Abstract
Background
Cytomegalovirus (CMV)–seropositive (R+) hematopoietic cell transplant (HCT) recipients have a survival disparity compared with CMV-seronegative recipient/donor (R–D–) pairs. We hypothesized that primary letermovir prophylaxis (LET) may abrogate this disparity. We investigated the relationship between LET and mortality at 1 year post-HCT.
Methods
In this retrospective cohort study, we included adult R–D– or R+ patients who received HCT pre-LET (between 1 January 2013 through 15 December 2017) and post-LET (between 16 December 2017 through December 2019). R+ were categorized by LET receipt as R+/LET or R+/no-LET. Cox proportional hazard models were used to estimate the association of LET with all-cause mortality at 1 year after transplantation.
Results
Of 848 patients analyzed, 305 were R–D–, 364 R+/no-LET, and 160 R+/LET. Because of similar mortality (adjusted hazard ratio [aHR], 1.29 [95% confidence interval {CI}, .76–2.18]; P = .353]) between pre-LET/R–D– and post-LET/R–D–, R–D– were combined into 1 group. Compared with R–D–, the aHR for mortality was 1.40 (95% CI, 1.01–1.93) for R+/no-LET and 0.89 (95% CI, .57–1.41) for R+/LET. Among R+, LET was associated with decreased risk of death (aHR, 0.62 [95% CI, .40–.98]); when conventional HCT and T-cell depleted HCT were analyzed separately, the aHR was 0.86 (95% CI, .51–1.43) and 0.21 (95% CI, .07–.65), respectively.
Conclusions
At 1 year post-HCT, LET was associated with closing the mortality disparity between R–D– and R+. Among all R+, LET was associated with decreased mortality, driven by 79% reduced incidence of death in T-cell depleted HCT.
Keywords: cytomegalovirus, CMV, prevention, letermovir, mortality, allogeneic hematopoietic cell transplant, HCT
At 1 year after hematopoietic cell transplant (HCT), letermovir prophylaxis (LET) was associated with closing the mortality disparity between cytomegalovirus R–D–and R+. Among all R+, LET was associated with decreased mortality; driven by 79% reduced incidence of death in T-cell–depleted HCT.
Cytomegalovirus (CMV) seropositivity of donor (D+) or recipient (R+) are associated with worse survival after hemopoietic cell transplantation (HCT) [1, 2]. CMV viremia is associated with an increased risk of overall mortality [3, 4], and CMV viral load kinetics may predict CMV disease and death [5, 6]. In a randomized study, letermovir primary CMV prophylaxis (LET) was associated with improved survival at week 24 post-HCT [7]. In a post hoc analysis, mortality at 48 weeks post-HCT was higher among placebo recipients compared with LET recipients, suggesting that reduction in mortality would be associated with reduction in clinically significant CMV infection (cs-CMVi); however, mortality was similar among LET recipients with and without cs-CMVi [8].
Ganciclovir prophylaxis is associated with decreased CMV-specific cellular responses [9]. Because letermovir works at late time-points in the viral life cycle, expression of “early” CMV antigens may enable generation of T-cell responses without cs-CMVi [10]. This hypothesis is also supported by less refractory cs-CMVi and CMV disease with LET compared with historical controls managed preemptively [11].
We hypothesized that LET may abrogate the survival disparity between CMV R+ and R–D– HCT recipients. We examined the relationship between LET and mortality in the first year after transplantation.
METHODS
Study Population
The cohort comprised of adult recipients of first peripheral blood or bone marrow HCT at Memorial Sloan Kettering Cancer Center (MSKCC) between 1 January 2013 and 31 December 2019. Patients with multiple myeloma, CMV R–D+, or unknown CMV serostatus were excluded from the analyses (Supplementary Figure 1).
Two timeframes were defined based on the date of LET implementation: pre-LET (between 1 January 2013 and 15 December 2017) and post-LET (from 16 December 2017 onward). Patients were followed until death or 1 year post-HCT, whichever occurred first.
Data were extracted from the electronic medical records and hospital databases. The study was approved by the MSKCC Institutional Review Board and Privacy Board.
Standards of Care
Representative conditioning regimens have been described [12, 13]. Per our institutional algorithm, patients with acute leukemia in first complete remission, or with myelodysplastic syndrome, received T-cell depleted (TCD) HCT unless excluded by comorbidities or declined by insurance. T-cell depletion/CD34+ selection was performed by the CliniMACS CD34+ reagent system (Miltenyi Biotec, Gladbach, Germany). Patients with lymphoma, or leukemia beyond first complete remission or not eligible for TCD, received conventional (CONV) HCT.
For graft-vs-host disease (GVHD) prophylaxis, TCD HCT did not receive pharmacologic immunosuppression. CONV HCT received tacrolimus and/or sirolimus and mycophenolate mofetil with or without methotrexate [14]; posttransplantation cyclophosphamide was mostly used for haploidentical HCT [12].
All patients received acyclovir 400 mg twice daily from admission for HCT through at least 1 year post-HCT and bacterial and fungal prophylaxis as described previously [15]. GVHD grading was based on consensus guidelines [16].
CMV Management
All patients received leukocyte-filtered blood products. R+ were monitored at least weekly through day (D) 100 post-HCT and at least once every 2 weeks through D180 by a quantitative CMV polymerase chain reaction assay in plasma (Cobas AmpliPrep/Cobas TaqMan, Roche Molecular Diagnostics, New Jersey). The lower limit of quantification (LLQ) was 136 IU/mL and the linear range was 137 to 9.1 × 106 IU/mL.
LET was administered at 480 mg daily. TCD and haploidentical or mismatched donor allograft recipients started LET on D7 through at least D180 or immune reconstitution. Matched related HCT without GVHD started by D28 and continued through D100 or off systemic GVHD treatment, whichever was longer.
