Summary
Background
Since August, 2019, US public health officials have been investigating a national outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI). A spectrum of histological patterns consistent with acute to subacute lung injury has been seen in biopsies; however, autopsy findings have not been systematically characterised. We describe the pathological findings in autopsy and biopsy tissues submitted to the US Centers for Disease Control and Prevention (CDC) for the evaluation of suspected EVALI.
Methods
Between Aug 1, 2019, and Nov 30, 2019, we examined lung biopsy (n=10 individuals) and autopsy (n=13 individuals) tissue samples received by the CDC, submitted by 16 US states, from individuals with: a history of e-cigarette, or vaping, product use; respiratory, gastrointestinal, or constitutional symptoms; and either pulmonary infiltrates or opacities on chest imaging, or sudden death from an undetermined cause. We also reviewed medical records, evaluated histopathology, and performed infectious disease testing when indicated by histopathology and clinical history.
Findings
21 cases met surveillance case definitions for EVALI, with a further two cases of clinically suspected EVALI evaluated. All ten lung biopsies showed histological evidence of acute to subacute lung injury, including diffuse alveolar damage or organising pneumonia. These patterns were also seen in nine of 13 (69%) autopsy cases, most frequently diffuse alveolar damage (eight autopsies), but also acute and organising fibrinous pneumonia (one autopsy). Additional pulmonary pathology not necessarily consistent with EVALI was seen in the remaining autopsies, including bronchopneumonia, bronchoaspiration, and chronic interstitial lung disease. Three of the five autopsy cases with no evidence of, or a plausible alternative cause for acute lung injury, had been classified as confirmed or probable EVALI according to surveillance case definitions.
Interpretation
Acute to subacute lung injury patterns were seen in all ten biopsies and most autopsy lung tissues from individuals with suspected EVALI. Acute to subacute lung injury can have numerous causes; however, if it is identified in an individual with a history of e-cigarette, or vaping, product use, and no alternative cause is apparent, a diagnosis of EVALI should be strongly considered. A review of autopsy tissue pathology in suspected EVALI deaths can also identify alternative diagnoses, which can enhance the specificity of public health surveillance efforts.
Introduction
Since Aug 1, 2019, the US Centers for Disease Control and Prevention (CDC), the US Food and Drug Administration, and state, territorial, local, and tribal health departments in the USA have been investigating a national outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI). As of Feb 18, 2020, 2807 hospitalised EVALI cases were reported in the USA.1 Among the 2668 hospitalised EVALI cases reported in the USA as of Jan 14, 2020, 66% were male and 76% were younger than 35 years.1 All patients with EVALI have a history of e-cigarette, or vaping, product use in the 90 days before symptom onset; present with respiratory, gastrointestinal, and constitutional symptoms; have evidence of pulmonary infiltrates or opacities seen on chest imaging; and have no alternative causes identified that could explain their clinical syndrome.2,3 Although the spectrum of clinical illness is not fully understood, serious outcomes of EVALI are common. As of Oct 18, 2019, 159 (47%) of 342 patients with EVALI were admitted to an intensive care unit and 74 (22%) of 338 required endotracheal intubation and mechanical ventilation.3 As of Feb 18, 2020, 68 deaths were reported with a median age of 49∙5 years (range 15–75 years).1
The exact pathophysiological mechanisms of EVALI are unknown. However, 1421 (80%) of 1782 patients with EVALI reported use of tetrahydrocannabinol (THC)-containing products, primarily from informal sources such as friends, family, or in-person or online dealers as opposed to commercial vendors.4 Additionally, vitamin E acetate has been strongly linked with EVALI;5–7 analysis of bronchoalveolar lavage fluid specimens identified vitamin E acetate in 48 of 51 patients with EVALI from 16 US states, but not in bronchoalveolar lavage fluid from a comparison group.7
An understanding of EVALI pathology is needed to improve diagnosis and therapy. A spectrum of acute to subacute lung injury patterns—such as diffuse alveolar damage, organising pneumonia, and acute fibrinous and organising pneumonia, accompanied by finely-vacuolated (foamy) macrophages—have been described in lung biopsies from patients with suspected EVALI.8,9 Similar findings in patients with e-cigarette, or vaping, product use have been noted in reports published before the current outbreak which was identified on Aug 1, 2019.10–13 The presence of lipid-laden macrophages in bronchoalveolar lavage fluid has also been described.14,15 Recent publications8,9,16 reviewed findings from lung biopsies of three patients who died, and autopsy lung findings from one case. However, autopsy findings from lung and other organs have not yet been systematically characterised.
To address this gap, we describe the pathological findings in tissue specimens from individuals with suspected EVALI submitted to the CDC Infectious Diseases Pathology Branch.
Methods
Specimen submission
This report describes pathological findings in lung biopsies and autopsy specimens for 23 individuals, from 16 US states, whose samples were received and evaluated by the CDC Infectious Diseases Pathology Branch between Aug 1, 2019, and Nov 30, 2019. We include all suspected EVALI cases submitted to our service for which evaluation was completed by Nov 30, 2019.
Cases were classified as confirmed or probable EVALI using the CDC 2019 Lung Injury Surveillance Primary Case Definition and the Lung Injury Surveillance Case Definition for Out-of-Hospital Deaths (appendix pp 3, 4). As part of the ongoing public health investigation into EVALI, the CDC aided state, territorial, local, and tribal health departments in the pathological evaluation of tissue specimens. Formalin-fixed, or formalin-fixed, paraffin-embedded tissues were requested from lung biopsies, and from the lung, upper airway, heart, liver, and kidney, which were collected during the autopsy. Specimens represent a convenience sample from individuals determined to meet CDC’s surveillance case definitions for confirmed or probable EVALI (EVALI cases; n=21) submitted by the health department,2 and from individuals with clinically suspected EVALI (n=2) due to: a history of recent (eg, in the 90 days before symptom onset) e-cigarette, or vaping, product use; antecedent respiratory, gastrointestinal, or constitutional symptoms; and sudden death from an undetermined cause but who did not meet the criteria for confirmed or probable EVALI according to the surveillance case definitions at the time of specimen submission to the CDC.
Pathological evaluation and infectious disease testing
Formalin-fixed tissues were processed for routine histology, sectioned, and stained with haematoxylin–eosin and for iron (appendix p 2). Separate representative lung sections from formalin-fixed tissues were also post-fixed with osmium tetroxide for lipid preservation before routine processing, haematoxylin-eosin staining, and sectioning (appendix p 2).
Histology of the lung and other organs was examined by a panel of six pathologists and characterised on the basis of the overall prominent pattern observed. If a histological pattern in the spectrum of acute to subacute lung injury was present, the diagnoses included: diffuse alveolar damage, either exudative (hyaline membranes) or proliferative and organising (type II pneumocyte hyperplasia and fibroblast proliferation), or a combination there of; acute fibrinous and organising pneumonia (intra-alveolar fibrin balls); and organising pneumonia (fibrosis and abundant fibroblastic proliferation).17–20
In cases for which little infectious disease testing was done before specimen submission, or for which the clinical history, inflammatory changes in tissues, or microbiological results suggested a possible infectious process, special immunohistochemical stains were used or molecular testing was done to assess for possible infectious agents (appendix pp 2–3).
Clinical and epidemiological data collection
For all individuals included in this analysis, medical records and surgical pathology or autopsy reports were reviewed for information regarding demographics, symptom history, comorbidities, physical examination findings, clinical laboratory and infectious disease testing, imaging study findings, treatment and advanced supportive care received, date of death, and pathological findings from the submitting institution. For individuals determined by the health department to meet the EVALI surveillance case definition, this information was supplemented by medical record abstraction and patient or proxy interview data.4 This investigation was reviewed in accordance with the CDC Procedures for Protection of Human Research Participants and was considered as non-research public health surveillance and diagnostic activity intended for the use in disease control or to inform policy during an emergency response.
