Abstract
Gastrulation is a fundamental and critical process of animal development whereby the mass of cells that results from the proliferation of the zygote transforms itself into a recognizable outline of an organism. The last few years have seen the emergence of a number of experimental models of early mammalian embryogenesis based on Embryonic Stem (ES) cells. One of this is the Gastruloid model. Gastruloids are aggregates of defined numbers of ES cells that, under defined culture conditions, undergo controlled proliferation, symmetry breaking, and the specification of all three germ layers characteristic of vertebrate embryos, and their derivatives. However, they lack brain structures and, surprisingly, reveal a disconnect between cell type specific gene expression and tissue morphogenesis, for example during somitogenesis. Gastruloids have been derived from mouse and human ES cells and several variations of the original model have emerged that reveal a hereto unknown modularity of mammalian embryos. We discuss the organization and development of gastruloids in the context of the embryonic stages that they represent, pointing out similarities and differences between the two. We also point out their potential as a reproducible, scalable and searchable experimental system and highlight some questions posed by the current menagerie of gastruloids.
Keywords: Embryos, Gastruloids, Experimental embryology, Systems biology
An embryo is a self-assembled, multicellular system that creates and develops the blueprint of an organism. It does so through controlled growth, the specification of diverse cell types and their spatial arrangement into tissues and organs. These events are tightly coordinated in space and time with reference to global and local coordinate systems that ensure the reproducible positioning of the different components of the organism into a functional whole. In animals, the first phase of this process is the generation of a body plan which follows a conserved sequence of events: the zygote divides to create a mass of seemingly identical cells, followed by a transformation of this isotropic structure into an outline of the organism. The process through which this transformation is achieved is called “gastrulation”.
The term gastrulation was introduced by Ernest Haeckel in his studies of the development of sponges to describe the transformation of a hollow ball of cells into a structure with two layers - the ectoderm and the endoderm (Haeckel, 1874). Since then, gastrulation has become a general term for a stage between the morula and the neurula, responsible for the generation of the three germ layers and associated with the outline of a body plan (Stern, 2004). Historically, our understanding of these events has been centred on large scale movements of cellular ensembles, but over the last forty years there has been an increased focus on the activities of individual cells and how these are coordinated to generate the primordia of tissues and organs.
In many organisms, including amphibia and fish, germ layers are specified before gastrulation, while in others, including mammals, they arise in step with the increase of cellular mass characteristic of very early stages of development. These differences highlight a variety of strategies to attain a body plan which appears conserved across vertebrates (Slack et al., 1993) and have led to the suggestion that gastrulation should not be considered as a ‘stage’ but, more properly, as a ‘process’ whereby organisms establish a coordinate system to position the primordia of the different tissues and organs that is implemented in different ways in different organisms (Sheng et al., 2021; Steventon et al., 2021).
The emergence of a coordinate system is crucial for the development of an organism, as this is the reference for the placement of the primordia of the different organs. In many arthropods, the coordinate system is provided maternally in the organization of the egg, and is translated directly to the body plan by the embryonic cells, that simply follow the cues inherited in the blastoderm (van Eeden and St Johnston, 1999). This strategy contrasts with that of other species, including some vertebrates, where the initial coordinate system is established by the zygote over time. In some cases, like ascidians, frogs, and fish, it is influenced by some asymmetries in the organization of the egg (Tuazon and Mullins, 2015). In amniotes, particularly in mammals, the coordinate system is generated de novo by the zygote, using interactions with and information provided by extraembryonic tissues (Takaoka and Hamada, 2012).
The emergence of model organisms during the XX century coincided with the implementation of two main approaches to study gastrulation. One, experimental embryology, is based on the interrogation of the organizational activities of groups of cells by physical manipulations of the embryo. Famously practiced on amphibia, it has provided many insights and fundamental principles of animal development (Hamburger, 1988). A different - though not mutually exclusive - avenue of research was pioneered in C. elegans and D. melanogaster and is based on the analysis of genetic mutants. It has led to the discovery and cataloguing of the elements that drive the generation of cell fates and morphogenesis (Anderson and Ingham, 2003; Garcia-Garcia et al., 2005). In both cases, the central object of the approach is the organism.
A different way of interrogating developmental processes was initiated by Ross Harrison in 1907. With an interest in the growth of axons during the development of the nervous system, he wondered whether this was a property of individual cells or of an ensemble of neural progenitors. To answer this question, Harrison felt he should try to observe cells dislodged from the organism and managed to maintain and develop in culture, blocks of embryonic nervous system taken out of a frog embryo (Harrison, 1907). In this context, he was able to follow the growth of individual axons in a manner that would not have been possible in the densely populated tissue embedded in the embryo. He showed that axons navigated as individual outgrowths with directional properties. With this very simple experiment, Harrison initiated cell culture and opened up new possibilities to ask questions such as, to what degree the development of an organism is reliant on intrinsic autonomous cues.
Harrison's work also provided a means to explore the properties of cells and tissues out of the context of the embryo, thus allowing experiments to test the ability of their component cells to recapitulate early, formative events in vitro. For instance, mammalian eutherian embryos present a special challenge to developmental studies because of their intrauterine development. In the case of human embryos there are added challenges because of ethical issues associated with their use for basic research (Cavaliere, 2017). Notwithstanding this, some aspects of the biology of very early mammalian embryos, such as the formation of the blastocyst, can be studied, because during the first week of development, the mammalian embryo is free-floating in the uterus which allows its study and, to a certain degree, experimentation (Gerri et al., 2020). Gastrulation, on the other hand, remains challenging to study and even observe since, in most mammalian species, it takes place after implantation, making its observation and experimentation very difficult.
Recently, advances in embryo culture and live-imaging techniques have opened a window into the multicellular dynamics of mammalian gastrulation (McDole et al., 2018). However, for the most part, research into the mechanisms of mammalian development has focussed on using genetic approaches to identify genes required for cell fates and cell movements and describing their phenotypes. This work is being expanded by the introduction of Embryonic Stem (ES) cell models of early mammalian development that allow experimental versatility and have revealed unforeseen experimental possibilities that, together with Genetics, are beginning to yield insights into how mammals put their body plan together (Fu et al., 2021; Shahbazi et al., 2019).
Here we discuss existing models of gastrulation with a special focus on ‘gastruloids’, a model of early mammalian development based on ES cells that reproduces many of the features of gastrulation. For this reason, it might be useful to first provide a brief account of the process as it happens in the embryo so that we can judge better the behaviour of ES cell-based models of gastrulation (Fig. 1).
Fig. 1. Schematic overview of mouse gastrulation.
(A) Mouse embryo development from fertilisation through implantation, and the establishment of the body plan through gastrulation. (B) Signalling gradients and biases from the extraembryonic ectoderm and visceral endoderm set up the localisation of the Primitive Streak in the proximal-posterior epiblast. T, Brachyury. (C) The ordered emergence of mesoderm from the primitive streak, in both a proximodistal and circumnavigation from posterior-to-anterior. Al, Allantois; YS, Yolk Sac; PGCs, Primordial Germ Cells; CM, Cardiac Mesoderm; LPM, Lateral Plate Mesoderm; IM, Intermediate Mesoderm; PM, Paraxial Mesoderm; AM, Axial Mesoderm.
1. Mammalian gastrulation: a primer
Most of what we know about gastrulation in mammals is based on the mouse embryo and we refer the reader to some excellent reviews for details of the process e.g (Arnold and Robertson, 2009; Bardot and Hadjantonakis, 2020; Tam and Gad, 2004; Tam and Behringer, 1997). Although the geometry of the pre-gastrulation mouse embryo, a cylinder, is different from the flat disc characteristic of most other mammalian species, including humans (Eakin and Behringer, 2004; Ghimire et al., 2021), the principles that have been established from the mouse are very likely to apply to other mammalian embryos. In all cases, mammalian gastrulation becomes evident with the appearance of the primitive streak, a dynamic cellular structure that outlines the anteroposterior axis at the midline of the organism (Ramkumar and Anderson, 2011; Sheng et al., 2021).
In the mouse, during implantation, the mass of cells in the blastocyst is transformed into a radially symmetric epithelial cylinder with a lumen, wrapped by a layer of Visceral Endoderm (VE) and attached to the uterus through the extraembryonic ectoderm, which is a derivative of the trophectoderm (TE). This organization creates a cup-shaped structure with a proximal-distal axis, where proximal is defined by the position of the extraembryonic ectoderm (Fig. 1A and (Rossant and Tam, 2009; Takaoka et al., 2007)). Genetic studies have shown how signalling from the two extraembryonic lineages (Visceral Endoderm, VE and Trophectoderm, TE) and their derivatives induce asymmetries on the epiblast (Fig. 1B). Thus, at E6.0, the VE exhibits an asymmetry that will impact the organization of the embryo: on one side, a specialized group of cells, the Anterior VE or AVE, secretes antagonists of BMP, Nodal and Wnt signalling into the epiblast. At about the same time, BMP4 from the extra-embryonic ectoderm initiates the expression of Wnt3 and Nodal that signal in the epiblast but, because of the antagonists secreted by the AVE, their signalling becomes restricted to the opposite side of the epiblast that becomes the posterior region of the embryo (Rivera-Perez and Hadjantonakis, 2014). The combined activities of Nodal and Wnt3 induce a localized source of Brachyury (T/Bra) expression in the proximal region of the embryo that initiates gastrulation by promoting EMT. At the opposite end, underneath the AVE, the embryonic cells remain epithelial.
