Abstract
Niclosamide is an FDA-approved oral anthelmintic drug currently being repurposed for COVID-19 infection. Its interesting applicability in multiple therapeutic indications has sparked interest in this drug/ scaffold. Despite its therapeutic use for several years, its detailed solubility information from Chemistry Manufacturing & Controls perspective is unavailable. Thus, the present study is intended to determine the mole fraction solubility of niclosamide in commonly used solvents and cosolvents at a temperature range of 298.15–323.15 K. The polymorphic changes from crystalline to monohydrate forms post-equilibration in various solvents were observed. The maximum mole fraction solubility of niclosamide at 323.15 K is 1.103 × 10-3 in PEG400, followed by PEG200 (5.272 × 10-4), 1-butanol (3.047 × 10-4), 2-propanol (2.42 × 10-4), ethanol (1.66 × 10-4), DMSO (1.52 × 10-4), methanol (7.78 × 10-5) and water (3.27 × 10-7). The molecular electrostatic potential showed a linear correlation with the solubility. PEG400 has higher electrostatic potential, and H-bond acceptor count, which forms a hydrogen bond with phenolic –OH of niclosamide and thus enhances its solubility. This data is valuable for the drug discovery and development teams working on the medicinal chemistry and process chemistry of this scaffold.
Keywords: Niclosamide, Thermodynamic solubility, Mole fraction solubility, Drug discovery and lead optimization, Chemistry manufacturing & controls, Process chemistry
1. Introduction
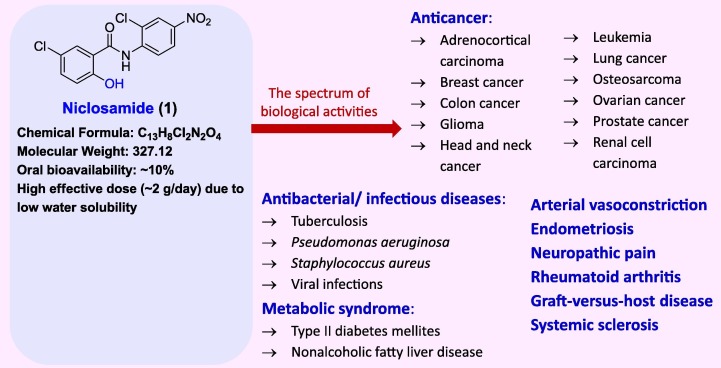
Niclosamide (1), [N-(2′-chloro-4′-nitrophenyl)-5-chlorosalicylamide], was approved by FDA in 1982 as an oral anthelmintic drug with a very high effective dose of around 2.0 g/day. The reason is its poor oral bioavailability due to low aqueous solubility. Niclosamide was discovered by Bayer laboratories and marketed as an emulsion concentrate formulation under the brand name, Bayluscide®. The molecule is lethal at 0.3 mg/L concentration in snails and thus was the standard molluscicide in the 1950 s [1], [2]. Niclosamide was first commercially available outside the US in 1961 [3]. Though approved as an anthelmintic drug, it is currently investigated in 30 clinical trials for various therapeutic indications. The objective of these clinical trials is to assess the clinical efficacy of niclosamide in alleviating COVID-19 (18 studies), prostate cancer (4 studies), colon cancer (2 studies), parasitic infections (2), diabetic nephropathy, ulcerative colitis, rheumatoid arthritis and familial adenomatous polyposis (1 study each) [4]. Furthermore, the pharmacological activities reported for niclosamide are apoptosis inducer in human breast cancer cell lines (MDA-MB-231) at concentration 1–10 µM/L, cell proliferation inhibitor (MCF-7) with IC50 of 1.06 µM/L [5], parasitic proliferation inhibitor (MIC50 = 200–250 nM/L) [6], hepatitis C virus replication inhibitor (0.16 µM/L) [7], acetylcholinesterase inhibitor [8], [9], and SARS-CoV-2 inhibitor [10]. The chemical structure and summary of pharmacological activities of niclosamide are depicted in Fig. 1 .
Fig. 1.
The chemical structure and the summary of pharmacological activities of niclosamide.
Niclosamide possesses low aqueous solubility (6–20 µg/mL) in water, phosphate-buffered saline [PBS (pH 7.4)], simulated gastrointestinal fluid (SGF), and simulated intestinal fluid (SIF). Thus, its low aqueous solubility at physiological conditions may be the limiting factor for its poor oral bioavailability, eventually a reason for the need for a very high dose.
