Significance
Animals display dimorphic sexual and aggressive behaviors. In Drosophila, the doublesex (dsx) gene encodes male-specific DsxM and female-specific DsxF transcription factors and controls sexual development and behaviors. In mammals, dsx-related Dmrt genes direct the differentiation of gonads that produce sex hormones, controlling both the organization and activation of neural circuits underlying sexually dimorphic behaviors. In this study, we find that sex-specific Dsx isoforms promote distinct sexual behaviors in both sexes, and oppositely regulate aggression to establish male-biased aggressiveness. We further show that Dsx functions both developmentally and acutely in sex-specific interneurons that control these behaviors. Our results reveal that a conserved sex-determination gene plays both organizational and activational roles in sexually dimorphic behaviors.
Keywords: sexual dimorphism, Drosophila, aggression, sexual behavior, doublesex
Abstract
Most animal species display dimorphic sexual behaviors and male-biased aggressiveness. Current models have focused on the male-specific product from the fruitless (fruM) gene, which controls male courtship and male-specific aggression patterns in fruit flies, and describe a male-specific mechanism underlying sexually dimorphic behaviors. Here we show that the doublesex (dsx) gene, which expresses male-specific DsxM and female-specific DsxF transcription factors, functions in the nervous system to control both male and female sexual and aggressive behaviors. We find that Dsx is not only required in central brain neurons for male and female sexual behaviors, but also functions in approximately eight pairs of male-specific neurons to promote male aggressiveness and approximately two pairs of female-specific neurons to inhibit female aggressiveness. DsxF knockdown females fight more frequently, even with males. Our findings reveal crucial roles of dsx, which is broadly conserved from worms to humans, in a small number of neurons in both sexes to establish dimorphic sexual and aggressive behaviors.
A central goal in neuroscience is to understand how innate behaviors are generated at the molecular and neuronal levels, and in particular how sexually dimorphic behaviors are established. A substantial body of work has revealed sex-specific or sex-biased expression of genes that are responsible for sexually dimorphic behaviors from worms to humans (1, 2).
The fruit fly, Drosophila melanogaster, is an excellent model system with valuable genetic tools that can be used to study dimorphic behaviors (3). Notably, Drosophila shows robust reproductive and aggressive behaviors with both qualitative and quantitative aspects of sexual dimorphism, as well as well-characterized dimorphic circuits (4, 5). For example, male flies display male-specific courtship behaviors, while virgin females evaluate the quality of male courtship and decide to be receptive or not (6). Aggressive dimorphic behaviors can also be analyzed. Qualitative differences in fighting patterns are observed between males and females, and fighting is also quantitatively different in that males fight more frequently than females (7–9).
Studies over several decades have led to the identification of two terminal genes in the sex determination hierarchy: fruitless (fru), encoding male-specific Fru (FruM) proteins, and doublesex (dsx), encoding sex-specific DsxM and DsxF proteins (10–12). Together, the fru and dsx gene products control most aspects of sexual dimorphism (5, 13). FruM is largely necessary and sufficient for building the potential for innate courtship behavior into the nervous system (14–16). In the absence of FruM, males are unable to court without prior social experience, but can acquire some courtship behaviors in an experience- and DsxM-dependent manner (17, 18). Indeed, FruM and DsxM coordinate to generate a subset of male-specific P1 neurons that integrate multiple sensory cues and initiate courtship (19–23). FruM is responsible for the dimorphic projections of aSP-f neurons in males and aSP-g neurons in females that underlie sex-specific pheromone responses and sexual behaviors (24). FruM is also responsible for the sex-specific fighting patterns and dominance (25), and specific subsets of FruM neurons are involved in generating sexually dimorphic aggression patterns (26). FruM is also responsible for the development of male-specific aggression-promoting neurons that express the neuropeptide tachykinin (Tk) (9, 27). Moreover, FruM is expressed in a subset of male-specific P1 neurons and aSP2 neurons that promote intermale aggression (28, 29). These findings reveal the crucial roles of FruM in establishing male-specific sexual behaviors and aggression patterns, and perhaps male-biased aggression intensity.
In contrast to the well-established role of FruM in controlling sexually dimorphic behaviors, studies of DsxM and DsxF have thus far mainly focused on sexual differentiation, as dsx and its related Dmrt genes control most aspects of morphological dimorphism in flies and other animals (30). Consequently, how dsx functions in the nervous system to mediate sexually dimorphic behaviors has rarely been studied (31). Previous studies of dsx function in behavior either directly used dsx mutants or dominate alleles that affect dsx function in all cells and are often intersexual (18, 32), or indirectly from manipulation of fru or transformer (tra), which acts upstream of both fru and dsx (18, 22, 26, 33). A recent study used RNA interference (RNAi) to knock down dsx expression in adulthood to investigate its role in behavior, but the manipulation was still carried out in all dsx-expressing cells (34). Despite the relatively understudied role of dsx in the nervous system, neurons expressing dsx are found to control most dimorphic behaviors, including male courtship and aggression (18, 22, 29, 33, 35–37), female precopulatory and postmating behaviors (38–42), as well as female aggression (37, 43–45). Importantly, a subset of fruM and dsxM coexpressing P1 neurons specifically promote male courtship but not aggression, whereas another subset of fruM-negative but dsxM-positive pC1 neurons specifically promote male aggression but not courtship (37), implicating a potential role of DsxM in regulating male courtship and aggression. Thus, it is of significant interest to investigate the role of DsxM in males and DsxF in females in regulating dimorphic sexual and aggressive behaviors.
In this study, we knocked down dsx function in the male and female nervous systems, from pan-neuronal to specific knockdown in as few as two pairs of neurons, and revealed crucial roles of DsxM in promoting male courtship and DsxF in promoting female receptivity in central brain neurons. Surprisingly, we found that DsxM and DsxF function oppositely in a small number of sex-specific aggression-promoting neurons to promote male aggression and inhibit female aggression, respectively. These results reveal the function of sex-specific DsxM and DsxF proteins in promoting reproductive behaviors in both sexes, while biasing aggression in males and females to establish sexually dimorphic aggression levels.
Results
Central Brain DsxM Regulates Male Sexual and Aggressive Behaviors.
A previous study found that dsx mutant males show a low level of courtship toward females (32), suggesting that dsx may be crucial for male courtship. To validate this finding, we tested courtship in dsx-null mutant males using three deficiency lines (18). In addition, we generated a dsxDBD allele, in which the first coding exon of dsx was replaced by the GAL4-DBD sequence (SI Appendix, Fig. S1A). The dsxDBD allele is a null mutant, as evidenced by the loss of Dsx immunostaining as well as intersexual phenotypes in dsxDBD/dsx1649-9625 males and females (SI Appendix, Fig. S1 B–E). We found that dsx-null mutant males (dsx683-7058/dsx1649-9625, dsx683-7058/dsxM+R15, and dsxDBD/dsx1649-9625) indeed showed low levels of courtship toward wild-type virgin females (courtship indices [CIs] < 7%), whereas heterozygous control males courted vigorously (CIs > 70%) (Fig. 1A). Although these results confirm that courtship is affected, one important caveat of these experiments is that dsx-null mutant males are intersex and have developmental defects in sexual organs. Thus, a specific role for dsx in the central nervous system (CNS) cannot be determined yet.
Fig. 1.