The main role of T/Bra is, together with Wnt signalling, to propagate an EMT along the midline of the epiblast from proximal to distal, laying down the antero-posterior (AP) axis of the embryo. At this stage (E6.5-E7.5) the embryo is growing at a fast rate (Mathiah et al., 2020; Snow, 1977) and the constraints of the extraembryonic tissues and the fluidity of the cells in the posterior epiblast, push many of the new cells from the epiblast towards the primitive streak (Williams et al., 2012). Throughout this process, cells traversing the streak evaginate and crawl over the epiblast, circumnavigating it towards the anterior region. Thus, as gastrulation proceeds there are two orthogonal large scale cell movements simultaneously in action: a proximo distal EMT wave that defines the AP axis and a posterior to anterior migration around the cylinder (Nowotschin and Hadjantonakis, 2010) (Fig. 1C). The coordination of these movements shapes the outline of the embryo's body plan. Fate mapping studies have shown that these movements are associated with an ordered assignment of cell fates that follows the proximo distal axis in the streak (Kinder et al., 1999, 2001). The first cells to express T/Bra move into the Extra Embryonic Ectoderm region and give rise to extraembryonic mesoderm derivatives (Allantois, yolk sac and primitive blood) and the Primordial Germ Cells (Saykali et al., 2019). Then, as the streak progresses distally, cells become specified sequentially as cranial, cardiac, lateral plate, intermediate, paraxial and axial mesoderm. It appears that this is not a clean sequence but that there are overlaps between fate assignments, meaning that feedback and additional influences can fine-tune the plastic embryonic cells at this stage of development (Fig. 1C) (Kinder et al., 1999, 2001).
As well as the emergence of the mesoderm at gastrulation, the VE also becomes gradually replaced by Definitive Endoderm (DE), an embryonic derivative of the epiblast (Kwon et al., 2008). The precise origin of the DE has been elusive, and it was previously believed that a precursor with bipotent potential towards both mesoderm and endoderm, so called ‘mesendoderm’, might mediate this lineage in mammalian gastrulation. However, recent evidence casts doubt on this hypothesis, and instead it appears that the endoderm emerges from a pool of cells that is distinct and separate from the mesoderm (Probst et al., 2021; Scheibner et al., 2021).
The first stage of gastrulation, which we refer to as ‘Primary gastrulation’ (to distinguish it from the events associated with the primitive streak during axial extension), ends when the Primitive streak reaches the most distal region of the epiblast where the node will appear at E7.5. During this time, opposite to the Primitive streak in the area under the influence of the AVE, the epiblast has been acquiring neuroectodermal fate, while the mesoderm that reaches the anterior proximal side of the embryo is fated towards cardiac mesoderm and, at this stage, lies at the most anterior position of the embryo.
At E7.5, the expression of Nodal disappears from the Primitive streak and becomes concentrated only at the node (Fig. 1). At this time, the endodermal and some mesodermal cells coalesce into an anterior projection of the node, the anterior mesendoderm, that mediates neural induction on the overlying anterior epiblast (Andoniadou and Martinez-Barbera, 2013; Camus et al., 2000). The effects of this process are the specification of the anterior epiblast into Fore- Mid- and Hind-Brain (FB, MB and HB) and a burst of proliferation in this region associated with the emergence of the neural folds that push the cardiac primordium posteriorly and inwards. Between 7.5 and 8.5, a sequence of multicellular movements transforms the cylinder into an elongated structure by convergent-extension. Coincident with these movements, the first somites appear and neurulation begins in the spinal cord. Also, the Primitive streak closes into the midline, except posterior to the node, in a region called the Caudal Epiblast (CE), where proliferation and ordered morphogenesis will fuel axial extension through to E10/E10.5 in association with what we suggest calling ‘Secondary Gastrulation’. This term accounts for the presence of a primitive streak throughout this process that acts a source of progenitors for lateral, intermediate and paraxial mesoderm as well as spinal cord. A special population of bipotential progenitors for the paraxial mesoderm and the spinal cord lies in the CE behind the node; this population is known as Neuromesodermal progenitor population (Wymeersch et al., 2021). This process of axial extension is associated with the progressive expression of Hox genes that act as positional markers and mediators of cellular identity along the anteroposterior axis and are laid down as spatial and temporal rulers of embryogenesis (Deschamps and Duboule, 2017).
The events associated with laying down the mammalian body plan can therefore be summarized in the following sequence of events: establishment of a multiaxial coordinate system, emergence of tissue and organ primordia with reference to the axial system, and subsequent axial extension. However, not much is known about the details of this process outside the mouse, whose cup-shaped epiblast might have specific effects on the morphogenesis of gastrulation that are likely to be absent in the flat epiblast of most other mammals. For example, gastrulation in rabbits, cows and pigs is initiated by a posterior extension of the epiblast associated with Bra expression that precedes the appearance of the Primitive Streak (Guillomot et al., 2004; Hassoun et al., 2009; Hue et al., 2001; Viebahn et al., 2002). In the case of humans, our knowledge is scant, mostly derived from anatomical studies of extant embryo collections (Ghimire et al., 2021) with a recent addition of gene expression analysis of one exceptional, gastrulation-stage embryo (Tyser et al., 2020). These studies suggest that, while many principles of gastrulation are likely to be general, details of gene expression, multicellular dynamics and morphological outcomes are likely to vary from one species to another.
2. Embryonic stem cells as models of early mammalian development
ES cells are clonal derivatives of mammalian epiblasts that self renew in culture and, under controlled conditions, can be differentiated into any cell type of the organism (Smith, 2001). Over the last twenty years, they have become a useful system to study the relationship between signals and cell fate assignments and, together with genetic approaches in the mouse, have created a framework to understand mammalian development.
One of the remarkable features of ES cells is how they recapitulate in vitro many events that occur in vivo. For instance, mouse ES cells in adherent culture that are differentiated into various mesodermal derivatives will follow the pattern that they would in the embryo and go through a transient phase of Bra expression as they exit pluripotency (Gadue et al., 2005; Kouskoff et al., 2005; Kubo et al., 2004; Turner et al., 2014b, 2014c). These differentiation events follow autonomous schedules of gene expression that mimic those of the embryo. On the other hand, the unconstrained growth of the cells in adherent culture leads to a lack of proportional control of the different cell types that is a significant characteristic of embryos.
If, instead of differentiating on top of substrates coating a flat dish, large numbers of ES cells (usually more than 1000) are placed in non-adherent conditions in the presence of Serum, they generate Embryoid Bodies (EBs) - unstructured three dimensional ensembles of different cell types that grow in a disorganized manner (Brickman and Serup, 2017). It is not rare to observe localization of T/Bra expression in these aggregates, a pattern that can be made more reliable by exposure to Wnt signalling (Boxman et al., 2016; ten Berge et al., 2008). EBs do not evolve further patterning, but become a useful source of some cell types that are not easy to obtain from adherent culture suggesting that cells gather information from their three-dimensional environment. Under more defined culture conditions, EBs are the starting point for neural patterned structures, including optic cups (Eiraku et al., 2011; Nakano et al., 2012) and brain organoids (Lancaster et al., 2013).
These observations suggest that the initial starting conditions, signalling exposure and 3D coordination between cells are crucial for the patterning and organization of germ layers and their derivatives that are the essence of gastrulation. This notion is reinforced by the behaviour of ES cells differentiated in high density on size-controlled adherent patterns that develop a concentric ring-like germ layer organization reminiscent of that in the embryo (Warmflash et al., 2014). Nevertheless, these cultures do not establish the full body plan organisation as they do in the embryo, leaving open the question of how the embryo achieves this goal.
3. Mouse gastruloids
The capacity of ES cells to integrate into a blastocyst and contribute to the development of a normal and fertile mouse, contrasts with their inability to organize a minimal body plan in culture, even when aggregated into EBs. This might be due to the lack of interactions between the ES cells and the extraembryonic tissues that play such a central role in the patterning of the epiblast in the embryo. To explore this possibility, a number of models have been developed combining ES cells with stem cells of extraembryonic lineages. Controlled combinations of ES and Trophoblast stem (TS) cells in suspension generate structures that resemble blastocysts, called blastoids, but these do not progress beyond an early epiblast stage and do not implant properly (Rivron et al., 2018) (Fig. 3). In the presence of either Matrigel, XEN or PrEnd cells, ES Cells can form epiblast like structures that develop an anteroposterior (AP) polarity and, at a low frequency, initiate gastrulation like movements but do not progress to a complete body plan (Amadei et al., 2021; Girgin et al., 2021a; Sozen et al., 2018). Occasionally these structures exhibit the initiation of a primitive streak (Fig. 3).
Fig. 3. Relationship between mouse embryo (A) and gastruloid (B) development.
Gastruloids exhibit the emergence and polarization of Bra and Nodal gene expression in the extending domain.
