Abstract
Background
There are few data on the utility of tenofovir diphosphate (TFV-DP) in dried blood spots (DBSs) to predict future viral load (VL) in postpartum women with HIV on antiretroviral therapy (ART).
Methods
We conducted a nested case-control study within a trial of postpartum ART delivery strategies. Participants started ART containing tenofovir disoproxil fumarate (TDF) in pregnancy, were <10 weeks postpartum, and had a VL <400 copies/mL. VL and TFV-DP samples were taken every 3–6 months over 24 months. Cases had ≥1 VL ≥20 copies/mL; controls were randomly sampled from women with persistent viral suppression (VS; VL <20 copies/mL). Generalized estimating equations were used to calculate likelihood odds ratios (LORs) for future VL ≥20 copies/mL by TFV-DP concentration at the preceding visit.
Results
61 cases and 20 controls contributed 365 DBS-VL pairs (median ART duration, 16 months). Sensitivity and specificity of TFV-DP <700 fmol/punch to detect future viremia were 62.9% (95% CI, 54.7–70.6%) and 89.7% (84.9–93.4%), respectively. Adjusting for age, ART duration, previous VL, and duration between the TFV-DP and VL measures, LORs of viremia for TFV-DP concentrations 350–699 and <350 fmol/punch versus TFV-DP ≥1850 fmol/punch were 3.5 (95% CI, 1.1–10.8; P = .033) and 12.9 (3.6–46.6; P < .0001), respectively. Including only samples taken during VS, the LOR of future viremia for TFV-DP concentration <350 fmol/punch versus TFV-DP ≥1850 fmol/punch was 9.5 (1.9–47.0).
Conclusions
TFV-DP concentrations in DBSs were strongly associated with future viremia and appear useful to identify nonadherence and predict future elevated VL.
Keywords: antiretroviral therapy, adherence, dried blood spots, tenofovir diphosphate, predictive value
Among South African women with HIV using tenofovir-containing antiretroviral therapy, tenofovir diphosphate concentrations were strongly associated with subsequent viremia occurring up to 6 months later, supporting use of this biomarker to identify nonadherence, predict potential viremia, and inform clinical management.
South Africa has the largest human immunodeficiency virus (HIV) epidemic in the world, with an estimated 7.5 million people living with HIV, of whom approximately 65% are women over the age of 15 years [1]. While 75% of women living with HIV in South Africa are estimated to be on antiretroviral therapy (ART), there are ongoing concerns regarding ART nonadherence, particularly in postpartum women [2–4]. Nonadherence to ART can lead to disease progression, HIV transmission, virological failure, and drug resistance [5, 6], and methods to measure adherence and identify those at high risk of poor treatment outcomes are needed in this population [2, 3, 7–9].
Assessment of adherence is challenging, however. Self-reported adherence usually overestimates actual adherence [10]. Pill counts, pharmacy refills, and electronic adherence measures, while more predictive of adherence, do not confirm pill ingestion [11–13]. Elevated HIV viral load (VL) is a delayed outcome of nonadherence and does not distinguish between nonadherence and ART drug resistance [12–14]. Objective adherence measures that identify patients with nonadherence prior to the development of elevated VL and related complications are urgently needed.
Pharmacologic measures, such as drug concentrations in dried blood spots (DBSs), can serve as objective measures of ART adherence. Recent studies have assessed the use of an assay to quantify tenofovir diphosphate (TFV-DP), the phosphorylated metabolite of tenofovir, in DBSs in persons with HIV taking tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) [15]. TFV-DP has an intracellular half-life of approximately 17 days in red blood cells with high accumulation (25-fold) to steady state, allowing quantification of average adherence in the preceding 2 months [16, 17]. TFV-DP concentrations in DBSs are strongly associated with concurrent viral suppression and may also be predictive of future viremia. The relative odds of future viremia increased with lower drug concentration categories in studies in the United States and South Africa, but data from different populations, including postpartum women, in whom nonadherence is a particular concern, are limited [18–21]. To address this, we examined whether TFV-DP in DBSs can predict future viremia in postpartum women living with HIV receiving TDF-containing ART in South Africa.
METHODS
Study Design and Participants
We conducted a nested case-control study within a trial of differentiated care for postpartum ART delivery (NCT03200054) in Cape Town, South Africa [20]. Women were enrolled in the trial if they were older than 18 years, less than 10 weeks postpartum, had a VL of less than 400 copies/mL in the preceding 3 months, and had no comorbidities requiring regular clinical follow-up. All women started TDF 300 mg, lamivudine (3TC) 300 mg or emtricitabine (FTC) 200 mg, and efavirenz (EFV) 600 mg taken once daily as a fixed-dose combination (FDC) during pregnancy. Follow-up visits in the trial were conducted at 3, 6, 12, 18, and 24 months postpartum. At each visit, face-to-face interviews including intercurrent medical history were completed, DBS specimens were collected, and blood samples for VL testing were drawn (Abbott Molecular RealTime HIV-1 assay; Abbott Molecular, Des Plaines, IL, USA).
