Abstract
This pathology PILOT study aims to define the role and feasibility of centralized pathology review in a cohort of 75 patients from different centers in the US and Europe using digital slide scanning. The pathologic material from 75 patients who had been diagnosed with MF/SS and were clinically staged as IIb or above was retrieved from 11 participating centers. Each pathology reviewer was provided with the pathologic diagnosis (by the referring pathologist), and the following list of histopathologic criteria (presence or absence) from the initial report: epidermotropism, folliculotropism, large cell transformation, syringotropism and granulomas. Patients with advance stage were selected for this study as this is a population where there is significant variability in the diagnosis of pathologic prognostic and predictive biomarkers. The slides were digitally scanned with an Aperio scanner and consensus review of cases occurred when major or minor discrepancies between the referral diagnosis and central pathology review occurred. Among the 75 cases, 70 (93.3%) had a final consensus diagnosis between the three central review pathologists. The overall agreement (OA) between the consensus review and the referring pathologist was 60%. The OA was also higher between the reviewers and consensus review, compared to the referring pathologist and consensus. 65.3% of cases had some type of discrepancy (major or minor) between the outside and consensus review. Major discrepancies were seen in 34 out of 73 cases (46.6%; 73 cases indicated a yes or no response). Minor discrepancies were seen in 32 out 75 (42.7%) of cases. Most of the major discrepancies were accounted by a difference in interpretation in the presence or absence of large cell transformation or folliculotropism. Most minor discrepancies were explained by a different interpretation in the expression of CD30. We found digital slide scanning to be a beneficial, reliable and practical for a methodical approach to perform central pathology review in the context of a large clinical prospective study.
Introduction
The cutaneous T-cell lymphomas (CTCL), mycosis fungoides (MF) and Sézary syndrome (SS), are extranodal forms of T-cell malignancies derived by mature post-thymic T-cells, and represent 4% of Non-Hodgkin lymphomas (NHL)[1]. Mycosis fungoides is the most common form of CTCL encountered in the clinical practice. SS is a less common subtype of CTCL with leukemic disease [1, 2]. Patients with early stage MF can be treated with skin-directed therapy, while the more advanced stages often require systemic treatment, typically handled by multidisciplinary groups (hematologists, dermatologists, radiation oncologists and pathologists)[3, 4]. One-third of patients present with advanced stage of MF or SS (stage IIB or above) and, of the patients who present with early stages of MF, a subset will progress to advanced stage, which carries a median survival time of 35–56 months [5, 6]. Patients with stage IIB or above include individuals with tumors, and/or presence of generalized erythroderma. The stage IIB cutoff is used in the vast majority of clinical trials to indicate which patients have advanced disease. However, an extraordinary high degree of clinical and pathologic heterogeneity exists, with a wide range of outcomes within clinical stages.
The Cutaneous Lymphoma International Consortium (CLIC) was an initiative developed by the International Society of Skin Lymphomas (ISCL) and endorsed from multiple cutaneous lymphoma programs around the world, including the European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Taskforce (EORTC), the US Cutaneous Lymphoma Consortium, and the United Kingdom Cutaneous Lymphoma Group to help develop a network of collaborations to improve the understanding of the clinico-pathologic and molecular aspects of these rare lymphomas on a large-scale basis. Following the recent publication of CLIC’s retrospective study of patients with advanced disease [7], a prospective cutaneous lymphoma international prognostic index (PROCLIPI) study was ensembled, with the goal of enrolling 2,000 patients over a 5-year course.
We postulate that because of the heterogeneity in the clinical and pathologic features of MF and SS, centralized pathology review is necessary to reach consensus on various pathological aspects of each case and better evaluate for distinctive predictive biomarkers in patients with advanced stage disease (e.g folliculotropism, granulomatous features, presence or absence of large cell transformation, discrepancies in the interpretation of immunohistochemical stains). Those variables are important in terms of prognosis and as predictive biomarkers to response to therapy (e.g. CD30 expression). This pathology PILOT study aims to define the role and feasibility of centralized pathology review in a cohort of 75 patients from different centers in the US and Europe using digital slide scanning.
Methods
The pathologic material from 75 patients who had been diagnosed with MF/SS and were clinically staged as IIB or above[2] was retrieved from 11 participating centers (Table 1) after obtaining approval from the University of Virginia Institutional Review Board (IRB). The slides were sent to the University of Virginia for digital slide scanning. The clinical information collected included the type of clinical lesion (patch, plaque, or tumor) that was biopsied, biopsy site, and TNMB/clinical staging. Each center was asked to submit their Hematoxylin & Eosin (H&E) stained slides, in addition to the following immunohistochemical stains: CD3, CD4, CD8, CD30 and Ki67 (when available).
