Abstract
Background
Myocardial infarction on non-occluded coronary artery represents a very specific subset of acute coronary syndrome (ACS). Coronary subclavian steal syndrome (CSSS) is defined by a left subclavian artery stenosis in case of (i) left internal mammary artery (LIMA) used to bypass left anterior descending artery (LAD) and (ii) >75% stenosis of the left subclavian artery prior to the origin of the LIMA to LAD graft. Here we report the case of a CSSS causing ACS.
Case summary
A 71-year-old man with history of LIMA to LAD coronary artery bypass surgery was admitted to the nephrology intensive care unit for acute kidney injury requiring dialysis. Due to rapid deterioration, altered left ventricular ejection fraction and elevated c-troponin levels, an urgent coronary angiography was performed. It revealed a subtotal occlusion of the left subclavian artery prior to the origin of the LIMA to LAD graft. This was responsible for a severely altered coronary flow in the LIMA and LAD. Revascularization of the proximal left subclavian artery with a stent was performed, enabling instant recovery of distal coronary flows.
Discussion
ACS due to CSSS in this report highlights the complexity of the cardio–renal interaction. Patients with coronary artery bypass graft and chronic kidney disease commonly exhibit a higher risk for severe progression of atherosclerosis at multiple sites. CSSS treatments include secondary prevention measures and revascularization (if indicated) such as an endovascular approach.
Keywords: Coronary subclavian steal syndrome, Acute coronary syndrome, Coronary artery bypass grafting, Left subclavian artery stenosis, Case report
Learning points.
Coronary subclavian steal syndrome (CSSS) is a presumed to be rare complication. It can only exist if the bypass is located behind a subclavian stenosis.
CSSS treatments include secondary prevention measures after coronary artery bypass graft and regular cardiovascular medical visits. If revascularization is indicated, the first-line strategy involves an endovascular approach.
Step-by-step angioplasty technique for CSSS is challenging. It requires multidisciplinary skills to both treat the vascular target lesion and control the bypass.
Introduction
Ischaemic trigger represents the most common cause of cardiogenic shock,1 and it occurs frequently in the setting of acute coronary syndrome (ACS) regardless of whether ST-segment elevation is present. Myocardial infarction with non-occluded coronary arteries (MINOCAs) represent a very specific subset of ACS that involves coronary dissection, coronary spasm, coronary thromboembolism, myocarditis, Takotsubo syndrome and supply–demand mismatch.2 Coronary subclavian steal syndrome (CSSS) is a cause of MINOCA, and it is characterized by inversion of flow in the internal thoracic artery due to subclavian artery’s stenosis ahead of the left internal mammary artery (LIMA). Stenosis of the subclavian artery is evidenced in 11.8% of patients with coronary artery disease that require coronary artery bypass graft (CABG) and known for peripheral arterial disease. CSSS occurs in 0.2–6.8% of CABG with LIMA. The most common cause for subclavian artery stenosis is atherosclerosis, and more rarely arteritis, radiation, fibromuscular dysplasia, and compression syndromes.3This report presents a case of a CSSS causing an ACS.