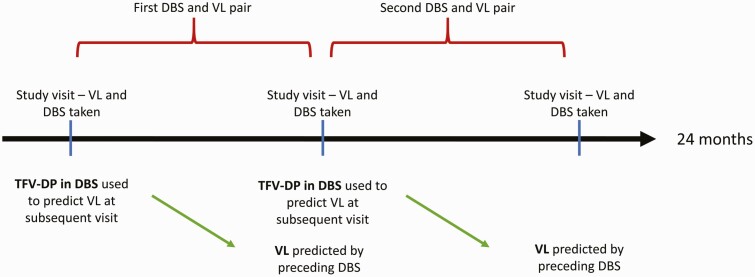
Excluding the VL measures taken at the first visit and the DBS samples taken at the final study visit, each DBS sample was linked with the VL specimen taken at the subsequent visit to create DBS and VL pairs (Figure 1). For this substudy, women with at least 2 DBS and VL pairs over the course of 24 months were eligible for inclusion. Among these, all women with at least 1 VL of 20 copies/mL or more were included as cases and a random sample of 20 women with persistent viral suppression (VL <20 copies/mL) were included as controls, providing a ratio of cases to controls of 3:1.
Figure 1.
Illustration of study visits and formation of DBS and VL pairs. Abbreviations: DBS, dried blood spot; HIV, human immunodeficiency virus; TFV-DP, tenofovir diphosphate; VL, viral load.
TFV-DP Quantification
To quantify TFV-DP in DBSs, 50 µL of venous blood ethylenediaminetetraacetic acid (EDTA) samples were pipetted onto DBS cards with up to 5 blood spots per card. The card was allowed to dry overnight at room temperature and was then stored at −80°C. TFV-DP concentrations in DBSs were assayed at the Clinical Pharmacology Laboratory in the Division of Clinical Pharmacology at the University of Cape Town using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method, modified from the original method developed by Bushman et al [21], using an AB Sciex API 5500 instrument. Dried blood spot specimens with TFV-DP concentrations of 350 fmol/punch or greater and with concurrent VL values of 1000 copies/mL or more were sent for HIV drug-resistance genotyping testing.
Analysis
Analyses were performed in Stata version 16.0 (StataCorp, College Station, TX, USA) or R (R Foundation, Vienna, Austria). Variables were summarized using means with standard deviations, medians with interquartile ranges (IQRs), or proportions, as appropriate. Concentrations of TFV-DP in DBSs below the lower limit of quantification (LLOQ; 16.6 fmol/punch) were assigned a value half that of the LLOQ (8.3 fmol/punch). TFV-DP concentrations were categorized based on previously established thresholds (<350, 350–699, 700–1249, 1250–1849, and ≥1850 fmol/punch), which were associated with concurrent viral suppression in a cohort of people with HIV in the United States and South African women [17, 22, 23]. Viremia was defined as a VL of 20 copies/mL or greater. Sensitivity and specificity of TFV-DP thresholds to predict viremia at subsequent visits were calculated in all specimens, and for DBS and VL pairs in which the DBS sample was taken during viral suppression (VL <20 copies/mL). Sensitivity and specificity were also calculated for DBS and subsequent VL pairs with more than 90 days between the 2 specimens. Logistic regression models using generalized estimating equations for repeated measures within individuals were used to calculate likelihood odds ratios (LORs) for viremia at subsequent visits, comparing each TFV-DP category with the highest reference category (≥1850 fmol/punch). In addition, we conducted a sensitivity analysis using TFV-DP thresholds of less than 350, 350–699, 700–1249, and more than 1250 fmol/punch, .with a concentration of less than 350, 350-699, 700-1249, and 1250 fmol/punch or more, with a concentration of 1250 fmol/punch or more as the reference category. Adjusted LORs were obtained from models including age, duration on ART at the point of VL, preceding VL result, and the duration between the DBS and VL result. Sensitivity analyses were done using thresholds for TFV-DP in DBSs (<800, 800–1649, and ≥1650 fmol/punch) that were predictive of future viremia (≥20 copies/mL) in a study in the United States [18] and using alternate VL thresholds to categorize viremia (VL ≥400 copies/mL and VL ≥1000 copies/mL). We also compared fluctuations in TFV-DP concentrations between cases (prior to an episode of viremia) and controls using the modified robust Brown-Forsythe Levene-type test [24].
