regulation by NarQ/P in A. pleuropneumoniae.
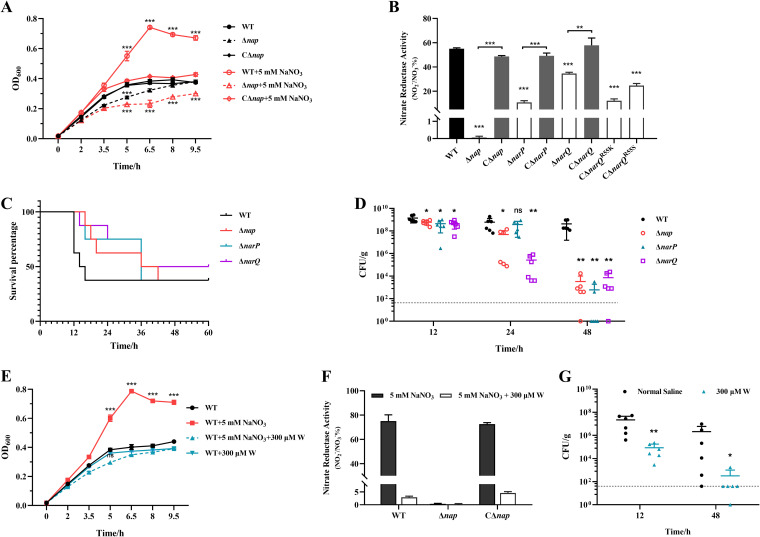
In the genome of A. pleuropneumoniae, there is only one predicted periplasmic nitrate reductase encoded by a seven-gene nap operon (napFDAGHBC). The results presented above have demonstrated that NarP directly upregulated the expression of the nap operon in response to nitrate under an anaerobic environment (Fig. 5). To explore its function, a knockout mutant of the nap operon (Δnap) and its complementary strain (CΔnap) were constructed (Fig. S2). Deletion of the nap operon resulted in growth defect compared with the WT strain under anaerobic conditions (Fig. 6A). The growth defect of Δnap was largely amplified compared with WT and CΔnap strains when nitrate was supplemented, with OD600 values showing significant difference between the WT/CΔnap and Δnap strains in the presence of nitrate from 5 h after subculture (Fig. 6A).
FIG 6.
Function of nitrate reductase Nap and the roles of narP, narQ, and nap in the pathogenicity of A. pleuropneumoniae. (A) Growth curves of the WT, Δnap, and CΔnap strains with or without nitrate supplementation; (B) nitrate-reducing activity of the WT, Δnap, ΔnarQ, and ΔnarP mutant strains, their complementary strains, and the single-residue-mutated complementary strains of the ΔnarQ mutant. The concentrations of NO2− in the supernatants of the bacterial strains were detected after 2 h of incubation of bacteria with 5 mM NaNO3. (C) Survival percentage curves of the mice infected by different A. pleuropneumoniae strains. Mice were intranasally infected with WT and mutant strains (20 μL) at 4 × 106 CFU (8 mice per group). The time of death of each mouse was recorded. (D) Bacterial loads in the mice infected by different A. pleuropneumoniae strains. Mice were intranasally infected with WT and mutant strains (20 μL) at 2 × 106 CFU (6 mice per group). The bacterial loads in the lungs of the mice were detected at 12, 24, and 48 h postinfection. (E) Antagonistic effect of molybdoenzyme inhibitor tungstate (W) on nitrate-induced growth of A. pleuropneumoniae; (F) inhibition of nitrate reductase activity of Nap by tungstate; (G) reduction in bacterial loads in mice infected with A. pleuropneumoniae by tungstate (6 mice per group). The bacterial strains were cultured anaerobically in NAD-supplemented TSB in a static anaerobic chamber. Data are shown as means ± SD from three independent replicates. The dotted line indicates the limit of detection (40 CFU/g). A two-tailed Mann-Whitney test was used for statistical analysis in animal experiments. In other experiments, the two-tailed t test was used for statistical analysis (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant).
Subsequently, the nitrate reductase activity of Nap was determined. After 2 h of incubation with additional nitrate in the medium, the concentrations of NO2− in the culture supernatants of the bacterial strains were determined. In comparison with the WT strain, the content of the nitrate reduction product NO2− in the supernatant of the Δnap strain was reduced to less than 1%, but was restored to the WT level in the complementary strain CΔnap (Fig. 6B). Additionally, the levels of NO2− in the supernatants of the ΔnarQ, ΔnarP, and ΔnarQ complementary strains with single-residue mutations (CΔnarQR55K and CΔnarQR55S) were significantly lower than those of the WT and fully complemented strains (Fig. 6B). Altogether, the results demonstrate that the Nap encoded by napFDAGHBC is the unique nitrate reductase in A. pleuropneumoniae, which is under the direct positive regulation of NarQ/P in response to nitrate.
NarP/Q and Nap play important roles in A. pleuropneumoniae pathogenicity.
To assess the contributions of the genes involved in nitrate response and metabolism in the virulence of A. pleuropneumoniae, the lethalities and bacterial loads of the WT strain and ΔnarP, ΔnarQ, and Δnap mutant strains were determined in the mouse infection model. The WT strain caused acute death between 12 and 16 h postinfection (Fig. 6C). Although no significant difference in final lethality was observed between the mutants and the WT strain, the death occurred more slowly in mice infected with the mutants, with the deaths occurring from 12 to 48 h postinfection (Fig. 6C). Bacterial loads in the lungs of the mice were also determined after using a lower infection dose that did not cause acute death of the mice. Compared with the high numbers of the WT strain recovered until 48 h postinfection, the three mutants were gradually cleared in vivo over time, with significantly lower bacterial numbers recovered compared with the WT strain at 48 h postinfection (Fig. 6D). Collectively, these data indicate that the proteins responsible for nitrate sensing and the corresponding regulator, NarQ/P, and the enzyme for nitrate utilization, Nap, contribute to the acute lethality of A. pleuropneumoniae and are critical for survival of this pathogen at a later infection stage in vivo.
