Abstract
The uptake of Ca2+ into mitochondria is thought to be an important signal communicating the need for increased energy production. However, dysregulated uptake leading to mitochondrial Ca2+ overload can trigger opening of the mitochondrial permeability transition pore and potentially cell death. Thus mitochondrial Ca2+ entry is regulated via the activity of a Ca2+-selective channel known as the mitochondrial calcium uniporter. The last decade has seen enormous momentum in the discovery of the molecular identities of the multiple proteins comprising the uniporter. Increasing numbers of studies in cultured cells and animal models have provided insight into how disruption of uniporter proteins affects mitochondrial Ca2+ regulation and impacts tissue function and physiology. This review aims to summarize some of these recent findings, particularly in the context of the heart.
Introduction
The body relies on the heart to pump blood and provide energy to the body, and this process consumes large amounts of ATP. To meet the energy demands of the heart, cardiomyocytes rely on mitochondria [1], which must constantly generate ATP for the heart even when the body is at rest. When the body needs to move, for instance to enable animals to chase prey and escape predators, even more energy is needed. The entry of Ca2+ into the mitochondria is thought to play a key role in signaling the mitochondria to increase ATP production [2]. Ca2+ has been shown to activate multiple matrix dehydrogenases and increase the flux through the Krebs cycle [3], as well as stimulate the electron transport chain including the ATP synthase [4,5], thereby matching ATP output to demand [6,7]. Thus the regulation of mitochondrial Ca2+ uptake is critical for cardiac and organismal physiology. Moreover, an excessive increase in matrix Ca2+ contributes to pathological events, including the opening of the mitochondrial permeability transition pore (PTP), which can lead to cell death [8]. Indeed, Ca2+-mediated PTP opening is thought to be an important step leading to injury after cardiac ischemia/reperfusion (I/R) [9].
Mitochondrial Ca2+ uptake is regulated by a Ca2+-selective channel in the inner mitochondrial membrane known as the mitochondrial calcium uniporter [10,11]. Many properties of the uniporter were mapped out starting in the 1960’s [12]: mitochondrial Ca2+ uptake was found to be an energy driven process [13], occur in an electrogenic manner [14], and be inhibitable by ruthenium red [15]. Furthermore, the uniporter was shown to be highly selective for Ca2+, binding it with high affinity [16]. The uniporter’s identity, however, remained elusive until 2011, when two groups were able to definitively identify the previously unstudied protein CCDC109A as the bona fide mitochondrial Ca2+ channel and renamed it Mitochondrial Calcium Uniporter (MCU) [17,18]. This discovery opened up the doors to molecular and genetic manipulation of the uniporter in attempts to test its physiological function and significance.
Uniporter structure
MCU contains two transmembrane domains spanning the inner mitochondrial membrane [17,18], oriented with its N- and C-termini inside the matrix [19]. The N-terminal domain (NTD) of MCU was found in two studies using x-ray crystallography to contribute toward modulation of Ca2+ uptake rates [20,21]. The Ca2+ channel is formed by oligomers of MCU, and structural studies have made substantial progress in elucidating the structure of MCU in a variety of organisms. Although an early study using electron microscopy and nuclear resonance on Caenorhabditis elegans MCU reported a five-fold symmetric pentameric structure [22], MCU has more recently been thoroughly characterized by several groups to adopt a tetrameric configuration. Several of these used cryo-electron microscopy (cryo-EM) on MCU from various fungi, including Neurospora crassa [23], Neosartorya fischeri [24], Cyphellophora europaea [25], Fusarium graminearum [26], and Metarhizium acridum, the last of which was also analyzed by x-ray crystallography [26]. These studies all agreed that MCU subunits assemble with tetrameric architecture. One among them also reported a cryo-EM structure from a metazoan organism, the zebrafish Danio rerio, and noted an interesting difference [25]. While fungal and metazoan MCU complexes similarly display four-fold symmetry in the transmembrane domain, the NTD within the mitochondrial matrix assembles as a dimer-of-dimers with two-fold symmetry in fungi [23–26] but not in zebrafish. Instead, the zebrafish structure showed an asymmetric arrangement of the four NTDs, forming a crescent shape to one side of the channel [25]. This side-by-side configuration was corroborated by a more recent structure of human MCU, and was proposed to mediate the dimerization of two MCU channels [27].
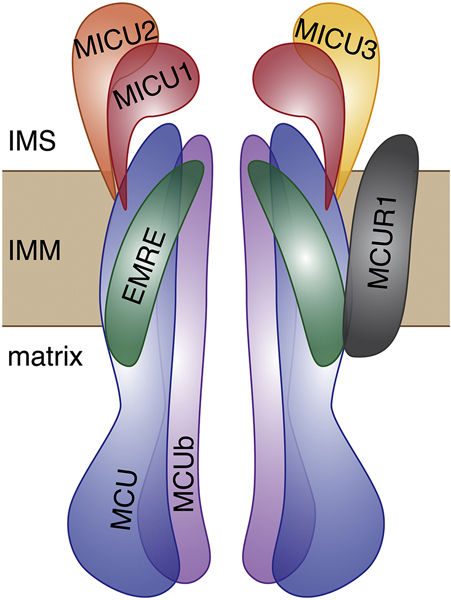
MCU does not act in isolation but as part of a multi-protein complex that collectively comprises the uniporter complex (Figure 1) [28]. In addition to MCU, a number of associated proteins impart the uniporter with its biochemical properties, and the expression of these subunits appears to be variable depending on tissue of origin in datasets from mouse [29] and human [30]. Hence, one might expect that the properties of mitochondrial Ca2+ uptake could vary in different tissues as a function of the uniporter’s composition, and it is important to consider how each component contributes to the activity of the complex as a whole. Among the uniporter subunits that also are transmembrane proteins are MCUb, MCUR1, and EMRE; other uniporter components in the MICU family reside in the intermembrane space. MCUb is an MCU homolog with close sequence similarity and was found to form heterooligomers with MCU and inhibit Ca2+ uptake in lipid bilayers [31]. In the heart, the ratio of MCUb to MCU mRNA expression is quite high relative to other tissues [30,31]. Another protein, MCUR1, has been found in some studies to bind to MCU and EMRE but not MCUb and affect mitochondrial Ca2+ uptake function and uniporter assembly [32–34], though its inclusion in the uniporter complex has been disputed [35]. Moreover, MCU was suggested to exist in a complex with either MCUR1 or MICU1, but not both simultaneously [32]. The final transmembrane protein known to bind to MCU is EMRE, which was found to be essential for uniporter function in cell culture [29,36]. Since its emergence in the metazoan lineage, EMRE has been conserved, and when co-expressed with MCU the two together form the minimal metazoan uniporter which confers mitochondrial Ca2+ uptake ability to the uniporter-lacking Saccharomyces cerevisiae [37,38]. The recent cryo-EM structure of human MCU was the first to show MCU in complex with EMRE [27], and resolved controversy over EMRE’s orientation [36,38,39] by demonstrating that EMRE is a single transmembrane protein oriented with its N-terminus in the matrix. Four EMRE subunits were shown to bind the MCU tetramer, with contact points between each EMRE and its two MCU neighbors [27]. The binding of EMRE to MCU was demonstrated to stabilize the pore exit in an open configuration such that Ca2+ can pass through. Because the metazoan uniporter appears to have evolved to require EMRE for MCU function, one might conjecture that EMRE exists for the purpose of conferring some regulatory advantage to the uniporter under as yet unclear conditions.
Figure 1.