Surprisingly, when mouse P19 embryo carcinoma cells are aggregated in Serum, they undergo a Wnt signalling-dependent polarized extension, with cells expressing Bra at one end, and patterns of gene expression characteristic of paraxial mesoderm along the extending domain (Marikawa et al., 2009). This tantalizing observation led to the development of an experimental protocol to explore the potential of mouse ES cells to form comparable structures (van den Brink et al., 2014).
When defined numbers of mouse ES cells are sown in small volumes of basal culture medium, they form spherical aggregates. After two days in culture, as they grow, some aggregates express low levels of Bra in a crescent shape domain at one pole. Further culture leads to a variety of structures, mostly with neural identity but some retaining polarized Bra expression, with a small number displaying irregular extensions (van den Brink et al., 2014) (Fig. 2). However, if, during the third day of culture, the aggregates are exposed to Wnt agonists e.g Chiron or Wnt3a, more than 80% of the aggregates display expression of Bra at one end, become teardrop shape and proceed to undergo elongation led by the group of cells with defined expression of Bra(Turner et al., 2014a, 2017b). After five days in culture these structures have an organization that resembles the mammalian body plan and, for this reason, we called them ‘gastruloids’ (Turner et al., 2017b; van den Brink et al., 2014) (Fig. 2). However, gastruloids do not exhibit any obvious morphological evidence of a primitive streak and, moreover, their cells do not undergo an EMT since they are not initially epithelial (van den Brink et al., 2014).
Fig. 2. Making Gastruloids.
(A, B) Schematic protocol for generating mouse and human gastruloids. Pluripotent stem cells are dissociated to a single cell suspension, and an particular cell number (typically 300–500 cells) are added to each well of a 96-well plate with rounded bottoms (A). The cells will settle and aggregate to form a 3D spherical structure that, over time, begins to break symmetry, polarize its gene expression, and undergo axial elongation. The timing of exposure to agonists of Wnt signalling differs for mouse and human ES cells (B). (C) Representative microscopy images of a developing mouse gastruloid timeline between about 72 and 126 h after aggregation. Images from a light sheet movie of a gastruloid bearing ubiquitously expressed GPI linked GFP (U.Fiuza unpublished). Scale bar: 100 μm. (D, E) Sample ollections of 120m hrs old gastruloids from E14 ES (D) and P19 embryo carcinoma (E) cells.
The difference between the organization of gastruloids and the disorderly arrangement of cell types in EBs is striking. The difference appears to lie in the initial number of cells which is critical for the polarization and elongation of the gastruloids; with too few cells they remain spherical and exhibit little growth while if the initial aggregates are made up of too many, they develop multiple foci of Bra expression, more than one elongation and eventually, for very large numbers, a disorganization similar to that seen in EBs(van den Brink et al., 2014). It is worth pointing out that the number of initial cells that gives rise to unipolar gastruloids is around 300, a number that is not far from the number of cells in an epiblast at the onset of gastrulation.
A survey of gene expression during the development of gastruloids reveals that the aggregates follow a schedule of gene expression that, in the embryo, is associated with the transition of the blastocyst to the epiblast and then through gastrulation to the emergence of the body plan (Beccari et al., 2018; Rossi et al., 2021a; Turner et al., 2017b). This suggests that the exposure to Wnt signalling on the third day of culture is mimicking the requirement for this activity during gastrulation where it plays a role coordinating and sustaining the expression of T/Bra(Tortelote et al., 2013). By 120 h in culture, the aggregates have developed a spatial organization of gene expression that is reminiscent of that in the E8.5 embryo (Beccari et al., 2018; van den Brink et al., 2014; Veenvliet et al., 2020). It is these features that led to the term ‘gastruloid’ for these structures.
Analysis of the cell types present in the gastruloid, in terms of gene expression signatures, reveals derivatives of all germ layers, including PGCs, neural crest, pharyngeal mesoderm and endoderm, endothelium, hematopoietic progenitors and, even some cells with placodal progenitor identify (Rossi et al., 2021a; Rossi et al., 2021b; van den Brink et al., 2020; Veenvliet et al., 2020). Most of these cell types are axially organized in a manner that mirrors the embryo, with a more robust and clear organization in the posterior, elongating region (Beccari et al., 2018; Veenvliet et al., 2020). At the anterior end, there is a clear signature of the cardiopharyngeal progenitors that will give rise to the heart tube and the anterior paraxial mesoderm (Rossi et al., 2021a) and, at the other end, a region very similar to the Caudal Lateral Epiblast harbouring a population of Neuromesodermal progenitors. Furthermore, longer culture reveals spatiotemporally organized expression of the four Hox clusters in a manner and with time scales comparable to mouse embryos (Beccari et al., 2018). Analysis of the early events of gastruloid formation reveals that they use similar Gene Regulatory Networks to break symmetry and polarize as embryos do (Beccari et al., 2018; Cermola et al., 2021; Turner et al., 2017b). Altogether these observations show that gastruloids faithfully execute spatial and temporal programs of gene expression associated with the emergence of the body plan.
A striking feature of gastruloids is that their multiaxial organization develops in the absence of any extraembryonic cell types that play a key role in this process in the embryo. This suggests the existence of an intrinsic symmetry-breaking ability of pluripotent cells in three dimensions that might then be further controlled by extraembryonic cues (Girgin et al., 2021a; Turner et al., 2017a). Two additional features of gastruloids are the absence of anterior neural (brain) cell types and of a primitive streak, both of which are probably linked to the lack of extraembryonic tissues. The AVE is a source of antagonists of Wnt and Nodal signalling which promote anterior neural development in the epiblast (Rivera-Perez and Hadjantonakis, 2014; Takaoka and Hamada, 2012) and the absence of VE in gastruloids leaves the cells in the aggregate unprotected to the posteriorizing effects of Chiron, thus suppressing the development of anterior neural structures. This interpretation is supported by the observation that exposure of the early aggregates to Wnt inhibitors in epiblast medium - FGF and Activin - elicits the appearance of anterior neural structures, though not of forebrain (Girgin et al., 2021b). Furthermore, culture of ES cells with VE stem cells, XEN cells, during gastruloid formation transforms the fate map and leads to a polarized but truncated body plan with anterior neural structures (Bérenger-Currias et al., 2020) (Fig. 3).
The absence of a primitive streak might also be a consequence of the lack of extraembryonic tissues as it has been suggested that this iconic structure is a consequence of interactions between extraembryonic membranes and the epiblast and reflects a pre-existing midline (Sheng et al., 2021). Gastruloids have a midline, reflected in the bilateral organization of the mesodermal derivatives, the gut tube and, significantly, a node region at the posterior end (Beccari et al., 2018; Xu et al., 2021), but they do not have any evidence of a morphological primitive streak.
Gastruloids have been validated by comparison with embryos in terms of single cell gene expression profiles (Rossi et al., 2021a; van den Brink et al., 2020; Veenvliet et al., 2020) and spatial organization of gene expression (van den Brink et al., 2020; Xu et al., 2021). Gastruloids not only recapitulate significant events of normal mouse development but they also are able to reproduce the effects of loss of gene function: Nodal (Turner et al., 2017b), Cripto (Cermola et al., 2021) and Tbx6 (Veenvliet et al., 2020). For this reason, they are likely to prove to be a good model system to explore aspects of early mammalian development in-depth with numbers that would not be easy to obtain from mice.
One significant feature of this experimental system is how robust the different events (symmetry breaking, localization of T/Bra expression and elongation) are in terms of timing and, by and large, spatial organization. In contrast, gastruloids do however exhibit a degree of variability in the degree of patterning of different germ layers. For example, the patterning, and in some instances the representation, of different mesodermal derivatives, the endoderm and the spinal cord can vary from 20% to 80% (Beccari et al., 2018; Veenvliet et al., 2020; Xu et al., 2021). While in some instances this can be ascribed to the cell line of origin used (Ortmann et al., 2020; van den Brink et al., 2020), it is likely that some of the variability is a consequence of the self-organizing nature of the patterning events. This possibility is supported by a recent adjustment in the protocol to generate gastruloids. This new method involves the juxtaposition of two aggregates at different developmental stages that, together, mimic the pre-gastrulation epiblast (Xu et al., 2021). First, a small aggregate is exposed to BMP4 which, similarly to the embryo, leads to the expression of Nodal and Wnt3. When this smaller aggregate is allowed to fuse with a larger one made up of pluripotent stem cells that have not experienced exogeneous BMP4 signalling. The combination recreates the body plan with less variability than the original gastruloids. Moreover, in this system, the more anterior cells express markers of midbrain and hindbrain, though they still lack forebrain tissue. It has been suggested that this is due to the presence of VE cells in the aggregate (Xu et al., 2021) but the evidence is lacking and this is not likely to be the case. It is not easy to distinguish visceral and definitive Endoderm and Gastruloids have been shown to lack this extraembryonic lineage (Turner et al., 2017b). Furthermore, ES cells do not generate VE spontaneously (Schroter et al., 2015) and the evidence presented by the authors relies on a Leptin that is not specific to VE (see (Vianello, 2021) for a discussion). Thus, the appearance of anterior neural structures in these gastruloids is unlikely to be due to any VE but, most likely, to the fact that these cells remain naïve and are not exposed to Wnt signalling, as they are in the standard gastruloid protocol. This allows them to develop these fates under the influence of an anterior notochord that is also present in this model. One interpretation of the observations from this model is that the Nodal/Wnt signalling centre creates an influence on the naïve cells that mimics, functionally, the primitive streak. For this reason, we refer to these structures as Directed gastruloids (D-gastruloids) to distinguish them from the original, self-organizing (SO-gastruloids).