The nitrate reductase inhibitor tungstate inhibits nitrate-promoted growth under anaerobic conditions and pathogenicity of A. pleuropneumoniae.
Since nitrate reductase activity is dependent on the coordination of the molybdenum cofactors (48), the molybdenase inhibitor tungstate in the form of Na2WO4 (49) was used to further confirm the role of nitrate reductase on the pathogenicity of A. pleuropneumoniae infection. An inhibition effect of Na2WO4 on A. pleuropneumoniae nitrate reductase activity was first confirmed. Addition of 300 μM Na2WO4 (50) had no effect on the growth of A. pleuropneumoniae in normal culture, but inhibited the growth promotion effect of nitrate on A. pleuropneumoniae, with OD600 values showing significant difference between Na2WO4-treated and untreated bacteria in the presence of nitrate from 5 h after subculture (Fig. 6E). Additionally, Na2WO4 significantly inhibited the activity of nitrate reductase activities of A. pleuropneumoniae WT and CΔnap strains (Fig. 6F). Next, the effect of Na2WO4 on the virulence of A. pleuropneumoniae was evaluated in the mouse infection model. Compared with the untreated group, the tungstate-treated group had significant lower pulmonary bacterial loads during the rapid infection phase (12 h postinfection) (Fig. 6G). At the later stage of infection (48 h postinfection), the recovered bacterial numbers were quite low in the tungstate-treated group, while a high recovery rate could still be observed in the control group (Fig. 6G).
DISCUSSION
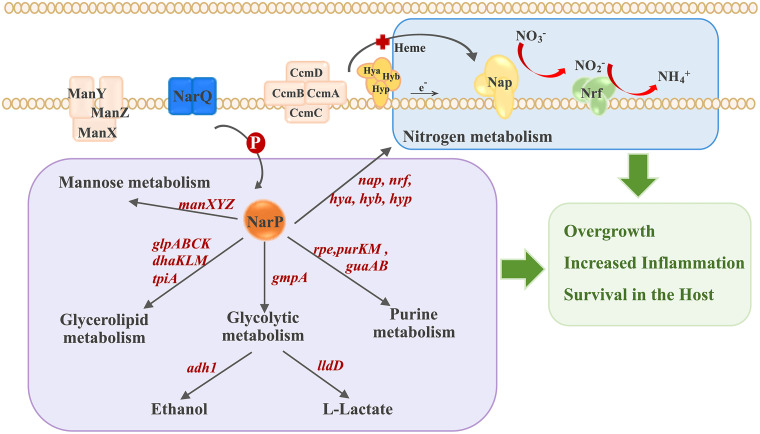
The adaptive survival and proliferation of pathogenic bacteria in different environments and inside the host are the basis for successful infection. Nitrate respiration is widely used by bacteria for energy acquisition to adapt to an anaerobic environment and is an integral part of the microbial nitrogen conversion network (51). As the most optimal terminal electron receptor for anaerobic respiration, nitrate is generated after the reaction between nitric oxide and superoxide during inflammation by iNOS, which can exist in various parts of the host (52). In individuals with intestinal inflammatory disease, the inflammatory by-product nitrate confers growth and colonization advantages for members of the Enterobacteriaceae, such as E. coli and Salmonella Typhimurium (4). However, the role of nitrate in host-interactive pathobiology in respiratory tracts is less well understood, although nitrate respiration supports hypoxic growth of P. aeruginosa at physiological levels of nitrate in cystic fibrosis airways (53). A. pleuropneumoniae is one of the causative pathogens of the pig respiratory disease, resulting in porcine pleuropneumonia. As a facultative anaerobic bacterium with an incomplete TCA cycle, A. pleuropneumoniae may rely on anaerobic respiration and fermentation as compensation for energy acquisition (37). In previous research, gene functional studies, characterization of mutant libraries, and transcription profiles during infection have revealed that adaptation of A. pleuropneumoniae to hypoxia is critical for lung infection (30–36, 40, 41). In this study, we found that nitrate promoted A. pleuropneumoniae growth both under oxygen-rich and anaerobic conditions, with a more significant effect occurring anaerobically (Fig. 1B to D). Nitrate also increased the lethality of A. pleuropneumoniae in mice (Fig. 1C). Furthermore, it was confirmed that nitrate signaling was mediated by the A. pleuropneumoniae TCS NarQ/P, which upregulated the unique nitrate reductase Nap and the genes involved in energy metabolism, including the glp operon (glgABC), dhaKLM, purKM, and the genes encoding the mannose (ManXYZ) and heme (CcmABC) transporters (Fig. 2), which likely contribute to nitrate-induced growth of this pathogen under hypoxia stress. Additionally, it was demonstrated that the crucial proteins functional in nitrate utilization and metabolism, the nitrate sensor (NarQ), gene regulator (NarP), and nitrate reductase (Nap), contributed to A. pleuropneumoniae survival in mice (Fig. 6). These results link nitrate respiration not only to growth but also to pathogenicity of pathogenic bacteria in vivo (Fig. 7).
FIG 7.
Regulatory model of A. pleuropneumoniae overgrowth and pathogenesis induced by nitrate.
The anaerobic conditions are most likely to occur at a later A. pleuropneumoniae infection stage in inflammatory and necrotic lung tissues. Consistent with this hypothesis, compared with the WT strain, ΔnarP, ΔnarQ, and Δnap mutants showed a delayed peak of mortality at higher infection doses in mice and were cleared more quickly at lower infection doses (Fig. 6). Transcriptomic analyses revealed that the most significant changes after exposure to nitrate were in metabolic pathways, with no specific virulence factors regulated by NarP after activation by nitrate. Therefore, we speculate that the major cause of the increased lethality after nitrate treatment (Fig. 1C)—the contributions of narP, narQ, and nap to the lethality and survival of A. pleuropneumoniae (Fig. 6C and D) in the mice—is the population expansion of bacteria promoted by nitrate through NarQ/P and Nap. As a result, rapidly proliferating A. pleuropneumoniae may further exacerbate inflammation in the host and continue to take advantage of the inflammation by-product nitrate, leading to increased tissue damage and prolonged time to be cleared (Fig. 7).