Cartoon diagram of the constituent proteins of the mitochondrial Ca2+ uniporter. Two MCU channel-forming proteins in the inner mitochondrial membrane (IMM) are shown in blue, along with two MCUb homologs in purple. Structural studies have shown MCU to form tetramers [23–26], and MCUb is thought to be able to substitute for MCU [31,81]. EMRE, a single-pass membrane protein that is thought to act as a scaffold for the complex [29,36], has recently been shown to bind 1:1 with MCU so that the juxtamembrane loop of MCU at the pore exit is stabilized in an open conformation, allowing the passage of Ca2+ [27]. MICU1, MICU2, and MICU3, the EF-hand proteins in the intermembrane space (IMS), are shown in red, orange, and yellow, respectively. The exact stoichiometry of these subunits is not definitively known and varies in different tissues [30,37,89]. They are reported to form homodimers as well as heterodimers, and their oligomerization state seems to depend on Ca2+ binding and can affect pore opening. The transmembrane protein MCUR1 is shown in gray; it is reported to bind to MCU and EMRE, although it does not appear to coexist in MCU complexes simultaneously with MICU1 [32].
The uniporter also includes a family of Ca2+-binding, EF-hand-containing proteins generally thought to localize to the intermembrane space and regulate mitochondrial Ca2+ uptake rates. Three paralogous MICU family members are known to exist in mice and humans, with differential expression across tissues [30,40]. MICU1 was the first uniporter protein to be identified [41] and is among the best studied, with the majority of studies agreeing that it both inhibits uniporter activity at low cytosolic Ca2+ concentrations and enhances uniporter activity at higher, above-threshold cytosolic Ca2+ levels [41–44]. Both MICU2 and MICU3 are thought to also function as activators of mitochondrial Ca2+ uptake and contribute to the cooperativity in Ca2+-binding exhibited by the uniporter under conditions of high cytosolic Ca2+ [30,40,44]. MICU2 is expressed and has been studied in the heart [45], while MICU3 thus far has been thought to be primarily found in neuronal tissues [30,40]. Crystal structures of MICU1 [46,47], MICU2 [48–50], and MICU3 [50] have recently been solved, providing critical insight into their ability to reorganize their oligomeric states when bound to Ca2+.
Controversy has emerged recently over a particular behavior of the uniporter for which MICU1 was given much credit. A wide body of literature has demonstrated that mitochondrial Ca2+ uptake is dependent on cytosolic Ca2+ levels [51–53] and that the uniporter opens only when cytosolic Ca2+ is elevated above a threshold concentration of ∼500 nM in rat cardiomyocytes [52]. In the heart, such concentrations would occur when release of Ca2+ from the sarcoplasmic reticulum (SR) forms localized Ca2+ microdomains adjacent to mitochondria [54]. The ability of the uniporter to remain closed at below-threshold cytosolic Ca2+ has been widely attributed to MICU1, often referred to as the gatekeeper of the uniporter [42,43,55]. However, this paradigm has come under scrutiny, as a recent study has provided evidence in the heart and skeletal muscle that a cytosolic Ca2+ threshold below which MCU is inactive cannot be detected [56]. Another study has suggested using patch-clamp analysis that MICU1 deletion does not affect the uniporter current and that the pore is constitutively active [57]. The lack of detectable Ca2+ uptake at low extramitochondrial Ca2+ seen in isolated mitochondria and cells is proposed to be due to altered activity of NCLX [57], the mitochondrial sodium-calcium exchanger that is a primary efflux pathway for matrix Ca2+ [58]. Currently, further data supporting these claims are slim. Despite a lone earlier study suggesting a matrix localization for MICU1 [59], that group nonetheless agrees that MICU1 acts as a gatekeeper [42,60]. Mounting evidence for non-uniporter roles for MICU1 [61–63] is not incompatible with a concurrent role in uniporter regulation. Ultimately, further evidence in a variety of experimental conditions will be needed to overturn the prevailing view on the existence of a cytosolic Ca2+ threshold for the uniporter and MICU1’s role therein.
Animal models of MCU deletion
To gain a more complete understanding of the physiological role of the uniporter, researchers have sought to delete MCU in model organisms. Recently, the genes encoding various uniporter subunit proteins were deleted in Drosophila melanogaster, with the unexpected result that introducing a null MCU mutation led to only a mild phenotype, despite eliminating mitochondrial Ca2+ uptake [63]. Flies lacking MCU were viable and developed to adult stages in expected Mendelian ratios. However, mitochondrial respiration rates were reduced, as was lifespan. Unexpectedly, MICU1 deletion was lethal before fly larvae reached the adult stage, and this phenotype was not rescued by MCU or EMRE deletion, suggesting a possible uniporter-independent role for MICU1 at least in flies.
Two independent models of MCU-deficient zebrafish have observed differing effects. One group found that silencing MCU via morpholino led to developmental alterations including notochord deviation and anteroposterior axis reduction that were present already at 26 hours post fertilization [64]. Another set of experiments undertaking a similar morpholino-induced knockdown of MCU in zebrafish noted no overt developmental or phenotypic effects until 5 days post fertilization, attributing this result to differences in background strain [65]. So while this evidence does not directly address the relevance of MCU in the heart specifically, it does suggest that MCU deletion has variable penetrance in physiological impact depending on at least in part on genetic background.
Genetic background also plays a role in modulating the effect of MCU loss in mice. The first characterization of MCU deletion in a mouse model used a gene-trap method to create a global germline MCU knockout (hereafter referred to as germline Mcu−/−) [66]. In an outbred, CD-1 background, mice lacking MCU were viable. Even so, fewer Mcu−/− mice than expected by Mendelian genetics were born; of the pups born from heterozygous breeder pairs, only 12–15% were Mcu−/− rather than the expected 25% [67]. These data strongly suggest that MCU deletion is deleterious during embryonic development, but with enough genetic diversity, some genetic backgrounds can tolerate MCU deletion without succumbing to lethality.
In terms of overall phenotype, the germline Mcu−/− mice were slightly smaller than WT counterparts, and the weights of organs including the heart were correspondingly smaller, proportional to body size [66]. Unexpectedly, despite a complete lack of Ca2+ uptake ability, Mcu−/− mitochondria exhibited normal oxygen consumption. Mcu−/− and WT MEFs were also found to have similar baseline and maximal oxidative capacity. Differences in skeletal muscle in matrix total calcium and levels of phosphorylation of the Ca2+-sensitive matrix enzyme pyruvate dehydrogenase (PDH) were only detected when mice were first fasted for 16 hours. MCU deletion did have an effect on skeletal muscle performance, reducing the maximal work performed on an inclined treadmill. Mcu−/− mice also displayed a small but significant decrease in grip strength, which was more pronounced in a modified vertical pull up test requiring concentric muscle contraction and entailing added weight at the base of the mouse’s tail. Thus, it was only under conditions of strenuous physical activity that impairment due to MCU deletion could be elicited.
In a follow-up study focused on the role of MCU in cardiac function, the germline Mcu−/− mice did in fact show reduced levels of basal matrix total calcium in the heart [68]. However, no significant changes were observed in Mcu−/− mice relative to WT in left or right ventricular ejection fraction or volume, at either 12 months or 20 months of age. To assess whether MCU plays a role in modulating heart function under increased workload, miniature pressure volume Millar catheters were used to measure cardiac output at baseline and following isoproterenol injection. WT and Mcu−/− mice showed no difference at baseline. Following isoproterenol, WT mice displayed increases in cardiac output, heart rate, and maximal dP/dt that were significantly different from baseline, but the increases in Mcu−/− mice did not reach statistical significance. However, the isoproterenol-stimulated responses in WT and Mcu−/− mice were not significantly different from each other. Thus the cardiac phenotype, if any, with loss of MCU was unexpectedly subtle, suggesting that perhaps mitochondrial Ca2+ uptake may not be as crucial for physiological cardiac responses as the literature had posited.