Role of the funding source
Authors did not receive specific funding for this study. The data in this study are collected and managed by the US CDC; however, the corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
Lung biopsies were received from ten individuals, including one death, and autopsy tissues were received from 13 individuals (table 1). Of all 23 individuals, 14 (61%) were younger than 35 years, and 15 (65%) were male. All ten individuals with lung biopsies met the EVALI surveillance case definition and were admitted to hospital; one individual died. Biopsies were collected at 14·5 median days (range 6–54) after illness onset. Nine (90%) individuals reported using THC-containing e-cigarette, or vaping, products, seven (70%) reported using nicotine-containing products, and six (60%) reported using both. Seven (70%) individuals had a mental health diagnosis (excluding substance use disorders), and five (50%) had other comorbidities including chronic pain, obesity, and chronic respiratory disease. Three individuals reported combustible marijuana use, but none had a history of other substance use.
Table 1:
Demographic, clinical, and pathological characteristics of individuals with suspected EVALI
| All individuals (n=23) | EVALI case (biopsy specimens; n=10) | EVALI case (autopsy specimens; n=11) | Not an EVALI case (autopsy specimens; n=2) | |
|---|---|---|---|---|
| Age group, years | ||||
| <35 | 14 (61%) | 7 (70%) | 5 (45%) | 2 (100%) |
| ≥35 | 9 (39%) | 3 (30%) | 6 (55%) | 0 (0%) |
| Sex | ||||
| Male | 15 (65%) | 6 (60%) | 8 (73%) | 1 (50%) |
| Female | 8 (35%) | 4 (40%) | 3 (27%) | 1 (50%) |
| Medical history | ||||
| Any comorbidity reported at diagnosis or identified at autopsy | 19 (83%) | 9 (90%) | 9 (82%) | 1 (50%) |
| Mental health diagnosis* | 16 (70%) | 7 (70%) | 8 (73%) | 1 (50%) |
| Medical comorbidity | 12 (52%) | 5 (50%) | 6 (55%) | 1 (50%) |
| Obesity (BMI ≥30 kg/m2) | 5 (22%) | 1 (10%) | 3 (27%) | 1 (50%) |
| Chronic pain† | 5 (22%) | 2 (20%) | 3 (27%) | 0 (0%) |
| Chronic respiratory disease‡ | 4 (17%) | 1 (10%) | 3 (27%) | 0 (0%) |
| Hypertension | 4 (17%) | 1 (10%) | 2 (18%) | 1 (50%) |
| Chronic liver disease§ | 3 (13%) | 0 (0%) | 3 (27%) | 0 (0%) |
| Diabetes | 3 (13%) | 1 (10%) | 2 (18%) | 0 (0%) |
| Obstructive sleep apnoea | 2 (9%) | 1 (10%) | 1 (9%) | 0 (0%) |
| Immune suppression | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Cardiac disease¶ | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Chronic kidney disease | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Tobacco and other substance use | ||||
| Current or former cigarette smoker | 15 (65%) | 4 (40%) | 10 (91%) | 1 (50%) |
| Combustible marijuana use | 7 (30%) | 3 (30%) | 4 (36%) | 0 (0%) |
| Use or misuse of other substances‖ | 6 (26%) | 0 (0%) | 5 (45%) | 1 (50%) |
| E-cigarette or vaping product use 90 days before symptom onset or death | ||||
| THC-containing products, any use | 20 (87%) | 9 (90%) | 10 (91%) | 1 (50%) |
| THC-containing products, exclusive use** | 8 (35%) | 3 (30%) | 4 (36%) | 1 (50%) |
| Nicotine-containing products, any use | 14 (61%) | 7 (70%) | 7 (64%) | 0 (0%) |
| Nicotine-containing products, exclusive use | 2 (9%) | 1 (10%) | 1 (9%) | 0 (0%) |
| Unknown type of e-cigarette or vaping product | 1 (4%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Clinical course | ||||
| Duration of symptoms before biopsy or death, days | 18 (6–140) | 14·5 (6–54) | 24 (11–140) | 20·5 (10–31) |
| Admission to hospital | 21 (91%) | 10 (100%) | 10 (91%) | 1 (50%) |
| Length of hospital admission before biopsy or death, days (n=21) | 7 (0–56) | 7 (1–10) | 17 (0–56) | 0 †† |
| Discharged and readmitted | 4 (17%) | 1 (10%) | 3 (27%) | 0 (0%) |
| Admission to intensive care unit | 18 (78%) | 7 (70%) | 10 (91%) | 1 (50%) |
| Intubation and mechanical ventilation | 15 (65%) | 4 (40%) | 10 (91%) | 1 (50%) |
| Extracorporeal membrane oxygenation | 3 (13%) | 1 (10%) | 2 (18%) | 0 (0%) |
| Death | 14 (61%) | 1 (10%) | 11 (100%) | 2 (100%) |
| Treatment during clinical course | ||||
| Antibiotics | 22 (96%) | 10 (100%) | 11 (100%) | 1 (50%) |
| Systemic corticosteroids | 20 (87%) | 9 (90%) | 10 (91%) | 1 (50%) |
| Initial chest CT or x-ray findings ‡‡ | ||||
| Any abnormality of lung parenchyma | 21/21 (100%) | 10/10 (100%) | 10/10 (100%) | 1/1 (100%) |
| Bilateral ground-glass opacities | 12/21 (57%) | 4/10 (40%) | 7/10 (70%) | 1/1 (100%) |
| Miliary or multinodular opacities | 3/21 (14%) | 2/10 (20%) | 1/10 (10%) | 0/1 (0%) |
| Hilar or mediastinal lymphadenopathy | 6/21 (29%) | 4/10 (40%) | 2/10 (20%) | 0/1 (0%) |
| Bilateral infiltrates, consolidation, or opacities excluding ground-glass opacities | 10/21 (48%) | 4/10 (40%) | 5/10 (50%) | 1/1 (100%) |
| CDC surveillance case definition §§ | ||||
| Confirmed cases | 15 (65%) | 7 (70%) | 8 (73%) | ·· |
| Probable cases | 6 (26%) | 3 (30%) | 3 (27%) | ·· |
| Not a case | 2 (9%) | ·· | ·· | 2 (100%) |
| Major pathological diagnoses | ||||
| Acute lung injury pattern | 19 (83%) | 10 (100%) | 8 (73%) | 1 (50%) |
| Diffuse alveolar damage | 16 (70%) | 8 (80%) | 7 (64%) | 1 (50%) |
| Exudative | 1 (4%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Exudative and proliferative | 6 (26%) | 4 (40%) | 2 (18%) | 0 (0%) |
| Proliferative | 6 (26%) | 4 (40%) | 2 (18%) | 0 (0%) |
| Organising | 3 (13%) | 0 (0%) | 3 (27%) | 0 (0%) |
| Organising pneumonia | 2 (9%) | 2 (20%) | 0 (0%) | 0 (0%) |
| Acute fibrinous and organising pneumonia | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Bronchopneumonia | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Chronic interstitial lung disease | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Bronchoaspiration | 1 (4%) | 0 (0%) | 1 (9%) | 0 (0%) |
| Pulmonary oedema | 1 (4%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Macrophage characteristics | ||||
| Foamy macrophages | 19 (83%) | 10 (100%) | 8 (73%) | 1 (50%) |
| Non-pigmented macrophages | 15 (65%) | 10 (100%) | 5 (45%) | 0 (0%) |
| Haemosiderin-laden macrophages | 14 (61%) | 5 (50%) | 7 (64%) | 2 (100%) |
| Other pigmented macrophages | 9 (39%) | 2 (20%) | 7 (64%) | 0 (0%) |
Data are n (%), median (range), or n/N (%). Individuals with biopsy or autopsy specimens submitted to the CDC for the evaluation of suspected EVALI. BMI=body-mass index. CDC=Centers for Disease Control and Prevention. EVALI=e-cigarette, or vaping, product use-associated lung injury. THC=tetrahydrocannabinol.