The clinical and pharmacological data highlight the repurposing opportunities for different indications. In fact, it is already being repurposed for various indications, as indicated by active clinical trials. The solubility data in different solvents is important to drive any drug discovery and development program. The process chemistry group can select the suitable solvent during scale-up and recrystallization [11] of niclosamide in a kilo-lab facility. Furthermore, this information may guide medicinal chemists in the lead optimization of this salicylamide scaffold. The use of cosolvents is one of the most common techniques to solubilize compounds for in vivo animal studies. The polarity of solvent plays an important role in compound solubilization. Cosolvents being less polar than water facilitates the solubilization of nonpolar molecules. The polar and semipolar solvents, for instance, ethanol (EtOH; log P, −0.31), isopropanol (IPA; log P, 0.05), methanol (MeOH; log P, −0.69), dimethyl sulfoxide (DMSO; log P, −1.35) improves the miscibility of a nonpolar drug resulting into enhanced solubility [12], [13]. The combination of cosolvents also reduces the polarity of bulk, hence used for the parenteral administration of lead molecules during the preclinical assessment. The most commonly used cosolvents are ethanol, low molecular weight propylene glycol, and DMSO. The equilibrium solubility of niclosamide in different solvents and cosolvents is not reported. Hence, this research aims to determine the mole fraction solubility of niclosamide at temperatures from 298.15 to 323.15 K in eight pure solvents, viz. water, DMSO, methanol, ethanol, 2-propanol, 1-butanol, propylene glycol 200 (PEG200), and propylene glycol 400 (PEG400). Additionally, differential scanning calorimetric (DSC) studies of pure and equilibrated niclosamide were conducted to evaluate polymorphic changes in the solid-state post experimentation. The experimental solubility data was assessed using non-linear multivariate analysis and linear regression.
2. Experimental
2.1. Materials and instruments
Niclosamide was purchased from Sigma Aldrich and characterized using 1H NMR, 13C NMR, and DSC analysis. The NMR analysis was performed on Bruker 400 MHz NMR instrument. HPLC-grade DMSO, methanol, ethanol, 2-propanol, and 1-butanol were used for the study. Polyethylene glycol (200 and 400) was purchased from Alfa Aesar, India. Millipore water was used in the study. The molecular weight of niclosamide is 327.12 g/mol. The molar mass of water, DMSO, methanol, ethanol, 2-propanol, 1-butanol, PEG200, and PEG400 are 18.015, 78.13, 32.04, 46.07, 60.1, 74.121, 200, and 400 g/mol, respectively. Additionally, experimental work was performed using micropipettes (Eppendorf research® plus) and a microplate shaker incubator (Eppendorf, ThermoMixer C). The DSC spectral data were recorded using Mettler Toledo’s DSC 1 STARe system. Schrodinger molecular modeling software was used to calculate the electrostatic potential of niclosamide and solvents.
2.2. Determination of equilibrium solubility of niclosamide
In eight mono solvents, the thermodynamic equilibrium solubility of niclosamide was measured at 298.15–323.15 K. The drug solubility was determined as per our previously reported miniaturized shake-flask method [14], [15], [16]. In brief, an excess amount of niclosamide was added to a 1.5 mL eppendorf tube containing solvent. The sealed tube containing a mixture was thoroughly vortexed and sonicated. To achieve equilibrium, these samples were shaken at specified temperatures for 24 h at 300 rpm. Post-equilibration, the samples were centrifuged at 25 °C for 20 min at 8000 rpm. The supernatant was diluted appropriately using a mixture of MeOH and water (8: 2 v/v). In all solvents, the concentration of niclosamide was estimated by recording the absorbance at 330 nm with a microplate reader (EPOCH2, BioTek). The calibration curve of niclosamide in the 5–35 µg/mL concentration range was linear (r2 = 0.999). Each analysis was performed in triplicates. The mole fraction solubility (Xe) of niclosamide was calculated by considering the average of three determinations. The experimental mole fraction solubility (Xe) of niclosamide was calculated in each solvent using Eq. (1) [17], [18]:
| (1) |
where, m1 = mass of niclosamide.
m2 = mass of pure solvents (water, DMSO, MeOH, EtOH, 2-propanol, 1-butanol, PEG 200, and PEG 400.
M1 = molar mass of niclosamide (g/mol).
M2 = molar masses of solvent (g/mol).
The thermodynamic equilibrium solubility of niclosamide in the mentioned solvents was obtained after supersaturation at the respective temperature. Here, the supernatant was analyzed for drug content, and bottom phases were analyzed for change in the solid-state of niclosamide, if any, using DSC and p-XRD studies. Here, the thermodynamic equilibrium solubility corresponds to the solubility of niclosamide in the bottom phase. The solubility profile of niclosamide in eight monosolvents was correlated using thermodynamic models such as non-linear multivariate regression, Van’t-Hoff and modified Apelblat equation.
2.3. DSC studies of niclosamide
The DSC spectra of the neat and equilibrated drug were recorded under isothermal conditions using Mettler Toledo’s DSC 1 STARe system. The solvent from the equilibrated sample was evaporated to dryness, and then the DSC spectra of the dried sample were recorded. Briefly, 3–5 mg of sample was sealed in an aluminum pan and heated from 30 to 350 °C at 10 °C/min.
2.4. Acetylcholinesterase inhibition
It is crucial to determine whether a change in the drug's solid state impacts the molecule's biological activity. Therefore the activity of various solid forms of niclosamide was evaluated in the Ellman assay, wherein the effect of neat drug and solvent-equilibrated niclosamide (NCL-DMSO and NCL-MeOH) on the inhibition of acetylcholinesterase enzyme was checked [19]. Based on the reported protocol [20], the stock solutions of electrical eel AChE (EeAChE), DTNB, and ATChI were prepared in 0.1 M phosphate buffer (pH 7.2). Niclosamide was dissolved in DMSO to get a 10 mM stock solution. It was diluted with PBS to get seven working stocks, 2000, 1000, 500, 250, 125, 62.5, and 31.2 µM. For the assay, 20 μL enzyme solution, 20 μL of niclosamide solution, and 140 μL 0.3 mM DTNB solution were added to the 96-well plate. The plate was incubated at 25 °C for 20 min, and then 20 μL of substrate, ATChI, was added. Absorbance was recorded at 412 nm, from which the percentage inhibition of enzyme was calculated. The dose–response (Log concentration vs percent inhibition) and IC50 value were obtained using GraphPad Prism 8.0.2 software. Donepezil was used as a positive control in the assay.