Another transgenic mouse model with disrupted MCU function was developed using Cre recombinase under the cardiomyocyte-specific α-myosin heavy chain promoter (αMHC-Cre) to drive the expression of a dominant-negative (DN) MCU with pore domain mutations. Like the germline Mcu−/− mice, these DN-Mcu mice were also bred in the CD1 outbred background, but in contrast were observed at Mendelian ratios [69]. This may suggest that loss of uniporter function during embryogenesis is better tolerated in the heart than in other tissues; however, the αMHC promoter becomes transcriptionally active shortly after birth [70], and recombination has been verified only as early as a few weeks after birth [71]. Thus it cannot be excluded that MCU could play a role in the developing heart prior to DN-MCU expression.
Like the global MCU−/− mice, the DN-Mcu mice also showed no differences from WT mice in baseline heart rate [69,72] or ejection fraction [69]. Cardiomyocytes from DN-Mcu mice, like those from Mcu−/− mice, did not display mitochondrial Ca2+ uptake activity [69,72]. Furthermore, an increase in the phosphorylation of PDH and decrease in PDH activity in DN-Mcu hearts suggested that basal levels of mitochondrial Ca2+ were lower than in WT [69], consistent with Ca2+ measurements in Mcu−/− hearts [68]. However, after isoproterenol injection, DN-Mcu mice displayed blunted increases in maximal dP/dt [69] and heart rate [72] compared to WT mice. Using telemetry to monitor heart rate in unrestrained and unsedated mice, similar findings were found upon activity [72]. Hence the data suggest that MCU activity has little effect on basal heart rate but is essential for fight or flight rate increases. This discrepancy between DN-Mcu mice and MCU−/− mice might be attributed to various differences in experimental conditions or perhaps to the contrast between loss of MCU protein which presumably destabilizes the complex and over-expression of non-functional MCU which might still bind other uniporter components.
One drawback to the use of germline knockout mice is that the gene deletion is chronic. In particular, because most Mcu−/− embryos appear to be unviable [67], the limited number of mice that do survive likely represent a fraction of the population that have undergone adaptation to the chronic absence of MCU. Thus, importantly, a few studies have examined the effects of acute MCU deletion in the heart [73,74]. In these studies, the evidence supported a number of the predictions regarding the importance of MCU in modulating energy production and mediating cell death. By crossing conditional Mcufl/fl mice with mice expressing the tamoxifen-inducible Cre recombinase driven by the α-MHC promoter (αMHC-MerCreMer), mice (hereafter Mcufl/fl-MCM) were generated to which tamoxifen could be administered via feed or injection to knock out MCU in the heart in adulthood [73,74]. Mitochondrial Ca2+ uptake was inhibited, as expected, but basal levels of mitochondrial total calcium [73] and free Ca2+ [73,74] appeared unaffected in Mcufl/fl-MCM hearts, unlike in the two germline models [68,69]. As excision by Cre was less than 100% complete, it cannot be entirely excluded that in adult hearts residual MCU might be enough to maintain matrix Ca2+ levels and presumably normal bioenergetic status at rest. The Mcufl/fl-MCM hearts showed no difference at baseline but strikingly were significantly impaired relative to WT in increasing LV contractility (maximal dP/dt) in response to either dobutamine [73] or isoproterenol [74]. A blunted response to isoproterenol was also observed in DN-Mcu mice in terms of heart rate [72]; in contrast, the Mcufl/fl-MCM mice did not exhibit significant differences in heart rate from controls pre- or post-isoproterenol [74]. Concurrently, mitochondrial total calcium appeared to be increased by short-term dobutamine treatment in control but not Mcufl/fl-MCM hearts [73], presumably because mitochondria without MCU were deficient in Ca2+ uptake. Interestingly, with sustained administration of dobutamine, mitochondrial total calcium levels in Mcufl/fl-MCM hearts eventually caught up to control levels [73]. Consistently with these data, sustained dobutamine also eventually led to indistinguishable maximal dP/dt values in Mcufl/fl-MCM and control mice. Moreover, Mcufl/fl-MCM mice overcame impairment relative to WT mice in treadmill performance when given a 30-minute warm-up period [73]. Hence, acute MCU deletion appeared to affect the cardiac response to adrenergic stimulation only in the short term, and another uniporter-independent pathway or pathways sufficed to restore long-term Ca2+ responses. These data suggest that while MCU is dispensable in homeostatic conditions, it is required for the “fight-or-flight” response.
The prediction that MCU loss would prevent the uptake of Ca2+ into mitochondria and thereby prevent the opening of the mPTP was also supported by acute but not chronic deletion of MCU. While pathological consequences of uniporter disruption will be discussed in depth in another review in this issue, it is worth noting here that a discrepancy between inducible and germline knockout of MCU manifested also in I/R injury. The global knockout model first reported the surprising result that Mcu−/− mice did not exhibit improved recovery or reduced infarct size post-I/R in a Langendorff ex vivo I/R model, and in fact while the WT mice were protected by cyclosporine A, the Mcu−/− mice were not [66]. Similarly, no decrease in infarct area in a similar I/R model was observed in DN-Mcu mice [69]. However, both studies using Mcufl/fl-MCM mice found that acute MCU deletion protected mice from I/R injury, using an in vivo model of I/R consisting of left descending coronary artery (LAD) ligation for 40 or 60 minutes and reperfusion for 24 hours [73,74]. Despite disparate experimental techniques, it appears that chronic versus acute loss of MCU in the heart can lead to functional differences. Taken together, the various mouse models of MCU deletion have thus far illuminated nuances in how the heart or whole animal responds to germline or adult-onset MCU deletion (Figure 2), spurring further research into the identification of compensatory pathways that impact cardiac function not only in normal physiology but also pathological conditions.
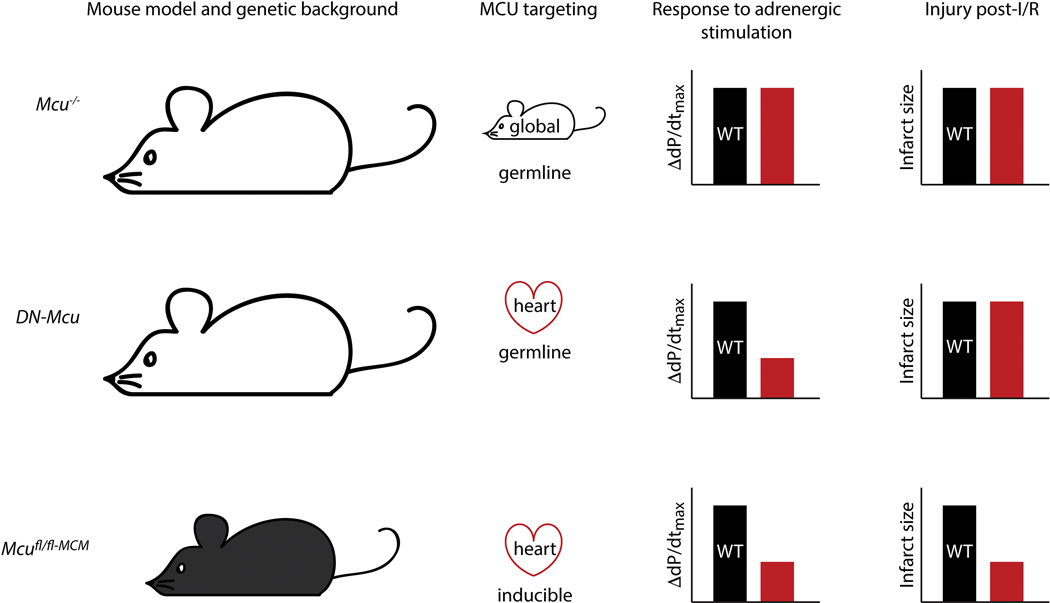
Figure 2.