As a ubiquitous cellular process in bacteria, nitrate metabolism involves multiple nitrate sensors, regulators, and enzymes (1, 54). E. coli and Salmonella Typhimurium possess two nitrate sensing TCSs, three nitrate reductases, two nitrate transporters, and two nitrite reductases (7, 12, 54). P. aeruginosa has two nitrate sensors (the NarX sensor kinase homologous to that in E. coli and the McpN chemoreceptor), three nitrate reductases, and the enzymes constituting a complete denitrification pathway (13, 55). These components endow bacteria with sophisticated regulatory strategies to sense different concentrations of NO3−, sometimes together with other metabolites (54). In the case of low NO3− concentrations, NarP is induced, enabling periplasmic reductase Nap expression, at which time the extracellular nitrate reduction pathway is active (56, 57). In contrast, NarX/L and the cytosolic nitrate reductase NarG play major roles in the presence of abundant nitrate (58). Compared with E. coli and P. aeruginosa, A. pleuropneumoniae has a concise system of nitrate sensing, reduction, and regulation, which includes a conservative NarQ with nitrate sensing sites, the cognate regulator NarP with a DNA binding site (42), and one reductase (Nap) containing a molybdenum factor binding site to convert nitrate to nitrite. Consistent with that in E. coli, Arg50 in the P-box region of A. pleuropneumoniae NarQ is most likely essential for nitrate sensing (Fig. 3). The NarP binding motif ATTTATATAACCTTATTTA was identified in our previous study and contains a sequence that is partially identical to that of E. coli NarP (42). In the transcriptome results, 74.7% of the 415 genes regulated by NarQ/P were found to contain the NarP binding motif in their upstream regions (see Table S2 in the supplemental material), indicating that most of the differentially expressed genes were directly regulated by NarP. As the sole nitrate reductase, Nap is essential for A. pleuropneumoniae growth and survival in vivo (Fig. 6). By occupying the active site of nitrate reductase, the molybdenase inhibitor sodium tungstate significantly reduced the enzyme activity of Nap. Also, it inhibited bacterial survival in mice, suggesting that Nap is a candidate drug target for treating A. pleuropneumoniae infection in vivo.
According to the hierarchical structure of metabolic energy production, the increased growth of A. pleuropneumoniae with additional nitrate should mainly come from upregulated nitrate metabolism (Fig. 2), which is the most energy-efficient metabolic pathway in the anaerobic respiration of bacteria. The operons or gene clusters related to the nitrate respiratory electron transport chain were all upregulated: for example, the napFDAGHBA operon, encoding nitrate reductase, the nrfABCD operon, encoding periplasmic nitrite reductase, and 10 out of 12 consecutive genes (APPSER1_RS07220 to -RS07270) encoding hydrogenases (Hya, Hyb, and Hyp). Electrons released from fermentation by hydrogenases shuttle across quinone pools to periplasmic nitrate and nitrite reductases, driving reduction of nitrate to ammonia (59). Accompanied by nitrate respiration, the upregulation of fermentation pathways mediated by alcohol dehydrogenase (encoded by adh1) and l-lactate dehydrogenase (encoded by lldD) should synergistically power the growth of A. pleuropneumoniae. The mannose transport system ManXYZ and rpe-encoded ribulose-phosphate 3-epimerase were upregulated, which are involved in the glycolysis pathway and the pentose phosphate pathways, respectively. The operons encoding glycerol uptake and phosphorylation (glpFK, glpQT, and glpABC), and the operon encoding PTS-dependent dihydroxyacetone kinase, dhaKLM, were also upregulated. Glycerol metabolism is coupled to nitrate respiration to maintain a constant balance of redox reactions (60). Therefore, nitrate may alleviate the catabolite inhibition of A. pleuropneumoniae, allowing the alternative carbon sources to be used in addition to glucose and fructose. Simultaneously, the catabolic pathways of the disaccharide maltose were inhibited, and the pathway leading to synthesis of polysaccharide starch/glycogen was upregulated, which may collectively increase carbon source reservoirs. These regulated pathways reveal that in response to nitrate in a hypoxic environment, A. pleuropneumoniae maximizes bacterial energy production capacity through nitrate metabolism and coordinates different metabolic pathways to enhance the metabolic state (Fig. 7).
Genes downregulated by nitrate under the regulation of NarP, a small subset of target genes, are mostly functionally associated with nitrate metabolism, being involved in either the balance of redox potential or competition for alternative electrons. These genes include those encoding the formate dehydrogenase accessory protein FdhE (61), formate/nitrite transporter family protein FocA_2 (62), the outer membrane protein OmpW, related to fumaric acid metabolism (63), and the molybdenum pterin conversion factor MoaD (64). The overall suppression of the assimilatory sulfate reduction pathway may be due to being accompanied by sulfur oxidation during nitrate reduction or to the competitive inhibition of electrons between the two oxide reduction reactions (48, 65, 66). In addition, downregulation of the pgaABCD operon, encoding poly-β-N-acetylglucosamine involved in A. pleuropneumoniae biofilm synthesis (67), suggests that nitrates may suppress the sessile status and trigger the planktonic growth of A. pleuropneumoniae.