Cs-CMVi was defined as any viral load (VL) treated with preemptive therapy (PET) by D180. Prior to LET (15 December 2017), PET was initiated for VL >LLQ for TCD and mismatched or haploidentical HCT or on methylprednisolone >0.5 mg/kg or equivalent. For all other patients, PET started for >2 consecutive VL >300 IU/mL [17]. For patients on LET, PET was initiated for >2 consecutive values of CMV VL >300 IU/mL. Patients with cs-CMVi (before or after LET) could receive secondary prophylaxis if after treatment with valganciclovir/ganciclovir (vGCV) or foscarnet (FCN) they remained at risk for CMV. Patients initiating methylprednisolone >0.5 mg/kg or equivalent after discontinuation of LET could restart LET for the duration of immunosuppression.
Study Design
The first objective was to assess if LET was associated with abrogation of survival disparity between R+ and R–D–. First, we compared the mortality between pre-LET and post-LET timeframes. Because the adjusted all-cause and nonrelapse mortality for R–D– were similar in pre-LET and post-LET timeframes, R–D– from both timeframes were combined as a single group (reference group). R+ were categorized by receipt of LET as no-LET and LET. The groups are summarized in Supplementary Figure 1.
The second objective was to evaluate LET as a risk factor for all-cause and nonrelapse mortality among all R+ patients and separately by HCT platform (CONV or TCD) in multivariable models.
Statistical Analysis
Descriptive statistics were used to tabulate demographics and transplant characteristics. Numeric data were expressed as median (interquartile range [IQR]) and compared using Mann-Whitney rank-sum tests or Kruskal-Wallis tests as appropriate. Categorical data were expressed as numbers and percentages and compared using χ2 tests or Fisher exact tests as appropriate. Pairwise comparisons were adjusted using Benjamini-Hochberg procedure. The cumulative incidence function was used to estimate the incidence of cs-CMVi. Death was treated as competing risk for cs-CMVi.
Antiviral days and live days by D180 were calculated for each patient as the number of days on a given antiviral(s) and alive, respectively. Antiviral days per 1000 person-days were calculated as (total antiviral days / total live days) × 1000 for each study group.
Univariable and multivariable Cox proportional hazard models and Fine-Gray subdistribution hazard models were performed to assess risk factors for all-cause and nonrelapse mortality, respectively. Second transplant, relapse, and progression of disease were treated as competing risk events for nonrelapse mortality. The time-independent variables included in the models were age, sex, race, and underlying disease, human leukocyte antigen match, stem cell source, conditioning regimen, HCT-comorbidity index, and GVHD prophylaxis. Acute GVHD grade by D100 was included as a time-dependent variable. The Pearson correlation coefficients were calculated, and coefficients >0.7 indicated the presence of multicollinearity. The redundant variables in pairs with correlation >0.7 were dropped based on clinicians’ decision. Any aforementioned variable with no correlation issue was included in the multivariable models. Variation inflation factor was further used to identify the correlations among variables in the multivariable models. Possible interactions between LET and other covariates were investigated by adding respective interaction terms. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated and visualized in forest plots. Adjusted cumulative incidence curves of 1-year all-cause and nonrelapse mortality calculated from corresponding multivariable models were shown across groups of interest. For analyses by HCT platform, GVHD prophylaxis was not included as posttransplantation cyclophosphamide was strongly related to matched/haploidentical donor among CONV, and TCD HCT did not receive pharmacologic immunosuppression. For TCD, HCT-comorbidity index and stem cell source were not included as these were prerequisites for TCD HCT. Race was not included due to uneven distribution of death at 1 year. All tests were 2-sided with a significance level of .05. All statistical analyses were performed using R software, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Of 848 patients analyzed, 564 (66.5%) were pre-LET and 284 (33.5%) post-LET. Table 1 shows the baseline characteristics of the cohort by study timeframe. Of the 305 R–D– patients analyzed, 200 (65.6%) were pre-LET and 105 (34.4%) post-LET. Pairwise comparisons of R–D– patients pre- and post-LET are provided in Supplementary Table 1.
Table 1.
Baseline Characteristics by Study Timeframe and Cytomegalovirus Serostatus
| Characteristics | Pre-LET Timeframe | Post-LET Timeframe | ||
|---|---|---|---|---|
| R–D– | R+ | R–D– | R+ | |
| (n = 200) | (n = 364) | (n = 105) | (n = 179) | |
| Demographic characteristics | ||||