A pictorial summary of several mouse models with MCU-null hearts. Mcu−/− [66,68], DN-Mcu [69,72], and Mcufl/fl-MCM [73,74] mice differ in terms of strain, with the former two raised in the CD-1 background (larger, white mice) and the latter in the C57BL6 background (smaller, black mice). The methods by which MCU was targeted are shown in the second column: global and germline for Mcu−/− [66]; heart-specific and germline for DN-Mcu [69,72]; heart-specific and tamoxifen inducible for Mcufl/fl-MCM [73,74]. The third column represents the response to isoproterenol stimulation in terms of increase in maximal dP/dt [68,69,74]. Note: Increase in heart rate also follows the same trend in the Mcu−/− [68] and the DN-Mcu [72] mice, and dobutamine stimulation in Mcufl/fl-MCM mice has a similar effect as isoproterenol [73]. The final column depicts the degree of injury, i.e. infarct size, after ischemia/reperfusion (I/R). Experiments were performed on ex vivo Langendorff-perfused hearts from the Mcu−/− [66] and DN-Mcu mice [69], and via in vivo LAD ligation in the Mcufl/fl-MCM mice [73,74]. Black bars represent WT mice, and red bars represent the MCU-null mice relative to WT.
MCU regulation of substrate selection
Although the requirement for uniporter activity in regulating increases in contractility is still somewhat ambiguous, some studies suggest that the presence or absence of MCU may play a role in the choice of metabolic substrates in the mitochondria. Mitochondrial Ca2+ uptake affects metabolism both in the short term and long term. On time scales as short as minutes, dynamic changes in matrix Ca2+ can rapidly stimulate the Krebs cycle by activating PDH, α-ketoglutarate dehydrogenase, and isocitrate dehydrogenase [6]. Indeed, isoproterenol stimulation of Mcufl/fl-MCM mice resulted in higher PDH phosphorylation levels and decreased PDH activity relative to controls [74]. No differences in PDH phosphorylation was observed at baseline [73,74]. A recent study distinguished between substrates that enabled mitochondrial Ca2+-sensitive ATP production (including glutamate and pyruvate), and those that did not (including lipids) [56]. Thus, rapid mitochondrial Ca2+ uptake plays a role in determining the utilization of particular substrates.
The longer-term consequences of inhibiting mitochondrial Ca2+ uptake through MCU deletion likely involve systemic changes to metabolism, comparable to what might occur in a sustained stressed or diseased state. Several studies in skeletal and cardiac muscle have investigated the molecular remodeling of mitochondrial and other cellular metabolic pathways due to loss of MCU. In skeletal muscle, both silencing of MCU via adeno-associated virus infection [75] and skeletal muscle-specific MCU deletion [76] were found to elevate basal phosphorylation levels of PDH. In the latter study, the Mcufl/fl-Mlc1f-Cre mice showed concurrent increases in blood lactate and glycolysis rate in muscle fibers [76]. Furthermore, in Mcufl/fl-Mlc1f-Cre fibers 80% of basal respiration was dependent on fatty acid oxidation, compared to 40% in control fibers. This switch toward increased fatty acid oxidation could be recapitulated by overexpression of PDH kinase 4 to increase PDH phosphorylation in control muscle fibers, and likewise overexpression of the PDH phosphatase PDP2 in Mcufl/fl-Mlc1f-Cre fibers to decrease PDH phosphorylation negated this metabolic switch [76]. Thus, the impairment of oxidative metabolism and increased reliance on fatty acid oxidation due to MCU deletion occurs via decreasing PDH activity. Moreover, these metabolic changes were accompanied by broad changes in gene expression.
Another study using an independent mouse model of skeletal muscle-specific MCU deletion, Mcufl/fl-MyoD-Cre, also found higher basal PDH phosphorylation and decreased PDH activity [77]. After running to exhaustion, by which point Mcufl/fl-MyoD-Cre and control mice relied fully on glucose, the Mcufl/fl-MyoD-Cre mice exhibited a faster shift toward fatty acid oxidation. Moreover, Mcufl/fl-MyoD-Cre muscle fibers demonstrated increased rates of respiration with fatty acids as substrates [77]. Overall, evidence suggests that loss of MCU in muscle results in preferential fatty acid metabolism. It is worth noting that these studies agree on this rewiring of metabolic substrate selection despite disagreeing in other areas, such as the role of the uniporter in regulating muscle size [75,76][77]. Thus, it seems that the role of the uniporter in determining mitochondrial substrate usage is an area of relatively greater consensus, at least in skeletal muscle.
How loss of the uniporter impacts metabolite selection in cardiac muscle is less clear. Surprisingly, isolated perfused hearts from DN-Mcu mice were shown to have higher oxygen consumption rates than wild-type controls, which was suggested to imply that DN-Mcu hearts were less efficient [69]. Although differences in glucose metabolite measurements were below the detection limit by NMR, evidence was shown for widespread gene expression changes in DN-Mcu hearts [69]. Thus, perturbation of uniporter function is associated with extensive transcriptional reprogramming that could provide an underlying mechanism for altered metabolic homeostasis.
More recently, a study using the tamoxifen-inducible cardiac-specific Mcufl/fl-MCM mouse model assessed cardiac function in an ex vivo perfusion system while measuring rates of glucose and palmitate oxidation via radioisotope tracing [78]. In basal conditions MCU deletion appeared not to affect fatty acid or glucose oxidation, but upon insulin addition Mcufl/fl-MCM hearts showed enhanced glucose oxidation to a greater extent than the increase in control hearts [78]. This suggests that PDH activity, which is rate-limiting in glucose oxidation, was not decreased in this context despite MCU loss. Notably, treatment with isoproterenol led to similar gains in glucose oxidation rate in Mcufl/fl-MCM and control hearts, but the rate of fatty acid oxidation was higher in Mcufl/fl-MCM hearts than in control hearts [78]. Because the Mcufl/fl-MCM hearts unexpectedly showed enhanced cardiac work at baseline and after isoproterenol, the study inferred that MCU deletion led to increased energy production and reserve, more heavily relying on fatty acid oxidation during acute adrenergic stress. This shift in substrate usage under conditions of stress is consistent with the afore-mentioned increase in fatty acid oxidation upon exercise to exhaustion in Mcufl/fl-MyoD-Cre mice, in which skeletal muscle lacking MCU was indeed observed to be less susceptible to fatigue [77]. However, the lack of MCU-dependent effects on glucose oxidation differed from an earlier study in neonatal cardiomyocytes. In these cells, simulated hyperglycemia reduced MCU gene expression and protein levels by half, concurrent with increased PDH phosphorylation, reduced glucose oxidation, and increased fatty acid oxidation [79]. Thus, despite some discrepancies in glucose oxidation changes, the evidence largely suggests that similarly to skeletal muscle, the heart becomes more reliant on fatty acid oxidation to produce energy in the absence of MCU. The molecular mechanisms underlying this remodeling of substrate preference and how they might be implicated in disease demand further study.
Other uniporter proteins in the heart
As aforementioned, activity of the uniporter depends not only on MCU but also on the regulatory subunits of the complex. Several mouse models examining the roles of other uniporter proteins in the heart have shed light on the roles of their contributions to uniporter function. For instance, the requirement for EMRE for mitochondrial Ca2+ uptake was recently verified in a mouse model of EMRE deletion [80]. Mitochondria from various tissues of global germline Emre−/− mice were unable to take up Ca2+, similarly to Mcu−/− mitochondria. Furthermore, Emre−/− mice were phenotypically similar in many ways to global Mcu−/− mice [66,68], manifesting reduced birth rate, decreased body weight, unimpaired cardiac response to isoproterenol, and no protection against I/R injury [80]. These data support an essential role for EMRE in uniporter function in vivo, not only in cell culture [29]. Moreover, muscle EMRE expression was strongly increased in a muscular dystrophy mouse model [80], suggesting that EMRE potentially contributes to modulation of the uniporter in conditions of stress or disease. Whether EMRE plays such a role in the heart is still unknown.
