Abstract
Background
This study was designed to compare outcomes of extended adjuvant temozolomide (TMZ) vs standard adjuvant TMZ following radiotherapy (RT) plus concurrent TMZ in newly diagnosed glioblastoma.
Methods
This systematic review and meta-analysis was carried out in accordance with Cochrane methodology. Only prospective clinical trials randomly assigning adults with newly diagnosed glioblastoma after concurrent RT/TMZ to 6 cycles of adjuvant TMZ (control arm) or extended (>6 cycles) adjuvant TMZ (experimental arm) were eligible. Primary outcome of interest was overall survival, while progression-free survival and toxicity were secondary endpoints. Hazard ratio (HR) for progression and death with corresponding 95% confidence interval (CI) were computed for individual primary study and pooled using random-effects model. Toxicity was defined as proportion of patients with ≥grade 3 hematologic toxicity and expressed as risk ratio (RR) with 95% CI. Any P-value <.05 was considered statistically significant.
Results
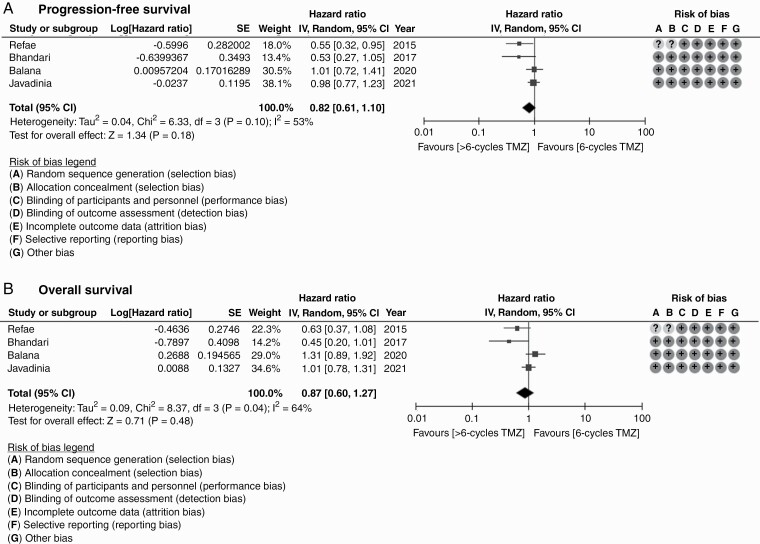
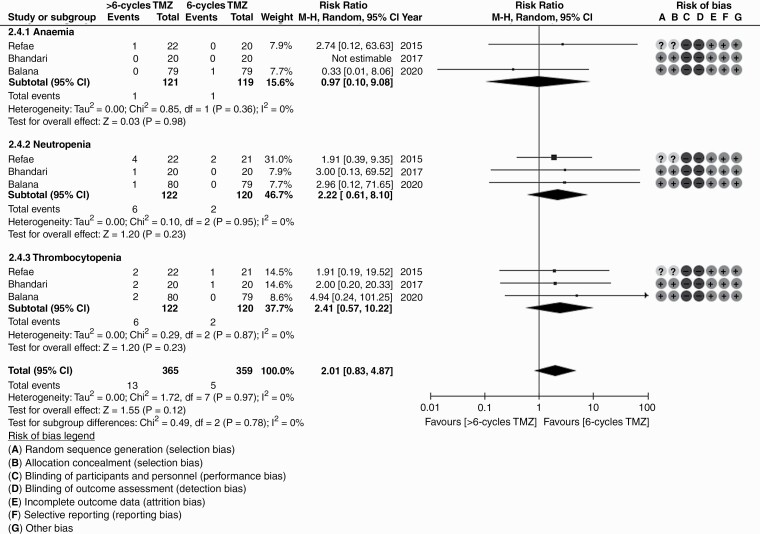
Systematic literature review identified five randomized controlled trials comparing standard (6 cycles) vs extended (>6 cycles) adjuvant TMZ in newly diagnosed glioblastoma. Outcome data could be extracted from 358 patients from four primary studies. Extended adjuvant TMZ was not associated with statistically significant reduction in the risk of progression (HR = 0.82, 95% CI: 0.61-1.10; P = .18) or death (HR = 0.87, 95% CI:0.60-1.27; P = .48) compared to standard adjuvant TMZ. Grade ≥3 hematologic toxicity though somewhat higher with extended adjuvant TMZ, was not significantly different between the two arms (RR = 2.01, 95% CI: 0.83-4.87; P = .12).
Conclusions
There is low-certainty evidence that extended adjuvant TMZ is not associated with significant survival benefit or increased hematologic toxicity in unselected patients with newly diagnosed glioblastoma compared to standard adjuvant TMZ.