| Age, y | ||||
| 18–39 | 36 (18.0) | 58 (15.9) | 9 (8.6) | 25 (14.0) |
| 40–64 | 102 (51.0) | 189 (51.9) | 54 (51.4) | 101 (56.4) |
| ≥65 | 62 (31.0) | 117 (32.1) | 42 (40.0) | 53 (29.6) |
| Sex | ||||
| Female | 63 (31.5) | 158 (43.4) | 36 (34.3) | 82 (45.8) |
| Male | 137 (68.5) | 206 (56.6) | 69 (65.7) | 97 (54.2) |
| Race | ||||
| White | 185 (92.5) | 281 (77.2) | 97 (92.4) | 127 (70.9) |
| Asian | 3 (1.5) | 27 (7.4) | 2 (1.9) | 21 (11.7) |
| African American | 5 (2.5) | 29 (8.0) | 3 (2.9) | 18 (10.1) |
| Other | 7 (3.5) | 27 (7.4) | 3 (2.9) | 13 (7.3) |
| Transplant characteristics | ||||
| Underlying disease | ||||
| AML/ALL/CML/MDS | 123 (61.5) | 274 (75.3) | 77 (73.3) | 129 (72.1) |
| Lymphoma/CLLa | 61 (30.5) | 66 (18.1) | 18 (17.1) | 36 (20.1) |
| MPD/Nonmalignantb | 16 (8.0) | 24 (6.6) | 10 (9.5) | 14 (7.8) |
| Donor type | ||||
| Matched related | 69 (34.5) | 111 (30.5) | 22 (21.0) | 61 (34.1) |
| Matched unrelated | 100 (50.0) | 199 (54.7) | 55 (52.4) | 81 (45.3) |
| Mismatched related/unrelated | 15 (7.5) | 32 (8.8) | 14 (13.3) | 17 (9.5) |
| Haploidentical | 16 (8.0) | 22 (6.0) | 14 (13.3) | 20 (11.2) |
| Stem cell source | ||||
| Bone marrow | 23 (11.5) | 51 (14.0) | 12 (11.4) | 31 (17.3) |
| Peripheral blood | 177 (88.5) | 313 (86.0) | 93 (88.6) | 148 (82.7) |
| Conditioning regimen | ||||
| Myeloablative | 119 (59.5) | 209 (57.4) | 56 (53.3) | 96 (53.6) |
| Reduced | 62 (31.0) | 122 (33.5) | 26 (24.8) | 61 (34.1) |
| Non-myeloablative | 19 (9.5) | 33 (9.1) | 23 (21.9) | 22 (12.3) |
| ATG | ||||
| No | 103 (51.5) | 189 (51.9) | 63 (60.0) | 110 (61.5) |
| Yes | 97 (48.5) | 175 (48.1) | 42 (40.0) | 69 (38.5) |
| HCT-comorbidity index | ||||
| 0 | 31 (15.5) | 56 (15.4) | 25 (23.8) | 36 (20.1) |
| 1–2 | 71 (35.5) | 107 (29.4) | 34 (32.4) | 57 (31.8) |
| 3+ | 98 (49.0) | 201 (55.2) | 46 (43.8) | 86 (48.0) |
| GVHD prophylaxis | ||||
| TAC/MTX + othersc | 101 (50.5) | 188 (51.6) | 49 (46.7) | 77 (43.0) |
| PTCy | 22 (11.0) | 33 (9.1) | 23 (21.9) | 39 (21.8) |
| Ex vivo T-cell depletion | 77 (38.5) | 143 (39.3) | 33 (31.4) | 63 (35.2) |
| HCT platform | ||||
| CONV | 123 (61.5) | 221 (60.7) | 72 (68.6) | 116 (64.8) |
| TCD | 77 (38.5) | 143 (39.3) | 33 (31.4) | 63 (35.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; ATG, antithymocyte globulin; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CONV, unmodified (conventional); GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; LET, letermovir prophylaxis; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; MTX, methotrexate; PTCy, posttransplantation cyclophosphamide; R–D–, cytomegalovirus-seronegative recipient/donor; R+, cytomegalovirus-seropositive recipient; TAC, tacrolimus; TCD, ex vivo T-cell – depleted/CD34+ - selected.
Including 155 lymphoma and 26 CLL.
Including 47 MPD and 17 nonmalignant hematologic disorders.
Others included 2 patients with no GVHD prophylaxis (syngeneic donors).
LET Administration
Pre-LET, 0 (0%) R+ received LET. Post-LET, 160 (89.4%) of R+ received LET and 19 (10.6%) did not receive LET due to clinical or insurance reasons.
LET was initiated at a median of 7 (IQR, 7–16) days post-HCT. The median duration of LET was 144 (IQR, 96–174) days. The duration of LET was longer for TCD than CONV HCT (median, 155 [IQR, 115–174] days and 136 [IQR, 92–167] days, respectively; P = .012).
Clinically Significant CMV Infection
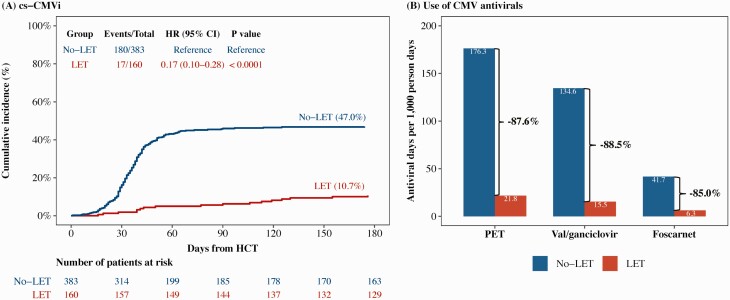
By D180, the cumulative incidence of cs-CMVi was 47.0% for no-LET and 10.7% for LET (P < .0001) (Figure 1A). Figure 1B shows the number of antiviral days per 1000 person-days for the no-LET and LET groups. Compared to no-LET, LET group had a reduction in total PET days, vGCV days, and FCN days by 87.6%, 88.5%, and 85.0%, respectively. The LET group received 721.4 LET days per 1000 person-days.
Figure 1.
Cumulative incidence of clinically significant cytomegalovirus infection (cs-CMVi) and use of cytomegalovirus (CMV) antivirals by receipt of letermovir prophylaxis (LET). A, Patients were censored at days 180 post–hematopoietic cell transplant or last follow-up, whichever occurred first. Death was treated as competing risk event. Univariable Fine-Gray subdistribution hazard model was used to compare the cumulative incidence of cs-CMVi between groups. B, Bars show estimated total days on antivirals per 1000 person-days across relevant groups. No-LET: CMV-seropositive recipients who did not receive LET. LET: CMV-seropositive recipients who received LET. Abbreviations: CI, confidence interval; CMV, cytomegalovirus; cs-CMVi, clinically significant cytomegalovirus infection; HCT, hematopoietic cell transplant; HR, hazard ratio; LET, letermovir prophylaxis; PET, preemptive therapy.
Association of LET With Mortality
Because patients who received no-LET or LET were in different timeframes, we examined mortality by study timeframes in multivariable models.