Includes reported mood, anxiety, psychotic, or trauma-related disorders, but not including substance use disorders (International Classification of Diseases F10–F19).
Includes reported chronic pain or chronic pain syndromes including fibromyalgia.
Includes chronic obstructive pulmonary disease or asthma.
Includes reported chronic liver disease or identification of cirrhosis at autopsy.
Includes one case with a reported medical history of underlying congestive heart failure.
Includes reported misuse of alcohol, opiates, or illicit substances including methamphetamine, cocaine, or intravenous drug use (unknown substance misuse either current, recent, or lifetime).
Exclusive use includes only THC-containing e-cigarette products with no reported nicotine or cannabidiol-containing e-cigarette product use.
Included only one patient who was admitted to hospital for less than 24 h (0 days), the other 1 of 2 cases was not admitted, thus there is no range.
These findings are not mutually exclusive.
EVALI cases identified using the CDC 2019 lung injury surveillance primary or out-of-hospital death case definitions (appendix p 3).2
Of the 13 individuals for whom autopsy specimens were submitted, 11 were classified as EVALI cases by the submitting health department according to the surveillance case definition. Of these 11 cases, death occurred at a median of 24 days (range 11–140 [IQR 19–50]) after illness onset. All 11 individuals had received antibiotics and ten (91%) received systemic corticosteroids. Ten (91%) were admitted to hospital at the time of death, including two who died within 1 day of admission, and three who died during readmission. Ten (91%) individuals had reported use of THC-containing e-cigarette, or vaping, products, seven (64%) of nicotine-containing products, and six (55%) of using both. Eight (73%) individuals had a mental health diagnosis (excluding substance use disorders), five (45%) had a history of alcohol or non-marijuana substance misuse, and six (55%) had one or more other comorbidities, including obesity and chronic respiratory disease. For the two deaths not classified as EVALI cases, one individual was found deceased at home and the other died within 1 day of hospitalisation.
In all ten biopsies, a spectrum of acute to subacute lung injury patterns was seen (table 2). Eight biopsies (80%), all collected within 8 weeks of illness onset, contained features consistent with diffuse alveolar damage, ranging from exudative and proliferative (four; figure 1A, B) to proliferative and organising with prominent fibroblastic proliferations (four; figure 1C). Six (60%) biopsies showed patchy neutrophilic infiltrates, and one of these six also showed intravascular leukocytosis with thrombi. The two remaining biopsies that did not show diffuse alveolar damage, collected in the second or third week after illness onset, showed features of organising pneumonia (figure 1D–F). Case 7 showed intra-alveolar and intra-bronchiolar fibroblastic proliferations with scattered eosinophils (figure 1D). Case 4 showed lymphocytic bronchiolitis and intra-bronchiolar fibroblastic proliferations with associated eosinophils (figure 1E, F). Infectious disease testing was done on five of ten biopsies. Case 5 showed patchy neutrophilic inflammation with focal immunostaining for Streptococcus spp by immunohistochemistry with negative Streptococcus pneumoniae-specific PCR; testing was negative in the other four biopsies.
Table 2:
Pathological findings in lung biopsy specimens submitted to the CDC for the evaluation of suspected EVALI
| Specimen type | Lung biopsy site | Weeks from illness onset to biopsy* | Pathological diagnosis in lung tissue | Intra-alveolar macrophages in lung tissue | Other pathological and infectious disease findings† | Findings from initial chest CT or x-ray‡ | Age group, years | E-cigarette or vaping product use | Medical comorbidities§ and smoking history | Surveillance case definition classification¶ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Surgical | Right lower lobe | 1 week | Diffuse alveolar damage (exudative and proliferative), multifocal (small samples)‖ | Foamy macrophages and non-pigmented macrophages, multifocal | Anthracosis, rare; neutrophilic inflammation, scattered | Innumerable pulmonary nodules with mediastinal lymphadenopathy | ≥35 | THC, nicotine | Conventional cigarette smoking | Probable | |
| Case 2 | Trans-bronchial | Right middle and right lower lobe | 1 week | Diffuse alveolar damage (proliferative), diffuse (small samples)‖ | Foamy macrophages and non-pigmented macrophages, multifocal | Anthracosis, rare; neutrophilic inflammation, scattered | Multifocal, patchy, and predominantly peripheral ground glass opacities; prominent mediastinal lymph nodes | <35 | THC | Chronic pain | Confirmed | |
| Case 3 | Surgical | Left upper lobe, lingula | 2 weeks | Diffuse alveolar damage (exudative and proliferative), diffuse | Foamy macrophages and non-pigmented macrophages, multifocal | Anthracosis, rare; mild neutrophilic inflammation; mild oedema; mild interstitial lymphocytic infiltrate; Gram stain and GMS: non-contributory; IHC for Streptococcus spp and select Gram-negative bacteria:** negative | Diffuse bilateral ground glass opacities | <35 | THC, nicotine, CBD | Not reported | Confirmed | |
| Case 4 | Surgical | Left lower lobe | 2 weeks | Organising pneumonia with bronchiolitis, airway-centred | Foamy macrophages and non-pigmented macrophages, multifocal, rare; foamy macrophages and non-pigmented macrophages, bronchiolar | Eosinophils within fibroblastic proliferations in the airways; Gram stain and GMS: negative; IHC for Streptococcus spp and select Gram-negative bacteria: negative | Innumerable pulmonary nodules, prominent bilateral hilar lymph nodes | <35 | THC, nicotine | Conventional cigarette smoking | Confirmed | |
| Case 5 | Surgical | Right middle lobe | 3 weeks | Diffuse alveolar damage (proliferative), multifocal, airway-centred | Foamy macrophages and non-pigmented macrophages, multifocal; pigmented macrophages (pigment not otherwise specified), moderate multifocal; pigmented macrophages (haemosiderin-laden), multifocal, rare | Anthracosis, rare; fibrin thrombi, rare; emphysema; neutrophilic inflammation, multifocal; eosinophils, scattered; chronic interstitial inflammation, multifocal; Gram stain: negative; Streptococcus spp IHC: positive; Streptococcus pneumoniae PCR: negative | Extensive ground glass opacities | ≥35 | THC | Obstructive sleep apnoea | Confirmed | |
| Case 6 | Surgical | Left upper lobe, lingula | 3 weeks | Diffuse alveolar damage (exudative and proliferative), multifocal | Foamy macrophages, rare; non-pigmented macrophages, moderate | Oedema; neutrophilic infiltrates, moderate; intravascular fibrin thrombi; intravascular leukocytosis, moderate; Gram stain and GMS: non-contributory; Streptococcus spp and select Gram-negative IHC: negative | Diffuse ground glass opacities and airspace opacities | <35 | THC, nicotine | Not reported | Confirmed | |
| Case 7 | Surgical | Right upper lobe, middle lobe, and lower lobe | 3 weeks | Organising pneumonia, diffuse | Foamy macrophages, multifocal; non-pigmented macrophages, extensive; non-pigmented macrophages, bronchiolar; pigmented macrophages (haemosiderin-laden), scattered | Anthracosis, rare; haemorrhage; eosinophils, scattered; fibrosis (severe) | Extensive bilateral infiltrates | <35 | THC, nicotine | Chronic respiratory disease; conventional cigarette smoking | Probable | |
| Case 8 | Surgical | Right upper lobe | 3 weeks | Diffuse alveolar damage (proliferative), diffuse | Foamy macrophages and non-pigmented macrophages, multifocal; pigmented macrophages (haemosiderin-laden and subset of macrophages with pigment not otherwise specified), occasional | Anthracosis, multifocal, rare; intra-alveolar neutrophils, scattered; interstitial lymphocytes, multifocal | Moderate, patchy, diffuse lung consolidation, lower lobe predominant; irregular mass-like process in left lower lobe; mediastinal and hilar borderline lymphadenopathy | ≥35 | Nicotine | Diabetes; conventional cigarette smoking | Confirmed | |
| Case 9 | Surgical | Left lower lobe | 4 weeks | Diffuse alveolar damage (proliferative), multifocal and geographic | Foamy macrophages, rare; non-pigmented macrophages, multifocal; pigmented macrophages (haemosiderin-laden), scattered | Anthracosis, multifocal, rare; Gram stain and GMS: negative; Pneumocystis jirovecii, Streptococcus spp, and select Gram-negative IHC: negative | Bilateral abnormality, infiltrates or opacities present, no subpleural sparing | <35 | THC, nicotine | Not reported | Probable | |
| Case 10 | Surgical | Right middle and right lower lobe | 6–8 weeks | Diffuse alveolar damage (proliferative and organising), multifocal (middle lobe) to diffuse (lower lobe) | Foamy macrophages and non-pigmented macrophages, multifocal; pigmented macrophages (haemosiderin-laden), rare, lower lobe | Anthracosis, rare; mild haemorrhage; oedema fluid (subpleural and intra-alveolar); mild chronic interstitial infiltrate; fibrin thrombi | Extensive patchy areas of consolidation and airspace disease in both lungs | <35 | THC | Obesity; hypertension; chronic pain | Confirmed | |
Pathology findings are described as “pathology (characteristic), distribution” unless otherwise specified. CBD=cannabidiol. CDC=Centers for Disease Control and Prevention. EVALI=e-cigarette, or vaping, product use-associated lung injury. GMS=Grocott methenamine silver stain. IHC=immunohistochemistry. THC=tetrahydrocannabinol.
