Abstract
Inflammation has been implicated in atrial fibrillation (AF), a very common and clinically significant cardiac rhythm disturbance, but its precise role remains poorly understood. Work performed over the past 5 years suggests that atrial cardiomyocytes have inflammatory signalling machinery — in particular, components of the NLRP3 (NACHT-, LRR- and pyrin domain-containing 3) inflammasome — that is activated in animal models and patients with AF. Furthermore, work in animal models suggests that NLRP3 inflammasome activation in atrial cardiomyocytes might be a sufficient and necessary condition for AF occurrence. In this Review, we evaluate the evidence for the role and pathophysiological significance of cardiomyocyte NLRP3 signalling in AF. We first summarize the evidence for a role of inflammation in AF and review the biochemical properties of the NLRP3 inflammasome, as defined primarily in studies of classic inflammation. We then briefly consider the broader evidence for a role of inflammatory signalling in heart disease, particularly conditions that predispose individuals to develop AF. We provide a detailed discussion of the available information about atrial cardiomyocyte NLRP3 inflammasome signalling in AF and related conditions and evaluate the possibility that similar signalling might be important in non-myocyte cardiac cells. We then review the evidence on the role of active resolution of inflammation and its potential importance in suppressing AF-related inflammatory signalling. Finally, we consider the therapeutic potential and broader implications of this new knowledge and highlight crucial questions to be addressed in future research.
Subject terms: Atrial fibrillation, Organelles
In this Review, Nattel and colleagues discuss the evidence suggesting a pathophysiological role of cardiomyocyte inflammatory signalling in atrial fibrillation, consider the therapeutic potential associated with these signalling pathways, including strategies promoting the resolution of inflammation, and highlight crucial questions to be addressed in future research.
Key points
Atrial fibrillation is the most common cardiac arrhythmia, and new insights into the pathophysiological mechanisms are required to improve the available therapeutic options.
Although inflammation has been thought to have a role in atrial fibrillation for ≥20 years, the precise nature of the association has proved elusive.
Work over the past 5 years has revealed a key role for inflammatory signalling in cardiomyocytes in atrial fibrillation development, revealing the importance of non-leukocyte cardiac cells in mediating disease-promoting cardiac inflammation.
The NLRP3 inflammasome in particular has been implicated in cardiomyocyte-mediated inflammatory signalling in atrial fibrillation.
The active resolution of inflammation is thought to be mediated by endogenous bioactive agents, which can be administered as pharmaceutical products to attenuate the development of chronic conditions such as atrial fibrillation.
Introduction
Atrial fibrillation (AF) is a common and important clinical condition, associated with substantial morbidity and mortality related to a host of potentially serious complications, such as cardiomyopathy, heart failure (HF) and stroke1. A variety of treatment approaches for AF are available, including antiarrhythmic drugs to promote maintenance of normal sinus rhythm, bradycardic agents to prevent excessively rapid heart rates, anticoagulant drugs to prevent thromboembolism and ablation procedures to promote maintenance of sinus rhythm. Although each of these approaches is useful, they all have potential limitations and adverse effects, leaving a substantial unmet need for therapeutic innovation2.
A role of inflammation in AF has been suspected for >20 years3, and considerable evidence has subsequently accrued4. Nevertheless, the benefits of traditional anti-inflammatory agents for the treatment of AF have been small and unconvincing overall4. Although the classic view is that inflammation results from the production of cytokines by a variety of infiltrating white cells in response to tissue injury and/or immune cell responses, evidence has begun to emerge that other cell types, including cardiomyocytes, can have potentially important inflammatory signalling that results in tissue pathology and remodelling. In this article, we review the evidence suggesting the pathophysiological relevance of cardiomyocyte inflammatory signalling in AF and consider the potential therapeutic opportunities associated with these signalling pathways. We also provide the essential contextual background, in terms of the relevant biochemical components, the evidence for a role of inflammation in heart disease and more specifically in AF, the possible involvement of inflammatory signalling in non-inflammatory, non-myocyte cardiac cells (such as fibroblasts and adipocytes) and the potential utility of promoting the resolution of inflammation in protecting against AF.
Inflammation, heart disease and AF
Inflammation is part of the response to infection and injury and is intimately involved in defence and healing processes. Distinct forms of inflammation result from sterile inflammatory signals (such as those generated by tissue injury and autoantigens, termed damage-associated molecular patterns (DAMPs))5 and signals derived from exogenous pathogens (termed pathogen-associated molecular patterns (PAMPs))6. Bioactive signals promote the recruitment of innate immune cells to confine and neutralize the targeted threat and to begin the healing process. However, when the inflammatory response persists beyond the original threat, chronic inflammation can cause adverse tissue remodelling and disease. Growing evidence suggests a direct link between systemic or local inflammation and AF development (Table 1).
Table 1.
Examples of inflammatory conditions associated with clinical AF
| Study | Study design | Number of patients | AF phenotype | Other clinical findings | Ref. |
|---|---|---|---|---|---|
| Sepsis | |||||
| Walkey et al. (2011) | Retrospective cohort from the California State Inpatient Database | 3,144,787 (patients with pre-existing AF were excluded) | Increase in new-onset AF in patients with versus without severe sepsis (OR 6.82); sepsis present in 14% of patients with new-onset AF | Risk factors for AF: advanced age, male sex, white ethnicity, HF, obesity, renal failure; increased risk of stroke (OR 2.7) and in-hospital death (OR 1.07) in patients with severe sepsis and new-onset AF | 11 |
| Walkey et al. (2013) | Representative 5% sample from Medicare beneficiaries in the USA | – | 25.5% of patients had an AF diagnosis during the hospitalization for sepsis; 7.2% had new-onset AF | Risk factors for AF: advanced age, white ethnicity; no association with cardiovascular risk factors | 12 |
| Kuipers et al. (2014) | Systematic review including 11 studies (studies primarily focused on postcardiotomy were excluded) | – | The incidence of new-onset AF was 8%, 10% and 23% in critically ill patients with sepsis, severe sepsis or septic shock, respectively | Risk factors for AF: advanced age, white ethnicity, organ failure, pulmonary artery catheter use; increased mortality (five studies) and risk of stroke (one study) in patients with new-onset AF and sepsis | 13 |
| Walkey et al. (2014) | Representative 5% sample from Medicare beneficiaries who survived hospitalization for sepsis in the USA | 133,722 | 24% had previous AF and 7% had new-onset AF | Increased long-term (5-year) risk of HF hospitalization, ischaemic stroke and death in patients with new-onset AF during sepsis | 14 |
| Walkey et al. (2016) | Retrospective cohort study in patients with AF during sepsis in the USA | 39,693 | – | Reduced in-hospital mortality when AF during sepsis was treated with β-blockers compared with Ca2+ channel blockers, digoxin or amiodarone | 241 |
| Koyfman et al. (2015) | Retrospective cohort study in patients with sepsis in an intensive care unit in Israel | 200 | 81 patients (40.5%) had new-onset AF, of whom 44 (54.3%) had a history of AF | In-hospital mortality was similar between patients with new-onset AF and those with previous AF | 10 |
| Klein Klouwenberg et al. (2017) | Retrospective cohort study in patients with sepsis in Netherlands | 1,782 | The cumulative risk of new-onset AF was 10%, 22% and 40% in patients with sepsis, severe sepsis and septic shock, respectively | Increased hospital stay and mortality in the intensive care unit in patients with new-onset AF during sepsis | 15 |
| Cheng et al. (2017) | Retrospective cohort study in survivors of septicaemia from the Taiwan National Health Insurance Database | 68,324 | 1.6% had pre-existing AF and 1.9% had new-onset AF | Risk factors for AF: advanced age, HF, respiratory failure; increased risk of stroke with new-onset AF during sepsis | 16 |
| Bosch et al. (2019) | Retrospective cohort study in patients with suspected infection in an intensive care unit in the USA | 9,528 | 2.5% had new-onset AF | Increased mortality with new-onset AF during sepsis | 17 |
| Fernando et al. (2020) | Retrospective cohort study in patients in an intensive care unit in Canada | 15,014 | 10.3% had new-onset AF; significant interaction between new-onset AF and sepsis | – | 18 |
| Ko et al. (2021) | Retrospective cohort study in patients with new AF diagnosis from UMass Memorial Health | 185 | 34.6% of patients with new-onset AF had sepsis as secondary precipitant | – | 19 |
| Long et al. (2021) | Retrospective cohort study in patients with sepsis from the MIMIC-II database | 7,528 | 16.5% had a history of AF | Risk factors for AF: advanced age, male sex, HF, chronic respiratory disease, liver disease, renal failure; increased mortality with new-onset AF during sepsis | 20 |
| Pericarditis | |||||
| Spodick (1976) | Prospective study of consecutive patients with acute pericarditis | 100 | 5% had new-onset AF (all among the 24 patients with definite heart disease) | – | 21 |
| Spodick (1984) | Study of consecutive patients in sinus rhythm with acute pericarditis and 24 h Holter monitoring; all patients received an anti-inflammatory agent (ibuprofen, aspirin or indomethacin) | 50 (patients with previous arrhythmias were excluded) | – | 40% had no cardiac disease and half of these patients had atrial ectopy; in patients with heart disease, 66% had atrial ectopy | 242 |
| Nagahama et al. (1998) | Study of consecutive patients with acute myocardial infarction | 398 | Of 105 patients with pericardial effusion, 36 (34%) developed new-onset AF | – | 22 |
| Ristić et al. (2000) | Study of patients with acute pericarditis | 40 | 20% had new-onset AF without predisposing myocardial or valvular disease | – | 23 |
| Talreja et al. (2003) | Study of patients with constrictive pericarditis | 143 | 31 patients (21%) had AF | – | 243 |
| Syed et al. (2012) | Study of patients with tuberculous pericarditis | 80 | 20 patients (25%) had AF at presentation; incidence of AF decreased to zero by 6 months of follow-up | Patients with AF were more likely to have left ventricular systolic dysfunction and higher serum levels of N-terminal pro-B-type natriuretic peptide | 244 |
| Imazio et al. (2015) | Prospective, multicentre study of patients with acute pericarditis | 822 | 4.3% developed new-onset AF within 24 h of pericarditis onset; 17% of patients with AF had structural heart disease; 74.3% of patients had spontaneous conversion to sinus rhythm within 24 h; in a 30-month follow-up, AF recurrence rate was higher in the AF (35%) than in the sinus rhythm (0.9%) group | – | 24 |
| Myocarditis | |||||
| Frustaci et al. (1991) | Study of patients with ‘lone’ AF resistant to conventional antiarrhythmic drugs | 14 | – | Three patients showed active myocarditis in left ventricular endomyocardial biopsy samples; LVEF ≥50%; LA ≤40 mm | 26 |
| Morgera et al. (1992) | Study of patients with myocarditis | 45 | Five patients had AF at presentation associated with HF and enlarged LA | – | 29 |
| Frustaci et al. (1997) | Study of 14 patients with ‘lone’ AF resistant to conventional antiarrhythmic drugs and 11 patients with Wolff–Parkinson–White syndrome (as controls) | 25 | – | Eight patients showed atrial myocarditis in biopsy samples from right atrial septum; LVEF ≥55%; LA ≤40 mm; no conduction abnormalities | 27 |
| Fuenmayor et al. (1997) | Study of eight patients with acute myocarditis caused by Chagas disease and 125 control patients | 133 | AF and atrial flutter could be induced by programmed electrical stimulation in four patients with Chagas disease | Normal LVEF and LA | 28 |
| Larsen et al. (2013) | Study of patients with atrial giant cell myocarditis | 6 | Four patients had AF with atrial dilatation, mitral or tricuspid regurgitation, or atrial mural thrombus | – | 245 |
| Deluigi et al. (2013) | Study of patients with biopsy-proven myocarditis | 84 | After hospital admission, four patients had AF associated with HF and atrial dilatation | – | 30 |
| Anderson et al. (2014) | Retrospective, multicentre study of children with myocarditis | 2,041 | 44 (2.2%) had reported AF or atrial flutter | – | 246 |
| Blagova et al. (2016) | Retrospective study of patients with idiopathic arrhythmias | 19 | 16 (84%) had AF (12 paroxysmal, 4 permanent) | 15 (79%) had biopsy-proven myocarditis | 247 |
| Subahi et al. (2019) | Retrospective study of patients with acute myocarditis from the US inpatient sample registry | 6,642 | 602 (9%) had reported AF | Patients with AF were older and more often were white and had HF, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, renal failure and coagulation disorder | 248 |
| Adegbala et al. (2019) | Retrospective study of patients with acute myocarditis from the US Nationwide Inpatient Survey Dataset | 32,107 | 26.9% had AF during hospitalization | Patients with AF were older, had more co-morbidities and higher mortality | 25 |
| Rasal et al. (2021) | Prospective study of children with acute myocarditis | 63 | 1.4% of children had AF during hospitalization | – | 249 |
AF, atrial fibrillation; HF, heart failure; LA, left atrium; LVEF, left ventricular ejection fraction.
Inflammatory conditions associated with clinical AF
Available evidence indicates a relationship between AF and systemic inflammation in patients with autoimmune diseases, such as rheumatoid arthritis7, psoriasis8, inflammatory bowel disease9 and sepsis10–19 (Table 1). In patients with sepsis, the incidence of new-onset AF ranges between 1.9% and 40%10–19, with patients with severe sepsis or septic shock having a higher incidence of new-onset AF than patients with milder forms of sepsis11,13,15. Risk factors associated with AF onset include advanced age11–13,15,20, male sex11,20, white ethnicity11–13, obesity11, HF11,16,20, renal failure11,20, chronic liver disease20, organ failure13, pulmonary catheter use13, and chronic respiratory disease and failure16,20, although new-onset AF also develops in patients with no cardiovascular co-morbidities12. In addition, in patients with sepsis, new-onset AF is associated with increased risks of stroke11,13,16 and in-hospital death11,13,15,17,20 and long-term (5-year) risks of hospitalization for HF, ischaemic stroke and death14.
Local cardiac inflammatory conditions, including pericarditis and myocarditis, also increase the incidence of AF (Table 1). The incidence of new-onset AF in patients with pericarditis ranges between 4.3% and 46%21–24, with spontaneous cardioversion to sinus rhythm occurring in 74.3% of patients within 24 h24, but with a significant rate of recurrence during 30 months of follow-up (34.3% in patients with AF at the initial episode versus 0.9% in patients with pericarditis without initial AF; P < 0.001)24.
During hospitalization, up to 26.9% of patients with acute myocarditis develop new-onset AF25. Among patients with myocarditis, mortality was higher in patients with AF than in those in sinus rhythm25. Although some studies found normal left ventricular (LV) function and left atrial size in patients with myocarditis and AF26–28, other work showed that myocarditis-related AF was more often associated with chronic HF and an enlarged left atrium29,30. Finally, serious coronavirus disease 2019 (COVID-19) causes a major systemic inflammatory response and is associated with the development of a wide variety of arrhythmias, of which AF is one of the most common31,32.
Insights from animal models on inflammation-associated AF
Animal models of systemic or local inflammation support the link between inflammation and AF (Table 2). Animals with sepsis, which have increased atrial infiltration of inflammatory macrophages and CD68+ cells33, have increased vulnerability to pacing-induced AF33,34. In animal models of lipopolysaccharide (LPS)-induced sepsis, the action potential duration and atrial effective refractory period decrease as a result of reduced L-type Ca2+ current (ICa,L) and increased IKr (rapid component of the delayed rectifier K+ current) and IKs (slow component of the delayed rectifier K+ current), together with slowed conduction velocity33–35, pointing to re-entry as a potential mechanism. The atrial activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) is higher during sepsis and causes hyperphosphorylation of cardiac ryanodine receptor 2 (RYR2) channels at Ser2814, which, together with increased Na+–Ca2+-exchanger levels, increases the likelihood of spontaneous sarcoplasmic reticulum Ca2+ release events34, promoting delayed afterdepolarizations (DADs) and triggered activity36,37.
Table 2.