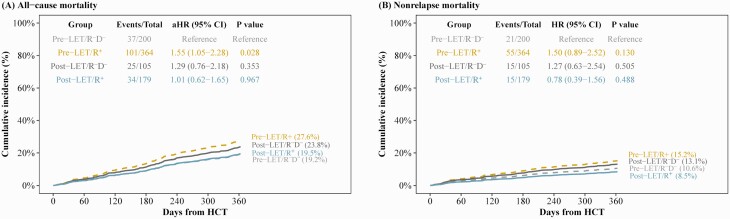
Figure 2 shows the adjusted cumulative incidence for all-cause and nonrelapse mortality by timeframe and CMV serostatus. Pre-LET/R–D– was set as the reference group. Pre-LET, R+ serostatus was associated with increased all-cause mortality (adjusted hazard ratio [aHR], 1.55 [95% CI, 1.05–2.28]; P = .028), whereas for post-LET/R–D– the aHR was 1.29 (95% CI, .76–2.18; P = .353) (Figure 2A).
Figure 2.
Adjusted cumulative incidence of all-cause and nonrelapse mortality by study timeframe and cytomegalovirus (CMV) serostatus. Adjusted cumulative incidences of all-cause mortality (A) and nonrelapse mortality (B) through 1 year post–hematopoietic cell transplant (HCT) by study timeframe and CMV serostatus. Patients were censored at 1 year post-HCT or last follow-up, whichever occurred first. For nonrelapse mortality, second transplant, relapse, and progression of disease were treated as competing risk events. The adjusted all-cause and nonrelapse mortality were calculated from multivariable Cox proportional hazard model and multivariable Fine-Gray subdistribution hazard model, respectively. Acute graft-vs-host disease grade was treated as a time-dependent variable in multivariable models. Pre-LET: prior to implementation of letermovir (from 1 January 2013 to 15 December 2017). Post-LET: after implementation of letermovir (from 16 December 2017 to 31 December 2019). Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCT, hematopoietic cell transplant; LET, letermovir prophylaxis; R–D–, cytomegalovirus-seronegative recipient/donor; R+, cytomegalovirus-seropositive recipient.
For nonrelapse mortality, the aHR was 1.50 (95% CI, .89–2.52; P = .130) for pre-LET/R+ and 1.27 (95% CI, .63–2.54; P = .505) for post-LET/R–D– (Figure 2B).
In summary, R+ was a predictor for all-cause mortality in the pre-LET timeframe. Importantly, the aHR for mortality for R–D– was similar in the 2 timeframes.
Next, we evaluated the association of LET with mortality in the multivariable models. R–D– from pre-LET and post-LET timeframes were combined into a single group (R–D–) and set as reference. R+ were categorized by receipt of LET into no-LET and LET groups. The baseline characteristics of the 3 groups are shown in Table 2. Pairwise comparisons are shown in Supplementary Table 2. The study groups and baseline characteristics were entered as time-independent variables. Acute GVHD was entered as a time-dependent variable. Antithymocyte globulin (ATG) was not included due to high correlation with GVHD prophylaxis (0.76).
Table 2.
Comparison of Baseline Characteristics Between Cytomegalovirus (CMV)–Seronegative Recipients/Donors (Reference) and CMV-Seropositive Recipients by Receipt of Letermovir Prophylaxis
| Characteristics | Overall | R–D– | R+ | P Valueb | |
|---|---|---|---|---|---|
| Referencea | No-LET | LET | |||
| (N = 848) | (n = 305) | (n = 383) | (n = 160) | ||
| Demographic characteristics | |||||
| Age, y | .885 | ||||
| 18–39 | 128 (15.1) | 45 (14.8) | 61 (15.9) | 22 (13.8) | |
| 40–64 | 446 (52.6) | 156 (51.1) | 202 (52.7) | 88 (55.0) | |
| ≥65 | 274 (32.3) | 104 (34.1) | 120 (31.3) | 50 (31.2) | |
| Sex | .002 | ||||
| Female | 339 (40.0) | 99 (32.5) | 163 (42.6) | 77 (48.1) | |
| Male | 509 (60.0) | 206 (67.5) | 220 (57.4) | 83 (51.9) | |
| Race | <.0001 | ||||
| White | 690 (81.4) | 282 (92.5) | 295 (77.0) | 113 (70.6) | |
| Asian | 53 (6.2) | 5 (1.6) | 29 (7.6) | 19 (11.9) | |
| African American | 55 (6.5) | 8 (2.6) | 29 (7.6) | 18 (11.2) | |
| Other | 50 (5.9) | 10 (3.3) | 30 (7.8) | 10 (6.2) | |
| Transplant characteristics | |||||
| Underlying disease | .116 | ||||
| AML/ALL/CML/MDS | 603 (71.1) | 200 (65.6) | 284 (74.2) | 119 (74.4) | |
| Lymphoma/CLLc | 181 (21.3) | 79 (25.9) | 73 (19.1) | 29 (18.1) | |
| MPD/Nonmalignantd | 64 (7.5) | 26 (8.5) | 26 (6.8) | 12 (7.5) | |
| Donor type | .226 | ||||
| Matched related | 263 (31.0) | 91 (29.8) | 119 (31.1) | 53 (33.1) | |
| Matched unrelated | 435 (51.3) | 155 (50.8) | 208 (54.3) | 72 (45.0) | |
| Mismatched related/unrelated | 78 (9.2) | 29 (9.5) | 33 (8.6) | 16 (10.0) | |
| Haploidentical | 72 (8.5) | 30 (9.8) | 23 (6.0) | 19 (11.9) | |
| Stem cell source | .300 | ||||
| Bone marrow | 117 (13.8) | 35 (11.5) | 56 (14.6) | 26 (16.2) | |
| Peripheral blood | 731 (86.2) | 270 (88.5) | 327 (85.4) | 134 (83.8) | |
| Conditioning regimen | .385 | ||||
| Myeloablative | 480 (56.6) | 175 (57.4) | 218 (56.9) | 87 (54.4) | |
| Reduced | 271 (32.0) | 88 (28.9) | 128 (33.4) | 55 (34.4) | |
| Non-myeloablative | 97 (11.4) | 42 (13.8) | 37 (9.7) | 18 (11.2) | |
| ATG | .528 | ||||
| No | 465 (54.8) | 166 (54.4) | 205 (53.5) | 94 (58.8) | |
| Yes | 383 (45.2) | 139 (45.6) | 178 (46.5) | 66 (41.2) | |