Table 1:
Participating Centers in Pathology PILOT study
| U01 – University of Virginia |
| U02 – Stanford |
| U03 – Memorial Sloan Ketterin Cancer Center |
| U04 – Washington University in St. Louis School of Medicine |
| U05 – Mayo Clinic (Scottsdale) |
| U06 – City of Hope |
| U07 – MD Anderson Cancer Center |
| U08 – Northwestern University |
| U09 – University of California in San Francisco (UCSF) |
| U10 – University of Washington (Seattle) |
| E01 – University Hospital Birmingham (UK) |
The slides were scanned using APERIO/Leica System (Leica SCN400-F, Germany). The H&E stained slides were scanned at 40x magnification, and each individual immunostain at 20x, after being de-identified. The digital scanned slides were uploaded to a central pathology database, and accessible to the pathologists involved in the study (JK, MP, AAG and JG) using the following link <https://btrfslide1.eservices.virginia.edu/dih/index.php>. Each pathology reviewer was provided with the pathologic diagnosis (by the referring pathologist), and the following list of histopathologic criteria (presence or absence) from the report: epidermotropism, folliculotropism, large cell transformation, syringotropism and granulomas. Large cell transformation was defined as the presence of more than 25% large cells among the total lymphoid infiltrate (large lymphocytes are cells four times or more the size of a normal lymphocyte by cross sectional area). The complete immunophenotypic profile was also available to each reviewer, including the interpretation of percentage of positive cells for Ki67 and CD30 by the referring pathologist (in many cases only a descriptive interpretation of expression of both markers was given without a specific percentage of expression). Additional information collected was the results of molecular studies done on each case (T-cell receptor gene rearrangement by PCR BIOMED studies, high throughput sequencing or flow cytometry V-beta repertoire), which were also accessible to the central pathology review panel. Each pathology reviewer (and also the referring pathologists) were provided with a PowerPoint presentation that listed definitions for each individual parameter being evaluated and photomicrographs with examples of each variable.
Each pathology reviewer provided an independent and blinded interpretation of each patient’s biopsy results regarding the diagnosis, presence or absence of large cell transformation, epidermotropism, folliculotropism, syringotropism, granulomas and immunohistochemistry results. The presence or absence of a hair follicle or adnexal structures was also entered in a database. The immunohistochemistry review for CD3, CD4 and CD8 was interpreted as positive or negative, in addition to providing a CD4:CD8 ratio. For Ki67 and CD30 a percentage of positive cells was also recorded, using the total number of lymphocytes as the denominator.
Cases with any discrepancy between the referring institution and one or more central reviewers were discussed in a consensus conference webinar. A consensus review interpretation of each one of these cases was then entered in the database. Two types of discrepancies were used in the analysis of the cases: 1) Major discrepancies included the evaluation of folliculotropism, syringotropism or large cell transformation that was different from the referring facility. Large cell transformation was defined in accordance to the World Health Organization (WHO) classification of tumors of the hematopoietic system (>25% of large cells – cells of more than 4 times the size of a normal lymphocyte – or presence of large cell nodules; 2) Minor discrepancies referred to a discordance of more than 10% in the rate of CD30 expression or Ki67 proliferation (provided that either CD30 or Ki67 were scored as <50% of cells); different interpretation of the remaining immunostains; presence or absence of granulomas and epidermotropism.
The statistical analysis focused on assessing correlation and agreement between a referring pathologist and the consensus interpretation by the three central panelist reviewers. For continuous measures, the Pearson correlation coefficient was used to assess the relationship between the referring pathologist and the consensus interpretation. For categorical variables, both the Cohen’s kappa coefficient of agreement and the overall percentage of agreement were used to describe agreement between the referring pathologist and the consensus interpretation. The Pearson correlation coefficient ranges from −1 to 1, where 0 indicates no relationship and values close to 1 and −1 indicate a strong linear relationship between variables. Landis and Koch[8] suggest the following guidance on interpretation of the kappa coefficient: values ≤ 0 as indicating no agreement, 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement. All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc, Cary, NC).
Results
The data used in this analysis consisted of results generated by 4 pathologists on 75 cases (the referring pathologist in addition to 3 pathologists that make up a central review). When diagnosis differed between the three central reviewers, a final consensus was determined between the pathologists. Table 2 lists the common diagnostic categories obtained from the referring institutions. Complete staging information was available in 46% of cases. The clinical description of the lesion and biopsy site was available in all cases. CD30 immunostain was performed in 87% of cases and Ki67 in 31% of cases. Among the 75 cases, 70 (93.3%) had a final consensus diagnosis between the three central review pathologists. The overall agreement between the consensus review and the referring pathologist was 60% for both major and minor criteria. 65.3% of cases had some type of discrepancy (major or minor) between the outside and consensus review. Major discrepancies were seen in 34 out of 73 cases (46.6%; 73 cases indicated a yes or no response). Minor discrepancies were seen in 32 out 75 (42.7%) of cases. Figures 1 and 2 show a percentage distribution of the major and minor discrepancies encountered between the consensus and referring facilities. Figure 3 shows a percentage distribution of major and minor discrepancies across all the participating institutions.