3. Results and discussion
3.1. Experimental solubility of niclosamide
The mole fraction solubilities (Xe) of niclosamide in the selected mono solvents at different temperatures (298.15–323.15 K) were assessed and given in Table 1 . In all mono solvents, a positive linear correlation of the mole fraction solubility, Xe, was obtained with the temperature. Niclosamide showed the highest solubility in PEG400 and lowest in water. At 323.15 K, the mole fraction solubility of niclosamide in all solvents was found to be maximum. The trend of Xe values was as follows: PEG400 (1.103 × 10-3) > PEG200 (5.272 × 10-4) > 1-butanol (3.047 × 10-4) > 2-propanol (2.42 × 10-4) > ethanol (1.66 × 10-4) > DMSO (1.52 × 10-4) > methanol (7.78 × 10-5) > water (3.27 × 10-7).
Table 1.
Experimental mole fraction solubility (Xe) of niclosamide in mono solvents at temperatures T = 298.15 to 323.15 K a.
|
Solvent |
Mole fraction solubility#(Xe ± STDEV) |
|||||
|---|---|---|---|---|---|---|
| T = 298.15 K | T = 303.15 K | T = 308.15 K | T = 313.15 K | T = 318.15 K | T = 323.15 K | |
| Water | 3.27 × 10-7 ± 1.34 × 10-8 | 4.12 × 10-7 ± 5.85 × 10-8 | 4.19 × 10-7 ± 1.08 × 10-8 | 4.27 × 10-7 ± 4.59 × 10-8 | 4.74 × 10-7 ± 8.99 × 10-8 | 6.04 × 10-7 ± 9.63 × 10-9 |
| DMSO | 1.52 × 10-4 ± 2.17 × 10-5 | 1.61 × 10-4 ± 1.01 × 10-5 | 1.65 × 10-4 ± 5.43 × 10-6 | 1.67 × 10-4 ± 8.77 × 10-6 | 1.70 × 10-4 ± 5.43 × 10-6 | 2.05 × 10-4 ± 1.34 × 10-5 |
| Methanol | 7.78 × 10-5 ± 8.39 × 10-6 | 7.69 × 10-5 ± 3.19 × 10-6 | 6.90 × 10-5 ± 5.82 × 10-6 | 6.75 × 10-5 ± 2.99 × 10-6 | 5.90 × 10-5 ± 6.56 × 10-6 | 5.35 × 10-5 ± 1.49 × 10-6 |
| Ethanol | 1.66 × 10-4 ± 8.40 × 10-6 | 1.83 × 10-4 ± 8.82 × 10-6 | 1.86 × 10-4 ± 1.47 × 10-5 | 1.86 × 10-4 ± 1.20 × 10-5 | 1.99 × 10-4 ± 7.09 × 10-6 | 2.00 × 10-4 ± 1.73 × 10-5 |
| 2-propanol | 2.42 × 10-4 ± 1.64 × 10-5 | 2.74 × 10-4 ± 5.52 × 10-6 | 2.79 × 10-4 ± 2.19 × 10-5 | 3.04 × 10-4 ± 1.12 × 10-5 | 3.45 × 10-4 ± 5.89 × 10-5 | 3.48 × 10-4 ± 3.31 × 10-5 |
| 1-butanol | 3.05 × 10-4 ± 4.44 × 10-5 | 3.09 × 10-4 ± 1.84 × 10-5 | 3.45 × 10-4 ± 1.03 × 10-5 | 3.47 × 10-4 ± 1.69 × 10-5 | 4.02 × 10-4 ± 2.38 × 10-5 | 5.91 × 10-4 ± 4.40 × 10-5 |
| PEG200 | 5.27 × 10-4 ± 4.31 × 10-5 | 5.56 × 10-4 ± 1.68 × 10-5 | 6.04 × 10-4 ± 8.91 × 10-5 | 6.66 × 10-4 ± 1.09 × 10-5 | 6.86 × 10-4 ± 1.63 × 10-5 | 7.90 × 10-4 ± 2.14 × 10-5 |
| PEG400 | 1.10 × 10-3 ± 5.92 × 10-5 | 1.29 × 10-3 ± 3.89 × 10-5 | 1.38 × 10-3 ± 7.36 × 10-5 | 1.59 × 10-3 ± 2.77 × 10-4 | 1.68 × 10-3 ± 1.16 × 10-4 | 1.93 × 10-3 ± 1.46 × 10-4 |
The standard uncertainty is 0.1 K for temperature measurement;
average of three determinations.