| HCT-comorbidity index | .150 | ||||
| 0 | 148 (17.5) | 56 (18.4) | 58 (15.1) | 34 (21.2) | |
| 1–2 | 269 (31.7) | 105 (34.4) | 113 (29.5) | 51 (31.9) | |
| ≥3 | 431 (50.8) | 144 (47.2) | 212 (55.4) | 75 (46.9) | |
| GVHD prophylaxis | .009 | ||||
| TAC/MTX + otherse | 117 (13.8) | 45 (14.8) | 38 (9.9) | 34 (21.2) | |
| PTCy | 415 (48.9) | 150 (49.2) | 199 (52.0) | 66 (41.2) | |
| Ex vivo T-cell depletion | 316 (37.3) | 110 (36.1) | 146 (38.1) | 60 (37.5) | |
| HCT platform | .856 | ||||
| CONV | 532 (62.7) | 195 (63.9) | 237 (61.9) | 100 (62.5) | |
| TCD | 316 (37.3) | 110 (36.1) | 146 (38.1) | 60 (37.5) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; ATG, antithymocyte globulin; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CONV, unmodified (conventional); GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; LET, letermovir prophylaxis; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; MTX, methotrexate; PTCy, posttransplantation cyclophosphamide; R–D–, cytomegalovirus-seronegative recipient/donor; R+, cytomegalovirus-seropositive recipient; TAC, tacrolimus; TCD, ex vivo T-cell depleted/CD34+ selected.
Reference: Includes R–D– from pre-LET (n = 200) and post-LET (n = 105) timeframes.
P values were calculated using χ2 tests.
Including 155 lymphoma and 26 CLL.
Including 47 MPD and 17 nonmalignant hematologic disorders.
Others included 2 patients with no GVHD prophylaxis (syngeneic donors).
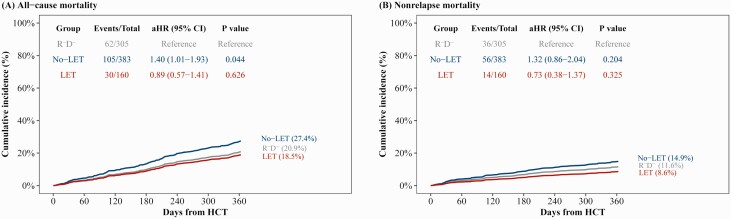
The aHR for all-cause mortality was 1.40 (95% CI, 1.01–1.93; P = .044) for no-LET and 0.89 (95% CI, .57–1.41; P = .626) for LET (Table 3). Male sex and HCT for myeloproliferative disorder (MPD)/nonmalignant disease were associated with decreased all-cause mortality, whereas higher HCT-comorbidity index was associated with incremental increase in mortality. The adjusted cumulative incidence for 1-year all-cause mortality was 20.9%, 27.4%, and 18.7% for the reference, no-LET, and LET groups, respectively (Figure 3A).
Table 3.
Multivariable Risk Factors for All-Cause and Nonrelapse Mortality Through 1 Year Post–Hematopoietic Cell Transplant
| Characteristics | All-Cause Mortalityc | Nonrelapse Mortalityd | ||||
|---|---|---|---|---|---|---|
| aHR | (95% CI) | P Value | aHR | (95% CI) | P Value | |
| Age, y | ||||||
| 18–39 | … | … | … | … | ||
| 40–64 | 1.18 | (.73–1.92) | .495 | 1.18 | (.62–2.24) | .615 |
| ≥65 | 1.42 | (.84–2.38) | .187 | 1.50 | (.76–2.99) | .246 |
| Sex | ||||||
| Female | … | … | … | … | ||
| Male | 0.73 | (.55–.97) | .029 | 0.65 | (.44–.96) | .032 |
| Race | ||||||
| White | … | … | … | … | ||
| Asian | 0.54 | (.25–1.17) | .120 | 0.28 | (.07–1.15) | .078 |
| African American | 0.84 | (.45–1.55) | .570 | 0.80 | (.36–1.79) | .586 |
| Other | 0.89 | (.48–1.63) | .701 | 0.37 | (.12–1.20) | .098 |
| Underlying disease | ||||||
| AML/ALL/CML/MDS | … | … | … | … | ||
| Lymphoma/CLL | 0.82 | (.52–1.30) | .409 | 1.33 | (.73–2.42) | .350 |
| MPD/Nonmalignant | 0.30 | (.12–.74) | .009 | 0.71 | (.28–1.78) | .464 |
| Donor type | ||||||
| Matched related | … | … | … | … | ||
| Matched unrelated | 1.23 | (.86–1.77) | .257 | 1.27 | (.75–2.15) | .368 |
| Mismatched related/unrelated | 1.53 | (.89–2.63) | .128 | 2.58 | (1.30–5.09) | .006 |
| Haploidentical | 0.95 | (.43–2.11) | .903 | 2.07 | (.71–6.05) | .185 |
| Stem cell source | ||||||
| Bone marrow | … | … | … | … | ||
| Peripheral blood | 0.87 | (.57–1.35) | .542 | 1.01 | (.55–1.87) | .970 |
| Conditioning regimen | ||||||
| Myeloablative | …— | …— | …— | …— | ||
| Reduced | 1.07 | (.71–1.61) | .744 | 1.51 | (.82–2.79) | .190 |
| Non-myeloablative | 0.62 | (.32–1.21) | .159 | 0.56 | (.22–1.40) | .212 |
| HCT-comorbidity index | ||||||
| 0 | … | … | … | … | ||
| 1–2 | 3.32 | (1.74–6.34) | .0003 | 2.25 | (1.07–4.74) | .033 |
| ≥3 | 3.76 | (2.02–7.01) | <.0001 | 2.54 | (1.25–5.19) | .010 |
| GVHD prophylaxis | ||||||
| TAC/MTX + others | … | … | … | … | ||
| PTCy | 1.63 | (.88–3.03) | .123 | 1.75 | (.78–3.94) | .178 |
| Ex vivo T-cell depletion | 0.71 | (.46–1.11) | .129 | 1.60 | (.84–3.06) | .153 |
| Acute GVHD gradec | ||||||
| 0–1 | … | … | … | … | ||
| 2–4 | 1.09 | (.80–1.49) | .576 | 1.25 | (.82–1.90) | .302 |
| Groupd | ||||||
| Reference | … | … | … | … | ||
| No-LET | 1.40 | (1.01–1.93) | .044 | 1.32 | (.86–2.04) | .204 |
| LET | 0.89 | (.57–1.41) | .626 | 0.73 | (.38–1.37) | .325 |
Abbreviations: aHR, adjusted hazard ratio; ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CI, confidence interval; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; LET, letermovir prophylaxis; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; MTX, methotrexate; PTCy, posttransplantation cyclophosphamide; TAC, tacrolimus.