Timeline
| Date | Event |
|---|---|
| Day 1 | A patient with a history of coronary artery bypass surgery was referred to the emergency department with deteriorating general condition and oliguria. |
| Day 3 | The patient was transferred to the nephrology intensive care unit for dialysis due to severe acute kidney injury. |
| Day 6 | Acute heart failure and worsening clinical condition required emergency haemodialysis and non-invasive ventilation. A coronary angiography was performed because of elevated cardiac troponin levels and a global impairment in left ventricular systolic function with left ventricular ejection fraction (LVEF) of 20%. It revealed significant stenosis of the middle left circumflex artery (LCX) and a subtotal occlusion stenosis of the left subclavian artery prior to the origin of the left internal mammary artery (LIMA) to left anterior descending artery (LAD) graft. The patient was treated with angioplasty of the middle LCX and angioplasty of the left subclavian artery. |
| Day 9 | Post-procedural echocardiography indicated a LVEF of 40%. Dobutamine was discontinued without complication. |
| Day 21 | Recovery of diuresis. Discontinuation of dialysis therapy. |
| Day 39 | The patient was discharged home on Day 39 due to marked socio-economic precarity, a lack of access to domiciliary care, and difficulties in access to rehabilitation and care services. |
Case presentation
A 71-year-old man was referred to the emergency department for deterioration of general condition, chest pain, abdominal pain, and oliguria evolving for 7 days. The patient had history of ischaemic heart disease with prior revascularization by percutaneous angioplasty with a bare metal stent of the right coronary artery (RCA) in 2009. Further coronary artery bypass grafting (CABG) involving the left internal mammary artery (LIMA) used to bypass an occlusion of the left anterior descending artery (LAD) was obtained later in 2009. Known left ventricular ejection function (LVEF) was 50%, and past medical history included a chronic kidney disease (stage G3B A3; creatinine level 149 µmol/L) of diabetic origin, chronic obstructive pulmonary disease GOLD Stage IIIA, hypertension, dyslipidemia, and Type 2 diabetes with insulin therapy. The patient had no history of peripheral vascular disease or vertebrobasilar insufficiency.