Examples of clear inflammatory settings leading to AF in experimental studies
| Model | Species | Phenotype | Intervention | Refs. |
|---|---|---|---|---|
| Sepsis | ||||
| LPS-induced sepsis | Guinea pig |
↓ Atrial cardiomyocyte APD ↓ Atrial Cav1.2 levels and ↓ ICa,L ↑ Atrial Kv11.1 levels and ↑ IKr ↑ Atrial Kv7.1 levels and ↑ IKs ↑ NO production in atrial cells ↑ iNOS, but not eNOS, levels |
Inhibition of NO production eliminated the changes in APD and ion currents | 35 |
| LPS-induced sepsis | Rat |
↑ Vulnerability to pacing-induced AF ↓ LVEF ↓ AERP, APD20, APD50 and APD90 ↑ Atrial conduction time ↑ CaMKII-mediated RYR2 phosphorylation at Ser2814 ↓ SERCA2A levels and ↓ CaMKII-mediated phospholamban phosphorylation at Thr17 ↑ NCX1 levels ↑ Total and autophosphorylated CaMKII at Thr287 ↑ ROS and MDA Abnormal iron metabolism (ferroptosis) |
Suppression of ferroptosis prevented AF and reduced oxidative stress and Ca2+-handling dysregulation | 34 |
| Pericarditis | ||||
| Sterile pericarditis | Dog |
Rapid atrial pacing-induced AF on days 2–14 after surgery Unstable re-entrant circuits with fibrillatory conduction Underlying cellular and molecular mechanisms of AF not studied |
AF duration and inducibility reduced or suppressed by administration of d,l-sotalol, JTV-519 (K201), AZD7009, ibuprofen, methylprednisolone, GAP-134, vanoxerine or ranolazine | 250–272 |
| Sterile pericarditis | Dog |
Rapid atrial pacing-induced AF on day 2 after surgery Prolonged intra-atrial conduction time Increased plasma CRP level Perimyocarditis and atrial inflammatory infiltrates with lipid degeneration Interstitial fibrosis |
AF and underlying tissue changes were suppressed by atorvastatin administration | 38 |
| Sterile pericarditis | Dog |
Rapid atrial pacing-induced AF on day 4 after surgery ↓ Atrial conduction velocity ↓ Atrial connexin 40 and connexin 43 levels ↓ Atrial α-actinin level (myolysis) ↑ Atrial vimentin level (fibrosis) |
– | 39 |
| Sterile pericarditis | Dog |
Rapid atrial pacing-induced AF on days 2–4 after surgery ↑ Atrial myeloperoxidase level |
– | 273 |
| Sterile pericarditis | Dog |
Rapid atrial pacing-induced AF on day 2 after surgery Shorter AERP ↑ CRP, IL-6 and TNF levels in plasma Perimyocarditis and atrial inflammatory infiltrates with lipid degeneration |
AF inducibility and duration and inflammatory indices were reduced by administration of polyunsaturated fatty acids | 43 |
| Sterile pericarditis | Goat |
Inducibility of AF by rapid atrial pacing was highest 72 h after surgery ↓ AERP 24 h to 21 days after surgery with loss of AERP rate adaptation Normal atrial conduction velocity ↑ CRP, IL-6 and TNF levels in plasma ↑ Lymphocyte atrial infiltration, epicardial thickening, myocardial rupture and necrosis |
↓ AF duration, but no increased AF inducibility; suppressed inflammatory indices with atorvastatin treatment | 42 |
| Sterile pericarditis | Rat |
Rapid atrial pacing-induced AF on days 1–4 after surgery ↑ Atrial levels of IL-6, IL-1β, IL-17A and TGFβ1 Perimyocarditis and atrial inflammatory infiltrates with lipid degeneration Interstitial fibrosis (↑ atrial levels of collagen 1, collagen 3, αSMA, MMP2 and MMP9 and ↓ levels of TIMP2 and glycosylated TIMP3) |
Neutralization of IL-17A reduced AF susceptibility and AF duration and suppressed inflammation and fibrosis | 49 |
| Sterile pericarditis | Rat |
Rapid atrial pacing-induced AF on day 3 after surgery Inhomogeneous conduction ↑ Atrial levels of IL-6, IL-1β, TGFβ1, TNF, STAT3 and miR-21 Interstitial fibrosis (↑ atrial levels of collagen 1, collagen 3 and αSMA) |
Administration of antagomir-21 and STAT3 inhibition reduced AF susceptibility and suppressed inflammation and fibrosis | 40 |
| Sterile pericarditis | Pig |
Rapid atrial pacing-induced AF 7 days after surgery ↓ Sinus cycle length Interstitial fibrosis Atrial myolysis Inflammation and leukocyte infiltration |
Amiodarone treatment prevented AF | 48 |
| Sterile pericarditis | Rabbit |
↑ Atrial premature contractions ↑ Spontaneous burst firing in pulmonary veins at baseline and with isoprenaline administration ↓ APD90 with reduced Cav1.2 and Kv11.1 levels Interstitial fibrosis |
Histone deacetylase inhibition prevented fibrosis and reduced atrial premature contraction and spontaneous burst firing | 44 |
| Sterile pericarditis | Rat |
Rapid atrial pacing-induced AF on day 3 after surgery Inhomogeneous conduction ↑ Atrial IL-6, TGFβ1, TNF and myeloperoxidase levels Interstitial fibrosis (↑ atrial collagen 1, collagen 3 and αSMA levels) ↑ Phosphorylation of STAT3, AKT and p38, but normal levels of phosphorylated ERK and phosphorylated JNK |
Colchicine administration suppressed inflammation and fibrosis | 41 |
| Sterile pericarditis | Rabbit |
↑ DADs and triggered activity in vitro in LA samples ↑ Pacing-induced burst firing in vitro in LA samples ↑ APD20 and APD50, normal APD90 in LA samples ↑ Atrial IL-1α, IL-8 and CCL4 levels, but normal IL-17A and IL-21 levels IL-1β, TNF and MMP9 not detectable |
Inhibition of TLR4 (with TAK-242), RYR2 (with ryanodine) or CaMKII (with KN-93) reduced pacing-induced burst firing | 47 |
| Sterile pericarditis | Rat |
Rapid atrial pacing-induced AF on day 3 after surgery ↑ Atrial ectopy; AERP unchanged ↑ APD20, APD50, APD90 (↓ K+ currents Ipeak, Isus and Ito) ↑ TRPV4 in atrial fibroblasts ↑ Atrial IL-6, TGFβ1 and TNF levels Interstitial fibrosis (↑ atrial levels of collagen 1, collagen 3, αSMA, and phosphorylated SMAD3, p38, AKT and STAT3) |
TRPV4 inhibition reduced AF susceptibility and suppressed inflammation and fibrosis | 45 |
| Sterile pericarditis | Minipig |
Rapid atrial pacing-induced AF 14 days after surgery Interstitial fibrosis |
– | 50 |
| Sterile pericarditis | Rat |
Rapid atrial pacing-induced AF on day 3 after surgery ↑ Atrial ectopy and re-entrant activity Normal AERP ↑ Atrial IL-6, IL-1β, MMP9 levels; plasma IL-6 level unchanged ↑ Myeloperoxidase level and CD68+ cell numbers Interstitial fibrosis (↑ atrial αSMA level) Heterogeneous Ca2+ transient prolongation ↑ Incidence of discordant alternans ↑ Relative level of Ser2808-phosphorylated RYR2 and Ser2814-phosphorylated RYR2 caused by downregulation of total RYR2 level ↓ Cav1.2 levels and SERCA activity; normal NCX1 levels |
Neutralization of IL-6 reduced atrial ectopy and susceptibility to AF and suppressed inflammation, fibrosis and Ca2+ handling abnormalities | 46 |
| Myocarditis | ||||
| Autoimmune or LPS-induced myocarditis | Rat |
11 out of 15 rats with chronic autoimmune myocarditis had inducible AF with atrial cardiomyocyte hypertrophy, increased LA and interstitial fibrosis LPS-induced acute myocarditis led to a reduction in atrial connexin 43 levels and did not cause inducible AF |
– | 51 |
AERP, atrial effective refractory period; AF, atrial fibrillation; APD, action potential duration; CaMKII, calcium/calmodulin-dependent kinase II; Cav1.2, voltage-gated L-type Ca2+ channel subunit-α; CCL4, C-C motif chemokine 4; CRP, C-reactive protein; DAD, delayed afterdepolarization; eNOS, endothelial nitric oxide synthase; IKr, rapid component of the delayed rectifier K+ current; IKs, slow component of the delayed rectifier K+ current; Ipeak, peak K+ current; Isus, sustained K+ current; Ito, transient outward K+ current; iNOS, inducible nitric oxide synthase; JNK, JUN amino-terminal kinase; Kv, voltage-gated K+ channel; LA, left atrial; LPS, lipopolysaccharide; LVEF, left ventricular ejection fraction; MDA, malondialdehyde; MMP, matrix metalloproteinase; NCX1, sodium–calcium exchanger 1; NO, nitric oxide; ROS, reactive oxygen species; RYR2, ryanodine receptor 2; SERCA2A, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2A; αSMA, α-smooth muscle actin; TGFβ1, transforming growth factor-β1; TIMP, tissue inhibitor of matrix metalloproteinases; TLR4, Toll-like receptor 4; TNF, tumour necrosis factor; TRPV4, transient receptor potential cation channel subfamily V member 4.
AF induced in animals with sterile pericarditis is associated with heterogeneous conduction slowing38–41, together with reduced connexin 40 and connexin 43 expression39. In these animals, the atrial effective refractory period and action potential duration are shorter42–44 or unchanged45–47. Increased atrial ectopy44–46 in experimental pericarditis is associated with DADs and cellular triggered activity47. Atrial structural remodelling in these animal models is characterized by myolysis39,48 and interstitial fibrosis38–41,44–46,48–50. In a rat model of chronic autoimmune myocarditis, increased susceptibility to AF was associated with increased left atrial size, cardiomyocyte hypertrophy and interstitial fibrosis together with reduced connexin 43 expression51. However, AF inducibility was not significantly increased in the acute phase51.
The NLRP3 inflammasome system
The production of pro-inflammatory cytokines central to inflammatory signalling is mainly controlled by the inflammasome, an intracellular multiprotein complex that functions as a molecular platform to activate the cysteine protease caspase 1 (Fig. 1), which subsequently cleaves pro-IL-1β and pro-IL-18 to generate pro-inflammatory IL-1β and IL-18. Although IL-1α triggers similar pro-inflammatory signalling to IL-1β and IL-18, IL-1α is produced in the active form and functions like an alarmin52. Inflammasome formation is initiated by PAMPs or DAMPs53, which are recognized by specific pattern-recognition receptors expressed in innate immune cells, such as macrophages and neutrophils, and in cardiac cells, including cardiomyocytes54 (Fig. 1). Pattern-recognition receptors can promote the formation of many inflammasomes, but NACHT-, LRR- and pyrin domain-containing 3 (NLRP3) is the main inflammasome linked to the pathology and progression of cardiovascular disease53,55,56. Extensive evidence shows that the adaptive immune response induced by NLRP3 inflammasome activation is involved in arrhythmogenesis, as discussed in detail below.
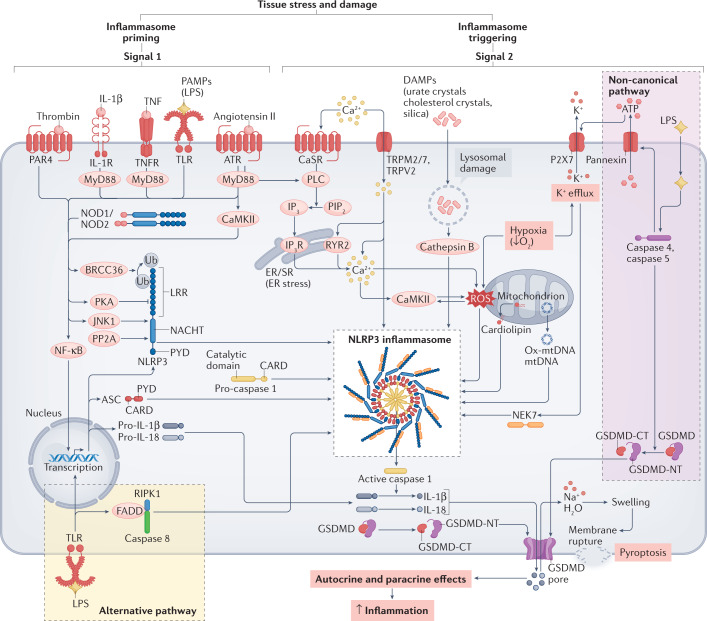
Fig. 1. Pathways involved in NLRP3 inflammasome activation.
Molecular pathways involved in NLRP3 inflammasome activation on the basis of information from classic models of inflammation. NLRP3 inflammasome activation in response to tissue stress and damage involves inflammasome priming (Signal 1), leading to increased expression of the major inflammasome components (NLRP3, ASC and caspase 1), and triggering (Signal 2), promoting assembly of the NLRP3 inflammasome. Subsequent activation of caspase 1 leads to activation of several proteins including IL-1β and IL-18, as well as gasdermin D (GSDMD) and IL-1β release through pores formed by the GSDMD amino terminus (NT) fragment. ATR, angiotensin receptor; BRCC36, Lys-63-specific deubiquitinase 36; CaMKII, Ca2+/calmodulin-dependent protein kinase II; CARD, caspase recruitment domain; CaSR, calcium-sensing receptor; DAMP, damage-associated molecular pattern; ER, endoplasmic reticulum; FADD, FAS-associated death domain protein; GSDMD-CT, gasdermin D carboxy terminus; IL-1R, IL-1 receptor type 1; IP3, inositol 1,4,5-trisphosphate; IP3R, inositol 1,4,5-trisphosphate receptor; JNK, JUN N-terminal kinase 1; LPS, lipopolysaccharide; LRR, leucine-rich repeat; mtDNA, mitochondrial DNA; MyD88, myeloid differentiation primary response protein 88; NEK7, NIMA related kinase 7; NF-κB, nuclear factor-κB; NLRP3, NACHT-, LRR- and pyrin domain-containing 3; NOD, nucleotide-binding oligomerization domain-containing protein; Ox-mtDNA, oxidized mitochondrial DNA; P2X7, P2X purinoceptor 7; PAMP, pathogen-associated molecular pattern; PAR4, proteinase-activated receptor 4; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PLC, phospholipase C; PP2A, protein phosphatase 2A; PYD, pyrin domain; ROS, reactive oxygen species; RIPK1, receptor-interacting serine/threonine-protein kinase 1; RYR2, ryanodine receptor 2; SR, sarcoplasmic reticulum; TLR, Toll-like receptor; TNF, tumour necrosis factor; TNFR, tumour necrosis factor receptor; TRP, transient receptor potential; Ub, ubiquitin.
The NLRP3 inflammasome comprises the sensor NLRP3, the adaptor protein ASC and the effector pro-caspase 1 (Fig. 1). The leucine-rich repeats (LRR) domain in the carboxy terminus of NLRP3 controls ligand sensing and autoregulation. The pyrin domain (PYD) in the amino terminus mediates the protein–protein interaction with the PYD of ASC. ASC recruits pro-caspase 1 via a caspase recruitment domain (CARD)57,58. Under basal conditions, NLRP3 resides in the endoplasmic reticulum59,60 and is in an inactive, ubiquitinated state until a priming signal induces deubiquitination61. ASC is predominantly located in the nucleus, but it can shuttle to the cytosol in close association with mitochondria59,60,62. ASC forms 1–2 µm structures called specks, which can leave the cell as large insoluble aggregates and propagate the inflammatory signal in phagocytic cells63,64.
The activation of the NLRP3 inflammasome requires two steps. The first step is ‘priming’ (which upregulates the expression of key inflammasome components and associated cytokines, typically via transcriptional activation) and the second step is ‘triggering’ (assembly and activation of the NLRP3 inflammasome). NLRP3 inflammasome priming involves transcriptional activation via Toll-like receptors (TLRs), tumour necrosis factor receptors (TNFRs), IL-1 receptors (IL-1Rs), angiotensin II receptors and/or protease-activated receptor 4, which stimulate the nuclear factor-κB (NF-κB) transcription pathway65–67 (Fig. 1). Non-transcriptional priming can occur through TLR and IL-1R type 1 (IL-1R1), which accelerate the deubiquitination of NLRP3, a process dependent on mitochondrial reactive oxygen species (ROS) production68. NF-κB also leads to deubiquitination of NLRP3 through the deubiquitinating enzyme BRCC3 (ref.61). Depending on the mechanisms of priming (transcriptional or non-transcriptional), increased expression of inflammasome components requires from 10–30 min to >3 h69–71.
