Abstract
Chromosomal DNAs of enterohemorrhagic, uropathogenic, and laboratory attenuated Escherichia coli strains differ in the rpoS-mutS region. Many uropathogens lack a deletion and an insertion characteristic of enterohemorrhagic strains. At the same chromosomal position, they harbor a 2.1-kb insertion of unknown origin with a base composition suggestive of horizontal gene transfer. Unlike virulence determinants associated with urinary tract infection and/or neonatal meningitis (pap or prs, sfa, kps, and hly), the 2.1-kb insertion is shared by all group B2 strains of the E. coli Reference Collection.
Genomic sequencing offers unprecedented opportunities for the identification of genetic polymorphisms related to bacterial evolution and virulence. The complete nucleotide sequence of Escherichia coli MG1655 (4), a representative laboratory-attenuated E. coli K-12 strain, provides a foundation for studies of the evolution and virulence of E. coli strains associated with diverse pathologies. The expanding list of E. coli virotypes includes diverse diarrheagenic organisms (labeled enterotoxigenic, enteropathogenic, enterohemorrhagic, enteroaggregative, enteroinvasive, and diffusely adherent) (28) as well as isolates associated with extraintestinal diseases, including neonatal meningitis (7) and urinary tract infections (UTIs) (including bacteriuria, cystitis, and pyelonephritis) (11). By complementing phenotypic analysis and multilocus enzyme electrophoresis, sequence comparisons are now providing profound insights into the pathogenesis and evolution of E. coli (5, 12, 19, 23–25, 28, 29, 37, 39–42). These and other studies (13, 15) reveal that the chromosomal DNA sequences of modern organisms reflect both their clonal origins and horizontal gene transfer.
A uropathogen-associated, rpoS-proximal DNA polymorphism in E. coli.
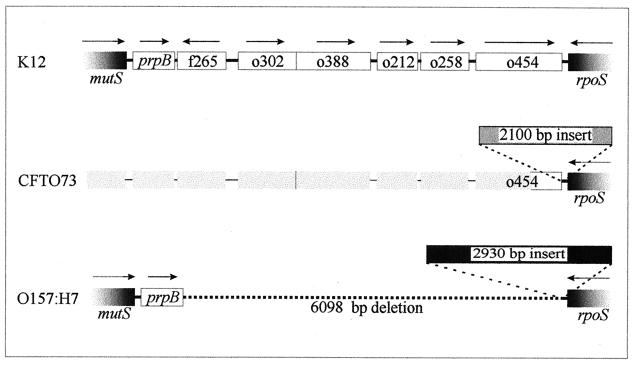
Recently, LeClerc et al. (21) reported that, in comparison to E. coli MG1655, E. coli O157:H7, related enterohemorrhagic E. coli strains, and Shigella dysenteriae lack 6.1 kb of chromosomal DNA and harbor a 2.9-kb DNA insertion in the rpoS-mutS intergenic region (61.5 to 61.7 map units) (Fig. 1). While deleting the rpoS locus from uropathogenic E. coli strains during a study of osmoregulation and virulence (9, 10), we discovered a different polymorphism at the same location. E. coli strain CFT073, a highly virulent isolate from a patient with pyelonephritis (27), appears to retain the full rpoS-mutS intergenic sequence characteristic of E. coli MG1655 (Fig. 1). In addition, a 2.1-kb DNA insert replaces the 2.9-kb sequence identified by LeClerc et al. (21). This insert was initially detected when attempts to PCR amplify rpoS failed to produce a DNA fragment of the expected size (primers A and G) (Fig. 2). Genomic DNA from E. coli CFT073 was prepared as follows (36). Bacteria harvested from a 1-ml overnight culture in Luria-Bertani medium (26) were washed once with 1 ml of saline (0.85% [wt/vol] NaCl), resuspended in 0.5 ml of distilled water, boiled for 10 min, and chilled on ice. Debris was removed by centrifugation, and the relevant sequences were PCR amplified with 5 μl of the resulting extract as a template (8). The insert sequence was determined by GenAlyTiC (University of Guelph, Guelph, Ontario). Additional primers were created as required to complete this 2.1-kb sequence (Fig. 2). The inserted DNA occurs at exactly the same location as that present in E. coli O157:H7 but differs in both size and sequence (the insert sequences are not related). It has a base composition of 40% G+C, a value much lower than the average for E. coli K-12 (50%) and for the immediately flanking sequences (52%). The insert may therefore have arrived in E. coli by horizontal gene transfer (29).