Multivariable Cox proportional hazard model was performed to assess aHRs for all-cause mortality.
Multivariable Fine-Gray subdistribution hazard model was performed to assess aHRs for nonrelapse mortality.
Acute GVHD grade was included as time-dependent covariate.
Reference group was composed of R–D–. No-LET and LET groups included R+ without and with letermovir, respectively.
Values in bold indicate significant P <.05.
Figure 3.
Adjusted cumulative incidence of all-cause and nonrelapse mortality for letermovir prophylaxis (LET) and no-LET groups compared with cytomegalovirus (CMV)–seronegative recipient/donor (R–D–; reference). Adjusted cumulative incidence of all-cause mortality (A) and nonrelapse mortality (B) through 1 year post– hematopoietic cell transplant recipient (HCT) by receipt of LET and CMV serostatus. Patients were censored at 1 year post-HCT or last follow-up, whichever occurred first. For nonrelapse mortality, second transplant, relapse, and progression of disease were treated as competing risk events. The adjusted all-cause and nonrelapse mortality were calculated from multivariable Cox proportional hazard model and multivariable Fine-Gray subdistribution hazard model, respectively. Acute graft-vs-host disease grade was treated as a time-dependent variable in multivariable models. No-LET: CMV-seropositive recipient without LET. LET: CMV-seropositive recipient with LET. Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCT, hematopoietic cell transplant recipient; LET, letermovir prophylaxis; R–D–, cytomegalovirus-seronegative recipient/donor.
For nonrelapse mortality, the aHR was 1.32 (95% CI, .86–2.04; P = .204) for no-LET, and 0.73 (95% CI, .38–1.37; P = .325) for LET (Table 3). Male sex was associated with decreased nonrelapse mortality, whereas receipt of HCT from mismatched related/unrelated donor and high HCT-comorbidity index was associated with increased nonrelapse mortality. The adjusted cumulative incidence curves for 1-year nonrelapse mortality were 11.6%, 14.9%, and 8.6% for the reference, no-LET, and LET groups, respectively (Figure 3B).
In summary, all-cause and nonrelapse mortality was similar between the LET group and R–D– after adjusting for covariates.
Association of LET With Mortality Among R+
Next, we examined the association between receipt of LET and mortality among R+ patients. Because of the large number of TCD HCT in our cohort and key differences with CONV, we also performed subgroup analyses for CONV and TCD HCT. No-LET was set as reference. In addition to variables examined for the entire cohort, cs-CMVi as time-dependent covariate and CMV donor serostatus were included. ATG was not included due to high correlation with GVHD prophylaxis (0.79).
All-Cause Mortality
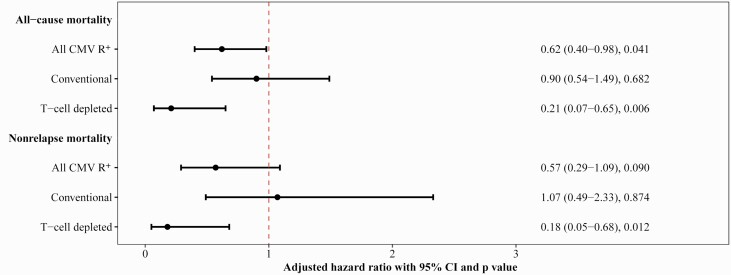
Of 543 R+ patients, 27.4% and 18.8% of patients in the no-LET and LET groups, respectively, had died by 1 year post-HCT. The aHR was 0.62 (95% CI, .40–.98; P = .041) (Figure 4, Supplementary Table 3). Male sex and underlying MPD/nonmalignant disease were associated with decreased all-cause mortality. Higher HCT-comorbidity index was associated with increased mortality.
Figure 4.
Results from multivariable models evaluating receipt of letermovir prophylaxis (LET) as a risk factor for all-cause and nonrelapse mortality in cytomegalovirus (CMV)–seropositive (R+) recipients. Number (%) of death from all causes (A) and death without second transplant, relapse, or progression of disease Adjusted hazard ratio (aHR) of all-cause mortality and nonrelapse mortality through 1 year post–hematopoietic cell transplant by receipt of LET among R+ patients. Patients were censored at 1 year post-HCT or last follow-up, whichever occurred first. For nonrelapse mortality, second transplant, relapse, and progression of disease were treated as competing risk events. Multivariable Cox proportional hazard models and Fine-Gray subdistribution hazard models were performed to assess aHR for all-cause and nonrelapse mortality, respectively. Acute graft-vs-host disease grade and clinically significant CMV infection were treated as a time-dependent variable in multivariable models. No-LET was treated as reference group. Bars show percentage; points show aHR and whiskers show 95% confidence interval. Abbreviations: CI, confidence interval; CMV, cytomegalovirus; R+, cytomegalovirus-seropositive recipient.