The triggering signal promotes the assembly of the sensor and effector proteins, facilitated by ASC, in a wheel-shaped hub that promotes the formation of the catalytically active caspase 1 in the centre of the NLRP3 inflammasome propeller72. Triggers of the NLRP3 inflammasome system that have been characterized in immunocompetent cells include ATP, urate crystals, cholesterol crystals, silica, ion flux (such as K+ efflux, Ca2+ fluxes and Na+ influx), mitochondrial dysfunction, excess ROS production, and lysosomal damage leading to cathepsin B release68,73,74. The spatial organization of NLRP3 inflammasome assembly is driven by the microtubule network60,75. The serine–threonine kinase NEK7 regulates NLRP3 inflammasome assembly, functioning downstream of K+ efflux76,77. Ca2+ signalling is also required for NLRP3 inflammasome triggering78. In immune cells, the Ca2+-sensing receptor can be sensitized by increasing extracellular Ca2+ levels to increase Ca2+ influx79 (Fig. 1). Influx of Ca2+ via the transient receptor potential channels TRPM2 (ref.80), TRPM7 (ref.81) and TRPV2 (ref.81) also triggers NLRP3 inflammasome assembly. In non-excitable cells, the inositol 1,4,5-trisphosphate receptor (IP3R) is the main Ca2+ release channel from the endoplasmic reticulum79. Inhibition of IP3R by 2-aminoethyl diphenylborinate reduces intracellular Ca2+ concentration and prevents IL-1β secretion in macrophages74,79. Ca2+ can also facilitate the interaction between NLRP3 and ASC79, and increases in cytosolic Ca2+ levels can lead to mitochondrial Ca2+ overload and the generation of mitochondria-derived ROS, with subsequent NLRP3 inflammasome activation78,82. Endoplasmic reticulum stress, partially caused by Ca2+ dysregulation, also triggers NLRP3 inflammasome assembly83. Mitochondrial recruitment of NLRP3 to the inner mitochondrial membrane, where it interacts with the mitochondrial protein cardiolipin, is followed by relocation from the inner to the outer mitochondrial membrane, promoting the interaction between NLRP3 and caspase 1 (ref.84) and NLRP3 inflammasome assembly. Multiple post-translational modifications (such as ubiquitination and phosphorylation) and endogenous modulators (such as CARD-only proteins and pyrin-only proteins) also regulate the activity of the NLRP3 inflammasome85. The regulation of NLRP3 activity is very complex and varies according to the type of cell and activating stimuli.
When the NLRP3 inflammasome is activated, caspase 1 triggers the cleavage of the inactive isoforms pro-IL-1β and pro-IL-18, releasing active IL-1β and IL-18. Caspase 1 also cleaves gasdermin D (GSDMD), removing its inhibiting C terminus (GSDMD-CT) and releasing the N terminus (GSDMD-NT), which forms pores in the plasma membrane (Fig. 1). These pores allow the products of the inflammasome cascade (such as IL-1β and IL-18) to leave the cell and propagate the activation of inflammatory signalling cascades to neighbouring cells86. The secretion of cytokines can be accompanied by inflammatory programmed cell death called pyroptosis. These effects can be mediated via caspase 1, known as the canonical NLRP3 pathway, or caspase 4 and caspase 5 (in humans; caspase 11 in mice), known as the non-canonical NLRP3 pathway.
Activation of the canonical NLRP3 inflammasome pathway in immune cells is mediated by cellular K+ depletion87. Pore formation via P2X purinoceptor 7 (P2X7), pannexin 1 channels or other pore-forming subunits allows K+ efflux (Fig. 1). Pannexin 1 channels release ATP, which rapidly opens P2X7, causing K+ efflux88 and NLRP3 inflammasome activation via the canonical pathway.
The non-canonical NLRP3 inflammasome pathway involves cleavage of caspase 4 and caspase 5 (refs.59,89,90) (Fig. 1), which recognize strong LPS signals via their CARD domain and cleave GSDMD59, leading to the formation of GSDMD-NT pores in the plasma membrane. Caspase 4 and caspase 5 are unique in terms of their very high selectivity for GSDMD, but cannot directly produce active cytokines from inactive precursors91. Caspase 4 and caspase 5 can also cleave pannexin 1 after LPS stimulation, causing ATP release with subsequent P2X7 activation and non-canonical NLRP3 inflammasome activation92,93. Triggering of NLRP3 inflammasome activation does not absolutely require K+ depletion and seems to be independent of K+ in human monocytes in vitro94. This alternative NLRP3 activation is mediated by the TRIF–RIPK1–FADD–caspase 8 pathway, and the release of IL-1β is much weaker than via canonical activation92. Caspase 8 suppresses RIPK3 activation, which participates in signalling processes leading to apoptosis95. Pathogens can facilitate the release of caspase 8-mediated suppression of RIPK3, increasing the generation of mitochondrial ROS that can activate the NLRP3 inflammasome95.
GSDMD pore formation can lead to inflammatory cell death or pyroptosis. The cell swelling, cell membrane disruption and release of cytoplasmic contents (including cytokines) that occur during pyroptosis can cause tissue damage, organ failure and shock96,97. Pyroptosis is primarily executed by caspase 4/caspase 5-mediated GSDMD cleavage with subsequent GSDMD-NT pore formation and cell membrane permeabilization98,99. IL-1β release mediated by canonical NLRP3 inflammasome activation can occur in the absence of pyroptosis100, which is likely to be the predominant mechanism in terminally differentiated cells such as cardiomyocytes.
Inflammatory signalling in heart disease
AF is promoted by numerous cardiovascular diseases and risk factors, several of which have been associated with activation of the NLRP3 inflammasome, as summarized below and illustrated in Fig. 2. A more detailed overview of the role of NLRP3 inflammasome activation in other cardiovascular diseases has been provided in previous reviews101–104.
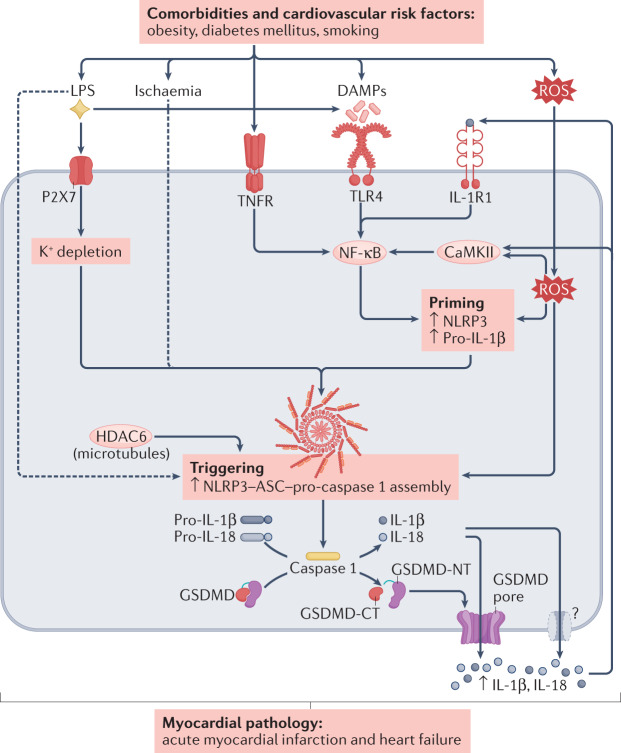
Fig. 2. Pathways leading to NLRP3 inflammasome activation induced by comorbidities and cardiovascular risk factors.
Main pathways through which risk factors, heart diseases and comorbidities can promote NLRP3 inflammasome activation, potentially leading to atrial fibrillation and contributing to myocardial pathology in conditions such as acute myocardial infarction and heart failure. Risk factors, including gut microbiota dysbiosis, obesity, diabetes mellitus, right heart disease and coronary artery disease, promote the production of several mediators that activate transmembrane receptors involved in NLRP3 inflammasome priming, including Toll-like receptor 4 (TLR4), tumour necrosis factor receptors (TNFRs) and IL-1 receptor type 1 (IL-1R1), as well as downstream activation of signalling pathways dependent on Ca2+/calmodulin-dependent protein kinase II (CaMKII), reactive oxygen species (ROS) and nuclear factor-κB (NF-κB). Assembly of inflammasome components (triggering) is mediated, among other pathways, by P2X purinoceptor 7 (P2X7) signalling and K+ depletion, as well as histone deacetylase 6 (HDAC6). Downstream activation of caspase 1 and formation of pores by the gasdermin D (GSDMD) amino terminus (NT) fragment subsequently allows the release of IL-1β and IL-18 from the cell. In parallel, these pathways promote pro-arrhythmic atrial remodelling, including spontaneous Ca2+ release events, delayed afterdepolarizations and triggered activity, as well as re-entry-promoting shortening of action potential duration and effective refractory period in combination with structural remodelling and associated conduction abnormalities. Dashed arrows indicate indirect effects. DAMP, damage-associated molecular pattern; GSDMD-CT, gasdermin D carboxy terminus; LPS, lipopolysaccharide; NLRP3, NACHT-, LRR- and pyrin domain-containing 3.
Acute myocardial infarction (MI) is a common trigger of AF1. The NLRP3 inflammasome is directly activated by intraplaque cholesterol crystals through leakage of the lysosomal protease cathepsin B105. NLRP3 is the predominant component of the inflammasome involved in the response to sterile injury, as occurs during acute MI101. Necrotic cell death releases cell contents, including several DAMPs, priming the NLRP3 inflammasome via activation of NF-κB102. In parallel, ischaemia-induced reductions in intracellular K+ levels act as a trigger for NLRP3 inflammasome activation102. The important role of NLRP3 inflammasome activation and subsequent pyroptosis in MI is supported by numerous preclinical studies showing smaller infarct sizes after ischaemia–reperfusion in mice with genetic deletion of NLRP3, IL-1R1, ASC or caspase 1 compared with wild-type mice, as well as with small interfering RNA-mediated or pharmacological inhibition of NLRP3 (summarized previously102) or P2X7 (ref.106) after the ischaemic insult. Although the NLRP3 system activation induced by ischaemia–reperfusion injury was initially shown to occur preferentially in cardiac fibroblasts (versus cardiomyocytes) in mice107, subsequent work clearly showed that the NLRP3 system is activated in both mouse ventricular and atrial cardiomyocytes106,108. Data on the clinical role of the NLRP3 inflammasome in MI are scarce and largely based on biomarkers or indirect evidence from clinical trials using non-selective NLRP3 inhibitors or IL‑1 blockers101. For example, colchicine treatment significantly reduced infarct size (measured as the area under the curve for biomarkers of necrosis or by cardiac MRI) in patients with ST-segment elevation acute MI compared with placebo109.
Inflammation is increasingly recognized as an important pathophysiological mediator of HF initiation and progression104, which is a major driver of AF-promoting atrial remodelling. IL-1β directly inhibits cardiac contractility102, in part as a result of alterations in cardiomyocyte Ca2+ handling, which can simultaneously promote cardiac arrhythmias110. Inhibition of NLRP3 inflammasome activation or downstream IL-1β signalling reduces adverse cardiac remodelling and ameliorates the HF phenotype in preclinical models of HF induced by MI without reperfusion, transverse aortic constriction leading to pressure overload, anthracycline toxicity, radiation injury or septic cardiomyopathy102. Moreover, data from rodent models of HF with reduced or preserved ejection fraction suggest that the beneficial cardiac effects of empagliflozin are associated with reduced activation of the NLRP3 inflammasome through a Ca2+-dependent mechanism111.
In addition, AF risk factors such as smoking1 are associated with increased NLRP3 activation112. Taken together, substantial evidence indicates a causative role of NLRP3 inflammasome activation, including in cardiomyocytes, in cardiovascular diseases associated with AF, ranging from stable coronary artery disease to acute MI and HF.
Atrial cardiomyocyte inflammatory signalling and AF
Inflammatory signalling component expression in atrial cardiomyocytes and AF
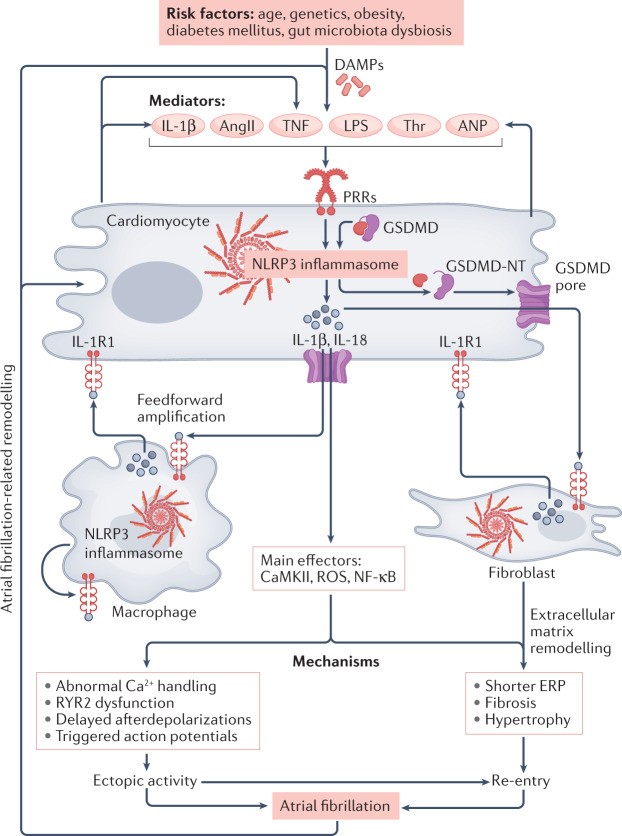
Although inflammatory signalling pathways are extensively studied in immune cells, emerging evidence supports the notion that atrial cardiomyocytes have the same inflammatory signalling pathways, the activation of which contributes to AF development33,106,110,113. Atrial cardiomyocytes not only produce potent pro-inflammatory cytokines such as IL-1β, IL-6, IL-18 and TNF, but also express the cytokine receptors (IL-1R1, IL-6R, IL-18R and TNFR) that mediate the effects of these inflammatory cytokines106,110,114–116. Activation of these cytokine receptors via autocrine or paracrine mechanisms stimulates several important AF-related signalling cascades, including those involving JAKs, STATs, MAPKs and NF-κB, which can in turn upregulate the transcription of genes encoding pro-inflammatory factors. Many stressors that increase the susceptibility to AF, such as ROS and endoplasmic reticulum stress68,83, can independently activate these signalling pathways, further amplifying the inflammatory signal and its propagation.
Accumulating evidence also indicates the engagement of the NLRP3 inflammasome in atrial cardiomyocytes. In the mouse atria-derived cardiomyocyte cell line HL-1, in vitro priming with LPS and triggering with ATP or nigericin promoted the formation of ASC aggregates106. These changes were accompanied by increased caspase 1 activity and caspase 1-mediated pyroptosis, both of which are prevented by caspase 1 inhibition106. These results demonstrate that canonical NLRP3 inflammasome activation is operative in atrial cardiomyocytes. In addition, pharmacological inhibition of P2X7 mimics the results obtained with caspase 1 inhibition106, suggesting that increased K+ efflux via P2X7 contributes to cardiomyocyte NLRP3 inflammasome activation. Simulated ischaemia in cardiomyocytes in vitro caused by exposure to hypoxia and an ‘ischaemic’ buffer similarly promoted the formation of ASC aggregates and increased caspase 1 activity, pointing to activation of the NLRP3 inflammasome in atrial cardiomyocytes in ischaemic conditions106. In vitro tachypacing of HL-1 cells (to mimic the atrial tachycardia that occurs during AF) increased the polarization and migration of co-cultured macrophages, features that are typically associated with inflammatory settings33. Conversely, LPS-treated macrophages caused IL-1β-mediated reduction of ICa,L in co-cultured HL-1 cells33. These data establish the potential for feedforward crosstalk between atrial cardiomyocytes and immune cells in the atria.
Evidence for a role of atrial cardiomyocyte inflammatory signalling in AF
Several studies have assessed the specific contributions to AF pathophysiology of the pro-inflammatory cytokine TNF, the IL-1β-maturation platform and the NLRP3 inflammasome113,117–119. Transgenic mice with cardiomyocyte-specific overexpression of TNF have enlarged atria, atrial fibrosis and abnormal atrial conduction together with downregulation of atrial connexin 40 levels, reduced atrial contractility and increased susceptibility to pacing-induced AF120,121. Conversely, genetic ablation of TNF or pharmacological inhibition of TNF with etanercept or a dominant-negative inhibitor of the soluble form of TNF (XPro1595) prevented exercise-induced adverse atrial remodelling and AF promotion in mice117,118. TNF-induced atrial arrhythmogenesis in mice is at least partly attributable to abnormalities in cardiomyocyte Ca2+ handling, including a higher frequency of triggered activity-promoting Ca2+ release events117,118,120. In vitro exposure of rabbit pulmonary vein cardiomyocytes to TNF for up to 10 h reduced ICa,L, led to larger amplitude DADs together with increased transient inward current and Na+–Ca2+-exchanger current, and increased diastolic Ca2+ levels, while reducing Ca2+ transient amplitude and sarcoplasmic reticulum Ca2+ content, and potentially causing AF-promoting triggered activity in the pulmonary vein cardiomyocyte sleeves122.