A recent study highlighted the ability of MCUb to alter uniporter stoichiometry and uptake kinetics [81], using knockout cells and a transgenic mouse overexpressing MCUb conditionally in the heart via the tamoxifen-inducible αMHC-MerCreMer. The transgenic mice exhibited reduced rates of mitochondrial Ca2+ uptake, consistent with cell culture data showing that MCUb is a negative regulator of the uniporter. Overexpression of MCUb allowed it to displace MCU, reducing MICU1 and MICU2 incorporation into the uniporter complex, thus preventing activation of Ca2+ uptake. In wild-type mouse hearts, despite previous data showing high mRNA expression [31], MCUb protein was not observed in the uniporter complex unless a stress such as I/R injury was applied [81], and correspondingly, a striking phenotypic consequence of MCUb overexpression was that it was protective against I/R injury. These data suggest that MCUb is critically involved in cardiac Ca2+ handling during stress and injury. However, it appears that under normal physiological conditions MCUb does not actively contribute to uniporter regulation in the heart.
The role of MICU2 in the heart has also been studied, based on bioinformatic screening data that showed elevation of MICU2 expression in human and mouse cardiomyopathy datasets [45]. A global germline Micu2−/− mouse was generated but was not observed to suffer from decrease in birth rate, size, or activity level. While overall cardiac histology was similar, mitochondria were 20% smaller in Micu2−/− hearts than in WT, suggesting that perhaps uniporter activity plays a role in mitochondrial morphology. In Micu2−/− mice, the development of left atrial enlargement was observed at 16 to 18 months of age, suggesting diastolic dysfunction. The Micu2−/− mice were then discovered to be susceptible to angiotensin II-induced decreases in fractional shortening as well as lethality due to aortic aneurysms. Thus, as in the case of MCUb, the role of MICU2 in the heart is more apparent under conditions of stress, including aging.
Somewhat surprisingly given the abundant literature on MICU1 in cell culture [41–44], relatively little is known about MICU1 in the heart specifically. Two independent studies have shown that whole-body knockout of MICU1 leads to complete or nearly complete postnatal lethality. Micu1−/− mice generated by breeding conditional Micu1fl/fl mice in the C57BL/6J background to germline eIIα-Cre mice uniformly died within several hours after birth [82]. However, at E18.5, Micu1−/− pups were shown by fetal echocardiography to have normal cardiac function, and no noticeable abnormalities in the heart were revealed by histology. In the second study, CRISPR-mediated global knockout of MICU1 in C57BL/6N mice resulted in postnatal lethality for nearly 6 out of 7 pups, and the few surviving Micu1−/− mice were notably smaller and weaker than WT littermates [55]. They also exhibited neurological and myopathic symptoms that were reminiscent of human patients with loss-of-function MICU1 mutations [83]. Overt cardiac defects were not noted to be a cause of impairment in these Micu1−/− mice, and in fact none of the 15 subjects in the human cohort were observed to have cardiomyopathy. Thus MICU1 or lack thereof appears to have less of an effect on physiological function in the heart compared to other tissues such as the nervous system and skeletal muscle. It deserves mentioning that the pathology of MICU1 loss was linked to mitochondrial Ca2+ overload [55,82,83], which could be largely rescued by reduced EMRE expression [55]. Given that elevated matrix Ca2+ contributes to PTP opening, it is not surprising that a study found that a roughly 50% decrease in MICU1 mediated by intramyocardial injection of siRNA was sufficient to significantly exacerbate I/R injury [84]. Taken together, normal heart function seems fairly robust to perturbations of the regulatory proteins of the uniporter, at least so long as MCU itself is present, and only under conditions of stress or disease do changes in mitochondrial Ca2+ kinetics significantly alter cardiac outcomes.
A role for MCU in development
The lower-than-expected birth rate for outbred CD-1 germline Mcu−/− was mentioned above [66], and in fact global MCU deletion is embryonic lethal in the inbred C57BL/6 background [67]. Because outbred mice by definition are genetically heterogeneous, one might speculate a small fraction of CD-1 embryos by chance are predisposed to tolerate the absence of MCU, resulting in reduced birth rate, whereas embryos in the genetically identical C57BL/6 background that lack MCU are uniformly nonviable. Since MCU deletion impacts birth rate in both backgrounds, mitochondrial Ca2+ uptake potentially plays an important role during development and tissue growth. A recent study has sought to explain how loss of MCU poses obstacles in embryogenesis. Systematic analysis of genotypes in many C57BL/6 litters revealed that Mcu−/− mice represented around 26.5% of embryos present at E9.5–10.5. However, the proportion of Mcu−/− embryos decreased between E11.5 and E13.5, such that no Mcu−/− pups were observed after E14.5 [85]. The percentage of TUNEL-positive, apoptotic cells was increased more than 5-fold relative to WT in Mcu−/− livers at E12.5. Though it is unclear that apoptosis in the liver is the precise cause of lethality in Mcu−/− embryos, it could conceivably contribute. Mitosis, a cellular process with high energy demands, was found to require a rapid mitochondrial Ca2+ transient to boost cellular ATP levels before anaphase onset. Depletion of MCU inhibited the mitochondrial Ca2+ transient, delaying the rise in ATP and delaying mitotic progression [85]. Though these processes were characterized in cultured HeLa cells, uniporter activity would likely also be important for cell proliferation and tissue growth in embryonic development.
Another study also demonstrated a role for MCU in meeting energy demand during the cell cycle, this time in regulating the G1-S transition [86]. MCU deletion was demonstrated in vivo to impair cellular proliferation; after a skin punch biopsy, wound healing was slower in Mcu−/− mice than in WT. In cultured vascular smooth muscle cells (VSMCs) transfected with MCU siRNA as well as in skin fibroblasts from Mcu−/− mice, slower cell growth was observed, corresponding to a delay in cell cycle progression through S phase. Entry into S phase coincided with increased mitochondrial Ca2+ uptake, increased oxygen consumption rate, and mitochondrial fusion, all of which were disrupted in Mcu−/− fibroblasts. These data suggest that without uniporter activity to coordinate ATP production and mitochondrial dynamics, cell division and thus tissue development would be unable to proceed normally.
While the uniporter clearly has roles in cell division in multiple cell types, whether it is essential for heart development is not entirely clear. To give rise to the heart, the first organ to form and function in embryogenesis, cardiac precursors at ∼E7.75 begin to display Ca2+ transients that are essential for differentiation into cardiomyocytes [87]. Global MCU loss led to embryonic lethality at around E11.5 [67,85], but does not provide evidence that MCU is required in early heart development specifically. Studies have shown that mice with constitutive heart-specific disruption of MCU via αMHC-Cre are viable [69,74], but as mentioned above αMHC-Cre expression does not occur early enough to capture what might be a critical period in cardiomyocyte differentiation [70,71]. To delete MCU in the heart during its formation, Cre would need to be driven by a developmental gene expressed in cardiac progenitor cells, such as the homeobox gene Nkx2.5 [88]. Nkx2.5-Cre expression begins ∼E7.5 [71], which likely would ensure MCU deletion early enough to determine its effects on heart development. Without such an experiment, resolving whether the heart requires MCU to form and function during embryogenesis remains difficult.