FIG. 1.
Comparison of the rpoS-mutS intergenic regions of E. coli K-12 (laboratory) (4), E. coli CFT073 (pyelonephritis) (this study), and E. coli O157:H7 (hemorrhagic colitis) (21). E. coli CFT073 lacks the 6.1-kb DNA deletion in the rpoS-mutS intergenic region that is characteristic of enterohemorrhagic strains (e.g., O157:H7). However the presence of the full 6.1-kb rpoS-mutS intergenic sequence found in E. coli MG1655 has not been verified for E. coli CFT073.
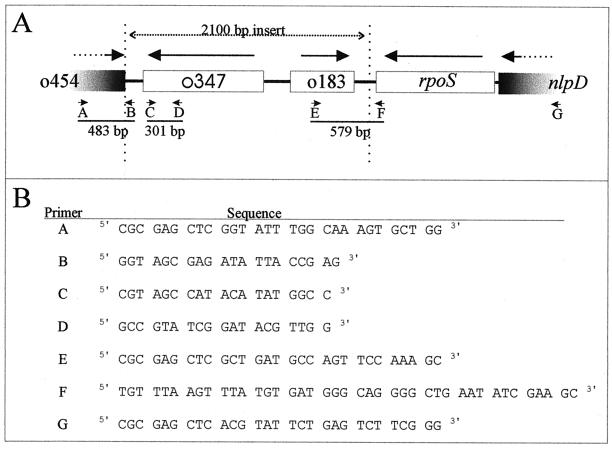
FIG. 2.
The rpoS-mutS intergenic region of E. coli CFT073 (accession no. AF270497) and its detection by PCR amplification. (A) Physical map, showing positions of ORFs (Table 2) and locations of DNA fragments amplified by PCR (Tables 1 and 3). (B) PCR primers used to perform the multiplex PCR analyses reported in Tables 1 and 3.
To determine the distribution of the inserted sequence, we applied PCR analysis to chromosomal DNA isolated from diverse clinical E. coli isolates (Table 1). DNA was prepared (36), and PCR was performed (8) using the primers listed in Table 1 and Fig. 2B (two pairs of primers per PCR). Production of a 301-bp amplicon during test 1 (primers C and D) indicated the presence of the inserted sequence. Production of 579- (primers E and F) and 483-bp (primers A and B) amplicons during test 2 indicated its location in the rpoS-mutS region. PCR amplification of the housekeeping locus putP, located at 23.3 map units, provided a positive control for the quality of the DNA templates. A DNA insert similar to that discovered in pyelonephritis isolate CFT073 was present in a majority of UTI isolates, including bacteria isolated from patients with uncomplicated pyelonephritis (7 of 7) or cystitis (8 of 12) and unspecified UTIs (4 of 4). It was present in approximately one-half of the tested isolates from patients with catheter-associated infections (3 of 7), and it was uncommon among bacteria isolated from patients with hemorrhagic colitis (0 of 5) or infantile diarrhea (2 of 21). When present, it was located in the rpoS-mutS region (Table 1).
TABLE 1.