Ethics
All participants provided written informed consent, including consent for specimen storage and drug assays, prior to completing any study procedures. The study was approved by the University of Cape Town Human Faculty of Health Sciences Human Research Ethics Committee.
RESULTS
Among 411 women in the parent trial, 389 had at least 2 DBS and subsequent VL pairs. Among these, 61 women had at least 1 VL of 20 copies/mL or more and were included as cases for this analysis. Of the remaining 328 who were virally suppressed at every visit, a random sample of 20 was selected, making up the final sample of 81 participants.
Of the 81 women, 3 (4%) women had 2 DBS and VL pairs included, 3 (4%) had 3 DBS and VL pairs, 25 (31%) had 4 DBS and VL pairs, and 50 (62%) had 5 DBS and VL pairs, for a total of 365 DBS and VL pairs. The median duration between each TFV-DP and VL measure was 175 days (IQR: 92–184 days). At the time of the first included DBS sample, median age was 29 (IQR: 26–32) years, median duration on ART was 16 (IQR: 14–20) months, and median duration postpartum was 19 (IQR: 9–88) days (Table 1). All women were receiving TDF, FTC, and EFV as an FDC taken once daily and had a VL less than 20 copies/mL at the time of the first DBS sample. Median TFV-DP concentration for the first included DBS sample was 1113 (IQR: 904–1432) fmol/punch, with 3 (4%), 8 (10%), 38 (47%), 22 (27%), and 10 (12%) samples in the less than 350-, 350–699-, 700–1249-, 1250–1849-, and 1850-fmol/punch or more categories, respectively. Among all 365 DBS specimens, the median TFV-DP concentration was 1051 (397–1466) fmol/punch. Overall, 308 (84%) DBS samples had concentrations of TFV-DP in the quantifiable range, with 90 (25%) in the less than 350-fmol/punch category (including specimens below the LLOQ), 27 (7%) in the 350–600-fmol/punch category, 109 (30%) in the 700–1249-fmol/punch category, 98 (27%) in the 1250–1849-fmol/punch category, and 41 (11%) in the 1850-fmol/punch or more category.
Table 1.
Characteristics of Included Women at Time of First Dried Blood Spot Sample
| TFV-DP Concentration in DBS (fmol/punch) | ||||||||
|---|---|---|---|---|---|---|---|---|
| All Participants (N = 81) | Cases (n = 61) | Controls (n = 20) | <350 (n = 3) | 350–699 (n = 8) | 700–1249 (n = 38) | 1250–1849 (n = 22) | ≥1850 (n = 10) | |
| Median age (IQR), years | 29 (26–32) | 28 (26–31) | 30 (26–33) | 28 (27–30) | 24 (21–27) | 30 (27–33) | 28 (25–33) | 29 (27–31) |
| Median months on ART (IQR) | 16 (14–20) | 16 (14–20) | 16 (14–20) | 15 (12–18) | 19 (15–22) | 15 (14–20) | 17 (14–20) | 17 (11–22) |
| Median days postpartum (IQR) | 19 (9 - 88) | 18 (9–89) | 23 (9–55) | 39 (6-93) | 55 (5-91) | 14 (9– 52) | 16 (10–42) | 89 (29–91) |
| Attended high school, n (%) | 80 (99) | 60 (98) | 20 (100) | 2 (67) | 8 (100) | 38 (100) | 22 (100) | 10 (100) |
| Employed and/or studying, n (%) | 25 (31) | 19 (32) | 6 (30) | 1 (33) | 4 (50) | 5 (13) | 10 (46) | 5 (50) |
| Married or cohabiting, n (%) | 35 (43) | 24 (39) | 11 (55) | 0 (0) | 4 (50) | 16 (42) | 12 (55) | 3 (30) |
| Previous ART use (ART or PMTCT), n (%) | 20 (25) | 13 (21) | 7 (35) | 0 (0) | 0 (0) | 14 (37) | 4 (18) | 2 (20) |
N = 81. All women were on tenofovir, emtricitabine, and efavirenz taken once daily as a fixed-dose combination and all women had a VL <20 copies/mL at first DBS by design.
Abbreviations: ART, antiretroviral therapy; DBS, dried blood spot; IQR, interquartile range; PMTCT, prevention of mother-to-child transmission; TFV-DP, tenofovir diphosphate.