Thus, MCU might plausibly be dispensable for the developing heart, and indeed in the Mcu−/− embryos, TUNEL staining was observed predominantly in the liver [85]. One possible explanation might be that uniporter stoichiometry is distinct among different tissues, including heart and liver. The heart has a lower MICU1 to MCU ratio, in contrast to the liver where the ratio is much higher [89]. These differences were shown to lead to disparate Ca2+ handling, with a higher cytosolic Ca2+ threshold for liver but also faster uptake and increased maximal uniporter activity once above threshold. Other studies also revealed a diminished Ca2+ response for the uniporter in cardiac mitochondria. When Ca2+ flux was measured on mitoplasts isolated from various mouse tissues using a patch-clamp technique, heart mitoplasts unexpectedly had by far the lowest current density compared to mitoplasts from the other tissues studied (liver, kidney, brown fat, and skeletal muscle) [90]. While the biophysical properties of the current were consistent between skeletal muscle and heart, confirming that the same channel was being studied, the heart current was as much as 30 times smaller than the skeletal muscle current. Collectively, these data suggest that relatively weak cardiac uniporter activity might be a plausible reason that loss of MCU seems less tolerated in the liver, for instance, than in the heart.
It should be kept in mind, however, that mitochondria occupy a large percentage of total cell volume in the heart, up to 37% in mice [91,92]. In skeletal muscle, for comparison, mitochondria comprise only ∼4% of cell volume in glycolytic fibers to ∼10% in oxidative fibers [92]. Furthermore, in cardiac muscle, uniporter complexes are enriched at the mitochondrial periphery, close to mitochondria-junctional SR interfaces [93] and away from NCLX [94], showing that Ca2+ uptake and extrusion are distributed to optimize energy efficiency and signal generation. Hence, with larger volumes of mitochondria, strategic uniporter spatial positioning, as well as continually occurring cytosolic transients, cardiac muscle likely employs mechanisms to protect itself against mitochondrial Ca2+ overload. Thus, it might be that each uniporter complex in the heart is constrained to be active only in a narrow physiological range.
Interestingly, however, the same analysis of current density in different tissues observed that currents in mitoplasts from neonatal hearts were roughly 5 times higher than those from adult hearts [90]. Cardiomyocytes undergo substantial changes as they mature in postnatal development [95], during which the mitochondrial reticulum develops [96] and mitochondrial volume expands from roughly 30% of cell volume in 3-day-old mouse hearts to nearly 40% in adults [97]. But a factor of five-fold lower MCU activity following postnatal development more than offsets this increase in mitochondrial volume. A similar ∼3.9-fold downregulation of MCU protein expression was found in mouse skeletal muscle between 1 and 42 days after birth [98]. Hence, high MCU activity might indeed be more important in the neonatal period or before, consistent with the findings that mitochondrial Ca2+ uptake is required to support active cell division [85,86].
Having learned that germline MCU deletion poses an obstacle to completing embryonic development but that acute knockout of MCU in adulthood has little effect on basal phenotype, we might speculate that perhaps mitochondrial Ca2+ uptake is critical during embryogenesis but decreases in importance as the animal progresses through development and reaches adulthood (Figure 3). Furthermore, it likely is no coincidence that MCU deletion causes embryonic lethality in inbred mice, but deletion of the gatekeeper MICU1 results in perinatal or postnatal lethality. Together these data suggest that mitochondrial Ca2+ uptake through the uniporter is required for embryonic development, but upon birth the adjustment to postnatal life requires appropriate regulation of the uniporter to prevent mitochondrial Ca2+ overload. This hypothesis may apply to a greater extent in other tissues than in the heart, but even in the heart the importance of keeping mitochondrial Ca2+ levels in check during adulthood is evident. Tamoxifen-induced heart-specific deletion in adult mice of NCLX led to mitochondrial Ca2+ overload, and 87% of male mice died within two weeks [99]. Hence one might speculate that the physiological importance of a functional uniporter might decline from the embryo to the adult, while regulation of mitochondrial Ca2+ to prevent overload becomes increasingly essential.
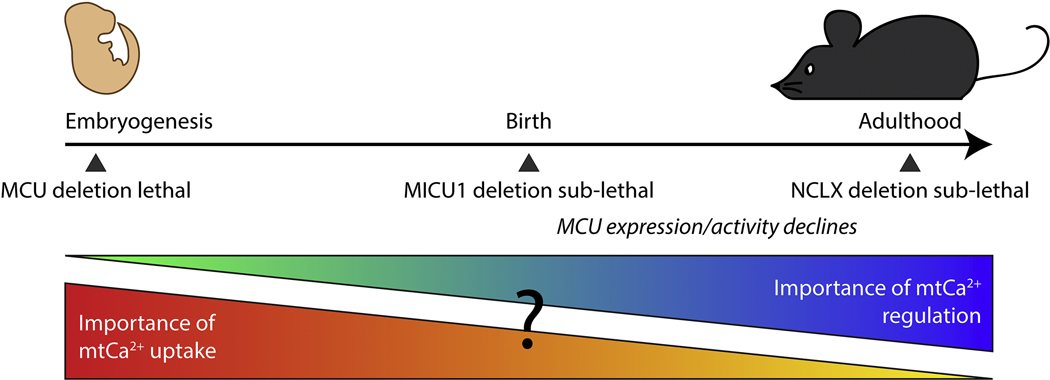
Figure 3.
Diagram illustrating a speculative estimate of the relative importance of mitochondrial Ca2+ uniporter activity versus regulation over a timeline of mouse development. Here, importance is used to denote essentiality to animal viability. In mouse embryogenesis, global MCU deletion is lethal in inbred mice [67], suggesting that uniporter activity is required during that period. After birth, a decline is seen in MCU current in the heart [90] and MCU expression in the skeletal muscle [98]. At birth, global MICU1 deletion, which has been shown to lead to mitochondrial Ca2+ overload, is near-lethal [55,82], and in adulthood heart-specific acute deletion of NCLX, which also leads to mitochondrial Ca2+ overload, is 87% lethal in males [99]. Together, these data suggest that tight regulation of the uniporter to prevent mitochondrial Ca2+ overload increases in importance postnatally.
Conclusions
Mice in which cardiac MCU was acutely deleted in adulthood were, as expected, unable to respond normally after acute adrenergic stimulation, implying that a sudden loss of hitherto functional uniporter activity disrupted the capacity to rapidly increase energy production to meet metabolic demand [73,74]. However, even acute deletion of MCU did not result in a marked deficit in heart function until the hearts were stimulated or stressed. MCU was required for flight-or-fight responses but dispensable for homeostatic cardiac function. Furthermore, Mcufl/fl-MCM animals were able to reach similar maximal rates of exercise if given a warm-up period, indicating that the uniporter is not the one and only pathway by which energy production in the heart can be stimulated.
Coming from a different angle, global deletion of MCU in the germline resulted in a surprisingly mild phenotype in the mice that were born [66]. The germline Mcu−/− model is not without limitations, and faces the criticism that because only a fraction of Mcu−/− mice are born, characterizing those survivors necessarily means analyzing animals in which compensatory pathways are likely activated, allowing deletion of MCU to be tolerated. But the very fact that such compensation can take place and enable mice to live with very little deficit, combined with the delayed but eventual response to stimulation in Mcufl/fl-MCM mice, highlights that we still have much to learn about how mitochondrial energy production can be regulated. Part of this adaptive pathway seems to involve reliance on fatty acids as alternative metabolic substrates, at least under increased workload. The mechanisms by which mitochondria might be able to circumvent MCU to restore response to energetic demand likely also have important roles in stress or disease conditions. Therefore, mapping out such pathways might provide clues toward potential unintended consequences of therapies designed to modulate uniporter activity or bioenergetics in cardiac disease.
Highlights.