Table 2:
Pathologic referring diagnosis
| Mycosis fungoides: 19% |
| Mycosis fungoides with folliculotropism: 9.5% |
| Mycosis fungoides with large cell transformation: 42% |
| Mycosis fungoides with large cell transformation and folliculotropism: 8% |
| Mycosis fungoides, granulomatous type: 3% |
| Sezary syndrome: 5% |
| Sezary syndrome with large cell transformation: 3% |
| Sezary syndrome with folliculotropism: 0% |
| Mycosis fungoides with granulomatous features and large cell transformation: 1% |
| Atypical lymphoid infiltrate: 4% |
| Mycosis fungoides with syringotropism: 1% |
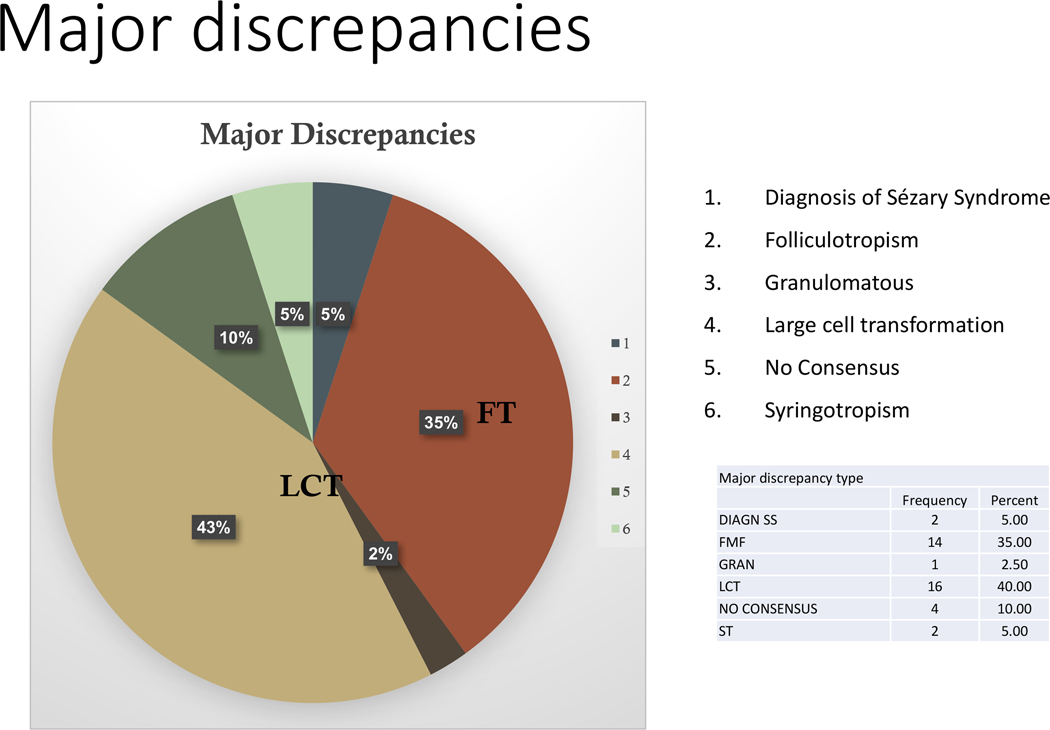
Figure 1: Distribution of major discrepancies in the study.
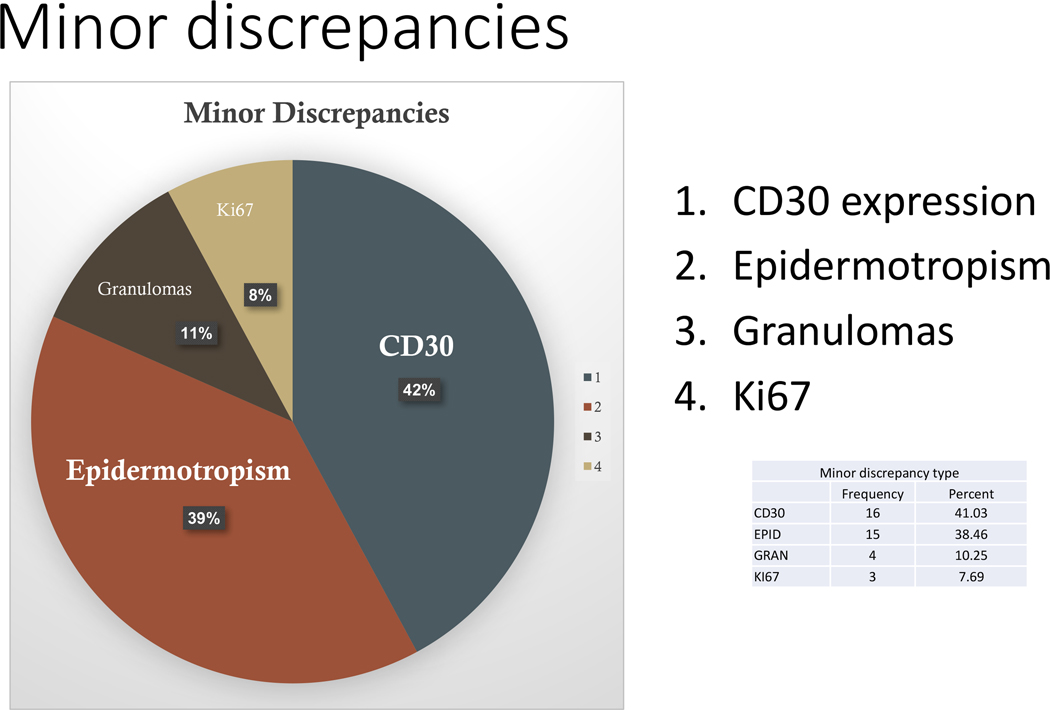
Figure 2: Distribution of minor discrepancies in the study.