Human atrial cardiomyocytes have all the key elements of the NLRP3 inflammasome system110,113. Most importantly, NLRP3 inflammasome activity is increased in atrial cardiomyocytes in patients with paroxysmal AF or long-lasting persistent AF113. Similarly, levels of ASC and pro-caspase 1, as well as the products of NLRP3 inflammasome activation, active caspase 1 and cleaved (pore-forming) GSDMD-NT, were significantly increased in atrial cardiomyocytes of patients undergoing cardiac surgery who subsequently developed postoperative AF compared with patients who remained in sinus rhythm110. These data suggest that NLRP3 inflammasome activation in atrial cardiomyocytes might be a common feature of many forms of AF.
The potential pathophysiological role of increased NLRP3 activity in atrial cardiomyocytes from patients with AF has been addressed by studies in animal models. Mice with cardiomyocyte-specific overexpression of a constitutively active form of NLRP3 (Myh6:Nlrp3A350V/+; CM-KI mice) develop atrial ectopy and a re-entry-promoting shortening of atrial refractoriness, together with atrial hypertrophy and fibrosis, associated with increased AF susceptibility113. These mice have substantially increased prevalence of spontaneous atrial ectopic activity, associated with an increased incidence of Ca2+-release events through RYR2 channels (Ca2+ sparks). The shortening of the atrial action potential duration and effective refractory period in CM-KI mice is likely to be due to upregulation of the expression of Kcna5 (which encodes the voltage-gated K+ channel Kv1.5) and the increase in the related repolarizing current IKur (ultra-rapid delayed rectifier K+ current)113. Although overall conduction velocity is unchanged, CM-KI mice have atrial fibrosis113, which is known to be associated with atrial arrhythmogenesis.
Atrial macrophage infiltration occurs in patients with AF33,123, possibly as a result of NLRP3 inflammasome activation and IL-1β and IL-18 signalling in atrial cardiomyocytes, with subsequent macrophage recruitment to the atria. The released IL-1β (and perhaps IL-18) acts on adjacent cells causing NF-κB-mediated transcriptional priming of NLRP3-components in the same and other cell types, thereby potentially leading to further NLRP3 inflammasome activation in surrounding cells, irrespective of the initial source. Acute application of IL-1β to human atrial cardiomyocytes in vitro increases the incidence of Ca2+ release events, particularly in cells from patients prone to develop postoperative AF110. Similarly, acute application of IL-1β increases the incidence of Ca2+ release events in HL-1 cells110, accompanied by increased CaMKII-mediated phosphorylation of RYR2 (which is an established molecular contributor to Ca2+ release events and AF36,37) together with activation of caspase 1 and the formation of pore-forming GSDMD-NT110.
The effects of IL-1β on Ca2+ handling are likely to be amplified by IL-6 (ref.46). IL-6 also rapidly induces reversible atrial electrical remodelling by downregulation of atrial connexin levels in HL-1 cells124. However, the precise actions of IL-6 in the atria require further delineation. Similarly, although the activation of caspase 1 in AF is expected to increase the atrial levels of IL-18, the relative contribution of IL-18 to the actions of IL-1β in the atria is unknown. In addition, whether IL-18 acts on the atria via mechanisms different from those of IL-1β is unclear. Given that selective IL-18 inhibitors are clinically available, future work should define the atrial effects of IL-18 and the therapeutic potential of IL-18 inhibition for AF management.
Histone deacetylase 6 (HDAC6) has an indispensable role in the microtubule transport and assembly of inflammasomes in macrophages60,75. HDAC6 activity increases with AF-related atrial remodelling in patients as well as in HL-1 cells subjected to in vitro high-frequency pacing125. Therefore, HDAC6 activation could contribute to Ca2+-dependent NLRP3 inflammasome activation. Ca2+-dependent activation of CaMKII in cardiomyocytes seems to contribute to NLRP3 inflammasome activation because in mouse models of cardiac remodelling induced by chronic angiotensin II infusion or transverse aortic constriction, cardiomyocyte-specific deficiency of CaMKII suppresses NLRP3 inflammasome activation66,126. This observation is consistent with findings that CaMKIIδ can activate NF-κB by phosphorylating (inactivating) the inhibitor of NF-κB (IκB kinase)127,128, thereby upregulating the transcription of multiple genes encoding NLRP3 inflammasome components. CaMKII can also increase mitochondrial Ca2+ entry via phosphorylation of the mitochondrial Ca2+ uniporter, which increases ROS production129, a key trigger of NLRP3 inflammasome activation and AF promotion130. Therefore, NLRP3 inflammasome-mediated CaMKII activation in atrial cardiomyocytes might produce Ca2+ leak from the sarcoplasmic reticulum and drive mitochondrial ROS production, creating a vicious cycle of arrhythmogenic inflammatory signalling.
Several known risk factors for AF such as obesity, diabetes mellitus, gut microbiota dysbiosis and right-sided heart disease can promote AF development via activation of inflammatory signalling in atrial cardiomyocytes131,132. Although the inflammatory signalling associated with risk factors is subtle compared with the more aggressive, well-recognized inflammation inducers such as acute tissue injury and pathogens, the chronic nature of risk factors could lead to substantial effects over the course of a lifetime. The activity of NLRP3 inflammasomes in atrial tissue of individuals with obesity correlates with increases in BMI119. In animal models of diet-induced obesity, increased atrial NLRP3 activity is required for obesity-induced atrial arrhythmogenesis119. Cardiomyocyte Ca2+ dysregulation, increased frequency of Ca2+ release events and atrial ectopy are key elements of the arrhythmogenic atrial substrate in obesity-driven AF in these animal models119.
Gut microbiota dysbiosis is also associated with upregulation of the NLRP3 system in the atria in mouse models131, with some gut microbiota-derived metabolites having direct effects on atrial arrhythmogenesis. For example, trimethylamine N-oxide (TMAO) upregulates NF-κB signalling, which leads to increased abundance of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF, and synthesis of nerve growth factor in atrial ganglionated plexi, which activate the cardiac autonomic nervous system131. TMAO also causes oxidative stress and activates the NLRP3 inflammasome and the transforming growth factor-β1 (TGFβ1)–SMAD3 pathway in ganglion plexus tissues in animal models133. Gut microbiota dysbiosis-derived LPS strongly activates the NLRP3 inflammasome, increases the expression and lateralization of connexin 43 and downregulates L-type Ca2+ channel expression131. Similarly, NLRP3 activity is increased in the right atria of patients with diabetes mellitus67. NLRP3 protein levels and caspase 1 activity in atrial tissue and serum IL-1β and IL-18 levels are increased in rabbits with alloxan-induced diabetes mellitus134. In this rabbit model, administration of glibenclamide (a hypoglycaemic agent that inhibits the NLRP3 inflammasome) reduces the diabetes-induced atrial structural remodelling and the related increases in AF inducibility134.
Right-sided heart disease is increasingly recognized as an important cause of AF135,136. In a rat model of right-sided heart disease induced by monocrotaline injection135,137, AF promotion was associated with increased expression of NLRP3 inflammasome-related components in the right atria, as evidenced by increased levels of active caspase 1 and IL-1β137.
Inflammatory signalling in non-myocyte heart cells
During sepsis, the NLRP3 inflammasome system is upregulated in mouse ventricular fibroblasts138. Cardiac dysfunction after MI in mice is also associated with upregulation of the NLRP3 inflammasome in ventricular fibroblasts107,108, with the major trigger of NLRP3 inflammasome activation being increased K+ efflux caused by ROS generation107. Similarly, in vitro treatment of mouse ventricular fibroblasts with LPS138 or human ventricular fibroblasts with thrombin67 activates the NLRP3 system, with increased secretion of IL-1β. These results support the notion that the NLRP3 inflammasome is activated in fibroblasts during cardiac injury.
Application of TNF to human cultured atrial fibroblasts from patients undergoing open heart surgery increased the expression and release of IL-6 and IL-1β via activation of TNFRs, with subsequent stimulation of p38–MAPK, PI3K–AKT and NF-κB signalling139, pointing to the production and functional regulation of pro-inflammatory cytokines in atrial fibroblasts. Similar increases in pro-inflammatory signalling were also noted after stimulation of human atrial fibroblasts with IL-1α52 and TGFβ1 (ref.140), together with downstream activation of pro-fibrotic signalling140. Abnormal inflammatory signalling in atrial fibroblasts has been implicated in AF development141. The AF-associated human variant p.Ile138Thr of NPPA (which encodes atrial natriuretic peptide) induced atrial inflammation and atrial fibrosis, causing both inducible and spontaneous AF, in a knock-in rat model141. RNA sequencing of Nppa-mutant rat atrial fibroblasts showed that the largest differences in expression were in genes related to TNF, NF-κB and NLRP3 signalling141. These data point to a role of atrial fibroblast inflammatory signalling in AF.
Obesity contributes to AF pathophysiology142. The highly secretory epicardial adipose tissue (EAT) surrounding the atria secrete a variety of pro-inflammatory and pro-fibrotic mediators that promote atrial remodelling, thereby increasing the susceptibility to AF142. EAT volume correlates with AF severity, postoperative AF incidence and AF recurrence rates after cardioversion or ablation143. In addition, EAT from patients with AF shows strong pro-inflammatory indices, as evidenced by a higher 18F-fluorodeoxyglucose PET signal intensity than that of EAT from patients without AF144. Extracellular vesicles derived from EAT from patients with AF contain larger amounts of pro-inflammatory and pro-fibrotic cytokines than EAT from control patients145. Extracellular vesicles derived from EAT from patients with AF induced sustained re-entry in human induced pluripotent stem cell-derived cardiomyocytes in vitro, whereas extracellular vesicles from EAT from control patients had no effect145. Therefore, the NLRP3 system in atrial adipocytes might constitute an important source of pro-inflammatory cytokines, a hypothesis that requires direct evaluation.
Resolution of inflammation in AF
Concept of inflammation resolution
Under physiological conditions, reparative inflammation is a complex and active process characterized by an initiation phase and a resolution phase, promoting healing and restoration of homeostasis146 (Fig. 3). The resolution of inflammation has traditionally been considered a passive process occurring when the stimulus to inflammation is no longer active. However, the discovery in the 1980s of the capacity of arachidonic acid derivatives, such as lipoxins and resolvins, to promote the resolution of inflammation led to a consensus that inflammation resolution is an active process following acute inflammation147. After infection or injury, the initiation of inflammation is followed by an augmentation of resolution-promoting signalling, arresting polymorphonuclear leukocyte (PMN) recruitment and suppressing pro-inflammatory signalling148. The active process of inflammation resolution involves specific enzymes (cytochrome P450, cyclooxygenase 1 (COX1), COX2, 5-lipoxygenase (5-LOX), 12-LOX and 15-LOX), endogenous lipid mediators (prostaglandins, leukotrienes, lipoxins and resolvins), cytokines (IL-10 and IL-13), immune cells (monocytes, anti-inflammatory M2 macrophages and dendritic cells) and biological processes designed to terminate acute inflammation, promote clearance of inflammatory cells and restore normal tissue and organ function147–150. Failure to undergo the complex shift from initial inflammatory signalling to pro-resolution signalling can contribute to abnormal accumulation of pro-inflammatory mediators and biomarkers (prostaglandins, leukotrienes, NF-κB, NLRP3 inflammasome, IL-1β, PMNs and pro-inflammatory M1 macrophages), which leads to chronic, unresolved inflammation, tissue fibrosis and impaired function151 (Fig. 3 and Table 3).
Fig. 3. Inflammation resolution concept.
In response to injury or infection, acute inflammation is activated to favour the return to homeostasis. During the initiation phase, damage signals (pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) promote phospholipase A2 (PLA2)-induced accumulation of arachidonic acid (AA), activation of the nuclear factor-κB (NF-κB) pathway and assembly of the NLRP3 inflammasome, leading to the release of pro-inflammatory substances, recruitment of polymorphonuclear leukocytes (PMNs) and monocyte polarization towards pro-inflammatory M1 macrophages. Apoptotic PMNs and M1 macrophages secrete 12-lipoxygenase (12-LOX) and 15-LOX, which triggers lipid-mediator class switching, signalled by increased production of resolution-promoting mediators from AA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Pro-resolution autacoids orchestrate the inhibition of PMN infiltration, the increased release of anti-inflammatory cytokines (IL-10 and IL-13) and increased macrophage polarization to anti-inflammatory M2 macrophages to phagocytize damaged cells. Inflammation resolution is characterized by efferocytosis, tissue repair, wound healing, preservation of functions and homeostasis. Failed resolution of inflammation is associated with dysfunctional triggering of lipid-mediator class switching, perpetuation of production and secretion of pro-inflammatory mediators (IL-1β, IL-6 and IL-18), aggravation and chronicity of the inflammatory status, development of fibrosis, myocardial damage, loss of myocardial function and arrhythmogenesis. CaMKII, calcium/calmodulin-dependent kinase II; COX, cyclooxygenase; CYP450, cytochrome P450; LTB4, leukotriene B4, LXA4, lipoxin A4; MAPK, mitogen-activated protein kinase; NLRP3, NACHT-, LRR- and pyrin domain-containing 3; PGE2, prostaglandin E2; PGI2, prostaglandin I2; ROS, reactive oxygen species; TXA2, thromboxane A2.
Table 3.
Anti-inflammatory therapeutics
| Agent | Specific targets | Beneficial effects | Limitations or adverse effects | Refs. |
|---|---|---|---|---|
| COX inhibitors | COX1 and COX2 |
Suppress the production of arachidonic acid metabolites (prostaglandins, leukotrienes, thromboxane A2) Reduce NLRP3 activity, inhibit caspase 1 and prevent IL-1β secretion Reduce pain |
Increase risk of clots, hypertension and cardiovascular events | 173,177 |
| Aspirin | COX1 and COX2 |
Irreversible acetylation of COXs Acetylated COX2 can metabolize docosahexaenoic acid and eicosapentaenoic acid Production of aspirin-triggered resolvins Restrains NLRP3 assembly and activity via blockade of reactive oxygen species release |
No evidence of atrial fibrillation prevention with chronic aspirin use Not recommended for patients with low risk of cardiovascular disease |
184,273 |
| Glucocorticoids | Immune cells and pro-inflammatory biomarkers |
Suppress pro-inflammatory biomarkers and activation of pro-inflammatory cells Promote clearance of apoptotic cells Reduce pain and swelling |
Increase NLRP3 activation and IL-1β secretion Delay wound healing Increase the risk of hypertension Increase atrial fibrillation incidence |
185 |
| NLRP3 inhibitors | NLRP3 inflammasome components and signalling |
Prevent maturation of NLRP3 components Prevent NLRP3 inflammasome assembly Reduce pro-inflammatory cytokine production Might promote lipid-mediator class switching |
Further studies are required to assess the potential of NLRP3 inhibitors in inflammatory conditions and clarify their safety and potential adverse effects | 195 |
| IL-1 inhibitors | IL-1R1 and IL-1β |
Prevent IL-1β interaction with its receptor IL-1R1 Inhibit IL-1β signalling Reduce IL-1β-induced production of pro-inflammatory interleukins (IL-6, IL-17, IL-18) |
Increase the risk of infection Increase cholesterol plasma levels Decrease circulating leukocyte numbers Might increase the risk of cancer |
102 |
| Specialized pro-resolving mediators | Pro-inflammatory processes and immune cells |
Prevent infiltration of polymorphonuclear leukocytes Activate M2 macrophage phagocytosis and clearance of cellular debris Resolvin D1 decreases atrial expression of NLRP3 components Non-toxic |
Few clinical and preclinical data on cardiac tissue currently available Various clinical trials are ongoing or have been completed: NCT04575753, NCT04997057, NCT03609541, NCT04697719, NCT02322073 |
274–278 |
COX, cyclooxygenase; IL-1R1, IL-1 receptor type 1; NLRP3, NACHT-, LRR- and pyrin domain-containing 3.