The mitochondrial calcium uniporter is a multi-protein complex in the inner mitochondrial membrane regulating energy production
Loss of cardiac MCU function impairs the fight-or-flight response in some mouse models
MCU loss causes shifts in metabolic substrate selection
MCU potentially plays a role in cell division and tissue development, though not necessarily in the heart
Acknowledgements
I thank Dr. Elizabeth Murphy and Dr. Toren Finkel for helpful discussions and feedback. This work was supported by NIH grant 1K22HL137901.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kohlhaas M, Nickel AG, Maack C. Mitochondrial energetics and calcium coupling in the heart. J Physiol (Lond) 2017;595:3753–63. doi: 10.1113/JP273609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Williams GSB, Boyman L, Lederer WJ. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 2015;78:35–45. doi: 10.1016/j.yjmcc.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Denton RM, McCormack JG. The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans 1980;8:266–8. [DOI] [PubMed] [Google Scholar]
- [4].Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol, Cell Physiol 2000;278:C423–435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- [5].Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 2013;52:2793– 809. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological Reviews 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- [7].Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012;51:2959–73. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lemasters JJ, Theruvath TP, Zhong Z, Nieminen A-L. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 2009;1787:1395–401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, et al. The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem 2013;288:10750–8. doi: 10.1074/jbc.R112.420752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Finkel T, Menazza S, Holmström KM, Parks RJ, Liu J, Sun J, et al. The ins and outs of mitochondrial calcium. Circ Res 2015;116:1810–9. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA 1961;47:1744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 1962;237:2670–7. [PubMed] [Google Scholar]
- [14].Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. American Journal of Physiology-Cell Physiology 1990;258:C755–86. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- [15].Moore CL. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochemical and Biophysical Research Communications 1971;42:298–305. doi: 10.1016/0006-291X(71)90102-1. [DOI] [PubMed] [Google Scholar]
- [16].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004;427:360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- [17].Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011;476:341–5. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011;476:336–40. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol 2012;30:1143–8. doi: 10.1038/nbt.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee Y, Min CK, Kim TG, Song HK, Lim Y, Kim D, et al. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep 2015;16:1318–33. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee SK, Shanmughapriya S, Mok MCY, Dong Z, Tomar D, Carvalho E, et al. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chemical Biology 2016;23:1157–69. doi: 10.1016/j.chembiol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, et al. Architecture of the mitochondrial calcium uniporter. Nature 2016;533:269–73. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yoo J, Wu M, Yin Y, Herzik MA, Lander GC, Lee S-Y. Cryo-EM structure of a mitochondrial calcium uniporter. Science 2018;361:506–11. doi: 10.1126/science.aar4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nguyen NX, Armache J-P, Lee C, Yang Y, Zeng W, Mootha VK, et al. Cryo-EM structure of a fungal mitochondrial calcium uniporter. Nature 2018;559:570–4. doi: 10.1038/s41586-018-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature 2018;559:580–4. doi: 10.1038/s41586018-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, et al. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature 2018;559:575–9. doi: 10.1038/s41586-0180-330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Y, Nguyen NX, She J, Zeng W, Yang Y, Bai X, et al. Structural Mechanism of EMRE-Dependent Gating of the Human Mitochondrial Calcium Uniporter. Cell 2019;177:1252–1261.e13. doi: 10.1016/j.cell.2019.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu JC, Parks RJ, Liu J, Stares J, Rovira II, Murphy E, et al. The In Vivo Biology of the Mitochondrial Calcium Uniporter. Adv Exp Med Biol 2017;982:49–63. doi: 10.1007/978-3-319-55330-6_3. [DOI] [PubMed] [Google Scholar]
- [29].Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 2013;342:1379–82. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death & Differentiation 2019;26:179–95. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J 2013;32:2362–76. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 2012;14:1336–43. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vais H, Tanis JE, Müller M, Payne R, Mallilankaraman K, Foskett JK. MCUR1, CCDC90A, Is a Regulator of the Mitochondrial Calcium Uniporter. Cell Metab 2015;22:533–5. doi: 10.1016/j.cmet.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tomar D, Dong Z, Shanmughapriya S, Koch DA, Thomas T, Hoffman NE, et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep 2016;15:1673–85. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab 2015;21:109–16. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- [36].Tsai M-F, Phillips CB, Ranaghan M, Tsai C-W, Wu Y, Willliams C, et al. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife 2016;5. doi: 10.7554/eLife.15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kovacs-Bogdan E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, et al. Reconstitution of the mitochondrial calcium uniporter in yeast. Proceedings of the National Academy of Sciences 2014;111:8985–90. doi: 10.1073/pnas.1400514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yamamoto T, Yamagoshi R, Harada K, Kawano M, Minami N, Ido Y, et al. Analysis of the structure and function of EMRE in a yeast expression system. Biochim Biophys Acta 2016;1857:831–9. doi: 10.1016/j.bbabio.2016.03.019. [DOI] [PubMed] [Google Scholar]
- [39].Vais H, Mallilankaraman K, Mak D- OD, Hoff H, Payne R, Tanis JE, et al. EMRE Is a Matrix Ca 2+ Sensor that Governs Gatekeeping of the Mitochondrial Ca 2+ Uniporter. Cell Reports 2016;14:403–10. doi: 10.1016/j.celrep.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 2010;467:291–6. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 2012;151:630–44. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 2013;17:976–87. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bick AG, Wakimoto H, Kamer KJ, Sancak Y, Goldberger O, Axelsson A, et al. Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proceedings of the National Academy of Sciences 2017;114:E9096–104. doi: 10.1073/pnas.1711303114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang L, Yang X, Li S, Wang Z, Liu Y, Feng J, et al. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. EMBO J 2014;33:594–604. doi: 10.1002/embj.201386523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park J, Lee Y, Park T, Kang JY, Mun SA, Jin M, et al. Structure of the MICU1-MICU2 heterodimer provides insights into the gatekeeping threshold shift. IUCrJ 2020;7:355–65. doi: 10.1107/S2052252520001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kamer KJ, Jiang W, Kaushik VK, Mootha VK, Grabarek Z. Crystal structure of MICU2 and comparison with MICU1 reveal insights into the uniporter gating mechanism. Proc Natl Acad Sci USA 2019;116:3546–55. doi: 10.1073/pnas.1817759116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu W, Shen Q, Lei Z, Qiu Z, Li D, Pei H, et al. The crystal structure of MICU2 provides insight into Ca2+ binding and MICU1-MICU2 heterodimer formation. EMBO Rep 2019;20:e47488. doi: 10.15252/embr.201847488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xing Y, Wang M, Wang J, Nie Z, Wu G, Yang X, et al. Dimerization of MICU Proteins Controls Ca2+ Influx through the Mitochondrial Ca2+ Uniporter. Cell Reports 2019;26:1203–1212.e4. doi: 10.1016/j.celrep.2019.01.022. [DOI] [PubMed] [Google Scholar]
- [51].Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J 1978;176:463–74. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol 1991;261:H1123–1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- [53].Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 2006;99:172–82. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kohlhaas M, Maack C. Calcium release microdomains and mitochondria. Cardiovascular Research 2013;98:259–68. doi: 10.1093/cvr/cvt032. [DOI] [PubMed] [Google Scholar]