Of 337 CONV, 29.5% and 26.0% of patients in the no-LET and LET groups, respectively, had died (Supplementary Table 4); the aHR was 0.90 (95% CI, .54–1.49; P = .682) (Figure 4, Supplementary Table 5). Underlying MPD/nonmalignant disease was associated with decreased mortality, whereas higher HCT-comorbidity index was associated with increased mortality.
Among 206 TCD, 24.0% and 6.7% of patients in the no-LET and LET groups, respectively, had died (Supplementary Table 4). The aHR was 0.21 (95% CI, .07–.65; P = .006) (Figure 4, Supplementary Table 5). Age ≥65 years and acute GVHD (grade 2–4) were associated with increased all-cause mortality.
Nonrelapse Mortality
Among all R+, 14.6% and 8.8% no-LET and LET patients, respectively, had died without second transplant, relapse, or progression of disease (POD) at 1 year (aHR, 0.57 [95% CI, .29–1.09]; P = .090) (Figure 4, Supplementary Table 3). Male sex was associated with decreased nonrelapse mortality, while HCT-comorbidity index was associated with increased mortality.
Among CONV, 13.9% and 11.0% of patients in the no-LET and LET groups, respectively, died without second transplant, relapse, or POD (Supplementary Table 4). The aHR was 1.07 (95% CI, .49–2.33; P = .874) (Figure 4, Supplementary Table 5). CMV-seropositive donor (D+) was associated with increased nonrelapse mortality.
Among TCD, 15.8% and 5.0% of patients in the no-LET and LET groups, respectively, died without second transplant, relapse, or POD (Supplementary Table 4). The aHR was 0.18 (95% CI, .05–.68; P = .012) (Figure 4, Supplementary Table 5). Age ≥65 and acute GVHD were associated with increased nonrelapse mortality.
In summary, among all R+, LET was associated with a significant decrease in all-cause mortality and a numerical decrease in nonrelapse mortality. In subgroup analyses, among conventional HCT, there was no significant decrease in the risk of death associated with LET. In contrast, among TCD, LET was associated with an incidence reduction of 79% for all-cause mortality (P = .006) and 82% for nonrelapse mortality (P = .012).
DISCUSSION
In this study, we examined the relationship between LET and mortality in the first year post-HCT. Our main findings are, first, that all-cause and nonrelapse mortality were similar between R–D– and R+ who received LET. In contrast, R+ who did not receive LET had approximately 40% higher risk of death compared to R–D–. Second, among R+, LET was associated with decreased risk of death compared with no-LET. When analyses were performed separately for conventional and T-cell depleted HCT, LET was associated with significant decrease in all-cause and nonrelapse mortality only for T-cell depleted HCT.
Implementation of LET at our center was associated with a decrease in the incidence of cs-CMVi from 47% to 10.7% and an 88% decrease in utilization of antivirals for preemptive therapy by D180. TCD comprised 37.3% of our cohort. While the incidence of CMV disease ranges between 1% and 5% in the general HCT population, higher rates of CMV disease and CMV-related mortality are reported in CD34+ selected/TCD HCT [17]. Treatment of CMV with vGCV and foscarnet have been associated with increased risk for neutropenia and acute kidney injury post-HCT [18]. Furthermore, CMV viremia serves as an independent risk factor for graft failure, GVHD, and fungal infections [19]. The effect of letermovir prophylaxis on long-term HCT outcomes is an important question clinically and programmatically. Given, however, the negative direct and indirect effects of CMV, toxicities of current CMV treatment, and safety and efficacy of LET, randomized clinical trials may not be feasible to address this question. Retrospective studies comparing outcomes between noncontemporaneous patient groups are challenging as observed differences could be due to multiple evolving practices rather than a single intervention [20].
In the present study, we first established that the mortality of R–D– was similar in the timeframes before and after LET. Using the combined group of R–D– as reference, we show that LET was associated with abrogation of the mortality disparity between R+ and R–D– recipients. In multivariable models, in the absence of LET, CMV recipient seropositivity (R+) was an independent predictor for mortality whereas with LET, R+ had similar risk of death with R–D–.
We next examined the association of LET with mortality among R+ recipients. Because of inherent differences between conventional and TCD HCT including patient selection and tempo of immune reconstitution, we also performed subgroup analyses for CONV and TCD HCT.
Among R+, LET was associated with a lower risk of death compared with no-LET. While an association between CMV VL and mortality has been reported prior to the availability of LET [3] in our study, cs-CMVi was not a predictor of death after adjusting for LET and other covariates.
Among CONV, no difference in mortality was detected between LET and no-LET. In contrast, among TCD, LET was associated with 79% and 82% reduction in all-cause mortality and in nonrelapse mortality, respectively. The randomized trial of the Blood and Marrow Transplant Clinical Trials Network 130, Progress II, was conducted without letermovir prophylaxis using the same method for TCD as in our patients. In Progress II, transplant-related mortality rates were 16.5% for TCD compared with 7% for CONV HCT with calcineurin inhibitor for GVHD prophylaxis (hazard ratio, 2.76; P = .01), but TCD was also associated with a significant reduction in chronic GVHD (16.4 % vs 31.1%) [21]. In the context of the findings of the present study, we speculate that if LET had been routinely used, the transplant-related mortality in TCD HCT may have been reduced. We believe it is essential that T-cell depletion continues to be explored with optimized standards of care.