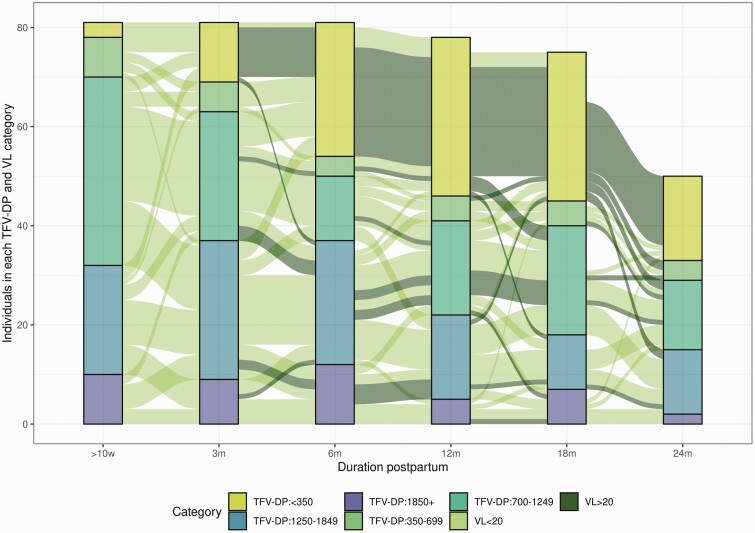
A dose–response relationship was found between decreasing TFV-DP concentrations in DBS and future viremia. Figure 2 shows the trajectory of TFV-DP concentrations by VL category. Of the 41 DBS samples with TFV-DP concentrations of 1850 fmol/punch or more,, 7 (17%) had a VL of 20 copies/mL or greater at the subsequent visit, compared with 22 of 98 (22%) with a TFV-DP concentration of 1250–1849 fmol/punch, 27 of 109 (25%) with a TFV-DP concentration of 700–1249 fmol/punch, 13 of 27 (48%) with a TFV-DP concentration of 350–699 fmol/punch, and 82 of 90 (91%) with a TFV-DP concentration less than 350 fmol/punch. There were 55 DBS samples with a TFV-DP concentration of less than 350 fmol/punch which had at least 2 subsequent visits at which VLs were taken; of these, 48 (87%) DBS samples were followed by 2 VL of 20 copies/mL or greater. (Supplementary Table 4). Variation in TFV-DP concentrations between cases and controls prior to the occurrence of viremia in cases indicated larger variances and lower median DBS values in cases compared with controls (P = .0002).
Figure 2.
HIV VL and TFV-DP trajectories over the study period (n = 81). The colored bars represent the proportion of individuals in each TFV-DP concentration category at each time point. The lines (paths) between the bars are colored by VL category and show the trajectory of TFV-DP concentrations over time. Abbreviations: HIV, human immunodeficiency virus; m, months; TFV-DP, tenofovir diphosphate; VL, viral load; w, weeks.
Specificity of TFV-DP in DBSs less than the LLOQ to predict future viremia in all specimens was 98% (95% confidence interval [CI]: 95.3–99.5%) (Table 2), but sensitivity was low at 35.1% (95% CI: 27.5–43.3%). Specificities remained high at a TFV-DP threshold of less than 700 fmol/punch (89.7%; 95% CI: 84.9–93.4%), while sensitivity increased to 62.9% (95% CI: 54.7–70.6%). Sensitivities were similar when the VL specimen was taken more than 90 days after the DBS. When including only specimens taking during viral suppression, specificity remained high but sensitivities were lower; in those with a VL less than 20 copies/mL, specificity to predict future viremia was 91.6% (95% CI: 86.7–95.1%), while sensitivity was 29.2% (95% CI: 18.6–41.8%).
Table 2.
Sensitivity and Specificity of Binary Drug Concentration Thresholds to Predict Future Viral Load ≥20 Copies/mL
| All Paired Specimens (n = 365), % | VL <20 Copies/mL at Preceding Visit (n = 255), % | DBS Done <90 Days Before VL (n = 85), % | ||||
|---|---|---|---|---|---|---|
| TFV-DP in DBS Concentration at Preceding Visit | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity |
| ≤LLOQ | 35.1 (27.5–43.3) | 98.1 (95.3– 99.5) | 0 (0–0.1) | 99.5 (97.1–99.9) | 36.1 (28.0–44.9) | 98.1 (94.6–99.6) |
| ≤350 fmol/punch | 54.3 (46.0–62.4) | 96.3 (92.8–98.4) | 12.3 (5.5–22.8) | 98.4 (95.5–99.7) | 56.4 (47.5–65.0) | 95.6 (91.2–98.2) |
| ≤700 fmol/punch | 62.9 (54.7–70.6) | 89.7 (84.9–93.4) | 29.2 (18.6–41.8) | 91.6 (86.7–95.1) | 66.2 (57.5–74.1) | 87.5 (81.4–92.2) |
| ≤1250 fmol/punch | 80.8 (73.6–86.7) | 51.4 (51.4–44.5–58.3) | 61.5 (48.6–73.4) | 52.1 (44.8 – 59.4) | 82.0 (74.4–88.1) | 52.5 (44.4–60.4) |
| ≤1850 fmol/punch | 95.4 (90.7–98.1) | 15.9 (11.3–21.5) | 89.2 (79.1–95.6) | 14.7 (14.7–10.0–20.6) | 96.2 (91.4–98.8) | 16.9 (11.4–23.6) |
Abbreviations: DBS, dried blood spot; LLOQ, lower limit of quantification; TDF-DP, tenofovir diphosphate; VL, viral load.