Endogenous pro-resolution biochemistry
In response to pathological insults or infection by viral or bacterial pathogens, phospholipase A2 accumulates in the affected tissue. This enzyme hydrolyses the sn-2 ester bond of cellular phospholipids to produce arachidonic acid, an essential n-6 polyunsaturated fatty acid (n-6 PUFA)152. Arachidonic acid is metabolized by cytochrome P450, COX1, COX2 and 5-LOX to produce numerous pro-inflammatory lipid mediators (thromboxane A2, prostaglandin A2 (PGA2), PGB2, PGE2 and PGI2) that activate pro-inflammatory signalling promoting PMN and M1 macrophage chemotaxis (NLRP3 inflammasome, IL-1β, IL-6, CXC chemokine ligand 1 (CXCL1) and CXCL2) and the increase in adhesion molecules (intercellular adhesion molecule 1, vascular cell adhesion molecule 1 and E-selectin) at the site of injury153–155. During neutrophil-induced apoptotic and phagocytic activity, intracellular and secreted levels of 12-LOX and 15-LOX increase153. Arachidonic acid interaction with 12-LOX and 15-LOX leads to the production of lipoxins, including lipoxin A4 and LXB4, which activate the LXA4 receptor N-formyl peptide receptor 2 to stimulate intracellular signals that initiate pro-resolution processes such as cessation of neutrophil chemotaxis, inhibition of NF-κB signalling, alteration of NLRP3 assembly and activity, and stimulation of non-phlogistic (that is, non-inflammatory) monocyte invagination156,157.
Shortly after the initiation of acute inflammation, the essential n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are released at the site of injury151. EPA and DHA compete with arachidonic acid for cytochrome P450, COX2, 5-LOX, 12-LOX and 15-LOX158. EPA and DHA are metabolized to produce E-series resolvins (RvEs) and D-series resolvins (RvDs), respectively, including RvE1 and RvD1 (refs.158,159). Production of anti-inflammatory PGD2 from arachidonic acid via COX2 is also increased159. These bioactive lipid mediators interact with specific receptors (G protein-coupled receptor 32 (GPR32), GPR18, chemerin-like receptor 1 (ChemR23), PGD2 receptor 1 and PGD2 receptor 2) expressed on endothelial cells, PMNs, monocytes, macrophages, dendritic cells and T helper 2 (TH2) cells, to stimulate the cessation of PMN chemotaxis, activation of M2 macrophage phagocytosis of apoptotic PMNs and cell debris, efferocytosis and clearance of inflammatory cells160.
Chronic inflammation probably involves a failure of inflammation resolution161. If 12-LOX and 15-LOX production is insufficient, the generation of the n-3 PUFA-derived pro-resolving mediators RvEs and RvDs is inadequate157. Failure of resolution leads to accumulation of arachidonic acid metabolites, including thromboxanes, prostaglandins and leukotrienes, which promote pro-inflammatory signalling. In addition, continued generation of DAMPs and other inflammatory triggers contributes to inflammation persistence. The active phase of inflammation persists and chronic inflammation results, leading to thrombogenesis, formation of fibrous tissue, and loss of tissue and organ function161,162. Treatment with molecules that promote inflammation resolution properties ameliorates disease in animal models of chronic inflammation137,163,164 (Fig. 3). Administration of RvD1 reduces NLRP3 inflammasome assembly by preventing the colocalization of NLRP3 with the inflammasome components ASC and pro-caspase 1 (ref.165).
Pathological and potential therapeutic relevance of inflammation resolution in heart disease
Evidence for a role of inflammation resolution in preventing chronic disease development is available for a range of cardiac conditions166. In a clinical study involving 27 patients with HF and 23 age-matched control individuals, patients with HF had significantly lower plasma levels of RvD1, together with decreased levels of the RvD1 receptor GPR32 in T cells and reduced 15-LOX activity in leukocytes167. In aortic valves from patients with aortic stenosis, the leukotriene B4 (LTB4) level was increased, whereas RvE1 and RvD3 levels were decreased in calcified regions compared with non-calcified regions168. In vitro treatment with RvE1 prevented human valvular interstitial cell calcification168. In addition, in Apoe-knockout mice that develop aortic valve disease, overexpression of the Caenorhabditis elegans gene fat-1 (which encodes a protein that enables endogenous synthesis of n-3 PUFAs) increased the production of n-3 PUFA derivatives in aortic valves and reduced valvular calcification and improved echocardiographic indices of valve disease168. By contrast, knockout of Cmklr1 (which encodes ChemR23) increased valve disease progression in these mice168.
In a mouse model of experimental autoimmune myocarditis, daily injections of the LXA4 analogue BML-111 (1 mg/kg per day, starting 7 days after myocarditis induction) prevented the myocarditis-induced overexpression of inflammation-related and fibrosis-related genes, including those encoding IL-1β, TNF, TGFβ1 and collagen 1A1 (ref.169). In addition, BML-111 treatment prevented myocarditis-induced reductions in LV ejection fraction, LV hypertrophy and cardiomyocyte apoptosis169. In rats with MI induced by coronary artery ligation, treatment with RvE1 decreased infarct size, attenuated leukocyte infiltration and reduced caspase 3 levels in the infarct area170. In a mouse model of MI, RvD1 treatment for 5 days after MI decreased M1 macrophage recruitment to the infarct region, reduced the myocardial expression of IL-1β, IL-6 and TNF, prevented myocardial fibrosis and ameliorated LV function171. In a rat model of right-sided heart disease, RvD1 administration attenuated right atrial fibrosis, decreased the expression of NLRP3 inflammasome components, promoted ChemR23 expression and prevented AF inducibility137. All these studies support the relevance of inflammation resolution in heart disease, but this area of research requires further study.
Therapeutic implications
Therapeutic strategies targeting the NLRP3 inflammasome to reduce the consequences of inflammation can involve traditional or novel NLRP3 inhibitors, glucocorticoids or classic anti-inflammatory medications such as non-steroidal anti-inflammatory drugs, as well as novel interventions that promote inflammation resolution172. Table 3 summarizes the main categories of anti-inflammatory interventions and presents some of the potential benefits and risks associated with these strategies.
COX2 inhibitors
The discovery of the role of COX1 and COX2 pathways in producing most of the pro-inflammatory metabolites from arachidonic acid was followed by the development of a range of now widely used non-steroidal anti-inflammatory COX inhibitors173. Genetic or pharmacological inhibition of PGE2 signalling reduces IL-1β secretion and caspase 1 activity in mice174. However, despite their anti-inflammatory properties, COX inhibitors can also disrupt inflammation resolution because, although they suppress COX-induced production of key pro-inflammatory lipid-mediators, such as PGE2 and LTB4, COX inhibitors also prevent the production of arachidonic acid-derived pro-resolution mediators such as PGD2 (which is involved in lipid-mediator class switching and efferocytosis)175,176. PGD2 signalling, via its interaction with PGD2 receptors 1 and 2 expressed on cells involved in inflammation termination (dendritic cells, mast cells and TH2 cells), promotes clearance of inflammatory cells, efferocytosis and resolution of inflammation175,176. This observation might explain some of the adverse effects of COX inhibitors. Over the past 15–20 years, it has been noted that COX inhibitors can increase the long-term risk of cardiovascular disease, including coronary atherothrombosis and HF and decrease endogenous cardioprotection against arrhythmogenic substrates and oxidative injury177. Moreover, COX inhibition increases systolic blood pressure and exacerbates the hypertensive effects of angiotensin II in animal models178,179. Combined medication involving COX inhibitors and angiotensin II inhibition revealed that COX inhibitors attenuate the vasodilatory properties of angiotensin-converting enzyme inhibitors and type 1 angiotensin II receptor blockers180. Perhaps because of these mixed effects, non-steroidal anti-inflammatory drugs do not seem to be effective for the treatment of AF181,182.
Aspirin
Aspirin is a classic non-steroidal anti-inflammatory agent that is widely used in cardiovascular therapeutics as an antiplatelet agent. Although aspirin-induced acetylation of COX2 blocks the production of pro-inflammatory lipid mediators (prostaglandins and leukotrienes) from arachidonic acid, acetylated COX2 can also metabolize EPA and DHA to produce a class of resolvin homologues called aspirin-triggered resolvins148. A study in mice showed that aspirin inhibits the release of ROS, thereby restraining NLRP3 inflammasome assembly and activation in the endothelium of coronary arteries183. However, a prospective, 6-year study involving 23,480 male patients found no association between chronic use of aspirin and AF incidence184.
Glucocorticoids
Glucocorticoids have well-known, broad-spectrum anti-inflammatory effects, promoting inflammation resolution via non-phlogistic monocyte polarization into M2 macrophages, stimulation of macrophage phagocytosis of apoptotic neutrophils and activation of pro-resolution processes185–188. Conversely, glucocorticoids increase NLRP3 mRNA and protein expression in human macrophages189. Glucocorticoids also sensitize cells to extracellular ATP and increase ATP-mediated release of pro-inflammatory molecules, including mature IL-1β, IL-6 and TNF189. Glucocorticoid use increases the risk of systemic hypertension, fluid retention, atherosclerotic vascular disease and atrial arrhythmias in a dose-dependent and duration-dependent manner190–192.
Selective NLRP3 inflammasome inhibitors
Oridonin, which is extracted from the Chinese medicinal herb Rabdosia rubescens, inhibits NLRP3 inflammasome assembly and NLRP3 inflammasome-induced production of IL-1β and activation of the pro-inflammatory transcription factor NF-κB193. MCC950, a diarylsulfonylurea-containing compound, selectively inhibits NLRP3 inflammasome activity by blocking ASC oligomerization and IL-1β secretion194,195. However, the commercial development of MCC950 was discontinued because of hepatotoxicity. In a mouse model of acute MI, oridonin and MCC950 treatment reduce cardiac fibrosis as well as the protein levels of NLRP3, IL-1β and IL-18 (ref.196). In a mouse model of MI, knockdown of Camk2n1, which encodes an endogenous inhibitor of CaMKII, led to increased CaMKII function as well as increased cardiac fibrosis and ventricular fibrillation susceptibility, supporting the notion that the CaMKII–p38–JNK–NLRP3 inflammasome pathway might be a potential therapeutic target197.
IL-1 inhibitors
IL-1β is among the most studied members of the IL-1 family. Evidence of the pro-inflammatory and cardiotoxic effects of IL-1β has led to the development of inhibitors of IL-1β signalling102. In the AIRTRIP trial198, anakinra, an IL-1R blocker that efficiently antagonizes the effects of IL-1α and IL-1β, administered for 2 months reduced the recurrence of pericarditis in patients with recurrent corticosteroid-dependent pericarditis. In the VCU-ART study199 in patients with ST-segment elevation MI, 14 days of treatment with anakinra improved ventricular function and decreased the risk of HF. Prevention of hospitalization for HF with anakinra treatment was also observed in the REDHART trial200. In the RHAPSODY trial201, treatment with rilonacept, an IL-1α and IL-1β cytokine trap, decreased the risk of pericarditis in patients with pericarditis resistant to conventional therapy. In the CANTOS trial202, therapy with canakinumab, a specific monoclonal antibody against IL-1β, reduced HF hospitalization in patients with previous MI and high-sensitivity C-reactive protein (hsCRP) levels of ≥2 mg/l compared with placebo.
Specialized pro-resolving mediators
In a study involving 27 patients with HF and 23 healthy individuals, plasma RvD1 levels, GPR32 levels in T cells and leukocyte 15-LOX activity were lower in patients with HF, suggesting that altered pro-resolution signalling might be involved in HF pathophysiology167. In a rat model of right-sided heart disease, RvD1 treatment decreased atrial expression of NLRP3 inflammasome-related genes (Asc, Casp1, Il1b and Nlrp3) and prevented the upregulation of genes encoding inflammatory cytokines (Ccl2, Cxcl1, Cxcl2 and Il6), together with reduced atrial fibrosis and AF susceptibility137. RvD1 also upregulates the expression of ChemR23, the receptor of the specialized pro-resolving mediators RvE1 and RvE2 (ref.137). In patients with aortic valve stenosis, the most common valvulopathy, RvE1 and RvD3 levels were lower whereas the LTB4 level was increased in calcified tricuspid aortic valve tissue168. Promotion of RvE1–ChemR23 signalling was associated with increased M2 macrophage recruitment and prevention of valvular calcification in animal models168. In patients without overt atherosclerosis, salivary levels of RvD1 were lower in those patients with greater intima–media thickness (which indicates early atherosclerosis)203. Patients with acutely symptomatic carotid atherosclerotic plaque rupture had lower circulating levels of RvD1 than patients with asymptomatic high-grade carotid stenosis204. Administration of specialized pro-resolving mediators (including RvE1, RvD1, RvD2 or maresin 1) promoting the increase in the ratio of specialized pro-resolving mediators to LTB4 is associated with decreased progression of atherosclerosis and activation of efferocytosis in mouse models205–207. RvE1 and RvD1 have been evaluated in mouse models of MI, revealing that specialized pro-resolving mediators promote inhibition of MAPK and NF-κB signalling in the infarcted myocardium to limit inflammation, reduce myocardial apoptosis and increase M2 macrophage recruitment208–210.
NLRP3 inhibitors currently in clinical trials
Several small molecules (<20 carbon–carbon bonds) have been developed that inhibit the NLRP3 inflammasome211 (Table 3). Inzomelid (MCC7840) inhibits NLRP3 inflammasome with half maximal inhibitory concentrations of <100 nmol/l211. Inzomelid was being tested in a phase I clinical trial in patients with Parkinson disease212 and in a phase II clinical trial in patients with cryopyrin-associated periodic syndromes213, but the studies have been withdrawn by the sponsor. In cardiovascular disease and arthritis, somalix has shown selective inhibition of the NLRP3 inflammasome, together with acceptable safety and tolerability in a phase I clinical trial in healthy individuals in Ireland211. In patients with HF with reduced LV ejection fraction, therapy with anakinra reduced circulating levels of CRP and IL-1 and improved cardiorespiratory function and quality of life compared with placebo214. Dapansutrile, also known as OLT1177, specifically targets NLRP3 inflammasome-induced release of IL-1β and IL-18 to prevent inflammation215. A phase Ib, randomized, double-blind, dose-escalation study of dapansutrile in patients with systolic HF found that treatment with dapansutrile for 14 days is safe, well tolerated and, at the highest dose of 2,000 mg, improves LV ejection fraction and exercise time compared with baseline216. Dapansutrile is currently being tested in phase II clinical trials in patients with Schnitzler syndrome217 and in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced cytokine storm218.
Tranilast (also known as rizaben), a well-known anti-allergic medication that has shown efficacy for the treatment of tissue fibrosis, is an antagonist of NLRP3 oligomerization via a specific interaction with the NACHT domain of NLRP3 (ref.219). The anti-inflammatory effects of tranilast are currently being tested in a phase I clinical trial in patients with mucinoses220 and in a phase II clinical trial in patients with cryopyrin-associated periodic syndromes221. Pirfenidone, which has anti-pulmonary fibrosis properties, inhibits NLRP3 inflammasome activation and NLRP3 inflammasome-induced fibrosis in mice222,223, and potential translational application in humans has been suggested223. Pirfenidone is currently being tested in several clinical trials in fibrosis-associated disorders, including pulmonary fibrosis218. Canakinumab blocks NLRP3 activity by inhibiting IL-1β. In the CANTOS trial224, canakinumab therapy decreased cardiovascular events compared with placebo in patients with previous MI and hsCRP levels of ≥2 mg/l. Canakinumab is currently being tested in a phase II clinical trial evaluating the effect of IL-1β inhibition on inflammation and the risk of cardiovascular disease217. Belnacasan (also known as VX-765), a specific inhibitor of caspase 1, prevents NLRP3 inflammasome-induced inflammation after ischaemia–reperfusion in mice225. Belnacasan is currently being tested in a phase II clinical trial aiming to decrease the inflammatory response in patients with SARS-CoV-2 infection226.
None of the clinically applicable specific NLRP3 inhibiting agents is currently under evaluation for AF, but several of them should be studied in both preclinical and clinical models in the future.
Colchicine, an anti-inflammatory medication that blocks NLRP3 oligomerization227, is commonly used to treat gout and has been shown to reduce the incidence of MI, stroke and coronary revascularization compared with placebo when administered for secondary prevention in patients with coronary artery disease228. On the basis of the anti-inflammatory effects of colchicine and the evidence for the importance of NLRP3 inflammasome-related inflammatory signalling in AF, colchicine has been suggested as a potential AF-preventing agent, with particular interest for contexts involving inflammation, such as postoperative AF229. However, available data suggest no effects of colchicine therapy on AF occurrence230,231. Large, randomized, double-blind trials currently underway, such as the COP-AF study232, will provide insights into this question.
Implications and future directions
Implications of the role of cardiomyocyte inflammatory signalling in AF
The observation that cells such as cardiomyocytes, fibroblasts and adipocytes, not previously considered to induce or maintain inflammation, have the biochemical machinery necessary to engage inflammatory signalling is interesting and potentially important. The observation that inflammatory signalling is increased in cardiomyocytes of patient populations with AF substrates and risk factors, and that cardiomyocyte-specific overexpression of active pro-inflammatory proteins creates the conditions for AF in animal models, points to the mechanistic relevance of this system in clinical AF.