In summary, this study has revealed that nitrate significantly promotes A. pleuropneumoniae growth in vitro and lethality in mice. By activating the TCS NarQ/P and upregulation of genes involved in nitrate respiration and multiple energy metabolism pathways, A. pleuropneumoniae can achieve an optimal metabolic state and growth in response to nitrate under anaerobic conditions. The genes involved in nitrate sensing, its cognate regulator, and the downstream-regulated nitrate reductase were essential for infection of A. pleuropneumoniae in vivo. These findings provide new insights into the adaptive survival of A. pleuropneumoniae to the stressed environment inside the host, especially the anaerobic conditions that can exist in inflammatory and necrotic tissues.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains, plasmids, and primers used in this study are listed in Table S3 in the supplemental material. All E. coli strains were cultured in Luria-Bertani (LB) broth (Oxoid, Hampshire, United Kingdom) or on LB agar plates, and 20 μg/mL chloramphenicol or 25 μg/mL kanamycin was added according to the resistance genes carried by the plasmid. All A. pleuropneumoniae strains were grown in tryptic soy broth (TSB) (Becton Dickinson and Company, NJ, USA) or on tryptic soy agar (TSA) plates supplemented with 10 μg/mL NAD at 37°C. Chloramphenicol (2 μg/mL) was added for A. pleuropneumoniae mutant screening and complemented strain cultivation.
For growth curve determination, RNA isolation, the infection assay and measurement of activity of nitrate reductase, A. pleuropneumoniae strains were cultured overnight in NAD-supplemented TSB at 180 rpm, and then the strains were subcultured from the starting OD600 value at 0.01 in the same medium. In bacterial subcultures, 5 mM NaNO3 and/or 300 μM Na2WO4 (for competitive substitution with Mo) were supplemented as needed, and the same concentration of NaCl was added as a control. Aerobic conditions were maintained by shaking bacterial culture at 180 rpm. Except as otherwise indicated, all bacterial cultures were performed under anaerobic conditions, which were maintained by static culture in an anaerobic environmental chamber (Bactron, Cornelius, OR, USA) with 5% CO2, 10% H2, and 85% N2 (39).
Construction of mutants and complemented strains.
The narP deletion mutant was constructed in our previous study (42). To construct deletion mutants of the narQ and nap operon, the A. pleuropneumoniae 4074 genomic DNA was used as the template to amplify the upstream and downstream flanking regions with primer pairs narQ1/2-narQ3/4 and nap1/2-nap3/4, respectively. The PCR products were cloned into the SalI/NotI-digested product of the suicide vector pEMOC2 by the ClonExpress MultiS one-step cloning kit (Vazyme, Nanjing, Jiangsu Province, China). The recombinant plasmids pEMOC2-narQ(U/D) and pEMOC2-nap(U/D) were transformed into E. coli β2155 and then transferred into the A. pleuropneumoniae 4074 WT strain by coincubation conjugation (68). Chloramphenicol (2 μg/mL) and sucrose (10%) were used to induce homologous recombination to generate mutants. Correct mutants were identified by amplification of the full-length and flanking regions of narQ and nap (Fig. S2). And it was confirmed that the upstream and downstream gene expression was not affected at the genomic DNA (gDNA) and cDNA levels.
To construct complementary strains, the complete gene/operon fragments were amplified from WT gDNA using primers pMC-narQ-F/R and pMC-napF/R, respectively. Primers pMC-narQ-F/R were paired with pMC_R55K-F/R and pMC_R55S-F/R to amplify narQ truncated fragments. The amplified fragments were cloned into the vector pMC-Express (69) linearized with restriction enzymes KpnI and NotI. The recombinant plasmids were electrotransformed into the corresponding mutant strains, and the complemented strains were screened on TSA plates supplemented with NAD (10 μg/mL) and chloramphenicol (2 μg/mL) and confirmed by amplification with primers amplifying gene coding sequences and the pMC-testF/R primers flanking the restriction site.
Transcriptome analysis by RNA-Seq.
As mentioned above, total RNA was isolated from the WT, ΔnarQ, and ΔnarP strains cultured to mid-log phase and supplemented or not with NaNO3 (5 mM) under anaerobic conditions. Total RNA was extracted using TRIzol lysis according to the protocol recommended by the manufacturer (Total RNA extraction kit; Tianmo Biotech, Beijing, China), with three independent biological replicates per group. After evaluating the quality and purity of the mRNA library by the Agilent 2100 system, sequencing was performed on the Illumina NextSeq 500 platform. Data filtering and quality control were performed with Cutadapt and FastQC, and high-quality clean reads were aligned to the A. pleuropneumoniae 4074 genome (NZ_CP029003.1) (70) with Bowtie2. The raw expression levels of the genes were counted using HTSeq 0.6.1p2, and the expression levels were normalized using FPKM. Genes with an expression fold change of ≥2 (|log2 fold change| of ≥1) and adjusted P value of <0.05 (P value adjusted by the Benjamini and Hochberg method) were identified as differentially expressed genes. The gene differential expression analysis and principal-component analysis were performed using DESeq. Two-way cluster analysis was performed on the differentially expressed genes for all comparison groups using Pheatmap. Clustering was performed according to the expression level of the same gene in different samples and the expression patterns of different genes in the same sample. The Euclidean method was used to calculate the distance, and the hierarchical clustering longest distance method (Complete Linkage) was used for clustering. Subsequently, KEGG enrichment analysis was performed for the differentially expressed genes to evaluate the effect of nitrate on biological functions. Shanghai Personalbio Technology Co., Ltd. (China), provided the technical support for RNA-Seq and analysis.
Quantitative real-time RT-PCR.
Processing of samples and extraction of RNA were performed as described as above. PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Beijing, China) was used to digest residual gDNA and synthesize cDNA. Using cDNA as the template and primers listed in Table S3, quantitative PCR (qPCR) was performed using TB Green Premix Ex Taq II kit (Tli RNaseH Plus) (TaKaRa, Beijing, China). Normalized to the 16S rRNA-encoding gene as an endogenous reference, the threshold cycle (2−ΔΔCT) was calculated for each gene to determine relative transcript levels.
Electrophoretic mobility shift assay.