The biological assessment showed acute renal failure of undetermined origin associated with hypokalaemia in an anuric patient. The physical examination was unremarkable, with an initial blood pressure (BP) of 125/79 mm Hg in the right arm, a heart rate of 98 beats/min and no sign of heart failure (no signs of right or left heart failure, no significant heart murmur and no chest pain). The electrocardiogram (ECG) showed no ST-segment changes. Laboratory examinations at admission revealed an acute kidney injury (AKI) with a markedly elevated creatinine level at 262 µmol/L (normal range: 49–90 µmol/L) and hypokalaemia (K+ = 2.12 mmol/L, normal range: 3.5–5 mmol/L). The initial high-sensitivity cardiac troponin I was 418 ng/L (normal range: <57 ng/L). Due to AKI and anuria, the patient was transferred to the nephrology intensive care unit.
The patient rapidly experienced acute heart failure and worsening clinical status that required emergent haemodialysis and non-invasive ventilation. Haemodynamic parameters (heart rate and BP) were still normal (99/61 mmHg in the right arm and 78 beats per minute, respectively), and the physical examination revealed orthopnea with signs of acute pulmonary oedema. Lactate measurement was still normal at this time. The relative kinetic change of high-sensitivity cardiac troponin I was 6.395% (249.930 ng/L; normal range: <57 ng/L). In contrast to the initial ECG, a repeat ECG showed new and discreet ST-segment depression in lateral leads (V5, V6, and aVL) and ST-segment elevation in aVR. A transthoracic echocardiography (TTE) revealed a severely impaired LVEF of 20%, a global hypokinesis of the LV with no specific regional wall motion abnormalities and a decreased cardiac index of 1.5 L/min/m² (normal: 2.8–4.2 L/min/m2).
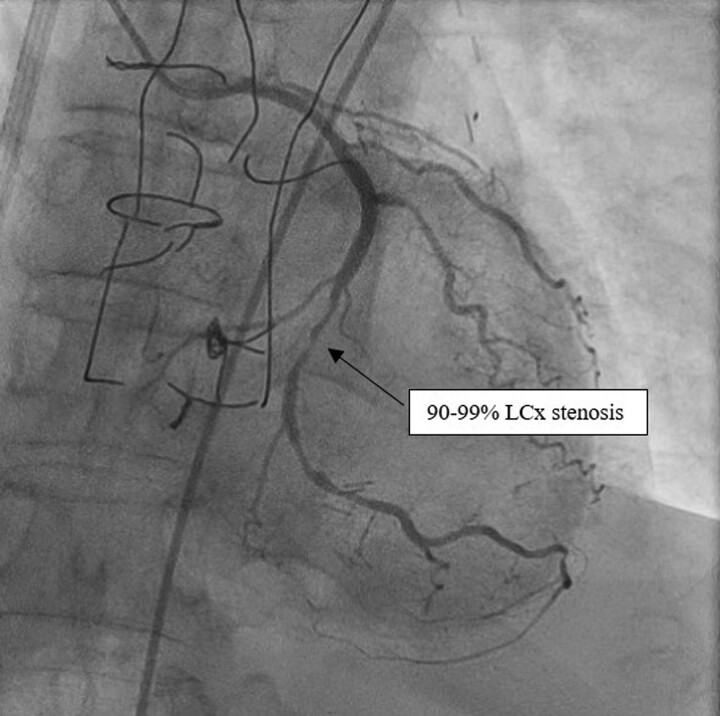
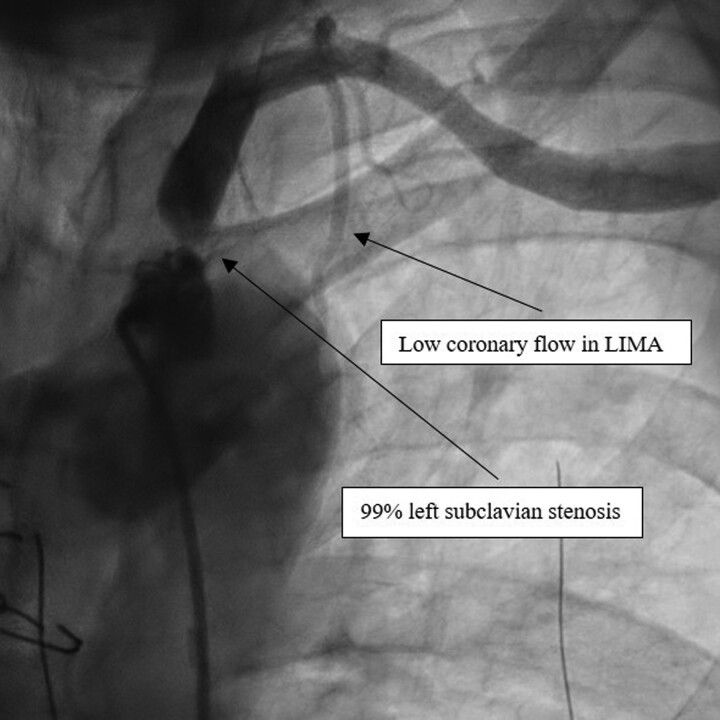
Due to rapid deterioration, medical management included aggressive medical therapy with inotropic support and urgent coronary angiography by right femoral access. The presence of myocardial ischaemia was not tested because of the low cardiac output, the very high level of troponin, and the severe impairment of LVEF highly suggestive of cardiogenic shock with an ongoing ischaemic trigger. The coronary angiography demonstrated a chronic occlusion of the proximal LAD, a significant stenosis of the middle left circumflex artery (LCX) (Figure 1 and Supplementary material online, Video S1) and a permeable RCA. A subtotal occlusion stenosis of the left subclavian artery prior to the origin of the LIMA to LAD graft was evidenced and responsible for a low coronary flow in the LIMA and LAD (Figure 2 and Supplementary material online, Video S2).
Figure 1.
Left coronary angiogram. Angiogram of the left coronary artery revealing a chronic occlusion of the proximal left anterior descending artery and a significant stenosis (90–99%) of the middle circumflex artery.
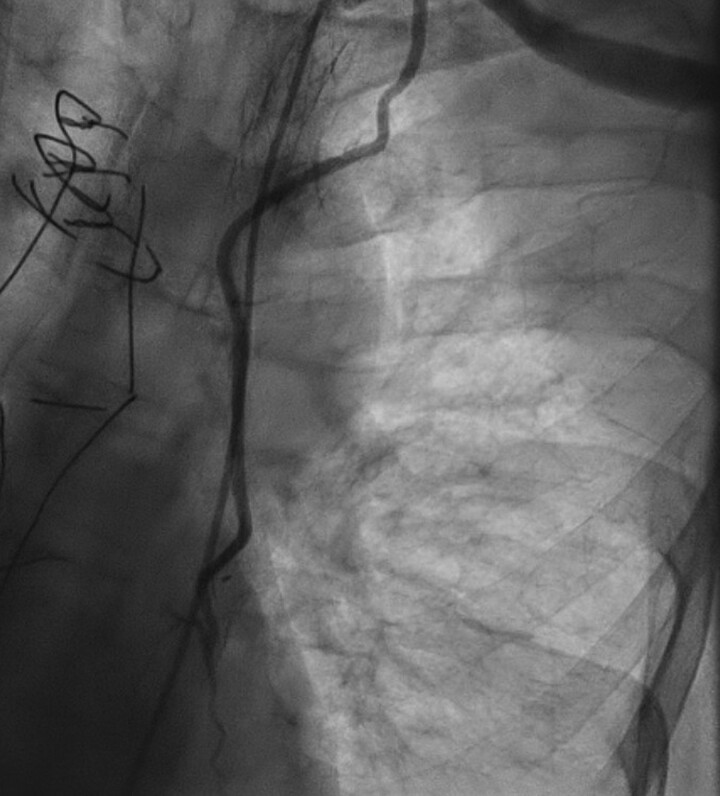
Figure 2.