These findings are of potential pathophysiological importance. Patients with so-called ‘secondary’ AF owing to acute illness, such as sepsis, acute infectious disease, recent surgery or MI, are likely to have recurrent AF in the future233. This observation has been largely unexplained. However, if atrial cardiomyocyte inflammatory signalling with consequent structural and/or electrophysiological changes is a common underlying theme, this phenomenon can be explained by the notion that the atrial substrate must reach a critical threshold to manifest AF. This critical threshold can be achieved by gradual progression to the critical state of substrate progression, but if intercurrent, acute pro-inflammatory contexts provide a transient increase in the atrial substrate, AF might manifest in a transient way before the underlying substrate reaches the threshold for arrhythmogenesis on its own. Following the resolution of the acute stress, the substrate will generally return to the previous subthreshold level, only to reach the threshold for AF development by gradual subsequent progression. This interesting possibility requires verification in future studies.
Extensive, but in some ways circumstantial, evidence for a role of inflammation in AF was available before the observation of the contribution of cardiomyocyte inflammatory signalling to AF4. However, the manifestations of atrial inflammation have been mild, and conventional anti-inflammatory drugs have had little or no efficacy in clinical trials in AF. Part of the explanation for the lack of efficacy of traditional anti-inflammatory agents in AF despite a role of atrial inflammation in this condition might be the localized and atypical nature of cardiomyocyte-derived inflammatory signalling and the lack of direct clinical proof of the anti-AF efficacy of selective NLRP3 inhibitors. Much more work is needed to characterize this phenomenon, identify its causes and clarify its pathophysiological and therapeutic relevance. The therapeutic implications relate to better understanding of the natural history of AF and its mechanisms, as well as specific therapeutic targets, as discussed above and tabulated in Table 3.
Key areas for future research
The study of inflammatory signalling in atrial cardiomyocytes is a rapidly evolving area, and many important questions remain to be addressed (Box 1). In the basic and translational arena, we need to understand the factors that prime and trigger (activate) the cardiomyocyte NLRP3 inflammasome. Do cardiac diseases and risk factors release DAMPs and/or other danger signals in atrial tissue? Does increased atrial pressure and/or volume affect cardiomyocyte inflammatory signalling? What is the precise role of atrial rate? What is the role of oxidative stress or other signals related to neurohormones (such as adrenergic neurotransmitters and angiotensin II)?
The role of NLRP3 inflammasome signalling in non-myocyte cardiac cells needs to be examined and, if validated, its effects on atrial electrophysiology, structure and function examined. We also need to know whether these systems are altered in different forms of AF in animal models and in patients. Cardiomyocyte NLRP3 inflammasome signalling has the capacity to release pro-inflammatory cytokines. It would be important to know whether such cytokine release can exert paracrine functions on neighbouring cells and whether it can result in more classic inflammatory cell recruitment and activation (Fig. 4). To identify which non-myocyte cell type is most relevant in different cohorts of patients or animal models with AF vulnerability, unbiased omics studies at the single-cell level could be performed. Single-cell and single-nucleus RNA sequencing or single-cell proteomics studies can help to identify discrete cell subtypes in atrial tissue from animal models and patients with AF234,235. Bioinformatic analysis can not only identify the differentially expressed genes or proteins within each cluster of cells, but also reveal which cell types have the most prominent changes at the transcriptional or protein level. Furthermore, analysis of cell-to-cell interactions can uncover networks that drive the development and progression of cardiac diseases236.
Fig. 4. Cardiomyocyte–immune cell interactions promote and maintain atrial fibrillation.
Dynamic interactions between cardiac cells and immune cells create a pro-inflammatory substrate for the promotion and maintenance of atrial fibrillation. Atrial fibrillation-promoting risk factors activate various mediators that signal through pattern-recognition receptors (PRRs) on cardiomyocytes to activate the cardiomyocyte NLRP3 inflammasome, leading to the release of IL-1β and IL-18 and activation of signalling pathways dependent on Ca2+/calmodulin-dependent protein kinase II (CaMKII), reactive oxygen species (ROS) and nuclear factor-κB (NF-κB). Subsequent activation of IL-1 receptor type 1 (IL-1R1) on macrophages promotes feedforward amplification of inflammatory signalling, whereas IL-1R1-mediated stimulation of fibroblasts causes extracellular matrix remodelling. Together, these processes result in cardiomyocyte Ca2+ handling abnormalities, including ryanodine receptor 2 (RYR2) channel remodelling, delayed afterdepolarizations, and triggered action potentials and ectopic activity, as well as re-entry-promoting effective refractory period (ERP) shortening and fibrotic structural remodelling, thereby inducing the principal mechanisms responsible for atrial fibrillation. AngII, angiotensin II; ANP, atrial natriuretic peptide; DAMPs, damage-associated molecular patterns; LPS, lipopolysaccharide; GSDMD, gasdermin D; GSDMD-NT, gasdermin D amino terminus; NLRP3, NACHT-, LRR- and pyrin domain-containing 3; Thr, thrombin; TNF, tumour necrosis factor.
The systemic mechanisms leading to cardiac activation of inflammatory signalling and of the NLRP3 system in particular are unknown. Ageing-related increases in levels of circulating LPS derived from gut microbiota dysbiosis, together with increased glucose intolerance, are potential primers and triggers of the atrial NLRP3 inflammasome system237. Hepatocyte-derived angiotensinogen can be taken up by cardiac fibroblasts and can activate ventricular NLRP3 inflammasome via an angiotensin II-independent pathway, thereby causing IL-1β-mediated suppression of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2A in cardiomyocytes238. Whether these mechanisms contribute to the activation of NLRP3 inflammasome in atrial cells of patients with AF is unknown and requires further study. The precise mechanisms of NLRP3 priming and triggering in heart cells need further assessment and potential atrial–ventricular differences in NLRP3 activation should be defined. For instance, at the ventricular level, IL-1β seems to be primarily released from cardiac fibroblasts rather than cardiomyocytes102,105,106. Conversely, GSDMD seems to be primarily synthesized and active in ventricular cardiomyocytes but not in fibroblasts, at least in mice239. GSDMD-mediated pyroptosis of ventricular cardiomyocytes is a key event during myocardial ischaemia–reperfusion injury239. Therefore, it seems that there is separation of labour between ventricular fibroblasts (IL-1β release) and cardiomyocytes (GSDMD-mediated pyroptosis) in the response to ischaemia–reperfusion injury240. Whether these principles apply to atrial cells is unknown and requires detailed investigation in future work.
The relative contributions of IL-1α, IL-1β and IL-18 to inflammatory signalling and cardiac dysfunction are unknown and should be studied in future work. Which atrial cells are the primary cell type for the synthesis and release of IL-1α, IL-1β and IL-18 remains to be determined.
The properties of inflammation resolution-promoting systems in cardiomyocytes and other cardiac cell types are very poorly understood. Further information is essential for a fuller appreciation of the role of inflammation resolution in the heart. We also need to understand whether the properties of these systems are affected by AF or AF-promoting conditions. The interactions between NLRP3 inflammasome signalling and resolution-promoting pathways need to be understood, and how activation of one system affects the other needs to be appreciated. Crucially, we need to understand whether atrial cardiomyocyte inflammatory signalling has specific properties that could be used to develop cardiac-specific or even atrial-specific anti-inflammatory interventions.
We need to understand how the properties of inflammatory signalling activation differ at various stages of AF. Does NLRP3 inflammasome activation differ in patients with paroxysmal, persistent or long-standing persistent AF? If there are differences, are parallel changes seen in relevant animal models? Similarly, does pro-resolution signalling differ at corresponding phases? Can blood biomarkers be identified that could be used to identify and quantitatively evaluate cardiomyocyte NLRP3 system activation in humans?
The ultimate key questions relate to therapeutic implications. Can we use this evolving basic science knowledge to develop compounds that target atrial cardiomyocyte inflammatory signalling with sufficient safety for long-term use in patients with AF and those at increased risk of AF, and thereby favourably alter the natural history of the condition? n-3 PUFAs are precursors of resolvins, but have not generally been found to be protective in AF, with some evidence for benefit, many studies suggesting no effect and other data suggesting adverse effects on AF occurrence228. Might the large number of bioactive products derived from n-3 PUFAs in humans be the explanation, with some being pro-inflammatory and others, such as resolvins, being anti-inflammatory? Could this explain the variable clinical results? What is the safety and toxicity profile of resolution-promoting compounds in humans? Can they be used in a practical way to prevent the promotion of the AF substrate and prevent recurrences after AF ablation, or even be used in primary prevention in patients at high risk of AF?
Box 1 Main areas to be addressed in future research.
Basic and translational studies
What are the factors leading to cardiomyocyte inflammatory signalling activation in atrial fibrillation and atrial fibrillation-promoting pathologies?
Do cardiac cells other than cardiomyocytes (such as adipocytes, fibroblasts and neural cells) have intact inflammasome systems and are they altered in atrial fibrillation?
Does activation of cardiomyocyte inflammatory signalling recruit and activate macrophages and other inflammation-associated leukocytes in the heart?
What are the characteristics of inflammation resolution signalling in cardiomyocytes and other cardiac cells and how is this system altered in atrial fibrillation?
Are there differences in pro-resolution mediators and signalling pathways between paroxysmal and persistent atrial fibrillation?
How do the properties of inflammatory signalling activation differ at various stages of atrial fibrillation?
How do resolution-promoting compounds affect NLRP3 inflammasome priming and triggering in animal models of atrial fibrillation?
Are there specific properties of atrial cardiomyocyte inflammatory signalling that could be used to develop cardiac-specific or even atrial-specific anti-inflammatory interventions?
Clinical investigation
What is the safety and toxicity profile of resolution-promoting compounds in humans?
Can compounds be developed that target atrial cardiomyocyte inflammatory signalling with sufficient safety for long-term use in patients with atrial fibrillation and individuals with increased risk of atrial fibrillation?
Are there biomarkers that can reliably identify and quantify cardiomyocyte NLRP3 inflammasome activation in humans?
Conclusions
The existence of inflammatory signalling in cardiomyocytes and its pathophysiological importance in AF have been recognized only for the past 5 years. This knowledge has great potential importance, both for understanding AF pathophysiology and for improving the treatment of AF. A great deal of additional work is needed before the relevance of this new knowledge can be fully evaluated, but careful attention to this promising area of research is warranted.
Acknowledgements
The authors thank T. Poppenborg (Institute of Pharmacology, University Duisburg-Essen, Germany) for help with preparing the figures for initial submission and A. Saljic (Institute of Pharmacology, University Duisburg-Essen, Germany) for critical review of the tables. The authors are supported by funding from the National Institutes of Health (R01HL136389, R01HL131517, R01HL089598 and R01HL163277 to D.D.; R01HL136389, R01HL147108 and R01HL163277 to N.L.), the German Research Foundation (DFG, Do 769/4-1 to D.D.), the European Union (large-scale integrative project MAESTRIA, no. 965286 to D.D.), the Netherlands Organization for Scientific Research (NWO/ZonMW Vidi 09150171910029 to J.H.), the Montreal Heart Institute Foundation (4800 to R.H.), the Canada Foundation for Innovation (42228 to S.N. and R.H.), the Canadian Institutes of Health Research (148401 to S.N.), and the AHA (Established Investigator Award 936111 to N.L).
Author contributions
All the authors contributed substantially to all aspects of the article.
Peer review
Peer review information
Nature Reviews Cardiology thanks Antonio Abbate, Shih-Ann Chen and David van Wagoner for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dobromir Dobrev, Stanley Nattel.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Heijman J, Guichard JB, Dobrev D, Nattel S. Translational challenges in atrial fibrillation. Circ. Res. 2018;122:752–773. doi: 10.1161/CIRCRESAHA.117.311081. [DOI] [PubMed] [Google Scholar]
- 3.Chung MK, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 4.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Zhao W. NLRP3 inflammasome – a key player in antiviral responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur. Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208. [DOI] [PubMed] [Google Scholar]
- 8.Ahlehoff O, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish nationwide cohort study. Eur. Heart J. 2012;33:2054–2064. doi: 10.1093/eurheartj/ehr285. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen SL, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. 2014;16:477–484. doi: 10.1093/europace/eut312. [DOI] [PubMed] [Google Scholar]
- 10.Koyfman L, et al. Epidemiology of new-onset paroxysmal atrial fibrillation in the general intensive care unit population and after discharge from ICU. A retrospective epidemiological study. Anaesthesiol. Intensive Ther. 2015;47:309–314. doi: 10.5603/AIT.a2015.0040. [DOI] [PubMed] [Google Scholar]
- 11.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkey AJ, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am. Heart J. 2013;165:949–955.e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit. Care. 2014;18:688. doi: 10.1186/s13054-014-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein Klouwenberg PM, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. a cohort study. Am. J. Respir. Crit. Care Med. 2017;195:205–211. doi: 10.1164/rccm.201603-0618OC. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CA, et al. New-onset atrial fibrillation-related ischemic stroke occurring after hospital discharge in septicemia survivors. QJM. 2017;110:453–457. doi: 10.1093/qjmed/hcx025. [DOI] [PubMed] [Google Scholar]
- 17.Bosch NA, et al. New-onset atrial fibrillation as a sepsis-defining organ failure. Ann. Am. Thorac. Soc. 2019;16:1332–1334. doi: 10.1513/AnnalsATS.201902-176RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernando SM, et al. New-onset atrial fibrillation and associated outcomes and resource use among critically ill adults – a multicenter retrospective cohort study. Crit. Care. 2020;24:15. doi: 10.1186/s13054-020-2730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko D, et al. Secondary precipitants of atrial fibrillation and anticoagulation therapy. J. Am. Heart Assoc. 2021;10:e021746. doi: 10.1161/JAHA.121.021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long Y, et al. Early coagulation disorder is associated with an increased risk of atrial fibrillation in septic patients. Front. Cardiovasc. Med. 2021;8:724942. doi: 10.3389/fcvm.2021.724942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA. 1976;235:39–41. doi: 10.1001/jama.1976.03260270025020. [DOI] [PubMed] [Google Scholar]
- 22.Nagahama Y, et al. The role of infarction-associated pericarditis on the occurrence of atrial fibrillation. Eur. Heart J. 1998;19:287–292. doi: 10.1053/euhj.1997.0744. [DOI] [PubMed] [Google Scholar]
- 23.Ristić AD, et al. Arrhythmias in acute pericarditis. An endomyocardial biopsy study. Herz. 2000;25:729–733. doi: 10.1007/PL00001990. [DOI] [PubMed] [Google Scholar]
- 24.Imazio M, et al. Incidence and prognostic significance of new onset atrial fibrillation/flutter in acute pericarditis. Heart. 2015;101:1463–1467. doi: 10.1136/heartjnl-2014-307398. [DOI] [PubMed] [Google Scholar]
- 25.Adegbala O, et al. Predictors, burden, and the impact of arrhythmia on patients admitted for acute myocarditis. Am. J. Cardiol. 2019;123:139–144. doi: 10.1016/j.amjcard.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Frustaci A, et al. Cardiac biopsy in patients with “primary” atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest. 1991;100:303–306. doi: 10.1378/chest.100.2.303. [DOI] [PubMed] [Google Scholar]
- 27.Frustaci A, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.CIR.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 28.Fuenmayor AJ, et al. Results of electrophysiologic studies in patients with acute chagasic myocarditis. Clin. Cardiol. 1997;20:1021–1024. doi: 10.1002/clc.4960201209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgera T, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am. Heart J. 1992;124:455–467. doi: 10.1016/0002-8703(92)90613-Z. [DOI] [PubMed] [Google Scholar]
- 30.Deluigi CC, et al. ECG findings in comparison to cardiovascular MR imaging in viral myocarditis. Int. J. Cardiol. 2013;165:100–106. doi: 10.1016/j.ijcard.2011.07.090. [DOI] [PubMed] [Google Scholar]
- 31.Coromilas EJ, et al. Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 2021;14:e009458. doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawalko M, Kaplon-Cieslicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int. J. Cardiol. Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Z, et al. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic. Res. Cardiol. 2016;111:63. doi: 10.1007/s00395-016-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang J, et al. Ferroportin-mediated ferroptosis involved in new-onset atrial fibrillation with LPS-induced endotoxemia. Eur. J. Pharmacol. 2021;913:174622. doi: 10.1016/j.ejphar.2021.174622. [DOI] [PubMed] [Google Scholar]
- 35.Aoki Y, et al. Role of ion channels in sepsis-induced atrial tachyarrhythmias in guinea pigs. Br. J. Pharmacol. 2012;166:390–400. doi: 10.1111/j.1476-5381.2011.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ. Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ. Res. 2020;127:51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc. Res. 2004;62:105–111. doi: 10.1016/j.cardiores.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Ryu K, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1231–H1241. doi: 10.1152/ajpheart.00607.2006. [DOI] [PubMed] [Google Scholar]
- 40.Huang Z, et al. Signal transducer and activator of transcription 3/microRNA-21 feedback loop contributes to atrial fibrillation by promoting atrial fibrosis in a rat sterile pericarditis model. Circ. Arrhythm. Electrophysiol. 2016 doi: 10.1161/CIRCEP.115.003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Q, et al. Colchicine prevents atrial fibrillation promotion by inhibiting IL-1β-induced IL-6 release and atrial fibrosis in the rat sterile pericarditis model. Biomed. Pharmacother. 2020;129:110384. doi: 10.1016/j.biopha.2020.110384. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Role of inflammation in the initiation and maintenance of atrial fibrillation and the protective effect of atorvastatin in a goat model of aseptic pericarditis. Mol. Med. Rep. 2015;11:2615–2623. doi: 10.3892/mmr.2014.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, et al. n-3 polyunsaturated fatty acids prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Int. J. Cardiol. 2011;153:14–20. doi: 10.1016/j.ijcard.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Chang CJ, et al. Histone deacetylase inhibition attenuates atrial arrhythmogenesis in sterile pericarditis. Transl. Res. 2018;200:54–64. doi: 10.1016/j.trsl.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Liao J, et al. TRPV4 blockade suppresses atrial fibrillation in sterile pericarditis rats. JCI Insight. 2020 doi: 10.1172/jci.insight.137528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao J, et al. Interleukin-6-mediated-Ca2+ handling abnormalities contributes to atrial fibrillation in sterile pericarditis rats. Front. Immunol. 2021;12:758157. doi: 10.3389/fimmu.2021.758157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin FJ, et al. Toll-like receptor 4 activation modulates pericardium-myocardium interactions in lipopolysaccharide-induced atrial arrhythmogenesis. Europace. 2021;23:1837–1846. doi: 10.1093/europace/euab073. [DOI] [PubMed] [Google Scholar]
- 48.Schwartzman D, et al. A plasma-based, amiodarone-impregnated material decreases susceptibility to atrial fibrillation in a post-cardiac surgery model. Innovations. 2016;11:59–63. doi: 10.1097/imi.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 49.Fu XX, et al. Interleukin-17A contributes to the development of post-operative atrial fibrillation by regulating inflammation and fibrosis in rats with sterile pericarditis. Int. J. Mol. Med. 2015;36:83–92. doi: 10.3892/ijmm.2015.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tubeeckx MRL, et al. Sterile pericarditis in Aachener minipigs as a model for atrial myopathy and atrial fibrillation. J. Vis. Exp. 2021 doi: 10.3791/63094. [DOI] [PubMed] [Google Scholar]