After adjusting for age, duration on ART at the point of VL, previous VL value, and duration between the TFV-DP and VL measurement, the LOR for a future VL of 20 copies/mL or greater with a TFV-DP concentration of 1250–1849 fmol/punch compared with TFV-DP of more than 1850 fmol/punch was 1.3 (95% CI: 0.5–3.3; P = .599) (Table 3). The magnitude of this association increased with decreasing TFV-DP concentration categories: compared with TFV-DP of 1850 fmol/punch or more, the adjusted LORs for TFV-DP concentrations of 700–1249, 350–699, and less than 350 fmol/punch were 1.5 (95% CI: .6–3.8; P = .391), 3.5 (95% CI: 1.1–10.8; P = .033), and 12.9 (95% CI: 3.6–46.6; P < .0001), respectively. Using a TFV-DP of 1250 fmol/punch or more as the reference category, the adjusted LORs for TFV-DP concentrations of 700–1249, 350–699, and less than 350 fmol/punch were 1.25 (95% CI: .68–2.30; P = .473), 2.87 (95% CI: 1.18–7.03; P = .021), and 10.74 (95% CI: 3.69–31.28; P < .0001), respectively (Supplementary Table 5).
Table 3.
Generalized Estimating Equations With Logistic Regression Predicting Future Viral Load ≥20 Copies/mL by Tenofovir Diphosphate Concentration
| OR (95% CI) | P | aOR (95% CI) | P | |
|---|---|---|---|---|
| TFV-DP in DBS Concentration at Preceding Visit | ||||
| ≥1850 fmol/punch | Ref | Ref | ||
| 1250–1849 fmol/punch | 1.41 (.55–3.61) | .478 | 1.29 (.50–3.35) | .599 |
| 700–1249 fmol/punch | 1.60 (.64–4.02) | .318 | 1.50 (.59–3.82) | .391 |
| 350–699 fmol/punch | 4.51 (1.49–13.68) | .008 | 3.46 (1.11–10.84) | .033 |
| <350 fmol/punch | 49.79 (16.73–148.12) | <.0001 | 12.94 (3.59–46.61) | <.0001 |
| Age, years | .93 (.89–.97) | .001 | .96 (.90–1.01) | .131 |
| Duration on ART at time of VL, months | 1.10 (1.07–1.12) | <.0001 | .97 (.92–1.02) | .193 |
| Previous VL result, log10 copies/mL | 5.48 (3.34–9.00) | <.0001 | 2.46 (1.37–4.42) | .003 |
| Duration between DBS and VL specimens, months | 1.18 (1.06–1.32) | .002 | 1.16 (.97–1.39) | .110 |
N = 365 DBS and VL samples in 81 women.
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; DBS, dried blood spot; OR, odds ratio; Ref, reference; TFV-DP, tenofovir diphosphate; VL, viral load.
The LORs for a VL of 400 copies/mL or greater at the subsequent visit were larger than for a VL of 20 copies/mL or greater: compared with TFV-DP concentrations of 1850 fmol/punch or more, the adjusted LORs for TFV-DP concentrations of 350–699 and less than 350 fmol/punch were 13.07 (95% CI: 1.5–116.8; P = .021) and 27.9 (95% CI: 3.0–263.8; P = .004), respectively (Supplementary Table 1). The LOR for a VL of 1000 copies/mL or greater for TFV-DP less than 350 fmol/punch was 20.9 (95% CI: 2.1–208.8) (Supplementary Table 2). Using TFV-DP in DBS thresholds established as predictive of future viremia in persons living with HIV on TDF-containing ART in the United States, the adjusted LORs of a VL of 20 copies/mL or more for TFV-DP concentrations of 800–1649 and less than 800 fmol/punch versus 1650 fmol/punch or more were 1.1 (95% CI: .5–2.2; P = .827) and 2.9 (95% CI: 1.3–6.4; P = .011), respectively (Supplementary Table 3).