Keywords: extended, glioblastoma, safety, survival, temozolomide
Glioblastoma is the commonest malignant primary tumor of the central nervous system (CNS) comprising nearly 40% of all primary brain tumors in adulthood.1 The contemporary post-surgical standard of care2 for patients with newly diagnosed glioblastoma is focal conformal radiotherapy (RT) to the tumor bed with margins using conventional fractionation (1.8-2 Gy per fraction for a total dose of 59.4-60 Gy in 30-33 fractions over 6-6.5 weeks) with concurrent oral temozolomide (TMZ) chemotherapy (75 mg/m2) followed by 6 cycles of adjuvant TMZ chemotherapy (150-200 mg/m2 D1-D5 every 4-weekly). The improvements in postoperative survival for newly diagnosed glioblastoma over time are clearly reflected in population-based analyses in the TMZ era.3 However, despite such aggressive multi-modality therapy, the prognosis of patients with glioblastoma remains dismal with an expected median survival of 15 months, 2-year survival of 27%, and 5-year survival barely reaching 10%.2,3 The benefit of adding TMZ (both concurrent and adjuvant) to RT was most pronounced in patients with epigenetic silencing of the O6-methylguanine DNA methyltransferase (MGMT) gene promoter via methylation,4 which has since been established as an independent prognostic factor as well as a predictive factor for response to TMZ chemotherapy.5 The benefit of adding TMZ to RT in patients with unmethylated MGMT has been marginal2,4 raising question marks over its usage in patients with unmethylated tumors.5,6 However, there exists considerable debate and controversy regarding the most appropriate technique for MGMT testing7 and defining the most optimal cutoff8 for classifying glioblastoma as lacking MGMT gene promoter methylation.
The optimal duration of adjuvant TMZ has remained controversial with wide variations in practice globally. Many patients with glioblastoma who remain progression-free after 6 cycles of TMZ receive further adjuvant TMZ till progression or 12 cycles (sometimes even more) based on personal, physician, and institutional preferences. Several physicians continue adjuvant TMZ (beyond 6 cycles) in patients demonstrating good response to TMZ (particularly with methylated MGMT) till clinico-radiological progression or toxicity. On the other hand, in most healthcare settings, TMZ is generally stopped after 6 cycles based on prevalent local standards. A meta-analysis9 involving 1018 patients with glioblastoma from seven studies reported statistically significant improvements in progression-free survival (PFS) (P < .001) and overall survival (OS) (P = .018) with extended adjuvant TMZ (>6 cycles) compared to standard 6 cycles. However, most such retrospective analyses and pooling of data are prone to inherent biases and cannot guide therapeutic decision making which relies on high-quality evidence generated through prospective randomized controlled trials (RCTs). The conduct and reporting of RCTs10–14 directly comparing extended adjuvant TMZ vs standard adjuvant TMZ in patients with newly diagnosed glioblastoma provides an opportunity to extract and pool the data to determine the efficacy and safety of such an experimental approach. The aim of this updated systematic review and meta-analysis was to compare the efficacy and safety of extended adjuvant TMZ (>6 cycles) vs standard adjuvant TMZ (6 cycles) in patients with newly diagnosed glioblastoma.
Materials and Methods
This systematic review was carried out in accordance with Cochrane methodology for systematic reviews of interventional studies15 and reported as per the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 Analysis, interpretation, and reporting included a risk of bias assessment using the Cochrane Risk of Bias tool that assigns studies as having low, unclear, or high risk of bias. Quality of evidence and strength of recommendation was based on the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach17 that involves consideration of methodological quality, directness of evidence, heterogeneity, precision of effect estimates, and publication bias.
Literature Search Strategy
An electronic search of Medline via PubMed was conducted on March 15, 2021 and updated again on October 1, 2021 with no language, year, or publication status restrictions. The Cochrane Central Register of Controlled Trials (CENTRAL) and Database of Abstracts of Reviews of Effectiveness (DARE) were also searched electronically. Details of the search strategy are presented in Supplementary file S1. Electronic search was further supplemented by hand-searching of review articles, cross-references, and conference proceedings.
Study Eligibility
Only RCTs testing the duration of adjuvant TMZ in newly diagnosed glioblastoma following postoperative concurrent RT/TMZ were considered eligible. Prospective clinical trials randomly assigning patients to extended (>6 cycles) adjuvant TMZ (experimental arm) or standard (6 cycles) adjuvant TMZ were included. Randomization in an individual study could have been done upfront before concurrent phase (RT/TMZ), after completion of concurrent RT/TMZ and before starting adjuvant phase, or after completion of standard adjuvant TMZ (6 cycles). Emulated RCTs, quasi-randomized trials, propensity-matched analyses, non-randomized comparative studies, or observational studies were not considered in this review.
Outcome Measures
Measures of efficacy included survival; the primary outcome of interest was OS with PFS being considered as a secondary outcome measure. OS was defined as the time interval between date of diagnosis (surgery) or date of inclusion in the study and last contact or death from any cause. PFS was calculated from diagnosis or inclusion in the study till documented clinico-radiological progression, last contact, or death whichever occurred earlier. Safety outcomes included the comparison of TMZ-induced grade 3 or worse hematologic toxicity (anemia, neutropenia, and thrombocytopenia) during adjuvant phase of therapy.