- 51.Hoyano M, et al. Inducibility of atrial fibrillation depends not on inflammation but on atrial structural remodeling in rat experimental autoimmune myocarditis. Cardiovasc. Pathol. 2010;19:e149–e157. doi: 10.1016/j.carpath.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Turner NA, et al. Interleukin-1α stimulates proinflammatory cytokine expression in human cardiac myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1117–H1127. doi: 10.1152/ajpheart.00372.2009. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, et al. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin. Transl. Med. 2020;10:91–106. doi: 10.1002/ctm2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L, et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell Mol. Med. 2015;19:2728–2740. doi: 10.1111/jcmm.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 2022;118:372–385. doi: 10.1093/cvr/cvab010. [DOI] [PubMed] [Google Scholar]
- 56.Scott L, Jr., Li N, Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 2019;287:195–200. doi: 10.1016/j.ijcard.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latz E. The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 59.Groslambert M, Py BF. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 2018;11:359–374. doi: 10.2147/JIR.S141220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Misawa T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 61.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 2009;182:3173–3182. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baroja-Mazo A, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 64.Dick MS, Sborgi L, Ruhl S, Hiller S, Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 2016;7:11929. doi: 10.1038/ncomms11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauernfeind FG, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suetomi T, et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation. 2018;138:2530–2544. doi: 10.1161/CIRCULATIONAHA.118.034621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fender AC, et al. Thrombin receptor PAR4 drives canonical NLRP3 inflammasome signaling in the heart. Basic. Res. Cardiol. 2020;115:10. doi: 10.1007/s00395-019-0771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 69.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin KM, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl Acad. Sci. USA. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schroder K, et al. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology. 2012;217:1325–1329. doi: 10.1016/j.imbio.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 72.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 73.Gong T, Yang Y, Jin T, Jiang W, Zhou R. Orchestration of NLRP3 inflammasome activation by ion fluxes. Trends Immunol. 2018;39:393–406. doi: 10.1016/j.it.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 2014;289:9158–9171. doi: 10.1074/jbc.M113.531202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magupalli VG, et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science. 2020 doi: 10.1126/science.aas8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharif H, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murakami T, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tseng HH, Vong CT, Kwan YW, Lee SM, Hoi MP. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci. Rep. 2016;6:35016. doi: 10.1038/srep35016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Compan V, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35:253–261. doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menu P, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iyer SS, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaidt MM, Hornung V. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J. 2016;35:2167–2169. doi: 10.15252/embj.201695415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munoz-Planillo R, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burnstock G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016;12:59–67. doi: 10.1007/s11302-015-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaidt MM, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 90.Netea MG, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 93.Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: toward identification of the effector molecules. Science. 2016;352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 96.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J. Leukoc. Biol. 2013;93:329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aglietti RA, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 100.Karmakar M, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun. 2020;11:2212. doi: 10.1038/s41467-020-16043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 102.Abbate A, et al. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silvis MJM, et al. Immunomodulation of the NLRP3 inflammasome in atherosclerosis, coronary artery disease, and acute myocardial infarction. J. Cardiovasc. Transl. Res. 2021;14:23–34. doi: 10.1007/s12265-020-10049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mezzaroma E, Abbate A, Toldo S. The inflammasome in heart failure. Curr. Opin. Physiol. 2021;19:105–112. doi: 10.1016/j.cophys.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mezzaroma E, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawaguchi M, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 108.Sandanger O, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 109.Deftereos S, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation. 2015;132:1395–1403. doi: 10.1161/CIRCULATIONAHA.115.017611. [DOI] [PubMed] [Google Scholar]
- 110.Heijman J, et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ. Res. 2020;127:1036–1055. doi: 10.1161/CIRCRESAHA.120.316710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Byrne NJ, et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ. Heart Fail. 2020;13:e006277. doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 112.Mehta S, Vijayvergiya R, Dhawan V. Activation of NLRP3 inflammasome assembly is associated with smoking status of patients with coronary artery disease. Int. Immunopharmacol. 2020;87:106820. doi: 10.1016/j.intimp.2020.106820. [DOI] [PubMed] [Google Scholar]
- 113.Yao C, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiao H, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur. Heart J. 2018;39:60–69. doi: 10.1093/eurheartj/ehx261. [DOI] [PubMed] [Google Scholar]
- 115.Zhuang YT, et al. IL-6 induced lncRNA MALAT1 enhances TNF-α expression in LPS-induced septic cardiomyocytes via activation of SAA3. Eur. Rev. Med. Pharmacol. Sci. 2017;21:302–309. [PubMed] [Google Scholar]
- 116.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J. Biol. Chem. 2005;280:4553–4567. doi: 10.1074/jbc.M411787200. [DOI] [PubMed] [Google Scholar]
- 117.Aschar-Sobbi R, et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat. Commun. 2015;6:6018. doi: 10.1038/ncomms7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lakin R, et al. Inhibition of soluble TNFα prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J. Mol. Cell Cardiol. 2019;129:165–173. doi: 10.1016/j.yjmcc.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 119.Scott L, Jr, et al. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc. Res. 2021;117:1746–1759. doi: 10.1093/cvr/cvab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saba S, et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-α. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1456–H1467. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 121.Sawaya SE, et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1561–H1567. doi: 10.1152/ajpheart.00285.2006. [DOI] [PubMed] [Google Scholar]
- 122.Lee SH, et al. Tumor necrosis factor-α alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80:1806–1815. doi: 10.1016/j.lfs.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 123.He G, et al. Increased M1 macrophages infiltration is associated with thrombogenesis in rheumatic mitral stenosis patients with atrial fibrillation. PLoS ONE. 2016;11:e0149910. doi: 10.1371/journal.pone.0149910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lazzerini PE, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J. Am. Heart Assoc. 2019;8:e011006. doi: 10.1161/JAHA.118.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li N, Brundel B. Inflammasomes and proteostasis novel molecular mechanisms associated with atrial fibrillation. Circ. Res. 2020;127:73–90. doi: 10.1161/CIRCRESAHA.119.316364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Willeford A, et al. CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight. 2018 doi: 10.1172/jci.insight.97054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ling H, et al. Ca2+/calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ. Res. 2013;112:935–944. doi: 10.1161/CIRCRESAHA.112.276915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yao Y, et al. Targeting CaMKII-δ9 ameliorates cardiac ischemia/reperfusion injury by inhibiting myocardial inflammation. Circ. Res. 2022 doi: 10.1161/CIRCRESAHA.121.319478. [DOI] [PubMed] [Google Scholar]
- 129.Joiner ML, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dobrev D, Dudley SC. Oxidative stress: a bystander or a causal contributor to atrial remodelling and fibrillation. Cardiovasc. Res. 2021;117:2291–2293. doi: 10.1093/cvr/cvab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gawalko M, et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 133.Yu L, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 2018;255:92–98. doi: 10.1016/j.ijcard.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 134.Wu X, et al. Role of NLRP3-inflammasome/caspase-1/galectin-3 pathway on atrial remodeling in diabetic rabbits. J. Cardiovasc. Transl. Res. 2020;13:731–740. doi: 10.1007/s12265-020-09965-8. [DOI] [PubMed] [Google Scholar]
- 135.Hiram R, et al. Right atrial mechanisms of atrial fibrillation in a rat model of right heart disease. J. Am. Coll. Cardiol. 2019;74:1332–1347. doi: 10.1016/j.jacc.2019.06.066. [DOI] [PubMed] [Google Scholar]
- 136.Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J. Cardiol. 2017;69:699–705. doi: 10.1016/j.jjcc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 137.Hiram R, et al. The inflammation-resolution promoting molecule resolvin-D1 prevents atrial proarrhythmic remodelling in experimental right heart disease. Cardiovasc. Res. 2021;117:1776–1789. doi: 10.1093/cvr/cvaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang W, et al. Cardiac fibroblasts contribute to myocardial dysfunction in mice with sepsis: the role of NLRP3 inflammasome activation. PLoS ONE. 2014;9:e107639. doi: 10.1371/journal.pone.0107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Turner NA, et al. Mechanism of TNFα-induced IL-1α, IL-1β and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc. Res. 2007;76:81–90. doi: 10.1016/j.cardiores.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 140.van Nieuwenhoven FA, Hemmings KE, Porter KE, Turner NA. Combined effects of interleukin-1α and transforming growth factor-β1 on modulation of human cardiac fibroblast function. Matrix Biol. 2013;32:399–406. doi: 10.1016/j.matbio.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 141.Cheng C, et al. Mutation in NPPA causes atrial fibrillation by activating inflammation and cardiac fibrosis in a knock-in rat model. FASEB J. 2019;33:8878–8891. doi: 10.1096/fj.201802455RRR. [DOI] [PubMed] [Google Scholar]
- 142.Gawalko M, et al. Adiposity-associated atrial fibrillation: molecular determinants, mechanisms and clinical significance. Cardiovasc Res. 2022 doi: 10.1093/cvr/cvac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wong CX, et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2016 doi: 10.1161/CIRCEP.116.004378. [DOI] [PubMed] [Google Scholar]
- 144.Mazurek T, et al. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am. J. Cardiol. 2014;113:1505–1508. doi: 10.1016/j.amjcard.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Shaihov-Teper O, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. 2021;143:2475–2493. doi: 10.1161/CIRCULATIONAHA.120.052009. [DOI] [PubMed] [Google Scholar]
- 146.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. 2013;63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weissmann G, Smolen JE, Korchak HM. Release of inflammatory mediators from stimulated neutrophils. N. Engl. J. Med. 1980;303:27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- 148.Serhan CN, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Houck, J. C. Chemical Messengers of the Inflammatory Process (Elsevier/North-Holland, 1979).
- 150.Samuelsson B. From studies of biochemical mechanism to novel biological mediators: prostaglandin endoperoxides, thromboxanes, and leukotrienes. Nobel Lecture, 8 December 1982. Biosci. Rep. 1983;3:791–813. doi: 10.1007/BF01133779. [DOI] [PubMed] [Google Scholar]
- 151.Serhan CN, et al. The atlas of inflammation resolution (AIR) Mol. Asp. Med. 2020;74:100894. doi: 10.1016/j.mam.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/S0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 153.Radmark O, Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. J. Lipid Res. 2009;50:S40–S45. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 155.Vestweber D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 156.Lee S, et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am. J. Respir. Crit. Care Med. 2017;196:713–726. doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh NK, Rao GN. Emerging role of 12/15-lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019;73:28–45. doi: 10.1016/j.plipres.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019;31:559–567. doi: 10.1093/intimm/dxz001. [DOI] [PubMed] [Google Scholar]
- 159.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl Acad. Sci. USA. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pirault J, Back M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 2018;9:1273. doi: 10.3389/fphar.2018.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Furman D, et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qu X, et al. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 2012;228:506–519. doi: 10.1002/path.4050. [DOI] [PubMed] [Google Scholar]
- 163.Al-Shaer AE, Pal A, Shaikh SR. Resolvin E1-ChemR23 axis regulates the hepatic metabolic and inflammatory transcriptional landscape in obesity at the whole genome and exon level. Front. Nutr. 2021;8:799492. doi: 10.3389/fnut.2021.799492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Markworth JF, et al. Metabolipidomic profiling reveals an age-related deficiency of skeletal muscle pro-resolving mediators that contributes to maladaptive tissue remodeling. Aging Cell. 2021;20:e13393. doi: 10.1111/acel.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li G, et al. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J. Lipid Res. 2017;58:1080–1090. doi: 10.1194/jlr.M072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Younes R, LeBlanc CA, Hiram R. Evidence of failed resolution mechanisms in arrhythmogenic inflammation, fibrosis and right heart disease. Biomolecules. 2022 doi: 10.3390/biom12050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chiurchiu V, et al. Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J. 2019;33:909–916. doi: 10.1096/fj.201801017R. [DOI] [PubMed] [Google Scholar]
- 168.Artiach G, et al. Omega-3 polyunsaturated fatty acids decrease aortic valve disease through the resolvin E1 and ChemR23 axis. Circulation. 2020;142:776–789. doi: 10.1161/CIRCULATIONAHA.119.041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jaen RI, et al. BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis. FASEB J. 2020;34:10531–10546. doi: 10.1096/fj.202000611R. [DOI] [PubMed] [Google Scholar]
- 170.Keyes KT, et al. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 171.Kain V, et al. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hiram R. Resolution-promoting autacoids demonstrate promising cardioprotective effects against heart diseases. Mol. Biol. Rep. 2022 doi: 10.1007/s11033-022-07230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 174.Hua KF, et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1β production. J. Cell Physiol. 2015;230:863–874. doi: 10.1002/jcp.24815. [DOI] [PubMed] [Google Scholar]
- 175.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 177.Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 2016;82:957–964. doi: 10.1111/bcp.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hocherl K, Endemann D, Kammerl MC, Grobecker HF, Kurtz A. Cyclo-oxygenase-2 inhibition increases blood pressure in rats. Br. J. Pharmacol. 2002;136:1117–1126. doi: 10.1038/sj.bjp.0704821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qi Z, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J. Clin. Invest. 2002;110:61–69. doi: 10.1172/JCI0214752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Izhar M, Alausa T, Folker A, Hung E, Bakris GL. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated blacks and Hispanics. Hypertension. 2004;43:573–577. doi: 10.1161/01.HYP.0000115921.55353.e0. [DOI] [PubMed] [Google Scholar]
- 181.Shiroshita-Takeshita A, Brundel BJ, Lavoie J, Nattel S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc. Res. 2006;69:865–875. doi: 10.1016/j.cardiores.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 182.Nomani H, Mohammadpour AH, Moallem SMH, Sahebkar A. Anti-inflammatory drugs in the prevention of post-operative atrial fibrillation: a literature review. Inflammopharmacology. 2020;28:111–129. doi: 10.1007/s10787-019-00653-x. [DOI] [PubMed] [Google Scholar]
- 183.Zhou X, et al. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm. Sin. B. 2019;9:711–723. doi: 10.1016/j.apsb.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ofman P, et al. Aspirin use and risk of atrial fibrillation in the Physicians’ Health Study. J. Am. Heart Assoc. 2014 doi: 10.1161/JAHA.113.000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu Y, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 1999;162:3639–3646. doi: 10.4049/jimmunol.162.6.3639. [DOI] [PubMed] [Google Scholar]
- 186.Giles KM, et al. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J. Immunol. 2001;167:976–986. doi: 10.4049/jimmunol.167.2.976. [DOI] [PubMed] [Google Scholar]
- 187.Birnbaum Y, et al. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 188.Lucas CD, et al. Downregulation of Mcl-1 has anti-inflammatory pro-resolution effects and enhances bacterial clearance from the lung. Mucosal Immunol. 2014;7:857–868. doi: 10.1038/mi.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Panoulas VF, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology. 2008;47:72–75. doi: 10.1093/rheumatology/kem311. [DOI] [PubMed] [Google Scholar]
- 191.Tripathi RC, Parapuram SK, Tripathi BJ, Zhong Y, Chalam KV. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15:439–450. doi: 10.2165/00002512-199915060-00004. [DOI] [PubMed] [Google Scholar]
- 192.Nashel DJ. Is atherosclerosis a complication of long-term corticosteroid treatment? Am. J. Med. 1986;80:925–929. doi: 10.1016/0002-9343(86)90639-X. [DOI] [PubMed] [Google Scholar]
- 193.Xu Y, et al. Multiple-modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS-activated microglia. Int. Immunopharmacol. 2009;9:360–365. doi: 10.1016/j.intimp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 194.Perregaux DG, et al. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 2001;299:187–197. [PubMed] [Google Scholar]
- 195.Zahid A, Li B, Kombe AJK, Jin T, Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gao RF, et al. The covalent NLRP3-inflammasome inhibitor Oridonin relieves myocardial infarction induced myocardial fibrosis and cardiac remodeling in mice. Int. Immunopharmacol. 2021;90:107133. doi: 10.1016/j.intimp.2020.107133. [DOI] [PubMed] [Google Scholar]
- 197.Wei Z, et al. Loss of Camk2n1 aggravates cardiac remodeling and malignant ventricular arrhythmia after myocardial infarction in mice via NLRP3 inflammasome activation. Free Radic. Biol. Med. 2021;167:243–257. doi: 10.1016/j.freeradbiomed.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 198.Brucato A, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316:1906–1912. doi: 10.1001/jama.2016.15826. [DOI] [PubMed] [Google Scholar]
- 199.Abbate A, et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharmacother. 2021 doi: 10.1093/ehjcvp/pvab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Van Tassell BW, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial) Circ. Heart Fail. 2017 doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Klein AL, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N. Engl. J. Med. 2021;384:31–41. doi: 10.1056/NEJMoa2027892. [DOI] [PubMed] [Google Scholar]
- 202.Everett BM, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010. [DOI] [PubMed] [Google Scholar]
- 203.Thul S, Labat C, Temmar M, Benetos A, Back M. Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: a novel biomarker of non-resolving vascular inflammation. Eur. J. Prev. Cardiol. 2017;24:903–906. doi: 10.1177/2047487317694464. [DOI] [PubMed] [Google Scholar]
- 204.Bazan HA, et al. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot. Essent. Fat. Acids. 2017;125:43–47. doi: 10.1016/j.plefa.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fredman G. Can inflammation-resolution provide clues to treat patients according to their plaque phenotype. Front. Pharmacol. 2019;10:205. doi: 10.3389/fphar.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Petri MH, et al. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E(-/-) mice. Br. J. Pharmacol. 2017;174:4043–4054. doi: 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Viola JR, et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 2016;119:1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- 208.Zhang J, et al. The anti-inflammatory mediator resolvin E1 protects mice against lipopolysaccharide-induced heart injury. Front. Pharmacol. 2020;11:203. doi: 10.3389/fphar.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging. 2016;8:2611–2634. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Halade GV, Lee DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. 2022;79:103992. doi: 10.1016/j.ebiom.2022.103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.El-Sharkawy LY, Brough D, Freeman S. Inhibiting the NLRP3 Inflammasome. Molecules. 2020 doi: 10.3390/molecules25235533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04338997 (2020).