The cosolvents may be employed during preclinical developmental assays to dissolve the molecules. The cosolvents disrupt intermolecular hydrogen bonding networks in the aqueous systems, which may be the reason for the enhanced solubility of the lipophilic molecule in the cosolvent combinations. Combining cosolvents may also decrease the system's polarity, resulting in more favorable physicochemical properties for drug dissolution. There are many FDA-approved formulations comprising cosolvent combinations that keep the drug molecule in solubilized form. For instance, Targretin is a soft gelatine capsule launched by Ligand Pharmaceuticals. It contains bexarotene, a retinoid analog that is insoluble in water, and hence it is dissolved in a combination of cosolvents comprising PEG400, polysorbate 80, povidone, butylated hydroxy anisole [21]. VePesid® is a soft gelatine capsule containing anticancer medication etoposide. The drug is dissolved in cosolvents comprising PEG400, citric acid, glycerine, and purified water. These marketed formulations signify cosolvents' importance in solubilizing lipophilic drug molecules [22], [23]. Thus for in vivo experiments of niclosamide or salicylamide scaffold, the solubility data will facilitate the selection of suitable cosolvent/solvent for administering compounds in animals [24]. Furthermore, the equilibrium solubility of niclosamide in different solvents will provide valuable information for the scale-up of a molecule. It may also facilitate the selection of appropriate solvent/s during the recrystallization and purification process.
3.2. Correlation of the Xe values of niclosamide
The non-linear multivariate regression analysis (Eq. (2)) was used to correlate mole fraction solubility, χe, of niclosamide.
| (2) |
where, A, B, C are parameters obtained using 2nd order polynomial non-linear fit.
The correlation of Xe with the temperature (298.15–323.15 K) for the studied mono solvents is depicted in Fig. 2 . The coefficients of A, B, and C for the solvents are shown in Table 2 . All solubility values in Table 2 correspond to the bottom phases, which are in equilibrium with the solution.
Fig. 2.
Mole fraction solubility (Xe) of niclosamide at different temperatures in various mono solvents.
Table 2.
Parameters of nonlinear multivariate regression analysis and modified Apelblat equation for niclosamide in eight solvents.
|
Nonlinear multivariate regression: | |||||
|---|---|---|---|---|---|
| Solvent | A (±STDEV) | B (±STDEV) | C (±STDEV) | R2 (±STDEV) | SEE |
| Water | 2.416 × 10-5 ± 4.832 × 10-6 | −1.618 × 10-7 ± 3.24 × 10-8 | 2.750 × 10-10 ± 5.5 × 10-11 | 0.8874 ± 0.0245 | 3.977 × 10-8 |
| DMSO | 8.327 × 10-3 ± 1.665 × 10-3 | −5.424 × 10-5 ± 1.08 × 10-5 | 9.000 × 10-8 ± 1.8 × 10-8 | 0.8556 ± 0.0207 | 8.948 × 10-6 |
| Methanol | −1.369 × 10-3 ± 2.74 × 10-4 | 1.026 × 10-5 ± 2.052 × 10-6 | −1.814 × 10-8 ± 3.63 × 10-9 | 0.9764 ± 0.0133 | 1.910 × 10-6 |
| Ethanol | −2.955 × 10-3 ± 5.91 × 10-4 | 1.900 × 10-5 ± 3.80 × 10-6 | −2.857 × 10-8 ± 5.71 × 10-9 | 0.9049 ± 0.0539 | 4.945 × 10-6 |
| 2-propanol | −1.134 × 10-3 ± 2.27 × 10-4 | 4.832 × 10-6 ± 9.664 × 10-7 | −7.143 × 10-10 ± 1.43 × 10-10 | 0.9558 ± 0.0118 | 1.139 × 10-5 |
| 1-butanol | 6.621 × 10-2 ± 1.324 × 10-2 | −4.339 × 10-4 ± 8.68 × 10-5 | 7.141 × 10-7 ± 1.428 × 10-7 | 0.9269 ± 0.0171 | 3.762 × 10-5 |
| PEG200 | 1.549 × 10-2 ± 3.098 × 10-3 | −1.058 × 10-4 ± 2.12 × 10-5 | 1.866 × 10-7 ± 3.732 × 10-8 | 0.9793 ± 0.0121 | 1.789 × 10-5 |
| PEG400 | 1.153 × 10-2 ± 2.306 × 10-3 | −9.624 × 10-5 ± 1.92 × 10-5 | 2.057 × 10-7 ± 4.114 × 10-8 | 0.9864 ± 0.0734 | 4.483 × 10-5 |
|
Modified Apelblat equation: | |||||
|---|---|---|---|---|---|
| Solvent | A (± STDEV) | B (± STDEV) | C (± STDEV) | R2 (± STDEV) | SEE |
| Water | 904.6 ± 150.766 | −326.6 ± 54.433 | 29.01 ± 4.835 | 0.8834 ± 0.0885 | 0.0235 |
| DMSO | 1368 ± 228.0 | −482.8 ± 80.466 | 42.33 ± 7.055 | 0.8607 ± 0.0074 | 0.0496 |
| Methanol | −1287 ± 214.5 | 450.1 ± 75.016 | −39.64 ± 6.606 | 0.9802 ± 0.0022 | 0.0269 |
| Ethanol | −593.4 ± 98.9 | 201.8 ± 33.633 | −17.40 ± 2.90 | 0.8986 ± 0.0023 | 0.0277 |
| 2-propanol | −273.8 ± 45.633 | 87.98 ± 14.663 | −7.263 ± 1.211 | 0.9600 ± 0.0041 | 0.03675 |
| 1-butanol | 4563 ± 760.5 | −1601 ± 266.833 | 140.1 ± 23.35 | 0.9425 ± 0.0175 | 0.0765 |
| PEG200 | 545.2 ± 545.2 | −197.5 ± 197.5 | 17.63 ± 17.63 | 0.9825 ± 0.0019 | 0.0254 |
| PEG400 | −305.5 ± 50.916 | 97.59 ± 16.265 | −7.926 ± 1.321 | 0.9868 ± 0.0027 | 0.0298 |
SEE, Standard error of estimate; STDEV, Standard deviation.