Data Extraction and Statistical Analyses
Two reviewers (R.T. and A.C.) independently read each abstract, pre-print, publication, protocol, or any other available study report and extracted relevant data from individual primary studies. Discrepancy, if any, was resolved through consensus interpretation by a third reviewer (T.G.). Extracted data included study characteristics, patient characteristics, number of participants randomized per arm, intervention details, and outcomes. Survival outcomes for individual patients were extracted manually from the published Kaplan-Meier survival curves using WebPlot digitizer.18 Using this individual participant level extracted data and published numbers at risk, survival curves for OS and PFS for each study were reconstructed.19 The number of events and the time points (t-risk and n-risk) were extracted from published data. If not reported explicitly, the same was derived from available graphs as precisely as possible to generate composite Kaplan-Meier survival curves that included individual-level data from all four RCTs.18,19 The hazard ratio (HR) with corresponding 95% confidence interval (CI) was computed for each individual primary study and also compared with the published HR (95% CI) if reported and reconciled as required prior to statistical pooling. Grade 3 or worse hematologic toxicity (anemia, neutropenia, and thrombocytopenia) was compared between the two arms and expressed as risk ratio (RR) with 95% CI. All data were pooled using the random-effects model and expressed as HR or RR as appropriate with corresponding 95% CI. Any P-value <.05 was considered as statistically significant. Sensitivity analysis (dropping one study at a time), subgroup analysis (based on MGMT methylation status), and publication bias (through funnel plot) were also assessed as appropriate. All analyses were done using Review Manager (RevMan) version 5.3 and GRADE profiler (GRADEpro) version 3.6.1 (The Nordic Cochrane Centre, Cochrane Collaboration, 2008), Stata 14.0 (StataCorp LP, College Station, TX, USA) and R Studio. The protocol is registered with the International Platform of Registered Systematic Reviews and Meta-analysis Protocols (INPLASY2021120114). No source of funding was involved in data extraction, analysis, interpretation, or reporting of results.
Results
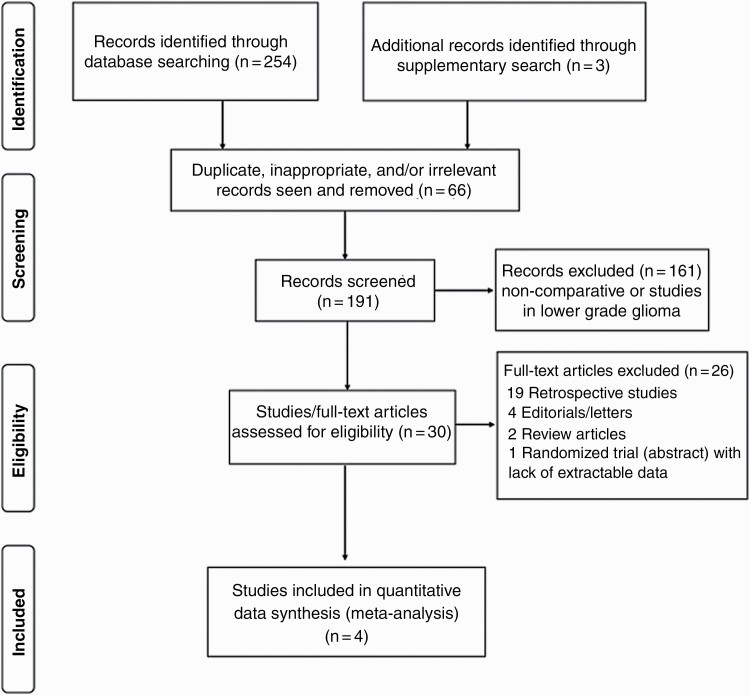
The flow diagram of study selection and inclusion in the meta-analysis as per PRISMA guidelines is depicted in Figure 1. Detailed PRISMA checklist is also provided in Supplementary file S2. Comprehensive and systematic search of the literature using the described search strategy identified a total of 257 potentially eligible records that were retrieved for further review. After removing duplicates, inappropriate, or irrelevant reports (n = 66), 191 abstracts were screened. Non-comparative studies and trials involving lower-grade glioma (n = 161) were excluded leaving 30 studies comparing extended adjuvant TMZ vs standard adjuvant TMZ in patients with newly diagnosed glioblastoma that were reviewed in greater detail. Finally, five RCTs (three full-text publications and two abstract presentations) were identified for inclusion; however, outcomes could not be extracted from one abstract publication,10 leaving 358 patients enrolled in four RCTs11–14 that were included in this updated systematic review and meta-analysis.
Figure 1.
Flow diagram of study selection and inclusion in the meta-analysis as per PRISMA guidelines. Abbreviation: PRISMA, Preferred Reporting of Systematic Reviews and Meta-Analyses.