Weeks from illness onset to biopsy are defined as 1 week meaning less than 7 days, 2 weeks meaning 7–13 days, etc.
Also results from the CDC tissue-based infectious disease testing.
Based on imaging findings reported to the CDC.
Includes reported history of one or more of the following: obesity, chronic pain, chronic respiratory disease, obstructive sleep apnoea, diabetes, or hypertension. Seven (70%) of ten individuals had a reported mental health diagnosis; these data are not included at an individual level in this table.
The CDC 2019 lung injury surveillance primary case definition (appendix p 3).2
Assessment limited by small size of the tissue sample.
Details to clarify cross-reactivity of the select Gram-negative IHC assay are in the appendix p 2.
Figure 1: Histopathological features of lung biopsies submitted for the evaluation of EVALI.
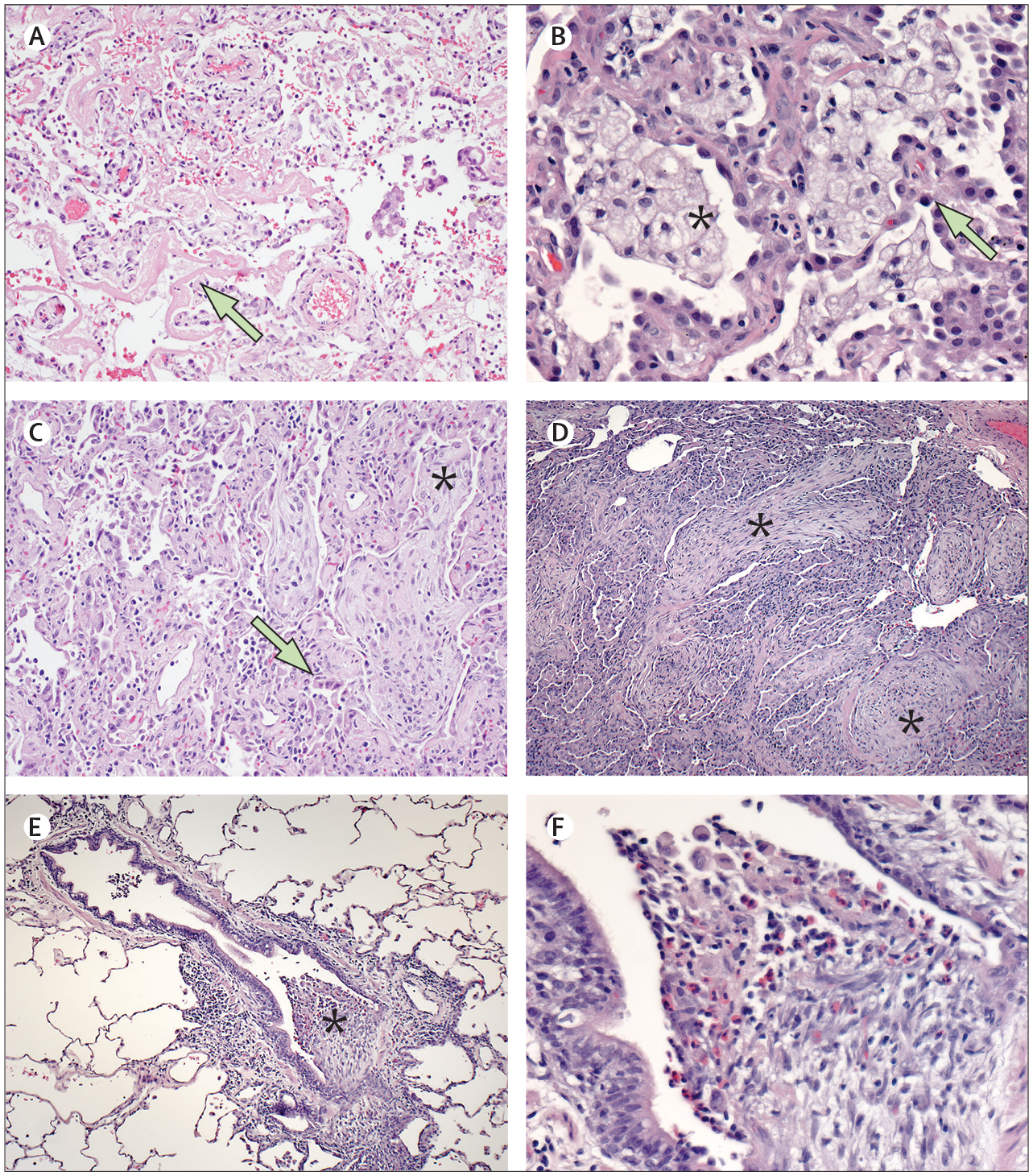
Lung biopsies submitted to the CDC were characterised on the basis of patterns of acute lung injury on haematoxylin and eosin stained slides. (A) Case 3 showed features of exudative and proliferative diffuse alveolar damage, with prominent hyaline membranes (arrow; 10× magnification). (B) Clusters of foamy macrophages (asterisk) were present in all ten biopsy lung samples and were abundant in case 5 (shown); type II pneumocyte hyperplasia was also prominent in case 5 (arrow; 40× magnification). (C) Features of proliferative and organising diffuse alveolar damage were seen in case 10, including type II pneumocyte hyperplasia (arrow) and fibroblastic proliferations (asterisk; 10× magnification). (D) Fibroblastic proliferations within airways and alveoli (asterisks), consistent with organising pneumonia, were a notable feature in case 7 (10× magnification). (E) Airway-centred inflammation and intrabronchiolar fibroblastic plugs (asterisks), consistent with organising pneumonia, were seen in case (4·5× magnification). (F) Higher magnification of figure 1E (case 4) showing fibroblastic plugs infiltrated by eosinophils and macrophages (40× magnification). CDC=Centers for Disease Control and Prevention. EVALI=e-cigarette, or vaping, product use-associated lung injury.
For the 11 autopsies of cases that met the surveillance case definition for EVALI, lung tissue was submitted from all 11, and other organ tissues were submitted from ten (table 3). Lung tissue from eight (73%) of these autopsies had histological findings consistent with the spectrum of acute to subacute lung injury, most often diffuse alveolar damage (figure 2). Two autopsies had features of exudative and proliferative diffuse alveolar damage. In Case 11, the patient was previously healthy and found deceased at home in the second week after illness onset.16 Similarly, tissue analysed by the CDC showed exudative and proliferative diffuse alveolar damage with extensive hyaline membranes and fibrin clumps in the lung. Additionally, the CDC noted microthrombi within glomeruli in the kidneys (appendix p 7). Case 15 had multiple comorbidities and died during week 6 after illness onset, after a rapid respiratory decompensation during week 3 of admission (figure 2A). In both cases, infectious disease testing done at the CDC was negative.