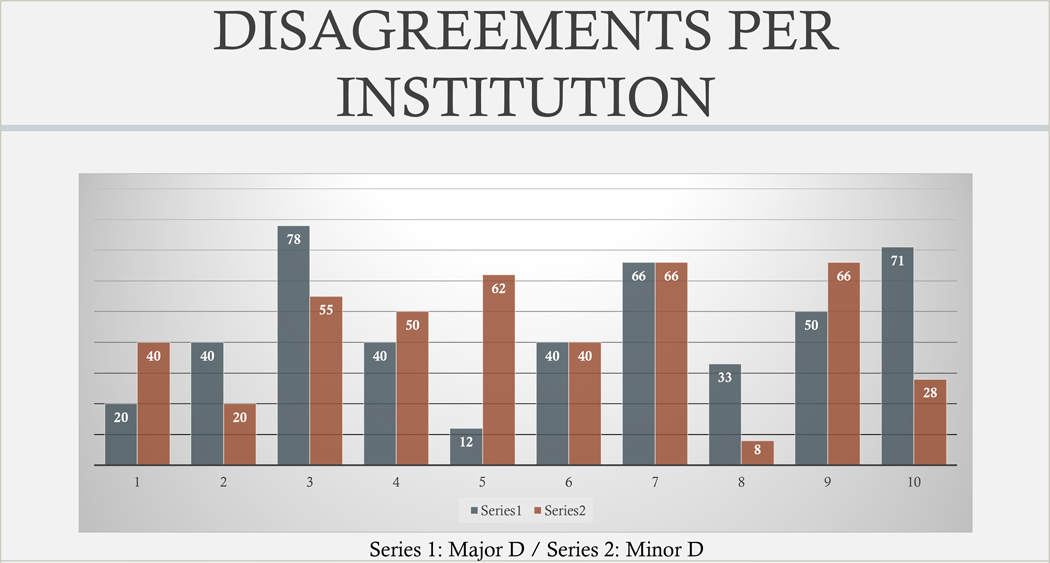
Figure 3: Disagreements per institution (%). Series 1 indicates % of major discrepancy and Series 2 % of minor discrepancy.
Notoriously, the different interpretations for the presence or absence of large cell transformation (43%) and folliculotropism (35%) accounted for most of the major diagnostic discrepancies. In most cases where a discrepancy of large cell transformation occurred, a different estimate of the total number of large cells was noted. In the case of discrepancies in folliculotropism, in most circumstances, the folliculotropism was a focal finding. Less common major diagnostic differences included the presence of syringotropism (5%).
Among the minor discrepancies, a different interpretation for CD30 was noted in 40% of cases (>10% difference when compared to the referring facility in cases with <50% expression), followed by a change in the presence or absence of epidermotropism (39% of cases). Discrepancies in CD30 interpretation occurred mainly because of different forms of scoring (positive versus negative without a percentage of staining, different denominator – neoplastic vs total infiltrate) and patterns of staining (cases with very weak staining were read as negative). Other minor discrepancies occurred upon the finding of granulomas (11%) or Ki67 proliferation (8%). It is noteworthy that while Ki67 accounted for a small proportion of the minor discrepancies, most cases lacked the evaluation of such marker. Figures 4–6 illustrate specific examples of major and minor discrepancies. For CD30, 53 cases had a percentage recorded from both the referring pathologist and the consensus diagnosis with a Pearson correlation of 0.66 (p-value < 0.0001). For Ki67, 14 cases had a percentage recorded from both the referring pathologist and the consensus diagnosis with a Pearson correlation of 0.86 (p-value < 0.0001). Figures 7 and 8 show the scatter plots of the correlation between the referring pathologist and central review consensus for CD30 and Ki67 interpretation.
Figure 4: Major discrepancy example. In this case, a diagnosis of tumor stage MF with no large cell transformation was established. Upon review, a consensus panelist agreed in the on the diagnosis of MF with LCT and granulomatous features. Granulomas can be a confounding factor in the diagnosis of LCT.
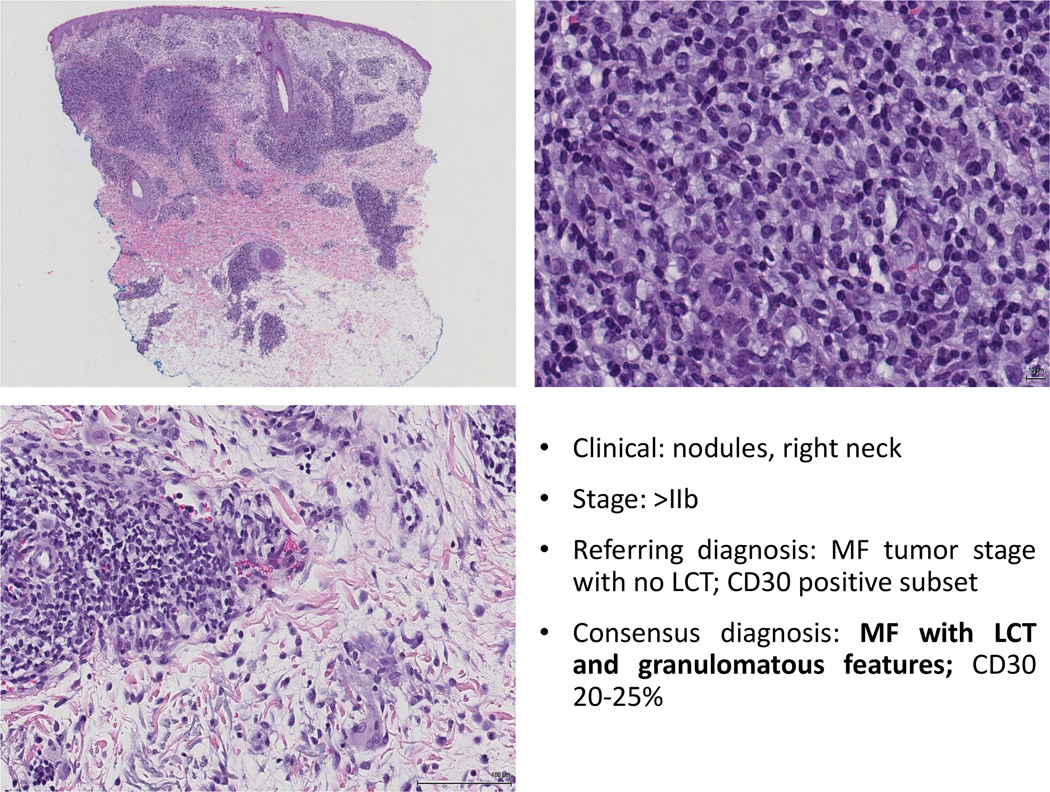
Figure 6: In this case a change in the interpretation of the CD30 immunostain can be noted. While the staining pattern is weak, there is no background staining, and most of the neoplastic cells appear to positive. A lack of consensus on the diagnosis of LCT was noted among the reviewers.