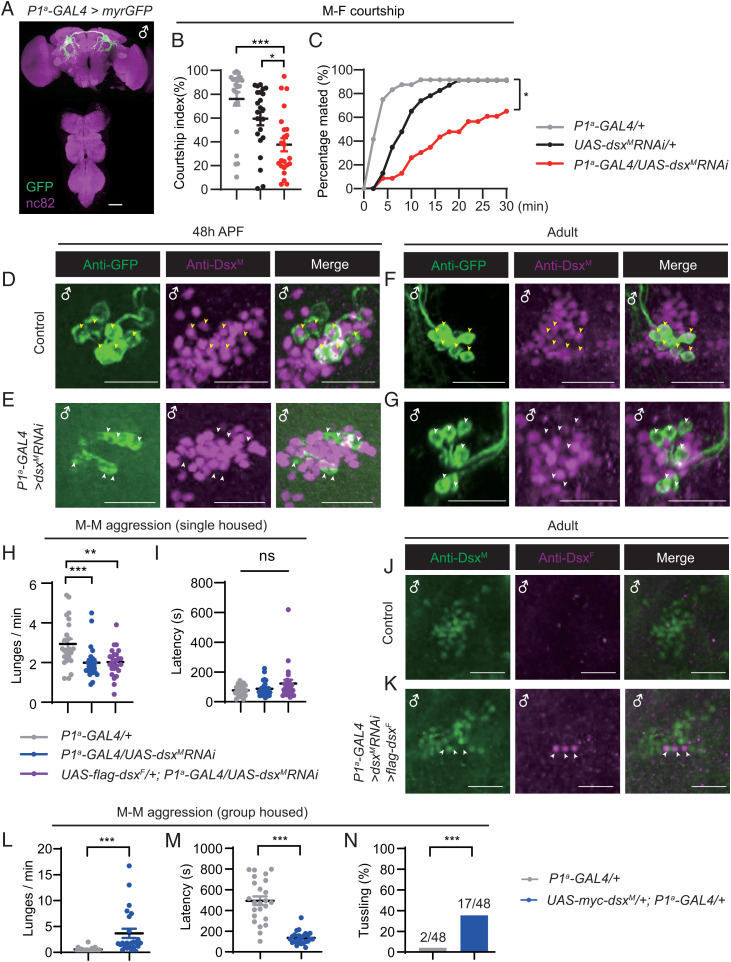
Central brain DsxM regulates male sexual and aggressive behaviors. (A and B) Courtship indices for dsx mutant (A) and RNAi-mediated dsxM knockdown males (B). Genotypes are indicated below. n = 24 for each. ***P < 0.001 and ns, not significant. Kruskal–Wallis test and post hoc Dunn’s multiple comparisons. (C and D) Expression pattern of dsxGAL4 (C) and dsxbrain (Otd-Flp;tub > GAL80 >, dsxGAL4) in male CNS (D). (Scale bars, 50 μm.) Representative of three samples each. (E–H) Anti-DsxM and anti-GFP signals in pupal (E and F) and adult (G and H) stages in control (UAS-myrGFP/+;dsxbrain/+) and dsxM knockdown (UAS-myrGFP/+;dsxbrain/UAS-dsxMRNAi) male brains. Yellow arrowheads indicate anti-DsxM signals in pC1 and pC2 neurons in control males (E and G), and white arrowheads indicate diminished anti-DsxM signals in the same brain region in dsxM knockdown males (F and H). (Scale bars, 50 μm.) Representative of five samples each. (I) Courtship indices for control and dsxM knockdown males. n = 23 for each. ***P < 0.001, Mann–Whitney U test. (J) The percentage of successful copulation within 30 min. n = 23 each. ***P < 0.001, χ2 test. (K) An example image of intermale aggression. (L) Number of lunges per minute for control and dsxM knockdown males. n = 24 for each. ***P < 0.001, Mann–Whitney U test. (M) Fighting latency of control and dsxM knockdown males. n = 24 for each. ***P < 0.001, Mann–Whitney U test. Error bars indicate SEM.
To address if neuronal dsx contributes to dimorphic behaviors, we set out to knock down dsx function specifically in the nervous system (SI Appendix, Fig. S2). We used two RNAi lines as described previously, one targeting the male-specific exon (UAS-dsxMRNAi) and the other the female-specific exon (UAS-dsxFRNAi) of the dsx gene (34, 46). Their efficiency was validated using real-time qPCR (SI Appendix, Fig. S2 B and C) and by confirming the presence of intersexual phenotypes. To knock down dsx expression specifically in the nervous system, we then combined these validated RNAi tools with the pan-neuronal driver R57C10-GAL4, and found that DsxM expression in the brain was almost undetectable in RNAi-mediated knockdown males, except for several false-positive cells that are likely due to the imperfect specificity of this antibody (SI Appendix, Fig. S2 D and E). We also found that knocking down dsxM in all dsx-expressing cells induced intersexual phenotypes like dsx mutant males, whereas knocking down dsxM pan-neuronally did not affect external sexual traits (SI Appendix, Fig. S2H). The knockdown of neuronal DsxM expression strongly impaired male courtship, almost to the level of dsx-null mutant males (CI ∼13%, control CIs > 80%) (Fig. 1B). These results indicate that neuronal DsxM is crucial for male courtship.
We next sought to identify the crucial subset of neurons in which DsxM is required for male courtship. First, we targeted dsx neurons specifically in the brain, hereafter referred to as dsxbrain neurons, by using the brain-specific flippase recombinase (Otd-Flp) (9, 17) (Fig. 1C for dsxGAL4 and Fig. 1D for dsxbrain [Otd-Flp;tub > GAL80 >, dsxGAL4]). We further examined the efficiency of dsx RNAi-mediated knockdown in control (UAS-myrGFP/+;dsxbrain/+) and experimental (UAS-myrGFP/+;dsxbrain/UAS-dsxMRNAi) males from different developmental stages (third-instar larval [L3], 48 h after pupal formation [48 h APF], 4- to 7-d-old adult) using both anti-GFP and anti-DsxM antibodies (Fig. 1 E–H and SI Appendix, Fig. S3 A and B). Whereas anti-DsxM signals mostly overlapped with the dsxbrain-driven GFP signals in all three developmental stages in control male brains (for pupal and adult expression, see Fig. 1 E and G; for larval expression, see SI Appendix, Fig. S3A), they were almost undetectable in UAS-myrGFP/+;dsxbrain/UAS-dsxMRNAi male brains regardless of the developmental stage (for pupal and adult expression, see Fig. 1 F and H; for larval expression, see SI Appendix, Fig. S3B). Notably, the knockdown was not perfect in some samples, as we observed anti-DsxM signals in a small number of cells with weak or no GFP signal, likely due to variation in expression from the dsxbrain driver in individual flies (SI Appendix, Fig. S3C). Nevertheless, these results demonstrated effective knockdown of dsxM in most, if not all, dsxbrain neurons throughout development.
We then tested the effect of knocking down dsxM in dsxbrain neurons on male courtship and aggressive behaviors. We found that compared to the control, dsxM knockdown males showed reduced courtship toward virgin females (Fig. 1I) (CI ∼23%, compared to ∼80% for control males) and less mating success (Fig. 1J) (∼20% mated within 30 min, compared to ∼90% for control males). To examine if DsxM function is also involved in male aggression, we tested intermale aggression in single-housed males (Fig. 1K). Knocking down dsxM in dsxbrain neurons significantly reduced intermale lunging frequency (Fig. 1L) and increased the latency to initiate lunging behavior (Fig. 1M). Taken together, these results indicate that DsxM functions in the central brain to promote female-directed courtship and intermale aggression.
DsxM Expression in the Male-Specific P1a Neurons Positively Correlates with Male Aggressiveness.
Central brain dsxM-expressing neurons include as many as ∼300 pC1, pC2, and pCd neurons (47–49). To further investigate the function of DsxM in regulating male courtship and aggression in a smaller number of neurons, we used a splitGAL4 driver to target a subset of male-specific P1 neurons (P1a neurons) (Fig. 2A) previously shown to be crucial for courtship initiation and aggression (21, 22, 29, 31, 50, 51). These P1a neurons are a subset of dsxM-expressing pC1 neurons (33). RNAi-mediated knockdown of dsxM specifically in P1a neurons significantly reduced male courtship toward virgin females (CI ∼36%) compared to two control lines (CIs > 60%) (Fig. 2B), and also reduced male mating success (∼60% mated in 30 min, compared to ∼90% for control males) (Fig. 2C), although the reduction was less than observed for males with dsxM knockdown in all dsxbrain neurons (Fig. 1 I and J).
Fig. 2.
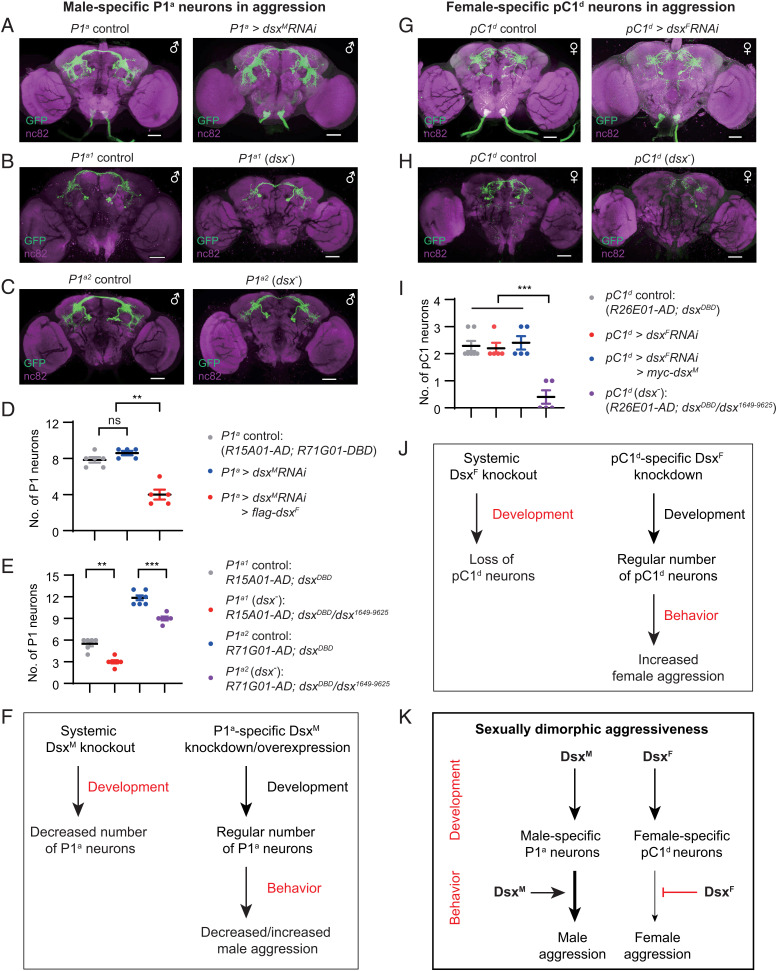
DsxM expression in P1a neurons positively regulates male aggressiveness. (A) Expression pattern of the P1a-GAL4 (R15A01-AD; R71G01-DBD) in male CNS. (Scale bars, 50 μm.) Representative of five samples. (B) Courtship indices for control and P1a-dsxM knockdown males. n = 24, 23, 23 from left to right. *P < 0.05, ***P < 0.001, Kruskal–Wallis test and post hoc Dunn’s multiple comparisons. (C) The percentage of successful copulation within 30 min. n = 24, 23, 23, respectively. *P < 0.05, χ2 test. (D–G) Anti-DsxM and anti-GFP signals in pupal (D and E) and adult (F and G) stages in control (UAS-myrGFP/+;P1a-GAL4/+) and P1a-dsxM knockdown (UAS-myrGFP/+;P1a-GAL4/UAS-dsxMRNAi) male brains. Yellow arrowheads indicate costaining of anti-DsxM and anti-GFP signals in control males (D and F), and white arrowheads indicate diminished anti-DsxM signals specifically in the GFP+ neurons in P1a-dsxM knockdown males (E and G). (Scale bars, 20 μm.) Representative of five samples each. (H and I) Lunging frequency (H) and fighting latency (I) for control and P1a-dsxM knockdown males, as well as males with dsxM knockdown and ectopic dsxF expression in P1a neurons. Genotypes are indicated. n = 23, 24, 24 from left to right. **P < 0.01, ***P < 0.001; ns, not significant, Mann–Whitney U test. (J and K) Costaining using anti-DsxM and anti-DsxF antibodies in control P1a-GAL4/+ (J) and experimental UAS-flag-dsxF/+;P1a-GAL4/UAS-dsxMRNAi (K) male adult brains. White arrowheads indicate ectopic expression of DsxF and knockdown of DsxM. (Scale bars, 20 μm.) Representative of five samples each. (L and M) Lunging frequency (L) is enhanced and fighting latency (M) is reduced by overexpression of DsxM in P1a neurons. n = 24 for each. ***P < 0.001, Mann–Whitney U test. (N) High-intensity aggression behavior (tussling) is induced with overexpression of DsxM in P1a neurons. n = 48 for each. ***P < 0.001, χ2 test. Error bars indicate SEM.