- [55].Liu JC, Liu J, Holmström KM, Menazza S, Parks RJ, Fergusson MM, et al. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep 2016;16:1561–73. doi: 10.1016/j.celrep.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wescott AP, Kao JPY, Lederer WJ, Boyman L. Voltage-energized calcium-sensitive ATP production by mitochondria. Nature Metabolism 2019;1:975–84. doi: 10.1038/s42255-019-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Garg V, Paranjpae I, Unsulangi T, Suzuki J, Milescu LS, Kirichok YV. Molecular Mechanism of Mitochondrial Calcium Uniporter Regulation. Biophysical Journal 2020;118:18a. doi: 10.1016/j.bpj.2019.11.283. [DOI] [Google Scholar]
- [58].Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 2010;107:436–41. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep 2013;5:1576–88. doi: 10.1016/j.celrep.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nemani N, Shanmughapriya S, Madesh M. Molecular regulation of MCU: Implications in physiology and disease. Cell Calcium 2018;74:86–93. doi: 10.1016/j.ceca.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gottschalk B, Klec C, Leitinger G, Bernhart E, Rost R, Bischof H, et al. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nature Communications 2019;10. doi: 10.1038/s41467-019-11692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tomar D, Thomas M, Garbincius JF, Kolmetzky DW, Salik O, Jadiya P, et al. MICU1 regulates mitochondrial cristae structure and function independent of the mitochondrial calcium uniporter channel. Cell Biology; 2019. doi: 10.1101/803213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tufi R, Gleeson TP, von Stockum S, Hewitt VL, Lee JJ, Terriente-Felix A, et al. Comprehensive Genetic Characterization of Mitochondrial Ca2+ Uniporter Components Reveals Their Different Physiological Requirements In Vivo. Cell Reports 2019;27:1541–1550.e5. doi: 10.1016/j.celrep.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Prudent J, Popgeorgiev N, Bonneau B, Thibaut J, Gadet R, Lopez J, et al. Bcl-wav and the mitochondrial calcium uniporter drive gastrula morphogenesis in zebrafish. Nat Commun 2013;4:2330. doi: 10.1038/ncomms3330. [DOI] [PubMed] [Google Scholar]
- [65].Soman S, Keatinge M, Moein M, Da Costa M, Mortiboys H, Skupin A, et al. Inhibition of the mitochondrial calcium uniporter rescues dopaminergic neurons in pink1 − / − zebrafish. European Journal of Neuroscience 2017;45:528–35. doi: 10.1111/ejn.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 2013;15:1464–72. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Murphy E, Pan X, Nguyen T, Liu J, Holmström KM, Finkel T. Unresolved questions from the analysis of mice lacking MCU expression. Biochem Biophys Res Commun 2014;449:384–5. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Holmström KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, et al. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol 2015;85:178–82. doi: 10.1016/j.yjmcc.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rasmussen TP, Wu Y, Joiner MA, Koval OM, Wilson NR, Luczak ED, et al. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci USA 2015;112:9129–34. doi: 10.1073/pnas.1504705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 1997;100:169–79. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Davis J, Maillet M, Miano JM, Molkentin JD. Lost in transgenesis: a user’s guide for genetically manipulating the mouse in cardiac research. Circ Res 2012;111:761–77. doi: 10.1161/CIRCRESAHA.111.262717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wu Y, Rasmussen TP, Koval OM, Joiner M- LA, Hall DD, Chen B, et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun 2015;6:6081. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, et al. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F, et al. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep 2015;10:1269–79. doi: 10.1016/j.celrep.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gherardi G, Nogara L, Ciciliot S, Fadini GP, Blaauw B, Braghetta P, et al. Loss of mitochondrial calcium uniporter rewires skeletal muscle metabolism and substrate preference. Cell Death Differ 2019;26:362–81. doi: 10.1038/s41418-018-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kwong JQ, Huo J, Bround MJ, Boyer JG, Schwanekamp JA, Ghazal N, et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 2018;3. doi: 10.1172/jci.insight.121689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Altamimi TR, Karwi QG, Uddin GM, Fukushima A, Kwong JQ, Molkentin JD, et al. Cardiac-specific deficiency of the mitochondrial calcium uniporter augments fatty acid oxidation and functional reserve. J Mol Cell Cardiol 2019;127:223–31. doi: 10.1016/j.yjmcc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- [79].Diaz-Juarez J, Suarez J, Cividini F, Scott BT, Diemer T, Dai A, et al. Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. American Journal of Physiology-Cell Physiology 2016;311:C1005–13. doi: 10.1152/ajpcell.00236.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu JC, Syder NC, Ghorashi NS, Willingham TB, Parks RJ, Sun J, et al. EMRE is essential for mitochondrial calcium uniporter activity in a mouse model. JCI Insight 2020;5. doi: 10.1172/jci.insight.134063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lambert JP, Luongo TS, Tomar D, Jadiya P, Gao E, Zhang X, et al. MCUB Regulates the Molecular Composition of the Mitochondrial Calcium Uniporter Channel to Limit Mitochondrial Calcium Overload During Stress. Circulation 2019;140:1720–33. doi: 10.1161/CIRCULATIONAHA.118.037968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Antony AN, Paillard M, Moffat C, Juskeviciute E, Correnti J, Bolon B, et al. MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun 2016;7:10955. doi: 10.1038/ncomms10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs A-M, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet 2014;46:188–93. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- [84].Xue Q, Pei H, Liu Q, Zhao M, Sun J, Gao E, et al. MICU1 protects against myocardial ischemia/reperfusion injury and its control by the importer receptor Tom70. Cell Death Dis 2017;8:e2923. doi: 10.1038/cddis.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhao H, Li T, Wang K, Zhao F, Chen J, Xu G, et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat Cell Biol 2019;21:476–86. doi: 10.1038/s41556-019-0296-3. [DOI] [PubMed] [Google Scholar]
- [86].Koval OM, Nguyen EK, Santhana V, Fidler TP, Sebag SC, Rasmussen TP, et al. Loss of MCU prevents mitochondrial fusion in G1-S phase and blocks cell cycle progression and proliferation. Sci Signal 2019;12. doi: 10.1126/scisignal.aav1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tyser RC, Miranda AM, Chen C, Davidson SM, Srinivas S, Riley PR. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. ELife 2016;5. doi: 10.7554/eLife.17113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Olson EN. Gene Regulatory Networks in the Evolution and Development of the Heart. Science 2006;313:1922–7. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Paillard M, Csordás G, Szanda G, Golenár T, Debattisti V, Bartok A, et al. Tissue-Specific Mitochondrial Decoding of Cytoplasmic Ca2+ Signals Is Controlled by the Stoichiometry of MICU1/2 and MCU. Cell Rep 2017;18:2291–300. doi: 10.1016/j.celrep.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Barth E, Stämmler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 1992;24:669–81. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- [92].Bleck CKE, Kim Y, Willingham TB, Glancy B. Subcellular connectomic analyses of energy networks in striated muscle. Nature Communications 2018;9. doi: 10.1038/s41467-018-07676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].De La Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, et al. Strategic Positioning and Biased Activity of the Mitochondrial Calcium Uniporter in Cardiac Muscle. J Biol Chem 2016;291:23343–62. doi: 10.1074/jbc.M116.755496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].De La Fuente S, Lambert JP, Nichtova Z, Fernandez Sanz C, Elrod JW, Sheu S-S, et al. Spatial Separation of Mitochondrial Calcium Uptake and Extrusion for Energy-Efficient Mitochondrial Calcium Signaling in the Heart. Cell Rep 2018;24:3099–3107.e4. doi: 10.1016/j.celrep.2018.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Piquereau J, Ventura-Clapier R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Frontiers in Physiology 2018;9. doi: 10.3389/fphys.2018.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Griffiths EJ, Balaska D, Cheng WHY. The ups and downs of mitochondrial calcium signalling in the heart. Biochim Biophys Acta 2010;1797:856–64. doi: 10.1016/j.bbabio.2010.02.022. [DOI] [PubMed] [Google Scholar]
- [97].Piquereau J, Novotova M, Fortin D, Garnier A, Ventura-Clapier R, Veksler V, et al. Postnatal development of mouse heart: formation of energetic microdomains: Energetic maturation of postnatal mouse heart. The Journal of Physiology 2010;588:2443–54. doi: 10.1113/jphysiol.2010.189670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kim Y, Yang DS, Katti P, Glancy B. Protein composition of the muscle mitochondrial reticulum during postnatal development. The Journal of Physiology 2019;597:2707–27. doi: 10.1113/JP277579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, et al. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 2017;545:93–7. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]