- 213.European Medicines Agency. EU Clinical Trials Registerhttps://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000489-40/GB (2020).
- 214.Van Tassell BW, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am. J. Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Marchetti C, et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA. 2018;115:E1530–E1539. doi: 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wohlford GF, et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J. Cardiovasc. Pharmacol. 2020;77:49–60. doi: 10.1097/FJC.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02272946 (2021).
- 218.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03939520 (2022).
- 219.Martin J, et al. Tranilast attenuates cardiac matrix deposition in experimental diabetes: role of transforming growth factor-β. Cardiovasc. Res. 2005;65:694–701. doi: 10.1016/j.cardiores.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 220.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03490708 (2018).
- 221.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03923140 (2019).
- 222.Li Y, et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol. Immunol. 2018;99:134–144. doi: 10.1016/j.molimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 223.Aimo A, et al. Pirfenidone as a novel cardiac protective treatment. Heart Fail. Rev. 2022;27:525–532. doi: 10.1007/s10741-021-10175-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ridker PM, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 225.Lyu H, et al. VX-765 prevents intestinal ischemia-reperfusion injury by inhibiting NLRP3 inflammasome. Tissue Cell. 2022;75:101718. doi: 10.1016/j.tice.2021.101718. [DOI] [PubMed] [Google Scholar]
- 226.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT05164120 (2022).
- 227.Toldo S, et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol. Ther. 2021;236:108053. doi: 10.1016/j.pharmthera.2021.108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Samuel M, et al. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Can. J. Cardiol. 2021;37:776–785. doi: 10.1016/j.cjca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 229.Varghese B, et al. Inflammation, atrial fibrillation, and the potential role for colchicine therapy. Heart Rhythm O2. 2021;2:298–303. doi: 10.1016/j.hroo.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tabbalat RA, et al. Effect of low-dose colchicine on the incidence of atrial fibrillation in open heart surgery patients: END-AF low dose trial. J. Int. Med. Res. 2020;48:300060520939832. doi: 10.1177/0300060520939832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tabbalat RA, et al. Effect of colchicine on the incidence of atrial fibrillation in open heart surgery patients: END-AF trial. Am. Heart J. 2016;178:102–107. doi: 10.1016/j.ahj.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 232.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03310125 (2022).
- 233.Aguilar M, Heijman J, Dobrev D, Nattel S. One ring to rule them all: continuous monitoring of patients with secondary atrial fibrillation points to a unifying underlying mechanism. Can. J. Cardiol. 2021;37:686–689. doi: 10.1016/j.cjca.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 234.Tucker NR, et al. Transcriptional and cellular diversity of the human heart. Circulation. 2020;142:466–482. doi: 10.1161/CIRCULATIONAHA.119.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Moreira LM, et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature. 2020;587:460–465. doi: 10.1038/s41586-020-2890-8. [DOI] [PubMed] [Google Scholar]
- 236.Litvinukova M, et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang Y, et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 2022;118:785–797. doi: 10.1093/cvr/cvab114. [DOI] [PubMed] [Google Scholar]
- 238.Rong J, et al. Loss of hepatic angiotensinogen attenuates sepsis-induced myocardial dysfunction. Circ. Res. 2021;129:547–564. doi: 10.1161/CIRCRESAHA.120.318075. [DOI] [PubMed] [Google Scholar]
- 239.Shi H, et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ. Res. 2021;129:383–396. doi: 10.1161/CIRCRESAHA.120.318629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Takahashi M. Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biol. Pharm. Bull. 2019;42:518–523. doi: 10.1248/bpb.b18-00369. [DOI] [PubMed] [Google Scholar]
- 241.Walkey AJ, Evans SR, Winter MR, Benjamin EJ. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a propensity-matched cohort study. Chest. 2016;149:74–83. doi: 10.1378/chest.15-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Spodick DH. Frequency of arrhythmias in acute pericarditis determined by Holter monitoring. Am. J. Cardiol. 1984;53:842–845. doi: 10.1016/0002-9149(84)90416-8. [DOI] [PubMed] [Google Scholar]
- 243.Talreja DR, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852–1857. doi: 10.1161/01.CIR.0000087606.18453.FD. [DOI] [PubMed] [Google Scholar]
- 244.Syed FF, et al. Atrial fibrillation as a consequence of tuberculous pericardial effusion. Int. J. Cardiol. 2012;158:152–154. doi: 10.1016/j.ijcard.2012.04.075. [DOI] [PubMed] [Google Scholar]
- 245.Larsen BT, et al. Atrial giant cell myocarditis: a distinctive clinicopathologic entity. Circulation. 2013;127:39–47. doi: 10.1161/CIRCULATIONAHA.112.128900. [DOI] [PubMed] [Google Scholar]
- 246.Anderson BR, Silver ES, Richmond ME, Liberman L. Usefulness of arrhythmias as predictors of death and resource utilization in children with myocarditis. Am. J. Cardiol. 2014;114:1400–1405. doi: 10.1016/j.amjcard.2014.07.074. [DOI] [PubMed] [Google Scholar]
- 247.Blagova OV, et al. Myocardial biopsy in “idiopathic” atrial fibrillation and other arrhythmias: nosological diagnosis, clinical and morphological parallels, and treatment. J. Atr. Fibrillation. 2016;9:24–30. doi: 10.4022/jafib.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Subahi A, et al. Impact of atrial fibrillation on patients hospitalized for acute myocarditis: insights from a nationally-representative United States cohort. Clin. Cardiol. 2019;42:26–31. doi: 10.1002/clc.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rasal G, et al. Arrhythmia spectrum and outcome in children with myocarditis. Ann. Pediatr. Cardiol. 2021;14:366–371. doi: 10.4103/apc.apc_207_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dunbar DN, et al. Intracavitary electrode catheter cardioversion of atrial tachyarrhythmias in the dog. J. Am. Coll. Cardiol. 1986;7:1015–1027. doi: 10.1016/S0735-1097(86)80219-4. [DOI] [PubMed] [Google Scholar]
- 251.Kumagai K, Yamanouchi Y, Tashiro N, Hiroki T, Arakawa K. Low energy synchronous transcatheter cardioversion of atrial flutter/fibrillation in the dog. J. Am. Coll. Cardiol. 1990;16:497–501. doi: 10.1016/0735-1097(90)90610-2. [DOI] [PubMed] [Google Scholar]
- 252.Ali IM, Butler CK, Armour JA, Murphy DA. Modification of supraventricular tachyarrhythmias by stimulating atrial neurons. Ann. Thorac. Surg. 1990;50:251–256. doi: 10.1016/0003-4975(90)90744-Q. [DOI] [PubMed] [Google Scholar]
- 253.Yamanouchi Y, Kumagai K, Tashiro N, Hiroki T, Arakawa K. Transesophageal low-energy synchronous cardioversion of atrial flutter/fibrillation in the dog. Am. Heart J. 1992;123:417–420. doi: 10.1016/0002-8703(92)90655-F. [DOI] [PubMed] [Google Scholar]
- 254.Ortiz J, et al. Mechanism of spontaneous termination of stable atrial flutter in the canine sterile pericarditis model. Circulation. 1993;88:1866–1877. doi: 10.1161/01.CIR.88.4.1866. [DOI] [PubMed] [Google Scholar]
- 255.Ortiz J, et al. Atrial defibrillation using temporary epicardial defibrillation stainless steel wire electrodes: studies in the canine sterile pericarditis model. J. Am. Coll. Cardiol. 1995;26:1356–1364. doi: 10.1016/0735-1097(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 256.Cmolik BL, et al. Successful atrial defibrillation with very-low-energy shocks by means of temporary epicardial wire electrodes. J. Thorac. Cardiovasc. Surg. 1996;111:392–397. doi: 10.1016/S0022-5223(96)70448-8. [DOI] [PubMed] [Google Scholar]
- 257.Kumagai K, Khrestian C, Waldo AL. Simultaneous multisite mapping studies during induced atrial fibrillation in the sterile pericarditis model. Insights into the mechanism of its maintenance. Circulation. 1997;95:511–521. doi: 10.1161/01.CIR.95.2.511. [DOI] [PubMed] [Google Scholar]
- 258.Sokoloski MC, et al. Safety of transvenous atrial defibrillation: studies in the canine sterile pericarditis model. Circulation. 1997;96:1343–1350. doi: 10.1161/01.CIR.96.4.1343. [DOI] [PubMed] [Google Scholar]
- 259.Kumagai K, Uno K, Khrestian C, Waldo AL. Single site radiofrequency catheter ablation of atrial fibrillation: studies guided by simultaneous multisite mapping in the canine sterile pericarditis model. J. Am. Coll. Cardiol. 2000;36:917–923. doi: 10.1016/S0735-1097(00)00803-2. [DOI] [PubMed] [Google Scholar]
- 260.Becker R, et al. Suppression of atrial fibrillation by multisite and septal pacing in a novel experimental model. Cardiovasc. Res. 2002;54:476–481. doi: 10.1016/S0008-6363(02)00231-6. [DOI] [PubMed] [Google Scholar]
- 261.Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 2003;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- 262.Goldstein RN, Khrestian C, Carlsson L, Waldo AL. Azd7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J. Cardiovasc. Electrophysiol. 2004;15:1444–1450. doi: 10.1046/j.1540-8167.2004.04354.x. [DOI] [PubMed] [Google Scholar]
- 263.Ryu K, et al. Characterization of the critical cycle length of a left atrial driver which causes right atrial fibrillatory conduction. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004;2004:3960–3963. doi: 10.1109/IEMBS.2004.1404106. [DOI] [PubMed] [Google Scholar]
- 264.Ryu K, Sahadevan J, Khrestian CM, Stambler BS, Waldo AL. Use of fast Fourier transform analysis of atrial electrograms for rapid characterization of atrial activation – implications for delineating possible mechanisms of atrial tachyarrhythmias. J. Cardiovasc. Electrophysiol. 2006;17:198–206. doi: 10.1111/j.1540-8167.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 265.Tselentakis EV, Woodford E, Chandy J, Gaudette GR, Saltman AE. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J. Surg. Res. 2006;135:68–75. doi: 10.1016/j.jss.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 266.Bui HM, Khrestian CM, Ryu K, Sahadevan J, Waldo AL. Fixed intercaval block in the setting of atrial fibrillation promotes the development of atrial flutter. Heart Rhythm. 2008;5:1745–1752. doi: 10.1016/j.hrthm.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 267.Ryu K, Sahadevan J, Khrestian CM, Stambler BS, Waldo AL. Frequency analysis of atrial electrograms identifies conduction pathways from the left to the right atrium during atrial fibrillation – studies in two canine models. J. Cardiovasc. Electrophysiol. 2009;20:667–674. doi: 10.1111/j.1540-8167.2008.01403.x. [DOI] [PubMed] [Google Scholar]
- 268.Rossman EI, et al. The gap junction modifier, GAP-134 [(2S,4R)-1-(2-aminoacetyl)-4-benzamido-pyrrolidine-2-carboxylic acid], improves conduction and reduces atrial fibrillation/flutter in the canine sterile pericarditis model. J. Pharmacol. Exp. Ther. 2009;329:1127–1133. doi: 10.1124/jpet.108.150102. [DOI] [PubMed] [Google Scholar]
- 269.Matsumoto N, et al. Vanoxerine, a new drug for terminating atrial fibrillation and flutter. J. Cardiovasc. Electrophysiol. 2010;21:311–319. doi: 10.1111/j.1540-8167.2009.01622.x. [DOI] [PubMed] [Google Scholar]
- 270.Cakulev I, et al. Oral vanoxerine prevents reinduction of atrial tachyarrhythmias: preliminary results. J. Cardiovasc. Electrophysiol. 2011;22:1266–1273. doi: 10.1111/j.1540-8167.2011.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bhimani AA, et al. Ranolazine terminates atrial flutter and fibrillation in a canine model. Heart Rhythm. 2014;11:1592–1599. doi: 10.1016/j.hrthm.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 272.Sadrpour SA, et al. Termination of atrial flutter and fibrillation by K201’s metabolite M-II: studies in the canine sterile pericarditis model. J. Cardiovasc. Pharmacol. 2015;65:494–499. doi: 10.1097/FJC.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 273.Goldstein RN, Ryu K, Khrestian C, van Wagoner DR, Waldo AL. Prednisone prevents inducible atrial flutter in the canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 2008;19:74–81. doi: 10.1111/j.1540-8167.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 274.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04575753 (2020).
- 275.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04997057 (2022).
- 276.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03609541 (2022).
- 277.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04697719 (2022).
- 278.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02322073 (2021).