Table 3:
Pathological findings in autopsy specimens submitted to the CDC for the evaluation of suspected EVALI
| Weeks from illness onset to death* | Pathological diagnosis in lung tissue | Intra-alveolar macrophages in lung tissue | Other pathological findings in lung tissue | CDC tissue-based infectious disease testing | Major findings in other organs | Findings from initial chest or abdomen CT or x-ray† | Age group, years | E-cigarette or vaping product use | Medical comorbidities at autopsy‡ and smoking history | Surveillance case definition classification § | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Autopsies with evidence of acute lung injury and no alternative cause | |||||||||||
| Case 11 | 2 weeks | Diffuse alveolar damage (exudative and proliferative), diffuse | Pigmented macrophages (pigment not otherwise specified) and non-pigmented macrophages, multifocal | Multifocal moderate anthracosis; mild haemorrhage; mild oedema | PCR for influenza A, B, RSV, and PIV1–4 negative; Gram stain negative; Streptococcus spp IHC negative, Streptococcus pyogenes, select Gram-negative bacteria:¶ IHC negative in the lung, liver, and kidney | Extensive glomerular fibrin thrombi in the kidney; multifocal arteriolosclerosis; mild oedema in the brain | Not done | <35 | THC, nicotine | Conventional cigarette smoking | Confirmed |
| Case 12 | 2 weeks | Acute fibrinous and organising pneumonia, diffuse | Abundant pigmented macrophages (pigment not otherwise specified) and non-pigmented macrophages | Multifocal moderate anthracosis; bronchoaspiration; haemorrhage | PCR for PIV1–4; positive for PIV1 in trachea, negative in the bronchus and lung; PCR for influenza A, B, and RSV negative in the trachea, bronchus, and lung | Diffuse small droplet steatosis in the liver; subcapsular cortical atrophy with chronic interstitial nephritis and glomerulosclerosis in the kidney; multifocal contraction band necrosis and interstitial haemorrhage (attributed to resuscitation) in the heart; mild multifocal perivascular haemorrhage in the brain; mild tracheobronchitis in the upper airway | Diffuse patchy ground glass opacities and consolidation, more prominent on the right lung and in the lung bases | <35 | THC, nicotine | Conventional cigarette smoking | Probable |
| Case 13 | 4 weeks | Diffuse alveolar damage (proliferative) | Multifocal abundant foamy macrophages, non-pigmented macrophages, and pigmented macrophages (pigment not otherwise specified) | Multifocal moderate anthracosis; intravascular leukocytosis; neutrophilic inflammation, focal | Not done | Glomerulosclerosis, mononuclear interstitial nephritis in the kidney | Diffuse ground glass opacities superimposed on paraseptal and centrilobular emphysema | ≥35 | THC | Immunosuppression; chronic kidney disease; hypertension; conventional cigarette smoking | Confirmed |
| Case 14 | 4 weeks | Diffuse alveolar damage (proliferative) | Multifocal non-pigmented macrophages; occasional pigmented macrophages (pigment not otherwise specified) | Anthracosis, rare; haemorrhage, focal; extensive intravascular leukocytosis | Gram stain and GMS non-contributory in the lung | Haemophagocytosis, sinus histiocytosis in the lymph node; fibrin thrombi, acute tubular necrosis in the kidney; central venous congestion in the liver | Moderate extensive patchy bilateral lower lobe consolidation, mediastinal and hilar lymphadenopathy | <35 | THC | Conventional cigarette smoking | Confirmed |
| Case 15 | 6–8 weeks | Patchy diffuse alveolar damage (exudative); and geographic (proliferative) | Abundant foamy macrophages and pigmented macrophages (haemosiderin-laden and subset of pigmented macrophages not otherwise specified) | Abundant anthracosis; squamous metaplasia; mild haemorrhage; emphysema; septal oedema | Gram stain, GMS, and AFB negative; Streptococcus spp and select Gram-negative bacteria: IHC negative in the lung | Glomerulosclerosis in the kidney; central venous congestion in the liver | Multifocal extensive bilateral patchy airspace disease | ≥35 | THC, nicotine | Chronic respiratory disease; chronic pain; conventional cigarette smoking | Confirmed |
| Case 16 | 6–8 weeks | Diffuse alveolar damage (organising), diffuse | Moderate foamy macrophages and pigmented macrophages (predominantly haemosiderin-laden macrophages, also pigment not otherwise specified) | Anthracosis, rare; bronchiolitis, neutrophilic and mononuclear; squamous metaplasia; necrosis in airways; inspissated mucus; pulmonary haemorrhage; alveolar oedema and necrosis; honeycomb cyst formation | Gram and GMS negative; PCR for influenza A, B, RSV, PIV1–4 negative in the lung | Neutrophils and mononuclear cells in the trachea; immunoblasts and neutrophils in red pulp in the spleen | Diffuse bilateral ground glass opacities, mediastinal and hilar lymphadenopathy | <35 | THC, nicotine | Not reported | Confirmed |
| Case 17 | 9–11 weeks | Diffuse alveolar damage (organising) | Foamy macrophages, rare; multifocal pigmented macrophages (haemosiderin-laden and pigment not otherwise specified) | Anthracosis, rare; squamous metaplasia; smooth muscle hyperplasia; bronchopneumonia; bronchiectasis or restructuring | Not done | Central venous necrosis in the liver; acute tubular kidney necrosis; heart congestion; tracheitis, neutrophilic | Ground glass opacities and right-sided pneumonia | ≥35 | THC, nicotine | Diabetes; obesity; chronic respiratory disease; chronic pain; hypertension; chronic liver disease; conventional cigarette smoking | Confirmed |
| Case 18 | 9–11 weeks | Diffuse alveolar damage (organising) | Multifocal moderate foamy macrophages; multifocal moderate pigmented macrophages (haemosiderin-laden) | Multifocal rare anthracosis; squamous metaplasia; mononuclear interstitial inflammation; bronchiectasis or restructuring; extensive haemorrhage | Not done | Liver steatosis and cirrhosis; myocyte loss and fibrosis in the heart | Extensive bilateral ground glass opacities | ≥35 | THC | Conventional cigarette smoking; chronic liver disease | Confirmed |
| Autopsies with no evidence of acute lung injury or with acute lung injury due to an alternative cause | |||||||||||
| Case 19 | 3 weeks | Multifocal bronchopneumonia | Rare foamy macrophages and pigmented macrophages (haemosiderin-laden); moderate non-pigmented macrophages | Anthracosis, rare; erythrophagocytosis; eosinophilic bronchiolitis; intravascular leukocytosis | Gram-variable cocci in pairs in the lung; Streptococcus spp IHC: positive in the lung, negative in the kidney; PCR for Streptococcus pneumoniae: positive in the lung | None seen | Multiple opacified bronchi in both lungs (greatest in lower lobe), patchy consolidation, nodular and ground glass opacities throughout the lungs (greatest in lower lobes) | <35 | Nicotine | Conventional cigarette smoking | Probable |
| Case 20 | 4 weeks | Increased alveolar macrophages, fibrin, haemorrhage, and bronchoaspiration | Multifocal rare foamy macrophages; locally extensive pigmented macrophages (haemosiderin-laden) | Multifocal rare anthracosis; multifocal bronchoaspiration; haemorrhage locally extensive and multifocal; intravascular leukocytosis | Streptococcus spp, select Gram-negative bacteria: IHC negative in the lung, liver, and kidney; PCR for influenza A, B, RSV, PIV1–4 negative in the trachea | Liver cirrhosis; myocardial fibrosis; necrotising tracheitis in the trachea | Extensive bilateral lung opacities on chest x-ray (no CT done) | ≥35 | THC, nicotine, CBD | Chronic respiratory disease; obesity; cardiac disease; chronic liver disease; conventional cigarette smoking | Probable |
| Case 21 | 12 weeks or more | Chronic interstitial lung disease (non-specific interstitial pneumonia), diffuse; mild bronchiolitis | Rare foamy macrophages; extensive pigmented macrophages (haemosiderin-laden) | Multifocal mild anthracosis; inspissated mucus, macrophages, and neutrophils in airways; squamous metaplasia; haemorrhage; honeycomb cysts | Gram stain and GMS: negative in the lung | Not submitted | Diffuse interstitial thickening with ground glass opacities | ≥35 | THC | Obesity; obstructive sleep apnoea; diabetes; chronic pain; conventional cigarette smoking | Confirmed |
| Case 22 | 2 weeks | Multifocal diffuse alveolar damage (exudative); mild focal bronchopneumonia | Rare foamy macrophages; abundant pigmented macrophages (haemosiderin-laden) | Multifocal moderate anthracosis; mild haemorrhage; septal oedema; intravascular leukocytosis; fibrin thrombi | Gram-negative rods in the kidney; Escherichia coli IHC positive in the kidney, negative in the liver, lung, heart, spleen | Severe kidney pyelonephritis; passive congestion and small droplet steatosis in the liver | Bilateral ground glass opacities and consolidative opacities with areas of peripheral clearing, air bronchograms, areas of superimposed interlobular septal thickening; abdominal ascites with mesenteric oedema and body wall anasarca | <35 | Unknown | Obesity; hypertension; conventional cigarette smoking | Not a case |
| Case 23 | 5 weeks | Pulmonary oedema and intra-alveolar haemorrhage | Extensive pigmented macrophages (haemosiderin-laden) | None noted | Not done | Myocardial fibrosis with myocyte injury | Not done | <35 | THC | Not reported | Not a case |
AFB=acid-fast bacilli. CBD=cannabidiol. CDC=Centers for Disease Control and Prevention. EVALI=e-cigarette, or vaping, product use-associated lung injury. GMS=Grocott methenamine silver stain. IHC=immunohistochemistry. PIV=parainfluenza virus. RSV=respiratory syncytial virus. THC=tetrahydrocannabinol.