The MEME-FIMO (i.e., Find Individual Motif Occurences) online tool (https://meme-suite.org/meme/tools/fimo) (P < 0.001) was used to search the promoter regions of selected genes for the specific binding motifs of NarP. The primers listed in Table S3 were used to amplify a 400-bp fragment upstream of the coding DNA sequence (CDS) containing the promoter of the candidate gene using A. pleuropneumoniae 4074 gDNA as the template. A. pleuropneumoniae NarP was expressed and purified in E. coli, and the EMSA procedure was performed as described in our previous study (42).
Measurement of nitrite to determine the activity of nitrate reductase.
The WT, Δnap, and CΔnap strains were incubated anaerobically for 2 h in TSB supplemented with NAD (10 μg/mL) and 5 mM NaNO3. The nitrite concentration in the culture supernatants was measured using the Griess reagent system on a 96-well plate according to the protocol recommended by the manufacturer (Beyotime nitrate/nitrite assay kit; Beyotime, Shanghai, China). According to the OD550 values measured after the Griess reaction, the nitrite concentrations of the experimental group with different strains and the control group without bacterial medium were obtained with reference to the nitrite standard curve.
In vivo infection experiments.
A Kunming mouse intranasal infection model was established to compare the virulence of A. pleuropneumoniae strains (WT and ΔnarQ, ΔnarP, and Δnap mutants). Four-week-old female mice were anesthetized with Avertin (1.25% tribromoethanol) at 20 μL/g. Mice were intranasally infected with bacterial cultures from the mid-log phase at a dose of 4 × 106 CFU in 20 μL, and survival was determined. Mice were infected intranasally with bacterial cultures from the mid-log phase at a dose of 2 × 106 CFU to determine the bacterial loads in the lungs. To test the effect of nitrate on the pathogenicity of A. pleuropneumoniae, the bacterial solution was combined with NaNO3 at 0, 50, or 500 μM. Mice were euthanized at 12, 24, and 48 h after infection. The lungs of mice were thoroughly homogenized and plated on TSA plates after dilution, and CFU were determined.
Kunming mice were purchased from Laboratory Animal Centre, Huazhong Agriculture University, Wuhan, China. All animal procedures abide by ethical principles of animal welfare and were performed according to protocols approved by the Ethic Committee of Huazhong Agricultural University (HZAUMO-2022-0016).
Alignment and data analysis.
The amino acid sequences of A. pleuropneumoniae NarQ (WP_005596587.1) were aligned with those from Escherichia coli (NarQ, WP_001300881.1; NarX, WP_000918073.1), Salmonella Typhimurium (NarQ, WP_000216812.1), and Haemophilus influenzae (NarQ, WP_014550495.1) obtained from the Protein database of the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/protein/). MAFFT (Multiple Alignment using Fast Fourier Transform: MAFFT ≤ Multiple Sequence Alignment ≤ EMBL-EBI) was used to perform multiple-sequence alignments (71).
The Venn diagram and Pearson spearman coefficient correlation were plotted by Bioinformatics (http://www.bioinformatics.com.cn), an open online platform for data analysis and visualization. Plotting and statistical analysis of growth curves, qPCR, and nitrite quantification were performed with GraphPad Prism v8. All quantified results were based on at least three replicates and were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using a two-tailed t test on at least three biological replicates, as indicated. For comparisons of the mortalities of mice, the log rank (Mantel-Cox) test was performed for statistical analysis. The bacterial burden in mice was statistically analyzed using the Mann-Whitney test. A P value of <0.05 was considered to be statistically significant.
Data availability.
The data discussed in this study have been deposited in NCBI's Gene Expression Omnibus (72) and are accessible through GEO series accession no. GSE207497.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2021YFD1800401), National Natural Science Foundation of China (31572535), Hubei Provincial Central Guidance Local Science and Technology Development Project of China (no. 2020ZYYD029), and the UK Biotechnology and Biological Sciences Research Council (BB/S019901/1).
Footnotes
Supplemental material is available online only.
Contributor Information
Lu Li, Email: lilu@mail.hzau.edu.cn.
Andreas J. Bäumler, University of California, Davis
REFERENCES
- 1.Unden G, Klein R. 2021. Sensing of O2 and nitrate by bacteria: alternative strategies for transcriptional regulation of nitrate respiration by O2 and nitrate. Environ Microbiol 23:5–14. 10.1111/1462-2920.15293. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ashton DS, Moncada S. 1988. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature 333:664–666. 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 3.Szabó C, Ischiropoulos H, Radi R. 2007. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680. 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 4.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. 2013. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–711. 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. 2007. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5:2177–2189. 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera-Chávez F, Winter SE, Lopez CA, Xavier MN, Winter MG, Nuccio SP, Russell JM, Laughlin RC, Lawhon SD, Sterzenbach T, Bevins CL, Tsolis RM, Harshey R, Adams LG, Bäumler AJ. 2013. Salmonella uses energy taxis to benefit from intestinal inflammation. PLoS Pathog 9:e1003267. 10.1371/journal.ppat.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez CA, Rivera-Chávez F, Byndloss MX, Bäumler AJ. 2015. The periplasmic nitrate reductase NapABC supports luminal growth of Salmonella enterica serovar Typhimurium during colitis. Infect Immun 83:3470–3478. 10.1128/IAI.00351-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin PA, Bettke JA, Tam JW, Leeds J, Bliska JB, Butler BP, van der Velden AWM. 2019. Inflammatory monocytes provide a niche for Salmonella expansion in the lumen of the inflamed intestine. PLoS Pathog 15:e1007847. 10.1371/journal.ppat.1007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabin RS, Stewart V. 1993. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J Bacteriol 175:3259–3268. 10.1128/jb.175.11.3259-3268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morozkina EV, Zvyagilskaya RA. 2007. Nitrate reductases: structure, functions, and effect of stress factors. Biochemistry (Mosc) 72:1151–1160. 10.1134/s0006297907100124. [DOI] [PubMed] [Google Scholar]