Distribution of the inserted sequence among clinical E. coli strains
| Strain or isolate(s) | Origin | PCR test 1a
|
PCR test 2b
|
Source or reference | ||
|---|---|---|---|---|---|---|
| putP (202 bp) | Internal fragment (301 bp) | rpoS border (579 bp) | o454 border (483 bp) | |||
| ATCC25992 | Clinical | + | + | + | + | G. Reid |
| BB593:2b | Fecal (child) | + | + | + | + | G. Reid |
| Co1 | Fecal (non-UTI) | + | − | − | − | G. Reid (35) |
| SM47c | Neonatal meningitis | + | − | − | − | G. Reid |
| 2 Isolatesd | Hemorrhagic colitis | + | − | − | − | C. L. Gyles |
| 3 Isolates | Hemorrhagic colitis | + | − | NT | NT | C. L. Gyles |
| 3 Isolatesd | Infantile diarrhea | + | − | − | − | C. L. Gyles |
| 16 Isolates | Infantile diarrhea | + | − | NT | NT | C. L. Gyles |
| 2 Isolates | Infantile diarrhea | + | + | + | + | C. L. Gyles |
| 1 Isolatec | Catheter | + | − | − | − | G. Reid |
| 3 Isolatesd | Catheter | + | − | − | − | G. Reid |
| 3 Isolates | Catheter | + | + | + | + | G. Reid |
| 431 | Bacteriuria | + | + | + | + | G. Reid (35) |
| 950 | UTI (bacteremia) | + | + | + | + | G. Reid (38) |
| 2239 | UTI | + | + | + | + | G. Reid |
| C1212 | UTI | + | + | + | + | G. Reid (31) |
| C1214 | UTI | + | + | + | + | G. Reid (31) |
| 2 Isolates | Cystitis | + | − | − | − | G. Reid |
| 2 Isolatesd | Cystitis | + | − | − | − | G. Reid (33, 34) |
| 8 Isolates | Cystitis | + | + | + | + | G. Reid (33, 34) |
| CFT073 | Acute pyelonephritis | + | + | + | + | H. Mobley (27) |
| HU734 | Acute pyelonephritis | + | + | + | + | G. Reid (16, 17) |
| 5 Isolates | Pyelonephritis | + | + | + | + | G. Reid |
Multiplex PCR test 1 was performed with putP primers plus primers C and D (Fig. 2). The putP primers were putP1, 5′-GGTTGCGTGTGCATACCGA-3′ (bp 287 to 305 of putP), and putP2, 5′-GCCGTTTCGTAGCTCATGC-3′ (bp 469 to 488 of putP). +, DNA fragments of the indicated sizes were obtained; −, PCR yielded no DNA fragment.
Multiplex PCR test 2 was performed using primers A, B, E, and F (Fig. 2). +, DNA fragments of the indicated sizes were obtained; −, PCR yielded no DNA fragment; NT, PCR test 2 was not performed.
Multiplex PCR test 2 yielded a single 526-bp DNA fragment (characteristic of E. coli K-12).
Multiplex PCR test 2 yielded a DNA fragment pattern unlike that of E. coli CFT073 or E. coli MC4100.
No 301-bp PCR product (internal to the DNA insert) was observed when PCR test 1 was applied with chromosomal DNA from Salmonella enterica serovar Typhimurium, Klebsiella oxytoca, Pseudomonas putida, Pseudomonas paucimobilis, Vibrio anguillarum, Yersinia ruckeri, Erwinia carotovora, Hafnia alvei, Enterobacter cloacae, or S. dysenteriae as a template. Like that from some E. coli isolates listed in Table 1, chromosomal DNA from S. dysenteriae supported DNA amplification with PCR test 2, but the resulting pattern of DNA fragments differed from that observed with E. coli CFT073 DNA as a template. Thus, the DNA insert observed in E. coli CFT073 was different from that found in E. coli O157:H7 and S. dysenteriae type 1 (21). It was more common among UTI isolates than among the other clinical E. coli isolates included in this study, and it was not detected by PCR amplification in an array of other organisms.
Analysis of the DNA sequence inserted in E. coli CFT073 revealed two open reading frames (ORFs) encoding proteins greater than 10 kDa in molecular mass for which similar sequences could be found (Fig. 2 and Table 2). ORF183 showed 26% identity to WrbA, a flavodoxinlike protein that is expressed by E. coli K-12 during stationary phase and whose sequence homologues have been found in bacteria, yeast, and plants (14). ORF347 showed comparable, limited levels of similarity to enzymes implicated in antibiotic hydrolysis (1) and synthesis (2). Though the base composition of the DNA encoding ORF183 was similar to that of E. coli K-12 (49%), the base composition of the DNA encoding ORF347 was much lower (39%), suggesting that they may differ in origin. No sequence similarity was sufficiently high to suggest the recent transfer of the entire 2.1-kb sequence or its subfragments from another organism.
TABLE 2.
Proteins related to ORFs encoded by the inserted sequence
| ORF
|
Similar sequence
|
||||||
|---|---|---|---|---|---|---|---|
| Name | %G+C | Name | Organism | Function | Size (aa)a | %IDb | Reference |
| o183 | 49 | WrbA | E. coli K-12 | Stationary phase; flavodoxinlike protein | 202 | 26 | 14 |
| o347 | 39 | IND-1 | Chryseobacterium indologenes | β-Lactamase precursor | 239 | 27 | 1 |
| ORF4 | Streptomyces roseofulvus | Polyketide antibiotic biosynthesis (cyclase) | 314 | 25 | 2 | ||
aa, amino acids.