Description of Included Studies
Baseline characteristics and outcomes of interest in the four RCTs are summarized in Table 1. The first RCT11 was reported from Egypt, wherein patients with glioblastoma without clinico-radiological evidence of progression following concurrent RT/TMZ and 6 cycles of adjuvant TMZ were randomized to observation (n = 29) or extended adjuvant TMZ (n = 30). The number of cycles in the extended adjuvant TMZ arm was not pre-defined, but continued till progression, with median number of TMZ cycles being 11. Survival outcomes in the study were reported on intention-to-treat basis from date of surgery. At a median follow-up of 15.2 months, median PFS (10.4 vs 13.2 months; P = .038) and OS (14.1 vs 18.8 months; P = .070) were better with extended adjuvant TMZ. The second RCT12 was conducted in India wherein patients with glioblastoma were randomized after concurrent RT/TMZ to either 6 cycles of adjuvant TMZ (n = 20) or 12 cycles of extended adjuvant TMZ (n = 20). Survival outcomes in the study were defined from date of surgery and reported on intention-to-treat basis. At a median follow-up of 17.25 months, both median PFS (12.6 vs 16.8 months; P = .069) and OS (15.4 vs 23.8 months; P = .044) favored extended adjuvant TMZ arm. The use of extended adjuvant TMZ was associated with increased grade 3 or worse hematologic toxicity (15 vs 5%) during the adjuvant phase of treatment. The largest dataset13 comparing standard adjuvant TMZ with extended adjuvant TMZ comes from a multicentric Spanish study (GEINO 14-01) wherein patients of newly diagnosed glioblastoma without evidence of progression after concurrent RT/TMZ plus 6 cycles of TMZ were randomized to no further TMZ (n = 79) vs continuing further TMZ up to a total of 12 cycles (n = 80), stratified by MGMT and measurable disease. Survival outcomes were defined and reported on intention-to-treat basis from date of inclusion in the study. At a median follow-up of 33.4 months, there were no significant differences in median PFS (7.7 vs 9.5 months; P = .95) or median OS (23.3 vs 18.2 months; P = .16) between the two arms. Toxicity of treatment, such as lymphopenia (P < .001), thrombocytopenia (P < .001), and nausea/vomiting (P = .001), although mild and self-limiting was more frequent with extended adjuvant TMZ. The most recent RCT14 reported from Iran in abstract form compared standard 6 cycles (n = 50) vs extended 12 cycles (n = 50) of TMZ in patients with newly diagnosed high-grade glioma. At a median follow-up of 16.5 months, there was no statistically significant difference in 2-year OS (55.5% vs 63.5%; P = .976) between the two arms.
Table 1.
Baseline Patient, Disease, and Treatment Characteristics With Summary Outcomes of Randomized Controlled Trials Comparing Standard Adjuvant TMZ (6 Cycles) vs Extended Adjuvant TMZ (>6 Cycles) in Patients With Newly Diagnosed Glioblastoma
| Author (Reference) | Age Range (years) | Males | RT Dose | Median FU (months) | Treatment Arm | Number of Patients | Median PFS (months) | Median OS (months) | ≥Grade 3 Hematologic Toxicity (Adjuvant TMZ) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | Neutropenia | Thrombocytopenia | |||||||||
| Refae11 | 19-72 | 79.7% | 59 Gy | 15.2 | 6-cycle TMZ | 29 | 10.4 | 14.1 | 0 | 9.5% | 4.7% |
| >6-cycle TMZ | 30 | 13.2 | 18.8 | 4.5% | 18% | 9% | |||||
| Bhandari12 | 19-65 | 60% | 60 Gy | 17.3 | 6-cycle TMZ | 20 | 12.8 | 15.4 | 0 | 0 | 5% |
| >6-cycle TMZ | 20 | 16.8 | 23.8 | 0 | 5% | 10% | |||||
| a , b Balana13 | 29-83 | 52.2% | NA/NR | 33.4 | 6-cycle TMZ | 79 | 7.7 | 23.3 | 1.2% | 0 | 0 |
| >6-cycle TMZ | 80 | 9.5 | 18.2 | 0 | 1.3% | 2.5% | |||||
| Javadinia14 | ≥18 | NA/NR | NA/NR | 16.5 | 6-cycle TMZ | 50 | 11.3 | 20.2 | NA/NR | NA/NR | NA/NR |
| >6-cycle TMZ | 50 | 13.0 | 23.2 | NA/NR | NA/NR | NA/NR | |||||
Abbreviations: FU, follow-up; NA/NR, not available/not reported; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; TMZ, temozolomide.
aSurvival outcomes were reported from date of inclusion in the study (after 6-cycle TMZ) and not from date of diagnosis (surgery).
bUpdated results (at ASCO 2021) reported median OS of 22.0 months in control arm (6-cycle TMZ) and 18.2 months in experimental arm (>6-cycle TMZ).