To validate the efficiency of dsxM knockdown specifically in the P1a neurons, we used anti-GFP to label P1a neurons and anti-DsxM to simultaneously examine knockdown efficiency and specificity (Fig. 2 D–G and SI Appendix, Fig. S3 D–I). We found no overlap of anti-GFP and anti-DsxM signals in the L3 stage in control UAS-myrGFP/+;P1a-GAL4/+ males, suggesting that P1a-GAL4 drives expression in dsxM-negative cells in the L3 stage (SI Appendix, Fig. S3D). Alternatively, these cells could be dsxM-positive P1a, but the DsxM expression was too weak to be detected by anti-DsxM antibody in this stage. In the pupal and adult stages, we observed well-overlapped anti-GFP and anti-DsxM signals in control UAS-myrGFP/+;P1a-GAL4/+ males, indicating consistent GAL4 expression in P1a neurons from pupal to adult stages (Fig. 2 D and F, yellow arrowheads, and SI Appendix, Fig. S3 F and H). Furthermore, anti-DsxM signals were almost diminished in the GFP+ P1a neurons but present in other pC1 neurons in both the pupal and adult brains of UAS-myrGFP/+;P1a-GAL4/UAS-dsxMRNAi (Fig. 2 E and G, white arrowheads, and SI Appendix, Fig. S3 G and I), indicating effective and specific knockdown of dsxM expression in P1a neurons. Together, these results demonstrate the effectiveness and specificity of dsxM knockdown in P1a neurons, and the crucial role of DsxM in these male-specific neurons for courtship intensity and mating success.
In addition to courtship behavior, male-specific P1a neurons also regulate aggressive behaviors (27, 29, 33). To test if DsxM in these P1a neurons is also involved in male aggression, we observed intermale aggression in single-housed males from both control and RNAi-mediated knockdown lines. Knocking down dsxM specifically in P1a neurons significantly reduced intermale lunging frequency (Fig. 2H). We then tested if expression of the female isoform DsxF would rescue or aggravate the aggression phenotype in dsxM knockdown males. We found similarly low levels of lunging frequency for dsxM knockdown males with or without ectopic expression of dsxF in P1a neurons compared to control males (Fig. 2H). However, when compared to controls, males with dsxM knockdown alone or together with DsxF expression did not show any significant difference in fighting latency (Fig. 2I). These results demonstrate that dsxM knockdown in P1a neurons reduces male aggression, but not as strongly as observed for dsxM knockdown in all dsxbrain neurons, suggesting the additional involvement of non-P1a dsxbrain neurons in regulating male aggression. Importantly, the reduction in male courtship and aggression in males with dsxM knockdown in either dsxbrain or P1a neurons was not due to locomotor defects as their walking speed was similar to control males (SI Appendix, Fig. S4 A and B).
To confirm the effectiveness of both dsxM knockdown and DsxF expression, we performed costaining of DsxM and DsxF in UAS-flag-dsxF/+;P1a-GAL4/UAS-dsxMRNAi male brains. We observed three to five pairs of anti-DsxF neurons within the pC1 cluster (ectopic expression of DsxF), in which there was no anti-DsxM signal (dsxM knockdown) (Fig. 2 J and K). Note that the number of P1a neurons was significantly smaller in males with ectopic DsxF expression, consistent with the involvement of DsxF in neural development (22, 48, 52) (for comparisons of neuronal numbers, see for example, Fig. 5).
Fig. 5.
Dsx isoforms promote development of aggression-promoting neurons in two sexes but oppositely modulate their function. (A) Expression pattern of P1a-GAL4 (R15A01-AD; R71G01-DBD) in control (Left) and dsxM knockdown males (Right). (B and C) Expression pattern of P1a1-GAL4 (B, R15A01-AD;dsxDBD) and P1a2-GAL4 (C, R71G01-AD;dsxDBD) in control (Left) and dsx mutant (dsxDBD/dsx1649-9625) males (Right). (Scale bars, 50 μm.) (D) Knocking down dsxM and overexpressing dsxF in P1a neurons, but not knocking down dsxM alone, affects cell numbers. n = 6, 5, 5 from left to right. **P < 0.01; ns, not significant, Mann–Whitney U test. (E) Systemic dsxM knockout reduces the number of P1 neurons. n = 6, 7, 7, 6 from left to right. **P < 0.01, ***P < 0.001, Mann–Whitney U test. (F) A summary of DsxM function on the development of P1a neurons and regulation of their aggression-promoting function. (G) Expression pattern of pC1d-GAL4 (R26E01-AD;dsxDBD) in control (Left) and dsxF knockdown females (Right). (H) Expression pattern of pC1d-GAL4 in control (Left) and dsxF mutant (dsxDBD/dsx1649-9625) females (Right). (Scale bars, 50 μm.) (I) Systemic dsxF knockout, but not restricted dsxF knockdown, impairs development of pC1d neurons. n = 7, 5, 5, 5 from left to right. ***P < 0.001, Mann–Whitney U test. (J) A summary of DsxF function on the development of pC1d neurons and regulation of their aggression-promoting function. (K) DsxM and DsxF promote the development of male-specific P1a in males and female-specific pC1d neurons in females respectively, while oppositely regulate their aggression-promoting function. Error bars in D, E, and I indicate SEM.
We then tested if acute knockdown of dsxM in P1a neurons during adulthood would reduce male aggression. We utilized the temperature-sensitive GAL4 repressor, GAL80ts, and raised tub-GAL80ts/+;P1a-GAL4/UAS-dsxMRNAi males at 18 °C throughout development. Adult males were heat-shocked at 30 °C for 4 d before aggression tests (SI Appendix, Fig. S5A). We found that knocking down dsxM expression specifically during adulthood did not affect male aggression (SI Appendix, Fig. S5 B and C), suggesting the crucial role of dsxM during development of P1a neurons.
To investigate whether the expression level of DsxM in P1a neurons would positively correlate with male aggression, we overexpressed dsxM specifically in P1a neurons and tested aggression in group-housed males. We found that these males lunged much more frequently (Fig. 2L) and quickly (Fig. 2M) than control males. In fact, 17 of 48 males with DsxM overexpression displayed tussling (SI Appendix and Movie S1), a high-intensity fighting pattern rarely seen in control males (2 of 48) (Fig. 2N).
We further tested if acute overexpression of dsxM in P1a neurons during adulthood would enhance male aggression. We utilized the above strategy using GAL80ts to restrict dsxM overexpression during adulthood (SI Appendix, Fig. S5A). We observed strong anti-Myc signals in UAS-myc-dsxM/+;P1a-GAL4/tub-GAL80ts but not in control males after heat shock (SI Appendix, Fig. S5D). Only weak anti-Myc signals were observed without heat shock (SI Appendix, Fig. S5 D–F), suggesting the successful but imperfect repression of GAL4 activity by GAL80ts at 18 °C, consistent with previous findings (17). We found that heat-shocked males with DsxM overexpression initiated fighting much quicker than all three groups of control males, although the lunging frequency was not consistently increased compared to each control line (SI Appendix, Fig. S5 G and H). Furthermore, a fraction of heat-shocked males with DsxM overexpression displayed the high-intensity tussling behavior, while none of the control males did (SI Appendix, Fig. S5I). These results indicate that acute overexpression of DsxM in P1a neurons during adulthood significantly enhances male aggression, although not as strongly as constitutive overexpression of DsxM. Together, the above results demonstrate that DsxM plays both developmental and acute roles in the male-specific P1a neurons for male aggressiveness.