In sensitivity analyses including only the 255 DBS samples taken during viral suppression, the LORs for a VL of 20 copies/mL or greater for TFV-DP concentrations of 1250–1849, 700–1249, and 350–699 fmol/punch compared with TFV-DP concentrations of 1850 fmol/punch or greater were not statistically significant (Table 4). However, compared with a TFV-DP concentration of 1850 fmol/punch or greater, a TFV-DP concentration less than 350 fmol/punch was associated with a statistically significant odds of viremia (LOR: 9.5; 95% CI: 1.9–47.0; P = .006).
Table 4.
Generalized Estimating Equations With Logistic Regression Predicting Future Viral Load ≥20 Copies/mL by Tenofovir Diphosphate Concentration for Dried Blood Spot Samples Taken During Viral Suppression
| OR (95% CI) | P | aOR (95% CI) | P | |
|---|---|---|---|---|
| TFV-DP in DBS Concentration at Preceding Visit | ||||
| ≥1850 fmol/punch | Ref | Ref | ||
| 1250-1849 fmol/punch | 1.01 (.38–2.69) | .978 | .86 (.31–2.37) | .772 |
| 700-1249 fmol/punch | 1.12 (.43–2.92) | .817 | 1.04 (.39–2.78) | .939 |
| 350-699 fmol/punch | 3.38 (1.07–10.73) | .038 | 2.47 (.74–8.22) | .140 |
| <350 fmol/punch | 10.67 (2.23–50.97) | .003 | 9.49 (1.92–47.00) | .006 |
| Age, years | .93 (.88–.99) | .0032 | .95 (.89–1.02) | .175 |
| Duration on ART at time of VL, months | 1.00 (.97–1.04) | .798 | .93 (.88–.99) | .031 |
| Duration between DBS and VL specimens, months | 1.22 (1.06–1.41) | .006 | 1.45 (1.16–1.81) | .001 |
N = 255 DBS and VL samples in 81 women.
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; DBS, dried blood spot; OR, odds ratio; Ref, reference; TFV-DP, tenofovir diphosphate; VL, viral load.
Two DBS samples with TFV-DP concentrations of 350 fmol/punch or greater, from separate participants, had concurrent VL values of 1000 copies/mL or more. An additional 2 samples, from a single participant, with TFV-DP concentrations of 700 fmol/punch or greater had concurrent VLs of 1000 copies/mL or more. These 4 samples were sent for HIV drug-resistance genotyping testing: 2 had no resistance mutations, 1 had a K103N mutation, and no amplification was obtained in the fourth sample.
DISCUSSION
In postpartum women on TDF-containing ART in South Africa, we observed a dose–response relationship between decreasing TFV-DP concentrations in DBSs and HIV viremia occurring up to 6 months in the future. In an adjusted model, the LOR for a subsequent VL of 20 copies/mL or greater was significantly higher for TFV-DP concentrations of 350–699 fmol/punch and less than 350 fmol/punch compared with TFV-DP concentrations of 1850 fmol/punch or more. TFV-DP concentrations less than 350 fmol/punch were persistently associated with a significant LOR of viremia when the analysis was restricted only to DBS specimens sampled during viral suppression. Taken together, these data point to the potential value of this objective adherence marker in populations on ART at high risk of viremia.
The findings here are in line with those of a study by Morrow et al [18] conducted in the United States, which found an association between lower drug concentration categories and future viremia, including in those virologically suppressed at the time of DBS sampling. In that study, compared with TFV-DP concentrations of 1650 fmol/punch or higher, both TFV-DP concentrations less than 800 and 800–1649 fmol/punch were significantly associated with a future VL of 20 copies/mL or greater (odds ratios: 4.7 and 2.1, respectively). While we found a similar dose–response relationship between TFV-DP concentrations and future viremia, only concentrations below 700 fmol/punch were significantly associated with future viremia in our analysis. Reasons for this difference require further investigation. The study by Morrow et al included participants on nonnucleoside reverse transcriptase inhibitor (NNRTI), integrase strand-transfer inhibitor, and boosted protease inhibitor-based ART regimens, while all participants in this analysis were on NNRTI-based ART. In addition, we lack data on body mass index, estimated glomerular filtration rate, hematocrit, and CD4 count, all of which may affect metabolism of tenofovir in plasma and of TFV-DP in red blood cells and DBSs [25, 26]. Future studies that include measurement of these potential confounders are required to confirm therapeutic thresholds.