Evidence Synthesis
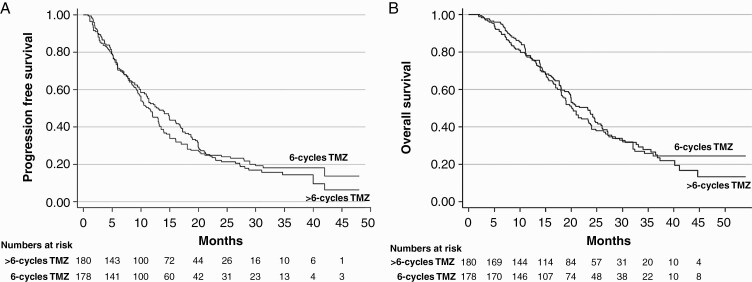
There was modest heterogeneity in included RCTs allowing statistical pooling of results. Overall quality of included studies was acceptable with low risk of bias for efficacy outcomes and unclear to high risk of bias for toxicity outcomes. Aggregate data from all four RCTs based on reconstructed Kaplan-Meier curves showed no significant difference between standard adjuvant TMZ vs extended adjuvant TMZ for PFS or OS (Figure 2). Extended adjuvant TMZ was not associated with statistically significant reduction in the risk of progression (HR = 0.82, 95% CI: 0.61-1.10; P = .18) or death (HR = 0.87, 95% CI:0.60-1.27; P = .48) compared to standard adjuvant TMZ (Figure 3). The overall rate of grade 3 or worse hematologic toxicity (Figure 4) during adjuvant phase though somewhat higher with extended adjuvant TMZ was not statistically significantly different between the two arms (RR = 2.01, 95% CI: 0.83-4.87; P = .12). Individual hematological toxicity during adjuvant TMZ, such as anemia (RR = 0.97, 95% CI: 0.10-9.08; P = .98), neutropenia (RR = 2.22, 95% CI: 0.61-8.10; P = .23), or thrombocytopenia (RR = 2.41, 95% CI: 0.57-10.22; P = .23) were also not significantly different (Figure 4). Sensitivity analysis (Supplementary Figure S3) demonstrated lack of influence of any single study on the overall treatment effect, inferences, and conclusions. Subgroup analysis based on MGMT methylation status could not be done due to lack of extractable data in published reports. A relatively symmetric funnel plot (Supplementary Figure S4) ruled out significant publication bias in the meta-analysis.
Figure 2.
Reconstructed Kaplan-Meier curves of progression-free survival (A) and overall survival (B) for individual-level participant data extracted from four randomized controlled trials comparing standard adjuvant temozolomide (6 cycles) vs extended adjuvant temozolomide (>6 cycles) in newly diagnosed glioblastoma. Note the completely overlapping curves suggesting no significant difference between the two arms.
Figure 3.
Forest plot for progression-free survival (A) and overall survival (B) including risk of bias in individual studies comparing standard adjuvant temozolomide (6 cycles) vs extended adjuvant temozolomide (>6 cycles) in newly diagnosed glioblastoma.
Figure 4.
Forest plot for grade 3 or worse hematologic toxicity (anemia, neutropenia, and thrombocytopenia) during adjuvant temozolomide in studies comparing standard adjuvant temozolomide (6 cycles) vs extended adjuvant temozolomide (>6 cycles) in newly diagnosed glioblastoma.
Strength of Recommendation
The quality of evidence and strength of recommendation for efficacy outcomes (PFS and OS) as well as safety outcomes (hematologic toxicity) in this updated systematic review and meta-analysis are summarized in Table 2. Despite pooling data only from prospective RCTs, quality of evidence was graded as being of low quality both for efficacy and safety, implying that further research was very likely to have an impact on the confidence in the estimate of effect and was very likely to change that estimate.
Table 2.
Summary of Findings With Quality of Evidence for the Benefits and Harms of Extended Adjuvant Temozolomide (>6 Cycles) Compared to Standard Adjuvant Temozolomide (6 Cycles) in Patients With Newly Diagnosed Glioblastoma
| Outcomes | Number of Participants (Studies) | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Risk With Control | Risk Difference With Extended Adjuvant Temozolomide (95% CI) | ||||
| Overall survival | 358 (4 studies) | ⊕⊕⊝⊝ LOWa due to inconsistency, imprecision |
HR = 0.87 (0.6-1.27) | 624 events per 1000 | 51 fewer events per 1000 (from 180 fewer to 87 more) |
| Progression-free survival | 358 (4 studies) | ⊕⊕⊝⊝ LOWa due to inconsistency, imprecision |
HR = 0.82 (0.61-1.1) | 837 events 1000 | 63 fewer events per 1000 (from 168 fewer to 27 more) |
| Overall grade 3 or worse hematological toxicity | 724 (3 studies) | ⊕⊕⊝⊝ LOWa due to risk of bias, imprecision |
RR = 2.01 (0.83-4.87) | 14 events per 1000 | 14 more events per 1000 (from 2 fewer to 54 more) |
| ≥Grade 3 anemia | 240 (3 studies) | RR = 0.97 (0.1-9.08) | 8 events per 1000 | 0 fewer events per 1000 (from 8 fewer to 68 more) | |
| ≥Grade 3 neutropenia | 242 (3 studies) | RR = 2.22 (0.61-8.1) | 17 events per 1000 | 20 more events per 1000 (from 7 fewer to 118 more) | |
| ≥Grade 3 thrombocytopenia | 242 (3 studies) | RR = 2.41 (0.57-10.22) | 17 events per 1000 | 24 more events per 1000 (from 7 fewer to 154 more) | |
Abbreviations: CI, confidence interval; HR, hazard ratio; RR, risk ratio.