4. Morphogenesis and gastruloids
A surprising feature of gastruloids is the contrast between their precise and proportioned spatial patterning of gene expression at the level of the body plan and, for the most part, the lack of morphogenesis and detailed patterning at the level of specific tissues (Fig. 4). This is particularly obvious in the neural progenitors in gastruloids, where their characteristic DV patterning is absent. This is probably due to the absence of a notochord, the source of Sonic Hedgehog that acts as a morphogen for this process (Sagner and Briscoe, 2017). Gastruloids display evidence of motorneuron progenitors, but they appear spatially jumbled (Beccari et al., 2018), a phenotype that has been described in Shh mutants (Bai et al., 2004). It will be interesting to see if the spinal cord in D-gastruloids, which have a notochord, exhibit some DV organization.
Fig. 4. Relationship between different embryo-like models and gastruloids.
Pictorial diagram of the relationship between various stem cell-based embryo-like models and the 3D gastruloids. ESC, Embryonic Stem Cells; TS, Trophoblast Stem cells; XEN,; Chi, Chiron; Epi, Epiblast-like Stem Cells; Mtg, Matrigel; WNTi, WNT signalling inhibition.
The paraxial mesoderm provides an intriguing example of the relationship between fate specification and morphogenesis. The T/Bra expressing domain in gastruloids exhibits an organization, in terms of gene expression, that is comparable to that of the tail bud in embryos (Beccari et al., 2018). Anterior to it, there is a clear signature of somitogenesis with sequential expression of Tbx6, Msgn, Ripply2, Tcf15, Pax3. Two features of this pattern are striking. One is that the relative proportions of the domains of gene expression correspond, approximately, to those in the embryo; most clearly seen in the localized expression of Ripply2 demarcating a narrow boundary between presomitic and somitic mesoderm. The second one is that there are no morphological differences between groups of cells: although gene expression identifies territories of paraxial (Tbx6) and somitic (Pax3, Tcf15) mesoderm, the cellular organization of these territories appears to be the same. Most strikingly, the domain expressing Tcf15 and Pax3 is not organized into somites. This suggests a disconnect between cell fate and morphogenesis in this tissue (Beccari et al., 2018; van den Brink et al., 2020; Veenvliet et al., 2020). Embedding gastruloids in Matrigel elicits segmentation in a significant number of cases (van den Brink et al., 2020) and, in some cell lines, to the appearance of somites that differentiate dermomyotome and sclerotome (Veenvliet et al., 2020). It is worth pointing out that the emergence of somites in gastruloids is associated with the presence of a relatively well-developed spinal cord like structure (Veenvliet et al., 2020).
As Matrigel provides a combination of mechanical and chemical signals (Kleinman and Martin, 2005), only further experiments with synthetic matrices functionalized chemically in different ways will resolve the contribution of each of these inputs to the morphogenesis of this tissue. In the meantime, this experiment suggests gastruloids represent a valuable system to probe the individual mechanochemical components of morphogenesis. As a proof of concept, analysis of the gene expression patterns in gastruloids reveals an upregulation of integrin signalling in the presence of matrigel that is consistent with an involvement of mechanochemical signalling in somite formation (Veenvliet et al., 2020).
In contrast with the patterning of the spinal cord and somite progenitors, morphogenesis of other tissues and organs may happen in gastruloids even in the absence of matrigel. For example, the formation of a cardiac primordium with directed interactions between the endoderm and the primary and the secondary fields (Rossi et al., 2021a). One example is the development of the endoderm, which exhibits a cell line dependent differentiation in adherent culture (Ortmann et al., 2020) and in gastruloids (van den Brink et al., 2020). In older gastruloids, e.g. 160 hrs, an endoderm derived gut tube can be observed ventral to the paraxial mesoderm and the neural progenitors, partially patterned along the AP axis. The formation of this tube can be followed from small islands of Cdh1/FoxA2 expressing cells at 72/96 h that coalesce into a continuous epithelium during the elongation of the gastruloids (Vianello, 2021). This process has not been described in the embryo although there is evidence for the presence of small clusters of FoxA2 expressing cells emerging from the posterior region of the epiblast and contributing to the endoderm (Probst et al., 2021; Scheibner et al., 2021). The formation of a gut tube in gastruloids furnishes an opportunity to study the emergence of this structure in a system that can be manipulated experimentally. In this regard, it is of interest that the proportion of gastruloids developing the endoderm can be stimulated by a hypoxic environment (López-Anguita et al., 2021).
Altogether, these examples highlight that because of their self organizing features and experimental versatility, gastruloids can be a useful tool to explore the connections between intrinsic and extrinsic influences in morphogenesis. One further example of this is the observation that D-gastruloids develop, at a low frequency, a neural plate and an extensive notochord which have never been observed in SO-gastruloids, thus making them ideal experimental subjects to unravel the mechanisms of morphogenesis.
4.1. A comparison with embryos
In addition to characterizing gastruloids in terms of their cell type composition and organization into different primordia, it is important to establish their relationship with embryos. The initial state of the ES cells, probably around E4.0, and the global patterns of gene expression throughout culture, suggest that by 48 h, the aggregates are at a stage very similar to E6.0, right at the onset of gastrulation (Beccari et al., 2018) (Fig. 4). This is likely to be the reason why the exposure to Wnt signaling at this time has such an impact on the progression of the aggregate. During the next 48 h (48–96 h), the gastruloid goes through stages that, from the perspective of gene expression, are very similar to those associated with gastrulation (see above and (Beccari et al., 2018; Rossi et al., 2021a; Veenvliet et al., 2020) (Fig. 4). It is very likely that the period of elongation (between 72 and 120 h, depending on the initial culture conditions) corresponds to the transformation of the initial cup-shaped structure into a recognizable outline of the organism in the embryo that takes place between E7.5 and E8.5 in the embryo. This correspondence is underpinned by the progressive onset of Hox gene expression. It is not clear at the moment what the timing and correspondence of events is later, but by 166 h, the Hox complexes are completely open which occurs in the mouse embryo by about E10 (Deschamps and Duboule, 2017).
Single cell analysis and spatial transcriptomics of gastruloids reveal a suite of cell types and organization of gene expression that, in the posterior region, closely resembles that of the embryo (Beccari et al., 2018; Rossi et al., 2021a; van den Brink et al., 2020; Veenvliet et al., 2020). Derivatives of the three germ layers, but particularly the different classes of mesoderm, can be seen organized along the AP and DV axes in the extending posterior region, with an even bilateral asymmetry across a clear midline. At the anterior end, the absence of neural cells results in the extension of somitogenesis to the most anterior limit where one can also find the heart primordium. In addition, during the early stages of gastruloid development, the anterior region displays a crescent of Tbx1 expression (Rossi et al., 2021a), a marker of the second heart field that also contains progenitors for the cranial mesoderm. Single cell analysis corroborates the presence of cardiopharyngeal mesoderm and also of a population of neural crest cells that tomo-sequencing allocates to the anterior region of the gastruloid. This regions also contain patches of genes defining placodal territory (Rossi et al., 2021a; van den Brink et al., 2020). This is a surprising finding as gastruloids lack brain cells which are thought to be closely associated with placodal development. Although the organization of the anterior region needs to be analyzed in more detail, there is an impression of a greater disorganization than in the extending end of the gastruloid. Perhaps this is due to the absence of anterior neural structures that play a central role in the organization of this part of the organism.
5. Human gastruloids
Early human embryos raise technical and, significantly, ethical issues that limit their use in research. The development of In Vitro Fertilization (IVF) (Johnson, 2019) opened up these studies by developing methods to culture fertilized eggs in vitro until the time of implantation. This was later used to maintain early embryos, thus enabling the study of the very early stages of development in humans and other primates. These studies, often done with surplus IVF embryos after consent, have provided valuable information about the first week of development, in particular, about the segregation of extraembryonic lineages and blastocyst formation (Gerri et al., 2020). However, studies beyond implantation have been restricted by our limited knowledge of the biology of human implantation and the difficulty of obtaining material for analysis.
Over the last few years, advances in human embryo culture have opened up the possibility of growing primate embryos ex vivo through the early stages of implantation and, maybe, even through gastrulation (Deglincerti et al., 2016; Popovic et al., 2019; Shahbazi et al., 2016; Xiang et al., 2020). However, in the case of humans, this is tightly regulated by the 14 day rule that limits human embryo culture to the initiation of gastrulation, approximately on the 14th day of development (Warnock, 1984). These advances have led to a discussion about the need to consider lifting the existing regulation, in certain circumstances, with an emerging consensus that this should be the case (Hyun et al., 2016; Lovell-Badge et al., 2021). While there is a swell of opinion in this direction and an understanding that the lifting of the rules represents a positive step for research, we also need to acknowledge that, at the moment, the evidence that such cultures can be implemented productively is feeble. After day 7, cultures of human embryos do not fare well ex vivo and the few embryos that reach day 14 are (in terms of numbers/frequencies) not in a healthy state. Studies with non-human Primates are an interesting alternative, though they present many of the same ethical issues as work with humans and for now, they suffer from similar drawbacks of low numbers of success and unclear state of the embryos at the end of the protocol (Ma et al., 2019; Niu et al., 2019).