- 11.Sparacino-Watkins C, Stolz JF, Basu P. 2014. Nitrate and periplasmic nitrate reductases. Chem Soc Rev 43:676–706. 10.1039/c3cs60249d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole JA, Richardson DJ. 18 August 2008, posting date. Respiration of nitrate and nitrite. EcoSal Plus 2008. 10.1128/ecosal.3.2.5. [DOI] [PubMed] [Google Scholar]
- 13.Scales BS, Dickson RP, Huffnagle GB. 2016. A tale of two sites: how inflammation can reshape the microbiomes of the gut and lungs. J Leukoc Biol 100:943–950. 10.1189/jlb.3MR0316-106R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Man WH, de Steenhuijsen Piters WA, Bogaert D. 2017. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15:259–270. 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffnagle GB, Dickson RP, Lukacs NW. 2017. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol 10:299–306. 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dedon PC, Tannenbaum SR. 2004. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys 423:12–22. 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Jones KL, Hegab AH, Hillman BC, Simpson KL, Jinkins PA, Grisham MB, Owens MW, Sato E, Robbins RA. 2000. Elevation of nitrotyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr Pulmonol 30:79–85. . [DOI] [PubMed] [Google Scholar]
- 18.Arai H. 2011. Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front Microbiol 2:103. 10.3389/fmicb.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sassu EL, Bossé JT, Tobias TJ, Gottschalk M, Langford PR, Hennig-Pauka I. 2018. Update on Actinobacillus pleuropneumoniae—knowledge, gaps and challenges. Transbound Emerg Dis 65(Suppl 1):72–90. 10.1111/tbed.12739. [DOI] [PubMed] [Google Scholar]
- 20.Chiers K, Donné E, Van Overbeke I, Ducatelle R, Haesebrouck F. 2002. Actinobacillus pleuropneumoniae infections in closed swine herds: infection patterns and serological profiles. Vet Microbiol 85:343–352. 10.1016/s0378-1135(01)00518-1. [DOI] [PubMed] [Google Scholar]
- 21.Bossé JT, Janson H, Sheehan BJ, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. 2002. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect 4:225–235. 10.1016/s1286-4579(01)01534-9. [DOI] [PubMed] [Google Scholar]
- 22.Chiers K, De Waele T, Pasmans F, Ducatelle R, Haesebrouck F. 2010. Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet Res 41:65. 10.1051/vetres/2010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahar N, Turni C, Tram G, Blackall PJ, Atack JM. 2021. Actinobacillus pleuropneumoniae: the molecular determinants of virulence and pathogenesis. Adv Microb Physiol 78:179–216. 10.1016/bs.ampbs.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Chen X, Tan C, Guo Y, Chen Y, Fu S, Bei W, Chen H. 2009. In vivo induced RTX toxin ApxIVA is essential for the full virulence of Actinobacillus pleuropneumoniae. Vet Microbiol 137:282–289. 10.1016/j.vetmic.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Lepesheva A, Osickova A, Holubova J, Jurnecka D, Knoblochova S, Espinosa-Vinals C, Bumba L, Skopova K, Fiser R, Osicka R, Sebo P, Masin J. 2021. Different roles of conserved tyrosine residues of the acylated domains in folding and activity of RTX toxins. Sci Rep 11:19814. 10.1038/s41598-021-99112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakarji L, Mikael LG, Srikumar R, Kobisch M, Coulton JW, Jacques M. 2006. Fhua and HgbA, outer membrane proteins of Actinobacillus pleuropneumoniae: their role as virulence determinants. Can J Microbiol 52:391–396. 10.1139/w05-135. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Cao S, Zhang L, Yuan J, Yang Y, Zhu Z, Wen Y, Wu R, Zhao Q, Huang X, Yan Q, Huang Y, Ma X, Wen X. 2017. TolC2 is required for the resistance, colonization and virulence of Actinobacillus pleuropneumoniae. J Med Microbiol 66:1170–1176. 10.1099/jmm.0.000544. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Cao Y, Gao L, Zhang L, Gong S, Yang J, Zhao H, Yang D, Zhao J, Meng J, Gao Q, Qi C. 2018. Outer membrane lipoprotein Lip40 modulates adherence, colonization, and virulence of Actinobacillus pleuropneumoniae. Front Microbiol 9:1472. 10.3389/fmicb.2018.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Cao S, Zhang L, Yuan J, Zhao Q, Wen Y, Wu R, Huang X, Yan Q, Huang Y, Ma X, Han X, Miao C, Wen X. 2019. A requirement of TolC1 for effective survival, colonization and pathogenicity of Actinobacillus pleuropneumoniae. Microb Pathog 134:103596. 10.1016/j.micpath.2019.103596. [DOI] [PubMed] [Google Scholar]