*The basis for the assumed risk (median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE: Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
aOpposing effects seen in two studies—Balana and Javadinia. Index of heterogeneity (I2) is high.
Discussion
The currently established practice of postoperative concurrent RT/TMZ followed by 6 cycles of adjuvant TMZ (Stupp regimen) in patients with newly diagnosed glioblastoma is based on a pivotal phase III trial demonstrating significant improvements in survival with combined modality treatment compared to RT alone.2 The benefit of adding TMZ to RT was most pronounced in patients with methylation of the MGMT gene promoter,4 with only a small minority of patients with unmethylated tumors deriving benefit as evidenced by tailing of the survival curves. However, MGMT testing itself is disputed with no “gold-standard” technique making it difficult and challenging to withhold TMZ in patients with unmethylated glioblastoma.6–8 The ability of MGMT methylation for prediction of response to alkylating TMZ chemotherapy coupled with longer survival of patients with methylated tumors and low risk of cumulative toxicity has prompted continuation of adjuvant TMZ beyond the standard 6-cycle regimen in different healthcare settings across the world without high-quality evidence based on local standards and personal/institutional preferences.
The benefit of extended adjuvant TMZ in patients with newly diagnosed glioblastoma has been a subject of controversy and debate for several years now. In a pooled analysis20 of 2214 patients with glioblastoma enrolled in four prospective controlled trials of the Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC), patients who were progression-free after 6 cycles of adjuvant TMZ were identified (n = 624). Of these, 291 patients received extended adjuvant TMZ till progression or 12 cycles while 333 patients were observed. After adjusting for various confounding factors, treatment beyond 6 cycles of TMZ was associated with somewhat improved PFS (HR = 0.80, 95% CI: 0.65-0.98; P = .03), which was more pronounced in patients with methylated MGMT (HR = 0.65, 95% CI: 0.50-0.80; P < .01). However, OS was not impacted by a number of TMZ cycles (HR = 0.91, 95% CI: 0.71-1.19; P = .52) regardless of the MGMT status, leading to the conclusion that continuation of TMZ beyond the standard 6 cycles does not improve survival in newly diagnosed glioblastoma. Similar analysis of the German Glioma Network cohort21 identified 61 patients with glioblastoma who were progression-free after 6 cycles of TMZ and received extended adjuvant TMZ compared to 81 patients who were observed after 6 cycles. There was no significant association of prolonged TMZ chemotherapy with PFS (HR = 0.8, 95% CI: 0.4-1.6; P = .56) or OS (HR = 1.6, 95% CI: 0.8-3.3; P = .22) after adjusting for age, performance status, extent of resection, and molecular markers questioning the practice of continuing TMZ beyond the standard 6 cycles. A questionnaire-based electronic survey22 from Spain reported that TMZ was continued for more than 6 cycles by 80.5% of neuro-oncologists; 44.4% only in the presence of residual disease, 27.8% for 12 cycles even in the absence of residual tumor, and 8.3% until progression with significant cost implications and economic burden to the society, prompting the design and conduct of a prospective multicenter study (GEINO 14-01) in Spain13 to assess the benefit of extended adjuvant TMZ in patients with newly diagnosed glioblastoma. Researchers from Germany compared the medical costs and clinical outcomes of short-term TMZ (6 cycles) vs long-term TMZ (12 cycles or until progression) using a time-dependent Markov model23 and reported incremental effectiveness of 0.022 quality-adjusted life years (QALY) with long-term use of TMZ at an incremental cost-effectiveness ratio (ICER) of 351 909/QALY, making it highly expensive treatment.
Secondary analysis of RTOG/EORTC trial database,20 post hoc analysis of registry data,21 and several retrospective non-randomized comparative studies24–27 have provided conflicting results on the efficacy and safety of continuing TMZ beyond 6 cycles. A systematic review and meta-analysis9 pooled data from 1018 patients with glioblastoma enrolled in seven comparative studies to assess the impact of extended adjuvant TMZ. The authors reported significant improvement in PFS (difference in means Z = 3.84 months, 95% CI: 2.559-7894; P < .001) and OS (difference in means Z = 2.375 months, 95% CI: 1.002-10.467; P = .18) with extended adjuvant TMZ (>6 cycles) compared to standard (6 cycles) adjuvant TMZ. However, the authors did acknowledge the methodological limitations of their meta-analysis warranting cautious interpretation of the findings. Very recently, another systematic review and meta-analysis28 of RCTs reported non-significant improvement in median PFS (12 vs 10 months, P = .27) without corresponding improvement in median OS (23 vs 24 months, P = .73) with extended adjuvant TMZ. However, over 70% (n = 624) patients included in that meta-analysis were from retrospective analysis of RTOG/EORTC trial database,7 which was erroneously described as a prospective trial randomly assigning patients to 6 cycles vs >6 cycles of TMZ introducing strong selection and performance bias with serious downgrading of the evidence-base.