Weeks from illness onset to autopsy are defined as 1 week meaning less than 7 days, 2 weeks meaning 7–13 days, etc.
Based on imaging findings reported to the CDC.
Includes reported history of one or more of the following: obesity, chronic pain, chronic respiratory disease, obstructive sleep apnoea, chronic kidney disease, immune suppression, chronic liver disease, diabetes, or hypertension; or autopsy findings consistent with chronic respiratory disease, chronic kidney disease, or cirrhosis. Nine (69%) of 13 individuals had a reported mental health diagnosis and six had a reported history of use or abuse of other substances, excluding marijuana; these data are not included at an individual level in this table.
The CDC 2019 lung injury primary or out-of-hospital death surveillance case definition (appendix p 3).2
Details to clarify cross-reactivity of the select Gram-negative IHC assay are in the appendix p 2.
Figure 2: Histopathological features of lung autopsies submitted for the evaluation of EVALI.

Sections of the lung from autopsies submitted to the CDC were characterised on the basis of patterns of acute lung injury on haematoxylin and eosin stained slides. (A) Hyaline membranes (arrow) and clusters of pigmented macrophages (asterisk) were seen in case 15 (10× magnification). (B) Prominent fibroblastic proliferations in airways and alveoli (asterisks) and type II pneumocyte hyperplasia were features of case 13 (10× magnification). (C) Case 12 had abundant intra-alveolar balls of fibrin (arrow) and abundant intra-alveolar macrophages (10× magnification). (D) Case 19 showed bronchopneumonia with immunohistochemical staining by an assay targeting Streptococcus spp where cocci are primarily seen phagocytosed by intra-alveolar macrophages and neutrophils (smaller inset; arrow; 40× magnification). (E) Chronic interstitial fibrosis (asterisk), bronchiectasis, chronic inflammation, haemorrhage, and restructuring were noted in case 21 (40× magnification). (F) Case 22 had prominent hyaline membranes (arrow; 10× magnification). CDC=Centers for Disease Control and Prevention. EVALI=e-cigarette, or vaping, product use-associated lung injury.
Five of the 11 EVALI autopsies had features of proliferative to organising diffuse alveolar damage with no exudative component. Two individuals died during the fourth week after illness onset: case 13 (figure 2B), in which the patient had comorbidities including chronic kidney disease (appendix p 7); and case 14, in which the patient was discharged and readmitted to hospital on the same day with cardiac arrest, then kept at hospital for 4 days before dying with multiorgan failure. In three individuals who died 6–11 weeks after illness onset (cases 16–18), the lung tissue samples from autopsy showed fibroblast proliferation and restructuring. Case 16 was previously healthy and readmitted to hospital 2 weeks after initial discharge with worsening respiratory symptoms and fever. The other two individuals had multiple comorbidities, including case 18, for whom cirrhosis was identified at autopsy. In case 18, mass spectrometry done at the CDC7 showed evidence of vitamin E acetate in bronchoalveolar lavage fluid collected 9 days after hospital admission, and our histopathological examination showed prominent foamy macrophages (appendix p 8). In two of five autopsies, infectious disease testing was negative.
Finally, case 12 showed features of acute fibrinous and organising pneumonia and bronchoaspiration (table 3, figure 2C). This patient was found unresponsive during the second week after illness onset and 2 days after hospital discharge for the treatment of EVALI. A mild tracheobronchitis was also noted (appendix p 7), and PCR for parainfluenza virus type 1 was positive for the trachea tissue sample but negative for the lung and bronchus tissue samples.
Autopsy lung tissue from three individuals, classified by the health department as EVALI cases according to the surveillance case definition, did not show histological features of acute to subacute lung injury (table 3). Case 19 showed bronchopneumonia (figure 2D) and features consistent with asthma. Antemortem sputum and blood cultures grew S pneumoniae, which was further confirmed by positive Streptococcus spp immunohistochemistry and S pneumoniae-specific PCR in the lung tissue by CDC testing. In case 20, histological features included intra-alveolar fibrin, patchy haemorrhage, intravascular leukocytosis, and bronchoaspiration. The patient died after 2 weeks of mechanical ventilation with multiple comorbidities and cirrhosis seen on autopsy (appendix p 7). Finally, in case 21, the patient died more than 12 weeks after illness onset; this patient was diagnosed with restrictive lung disease as an outpatient, with evidence of fibrosis on imaging before acute worsening of symptoms 3 days before admission to hospital. Autopsy findings included histological features indicating chronic fibrosing interstitial lung disease, consistent with non-specific interstitial pneumonia (figure 2E). This case might have represented an acute exacerbation of a chronic underlying lung disease.
Autopsy tissues from two individuals with clinically suspected EVALI were evaluated, and subsequently not classified as EVALI cases. Case 22 presented with severe respiratory distress and a chest CT showed bilateral ground glass opacities; the patient died within one day of admission to the hospital. Autopsy findings included exudative diffuse alveolar damage (figure 2F), and gross and histological evidence of extensive pyelonephritis (appendix p 7) with Escherichia coli detected from a kidney tissue culture; CDC testing found Gram-negative rods with E coli immunohistochemistry in kidney tissue (appendix p 7). There was no evidence of E coli found by immunohistochemical staining in the other tissues that were submitted. On the basis of severe pyelonephritis seen at autopsy, this patient most likely died of urosepsis with possible associated acute respiratory distress syndrome, and not EVALI. Additionally, using the surveillance case definition, the submitting health department and medical examiner determined that infection was sole cause of the underlying lung injury. Case 23, an individual who was found deceased at home, showed pulmonary oedema and an intra-alveolar haemorrhage, with abundant siderophages and evidence of cardiac disease consistent with heart failure.