Left subclavian angiogram. Left subclavian angiogram revealing a subtotal occlusion of the left subclavian artery responsible for a low coronary flow in the left internal mammary artery and distal left anterior descending artery.
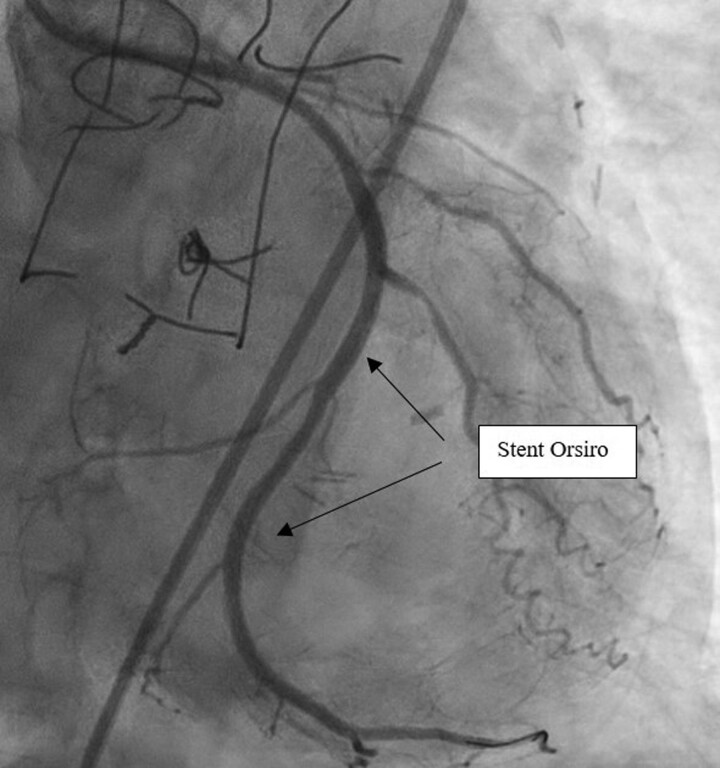
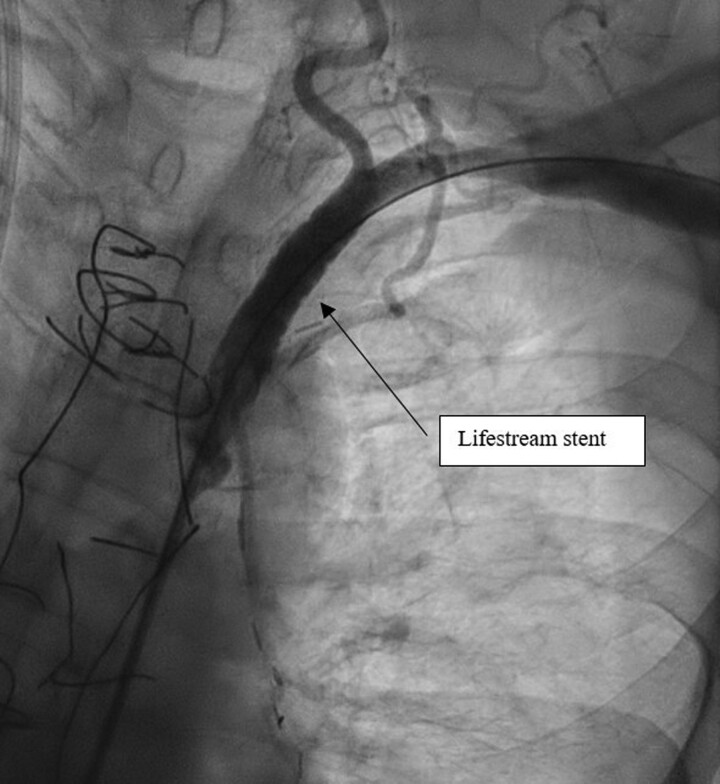
Successful angioplasty of the middle LCX was performed with two Orsiro stents (Biotronik®) (Figure 3 and Supplementary material online, Video S3). The angioplasty was performed using a 3.75 6 French Extra Back Up catheter. A Balance Middleweight (Abbott®) 0.014 guidewire was used to catheterize the lesion in the middle LCX over which pre-dilation of the coronary lesion was conducted using by a Ryurei (Terumo®) 2.5*20 mm balloon inflated to 14 atmospheres for a maximum of 10 s. Then, the two Orsiro stents (Biotronik®) were deployed using the same guidewire. The left subclavian artery stenosis was crossed with a Terumo Angled 0.035 mm guidewire (Terumo®) in a JR4 catheter 6F 125 cm catheter (Medtronic®). The guidewire was changed by a Starter J 0.35 260 cm (Boston Scientific®) to facilitate the change to a Performer 12F X 80 cm catheter (Cook®). The stenosis was predilated using a Mustang balloon of 8 mm × 40 mm × 75 cm (Boston Scientific®). Then, implantation of a 10*38 mm Lifestream stent (Bard Medical®) impacted at 16 atmospheres was performed. This helped restoring blood flow in the LIMA (Figure 4 and Supplementary material online, Video S4). LIMA angiography was finally performed and showed no occlusion, stenosis nor thrombosis in the LIMA and the distal LAD (Figure 5 and Supplementary material online, Video S5).
Figure 3.
Left coronary angiogram after circumflex stent implantation. Left coronary angiogram after circumflex angioplasty showed a successful implantation of active stent with TIMI 3 perfusion.
Figure 4.
Left subclavian angiogram after angioplasty. Left subclavian angiogram after successful angioplasty [10*38 mm Lifestream stent (Bard Medical®)].
Figure 5.
Left internal mammary artery angiogram. Permeable left internal mammary artery.
Post-procedural echocardiography indicated a LVEF of 40%. Rapid recovery of diuresis and baseline renal function were observed. The patient was discharged 33 days after the procedure. Dual antithrombotic therapy at discharge included aspirin (75 mg/day continuously) and a P2Y12 inhibitor (Clopidogrel 75 mg/day) for 12 months.
Discussion