%ID, percent identity.
The inserted sequence is present in all members of the ECOR group B2.
The E. coli Reference (ECOR) Collection, a set of E. coli strains isolated from diverse hosts and geographic locations, was designed to represent the variation and genetic structure of E. coli (30). Studies of housekeeping loci, applied to these strains and others, clearly define the clonal nature of natural E. coli populations (18, 40). We explored the evolutionary origin of the 2.1-kb rpoS-proximal inserted sequence by examining its occurrence among the 72 ECOR strains (Table 3). PCR test 1 (Table 1) detected this sequence in ECOR strains EC23 and EC32 and in each of the ECOR group B2 strains. UTI-related virulence determinants, believed to have arrived by horizontal gene transfer, occur at higher frequency within ECOR group B2 than among other ECOR strains (Table 3) (6, 20, 22). The presence of the rpoS-proximal 2.1-kb insertion within group B2 members is therefore consistent with its presence in many UTI isolates (Table 1). This insertion is present in all group B2 isolates, whereas few contain all of the tested UTI-linked virulence determinants (pap, prs, sfa, kps, and hly), each of which varies in chromosome map position among E. coli isolates. It is thus likely that the 2.1-kb insertion arrived earlier than these virulence determinants during the evolution of group B2.
TABLE 3.
Properties of ECOR strains harboring the inserted sequencea
| Strain | ECOR group | Distribution of UTI-associated virulence determinantsb
|
|||
|---|---|---|---|---|---|
| pap or prs | sfa | kps | hly | ||
| EC23 | A | − | − | − | − |
| EC32 | B1 | − | − | − | − |
| EC51 | B2 | + | + | + | + |
| EC52 | B2 | + | + | + | + |
| EC54 | B2 | − | + | + | + |
| EC56 | B2 | + | − | + | + |
| EC57 | B2 | + | + | + | + |
| EC55 | B2 | + | − | + | − |
| EC65 | B2 | − | + | − | + |
| EC61 | B2 | − | − | + | − |
| EC62 | B2 | + | − | + | − |
| EC63 | B2 | + | + | + | + |
| EC64 | B2 | + | + | + | − |
| EC53 | B2 | + | + | + | + |
| EC59 | B2 | − | − | − | − |
| EC60 | B2 | + | + | − | + |
| EC66 | B2 | + | + | + | − |
Evidence for the presence and location of the rpoS-proximal inserted sequence in the ECOR strains was sought by multiplex PCR test 1 with PCR-based detection of putP as a control (see the text and Table 1). The listed strains, ordered according to their evolutionary relationships (18), contained both putP and the rpoS-proximal insertion sequence as indicated by both tests. The other 55 ECOR strains (not listed) contained the former but not the latter sequence.
The mnemonics refer to genetic loci encoding the following virulence determinants: pap, pyelonephritis-associated or P pili with class I adhesins; prs, pyelonephritis-associated or P pili with class III adhesins; sfa, S-fimbrial adhesins; kps, type II capsule; hly, α-hemolysin (6, 22). +, present; −, absent.
Some data suggest that genomic sequences common to group B2 organisms diverge deeply from those of commensal E. coli strains in ECOR groups A and B1 and have provided an essential context for the evolution of extraintestinal virulence (3, 32). Bingen et al. compared the distribution of ribotypes and virulence markers associated with extraintestinal infections for 69 neonatal meningitis isolates and for the ECOR strains (3). The neonatal meningitis isolates were concentrated in phylogenetic group B2. Though present in all phylogenetic groups, virulence markers linked to neonatal meningitis (including sfa or foc and ibe-10) were also present at the highest frequency in group B2. In contrast, the UTI-associated marker pap was present at the highest frequencies in non-B2 neonatal meningitis isolates and in group B2 ECOR strains. The 2.1-kb rpoS-proximal DNA insertion present in group B2 ECOR strains and many uropathogens was not detected in the single neonatal meningitis isolate included in this study (Table 1). Given their concentration in group B2, the 2.1-kb sequence may be found within other neonatal meningitis isolates.
Nucleotide sequence accession number.