Varying amounts of foamy macrophages were seen in lung tissues from 19 (83%) of 23 individuals, including all biopsies and nine autopsies, and in 16 of 19 individuals with histological features of acute to subacute lung injury (appendix p 8). Lung tissues post-fixed with osmium tetroxide were evaluated in 12 autopsies, of which four showed dark brown variably sized globules suggestive of a lipid within macrophages and intra-alveolar fibrin (appendix p 8), including two with bronchoaspiration.
Intra-alveolar macrophages containing golden to granular brown pigments were seen in 18 (78%) of 23 individuals, including all 13 autopsies. 14 of these 18 showed iron stain-positive pigments consistent with haemosiderin (appendix p 8). Pigmented macrophages that did not stain using iron staining but contained a fine granular pigment, similar to so-called smoker’s macrophages described elsewhere,8,21 were also seen in nine of 18 individuals (appendix p 8). Varying amounts of anthracosis was seen in the lung tissue of all 23 individuals, although the amount did not correlate with age of the individual or the reported amount of cigarette smoking.22
Discussion
Acute to subacute lung injury can be caused by a range of factors including toxic inhalants and ingestants, drug reactions, infections, and others;17,18 however, the lung has a limited range of responses to injury, so differentiating these causes on the basis of histology alone can be challenging. In this investigation into the pathology of lung injury in tissue samples received by the CDC, we have focused on describing the patterns of injury observed in relation to clinical and exposure history, and identifying comorbidities, co-infections, and other possible causes. To our knowledge, this Article includes the first description of findings in a series of autopsies evaluated for suspected EVALI.
In this case series, lung tissue samples from all biopsies and most autopsies of confirmed or probable EVALI cases had features of acute to subacute lung injury, which is similar to findings from previous literature.8,9 Diffuse alveolar damage was the most common acute to subacute lung injury pattern in this series, particularly among autopsies. In autopsies, the stage of diffuse alveolar damage was generally consistent with the duration of illness, with proliferative and organising features seen in individuals with longer clinical courses. However, in some individuals determining a clear correlation between duration of illness and stage of diffuse alveolar damage was complicated by tissue sampling, hospital readmission, mechanical ventilation, infections, and possible continued e-cigarette, or vaping, product use. Additionally, autopsy lung tissue from three individuals classified as EVALI cases using the surveillance case definition did not have features of acute to subacute lung injury and instead showed bronchopneumonia, bronchoaspiration, and chronic interstitial lung disease. Although the contribution of e-cigarette, or vaping, product use to the lung pathology cannot be excluded, the discordance between pathological findings and the EVALI case classification shows that the surveillance case definition lacks specificity and reinforces the importance of careful clinical diagnosis and, where relevant, autopsies.
EVALI is a diagnosis of exclusion and clinical features are similar to those seen in influenza, other respiratory viral infections, and community-acquired pneumonia.3 In addition, histologically, acute to subacute lung injury can be seen in influenza,23 bacterial sepsis, and other infections. The CDC identified infectious agents in four individuals, including two agents that appeared to be the primary contributors to the observed lung pathology. One individual had bronchopneumonia due to S pneumoniae. Of note, the association between e-cigarette, or vaping, product use and streptococcal infection has been shown in animal models.24,25 In the autopsy showing E coli pyelonephritis with exudative diffuse alveolar damage, acute to subacute lung injury could have arisen from urosepsis or from e-cigarette, or vaping, product use, but was eventually attributed to the infectious process. Differentiating between these causes is not possible histologically, and as in this case, requires careful clinicopathological correlation.
The presence of lipid-laden macrophages in bronchoalveolar lavage fluid,14,15 and foamy macrophages in lung biopsy specimens have been previously described in suspected EVALI cases.8,9 Additionally, findings from animal models suggest that exposure to propylene glycol and vegetable glycerine in e-cigarette aerosol might impair lipid homoeostasis and alveolar macrophage function.24 Osmium tetroxide, a post-fixation method that stains lipids,26 has shown lipid staining in macrophages in a small subset of lung tissues evaluated by the CDC; however, the nature and significance of this lipid is unknown, especially in the setting of concomitant bronchoaspiration. Foamy macrophages can be an incidental finding, but they are more frequently associated with lung pathology.21 As such, these macrophages might be a feature of acute to subacute lung injury, instead of a diagnostic clue suggesting EVALI. Similarly, post-fixation with osmium tetroxide and characterisation of pigment in macrophages by iron staining did not reveal findings that could be used to specifically diagnose EVALI. However, continued efforts are needed to explore the potential role of macrophages and endogenous and exogenous lipids in the pathogenesis of EVALI.
Among autopsy submissions, findings consistent with medical comorbidities were seen or reported in the medical record. For some, these findings included obesity and chronic lung disease, which are independent risk factors for severe outcomes of acute respiratory failure.23,27,28 Four deaths occurred in individuals without substantial medical risk factors for poor outcomes of acute to subacute lung injury. Histologically, these cases showed diffuse acute to subacute lung injury patterns, including acute fibrinous and organising pneumonia. Understanding of the histological correlates and risk factors for serious outcomes of EVALI, among previously healthy individuals and those with medical comorbidities, is crucial.
Our study has limitations. First, only a small number of biopsies and autopsies were evaluated at the CDC and findings might not be representative of all EVALI cases; cases necessitating biopsy or autopsy represent the more severe end of the clinical spectrum. Second, findings within a lung sample, particularly in small biopsies, might not be representative of the process within the remaining lung. Third, factors such as extended formalin fixation, previous antimicrobial treatment, and tissue sampling can affect the sensitivity of tissue-based infectious disease testing. Finally, individuals could have been misclassified as EVALI cases, and misclassification of reported e-cigarette, or vaping, product use, and medical history might occur.
Similarly to previously-reported surveillance findings, most of the patients with EVALI in this case series used THC-containing e-cigarette, or vaping products.4,5,29 The semi-solid resinous extracts of cannabis buds, used to make THC-containing e-liquids, are not miscible in the propylene glycol and glycerol mixture typically used to make nicotine-containing e-liquids.30 Other ingredients including terpenes, medium chain triglycerides, and vitamin E acetate are often added to illicit or counterfeit THC-containing e-liquids to make them appear of higher quality. Inhalation of these and other ingredients including diacetyl and other chemical flavorings,30–32 heavy metals, or e-liquid thermal decomposition products might contribute to the development of EVALI.33 Vitamin E acetate has been strongly linked with EVALI, as it was identified in bronchoalveolar lavage fluid from EVALI cases, but not found in controls.7 Vitamin E acetate was present in one patient who died. Only one of the patients that was positive had bronchoalveolar lavage fluid tested at the CDC. However, the contribution of vitamin E acetate or other potential toxicants to the pathology seen in this case series is unclear.
The cause of sudden unexpected deaths in individuals with a history of e-cigarette, or vaping, product use might not be readily apparent. For individuals who are found dead or who decompensate precipitously, in whom extensive clinical evaluation is not possible, autopsy findings can shed light on the cause of death. If an acute to subacute lung injury pattern is identified histologically, and an alternative cause is not identified, a diagnosis of EVALI should be considered. For one case in this series (case 11), autopsy findings of acute to subacute lung injury in the absence of an alternative cause enabled the case to meet the surveillance case definition. This case was an out-of-hospital death, for which the Lung Injury Surveillance Case Definition for Out-of-Hospital Deaths was used (appendix p 3), which relies on identifying pathological evidence of acute lung injury. The other out-of-hospital deaths in this case series did not have evidence of acute lung injury. However, cases might be complex when more than one process could possibly contribute to acute to subacute lung injury. Additionally, a history of e-cigarette, or vaping, product use should be ascertained for decedents who at autopsy have acute to subacute lung injury but have an undetermined cause of death. US state, territorial, local, and tribal health departments should continue to work closely with medical examiners, coroners, and clinicians in their jurisdictions to raise awareness for the clinical and pathological findings of EVALI and to facilitate reporting of such cases.