The present updated systematic review and meta-analysis provides low-certainty evidence that extended adjuvant TMZ is not associated with significant benefits or harms in unselected patients with newly diagnosed glioblastoma and should not be offered outside the context of prospective clinical trials. It is possible that patients with methylated tumors might stand to benefit with continuation of TMZ beyond 6 cycles; however, none of the included RCTs comparing standard adjuvant TMZ with extended adjuvant TMZ used MGMT gene promoter methylation to enrich their patient population. The GEINO 14-01 study13 was stratified on MGMT status but did not find any survival benefit even in patients with methylated tumors possibly due to low power inherent to much smaller sample size (n = 97) for such comparison. Based on previously published data,4,29 it is quite reasonable to believe that patients with glioblastoma with unmethylated MGMT derive very little benefit if at all with standard adjuvant TMZ, and it would be naïve to assume that this cohort would demonstrate any benefit with protracted duration of TMZ. Hence, the lack of significant benefit in unselected cohorts of newly diagnosed glioblastoma is quite expected. However, there still might be some merit in investigating the role of extended adjuvant TMZ in patients with methylation of the MGMT gene promoter. Two ongoing Australian trials EX-TEM (ACTRN12618001944224) and MAGMA (ACTRN12620000048987) are currently testing the safety and efficacy of extended adjuvant TMZ in patients with newly diagnosed glioblastoma using MGMT status for stratification. Biomarker-based Optimization of Adjuvant Therapy (BOAT) study is the lone ongoing prospective trial (CTRI/2018/11/016349) that randomly assigns glioblastoma patients only with methylated MGMT to standard 6 cycles of TMZ vs extended adjuvant TMZ.30
Strengths and Limitations
Despite including only prospective RCTs for statistical pooling using modern meta-analytic methods, several caveats and limitations remain. All included primary studies enrolled a small number of patients that were not adequately powered to demonstrate differences in survival between the two arms. Even after pooling of data from four RCTs, the overall sample size still remained quite small (N = 358) with low power to detect any meaningful difference in outcomes precluding robust conclusions. Although patients in the largest study (GEINO 14-01) were stratified on MGMT gene promoter methylation status, none of the trials were limited to biomarker-enriched patient population (methylated MGMT), which is expected to derive the most benefit with extended adjuvant TMZ. All included trials were open-label without any placebo control or blinding of patients/physicians with potential for significant performance and detection bias. Evidence synthesis was primarily based on data reported in publications without access to individual patient data that could provide more methodologic robustness. Finally, this review did not assess the impact of extended adjuvant TMZ on health-related quality of life or its cost-effectiveness due to lack of reporting of such data in individual primary studies.
Conclusion
This meta-analysis suggests that extended adjuvant TMZ is not associated with significant survival benefit or increased hematologic toxicity in unselected patients with newly diagnosed glioblastoma compared to standard adjuvant TMZ. However, based on the quality of data, level of evidence is classified as low certainty for this statement.
Supplementary Material
Contributor Information
Tejpal Gupta, Department of Radiation Oncology, ACTREC/TMH, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
Riddhijyoti Talukdar, Department of Radiation Oncology, ACTREC/TMH, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
Sadhana Kannan, Department of Clinical Research Secretariat, ACTREC/TMH, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
Archya Dasgupta, Department of Radiation Oncology, ACTREC/TMH, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
Abhishek Chatterjee, Department of Radiation Oncology, ACTREC/TMH, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
Vijay Patil, Department of Medical Oncology, ACTREC/TMH, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
Funding
No funding support was involved in this systematic review and meta-analysis.
Registration. The protocol is registered with the International Platform of Registered Systematic Reviews and Meta-analysis Protocols (INPLASY2021120114).
Institutional Ethics Committee approval. Not applicable.
Conflict of interest statement. None of the authors have any conflicts of interest to declare.
Authorship statement. Study concept, design, and protocol writing: T.G. Literature search: R.T., A.D., and T.G. Screening records and data extraction: R.T. and A.C. Statistical analysis: S.K. and T.G. Manuscript writing: first draft—R.T., final draft—T.G. Critical review and editing of manuscript: V.P. Final approval of manuscript: all authors.
Data Availability
All extracted data from individual primary studies are vested with the principal investigator and corresponding author that can be made available on reasonable request.