Central Brain DsxF Regulates Female Sexual and Aggressive Behaviors.
Since we found that DsxM plays a crucial role in male courtship and aggression, we next tested the role of DsxF in female behaviors. Knocking down neuronal DsxF expression using the pan-neuronal driver R57C10-GAL4 nearly eliminated female receptivity to courting males (∼2% mated in 30 min, controls > 75% in 30 min) (Fig. 3A). DsxF expression in the brain was reduced to an undetectable level in these RNAi-mediated virgin females (SI Appendix, Fig. S2 F and G). Furthermore, knocking down dsxF in all dsx-expressing cells induced intersexual phenotypes, whereas knocking down dsxF pan-neuronally did not affect external sexual traits (SI Appendix, Fig. S2I).
Fig. 3.
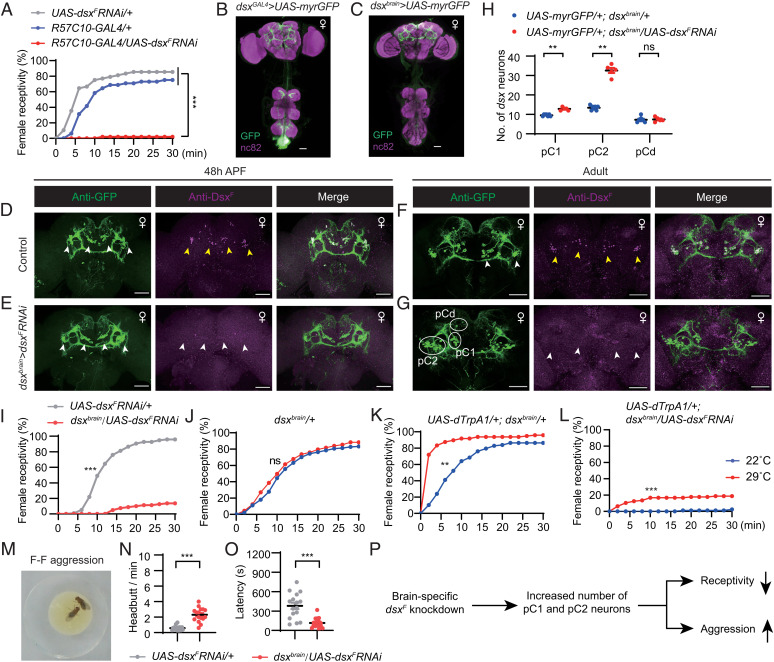
Central brain DsxF regulates sexual and aggressive behaviors in females. (A) Female receptivity by control and DsxF knockdown females, n = 48 for each. ***P < 0.001, χ2 test. (B and C) Expression pattern of dsxGAL4 (B) and dsxbrain-GAL4 (C) in female CNS. (Scale bars, 50 μm.) Representative of three samples each. (D–G) Anti-DsxF and anti-GFP signals in pupal (D and E) and adult (F and G) stages in control (UAS-myrGFP/+;dsxbrain/+) and dsxF knockdown (UAS-myrGFP/+;dsxbrain/UAS-dsxFRNAi) female brains. Yellow arrowheads indicate anti-DsxF signals in pC1 and pC2 neurons in control females (D and F), and white arrowheads indicate the diminished anti-DsxF signals in the same brain region in dsxF knockdown females (E and G). White circles indicate GFP+ pC1, pC2, and pCd cell bodies. (Scale bars, 50 μm.) Representative of five samples each. (H) Knocking down dsxF in dsxbrain neurons significantly increases numbers of pC1 and pC2, but not pCd neurons in females. n = 7 and 5 for control and dsxF knockdown females, respectively. **P < 0.01; ns, not significant, Mann–Whitney U test. (I) Female receptivity for control UAS-dsxFRNAi/+ (n = 96) and dsxbrain/UAS-dsxFRNAi (n = 80) females. ***P < 0.001, χ2 test. (J–L) Activation of dsxbrain neurons promotes receptivity in control females but does not restore receptivity in dsxF knockdown females. For dsxbrain/+ females, n = 144 (22 °C) and 137 (29 °C); for dTrpA1/+; dsxbrain/+ females, n = 117 (22 °C), and 144 (29 °C); for UAS-dTrpA1/+;dsxbrain/UAS-dsxFRNAi females, n = 80 (22 °C) and 96 (29 °C). **P < 0.01, ***P < 0.001; ns, not significant, χ2 test. (M) An example image of headbutt aggression between two females. (N and O) Number of headbutts per minute (N) and fighting latency (O) for control and dsxF knockdown females. n = 16 pairs for each. ***P < 0.001, Mann–Whitney U test. (P) A summary of how brain-specific dsxF knockdown affects development and consequently sexual and aggressive behaviors in females. Error bars indicate SEM.
We then narrowed down the subsets of neurons in which DsxF is required for female receptivity by targeting dsx neurons specifically in the female brain (dsxbrain) using the same genotype described above (Fig. 3 B and C). The efficiency of RNAi in control (UAS-myrGFP/+;dsxbrain/+) and experimental (UAS-myrGFP/+;dsxbrain/UAS-dsxFRNAi) females in the L3, 48 h APF, and adult stages was validated using anti-GFP and anti-DsxF antibodies (Fig. 3 D–G and SI Appendix, Fig. S6 A and B). We found that anti-DsxF signals largely overlapped with the anti-GFP signals in all the three developmental stages in control female brains (for pupal and adult expression, see Fig. 3 D and F; for larval expression, see SI Appendix, Fig. S6A); however, anti-DsxF signals were diminished in UAS-myrGFP/+;dsxbrain/UAS-dsxFRNAi female brains regardless of the developmental stage (for pupal and adult expression, see Fig. 3 E and G; for larval expression see SI Appendix, Fig. S6B). Interestingly, we observed an increased number of GFP+ neurons in the dsxF knockdown females, especially pC1 (9.6 neurons per hemisphere in control and 12.8 in dsxF knockdown females) and pC2 (13.4 neurons per hemisphere in control and 32.6 in dsxF knockdown females) neurons (Fig. 3H), consistent with the finding that DsxF generally promotes programmed cell death. Furthermore, females with DsxF knockdown in dsxbrain neurons were rarely receptive to courting males (∼14% mated in 30 min, ∼95% for controls in 30 min) (Fig. 3I). These results indicate that DsxF expression in dsxbrain neurons is crucial for female receptivity to courting males, probably through its role in the development of pC1 and pC2 neurons.
Since central brain dsx-expressing neurons promote female receptivity (38), we set out to test if artificial activation of dsxbrain neurons could restore female receptivity in DsxF knockdown females. Activation of dsxbrain neurons expressing the temperature-dependent cation channel dTrpA1 (53) at 29 °C significantly increased female receptivity (>80% mated in 6 min at 29 °C, and ∼40% mated in 6 min for control females) (Fig. 3 J and K); however, activation of dsxbrain neurons together with DsxF knockdown only partially increased female receptivity (∼20% at 29 °C and ∼2% at 22 °C in 30 min) (Fig. 3L) and was still far less than that in control females. These results suggest that the low level of female receptivity in DsxF knockdown females is not only an issue of neuronal activity but may also be due to organizational changes in the receptivity circuit (e.g., development of more pC1 and pC2 neurons, as shown in Fig. 3H).
We next tested aggressive behaviors, as dsx-expressing neurons are also involved in regulating female aggression (45). Surprisingly, we found that knocking down DsxF expression in dsxbrain neurons significantly enhanced the frequency of female aggression (Fig. 3 M and N) and also reduced fighting latency (Fig. 3O). These results indicate that DsxF in dsxbrain neurons promotes female receptivity but suppresses female aggression (Fig. 3P).
DsxF in Two Pairs of pC1d Neurons Inhibits Female Aggression.