Supplementary Material
Research in context.
Evidence before this study
Since August, 2019, the US Centers for Disease Control and Prevention (CDC); Food and Drug Administration; and state, territorial, local, and tribal health departments have been investigating a national outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI). Journal articles published before Dec 15, 2019, have described pathological findings in biopsy tissues from one or more patients with a history of e-cigarette, or vaping, product use. Acute to subacute lung injury patterns including organising pneumonia, acute fibrinous and organising pneumonia, and diffuse alveolar damage, have been described in lung biopsies from two series of suspected EVALI patients. Similar findings were described in individual case reports published before the current outbreak, which was identified on Aug 1, 2019, regarding patients with evidence of lung injury and a history of e-cigarette, or vaping, product use. Finally, although biopsies from patients who ultimately died were included in this case series, to our knowledge only one case report describing autopsy findings in a patient with EVALI has been published.
Added value of this study
We describe pathological findings in tissue specimens submitted to the CDC, by 16 US states, for the evaluation of suspected EVALI, including findings from 13 autopsies in patients with diverse clinical courses. This study reiterates the histological spectrum of acute lung injury patterns in severe and fatal cases of this emerging public health threat and highlights the importance of thorough clinical evaluation and multidisciplinary discussions to exclude alternative diagnoses. Autopsy lung tissues showed acute to subacute lung injury in nine of 13 autopsies, and the most common finding was diffuse alveolar damage. Additionally, pathological evaluation facilitated the identification of alternative diagnoses in suspected EVALI cases, including bronchopneumonia, bronchoaspiration, acute lung injury caused by an infectious process, and chronic interstitial lung disease. Identification of these alternative diagnoses enhanced the specificity of public health surveillance efforts and provide a rationale for histological evaluation of lung samples in suspected cases.
Implications of all the available evidence
Our findings reinforce that if an acute to subacute lung injury pattern is identified histologically in a biopsy or at autopsy in an individual with a history of e-cigarette, or vaping, product use, and an alternative cause is not identified, a diagnosis of EVALI should be considered. However, cases might be more complex if more than one process contributed to the lung injury. Additionally, a history of e-cigarette, or vaping, product use should be ascertained for decedents who have lesions suggesting acute lung injury at autopsy but have an undetermined cause of death. Given that some cases with suspected EVALI had alternative diagnoses, the discordance between pathological findings and the EVALI case classification shows that the surveillance case definition lacks specificity and reinforces the importance of a careful clinical diagnosis and accurate autopsies. EVALI cases continue to be reported, and continued surveillance, laboratory evaluation, and research into disease pathophysiology are crucial.
Acknowledgments
For their thoughtful work in supporting the identification of suspected EVALI cases, and evaluation of autopsy and biopsy tissue specimens, we thank: US Centers for Disease Control and Prevention (CDC) Infectious Diseases Pathology Branch, Histology, Immunohistochemistry, Electron Microscopy and Microbiology, Epidemiology and Operations, and Molecular Pathology teams; CDC’s Lung Injury Response Clinical Task Force, Laboratory Task Force, and Epidemiology and Surveillance Task Force; Laura Williamson, Lisa Mannix, Mary Case, David Arboe III, David Zimmerman, Gulpreet Bowman, Thomas Coyne, Aldo Fusaro, Kathryn Taylor, Gary A Walter, Carla Boutwell, Jennifer Hanson, Timothy Casey, Carlos Galliani, Damon R Olson, Rebecca Asch-Kendrick, Leslie Kollmann, Adele Lewis, and Brittney A Imblum; Georgia Department of Public Health; Wisconsin Department of Health Services; Wisconsin State Laboratory of Hygiene; Kentucky Department of Public Health; Florida Department of Health; and other State and Local Health Departments, clinicians, pathologists, medical examiners, and coroners.
Funding
US Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests. The findings and conclusions in this Article do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.US Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping. 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed June 6, 2020).
- 2.US Centers for Disease Control and Prevention. Smoking & tobacco use. 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html (accessed June 6, 2020).
- 3.Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury - United States, October 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozier MJ, Wallace B, Anderson K, et al. Update: demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injuries - United States, December 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 1142–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis N, McCaffrey K, Sage K, et al. E-cigarette use, or vaping, practices and characteristics among persons with associated lung injury - Utah, April-October 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 953–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med 2020; 382: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med 2019; 381: 1780–81. [DOI] [PubMed] [Google Scholar]
- 9.Mukhopadhyay S, Mehrad M, Dammert P, et al. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping). Am J Clin Pathol 2020; 153: 30–39. [DOI] [PubMed] [Google Scholar]
- 10.Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep 2017; 5: e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He T, Oks M, Esposito M, Steinberg H, Makaryus M. “Tree-in-bloom”: severe acute lung injury induced by vaping cannabis oil. Ann Am Thorac Soc 2017; 14: 468–70. [DOI] [PubMed] [Google Scholar]
- 12.Itoh M, Aoshiba K, Herai Y, Nakamura H, Takemura T. Lung injury associated with electronic cigarettes inhalation diagnosed by transbronchial lung biopsy. Respirol Case Rep 2017; 6: e00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin Respir J 2018; 12: 1295–99. [DOI] [PubMed] [Google Scholar]
- 14.Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia - North Carolina, July–August 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 784–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med 2019; 381: 1488–89. [DOI] [PubMed] [Google Scholar]
- 16.Marsden L, Michalicek ZD, Christensen ED. More on the pathology of vaping-associated lung injury. N Engl J Med 2020; 382: 387–88. [DOI] [PubMed] [Google Scholar]
- 17.Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med 2017; 141: 916–22. [DOI] [PubMed] [Google Scholar]
- 18.Katzenstein AA. Diagnostic atlas of non-neoplastic lung disease: a practical guide for surgical pathologists, 1st edn. New York, NY: Demos Medical, 2016. [Google Scholar]
- 19.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araya J, Kawabata Y, Jinho P, Uchiyama T, Ogata H, Sugita Y. Clinically occult subpleural fibrosis and acute interstitial pneumonia a precursor to idiopathic pulmonary fibrosis? Respirology 2008; 13: 408–12. [DOI] [PubMed] [Google Scholar]
- 21.Rossi G, Cavazza A, Spagnolo P, et al. The role of macrophages in interstitial lung diseases: number 3 in the series “pathology for the clinician” edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev 2017; 26: 170009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takano APC, Justo LT, Dos Santos NV, et al. Pleural anthracosis as an indicator of lifetime exposure to urban air pollution: an autopsy-based study in Sao Paulo. Environ Res 2019; 173: 23–32. [DOI] [PubMed] [Google Scholar]
- 23.Shieh WJ, Blau DM, Denison AM, et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 2010; 177: 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 2019; 129: 4290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 2015; 10: e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wigglesworth VB. Histological staining of lipids for the light and electron microscope. Biol Rev Camb Philos Soc 1988; 63: 417–31. [DOI] [PubMed] [Google Scholar]
- 27.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med 2008; 36: 151–58. [DOI] [PubMed] [Google Scholar]
- 28.Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011; 52: 301–12. [DOI] [PubMed] [Google Scholar]
- 29.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - final report. N Engl J Med 2020; 382: 903–16. [DOI] [PubMed] [Google Scholar]
- 30.Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B. E-cigarettes: a review of new trends in cannabis use. Int J Environ Res Public Health 2015; 12: 9988–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbs AF, Kreiss K, Cummings KJ, et al. Flavorings-related lung disease: a brief review and new mechanistic data. Toxicol Pathol 2019; 47: 1012–26. [DOI] [PubMed] [Google Scholar]
- 32.Landman ST, Dhaliwal I, Mackenzie CA, Martinu T, Steele A, Bosma KJ. Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. CMAJ 2019; 191: E1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Academies of Sciences, Engineering, and Medicine. Public health consequences of e-cigarettes. Washington, DC: National Academies Press, 2018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