There is only a limited number of case reports or case series in the available literature regarding CCCS. The present case is unique in the way that we report a CSSS responsible for cardiogenic shock, and such clinical presentation has not been described before to our knowledge. Finally, we discuss the step-by-step angioplasty technique for CSSS with high quality imaging, highly relevant to fellows and EHJ readers involved in everyday practice in Cath labs. CSSS is defined by left subclavian artery stenosis in the case of (i) the LIMA being used to bypass a LAD stenosis or occlusion, and (ii) >75% stenosis of the left subclavian artery prior to the origin of the LIMA to LAD graft.4 CSSS is a presumed to be rare complication, but can occur in 0.2–6.8% of patients with a LIMA graft.3
First, this case report highlights the complexity of the cardiorenal interaction in patients presenting with cardiogenic shock and the importance of measuring BP sequentially or simultaneously in both arms. Indeed, measurement of BP was only performed in the right arm in the present case. A substantial difference in BP from one arm to the other (right to left gradient) would have been a warning sign for a stenosis of the left subclavian artery.5 Second, patients with CABG and chronic kidney disease commonly exhibit a higher risk for severe progression of atherosclerosis at multiple sites .6,7 In this report, the patient underwent careful duplex evaluation of the carotid, vertebral and subclavian arteries as part of the pre-operative vascular assessment before CABG. Pre-operative vascular assessment was normal in 2009. Chronic kidney disease is commonly associated with an increase in atherosclerotic burden,8 and the quick progression of atherosclerosis in this case was likely multifactorial (diabetes, hypertension, dyslipidaemia, overweight and coronary artery disease). Finally, CSSS treatments include secondary prevention measures after CABG such as controlling risk factors, adhering to evidence-based secondary preventive medications (e.g. antithrombotic therapy and statins) and regular cardiovascular medical visits.9 If revascularization is indicated: the first-line strategy involves an endovascular approach,3 and surgery should only be considered as a rescue strategy.9
Conclusions
Patients with CABG and chronic kidney disease commonly exhibit a higher risk of progression for non-coronary atherosclerosis. The present case highlights the clinical scenario of a de novo left subclavian artery stenosis responsible for CSSS.