- 30.Baltes N, N'Diaye M, Jacobsen ID, Maas A, Buettner FF, Gerlach GF. 2005. Deletion of the anaerobic regulator HlyX causes reduced colonization and persistence of Actinobacillus pleuropneumoniae in the porcine respiratory tract. Infect Immun 73:4614–4619. 10.1128/IAI.73.8.4614-4619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buettner FF, Bendallah IM, Bossé JT, Dreckmann K, Nash JH, Langford PR, Gerlach GF. 2008. Analysis of the Actinobacillus pleuropneumoniae ArcA regulon identifies fumarate reductase as a determinant of virulence. Infect Immun 76:2284–2295. 10.1128/IAI.01540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buettner FF, Bendalla IM, Bossé JT, Meens J, Nash JH, Härtig E, Langford PR, Gerlach GF. 2009. Analysis of the Actinobacillus pleuropneumoniae HlyX (FNR) regulon and identification of iron-regulated protein B as an essential virulence factor. Proteomics 9:2383–2398. 10.1002/pmic.200800439. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan BJ, Bossé JT, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. 2003. Identification of Actinobacillus pleuropneumoniae genes important for survival during infection in its natural host. Infect Immun 71:3960–3970. 10.1128/IAI.71.7.3960-3970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lone AG, Deslandes V, Nash JH, Jacques M, Macinnes JI. 2009. Modulation of gene expression in Actinobacillus pleuropneumoniae exposed to bronchoalveolar fluid. PLoS One 4:e6139. 10.1371/journal.pone.0006139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klitgaard K, Friis C, Jensen TK, Angen Ø, Boye M. 2012. Transcriptional portrait of Actinobacillus pleuropneumoniae during acute disease—potential strategies for survival and persistence in the host. PLoS One 7:e35549. 10.1371/journal.pone.0035549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P, Xu Z, Sun X, Yin Y, Fan Y, Zhao J, Mao X, Huang J, Yang F, Zhu L. 2017. Transcript profiling of the immunological interactions between Actinobacillus pleuropneumoniae serotype 7 and the host by dual RNA-seq. BMC Microbiol 17:193. 10.1186/s12866-017-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z, Zhou Y, Li L, Zhou R, Xiao S, Wan Y, Zhang S, Wang K, Li W, Li L, Jin H, Kang M, Dalai B, Li T, Liu L, Cheng Y, Zhang L, Xu T, Zheng H, Pu S, Wang B, Gu W, Zhang XL, Zhu GF, Wang S, Zhao GP, Chen H. 2008. Genome biology of Actinobacillus pleuropneumoniae JL03, an isolate of serotype 3 prevalent in China. PLoS One 3:e1450. 10.1371/journal.pone.0001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konze SA, Abraham WR, Goethe E, Surges E, Kuypers MMM, Hoeltig D, Meens J, Vogel C, Stiesch M, Valentin-Weigand P, Gerlach GF, Buettner FFR. 2019. Link between heterotrophic carbon fixation and virulence in the porcine lung pathogen Actinobacillus pleuropneumoniae. Infect Immun 87:e00768-18. 10.1128/IAI.00768-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Zhu J, Yang K, Xu Z, Liu Z, Zhou R. 2014. Changes in gene expression of Actinobacillus pleuropneumoniae in response to anaerobic stress reveal induction of central metabolism and biofilm formation. J Microbiol 52:473–481. 10.1007/s12275-014-3456-y. [DOI] [PubMed] [Google Scholar]
- 40.Baltes N, Hennig-Pauka I, Jacobsen I, Gruber AD, Gerlach GF. 2003. Identification of dimethyl sulfoxide reductase in Actinobacillus pleuropneumoniae and its role in infection. Infect Immun 71:6784–6792. 10.1128/IAI.71.12.6784-6792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobsen I, Hennig-Pauka I, Baltes N, Trost M, Gerlach GF. 2005. Enzymes involved in anaerobic respiration appear to play a role in Actinobacillus pleuropneumoniae virulence. Infect Immun 73:226–234. 10.1128/IAI.73.1.226-234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Huang Q, Fang Q, Li H, Tang H, Zou G, Wang D, Li S, Bei W, Chen H, Li L, Zhou R. 2020. Identification of genes regulated by the two-component system response regulator NarP of Actinobacillus pleuropneumoniae via DNA-affinity-purified sequencing. Microbiol Res 230:126343. 10.1016/j.micres.2019.126343. [DOI] [PubMed] [Google Scholar]
- 43.Olive AJ, Sassetti CM. 2016. Metabolic crosstalk between host and pathogen: sensing, adapting and competing. Nat Rev Microbiol 14:221–234. 10.1038/nrmicro.2016.12. [DOI] [PubMed] [Google Scholar]
- 44.Cavicchioli R, Chiang RC, Kalman LV, Gunsalus RP. 1996. Role of the periplasmic domain of the Escherichia coli NarX sensor-transmitter protein in nitrate-dependent signal transduction and gene regulation. Mol Microbiol 21:901–911. 10.1046/j.1365-2958.1996.491422.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheung J, Hendrickson WA. 2009. Structural analysis of ligand stimulation of the histidine kinase NarX. Structure 17:190–201. 10.1016/j.str.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gushchin I, Melnikov I, Polovinkin V, Ishchenko A, Yuzhakova A, Buslaev P, Bourenkov G, Grudinin S, Round E, Balandin T, Borshchevskiy V, Willbold D, Leonard G, Buldt G, Popov A, Gordeliy V. 2017. Mechanism of transmembrane signaling by sensor histidine kinases. Science 356:eaah6345. 10.1126/science.aah6345. [DOI] [PubMed] [Google Scholar]
- 47.Gushchin I, Orekhov P, Melnikov I, Polovinkin V, Yuzhakova A, Gordeliy V. 2020. Sensor histidine kinase NarQ activates via helical rotation, diagonal scissoring, and eventually piston-like shifts. Int J Mol Sci 21:3110. 10.3390/ijms21093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cerqueira NM, Gonzalez PJ, Fernandes PA, Moura JJ, Ramos MJ. 2015. Periplasmic nitrate reductase and formate dehydrogenase: similar molecular architectures with very different enzymatic activities. Acc Chem Res 48:2875–2884. 10.1021/acs.accounts.5b00333. [DOI] [PubMed] [Google Scholar]