The development of human blastoids provides a promising alternative to blastocysts as a subject of study that could be used to explore perigastrulation stages in vitro (Kagawa et al., 2021; Liu et al., 2021; Yanagida et al., 2021; Yu et al., 2021). However, so far, and despite large publicity surrounding these entities, what has been reported only partially reflects the natural blastocysts (Zhao et al., 2021). In the context of postimplantation studies, micropatterns have provided insights into the role of signals in the organization of early fate decisions (Camacho-Aguilar and Warmflash, 2020; Liu and Warmflash, 2021) but they lack a higher level of dynamic organization required for studies of gastrulation. Attempts to obtain this are being attempted by the assembly of a postimplantation epiblast from ES, TS and XEN cells but are still preliminary (Zhu et al., 2021).
An alternative line of enquiry is provided by the observation that, under controlled culture conditions, human ES cells develop structures that mimic the formation of the amnion and the initiation of gastrulation (Shao et al., 2017; Zheng et al., 2019). Although these structures collapse after a polarized initiation of gastrulation, they are providing useful information about the formation of the epiblast and highlighting the differences between primates and mouse embryos.
Another model is provided by the human equivalent of SO-gastruloids that has been developed from human ES cells using mouse gastruloids as a reference (Marikawa et al., 2020; Moris et al., 2020a). Human gastruloids are strikingly similar to their mouse counterparts: they lack anterior neural and extraembryonic cell types but include a cardiac primordium at one end and a signature of the caudal lateral epiblast with a node, at the other. Detailed analysis of the spatial expression of the genome reveals the outline of a genetic body plan with many similarities to the mouse and also some differences.
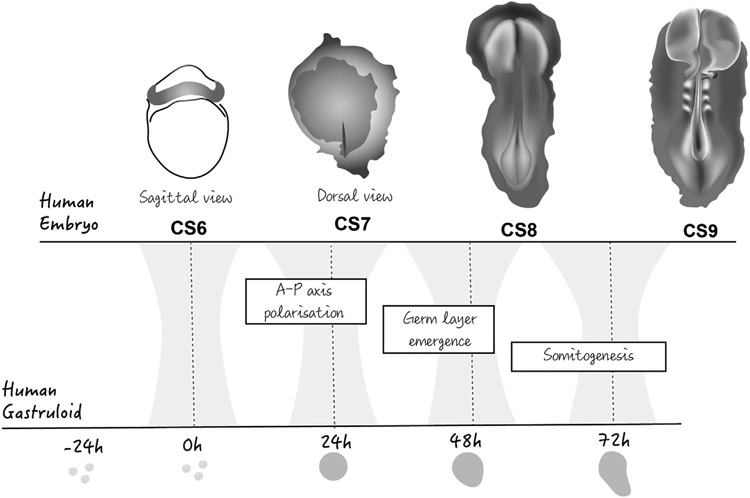
Human gastruloids appear to be extremely sensitive to the initial culture conditions, but they recapitulate some of the known germ layer interactions that have been described in the mouse e.g loss of endoderm due to loss of Nodal signalling results in a loss of early cardiac mesodermal differentiation (Moris et al., 2020a). Significantly, after 72 h in culture, human gastruloids exhibit a spatially patterned gene expression signature of somitogenesis, though as in the case of the mouse gastruloids, without somites. This suggests that, at this time, human gastruloids have started the process of somitogenesis, a feature that can be used to map them onto embryos from the Carnegie collection. With this in mind, it has been suggested that 72 h old human gastruloids are at a stage equivalent to Carnegie stage 9 or 19-21-day-old embryos, which contain 3/4 somites that are absent in Carnegie stage 8 (Fig. 6) (Moris et al., 2020a). This is not incompatible with ‘normal’ developmental timing in vitro since, despite being derived from blastocysts around day 6 or 7 of development, hES cells appear to be in a heterogeneous state but biased towards day 13–15, as they react to Wnt signalling with an immediate expression of gastrulation-associated genes (Loh et al., 2016). Thus, taking 13/15 days as the average starting age of hES cells and the formation of human gastruloids starting with pretreatment day as day 1, 72 h from aggregation would be 18/19 days of development, consistent with their being at CS9 (Fig. 5).
Fig. 6. Human embryos and human gastruloids.
Comparison of human embryogenesis and human gastruloid development (see text for details).
Fig. 5. Relationship between mouse embryos and gastruloids.
Outline of a mouse embryo at E8.5 with axial organization and germ layer derivatives indicated (A) compared to a 120 h old gastruloid (B and see text for details). (C) Examples of patterns of gene expression in 120 hrs old gastruloids. TLC2 is a reporter for canonical Wnt signalling.
It is early days, but human gastruloids have the potential to serve as a model to explore the causes of miscarriages and birth defects arising around the stage of gastrulation. In this regard, they have been shown to be effective as a test for teratology that bypasses animal testing (Kirkwood-Johnson et al., 2021; Mantziou et al., 2021; Marikawa et al., 2020). Furthermore, it will be interesting to explore their sensitivity to starting conditions, and whether they can, like their mouse counterparts, be exploited to generate organ-specific gastruloids as was recently shown in a model of posterior neural development and interactions between the peripheral nervous system and the gut (Olmsted and Paluh, 2021).
6. What is (and what is not) a gastruloid?
The above discussions and the ever-increasing number of embryonic structures derived from PSCs require a rationalization. Here we want to focus on gastruloids. Not to suggest any umbrella terminology for them, which should be done by wide discussion and consensus, but to provide an overview of current existing models and to express our view of what we understand to be a gastruloid.
We see gastruloids as three dimensional aggregates of PSCs which recapitulate what we perceive as the essence of gastrulation: symmetry breaking, multiaxial organization, germ layer specification and the laying down of a vertebrate body plan (van den Brink et al., 2014). This would, in principle, exclude the micropatterned 2D structures that display radially symmetrical germ layer organisation, but no axial organisation. However, these structures exhibit some features of gastrulation and have already adopted the term gastruloid (Simunovic and Brivanlou, 2017) For this reason, and in agreement with an emerging trend, we suggest the name 2D-gastruloid for them. We also propose a further subdivision within the overall definition of a gastruloid that we have used here between the original Self Organized (SO) model (van den Brink et al., 2014) and what we have called here a ‘directed’ (D) model (Xu et al., 2021). As for the other structures described that use gastruloids as a starting state, e.g TLS or cardiac-biased gastruloids, we see them as specialized subsets of (SO-)gastruloids (Fig. 3).
Gastruloids are not embryos, nor do they aim to fully reproduce the development and organization of embryos as, for example, blastoids do. Gastruloids are models of early mammalian development that allow us to set probing questions about how cells build tissues and organs outside an embryonic context. They are a way to explore multicellular self-organization; the relationship between genes and cells as well as between cells and tissues, and an opportunity to do experimental embryology in mammals by combining it with Genetics (Moris et al., 2020b; Steventon et al., 2021). For these reasons, it is important to always ensure that we understand what aspect(s) of embryos are being modelled in our experiments, and to chose a model that is suitable for the questions we are asking.
The notion that one can learn from deconstructing and reconstructing organs and tissues may be alien to modern developmental geneticists but this is the gist of the experiments of Driesch, Spemann and Mangold, Holtfretter and others, which have been profoundly insightful for our understanding of the mechanisms of animal development (Hamburger, 1988). For this approach to be as useful as related ones have been in the past, we need a combination of quantification and reproducibility, because we do not want to simply learn that something happens, we also want to know why and how it happens. Towards this, numbers provide statistical power and represent one of the advantages of the ES cell-based models, together with the high reproducibility of the experiments. After all, the reason we have learnt so much from embryos is because they have a higher than 90% rate of success and, for the most part and for a given species and batch, they look like each other; this allows us to pick on subtle differences and learn from them, which is the basis of mutant screens. Gastruloids fulfil many of these requirements for an experimental system.
7. Lessons and questions for the future
Gastruloids can be considered as complex, cell-based reagents to ask questions about how genes and cells interact to build embryos. From this perspective, it is important to acknowledge that it is early days and that there is much room for improvement in the technical development of the system. For example, we need to acknowledge that the current protocols to make gastruloids, particularly those from human ES cells, are open to modification and protocol improvement and we should explore those possibilities. Examples of this can be found in the D-gastruloids (Xu et al., 2021) and in the combinations with extraembryonic progenitors (Bérenger-Currias et al., 2020; Girgin et al., 2021a) (see Fig. 3). What these variations highlight is the modularity and versatility of the system that, we hope, will be explored to address specific questions.