Numerous studies have documented a high risk of nonadherence and loss of virologic control in the postpartum period, including in South Africa, and development of management strategies for this group is a public health priority [2, 3, 7, 9]. The use of TFV-DP in DBSs in combination with VL results could facilitate appropriate management by detecting nonadherence early and helping to differentiate nonadherence from drug resistance. Detection of low TFV-DP concentrations in individuals with suppressed VLs would suggest nonadherence and allow earlier intervention than when relying on VL alone [27]. However, it is important to note that, when TFV-DP concentrations were taken during viral suppression, the sensitivities of TFV-DP concentrations below 350 fmol/punch and below 700 fmol/punch to detect future viremia were high but specificities were low. Women with moderately high TFV-DP concentrations during viral suppression may thus still be at risk of future viremia and should continue to receive adherence counselling and other methods of adherence assessment. Viremia in the presence of relatively high TFV-DP concentrations could indicate possible ART drug resistance and identify those in need of drug-resistance testing [28, 29]. In this analysis, 4 DBS specimens with TFV-DP concentrations of 350 fmol/punch or greater had concurrent VLs of 1000 copies/mL or more and were sent for HIV drug-resistance genotyping: a K103N mutation was identified in 1 sample. Early management of drug resistance is vital to prevent the accumulation of further mutations, but drug-resistance testing is costly. Thus, the quantification of TFV-DP concentrations in DBSs could help prioritize individuals in need of drug-resistance testing.
A strength of this study is that tenofovir-based ART is extensively used as part of first-line ART regimens, including in South Africa [30]. Limitations include that participants were seen in a research setting and inclusion in the analysis was dependent on study visit attendance. The outcome in this analysis was the occurence of 1 VL of 20 copies/mL or more and further research should assess the ability of TFV-DP in DBSs to predict persistent viremia. In addition, the utility of TFV-DP in DBSs in diverse clinical settings, and the effect of its use on clinical outcomes, requires additional investigation. Further, TFV-DP in DBS assays remain costly and require laboratory facilities. Thus, cost-effectiveness and feasibility require investigation, particularly in resource-limited settings, as well as the development of point-of-care tests.
In summary, we found that TFV-DP concentrations in DBSs were predictive of future viremia in postpartum South African women on TDF-containing ART, including those virally suppressed at DBS sampling. Used in conjunction with VL testing, TFV-DP concentrations in DBSs could be valuable to identify individuals at risk of future viremia and help distinguish nonadherence from ARV drug resistance, thereby guiding clinical management. Further research is required to confirm therapeutic thresholds in different populations and assess its use in clinical practice.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Conceptualization and design: L. M., M. L., J. C.-M., C. O.; data curation: J. O., S. K.; analysis: J. O., M. L.; funding acquisition: L. M.; trial conduct: J. A., S. K., J. O., T. R. M., N.-C. H., L. W.; writing, original draft: J. O.; writing, review and editing: C. O., T. K. P., N.-C. H., S. K., T. R. M., J. A., L. W., N.-y.H. , J. C.-M., M. L.
Acknowledgments. The authors thank all study participants and staff for their contribution to this work.
Financial support. This work was supported by the Medical Research Council (grant number MR/M007464/1). J. O. received training in research that was supported by the Fogarty International Centre of the National Institutes of Health (NIH) under award numbers D43 TW010559 and D43 TW00934D. The University of Cape Town Clinical PK Laboratory is supported in part via the Adult Clinical Trial Group (ACTG), by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701, as well as the Infant Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT), with funding provided by the NIAID (award number U01 AI068632), The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Mental Health grant AI068632. J. C.-M. is supported by NIH/NIAID award number R01AI145453.
Contributor Information
Jasantha Odayar, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Catherine Orrell, Desmond Tutu HIV Centre, Department of Medicine and Institute of Infectious Diseases and Molecular Medicine, University of Cape Town Medical School, Cape Town, South Africa.
Tamsin K Phillips, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Nai Chung Hu, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Siti Kabanda, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Thokozile R Malaba, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Joanna Allerton, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Lubbe Wiesner, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa.
Nei yuan Hsiao, Division of Medical Virology, National Health Laboratory Service, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africaand.
Jose Castillo-Mancilla, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Maia Lesosky, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
Landon Myer, Division of Epidemiology and Biostatistics, School of Public Health & Family Medicine, University of Cape Town, Cape Town, South Africa.
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS). Country factsheets South Africa 2019. Available at: https://www.unaids.org/en/regionscountries/countries/southafrica. Accessed 22 May 2021.