3.3. Apparent thermodynamics of niclosamide
In the investigated solvents, the dissolution performance of niclosamide was determined by ‘apparent thermodynamic analysis’. It is measured using the Van’t-Hoff equation (3):
| (3) |
where, R = universal gas constant, ΔH° = enthalpy, ΔS° = entropy.
A graph of experimental solubility expressed as ln (Xe) was plotted against 1/T.
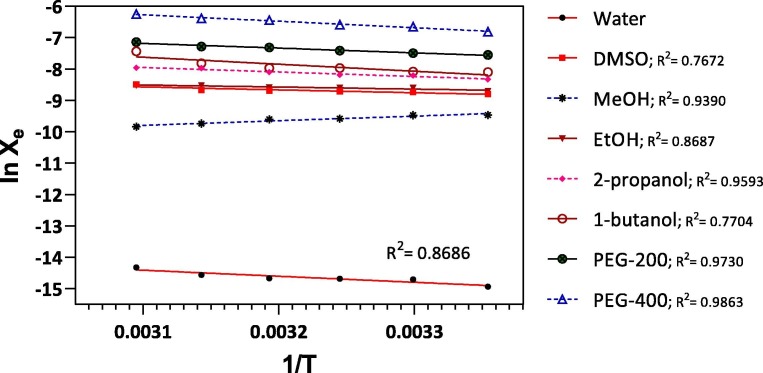
The linear regression of niclosamide in the solvents is shown in Fig. 3 . The R2 values ranged from 0.7672 to 0.9863. The goodness of fit supported that the dissolution of niclosamide in all investigated solvents is an entropy-driven process. The change in Gibbs energy (ΔG) is calculated using equation (4):
| (4) |
Fig. 3.
Correlation of lnXe in various solvents against 1/T.
Where ΔH = change in enthalpy;
ΔS = change in entropy;
T = Temperature in Kelvin.
Xe = mole fraction solubility of the component.
Table 1 shows that Xe of niclosamide in all studied solvents is less than unity; therefore, lnXe is negative; thus, overall ΔS is positive. When ΔS is positive, the dissolution process is always entropy-driven. The dissolution properties of niclosamide in 8 monosolvents were calculated and presented in Table 3 . The dissolution Gibbs free energy (ΔG) is positive for water, DMSO, MeOH, and EtOH, indicating that the dissolution process is nonspontaneous and requires external energy such as heat. On the contrary, ΔG of niclosamide in 2-propanol, 1-butanol, PEG-200, and PEG-400 was negative, indicating the spontaneous and favorable dissolution. This could be the reason for the comparatively high solubility of niclosamide in PEG-200 and PEG-400.
Table 3.
The dissolution Gibbs free energy of niclosamide in eight monosolvnets.
| T/K | ΔdisG (J/mol) | T/K | ΔdisG (J/mol) | T/K | ΔdisG (J/mol) | T/K | ΔdisG (J/mol) |
|---|---|---|---|---|---|---|---|
| Water | Methanol | 2-propanol | PEG-200 | ||||
| 298.15 | 588.5 | 298.15 | 5761.0 | 298.15 | −394.7 | 298.15 | −768.4 |
| 303.15 | 630.7 | 303.15 | 5833.0 | 303.15 | −377.2 | 303.15 | −756.0 |
| 308.15 | 672.9 | 308.15 | 5905.0 | 308.15 | −359.7 | 308.15 | −743.5 |
| 313.15 | 715.0 | 313.15 | 5977.0 | 313.15 | −342.2 | 313.15 | −731.0 |
| 318.15 | 757.2 | 318.15 | 6049.0 | 318.15 | −324.8 | 318.15 | −718.5 |
| 323.15 | 799.4 | 323.15 | 6121.0 | 323.15 | −307.3 | 323.15 | −706.0 |
| DMSO | Ethanol | 1-butanol | PEG-400 | ||||
| 298.15 | 768.6 | 298.15 | 1291.2 | 298.15 | −2026.0 | 298.15 | −2092.5 |
| 303.15 | 797.0 | 303.15 | 1323.7 | 303.15 | −2022.6 | 303.15 | −2093.0 |
| 308.15 | 825.5 | 308.15 | 1356.2 | 308.15 | −2019.0 | 308.15 | −2093.6 |
| 313.15 | 854.0 | 313.15 | 1388.7 | 313.15 | −2015.7 | 313.15 | −2094.0 |
| 318.15 | 882.4 | 318.15 | 1421.2 | 318.15 | −2012.0 | 318.15 | −2094.8 |
| 323.15 | 910.9 | 323.15 | 1453.7 | 323.15 | −2008.7 | 323.15 | −2095.3 |
The modified Apelblat equation is a simple empirical model to link the solubility of a compound in different solvents at varying temperatures computed using equation (5):
| (5) |
where Xe = mole fraction solubility of niclosamide in different solvents;
T = absolute temperature;
A, B, C = empirical constants.