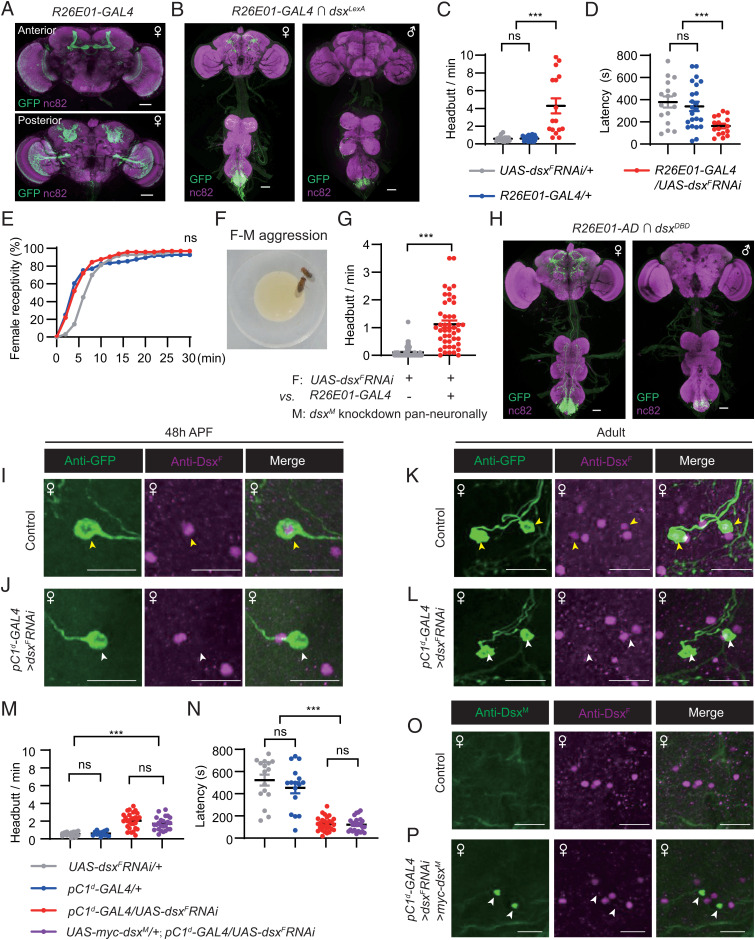
Two pairs of female-specific dsx-expressing pC1 neurons were previously shown to promote female aggression (43–45). To test if DsxF modulates female aggression in these pC1 neurons, we utilized the R26E01-GAL4 driver that labels the aggression-promoting pC1 neurons as well as many other neurons in females (45) (Fig. 4A). We then checked for overlapping expression with dsxLexA using an intersectional strategy (21). Two pairs of female-specific pC1 neurons, hereafter referred to as pC1d neurons according to previous studies (43, 44, 54), as well as some neurons in the abdominal ganglion were labeled (Fig. 4B). Females with DsxF knockdown in R26E01-GAL4 neurons showed an increase in aggressive behaviors, including high levels of headbutt frequency (Fig. 4C, SI Appendix, and Movie S2), and quicker initiation of the behavior (Fig. 4D).
Fig. 4.
DsxF in two pairs of pC1d neurons suppresses female aggression. (A) Expression pattern of R26E01-GAL4 in the female brain. (Scale bars, 50 μm.) Representative of three samples. (B) Intersectional expression between R26E01-GAL4 and dsxLexA (LexAop2-Flp/UAS > stop > myrGFP;dsxLexA/R26E01-GAL4) in the female and male CNS. (Scale bars, 50 μm.) Representative of five samples. (C and D) Knocking down DsxF expression in R26E01-GAL4 neurons enhances female headbutt frequency (C) and reduces fighting latency (D). n = 16, 22, 16 pairs from left to right. ***P < 0.001; ns, not significant, Mann–Whitney U test. (E) Knocking down DsxF expression in R26E01-GAL4 neurons does not affect female receptivity. n = 96 for each. ns, not significant. χ2 test. (F) An example image of a female fighting against a male. (G) Knocking down DsxF expression in R26E01-GAL4 neurons induces female fighting toward males with pan-neuronal knockdown of DsxM (R57C10-GAL4/UAS-dsxMRNAi). n = 48 and 45, respectively. ***P < 0.001, unpaired Student’s t test. (H) Expression pattern of pC1d-GAL4 (R26E01-AD;dsxDBD) in female and male CNS. (Scale bars, 50 μm.) Representative of five samples. (I–L) Anti-DsxF and anti-GFP signals in pupal (I and J) and adult (K and L) stages in control (UAS-myrGFP/+;pC1d-GAL4/+) and pC1d-dsxF knockdown (UAS-myrGFP/+;pC1d-GAL4/UAS-dsxFRNAi) female brains. Yellow arrowheads indicate costaining of anti-DsxF and anti-GFP signals in control females (I and K), and white arrowheads indicate diminished anti-DsxF signals specifically in the GFP+ neurons in pC1d-dsxF knockdown females (J and L). (Scale bars, 20 μm.) Representative of five samples each. (M and N) Headbutt frequency (M) and fighting latency (N) by control and pC1d-dsxF knockdown females, as well as females with dsxF knockdown and ectopic dsxM expression in pC1d neurons. Genotypes are indicated. n = 16, 16, 28, 24 from left to right. ***P < 0.001; ns, not significant, Mann–Whitney U test. (O and P) Costaining of anti-DsxM and anti-DsxF signals in control pC1d-GAL4/+ (O) and experimental UAS-myc-dsxM/+;pC1d-GAL4/UAS-dsxFRNAi (P) female adult brains. White arrowheads indicate ectopic expression of DsxM and knockdown of DsxF. (Scale bars, 20 μm.) Representative of five samples each. Error bars indicate SEM.
As these females displayed strong aggression toward each other, we wondered if they would also fight with males. We found that DsxF knockdown females did not fight with courting wild-type males, and were equally receptive to them compared to control females (Fig. 4E). When paired with the DsxM knockdown males that we found rarely courted (Fig. 1B), DsxF knockdown females fought with the males more frequently than the control females did (1.1 headbutts vs. 0.1 headbutt per min) (Fig. 4 F and G, SI Appendix, and Movie S3). These results demonstrate that DsxF expression in two pairs of female-specific pC1d neurons inhibits female aggression toward both females and males, but does not affect receptivity to effectively courting males. Alteration of receptivity and aggressive behaviors in females with DsxF knockdown in either dsxbrain or pC1d neurons is not due to potential locomotor defect as these females displayed similar walking speed compared with control females (SI Appendix, Fig. S4 C and D).
To further confirm these findings, we utilized the splitGAL4 system (55) and obtained R26E01-AD (56) with above-mentioned dsxDBD, and then successfully labeled the two pairs of female-specific pC1d neurons in the R26E01-AD;dsxDBD females (hereafter referred to as pC1d-GAL4) (Fig. 4H). We also validated the efficiency of dsxF RNAi in these pC1d neurons in the L3, 48 h APF, and adult stages as we did above (Fig. 4 I–L and SI Appendix, Fig. S6 C–H). We found that the pC1d-GAL4 showed no expression in the L3 stage in control UAS-myrGFP/+;pC1d-GAL4/+ females (SI Appendix, Fig. S6 C and D), but labeled a pair of pC1d neurons in the pupal stage (Fig. 4I and SI Appendix, Fig. S6E) and two pairs of pC1d neurons in the adult stage (Fig. 4K and SI Appendix, Fig. S6G) that overlapped with anti-DsxF (Fig. 4 I and K, yellow arrowheads). In the RNAi-mediated UAS-myrGFP/+;pC1d-GAL4/UAS-dsxFRNAi females, anti-DsxF signals were specifically diminished in the GFP+ pC1d neurons (Fig. 4 J and L, white arrowheads), indicating efficient and specific knockdown of dsxF in pC1d neurons. We then tested aggression in these RNAi-mediated knockdown females and found significantly enhanced headbutt frequency (Fig. 4M) and reduced latency to initiate fighting compared to two control lines (Fig. 4N).
We then asked if expression of the male isoform DsxM would rescue or enhance the aggression phenotype in dsxF knockdown females. We found that dsxF knockdown females with or without ectopic expression of dsxM in pC1d neurons showed a similarly high level of aggression (Fig. 4 M and N). Costaining of DsxM and DsxF in UAS-myc-dsxM/+;pC1d-GAL4/UAS-dsxFRNAi female brains confirmed the effectiveness of both dsxF knockdown and DsxM expression: two pairs of anti-DsxM neurons were observed within the pC1 cluster (ectopic expression of DsxM), in which there was no anti-DsxF signal (dsxF knockdown) (Fig. 4 O and P). These results indicate that DsxF is crucial in the female-specific pC1d neurons to inhibit aggression, whereas ectopic expression of DsxM in these neurons has no further effect, likely due to the already enhanced aggressiveness.
We further tested if acute knockdown of dsxF in pC1d neurons during adulthood using the above-mentioned GAL80ts strategy would enhance female aggression (SI Appendix, Fig. S7A). We found that knocking down dsxF expression in pC1d neurons for 4 d during adulthood significantly enhanced female headbutt frequency (SI Appendix, Fig. S7B) and reduced fighting latency (SI Appendix, Fig. S7C). These results indicate that DsxF plays an acute role in pC1d neurons to inhibit female aggression.
Dsx Isoforms Promote the Development of Aggression-Promoting Neurons in Both Sexes while Oppositely Regulate Their Functional Output.
The above results revealed an aggression-promoting role for DsxM in the male-specific P1a neurons, and an aggression-inhibiting role for DsxF in the female-specific pC1d neurons. Dsx is also crucial for neuronal development, as we found for females with dsxF knockdown in dsxbrain neurons. We therefore tested if knocking down dsx in P1a or pC1d neurons would affect the development of these neurons (Fig. 5 A–E and G–I). However, no changes were observed in either neuronal number or apparent morphology of P1a neurons in UAS-myrGFP/+;P1a-GAL4/UAS-dsxMRNAi males (7.8 neurons per hemisphere in control males and 8.6 in P1a DsxM knockdown males) (Fig. 5 A and D). Notably, we only observed approximately four neurons per hemisphere in UAS-flag-dsxF/+;P1a-GAL4/UAS-dsxMRNAi males with anti-DsxF expression (Figs. 2K and 5D). These results indicate that dsxM knockdown with overexpression of dsxF in P1a neurons, but not dsxM knockdown alone, impairs the development of these neurons.