- 2. Nachega JB, Uthman OA, Anderson J, et al. . Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries. AIDS 2012; 26:2039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myer L, Dunning L, Lesosky M, et al. . Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis 2017; 64:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsen A, Magasana V, Dinh T, et al. . Longitudinal adherence to maternal antiretroviral therapy and infant nevirapine prophylaxis from 6 weeks to 18 months postpartum amongst a cohort of mothers and infants in South Africa. BMC Infect Dis 2019; 19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Press N, Tyndall M, Wood E, Hogg R, Montaner J.. Virologic and immunologic response, clinical progression, and highly active antiretroviral therapy adherence. J Acquir Immune Defic Syndr 2002; 31:S112–7. [DOI] [PubMed] [Google Scholar]
- 6. Chi BH, Cantrell R, Zulu I.. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol 2009; 38:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffman RM, Warshaw MG, Amico KR, et al. . Predictors of viremia in postpartum women on antiretroviral therapy. J Acquir Immune Defic Syndr 2020; 83:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myer L, Phillips TK.. Beyond “Option B+”: understanding antiretroviral therapy (ART) adherence, retention in care and engagement in ART services among pregnant and postpartum women initiating therapy in sub-Saharan Africa. J Acquir Immune Defic Syndr 2017; 75:S115–22. [DOI] [PubMed] [Google Scholar]
- 9. Myer L, Essajee S, Broyles LN, et al. . Pregnant and breastfeeding women: a priority population for HIV viral load monitoring. PLoS Med 2017; 14:e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA.. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006; 10:227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. . Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015; 5:470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinelli M, Haberer J, Chai P, Castillo-Mancilla J, Anderson P, Gandhi M.. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep 2020; 17:301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKinney O, Pearce D, Banta J, et al. . Evaluation of pill counts adherence with self-reported adherence in assessing antiretroviral therapy behavior of women living with HIV at a faith-based clinic in Malawi. HIV Curr Res 2017; 02:1–8. [Google Scholar]
- 14. Stöhr W, Fidler S, McClure M, et al. . Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy. PLoS One 2013; 8:e782878–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo-Mancilla J, Coyle RP, Zheng JH, et al. Tenofovir diphosphate arising from TAF is quantifiable in dried blood spots. [Abstract 405]. Presented at: Conference on Retroviruses and Opportunistic Infections 2017 (Seattle, WA).
- 16. Castillo-Mancilla JR, Zheng JH, Rower JE, et al. . Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. . Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62:e01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips TK, Sinxadi P, Abrams EJ, et al. . A comparison of plasma efavirenz and tenofovir, dried blood spot tenofovir-diphosphate, and self-reported adherence to predict virologic suppression among South African women. J Acquir Immune Defic Syndr 2019; 81:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. . Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2019; 68:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrow M, Mawhinney S, Coyle RP, et al. . Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2020; 221:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robbins RN, Jennings L, Nguyen N, et al. . Tenofovir diphosphate in dried blood spots predicts future viraemia in South Africa [Abstract 398]. Presented at: Conference on Retroviruses and Opportunistic Infections 2021. (Boston, MA: ). [Google Scholar]
- 22. Odayar J, Malaba TR, Allerton J, Lesosky M, Myer L.. Delivery of antiretroviral therapy to HIV-infected women during the postpartum period: the Postpartum Adherence Clubs for Antiretroviral Therapy (PACART) trial. Contemp Clin Trials Commun 2019; 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bushman L, Kiser J, Rower J, et al. . Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011; 56:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown M. B., Forsythe A. B. Robust tests for the equality of variances. J Am Stat Assoc 1974; 69:364–7. [Google Scholar]
- 25. Baxi SM, Greenblatt RM, Bacchetti P, et al. . Common clinical conditions-age, low BMI, ritonavir use, mild renal impairment-affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS 2014; 28:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilhelm AJ, den Burger JCG, Swart EL.. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet 2014; 53:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kristofich M, Anderson P, Castillo-Mancilla J.. Beyond HIV viral load: application of pharmacologic measures to identify ART adherence mismatch. Ther Adv Infect Dis 2021; 8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yager JL, Coyle RP, Coleman SS, et al. . Moderately high tenofovir diphosphate in dried blood spots indicates drug resistance in viremic persons living with HIV. J Int Assoc Provid AIDS Care 2019; 18:2325958219888451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castillo-Mancilla J, Zhao Y, Brijkumar J.. Tenofovir diphosphate in dried blood spots predicts virologic failure and resistance [Abstract 520]. Presented at: Conference on Retroviruses and Opportunistic Infections 2020. Boston, MA. [Google Scholar]
- 30. South African National Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria; 2013. Available at: https://sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf. Accessed 25 August 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.