The parameters obtained from the modified Apelblat equation are given in Table 2. The correlation coefficient values obtained from lnXe and ln(T/K) showed a linear correlation with R2 ranging from 0.8834 to 0.9868.
3.4. DSC analysis of solvated forms of niclosamide post-equilibration in various solvents
It is crucial to determine whether the drug exists in two or more crystalline phases as per the regulatory guidance. Understanding the polymorphism or pseudo-polymorphism of API provides valuable information to process chemistry groups on the process parameters and solvents employed during the scale-up process. Furthermore, it also guides formulation scientists in selecting various pharmaceutical unit operations, thereby assuring the stability and bioavailability of the drug in the finished formulation [25], [26]. For instance, the soft gelatine capsules of ritonavir were recalled from the market due to a new polymorph. The product failed the dissolution studies because of the low solubility of ritonavir’s new polymorph [27]. Schwarz Pharma withdrew the Neupro® patch in 2008, a transdermal patch of dopamine agonist rotigotine. The reason for this product recall was the formation of less soluble crystals of rotigotine, eventually responsible for low bioavailability and thus efficacy of the product [28]. These incidences highlight the importance of polymorphic studies of drug molecules during drug discovery and development. DSC analysis is one of the important techniques indicating polymorphic or pseudo-polymorphic changes within the drug.
Bhavana and co-workers have reported the polymorphic forms of niclosamide. The study reports the conversion of crystalline niclosamide to two monohydrates with different dehydration peaks. During the high-energy milling process, niclosamide is converted to (a) metastable monohydrate with a dehydration peak at ∼ 180 °C and (b) stable monohydrate with a dehydration peak at ∼ 90 °C. The authors also compared the impact of ball milling and wet granulation on the polymorphic transformation of niclosamide. The results indicated that the ball milling resulted in the formation of metastable niclosamide. On the contrary, the wet granulation process yielded a product with anhydrous niclosamide. The reason being during the drying process, the metastable form was reconverted to the anhydrous niclosamide. Thus, this study highlights that high-energy milling should be avoided to prohibit the formation of solvated forms of niclosamide [29].
The DSC analysis of niclosamide in pure and post-equilibrated form was conducted to investigate its conversion into another solid state. The post-equilibrated form here refers to the bottom phases, which are in equilibrium and the thermodynamically stable state with the solution(s). Fig. 4 a-4c depicts the DSC curve of niclosamide in pure and post-equilibration in different solvents. The sharp peak of niclosamide was observed at 228.29 °C, indicating its anhydrous crystalline nature. However, niclosamide is converted entirely to a metastable state (melting endotherm at ∼ 171.19 °C) in DMSO (Fig. 4a). Furthermore, as depicted in Fig. 4b and 4c, a partial conversion of niclosamide to metastable form was noticed in 1-butanol (168.98 °C), water (162.47 °C), and MeOH (175.22 °C). Our results also indicated the formation of the stable monohydrate of niclosamide in solvents, namely 1-butanol, PEG200, and PEG400, with melting endotherm at 104.55, 105.32, and 94.04 °C, respectively. The heat capacity (Cp) of the pure, metastable monohydrate, and stable monohydrate niclosamide was −132.88, −114.29, and −68.42 J/g. Ethanol and 2-propanol prevented the polymorphic transformation of niclosamide (melting endotherm at 229.47 and 228.94 °C). Thus, during kilo-lab scale-up studies, ethanol and 2-propanol may be used for reaction work-up and recrystallization, which will prohibit the formation of monohydrates of niclosamide. Additionally, for in vivo animal experiments, cosolvents such as PEG200 and PEG400 may be preferred due to the high solubility of niclosamide in them. The enhanced solubility may be attributed to the conversion of crystalline drug to stable monohydrate.
Fig. 4.
DSC analysis. (a). overlay DSC thermographs of pure niclosamide and equilibrated niclosamide in DMSO, 1-butanol, PEG200, and PEG400; (b). overlay DSC thermographs of pure niclosamide and equilibrated niclosamide in water, methanol, ethanol, and 2-propanol; (c) overlay DSC thermographs of pure niclosamide and equilibrated niclosamide in water and methanol.
The DSC analysis showed a difference in the melting endotherm of niclosamide API and the samples post-equilibration in DMSO, MeOH, and EtOH. Therefore, the bottom phases of niclosamide equilibrated in DMSO, MeOH, and EtOH were analyzed to monitor change in the crystallinity compared to the pure API using the powder X-ray diffraction (p-XRD) studies. The p-XRD pattern of niclosamide with and without equilibration is presented in Fig. 5 . The data correlated with the DSC results wherein new peaks were observed in niclosamide equilibrated with DMSO, MeOH, and EtOH. This supported the conversion of niclosamide in another solid state/s (metastable and stable monohydrate of niclosamide). The peak shift in p-XRD indicates the structural changes indicative of the polymorphic transformations. The reason is that each polymorph, salt, or cocrystal will have its specific p-XRD pattern.
Fig. 5.
p-XRD results of niclosamide alone and after equilibration in organic solvents. (a). niclosamide; (b). niclosamide equilibrated in DMSO; (c) niclosamide equilibrated in methanol; (d). niclosamide equilibrated in ethanol.