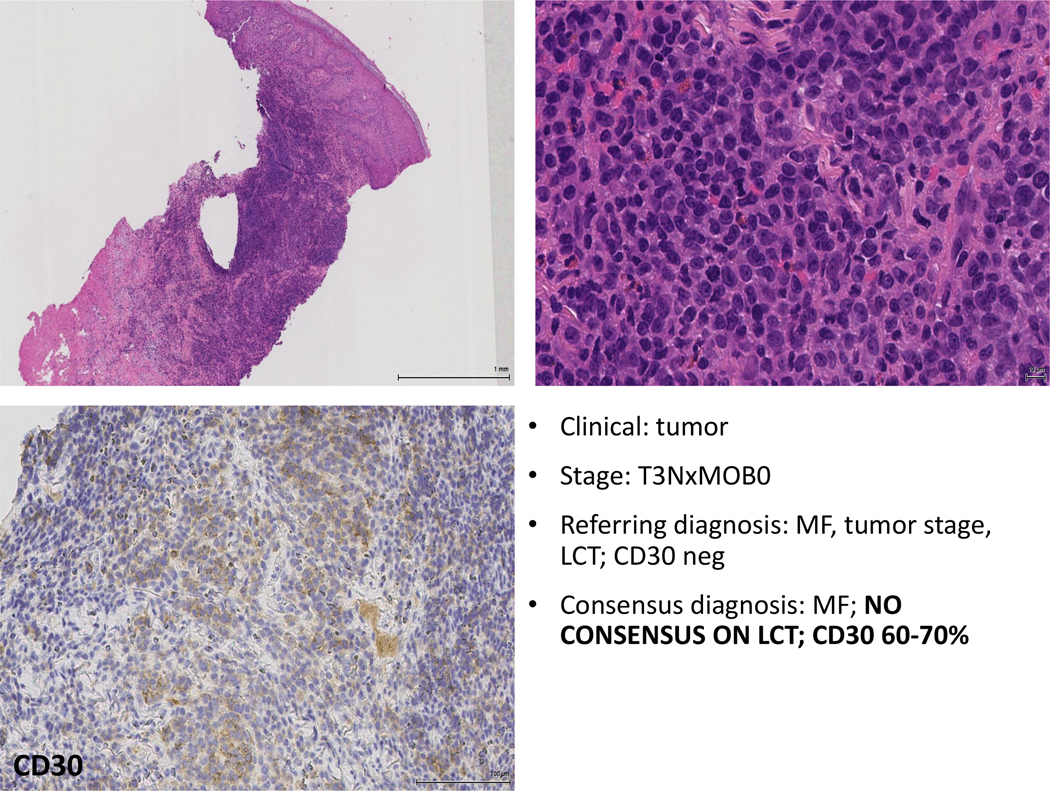
Table 3 shows a comparison of the kappa interobserver agreements for final diagnosis, LCT, folliculotropism, syringotropism, epidermotropism and granulomas, evaluated in the study between the referring pathologist and consensus review. The overall agreement (OA) between the reviewer pathologist and consensus review was significantly higher compared to the OA between the referring pathologist and consensus review. Interestingly, most of the parameters evaluated show only a modest degree of interobserver agreement (with the exception of syringotropism that had a fair level of agreement). In addition, percentage evaluation of interobserver agreements were done among the central panelist reviewers: briefly, both LCT and CD30 expression show only a modest degree of interobserver reproducibility (68% and 75%, respectively). Folliculotropism, syringotropism and evaluation for the presence of granulomas had a higher level of agreement (Figure 9).
Table 3:
Comparing the outside diagnosis and the consensus review
| Kappa (95% CI) | Agreement proportion (95% CI) | |
|---|---|---|
| Diagnosis | 0.49 (0.35,0.63) | 0.60 (0.49,0.71) |
| LCT | 0.43 (0.24,0.62) | 0.70 (0.59,0.80) |
| FMF | 0.49 (0.28,0.70) | 0.79 (0.70,0.89) |
| ST | 0.25 (−0.20,0.70) | 0.93 (0.87,0.99) |
| EPID | 0.49 (0.28,0.71) | 0.79 (0.70,0.89) |
| GM | 0.41 (0.00,0.83) | 0.93 (0.87,0.99) |
LCT: large cell transformation / FMF: MF with folliculotropism / ST: syringotropism / EPID: Epidermotropism / GM: granulomatous features
To summarize the results, we noted a very large number of discrepant results between referring pathologist from major academic centers with well-established cutaneous lymphoma programs and the consensus pathology review. Poor level of interobserver agreements were seen between the consensus and referring pathologist for major pathologic findings (e.g. LCT, FT, CD30 expression) believed to be important biomarkers to determine prognosis of the disease.