Supplementary Material
Contributor Information
Adrien Carmona, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Benjamin Marchandot, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Mylene Sagnard, Division of Nephrology Critical Care and Transplant Nephrology, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Olivier Morel, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Lead author biography
 Dr Adrien Carmona studied at the University of Paris in the Faculty of Medicine of Paris Descartes during the years 2006–2013. He took his first step as a medical student in the Parisian hospitals of Cochin, Hotel Dieu, Georges Pompidou and Necker. His years of faculties pushed him to choose the specialty of Cardiology at the University Hospital of Strasbourg. He graduated MD in 2019 and since then has held a position as Head of Clinic in the Interventional Cardiology Department of the University Hospitals of Strasbourg.
Dr Adrien Carmona studied at the University of Paris in the Faculty of Medicine of Paris Descartes during the years 2006–2013. He took his first step as a medical student in the Parisian hospitals of Cochin, Hotel Dieu, Georges Pompidou and Necker. His years of faculties pushed him to choose the specialty of Cardiology at the University Hospital of Strasbourg. He graduated MD in 2019 and since then has held a position as Head of Clinic in the Interventional Cardiology Department of the University Hospitals of Strasbourg.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online
Slide sets: A fully edited slide set detailing this case and suitable for local presentations is avaialble online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with the COPE guidelines.
Conflict of interest: None declared.
Funding: This work was supported by GERCA (Groupe pour l’Enseignement, la prévention et la Recherche Cardiologique en Alsace).
References
- 1. Delmas C, Puymirat E, Leurent G, Elbaz M, Manzo-Silberman S, Bonello L, Gerbaud E, Bataille V, Levy B, Lamblin N, Bonnefoy E, Henry P, Roubille F. Design and preliminary results of FRENSHOCK 2016: a prospective nationwide multicentre registry on cardiogenic shock. Arch Cardiovasc Dis 2019;112:343–353. [DOI] [PubMed] [Google Scholar]
- 2. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JF. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American heart association. Circulation 2019;139. [DOI] [PubMed] [Google Scholar]
- 3. Cua B, Mamdani N, Halpin D, Jhamnani S, Jayasuriya S, Mena-Hurtado C. Review of coronary subclavian steal syndrome. J Cardiol 2017;70:432–437. [DOI] [PubMed] [Google Scholar]
- 4. Lak HM, Shah R, Verma BR, Roselli E, Caputo F, Xu B. Coronary subclavian steal syndrome: a contemporary review. Cardiology 2020;145:601–607. [DOI] [PubMed] [Google Scholar]
- 5. Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, McDermott MM. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol 2004;44:618–623. [DOI] [PubMed] [Google Scholar]
- 6. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 7. Cooper WA, O’Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the society of thoracic surgeons national adult cardiac database. Circulation 2006;113:1063–1070. [DOI] [PubMed] [Google Scholar]
- 8. Mok Y, Ballew SH, Matsushita K. Chronic kidney disease measures for cardiovascular risk prediction. Atherosclerosis 2021;335:110–118. [DOI] [PubMed] [Google Scholar]
- 9. European Stroke Organisation, Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Röther J, Sievert H, van Sambeek M, Zeller T. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European society of cardiology (ESC). Eur Heart J 2011;32:2851–2906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.