The effect of the solid-state changes in niclosamide on its physical, biological, and chemical properties was assessed. The niclosamide equilibrated in methanol, and DMSO (NCL-MeOH and NCL-DMSO, respectively) was obtained as a dry powder by evaporating the solvent. The neat drug (NCL), NCL-MeOH, and NCL-DMSO were tested for their inhibitory activity on acetylcholinesterase (AChE). The dose–response curves (Fig. 6 a-c) indicated no significant effect on the AChE inhibitory activity of niclosamide after converting it from crystalline to metastable form. We also checked the chemical nature of equilibrated niclosamide using 1H NMR analysis. The 1H NMR spectra shown in Fig. 6d-f indicate no chemical change. Finally, we also checked whether the solid-state change observed here impacts the aqueous solubility. The water solubility of neat and equilibrated powders (NCL-MeOH and NCL-DMSO) was found to be 20.762 ± 0.099, 16.261 ± 0.082, and 18.082 ± 0.045 µg/mL, respectively. Although the solvents introduced solid-state changes in niclosamide post-equilibration, it does not impact its biological activity, physical nature, and chemical structure.
Fig. 6.
Investigating the biological activity (AChE inhibition) and chemical nature (1H NMR) of niclosamide powder obtained after equilibrating in methanol and DMSO at 323.15 K (a-c). The dose–response curves and IC50 values for inhibition of AChE by neat drug niclosamide (NCL) equilibrated in methanol (NCL-MeOH) and niclosamide equilibrated in DMSO (NCL-DMSO), respectively. Three curves in each plot correspond to the triplicate data; (d-f). 1H NMR of neat drug niclosamide, niclosamide equilibrated in methanol (NCL-MeOH), and niclosamide equilibrated in DMSO (NCL-DMSO), respectively.
3.5. Molecular electrostatic potential and solubility
Using the Poisson-Boltzmann electrostatic method, the electrostatic potential of niclosamide and solvents was calculated using the Schrodinger software. The 3D structures and Poisson-Boltzmann electrostatic surface maps of niclosamide and PEGs are shown in Fig. 7 A. The reddish and blue zones represent electron concentrated areas with a negative and positive potential, respectively. The increase in temperature is correlated directly with the electrostatic potential and solubility. PEG400 has higher electrostatic potential among all the solvents used, and niclosamide displayed its highest solubility. Niclosamide solubility is higher in the solvent with high H-bond acceptors. A high hydrogen bond acceptor is highly favorable in the dissolution process of niclosamide because it provides more binding sites for the hydroxyl group of niclosamide to form hydrogen bonds with solvent and improve its solubility. The HBA number of PEG400 (17.0) and PEG200 (8.5) is higher than DMSO (4.0), water (1.7), and other solvents studied, which is reflected in the solubility values of niclosamide in these solvents. Niclosamide displayed the highest solubility in PEG400 solvent as it has the highest HBA count (Fig. 7B).
Fig. 7.
The correlation of molecular electrostatic potential, hydrogen-bond acceptor (HBA) count of solvents with niclosamide solubility. (A). 3D structures and electrostatic potential surfaces of niclosamide, PEG200, and PEG400; (B). Effect of HBA count of solvent/ electrostatic potential changes on mole fraction solubility of niclosamide.
4. Conclusion
The thermodynamic equilibrium solubilities of niclosamide in eight commonly used solvents at ‘T = 298.15 to 323.15 K’ was determined. The solubility trend of niclosamide was linear and temperature-dependent. The highest mole fraction solubility of niclosamide was observed in PEG400 and PEG200. The molecular interactions between niclosamide and PEGs were estimated by calculating electrostatic potential. The reason for its high solubility in PEGs may be attributed to the formation of ‘hydrogen bonds’. Our analysis confirmed that the H-bond acceptor count is important as it affects the solubility of niclosamide. The prominent molecular interactions between niclosamide and PEGs were noted. Furthermore, the study confirmed the conversion of crystalline niclosamide to its metastable form in the solvents in the study except for ethanol and 2-propanol. The solid-state changes observed do not affect its biological, chemical, and physical properties. The obtained mole fraction solubility data of niclosamide may guide the process chemistry group and medicinal chemists in selecting appropriate solvents for purification and recrystallization. This data will also provide valuable inputs to the formulation and DMPK groups working on this scaffold.
CRediT authorship contribution statement
Jigar S. Bhanushali: Methodology. Sonali S. Bharate: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank the central instrumentation laboratory (CIL) of Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM’s NMIMS, Mumbai, for the DSC analysis of samples.
Funding
Financial support from SVKM’s NMIMS Seed Grant (2021-22) is gratefully acknowledged.
Data availability
Data will be made available on request.
References
- 1.Andrews P., Thyssen J., Lorke D. Pharmacol. Ther. 1982;19:245. doi: 10.1016/0163-7258(82)90064-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Mook R.A., Jr., Premont R.T., Wang J. Cell Signal. 2018;41:89. doi: 10.1016/j.cellsig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson R.D., Hewlett E.L. Ann. Intern. Med. 1985;102:550. doi: 10.7326/0003-4819-102-4-550. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous, https://clinicaltrials.gov/ct2/results?cond=&term=Niclosamide&cntry=&state=&city=&dist= accessed on 17-December 2021.