Discussion
Patients with advanced stage MF or SS have unfavorable prognosis with survivals that range 1–7+ years. Currently, we lack prospectively validated prognostic models that augment clinical staging and can be utilized for risk stratification in clinical management or therapeutic trials. Because of the uncommon nature of the disease, and the even rarer occurrence of the advanced forms, large-scale prospective studies are in need for the evaluation of prognostic and predictive biomarkers in the advanced stages. Equally important is the fact that there is a need for a reliable and reproducible method to evaluate pathologic parameters to assure an accurate collection of the information from these patients. The PROCLIPI study was designed in the context of the CLIC to study patients with advanced disease in a prospective way with the help of centralized pathology review. This study is the largest international project of its kind ever performed in the history of the disease. This pathology PILOT study was performed to evaluate the efficacy and suitability of digital slide scanning for the evaluation of central pathology review in patients with advanced disease, and its potential utility in the prospective study.
One of the main issues that have become apparent in the diagnosis of this type of malignancies is that, even in the context of an appropriate panel of immunohistochemistry and molecular testing, the low prevalence of these disorders results in a lack of confidence by most dermatopathologists and hematopathologists to establish a correct diagnosis. Thus, expert review is essential when dealing with these tumors. The International T-cell Lymphoma Project evaluated a cohort of 1314 cases of PTCL and NKTCL during a period of 12 years in an international scale using central pathology review[9]. They reported that 10.4% of cases were misclassified by the referring pathologists. In that study, the interobserver agreement (among experts) for the diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) and subcutaneous panniculitis-like T-cell lymphoma (SPTCL) were 66% and 75%, respectively (the lowest among all cases of PTCLs in the study). Herrera et al[10] compared the referring and centralized diagnoses of PTCL in a cohort of 131 patients with PTCL (in National Comprehensive Cancer Network Centers). Discrepant diagnoses occurred in 36% of cases, and approximately half of the discrepancies occurred after a new interpretation of the existent immunostains was done. They estimated that expert reclassification could have led to a change in treatment in approximately 44% of cases. More recently, Bellei et al[11] reported incorrect diagnosis of PTCLs in 13.1% of cases upon review of the slides in a cohort of 573 cases.
The evaluation of the interobserver agreements among the diagnosis of the CTCL, MF and SS, is much more limited. Guitart et al[12] proposed a grading system to evaluate biopsies of MF/SS. Upon the evaluation of 50 cases by 4 independent dermatopathologists using specific diagnostic categories, but without specific criteria, consensus diagnosis was achieved in 48% of cases, nearly the equivalent to flipping a coin (kappa 0.63). The re-evaluation of the same cases providing the proposed criteria led to only a very mild increase in the consensus agreement to 58% (with a modest kappa score 0.74). Florell et al[13] reviewed the interobserver agreement in the diagnosis of 138 cases of benign dermatoses, ‘atypical dermatoses’, and MF. Significant interobserver discrepancies were noted, accounting for 50% of cases of atypical dermatoses and 36% of cases of MF. The initial kappa agreement was only fair to moderate (0.33–0.44). In the present study, we identified an overall agreement with the referring facilities of only 35% among central pathology reviewers and major academic centers with well-established cutaneous lymphoma programs. Most of the major disagreements were particularly associated with the presence or absence of LCT and FT. While this might not be a surprise, we speculate that pathologists might be more confident in the determination of LCT in the context of tumoral lesions, but not in a patch, plaque, or erythrodermic skin disease. Nonetheless, the strict definition of large cell transformation is arbitrary relying on the finding of more than 25% of large cells or large cell nodules[14]. Similarly, the ‘nodular’ definition of large cells is vague and most pathologist do not use it. Such tumoral nodules can be hard to identify and focal in the biopsies.
The diagnosis of large cell transformation in MF/SS has important clinical implications. LCT is associated with more advanced disease and worse prognosis. In the retrospective analysis by Scarisbrick et al[7] on 1275 patients with advanced stage disease, LCT was one of the four variables that were independent predictors of survival (in addition to stage IV, elevated LDH or age above 60). LCT was associated with an overall survival of 49.8 months, and 5-year survival of 39%. CD30 expression and Ki67 were also associated with worse prognosis in patients with T3 disease (presence of tumors). However, LCT is currently not part of the staging systems used to classify MF/SS, and many studies have found no survival differences (when comparing LCT vs non-LCT cases)[15–17]. The authors speculate that such significant differences across the literature are due, to at least in part, to variable interpretation of the pathology data, and the significant disagreements present in the diagnosis of LCT. Indeed, there is also no current consensus in the way of interpreting CD30 expression. We found that discrepancies in CD30 interpretation were due to different forms of scoring (positive versus negative without a percentage of staining, different denominator – neoplastic vs total infiltrate) and patterns of staining (cases with very weak staining were read as negative). Additionally, contaminating cells (e.g. macrophages, suspected reactive immunoblasts) can be CD30-positive and make an accurate interpretation of the percentage of staining more difficult.