To further examine if systemic dsx knockout would affect the development of P1a neurons, we used the P1a-splitGAL4 reagents (R15A01-AD and R71G01-DBD) and dsxDBD to generate two additional splitGAL4 lines, P1a1-splitGAL4 (R15A01-AD;dsxDBD) and P1a2-splitGAL4 (R71G01-AD;dsxDBD), which could be easily assayed in the dsx mutant background (dsxDBD/dsx1649-9625). We found that systemic dsx knockout (dsxDBD/dsx1649-9625) significantly reduced the number of GAL4-labeled P1 neurons (for P1a1-splitGAL4, 5.5 neurons per hemisphere in control males, and 3 neurons in dsx mutant males; for P1a2-splitGAL4, 11.8 neurons per hemisphere in control males, and 9 neurons in dsx mutant males) (Fig. 5 B–E). These results indicate that systemic dsxM knockout, but not restricted knockdown of expression, impairs the development of P1a neurons. Taken together with our behavioral experiments described above, these findings show that DsxM promotes both the development of the male-specific P1a neurons and their aggression-promoting function (Fig. 5F).
We next tested whether knocking down dsxF in pC1d neurons would affect the development of these neurons, for example by increasing the number of these pC1d neurons as we observed with the dsxbrain driver (Fig. 5 G–I). However, knocking down dsxF specifically in pC1d neurons driven by the pC1d-GAL4 did not change either the number or projection of these neurons (2.3 neurons per hemisphere in control females and 2.2 in pC1d-dsxF knockdown females) (Fig. 5 G and I). Simultaneous DsxF knockdown and DsxM expression in these pC1d neurons also did not affect the cell number (Figs. 4O and 5I). Surprisingly, we found that systemic dsx knockout (dsxDBD/dsx1649-9625), as we found with P1a neurons in males, severely impaired the development of pC1d neurons (0.5 pC1d neuron per hemisphere in the dsx mutant background, compared to ∼2 neurons in control females) (Fig. 5 H and I). Thus, systemic dsxF knockout, but not restricted knockdown of dsxF, affects the development of pC1d neurons. Combined with the above behavioral experiments, these results reveal that DsxF promotes the development of female-specific pC1d neurons while inhibiting their aggression-promoting function (Fig. 5J).
Taken together, our findings show that DsxM and DsxF promote the development of male-specific P1a neurons and female-specific pC1d neurons, respectively, but oppositely regulate the functional output of these aggression-promoting neurons (Fig. 5K).
Discussion
In this study, we investigated the function of sex-specific DsxM and DsxF proteins in the nervous system, and revealed crucial roles of DsxM in promoting male courtship and DsxF in promoting female receptivity. Most importantly, we found opposite regulatory roles for DsxM and DsxF in a subset of male- or female-specific aggression-promoting neurons, respectively. Whereas DsxM promotes male aggression, DsxF suppresses female aggression, indicating a potential mechanism underlying male-biased aggression across animal species.
For decades, the male-specific FruM was considered a master gene controlling most aspects of male courtship and male-specific aggressive pattern and dominance (14–16, 25, 26), whereas the roles of DsxM in males and DsxF in females were largely focused on sexual differentiation (12). Given that DsxM is expressed in ∼900 neurons that largely overlap with FruM in males, and DsxF is expressed in ∼700 neurons where there is no recognized or functional Fru protein, the potential involvement of DsxM and DsxF in these neurons in controlling male and female sexual and aggressive behaviors has been largely underestimated and rarely studied (47–49). Previous studies either used dsx mutant or dominant alleles that affect all dsx-expressing cells other than the nervous system (18, 32), manipulated tra and fruM to infer dsx function indirectly (18, 22, 33), or focused on dsx-expressing neurons but not the function of the gene (37–40, 42–45, 48, 57).
Our study of the role of neuronal Dsx expression in controlling dimorphic sexual and aggressive behaviors is a crucial step forward in reevaluating the genetic basis underlying these behaviors. Importantly, our study has revealed that Dsx function in the nervous system is crucial for both male and female sexual and aggressive behaviors and is responsible for at least some dimorphic features. Knocking down dsx in the nervous system or specifically in dsxbrain neurons reduced courtship intensity and mating success by ∼80% in males that still have normal FruM function. Furthermore, males lacking FruM but retaining DsxM function could acquire courtship through social experience (17, 18, 58). These results indicate that neuronal DsxM is crucial, together with FruM, in establishing male courtship behavior. Unlike males that have both FruM and DsxM function, females have functional DsxF but no FruF, suggesting an even more fundamental role of DsxF in regulating female behaviors. Indeed, knocking down dsxF pan-neuronally nearly abolished receptivity in virgin females (only 1 out 48 mated in 30 min), and knocking down dsxF specifically in dsxbrain neurons reduced female receptivity by nearly 90%. Thus, while FruM is necessary for innate expression of male courtship, neuronal DsxM is crucial for courtship robustness and the experience-dependent courtship acquisition in the absence of FruM, and neuronal DsxF is necessary for female receptivity.
To date, the study of aggressive behaviors has focused on FruM, which has been found to regulate sex-specific fighting patterns (e.g., male lunge vs. female headbutt) and social dominance through fighting (25, 26). However, this role is limited to male-specific aggressiveness. Moreover, although subsets of aggression-promoting neurons including P1a, TkFru, and aSP2 are fruM-positive (9, 27, 28), the function of FruM in P1a and aSP2 neurons regarding aggression has not yet been determined. Our results showing that DsxM in the male-specific P1a neurons promotes male aggression, while DsxF in the female-specific pC1d neurons suppresses female aggression, provide a mechanistic model involving both sexes that can explain male-biased aggressiveness.
How would neuronal Dsx proteins regulate sexual and aggressive behaviors in both sexes? There are at least two possibilities: 1) Dsx regulates neuronal development (e.g., the number of neurons through cell proliferation or cell death), as suggested previously (22, 48, 49, 52), and fine neuronal projections (59); or 2) Dsx regulates neuronal physiology, (e.g., sensitivity or excitability), as recently found for FruM function (60, 61). Our results suggest both possibilities.
First, females with dsxF knockdown in all dsxbrain neurons displayed reduced receptivity and increased aggression, probably due to DsxF function in neuronal development, as these females have an increased number of pC1 and pC2 neurons (Fig. 3H). The ectopically generated pC1 and pC2 neurons would affect the organization or capability of the dsx circuitry that is crucial for female behaviors (38–41). Indeed, while activation of dsxbrain neurons promotes receptivity in control females, activating these dsxbrain neurons (with more pC1 and pC2) fails to restore receptivity in dsxF knockdown females.
Second, knocking down dsxM specifically in P1a neurons in males, or dsxF specifically in pC1d neurons in females, did not affect either the cell number or gross morphology of these neurons, but whether there is any fine dendritic change is not yet conclusive. Interestingly, we found fewer P1a neurons in dsx mutant males, and pC1d neurons were almost absent in dsx mutant females, indicating crucial roles for DsxM and DsxF in the development of P1a neurons in males and pC1d neurons in females, respectively. The discrepancy between systemic dsx knockout and restricted dsx knockdown on the development of P1a/pC1d neurons might be due to: 1) the late onset of dsx knockdown, as no faithful P1a/pC1d expression was observed in the larval stage; 2) dose-dependent mechanisms (knockout vs. knockdown); or 3) noncell autonomous mechanisms (restricted vs. systemic). Nevertheless, the decreased/increased aggression in P1a-dsxM knockdown/overexpression males and increased aggression in pC1d-dsxF knockdown females are potentially due to nondevelopmental mechanisms. Indeed, acutely overexpressing DsxM in P1a neurons during adulthood also promoted male aggression. In addition, acutely knocking down DsxF in pC1d neurons during adulthood enhanced female aggression. Thus, we propose that DsxM and DsxF might function to regulate neuronal physiology through their function as transcription factors during adulthood, in addition to their function in neuronal development. Future studies should reveal both developmental and nondevelopmental aspects of Dsx function in distinct subsets of dsx-expressing neurons in males and females. Intriguingly, the dsx-related Dmrt genes control the differentiation of gonads in mammals, which produce sex-specific hormones and play both organizational (developmental) and activational (nondevelopmental) roles in sexually dimorphic behaviors (2, 30, 62, 63). Thus, our findings reveal how a conserved sex determination gene functions cell autonomously in flies, or cell nonautonomously via hormones in mammals, to fulfill both organizational and activational functions in sexually dimorphic behaviors.