- 5.Chen H., Yang Z., Ding C., Chu L., Zhang Y., Terry K., Liu H., Shen Q., Zhou J. ACS Med. Chem. Lett. 2013;4:180. doi: 10.1021/ml3003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fomovska A., Wood R.D., Mui E., Dubey J.P., Ferreira L.R., Hickman M.R., Lee P.J., Leed S.E., Auschwitz J.M., Welsh W.J., Sommerville C., Woods S., Roberts C., McLeod R. J. Med. Chem. 2012;55:8375. doi: 10.1021/jm3007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stachulski A.V., Pidathala C., Row E.C., Sharma R., Berry N.G., Lawrenson A.S., Moores S.L., Iqbal M., Bentley J., Allman S.A., Edwards G., Helm A., Hellier J., Korba B.E., Semple J.E., Rossignol J.F. J. Med. Chem. 2011;54:8670. doi: 10.1021/jm201264t. [DOI] [PubMed] [Google Scholar]
- 8.He P., Wang W., Sanogo B., Zeng X., Sun X., Lv Z., Yuan D., Duan L., Wu Z. Parasit. Vectors. 2017;10:383. doi: 10.1186/s13071-017-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W., Qin Z., Zhu D., Wei Y., Li S., Duan L. Antimicrob. Agents Chemother. 2016;60:323. doi: 10.1128/AAC.01539-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.C. Prabhakara, R. Godbole, P. Sil, S. Jahnavi, S.E. Gulzar, T.S. van Zanten, D. Sheth, N. Subhash, A. Chandra, A. Shivaraj, P. Panikulam, I. U, V.K. Nuthakki, T.P. Puthiyapurayil, R. Ahmed, A.H. Najar, S.M. Lingamallu, S. Das, B. Mahajan, P. Vemula, S.B. Bharate, P.P. Singh, R. Vishwakarma, A. Guha, V. Sundaramurthy, S. Mayor, PLoS Pathog. 17 (2021) e1009706. [DOI] [PMC free article] [PubMed]
- 11.Sadeghi M., Cascella F., Tenberg V., Seidel-Morgenstern A., Lorenz H. J. Mol. Liq. 2021;343 [Google Scholar]
- 12.Hansen C.J., Siricilla S., Boatwright N., Rogers J.H., Kumi M.E., Herington J. Reprod. Sci. 2021 doi: 10.1007/s43032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen A.M., Friedl J.D., Wibel R., Chamieh J., Cottet H., Bernkop-Schnurch A. Mol. Pharm. 2020;17:3236. doi: 10.1021/acs.molpharmaceut.0c00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R., Kumar V., Bharate S.S., Vishwakarma R.A. Bioorg. Med. Chem. 2017;25:5513. doi: 10.1016/j.bmc.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Kumar V., Bharate S.S., Vishwakarma R.A. Eur. J. Pharm. Sci. 2016;92:203. doi: 10.1016/j.ejps.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Bharate S.S., Vishwakarma R.A. Bioorg. Med. Chem. Lett. 2015;25:1561. doi: 10.1016/j.bmcl.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Ahad A., Shakeel F., Alfaifi O.A., Raish M., Ahmad A., Al-Jenoobi F.I., Al-Mohizea A.M. Int. J. Pharm. 2018;544:165. doi: 10.1016/j.ijpharm.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Vakili K., Rezaei H., Poturcu K., Jouyban A., Hanaee J., Martinez F., Rahimpour E. J. Mol. Liq. 2021;344 [Google Scholar]
- 19.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Stone R.M. Biochem. Pharmacol. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 20.Nuthakki V.K., Sharma A., Kumar A., Bharate S.B. Drug Dev. Res. 2019;80:655. doi: 10.1002/ddr.21544. [DOI] [PubMed] [Google Scholar]
- 21.Anonymous, https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021055s006lbl.pdf assessed on 18-December 2021.
- 22.Narazaki R., Sanghvi R., Yalkowsky S.H. Mol. Pharm. 2007;4:550. doi: 10.1021/mp070007o. [DOI] [PubMed] [Google Scholar]
- 23.Pole D.L. J. Pharm. Sci. 2008;97:1071. doi: 10.1002/jps.21060. [DOI] [PubMed] [Google Scholar]
- 24.Kapetanovic I.M., Muzzio M., Hu S.C., Crowell J.A., Rajewski R.A., Haslam J.L., Jong L., McCormick D.L. Cancer Chemother. Pharmacol. 2010;65:1109. doi: 10.1007/s00280-009-1116-4. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia A., Chopra S., Nagpal K., Deb P.K., Tekade M., Tekade R.K. Advances in Pharmaceutical Product Development and Research, Dosage Form Design Parameters. Academic Press; 2018. pp. 31–65. [Google Scholar]
- 26.Raw A.S., Furness M.S., Gill D.S., Adams R.C., Holcombe F.O., Jr., Yu L.X. Adv. Drug Deliv. Rev. 2004;56:397. doi: 10.1016/j.addr.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Bharate S.S., Vishwakarma R.A. Expert Opin. Drug Deliv. 2013;10:1239. doi: 10.1517/17425247.2013.783563. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhuri K.R. Expert Opin. Drug Deliv. 2008;5:1169. doi: 10.1517/17425240802500870. [DOI] [PubMed] [Google Scholar]
- 29.Bhavana V., Chavan R.B., Mannava M.K.C., Nangia A., Shastri N.R. Talanta. 2019;199:679. doi: 10.1016/j.talanta.2019.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.