References
- 1. Ostrom QT, Patil N, Cioffi G, et al. Statistical report: CBTRUS: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;12(Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason W, van den Bent M, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Johnston A, Creighton N, Parkinson J, et al. Ongoing improvements in postoperative survival of glioblastoma in the temozolomide era: a population-based data linkage study. Neurooncol Pract. 2020;7(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hegi M, Diserens A, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Eng J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 5. Alnahhas I, Alsawas M, Rayi A, et al. Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis. Neurooncol Adv. 2020;2(1):vdaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hegi ME, Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter—still a dilemma? Neuro Oncol. 2015;17(11):1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malmstrom A, Lysiak M, Kristensen BW, et al. Do we really know who has an MGMT methylated glioblastoma: results of an international survey regarding use of MGMT analyses for glioma. Neurooncol Pract. 2020;7(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hegi M, Genbrugge E, Gorlia T, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alimohammadi E, Bagheri SR, Taheri S, et al. The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma multiforme: a meta-analysis and systematic review. Oncol Rev. 2020;14(1):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Renard L, Clement P, Hammouch F, et al. Safety analysis of randomized Belgian phase II trial of extended use of adjuvant temozolomide in newly diagnosed glioblastoma patients. Neuro Oncol. 2010;12:46–47. http://hdl.handle.net/2078.1/58558. [Google Scholar]
- 11. Refae A, Ezzat A, Salem D, et al. Protracted adjuvant temozolomide in glioblastoma multiforme. J Can Ther. 2015;6:748–758. [Google Scholar]
- 12. Bhandari M, Gandhi AK, Devnani B, et al. Comparative study of adjuvant temozolomide six cycles versus extended 12 cycles in newly diagnosed glioblastoma multiforme. J Clin Diagn Res. 2015;11(5):XC04–XC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balana C, Vaz MA, Manuel Sepúlveda J, et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14-01). Neuro Oncol. 2020;22(12):1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Javadinia S, Anvari K, Seilaniantousi M, et al. 354P Extended dosing (12 cycles) vs. conventional dosing (6 cycles) of adjuvant temozolomide in adults with newly diagnosed high grade gliomas: a randomized, single-blind, two-arm, parallel-group controlled trial. Ann Oncol. 2021;32:S520–S521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2011. https://www.handbook.cochrane.org
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings table. J Clin Epidemiol. 2011;64(4):383–394. [DOI] [PubMed] [Google Scholar]
- 18. Rohtagi A. WebPlot digitizer. Pacifica, California, USA, 2021. https://automeris.io/WebPlotDigitizer
- 19. Guyot P, Ades AE, Ouwens NJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumenthal DT, Gorlia T, Gilbert MR, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19(8):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gramatzki D, Kickingereder P, Hentschel B, et al. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology. 2017;88(15):1422–1430. [DOI] [PubMed] [Google Scholar]
- 22. Balana C, Vaz MA, Lopez D, et al. Should we continue temozolomide beyond 6 cycles in the adjuvant treatment of glioblastoma without an evidence of clinical benefit? A cost analysis based on prescribing patterns in Spain. Clin Transl Oncol. 2014;16:273–279. [DOI] [PubMed] [Google Scholar]
- 23. Waschke A, Arefian H, Walter J, et al. Cost-effectiveness of the long-term use of temozolomide for treating newly diagnosed glioblastoma in Germany. J Neurooncol. 2018;138(2):359–367. [DOI] [PubMed] [Google Scholar]
- 24. Roldán Urgoiti GB, Singh AD, Easaw JC. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol. 2012;108:173–177. [DOI] [PubMed] [Google Scholar]
- 25. Darlix A, Baumann C, Lorgis V, et al. Prolonged administration of adjuvant temozolomide improves survival in adult patients with glioblastoma. Anticancer Res. 2013;33(8):3467–3474. [PubMed] [Google Scholar]
- 26. Skardelly M, Dangel E, Gohde J, et al. Prolonged temozolomide maintenance therapy in newly diagnosed glioblastoma. Oncologist. 2017;22(5):570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quan R, Zhang H, Li Z, et al. Survival analysis of patients with glioblastoma treated by long-term administration of temozolomide. Medicine. 2020;99(2):e18591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Attarian F, Taghizadeh-Hesary F, Fanipakdel A, et al. A systematic review and meta-analysis on the number of adjuvant temozolomide cycles in newly diagnosed glioblastoma. Front Oncol. 2021;11:779491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamson DO, Grossman SA. The role of temozolomide in patients with newly diagnosed wild-type IDH, unmethylated MGMTp glioblastoma during the COVID-19 pandemic. JAMA Oncol. 2021;7(5):675–676. [DOI] [PubMed] [Google Scholar]
- 30. Gupta T, Chatterjee A, Patil V. Extended adjuvant temozolomide in newly diagnosed glioblastoma: is more less? Comment on “A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14-01)”. Neuro Oncol. 2020;22(12):1887–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All extracted data from individual primary studies are vested with the principal investigator and corresponding author that can be made available on reasonable request.