In this exploration of the ‘phase space’ of what can be construed as a gastruloid, we should bear in mind two details. One, that we are dealing with cells which, as the complex system that they are, have many variables that we cannot control; for this reason, the current work is very much empirical. Also, and most importantly, when comparing results across laboratories we need to bear in mind that no embryonic stem cell line is a tabula rasa. Different cell lines have been shown to exhibit biases in differentiation potential (Ortmann et al., 2020), and this is most obvious in the case of iPSCs(Cuomo et al., 2020; Schwartzentruber et al., 2018). This is likely to be reflected in the development of gastruloids and organoids at large but is rarely acknowledged and yet, it is essential information when transferring protocols from one lab to another.
We also feel that the main aim of working with gastruloids and other ES cell-based embryo-like model systems, should not be to make them look as similar to embryos as possible, but to notice the differences and ponder the reasons for them. It might well be that they are experimental artifacts, but they could also reflect some fundamental property of the embryo revealed by the ES cell-based system. A good example relates to the disconnect between cell fate specification and morphogenesis observed during somitogenesis in gastruloids (see above). If we do this, we shall learn much about the engineering of mammalian development, and maybe even vertebrate embryos in general.
At present, Gastruloids have already raised a number of questions related to fundamental features of embryos that cannot be observed, and much less explored, with more classical experimental approaches and require deep investigation. For example the dissociation of morphogenesis and patterned gene expression during somitogenesis. Here are we want to highlight some others:
The requirement for a defined number of cells in the initial aggregate to form a proper gastruloids. Addressing this observation is will provide insights into the general question of the relationship between the size of tissue and organ primordia and competence to differentiate.
The emergence of a body plan in the absence of a primitive streak. On this, gastruloids represent a promising experimental system to investigate the requirement and role for this structure in organizing the germ layers and laying down the primordia for tissues and organs.
The de novo multiaxial organization of gastruloids, provides a unique ground to explore the origin of the coordinate systems that serve as a central reference for the organization and development of an embryo.
The observed disconnect between fates and morphogenesis in certain tissues in gastruloids represents a useful experimental ground to probe the role of mechanochemical signalling and geometry, as separate from that of GRNs, in morphogenesis. This should allow the identification of the gene and cell regulatory networks mediating morphogenesis.
The synchronicity of individual gastruloid development and, as we gather information, the comparison between their ‘developmental time’ and that of embryos, should allow an in-depth investigation of the basis for these dynamics.
The differential response of epithelial and non-epithelial gastruloids to the same signal will allow the study of the relationship between cell organization and signal interpretation in fate assignments and morphogenesis.
The causes for the differences in organization between SO- and D-gastruloids.
In summary, we believe Gastruloids are a useful model to understand normal mammalian development. In the case of human gastruloids they represent the only currently available window into gastrulation and the construction of the body plan. The robustness, reproducibility and scalability of the system will empower these studies, as these features makes gastruloids a suitable substrate for genetic, phenotypic and drug screens. At this, it is important to emphasize that gastruloids are not a substitute for embryo work but a useful and complementary tool, particularly where large numbers of embryos would need to be used to get the same dataset e.g molecular studies or genetic and drug screens, with implications for the 3Rs principles.
In the long term, and in addition to being tools for basic research, we also see gastruloids as a vehicle for the development of new organoid systems. Often, the emergence of an organ, a tissue or a stem cell niche, requires the interactions of several cell types and even germ layers. These interactions can be engineered successfully in the lab (Eicher et al., 2022; Koike et al., 2019) but they occur ‘for free’ in gastruloids. It will be interesting to see whether and how this fundamental feature of gastruloids can be harnessed for organ construction. However, for these endeavours to be successful, we need to remain critical of the system, acknowledge that gastruloids are not embryos but that they are models, minimal cellular models, and use them to answer specific questions concerning the emergence of tissues and organs.
Acknowledgements
We want to thank Eddy de Robertis for the opportunity to contribute to this collection and to Denis Duboule, Matthias Lutolf, David Turner, Ben Steventon, and members of our labs for discussions about gastruloids over the years and to Chaitanya Dingare for panel D in Fig. 2. AMA research is funded by an ERC AdG (MiniEmbryoBlueprint_ 834580) and the “Maria de Maeztu” Programme for Units of Excellence in R&D (Grant No. CEX2018-000792-M). YM is supported by the National Institutes of Health (R03 HD101735 and R03 HD102502) and NM is supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC011181), the UK Medical Research Council (FC011181), and the Wellcome Trust (FC011181).
References
- Amadei G, Lau KYC, De Jonghe J, Gantner CW, Sozen B, Chan C, Zhu M, Kyprianou C, Hollfelder F, Zernicka-Goetz M, 2021. Inducible stem-cell-derived embryos capture mouse morphogenetic events in vitro. Dev. Cell 56, 366–382 e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KV, Ingham PW, 2003. The transformation of the model organism: a decade of developmental genetics. Nat. Genet 33 (Suppl. l), 285–293. [DOI] [PubMed] [Google Scholar]
- Andoniadou CL, Martinez-Barbera JP, 2013. Developmental mechanisms directing early anterior forebrain specification in vertebrates. Cell. Mol. Life Sci 70, 3739–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Robertson EJ, 2009. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol 10, 91–103. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL, 2004. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6, 103–115. [DOI] [PubMed] [Google Scholar]
- Bardot ES, Hadjantonakis AK, 2020. Mouse gastrulation: coordination of tissue patterning, specification and diversification of cell fate. Mech. Dev 163, 103617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, Lutolf MP, Duboule D, Arias AM, 2018. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276. [DOI] [PubMed] [Google Scholar]
- Bérenger-Currias N, Mircea M, Adegeest A, van den Berg PR, Feliksik M, Hochane M, Idema T, Tans SJ, Semrau S, 2020. Early neurulation recapitulated in assemblies of embryonic and extraembryonic cells. bioRxiv. 10.1101/2020.02.13.947655. [DOI] [Google Scholar]
- Boxman J, Sagy N, Achanta S, Vadigepalli R, Nachman I, 2016. Integrated live imaging and molecular profiling of embryoid bodies reveals a synchronized progression of early differentiation. Sci. Rep. 6, 31623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman JM, Serup P, 2017. Properties of Embryoid Bodies, vol. 6. Wiley Interdiscip Rev Dev Biol. [DOI] [PubMed] [Google Scholar]
- Camacho-Aguilar E, Warmflash A, 2020. Insights into mammalian morphogen dynamics from embryonic stem cell systems. Curr. Top. Dev. Biol 137, 279–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus A, Davidson BP, Billiards S, Khoo P, Rivera-Perez JA, Wakamiya M, Behringer RR, Tam PP, 2000. The morphogenetic role of midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse embryo. Development 127, 1799–1813. [DOI] [PubMed] [Google Scholar]
- Cavaliere G, 2017. A 14-day limit for bioethics: the debate over human embryo research. BMC Med. Ethics 18, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermola F, D'Aniello C, Tate R, De Cesare D, Martinez-Arias A, Minchiotti G, Patriarca EJ, 2021. Gastruloid development competence discriminates different states of pluripotency. Stem Cell Rep. 16, 354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo ASE, Seaton DD, McCarthy DJ, Martinez I, Bonder MJ, Garcia-Bernardo J, Amatya S, Madrigal P, Isaacson A, Buettner F, Knights A, Natarajan KN, HipSci C, Vallier L, Marioni JC, Chhatriwala M, Stegle O, 2020. Publisher Correction: single-cell RNA-sequencing of differentiating iPS cells reveals dynamic genetic effects on gene expression. Nat. Commun 11, 1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglincerti A, Croft GF, Pietila LN, Zernicka-Goetz M, Siggia ED, Brivanlou AH, 2016. Self-organization of the in vitro attached human embryo. Nature 533, 251–254. [DOI] [PubMed] [Google Scholar]
- Deschamps J, Duboule D, 2017. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Dev. 31, 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin GS, Behringer RR, 2004. Diversity of germ layer and axis formation among mammals. Semin. Cell Dev. Biol 15, 619–629. [DOI] [PubMed] [Google Scholar]
- Eicher AK, Kechele DO, Sundaram N, Berns HM, Poling HM, Haines LE, Sanchez JG, Kishimoto K, Krishnamurthy M, Han L, Zorn AM, Helmrath MA, Wells JM, 2022. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell 29, 36–51 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y, 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56. [DOI] [PubMed] [Google Scholar]