The 2.1-kb insert in the rpoS-mutS intergenic region of E. coli CFT073 was registered with GenBank under accession no AF270497.
Acknowledgments
We are grateful to R. M. W. Stevenson for chromosomal DNAs isolated from diverse bacteria, to C. Whitfield and Karen Amor for chromosomal DNA isolated from the ECOR strains, and to C. L. Gyles for S. dysenteriae and for comments on the manuscript.
We thank the Medical Research Council of Canada for Research Operating Grant MT-15113.
REFERENCES
- 1.Bellais S, Leotard S, Poirel L, Naas T, Nordmann P. Molecular characterization of a carbapenem-hydrolyzing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol Lett. 1999;171:127–132. doi: 10.1111/j.1574-6968.1999.tb13422.x. [DOI] [PubMed] [Google Scholar]
- 2.Bibb M J, Sherman D H, Omura S, Hopwood D A. Cloning, sequencing and deduced functions of a cluster of Streptomyces genes probably encoding biosynthesis of the polyketide antibiotic frenolicin. Gene. 1994;142:31–39. doi: 10.1016/0378-1119(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 3.Bingen E, Picard B, Brahimi N, Mathy S, Desjardins P, Elion J, Denamur E. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J Infect Dis. 1998;177:642–650. doi: 10.1086/514217. [DOI] [PubMed] [Google Scholar]
- 4.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Bonacorsi S P P, Clermont O, Tinsley C, LeGall I, Beaudoin J-C, Elion J, Nassif X, Bingen E. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect Immun. 2000;68:2096–2101. doi: 10.1128/iai.68.4.2096-2101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd E F, Hartl D L. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J Bacteriol. 1998;180:1159–1165. doi: 10.1128/jb.180.5.1159-1165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley J S. Neonatal meningitis. Pediatr Infect Dis. 1985;4:315–320. doi: 10.1097/00006454-198505000-00047. [DOI] [PubMed] [Google Scholar]
- 8.Brown E D, Wood J M. Redesigned purification yields a fully functional PutA protein dimer from Escherichia coli. J Biol Chem. 1992;267:13086–13092. [PubMed] [Google Scholar]
- 9.Culham D E, Dalgado C, Gyles C L, Mamelak D, MacLellan S, Wood J M. Osmoregulatory transporter ProP influences colonization of the urinary tract by Escherichia coli. Microbiology. 1998;144:91–102. doi: 10.1099/00221287-144-1-91. [DOI] [PubMed] [Google Scholar]
- 10.Culham D E, Emmerson K S, Lasby B, Mamelak D, Steer B A, Gyles C L, Villarejo M, Wood J M. Genes encoding osmoregulatory proline/glycine betaine transporters and the proline catabolic system are present and expressed in diverse clinical Escherichia coli isolates. Can J Microbiol. 1994;40:397–402. doi: 10.1139/m94-065. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C.: ASM Press; 1996. pp. 135–174. [Google Scholar]
- 12.Feng P, Lampel K A, Karch H, Whittam T S. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J Infect Dis. 1998;177:1750–1753. doi: 10.1086/517438. [DOI] [PubMed] [Google Scholar]
- 13.Frankel G, Phillips A D, Rosenshine I, Dougan G, Kaper J B, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 14.Grandori R, Khalifah P, Boice J A, Fairman R, Giovanielli K, Carey J. Biochemical characterization of WrbA, founding member of a new family of multimeric flavodoxin-like proteins. J Biol Chem. 1998;273:20960–20966. doi: 10.1074/jbc.273.33.20960. [DOI] [PubMed] [Google Scholar]
- 15.Hacker J, Blumoehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 16.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagberg L, Hull R, Hull S, Falkow S, Freter R, Svanborg Eden C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983;40:265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzer P J, Inouye S, Inouye M, Whittam T S. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J Bacteriol. 1990;172:6175–6181. doi: 10.1128/jb.172.11.6175-6181.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurtado A, Rodríguez-Valera F. Accessory DNA in the genomes of representatives of the Escherichia coli reference collection. J Bacteriol. 1999;181:2548–2554. doi: 10.1128/jb.181.8.2548-2554.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai X H, Wang S Y, Uhlin B E. Expression of cytotoxicity by potential pathogens in the standard Escherichia coli collection of reference (ECOR) strains. Microbiology. 1999;145:3295–3303. doi: 10.1099/00221287-145-11-3295. [DOI] [PubMed] [Google Scholar]