Our study has several limitations inherent to real-world studies. First, our use of LET was different from the phase 3 registration study. We used a risk-adaptive approach for the duration of LET. This may at least partially account for the relatively flat incidence curve of cs-CMVi through D180. Second, transient low-level CMV viremia on LET was not treated with preemptive therapy. Transient CMV viremia may have led to peripheral T-cell expansion [21, 22]. The observation that LET recipients, especially TCD, had numerically lower mortality than R–D– is of interest in view of recent studies demonstrating a survival benefit with CMV reactivation [22–24].
In summary, letermovir prophylaxis was associated with leveling the mortality disparity between CMV R+ and R–D– recipients in the first year post-HCT. Among CMV R+, LET was associated with decreased all-cause mortality. When conventional HCT and T-cell depleted HCT were analyzed separately, the reduction in risk of death was significant only for TCD. The impact of letermovir on long-term immune signatures and clinical outcomes post-HCT merits further study.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Y. S. designed the research, collected, analyzed and interpreted data, and wrote the manuscript. A. S. designed the research, collected and interpreted data, and wrote the manuscript. E. K., T. N., G. H., P. Z., and H. D. helped in data collection and quality check. C. C., R. T., B. S., S. G., A. J., and M.-A. P. provided critical review of the manuscript. G. P. contributed to and supervised all aspects of the study. The final version of the manuscript was approved by all coauthors.
Financial support. This work was in part supported by the National Cancer Institute at the National Institutes of Health (grant number P30 CA008748 to C. C., R. T., B. S., S. G., A. J., M.-A. P., and G. P.).
Contributor Information
Yiqi Su, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Anat Stern, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Rambam Health Care Campus, Haifa, Israel.
Eleni Karantoni, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Air Force General Hospital, Athens, Greece.
Tamara Nawar, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Gyuri Han, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Phaedon Zavras, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Henry Dumke, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Christina Cho, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Roni Tamari, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Brian Shaffer, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Sergio Giralt, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Ann Jakubowski, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Miguel Angel Perales, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Genovefa Papanicolaou, Infectious Disease Service, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Weill Cornell Medical College, New York, New York, USA.
References
- 1. Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 2013; 122:3359–64. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt-Hieber M, Tridello G, Ljungman P, et al. The prognostic impact of the cytomegalovirus serostatus in patients with chronic hematological malignancies after allogeneic hematopoietic stem cell transplantation: a report from the Infectious Diseases Working Party of EBMT. Ann Hematol 2019; 98:1755–63. [DOI] [PubMed] [Google Scholar]
- 3. Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016; 127:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duke ER, Williamson BD, Borate B, et al. CMV viral load kinetics as surrogate endpoints after allogeneic transplantation. J Clin Invest 2021; 131:e133960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stern A, Su Y, Dumke H, et al. CMV viral load kinetics predict CMV end-organ disease and mortality after hematopoietic cell transplant (HCT). J Infect Dis 2021; 224:620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377:2433–44. [DOI] [PubMed] [Google Scholar]
- 8. Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis 2020; 70:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101:407–14. [DOI] [PubMed] [Google Scholar]
- 10. Verghese PS, Schleiss MR.. Letermovir treatment of human cytomegalovirus infection antiinfective agent. Drugs Future 2013; 38:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sassine J, Khawaja F, Shigle TL, et al. Refractory and resistant cytomegalovirus after hematopoietic cell transplant in the letermovir primary prophylaxis era. Clin Infect Dis 2021; 73:1346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montoro J, Ceberio I, Hilden P, et al. Ex vivo T cell-depleted hematopoietic stem cell transplantation for adult patients with acute myelogenous leukemia in first and second remission: long-term disease-free survival with a significantly reduced risk of graft-versus-host disease. Biol Blood Marrow Transplant 2020; 26:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceberio I, Devlin SM, Sauter C, et al. Sirolimus, tacrolimus and low-dose methotrexate based graft-versus-host disease prophylaxis after non-ablative or reduced intensity conditioning in related and unrelated donor allogeneic hematopoietic cell transplant. Leuk Lymphoma 2015; 56:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo SK, Xiao K, Huang YT, et al. Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: a 12-year single institution study. J Infect 2014; 69:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15:825–8. [PubMed] [Google Scholar]
- 17. Huang YT, Kim SJ, Lee YJ, et al. Co-infections by double-stranded DNA viruses after ex vivo T cell-depleted, CD34(+) selected hematopoietic cell transplantation. Biol Blood Marrow Transplant 2017; 23:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zavras P, Su Y, Fang J, et al. Impact of preemptive therapy for cytomegalovirus on toxicities after allogeneic hematopoietic cell transplantation in clinical practice: a retrospective single-center cohort study. Biol Blood Marrow Transplant 2020; 26:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Annaloro C, Serpenti F, Saporiti G, et al. Viral infections in HSCT: detection, monitoring, clinical management, and immunologic implications. Front Immunol 2020; 11:569381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald GB, Sandmaier BM, Mielcarek M, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med 2020; 172:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldberg JD, Zheng J, Ratan R, et al. Early recovery of T-cell function predicts improved survival after T-cell depleted allogeneic transplant. Leuk Lymphoma 2017; 58:1859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain T, Cho C, Hilden P, et al. Cytomegalovirus reactivation promotes CD8+ T cell subset recovery after unmodified allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019; 25:S326–7. [Google Scholar]
- 23. Elmaagacli AH, Koldehoff M.. Cytomegalovirus replication reduces the relapse incidence in patients with acute myeloid leukemia. Blood 2016; 128:456–9. [DOI] [PubMed] [Google Scholar]
- 24. Litjens NHR, van der Wagen L, Kuball J, Kwekkeboom J.. Potential beneficial effects of cytomegalovirus infection after transplantation. Front Immunol 2018; 9:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.