- Fu J, Warmflash A, Lutolf MP, 2021. Stem-cell-based embryo models for fundamental research and translation. Nat. Mater 20, 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM, 2005. Germ layer induction from embryonic stem cells. Exp. Hematol 33, 955–964. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Eggenschwiler JT, Caspary T, Alcorn HL, Wyler MR, Huangfu D, Rakeman AS, Lee JD, Feinberg EH, Timmer JR, Anderson KV, 2005. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc. Natl. Acad. Sci. U. S. A 102, 5913–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerri C, Menchero S, Mahadevaiah SK, Turner JMA, Niakan KK, 2020. Human embryogenesis: a comparative perspective. Annu. Rev. Cell Dev. Biol 36, 411–440. [DOI] [PubMed] [Google Scholar]
- Ghimire S, Mantziou V, Moris N, Arias AM, 2021. Human gastrulation: the embryo and its models. Dev. Biol 474, 100–108. [DOI] [PubMed] [Google Scholar]
- Girgin MU, Broguiere N, Hoehnel S, Brandenberg N, Mercier B, Arias AM, Lutolf MP, 2021a. Bioengineered embryoids mimic post-implantation development in vitro. Nat. Commun 12, 5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgin MU, Broguiere N, Mattolini L, Lutolf MP, 2021b. Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. Stem Cell Rep. 16, 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillomot M, Turbe A, Hue I, Renard JP, 2004. Staging of ovine embryos and expression of the T-box genes Brachyury and Eomesodermin around gastrulation. Reproduction 127, 491–501. [DOI] [PubMed] [Google Scholar]
- Haeckel E, 1874. Die Gastrae Theorie, die phylogenetische Classification des Thierreichs und die Homologie der Keimblatter. Jena Ztschr. Naturwiss 8, 1–55. [Google Scholar]
- Hamburger V, 1988. The Heritage of Experimental Embryology. Oxford University Press. [Google Scholar]
- Harrison RG, 1907. Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med 4, 104–143. [Google Scholar]
- Hassoun R, Schwartz P, Feistel K, Blum M, Viebahn C, 2009. Axial differentiation and early gastrulation stages of the pig embryo. Differentiation 78, 301–311. [DOI] [PubMed] [Google Scholar]
- Hue I, Renard JP, Viebahn C, 2001. Brachyury is expressed in gastrulating bovine embryos well ahead of implantation. Dev. Gene. Evol 211, 157–159. [DOI] [PubMed] [Google Scholar]
- Hyun I, Wilkerson A, Johnston J, 2016. Embryology policy: revisit the 14-day rule. Nature 533, 169–171. [DOI] [PubMed] [Google Scholar]
- Johnson MH, 2019. A short history of in vitro fertilization (IVF). Int. J. Dev. Biol 63, 83–92. [DOI] [PubMed] [Google Scholar]
- Kagawa H, Javali A, Khoei HH, Sommer TM, Sestini G, Novatchkova M, Scholte Op Reimer Y, Castel G, Bruneau A, Maenhoudt N, Lammers J, Loubersac S, Freour T, Vankelecom H, David L, Rivron N, 2021. Human blastoids model blastocyst development and implantation. Nature 601, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP, 1999. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126, 4691–4701. [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, Tam PP, 2001. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development 128, 3623–3634. [DOI] [PubMed] [Google Scholar]
- Kirkwood-Johnson L, Katayama N, Marikawa Y, 2021. Dolutegravir impairs stem cell-based 3D morphogenesis models in a manner dependent on dose and timing of exposure: an implication for its developmental toxicity. Toxicol. Sci 184, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR, 2005. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol 15, 378–386. [DOI] [PubMed] [Google Scholar]
- Koike H, Iwasawa K, Ouchi R, Maezawa M, Giesbrecht K, Saiki N, Ferguson A, Kimura M, Thompson WL, Wells JM, Zorn AM, Takebe T, 2019. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 574, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G, 2005. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc. Natl. Acad. Sci. U. S. A 102, 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G, 2004. Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651–1662. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK, 2008. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev. Cell 15, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA, 2013. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Warmflash A, 2021. Self-organized signaling in stem cell models of embryos. Stem Cell Rep. 16, 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tan J, Schrödrr J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun B, Chen K, Sun G, Zhou Y, Poppe D, Lister R, Clark AT, Rackham O, Zenker J, Polo JM, 2021. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature. 10.1038/s41586-021-03372-y. [DOI] [PubMed] [Google Scholar]
- Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, Shyh-Chang N, Fernhoff NB, George BM, Wernig G, Salomon RE, Chen Z, Vogel H, Epstein JA, Kundaje A, Talbot WS, Beachy PA, Ang LT, Weissman IL, 2016. Mapping the pairwise choices leading from pluripotency to human bone, heart, and other mesoderm cell types. Cell 166, 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Anguita N, Ipek Gassaloglu S, Stötzel M, Typou M, Virta I, Hetzel S, Buschow R, Koksal B, Atilla D, Maitschke-Rajasekharan R, Chen R, Mattei AL, Bedzhov I, Meierhofer D, Meissner A, Veenvliet JV, Bulut-Karslioglu A, 2021. Hypoxia induces a transcriptional early primitive streak signature in pluripotent cells enhancing spontaneous elongation and lineage representation in gastruloids. bioRxiv. 10.1101/2021.07.21.452906. [DOI] [Google Scholar]
- Lovell-Badge R, Anthony E, Barker RA, Bubela T, Brivanlou AH, Carpenter M, Charo RA, Clark A, Clayton E, Cong Y, Daley GQ, Fu J, Fujita M, Greenfield A, Goldman SA, Hill L, Hyun I, Isasi R, Kahn J, Kato K, Kim JS, Kimmelman J, Knoblich JA, Mathews D, Montserrat N, Mosher J, Munsie M, Nakauchi H, Naldini L, Naughton G, Niakan K, Ogbogu U, Pedersen R, Rivron N, Rooke H, Rossant J, Round J, Saitou M, Sipp D, Steffann J, Sugarman J, Surani A, Takahashi J, Tang F, Turner L, Zettler PJ, Zhai X, 2021. ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Rep. 16, 1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhai J, Wan H, Jiang X, Wang X, Wang L, Xiang Y, He X, Zhao ZA, Zhao B, Zheng P, Li L, Wang H, 2019. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science 366. [DOI] [PubMed] [Google Scholar]
- Mantziou V, Baillie-Benson P, Jaklin M, Kustermann S, Arias AM, Moris N, 2021. In vitro teratogenicity testing using a 3D, embryo-like gastruloid system. Reprod. Toxicol 105, 72–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Chen HR, Menor M, Deng Y, Alarcon VB, 2020. Exposure-based assessment of chemical teratogenicity using morphogenetic aggregates of human embryonic stem cells. Reprod. Toxicol 91, 74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Tamashiro DA, Fujita TC, Alarcon VB, 2009. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiah N, Despin-Guitard E, Stower M, Nahaboo W, Eski ES, Singh SP, Srinivas S, Migeotte I, 2020. Asymmetry in the frequency and position of mitosis in the mouse embryo epiblast at gastrulation. EMBO Rep. 21, e50944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole K, Guignard L, Amat F, Berger A, Malandain G, Royer LA, Turaga SC, Branson K, Keller PJ, 2018. In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell 175, 859–876 e833. [DOI] [PubMed] [Google Scholar]
- Moris N, Anlas K, van den Brink S, Alemany A, Balayo T, van Oudenaarden A, Martinez Arias A, 2020a. An in vitro model of early anterior posterior organization during human development. Nature 582, 410–415. [DOI] [PubMed] [Google Scholar]
- Moris N, Martinez Arias A, Steventon B, 2020b. Experimental embryology of gastrulation: pluripotent stem cells as a new model system. Curr. Opin. Genet. Dev 64, 78–83. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y, 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785. [DOI] [PubMed] [Google Scholar]
- Niu Y, Sun N, Li C, Lei Y, Huang Z, Wu J, Si C, Dai X, Liu C, Wei J, Liu L, Feng S, Kang Y, Si W, Wang H, Zhang E, Zhao L, Li Z, Luo X, Cui G, Peng G, Izpisua Belmonte JC, Ji W, Tan T, 2019. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science 366. [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Hadjantonakis AK, 2010. Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr. Opin. Genet. Dev 20, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted ZT, Paluh JL, 2021. Co-development of central and peripheral neurons with trunk mesendoderm in human elongating multi-lineage organized gastruloids. Nat. Commun 12, 3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann D, Brown S, Czechanski A, Aydin S, Muraro D, Huang Y, Tomaz RA, Osnato A, Canu G, Wesley BT, Skelly DA, Stegle O, Choi T, Churchill GA, Baker CL, Rugg-Gunn PJ, Munger SC, Reinholdt LG, Vallier L, 2020. Naive pluripotent stem cells exhibit phenotypic variability that is driven by genetic variation. Cell Stem Cell 27, 470–481 e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Bialecka M, Gomes Fernandes M, Taelman J, Van Der Jeught M, De Sutter P, Heindryckx B, Chuva De Sousa Lopes SM, 2019. Human blastocyst outgrowths recapitulate primordial germ cell specification events. Mol. Hum. Reprod 25, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst S, Sagar, Tosic J, Schwan C, Grun D, Arnold SJ, 2021. Spatiotemporal Sequence of Mesoderm and Endoderm Lineage Segregation during Mouse Gastrulation. Development, vol. 148. [DOI] [PubMed] [Google Scholar]
- Ramkumar N, Anderson KV, 2011. SnapShot: mouse primitive streak. Cell 146, 488–488 e482. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Hadjantonakis AK, 2014. The dynamics of morphogenesis in the early mouse embryo. Cold Spring Harbor Perspect. Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivie J, Truckenmuller RK, van Oudenaarden A, van Blitterswijk CA, Geijsen N, 2018. Blastocyst-like structures generated solely from stem cells. Nature 557, 106–111. [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP, 2009. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713. [DOI] [PubMed] [Google Scholar]