In a similar way to what has been reported with LCT, the presence of FT has historically been associated with a more aggressive clinical course and worse outcome than conventional MF[18, 19]. FMF (folliculotropic MF) is designated as a variant of the disease that is characterized by distinct clinical features (follicular based papules, alopecia, cysts and comedones). Patients with FT may be refractory to standard therapy and have a worse survival. Patients with stage IA/B FMF have been reported to show a similar 5-year outcome to tumor stage disease and worse 10-year survival when compared to conventional MF[5]. In the UK data from 1522 patients with MF/SS, 189 patients had FMF, on multivariate analysis FT was an independent predictor of poor survival and had increased risk of disease progression[20]. We found a poor level of interobserver agreement for the pathologic diagnosis of FT within advanced stage patients. We speculate that small foci of folliculotropism can be sometimes missed in the biopsy, and that cases with follicular destruction, where only remnants of keratinous debris in the dermis and have minimal infiltration of the hair follicle epithelium, can be easily missed. In addition, conventional MF may have subtle involvement of the infundibular portion of the follicle which may be misinterpreted as folliculotropism. Another point of uncertainty is whether folliculotropic MF or mycosis fungoides with focal folliculotropism should be considered separately.
Like the variability in the interpretation of large cell transformation and folliculotropism, differences in the percentage of CD30 and Ki67 expression were also seen between the referring pathologist and central reviewers. Because CD30 is an actionable targeted therapy in CTCL[21, 22], an accurate assessment of the percentage of expression of the antigen in the tumor cells appears to be important in the decision-making process of treating patients with brentuximab vedotin. Kim et al[23] enrolled 32 patients with advanced stage MF and SS in a phase II study looking at responses to brentuximab vedotin. Patients with <5% CD30 expression had a lower likelihood of global response than did those with >5% CD30 expression (p<.005). Notably, most patients with >30% expression had significant and durable responses. In counterpart, Duvic et al[24] found no significant differences in treatment responses in MF/SS according to the levels of CD30 expression. Nonetheless, the patients with higher CD30 had a more durable response compared to those who did not. In addition to treatment efficacy, CD30 expression has been linked to a difference in prognosis of the disease (a feature that has been a matter of significant debate in terms of whether CD30 expression confers a better or worse prognosis)[25–27]. Because the role of CD30 in both prognosis and treatment-related therapies, an accurate centralized evaluation of expression is capital to understand the role of this marker in the disease.
In summary, we found digital slide scanning to be beneficial, reliable and practical for a methodical approach to perform central pathology review in the context of a large clinical prospective study. We found significant differences in the interpretation of common pathologic parameters in the advanced stages. The additional benefit that derive from the slide scanning is the construction of a digitized biobank where the slides data can be permanently preserved, stored, and directly linked to rigorously collected clinical and molecular information. Digital slide scanning is also a very feasible way of performing centralized pathology review, given the broad access of academic medical centers to its use. It provides a solid template for the development of future translational studies that link molecular, clinical and pathologic information. Therefore, pathology central review is highly advised in the context of the diagnosis of CTCL for prospective research studies and clinical trials.
Supplementary Material
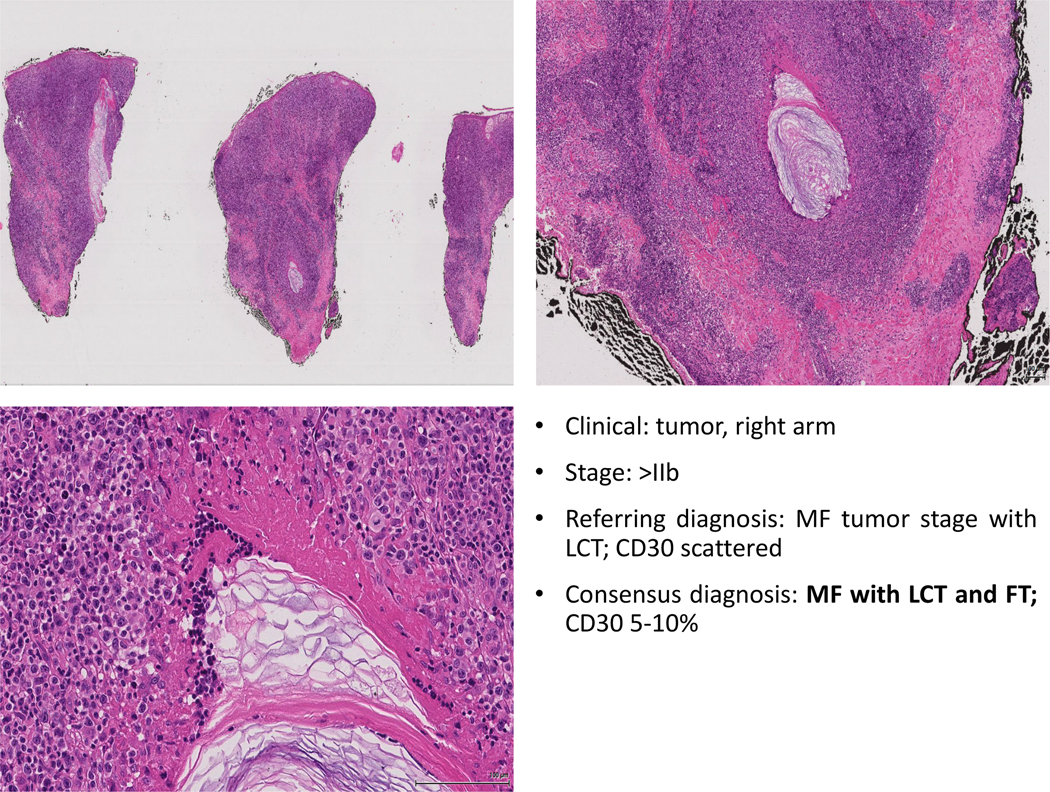
Figure 5: In this particular case the biopsy illustrates an example of an advanced lesion of FT MF with acneiform features and LCT. Some cases of acneiform FT MF might not reveal significant infiltration of the hair follicle epithelium. In this case, the hint to the presence of FT is marked by the presence of hair destruction.