- 21.LeClerc J E, Li B, Payne W L, Cebula T A. Promiscuous origin of a chimeric sequence in the Escherichia coli O157:H7 genome. J Bacteriol. 1999;181:7614–7617. doi: 10.1128/jb.181.24.7614-7617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marklund B I, Tennent J M, Garcia E, Hamers A, Båga M, Lindberg F, Gaastra W, Normark S. Horizontal gene transfer of the Escherichia coli pap and prs pili operons as a mechanism for the development of tissue-specific adhesive properties. Mol Microbiol. 1992;6:2225–2242. doi: 10.1111/j.1365-2958.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 23.Milkman R. Recombinational exchange among clonal populations. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2663–2684. [Google Scholar]
- 24.Milkman R. Recombination and population structure in Escherichia coli. Genetics. 1997;146:745–750. doi: 10.1093/genetics/146.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milkman R, Bridges M M. Molecular evolution of the Escherichia coli chromosome. III. Clonal frames. Genetics. 1990;126:505–517. doi: 10.1093/genetics/126.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 27.Mobley H L, Jarvis K G, Elwood J P, Whittle D I, Lockatell C V, Russell R G, Johnson D E, Donnenberg M S, Warren J W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–155. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 28.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochman H, Lawrence J G. Phylogenetics and the amelioration of bacterial genomes. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2627–2637. [Google Scholar]
- 30.Ochman H, Selander R K. Standard reference strains of Escherichia coli from natural populations. J Bacteriol. 1984;157:690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orskov I, Orskov F, Birch-Anderson A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980;27:657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picard B, Garcia J S, Gouriou S, Duriez P, Brahimi N, Bingen E, Elion J, Denamur E. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun. 1999;67:546–553. doi: 10.1128/iai.67.2.546-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid G, Brooks H J L. In vitro attachment of E. coli to human epithelial cells. NZ Med J. 1984;97:439–442. [PubMed] [Google Scholar]
- 34.Reid G, Brooks H J L, Bacon D F. In vitro attachment of Escherichia coli to human epithelial cells: variation in receptivity during the menstrual cycle and pregnancy. J Infect Dis. 1983;148:412–421. doi: 10.1093/infdis/148.3.412. [DOI] [PubMed] [Google Scholar]
- 35.Reid G, Zorzitto M L, Bruce A W, Jewett M A S, Chan R C Y, Costerton J W. Pathogenesis of urinary tract infection in the elderly: the role of bacterial adherence to uroepithelial cells. Curr Microbiol. 1984;11:67–72. [Google Scholar]
- 36.Reid S. M.Sc. thesis. Guelph, Canada: University of Guelph; 1991. [Google Scholar]
- 37.Rode C K, Melkerson-Watson L J, Johnson A T, Bloch C A. Type-specific contributions to chromosome size differences in Escherichia coli. Infect Immun. 1999;19:230–236. doi: 10.1128/iai.67.1.230-236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salit I E, Hanley J, Chubb L, Fleming S. Detection of pilus subunits (pilins) and filaments by using anti-P pilin antisera. Infect Immun. 1988;56:2330–2335. doi: 10.1128/iai.56.9.2330-2335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperandio V, Kaper J B, Bortolino M R, Neves B C, Keller R, Trabulsi L R. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli (STEC) serotypes. FEMS Microbiol Lett. 1998;164:133–139. doi: 10.1111/j.1574-6968.1998.tb13078.x. [DOI] [PubMed] [Google Scholar]
- 40.Whittam T S. Genetic variation and evolutionary processes in natural populations of Escherichia coli. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2708–2722. [Google Scholar]
- 41.Whittam T S, Reid S D, Selander R K. Mutators and long-term molecular evolution of pathogenic Escherichia coli O157:H7. Emerg Infect Dis. 1998;4:615–617. doi: 10.3201/eid0404.980411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wieler L H, McDaniel T K, Whittam T S, Kaper J B. Insertion site of the locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia coli differs in relation to the clonal phylogeny of the strains. FEMS Microbiol Lett. 2000;156:49–53. doi: 10.1111/j.1574-6968.1997.tb12704.x. [DOI] [PubMed] [Google Scholar]