- Rossi G, Broguiere N, Miyamoto M, Boni A, Guiet R, Girgin M, Kelly RG, Kwon C, Lutolf MP, 2021a. Capturing cardiogenesis in gastruloids. Cell Stem Cell 28, 230–240 e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Giger S, Hübscher T, Lutolf M, 2021b. Gastruloids as in vitro models of embryonic blood development with spatial and temporal resolution. bioRxiv. 10.1101/2021.03.21.436320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagner A, Briscoe J, 2017. Morphogen Interpretation: Concentration, Time, Competence, and Signaling Dynamics, vol. 6. Wiley Interdiscip Rev Dev Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykali B, Mathiah N, Nahaboo W, Racu ML, Hammou L, Defrance M, Migeotte I, 2019. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibner K, Schirge S, Burtscher I, Buttner M, Sterr M, Yang D, Bottcher A, Ansarullah, Irmler M, Beckers J, Cernilogar FM, Schotta G, Theis FJ, Lickert H, 2021. Epithelial cell plasticity drives endoderm formation during gastrulation. Nat. Cell Biol 23, 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroter C, Rue P, Mackenzie JP, Martinez Arias A, 2015. FGF/MAPK signaling sets the switching threshold of a bistable circuit controlling cell fate decisions in embryonic stem cells. Development 142, 4205–4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber J, Foskolou S, Kilpinen H, Rodrigues J, Alasoo K, Knights AJ, Patel M, Goncalves A, Ferreira R, Benn CL, Wilbrey A, Bictash M, Impey E, Cao L, Lainez S, Loucif AJ, Whiting PJ, Consortium H, Gutteridge A, Gaffney DJ, 2018. Molecular and functional variation in iPSC-derived sensory neurons. Nat. Genet 50, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NM, Campbell A, Devito LG, Ilic D, Khalaf Y, Niakan KK, Fishel S, Zernicka-Goetz M, 2016. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol 18, 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi MN, Siggia ED, Zernicka-Goetz M, 2019. Self-organization of stem cells into embryos: a window on early mammalian development. Science 364, 948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Taniguchi K, Townshend RF, Miki T, Gumucio DL, Fu J, 2017. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun 8, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, Martinez Arias A, Sutherland A, 2021. The primitive streak and cellular principles of building an amniote body through gastrulation. Science 374, abg1727. [DOI] [PubMed] [Google Scholar]
- Simunovic M, Brivanlou AH, 2017. Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis. Development 144, 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM, Holland PW, Graham CF, 1993. The zootype and the phylotypic stage. Nature 361, 490–492. [DOI] [PubMed] [Google Scholar]
- Smith AG, 2001. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol 17, 435–462. [DOI] [PubMed] [Google Scholar]
- Snow MH, 1977. Gastrulation in the mouse: growth and regionalization of the epiblast. J. Embryol. exp. Morph 42, 293–303. [Google Scholar]
- Sozen B, Amadei G, Cox A, Wang R, Na E, Czukiewska S, Chappell L, Voet T, Michel G, Jing N, Glover DM, Zernicka-Goetz M, 2018. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol 20, 979–989. [DOI] [PubMed] [Google Scholar]
- Stern CD, 2004. Gastrulation: from Cells to Embryos. Cold Spring Harbour Laboratory Press, CSH; NY. [Google Scholar]
- Steventon B, Busby L, Arias AM, 2021. Establishment of the vertebrate body plan: rethinking gastrulation through stem cell models of early embryogenesis. Dev. Cell 56, 2405–2418. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Hamada H, 2012. Cell fate decisions and axis determination in the early mouse embryo. Development 139, 3–14. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Hamada H, 2007. Origin of body axes in the mouse embryo. Curr. Opin. Genet. Dev 17, 344–350. [DOI] [PubMed] [Google Scholar]
- Tam P, Gad J, 2004. Gastrulation in the mouse embryo. In: Stern CD (Ed.), Gastrulation; from Cells to Embryos. Cold Spring Harbor Laboratory Press, pp. 233–262. [Google Scholar]
- Tam PP, Behringer RR, 1997. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev 68, 3–25. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R, 2008. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortelote GG, Hernandez-Hernandez JM, Quaresma AJ, Nickerson JA, Imbalzano AN, Rivera-Perez JA, 2013. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev. Biol 374, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon FB, Mullins MC, 2015. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin. Cell Dev. Biol 42, 118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D, Alonso-Crisostomo L, Girgin M, Baillie-Johnson P, Glodowski C, Hayward P, Collignon J, Gustavsen MW, Serup P, Steventon B, Lutolf M, Martinez Arias A, 2017a. Gastruloids Develop the Three Body Axes in the Absence of Extraembryonic Tissues and Spatially Localised Signalling bioRxiv, doi.org/ 10.1101/104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DA, Girgin M, Alonso-Crisostomo L, Trivedi V, Baillie-Johnson P, Glodowski CR, Hayward PC, Collignon J, Gustavsen C, Serup P, Steventon B, M PL, Arias AM, 2017b. Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development 144, 3894–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DA, Hayward PC, Baillie-Johnson P, Rue P, Broome R, Faunes F, Martinez Arias A, 2014a. Wnt/beta-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development 141, 4243–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DA, Rue P, Mackenzie JP, Davies E, Martinez Arias A, 2014b. Brachyury cooperates with Wnt/ss-Catenin signalling to elicit Primitive Streak like behaviour in differentiating mouse ES cells. BMC Biol. 12, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DA, Trott J, Hayward P, Rue P, Martinez Arias A, 2014c. An interplay between extracellular signalling and the dynamics of the exit from pluripotency drives cell fate decisions in mouse ES cells. Biol. Open 3, 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyser RCV, Mahammadov E, Nakanoh S, Vallier L, Srinivas S, 2020. A spatially resolved single cell atlas of human gastrulation. bioRxiv. 10.1101/2020.07.21.213512. [DOI] [Google Scholar]
- van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivie J, Baillie-Johnson P, Nichols J, Sonnen KF, Martinez Arias A, van Oudenaarden A, 2020. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409. [DOI] [PubMed] [Google Scholar]
- van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, Martinez Arias A, 2014. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden F, St Johnston D, 1999. The polarisation of the anterior-posterior and dorsalventral axes during Drosophila oogenesis. Curr. Opin. Genet. Dev 9, 396–404. [DOI] [PubMed] [Google Scholar]
- Veenvliet JV, Bolondi A, Kretzmer H, Haut L, Scholze-Wittler M, Schifferl D, Koch F, Guignard L, Kumar AS, Pustet M, Heimann S, Buschow R, Wittler L, Timmermann B, Meissner A, Herrmann BG, 2020. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 370. [DOI] [PubMed] [Google Scholar]
- Vianello SLM, 2021. In vitro endoderm emergence and self-organisation in the absence of extraembryonic tissues and embryonic architecture. bioRxiv. 10.1101/2020.06.07.138883. [DOI] [Google Scholar]
- Viebahn C, Stortz C, Mitchell SA, Blum M, 2002. Low proliferative and high migratory activity in the area of Brachyury expressing mesoderm progenitor cells in the gastrulating rabbit embryo. Development 129, 2355–2365. [DOI] [PubMed] [Google Scholar]
- Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH, 2014. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock M, 1984. In: Office, H.S. (Ed.), Report of the Committee of Inquiry into Human Fertilisation and Embryology, p. 9314. [Google Scholar]
- Williams M, Burdsal C, Periasamy A, Lewandoski M, Sutherland A, 2012. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dynam 241, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymeersch FJ, Wilson V, Tsakiridis A, 2021. Understanding Axial Progenitor Biology in Vivo and in Vitro. Development, vol. 148. [DOI] [PubMed] [Google Scholar]
- Xiang L, Yin Y, Zheng Y, Ma Y, Li Y, Zhao Z, Guo J, Ai Z, Niu Y, Duan K, He J, Ren S, Wu D, Bai Y, Shang Z, Dai X, Ji W, Li T, 2020. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577, 537–542. [DOI] [PubMed] [Google Scholar]
- Xu PF, Borges RM, Fillatre J, de Oliveira-Melo M, Cheng T, Thisse B, Thisse C, 2021. Construction of a mammalian embryo model from stem cells organized by a morphogen signalling centre. Nat. Commun 12, 3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida A, Spindlow D, Nichols J, Dattani A, Smith A, Guo G, 2021. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28, 1016–1022 e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, Wang L, Wang K, Zhao S, Hon GC, Wu J, 2021. Blastocyst-like structures generated from human pluripotent stem cells. Nature 591, 620–626. [DOI] [PubMed] [Google Scholar]
- Zhao C, Plaza Reyes A, Schell J, Weltner J, Ortega J, Zheng Y, Björklund A, Rossant J, Fu J, Petropoulos S, Lanner F, 2021. Reprogrammed blastoids contain amnion-like cells but not trophectoderm. bioRxiv. 10.1101/2021.05.07.442980. [DOI] [Google Scholar]
- Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL, Fu J, 2019. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Shahbazi M, Martin A, Zhang C, Sozen B, Borsos M, Mandelbaum RS, Paulson RJ, Mole MA, Esbert M, Titus S, Scott RT, Campbell A, Fishel S, Gradinaru V, Zhao H, Wu K, Chen ZJ, Seli E, de Los Santos MJ, Zernicka Goetz M, 2021. Human embryo polarization requires PLC signaling to mediate trophectoderm specification. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]