The male brain has ∼300 dsxM neurons, whereas the female brain has only ∼60 dsxF neurons (47, 48), and DsxF has been found to generally reduce cell number through programmed cell death (22, 48, 52). Interestingly, we found that DsxF promotes the development of approximately two pairs of female-specific pC1d neurons, which seems contradictory to its function on other neurons. Thus, there could be a distinct mechanism by which DsxF regulates the development of pC1d neurons, which awaits future investigation. The fact that DsxF promotes the development of the aggression-promoting pC1d neurons while inhibiting their functional output is also intriguing. Such a mechanism does not simply shut down aggressive behaviors by inducing cell death of aggression-promoting neurons, but instead develops the neural substrates underlying the potential for female aggressive behaviors, while simultaneously modulating the intensity of aggression.
Materials and Methods
Fly Stocks.
Flies were maintained at 22 °C or 25 °C in a 12-h light/12-h dark cycle at ∼60% humidity. Canton-S (wtcs) were used as wild-type strains. dsx mutant lines used in Fig. 1A include dsx683-7058, dsx1649-9625, and dsxM+R15, which were used as previously described (18). dsxGAL4 (48), dsxLexA (64), Otd-Flp (17), P1a-GAL4 (w-; R15A01-AD attP40; R71G01-DBD attP2) (50), and UAS-dTrpA1 (53) were used as previously described. actin-GAL4 (BDSC_25374), R57C10-GAL4 (attP2, BDSC_39171), R26E01-GAL4 (attP2, BDSC_60510), R26E01-AD (attP40, BDSC_75740), R15A01-AD (attP40, BDSC_68837), R71G01-AD (attP40, BDSC_70798), R71G01-DBD (attP2, BDSC_69507), tub > GAL80> (BDSC_38881), tub-GAL80ts (BDSC_7018), UAS-myrGFP attP40 (BDSC_32198), and UAS-myrGFP su(Hw)attP5 (BDSC_32199) were obtained from Bloomington Drosophila Stock Center. UAS-dsxMRNAi (attP2) (34), UAS-dsxFRNAi (attP2) (34), UAS-myc-dsxM (attP40) (46), and UAS-flag-dsxF (attP40) (46) lines were used as previously described and as also described below. dsxDBD was generated in this study and described below in detail. Detailed information about fly stocks and other materials used in this study is listed as SI Appendix, Table S1.
Transgenic Flies.
dsxDBD was generated by replacing the first coding exon of dsx with the GAL4-DBD fragment via CRISPR/Cas9-mediated homology-directed repair. UAS-dsxMRNAi and UAS-dsxFRNAi lines were recently generated by the Tsinghua Fly Center at Tsinghua University based on pVALIUM20 plasmids (65) and were used previously (34). UAS-flag-dsxF and UAS-myc-dsxM lines were generated previously in our laboratory (46).
DsxM and DsxF Antibodies.
DsxM and DsxF antibodies were generated in our laboratory with help from GenScript and were used previously (34). In brief, the rabbit polyclonal anti-DsxM antibody was generated using the male-specific Dsx fragment N′-ARVEINRTVA QIYYNYYTPM ALVNGAPMYL TYPSIEQGRY GAHFTHLPLT QICPPTPEPL ALSRSPSSPS GPSAVHNQKP SRPGSSNGTV HSAASPTMVT TMATTSSTPT LSRRQRSRSA TPTTPPPPPP AHSSSNGAYH HGHHLVSSTA AT-C′. The mouse monoclonal anti-DsxF antibody was generated using the peptide N′-PLGQDVFLDYCQKLLEKFRYPWELMPLMYVILKDADANIEEASRRIEEGQYVVNEYSRQHNLNIYDGGELRNTTRQCG-C′ (the underline marks the female-specific sequence).
Male Courtship Assay.
Newly enclosed virgin males were collected and single housed for 4 to 7 d until testing. Virgin wtcs females were collected and group-housed for 4 to 7 d as courtship targets. To measure courtship, small round two-layer chambers (diameter: 1 cm; height: 3 mm per layer) were used. Tester males and target females were loaded individually into the two-layer food contained chambers and were separated by a plastic transparent film until the courtship test for 30 min. CI, which is the percentage of observation time that a fly performs any courtship step in the first 10 min of the video, was used to measure courtship, and scored manually using the LifeSongX software (lifesong.bio.brandeis.edu/).
Female Receptivity Assay.
Virgin females were collected and group-housed for 4 to 7 d until the test. Newly enclosed wtcs virgin males were collected and group-housed for 4 to 7 d as courters unless otherwise described. Males and females were loaded and tested as described for the male courtship assay. Female receptivity was manually measured every 2 min as the cumulative percentage of females with successful copulation with males.
For dTrpA1-based neuronal activation experiments (Fig. 3 J–L), flies were raised at 22 °C until behavioral tests. Behavioral chambers containing flies were placed at control (22 °C) or experimental temperatures (29 °C) for 30 min before behavioral tests.
Male and Female Aggression Assays.
All aggression assays were carried out at 25 °C and ∼60% humidity between 11:00 AM and 5:00 PM. The aggression chamber is made up of four acrylic plates. The bottom plate has 24 wells, containing food substrates (regular food plus apple juice and yeast, diameter: 8 mm; depth: 3 mm). The second and third plates have 24 cylindrical arenas (diameter: 15 mm; height of each plate: 3 mm), in which two tester flies were gently aspirated and separated by a transparent film. Flies were allowed to recover for ∼60 min and video was recorded for ∼30 min after removing the transparent film. For M–M and F–F aggression, two males or females of the same-genotype were used. All aggression tests were performed at least twice for each genotype and were manually analyzed. For detailed information about the aggression assay, see SI Appendix, Materials and Methods.
Spontaneous Locomotion Assay.
Four- to 7-d-old males and females were raised using the same condition for aggression tests. Individual flies were loaded into round chambers (diameter: 2 cm; height: 3 mm) with regular food at the bottom and recorded for 24 h starting from 9:00 AM under constant light condition. The average walking velocity during the 24-h recording was quantified using the ZebraLab software system (ViewPoint Life Sciences), as previously described (50).
Quantitative Real-Time PCR.
qPCR was performed using a LightCycler 96 Real-Time PCR System (Roche). Total RNA was extracted from ∼30 adult flies using a commercial TRIZOL reagent (15596026, Invitrogen) and purified with DNA-free kit (AM1906, Ambion) according to the manufacturer’s protocol. First-strand cDNA was synthesized for each RNA sample using Prime Script reagent kit (18091050, Invitrogen). EvaGreen Dye (31000, Biotium) and High Fidelity PCR SuperMix (AS131-21, Transgen) were used to conduct qPCR. actin was used as control for normalization. The primers used were as follows:
actin-forward: 5′- CAGGCGGTGCTTTCTCTCTA -3′,
actin-reverse: 5′-AGCTGTAACCGCGCTCAGTA-3′;
dsxF-forward: 5′- TTCCGCTATCCTTGGGAGCT -3′;
dsxF-reverse: 5′- CATCCACATTGCCGCGTTGT -3′;
dsxM-forward: 5′-GAAGAGGCTTCCCGGCGAAT-3′;
dsxM-reverse: 5′- GGACAAATCTGTGTGAGCGG -3′.
Tissue Dissection, Staining, and Imaging.
Flies were dissected at three developmental stages (L3, 48 h APF, and 4- to 7-d-old adult). Larval gender was determined by taking advantage of the translucence of the cuticle and looking for the absence or presence of gonads. All flies were dissected in Schneider’s insect medium (Thermo Fisher Scientific) and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 to 30 min at room temperature. After 4 × 15-min washing in PAT3 (0.5% Triton X-100, 0.5% bovine serum albumin in PBS), tissues were blocked in 3% normal goat serum (NGS) for 60 min at room temperature, then incubated in primary antibodies diluted in 3% NGS overnight at 4 °C, then washed four times in PAT3, and incubated in secondary antibodies diluted in 3% NGS for 1 to 2 d at 4 °C. Tissues were then washed four times thoroughly in PAT3 and mounted for imaging. Primary antibodies used: rabbit anti-GFP (Invitrogen A11122; 1:1,000), mouse anti-GFP (Invitrogen A11120; 1:1,000), mouse anti-Bruchpilot (Developmental Studies Hybridoma Bank nc82; 1:50), mouse anti-Myc (MBL, M047-3; 1:600), mouse anti-DsxF and rabbit anti-DsxM (see Materials and Methods for details of antibody generation; 1:400). Secondary antibodies used: donkey anti-rabbit IgG conjugated to Alexa 488 (Invitrogen, A21206; 1:500), donkey anti-mouse IgG conjugated to Alexa 488 (Invitrogen, A21202; 1:500), donkey anti-rabbit IgG conjugated to Alexa 555 (Invitrogen, A31572; 1:500), donkey anti-mouse IgG conjugated to Alexa 555 (Invitrogen, A31570; 1:500). Samples were imaged at 10× or 20× magnification on Zeiss 700 confocal microscope and processed with ImageJ software. For quantification of anti-Myc fluorescence intensity (SI Appendix, Fig. S5D), all control and experimental samples were imaged using the same parameter and analyzed using ImageJ.
For visualizing morphological appearances of flies (SI Appendix, Figs. S1 B–E and S2 H and I), 2- to 4-d-old adult males and females were frozen at −80 °C for 30 min and later imaged by a Nikon Shuttle pix P400RV stereoscopic microscope.