References
- 1.Willemze R, et al. , WHO-EORTC classification for cutaneous lymphomas. Blood, 2005. 105(10): p. 3768–85. [DOI] [PubMed] [Google Scholar]
- 2.Olsen E, et al. , Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood, 2007. 110(6): p. 1713–22. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker S, Hoppe R, and Prince HM, How I treat mycosis fungoides and Sezary syndrome. Blood, 2016. 127(25): p. 3142–53. [DOI] [PubMed] [Google Scholar]
- 4.Virmani P, et al. , Systemic therapy for cutaneous T-cell lymphoma: who, when, what, and why? Expert Rev Hematol, 2017. 10(2): p. 111–121. [DOI] [PubMed] [Google Scholar]
- 5.Scarisbrick JJ, et al. , Prognostic factors, prognostic indices and staging in mycosis fungoides and Sezary syndrome: where are we now? Br J Dermatol, 2014. 170(6): p. 1226–36. [DOI] [PubMed] [Google Scholar]
- 6.Benton EC, et al. , A cutaneous lymphoma international prognostic index (CLIPi) for mycosis fungoides and Sezary syndrome. Eur J Cancer, 2013. 49(13): p. 2859–68. [DOI] [PubMed] [Google Scholar]
- 7.Scarisbrick JJ, et al. , Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sezary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol, 2015. 33(32): p. 3766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis JR and Koch GG, The measurement of observer agreement for categorical data. Biometrics, 1977. 33(1): p. 159–74. [PubMed] [Google Scholar]
- 9.Vose J, et al. , International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol, 2008. 26(25): p. 4124–30. [DOI] [PubMed] [Google Scholar]
- 10.Herrera AF, et al. , Comparison of referring and final pathology for patients with T-cell lymphoma in the National Comprehensive Cancer Network. Cancer, 2014. 120(13): p. 1993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellei M, et al. , Pitfalls and major issues in the histologic diagnosis of peripheral T-cell lymphomas: results of the central review of 573 cases from the T-Cell Project, an international, cooperative study. Hematol Oncol, 2016. [DOI] [PubMed]
- 12.Guitart J, et al. , Histologic criteria for the diagnosis of mycosis fungoides: proposal for a grading system to standardize pathology reporting. J Cutan Pathol, 2001. 28(4): p. 174–83. [DOI] [PubMed] [Google Scholar]
- 13.Florell SR, et al. , Usefulness (or lack thereof) of immunophenotyping in atypical cutaneous T-cell infiltrates. Am J Clin Pathol, 2006. 125(5): p. 727–36. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow SH, International Agency for Research on Cancer., and World Health Organization., WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. World Health Organization classification of tumours. 2008, Lyon, France: International Agency for Research on Cancer. 439 p. [Google Scholar]
- 15.Benner MF, et al. , Prognostic factors in transformed mycosis fungoides: a retrospective analysis of 100 cases. Blood, 2012. 119(7): p. 1643–9. [DOI] [PubMed] [Google Scholar]
- 16.Diamandidou E, et al. , Prognostic factor analysis in mycosis fungoides/Sezary syndrome. J Am Acad Dermatol, 1999. 40(6 Pt 1): p. 914–24. [DOI] [PubMed] [Google Scholar]
- 17.Pulitzer M, et al. , Mycosis fungoides with large cell transformation: clinicopathological features and prognostic factors. Pathology, 2014. 46(7): p. 610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YH, et al. , Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol, 2003. 139(7): p. 857–66. [DOI] [PubMed] [Google Scholar]
- 19.Gerami P, et al. , Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol, 2008. 144(6): p. 738–46. [DOI] [PubMed] [Google Scholar]
- 20.Agar NS, et al. , Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol, 2010. 28(31): p. 4730–9. [DOI] [PubMed] [Google Scholar]
- 21.Bagot M, New Targeted Treatments for Cutaneous T-cell Lymphomas. Indian J Dermatol, 2017. 62(2): p. 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger GK, et al. , Brentuximab vedotin for treatment of non-Hodgkin lymphomas: A systematic review. Crit Rev Oncol Hematol, 2017. 109: p. 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YH, et al. , Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sezary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J Clin Oncol, 2015. 33(32): p. 3750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvic M, et al. , Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J Clin Oncol, 2015. 33(32): p. 3759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barberio E, et al. , Transformed mycosis fungoides: clinicopathological features and outcome. Br J Dermatol, 2007. 157(2): p. 284–9. [DOI] [PubMed] [Google Scholar]
- 26.Edinger JT, et al. , CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol, 2009. 33(12): p. 1860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadin ME, et al. , High soluble CD30, CD25, and IL-6 may identify patients with worse survival in CD30+ cutaneous lymphomas and early mycosis fungoides. J Invest Dermatol, 2012. 132(3 Pt 1): p. 703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.