- 49.Gates AJ, Hughes RO, Sharp SR, Millington PD, Nilavongse A, Cole JA, Leach ER, Jepson B, Richardson DJ, Butler CS. 2003. Properties of the periplasmic nitrate reductases from Paracoccus pantotrophus and Escherichia coli after growth in tungsten-supplemented media. FEMS Microbiol Lett 220:261–269. 10.1016/S0378-1097(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, Hughes ER, Büttner L, de Lima Romão E, Behrendt CL, Lopez CA, Sifuentes-Dominguez L, Huff-Hardy K, Wilson RP, Gillis CC, Tükel Ç, Koh AY, Burstein E, Hooper LV, Bäumler AJ, Winter SE. 2018. Precision editing of the gut microbiota ameliorates colitis. Nature 553:208–211. 10.1038/nature25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuypers MMM, Marchant HK, Kartal B. 2018. The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276. 10.1038/nrmicro.2018.9. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Torres A, Bäumler AJ. 2016. Nitrate, nitrite and nitric oxide reductases: from the last universal common ancestor to modern bacterial pathogens. Curr Opin Microbiol 29:1–8. 10.1016/j.mib.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Line L, Alhede M, Kolpen M, Kühl M, Ciofu O, Bjarnsholt T, Moser C, Toyofuku M, Nomura N, Høiby N, Jensen P. 2014. Physiological levels of nitrate support anoxic growth by denitrification of Pseudomonas aeruginosa at growth rates reported in cystic fibrosis lungs and sputum. Front Microbiol 5:554. 10.3389/fmicb.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gushchin I, Aleksenko VA, Orekhov P, Goncharov IM, Nazarenko VV, Semenov O, Remeeva A, Gordeliy V. 2021. Nitrate- and nitrite-sensing histidine kinases: function, structure, and natural diversity. Int J Mol Sci 22:5933. 10.3390/ijms22115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-Mora D, Ortega A, Matilla MA, Martinez-Rodriguez S, Gavira JA, Krell T. 2019. The molecular mechanism of nitrate chemotaxis via direct ligand binding to the PilJ domain of McpN. mBio 10:e02334-18. 10.1128/mBio.02334-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Tseng CP, Gunsalus RP. 1999. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J Bacteriol 181:5303–5308. 10.1128/JB.181.17.5303-5308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter LC, Millington P, Griffiths L, Thomas GH, Cole JA. 1999. Competition between Escherichia coli strains expressing either a periplasmic or a membrane-bound nitrate reductase: does Nap confer a selective advantage during nitrate-limited growth? Biochem J 344:77–84. 10.1042/bj3440077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noriega CE, Lin HY, Chen LL, Williams SB, Stewart V. 2010. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol 75:394–412. 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes ER, Winter MG, Alves da Silva L, Muramatsu MK, Jimenez AG, Gillis CC, Spiga L, Chanin RB, Santos RL, Zhu W, Winter SE. 2021. Reshaping of bacterial molecular hydrogen metabolism contributes to the outgrowth of commensal E. coli during gut inflammation. eLife 10:e58609. 10.7554/eLife.58609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unden G, Bongaerts J. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320:217–234. 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 61.Lüke I, Butland G, Moore K, Buchanan G, Lyall V, Fairhurst SA, Greenblatt JF, Emili A, Palmer T, Sargent F. 2008. Biosynthesis of the respiratory formate dehydrogenases from Escherichia coli: characterization of the FdhE protein. Arch Microbiol 190:685–696. 10.1007/s00203-008-0420-4. [DOI] [PubMed] [Google Scholar]
- 62.Lü W, Du J, Schwarzer NJ, Gerbig-Smentek E, Einsle O, Andrade SL. 2012. The formate channel FocA exports the products of mixed-acid fermentation. Proc Natl Acad Sci USA 109:13254–13259. 10.1073/pnas.1204201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao M, Lai Y, Sun J, Chen G, Yan A. 2016. Transcriptional regulation of the outer membrane porin gene ompW reveals its physiological role during the transition from the aerobic to the anaerobic lifestyle of Escherichia coli. Front Microbiol 7:799. 10.3389/fmicb.2016.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leimkühler S. 2020. The biosynthesis of the molybdenum cofactors in Escherichia coli. Environ Microbiol 22:2007–2026. 10.1111/1462-2920.15003. [DOI] [PubMed] [Google Scholar]
- 65.Thorup C, Schramm A, Findlay AJ, Finster KW, Schreiber L. 2017. Disguised as a sulfate reducer: growth of the Deltaproteobacterium Desulfurivibrio alkaliphilus by sulfide oxidation with nitrate. mBio 8:e00671-17. 10.1128/mBio.00671-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Xing J. 2021. Haloalkaliphilic denitrifiers-dependent sulfate-reducing bacteria thrive in nitrate-enriched environments. Water Res 201:117354. 10.1016/j.watres.2021.117354. [DOI] [PubMed] [Google Scholar]
- 67.Bossé JT, Sinha S, Li MS, O'Dwyer CA, Nash JH, Rycroft AN, Kroll JS, Langford PR. 2010. Regulation of pga operon expression and biofilm formation in Actinobacillus pleuropneumoniae by sigmaE and H-NS. J Bacteriol 192:2414–2423. 10.1128/JB.01513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oswald W, Tonpitak W, Ohrt G, Gerlach G. 1999. A single-step transconjugation system for the introduction of unmarked deletions into Actinobacillus pleuropneumoniae serotype 7 using a sucrose sensitivity marker. FEMS Microbiol Lett 179:153–160. 10.1111/j.1574-6968.1999.tb08721.x. [DOI] [PubMed] [Google Scholar]
- 69.Bossé JT, Durham AL, Rycroft AN, Kroll JS, Langford PR. 2009. New plasmid tools for genetic analysis of Actinobacillus pleuropneumoniae and other Pasteurellaceae. Appl Environ Microbiol 75:6124–6131. 10.1128/AEM.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Z, Chen X, Li L, Li T, Wang S, Chen H, Zhou R. 2010. Comparative genomic characterization of Actinobacillus pleuropneumoniae. J Bacteriol 192:5625–5636. 10.1128/JB.00535-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R. 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edgar R, Domrachev M, Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210. 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00239-22-s0001.pdf, PDF file, 5.1 MB (5.1MB, pdf)
Data Availability Statement
The data discussed in this study have been deposited in NCBI's Gene Expression Omnibus (72) and are accessible through GEO series accession no. GSE207497.