Statistics.
For all experiments, control and experimental flies were tested together under the same conditions, and data were collected from at least two independent experiments. For CNS imaging data, we dissected 5∼10 flies for each condition. For assaying courtship and aggression, which have a performance index for individual flies, we tested ∼20 flies. For assaying female receptivity, which used a single index for all samples (percentage mated successfully), we tested up to 100 pairs of flies. Statistical analyses were performed using GraphPad Prism 8 and are indicated in each figure legend. Data presented in this study were first verified for normal distribution by D’Agostino–Pearson normality test. If normally distributed, Student’s t test was used for pairwise comparisons, and one-way ANOVA was used for multiple group comparisons, followed by Tukey’s multiple comparisons. If not normally distributed, Mann–Whitney U test was used for pairwise comparisons, and Kruskal–Wallis test was used for comparisons among multiple groups, followed by Dunn’s multiple comparisons. For male mating success and female receptivity, χ2 tests were performed to compare two different groups at the 30-min time point, if not otherwise mentioned.
Supplementary Material
Acknowledgments
We thank the Bloomington Drosophila Stock Center and Tsinghua Fly Center for fly stocks. This work was supported by grants from the National Key R&D Program of China (2019YFA0802400), the National Natural Science Foundation of China (31970943 and 31700905), and the Jiangsu Innovation and Entrepreneurship Team Program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. B.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201513119/-/DCSupplemental.
Data, Materials, and Software Availability
All data generated or analyzed during this study are included in the main text and supporting information. This study does not involve new code or sequence data. Fly stocks and reagents used in this study are available from the corresponding author upon reasonable request.
References
- 1.Jazin E., Cahill L., Sex differences in molecular neuroscience: From fruit flies to humans. Nat. Rev. Neurosci. 11, 9–17 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Yang C. F., Shah N. M., Representing sex in the brain, one module at a time. Neuron 82, 261–278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo C., Pan Y., Gong Z., Recent advances in the genetic dissection of neural circuits in Drosophila. Neurosci. Bull. 35, 1058–1072 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cachero S., Ostrovsky A. D., Yu J. Y., Dickson B. J., Jefferis G. S., Sexual dimorphism in the fly brain. Curr. Biol. 20, 1589–1601 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahina K., Sex differences in Drosophila behavior: Qualitative and quantitative dimorphism. Curr. Opin. Physiol. 6, 35–45 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson B. J., Wired for sex: The neurobiology of Drosophila mating decisions. Science 322, 904–909 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Nilsen S. P., Chan Y. B., Huber R., Kravitz E. A., Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 101, 12342–12347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S., Lee A. Y., Bowens N. M., Huber R., Kravitz E. A., Fighting fruit flies: A model system for the study of aggression. Proc. Natl. Acad. Sci. U.S.A. 99, 5664–5668 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahina K., et al. , Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221–235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryner L. C., et al. , Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Ito H., et al. , Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. U.S.A. 93, 9687–9692 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burtis K. C., Baker B. S., Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56, 997–1010 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Siwicki K. K., Kravitz E. A., Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr. Opin. Neurobiol. 19, 200–206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockinger P., Kvitsiani D., Rotkopf S., Tirián L., Dickson B. J., Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Manoli D. S., et al. , Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Demir E., Dickson B. J., fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Chen J., et al. , fruitless tunes functional flexibility of courtship circuitry during development. eLife 10, e59224 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y., Baker B. S., Genetic identification and separation of innate and experience-dependent courtship behaviors in Drosophila. Cell 156, 236–248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallman B. R., Kim H., Scott K., Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 4, e11188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clowney E. J., Iguchi S., Bussell J. J., Scheer E., Ruta V., Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y., Meissner G. W., Baker B. S., Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc. Natl. Acad. Sci. U.S.A. 109, 10065–10070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura K., Hachiya T., Koganezawa M., Tazawa T., Yamamoto D., Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759–769 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Wu S., et al. , Drosulfakinin signaling in fruitless circuitry antagonizes P1 neurons to regulate sexual arousal in Drosophila. Nat. Commun. 10, 4770 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohl J., Ostrovsky A. D., Frechter S., Jefferis G. S., A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 155, 1610–1623 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrontou E., Nilsen S. P., Demir E., Kravitz E. A., Dickson B. J., fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 9, 1469–1471 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Chan Y. B., Kravitz E. A., Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 104, 19577–19582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohl M., Ishii K., Asahina K., Layered roles of fruitless isoforms in specification and function of male aggression-promoting neurons in Drosophila. eLife 9, e52702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K., et al. , A circuit node that integrates convergent input from neuromodulatory and social behavior-promoting neurons to control aggression in Drosophila. Neuron 95, 1112–1128.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoopfer E. D., Jung Y., Inagaki H. K., Rubin G. M., Anderson D. J., P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, e11346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp A., Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 28, 175–184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen D., et al. , Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat. Commun. 8, 154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villella A., Hall J. C., Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics 143, 331–344 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii K., Wohl M., DeSouza A., Asahina K., Sex-determining genes distinctly regulate courtship capability and target preference via sexually dimorphic neurons. eLife 9, e52701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng Q., et al. , The sex determination gene doublesex is required during adulthood to maintain sexual orientation. J. Genet. Genomics 49, 165–168 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Pan Y., Robinett C. C., Baker B. S., Turning males on: Activation of male courtship behavior in Drosophila melanogaster. PLoS One 6, e21144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavlou H. J., et al. , Neural circuitry coordinating male copulation. eLife 5, e20713 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koganezawa M., Kimura K., Yamamoto D., The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Curr. Biol. 26, 1395–1403 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Zhou C., Pan Y., Robinett C. C., Meissner G. W., Baker B. S., Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Wang K., et al. , Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature 589, 577–581 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Wang F., et al. , Neural circuitry linking mating and egg laying in Drosophila females. Nature 579, 101–105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F., Wang K., Forknall N., Parekh R., Dickson B. J., Circuit and behavioral mechanisms of sexual rejection by Drosophila females. Curr. Biol. 30, 3749–3760.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Rezával C., Nojima T., Neville M. C., Lin A. C., Goodwin S. F., Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 24, 725–730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schretter C. E., et al. , Cell types and neuronal circuitry underlying female aggression in Drosophila. eLife 9, e58942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deutsch D., et al. , The neural basis for a persistent internal state in Drosophila females. eLife 9, e59502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palavicino-Maggio C. B., Chan Y. B., McKellar C., Kravitz E. A., A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc. Natl. Acad. Sci. U.S.A. 116, 17029–17038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng Q., et al. , The sex determination gene doublesex regulates expression and secretion of the basement membrane protein Collagen IV. J. Genet. Genomics 49, 636–644 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Lee G., Hall J. C., Park J. H., Doublesex gene expression in the central nervous system of Drosophila melanogaster. J. Neurogenet. 16, 229–248 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Rideout E. J., Dornan A. J., Neville M. C., Eadie S., Goodwin S. F., Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinett C. C., Vaughan A. G., Knapp J. M., Baker B. S., Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8, e1000365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Guo C., Chen D., Peng Q., Pan Y., Hierarchical control of Drosophila sleep, courtship, and feeding behaviors by male-specific P1 neurons. Neurosci. Bull. 34, 1105–1110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohatsu S., Koganezawa M., Yamamoto D., Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Sanders L. E., Arbeitman M. N., Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev. Biol. 320, 378–390 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamada F. N., et al. , An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu H., et al. , A circuit logic for sexually shared and dimorphic aggressive behaviors in Drosophila. Cell 184, 847 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luan H., Peabody N. C., Vinson C. R., White B. H., Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dionne H., Hibbard K. L., Cavallaro A., Kao J. C., Rubin G. M., Genetic reagents for making split-GAL4 lines in Drosophila. Genetics 209, 31–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rezával C., et al. , Activation of latent courtship circuitry in the brain of Drosophila females induces male-like behaviors. Curr. Biol. 26, 2508–2515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng Q., Chen J., Pan Y., From fruitless to sex: On the generation and diversification of an innate behavior. Genes Brain Behav. 20, e12772 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Shirangi T. R., Wong A. M., Truman J. W., Stern D. L., Doublesex regulates the connectivity of a neural circuit controlling Drosophila male courtship song. Dev. Cell 37, 533–544 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Zhao S., et al. , Chromatin-based reprogramming of a courtship regulator by concurrent pheromone perception and hormone signaling. Sci. Adv. 6, eaba6913 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sethi S., et al. , Social context enhances hormonal modulation of pheromone detection in Drosophila. Curr. Biol. 29, 3887–3898.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz K. M., Molenda-Figueira H. A., Sisk C. L., Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 55, 597–604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang S. X., Glantz E. H., Miner L. E., Rogulja D., Crickmore M. A., Hormonal control of motivational circuitry orchestrates the transition to sexuality in Drosophila. Sci. Adv. 7, eabg6926 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou C., et al. , Central neural circuitry mediating courtship song perception in male Drosophila. eLife 4, e08477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni J. Q., et al. , A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the main text and supporting information. This study does not involve new code or sequence data. Fly stocks and reagents used in this study are available from the corresponding author upon reasonable request.