Abstract
Hund’s multiplicity rule states that a higher spin state has a lower energy for a given electronic configuration1. Rephrasing this rule for molecular excited states predicts a positive energy gap between spin-singlet and spin-triplet excited states, as has been consistent with numerous experimental observations over almost a century. Here we report a fluorescent molecule that disobeys Hund’s rule and has a negative singlet–triplet energy gap of −11 ± 2 meV. The energy inversion of the singlet and triplet excited states results in delayed fluorescence with short time constants of 0.2 μs, which anomalously decrease with decreasing temperature owing to the emissive singlet character of the lowest-energy excited state. Organic light-emitting diodes (OLEDs) using this molecule exhibited a fast transient electroluminescence decay with a peak external quantum efficiency of 17%, demonstrating its potential implications for optoelectronic devices, including displays, lighting and lasers.
Subject terms: Physical chemistry, Organic LEDs
A fluorescent molecule is described that does not follow Hund’s rule and instead shows singlet and triplet excited states with inverted energy levels, leading to high-efficiency OLEDs with potential implications for optoelectronic devices.
Main
The spin multiplicity of molecular excited states plays a crucial role in organic optoelectronic devices. In the case of OLEDs, recombination of charge carriers leads to the formation of singlet and triplet excited states in a 1:3 ratio. This spin statistics limits the internal quantum efficiency of OLEDs and leads to the energy loss owing to the spin-forbidden nature of triplet excited states to emit photons. To overcome this issue, two strategies for harvesting the ‘dark’ triplet excited states as photons have been established. The first relies on organometallic complexes with transition metals, such as iridium and platinum, which induce a large spin–orbit coupling to allow triplet states to emit photons as phosphorescence2–4. The other uses organic molecules that exhibit thermally activated delayed fluorescence (TADF)5–7. This class of materials has energetically close singlet and triplet excited states, in which ambient thermal energy upconverts the triplet states into the singlet states through reverse intersystem crossing (RISC). Although the concept of TADF has the advantage of eliminating the need for transition metals, the resultant temporally delayed fluorescence typically has a time constant in the microsecond or even millisecond range, which is long enough for detrimental bimolecular annihilations, such as triplet–triplet annihilation and triplet–polaron annihilation, to compete with delayed fluorescence. These bimolecular annihilations lead to the decrease in device efficiency under high current densities, known as efficiency roll-off in OLEDs8,9, and also generate high-energy excitons that are suspected to cause chemical degradation of materials, particularly in blue OLEDs10. The research community has thus focused on minimizing the singlet–triplet energy gap (ΔEST) to accelerate the upconversion by thermal activation7. Alternatively, an ideal case would be thermodynamically favourable downconversion with negative ΔEST, which is not expected if applying Hund’s multiplicity rule to the lowest-energy excited state. Herein, we demonstrate experimental evidence of the existence of highly fluorescent organic molecules that disobey Hund’s rule and possess negative ΔEST for constructing efficient OLEDs.
Numerous observations of positive ΔEST in molecular excited states are generally understood by the exchange interaction, the quantum-mechanical effect involving Pauli repulsion, which stabilizes triplet states relative to singlet states11. ΔEST is simply equal to twice the positive exchange energy if the lowest-energy singlet and triplet excited states (S1 and T1) have the same single-excitation configuration11. Although there is general agreement that ΔEST must be positive, potentially negative ΔEST has been discussed in nitrogen-substituted phenalene analogues, such as cycl[3.3.3]azine and heptazine, during the past two decades12–21. Recent theoretical studies have also suggested the possibility of negative ΔEST in these molecules by accounting for double-excitation configurations in which two electrons of occupied orbitals have been promoted out to virtual orbitals15–19 (Supplementary Fig. 1). Because the Pauli exclusion principle restricts the accessible double-excitation configurations in T1, an effective admixture of such configurations stabilizes S1 relative to T1. If this stabilization overcomes the exchange energy, ΔEST could be a negative value (Fig. 1a). However, to the best of our knowledge, none of the molecules has been experimentally identified with negative ΔEST and the resultant delayed fluorescence from inverted singlet and triplet excited states (DFIST). We note that the accounting for double-excitation configurations has proved crucial to theoretically reproduce the small but positive ΔEST of 5,9-diphenyl-5,9-diaza-13b-boranaphtho[3,2,1-de]anthracene (DABNA-1) (0.15 eV)22,23.
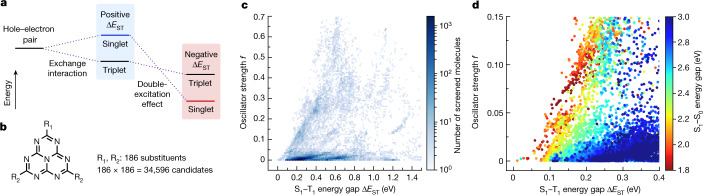
Fig. 1. Computational screening of heptazine analogues.
a, Schematic diagram of singlet and triplet excited states split in energy by the exchange interaction (middle) and then inverted by including the double-excitation effect (right). b, Molecular structures of the heptazine analogues examined in the computational screening. c, Number of screened molecules as a function of ΔEST and f calculated by TDDFT. d, S1–S0 energy gaps as a function of ΔEST and f calculated by TDDFT.
Pioneering computational calculations15 inspired us to focus on heptazine as a potential class of molecules that exhibit DFIST. Correlated wave function theories suggested that S1 of heptazine lies 0.2–0.3 eV below T1, although S1 is a ‘dark’ state, meaning that the electronic transition to the ground state (S0) is dipole-forbidden and the oscillator strength (f) is zero in the D3h symmetry point group. Notably, the heptazine core is shared by several synthesized molecules that exhibit intense TADF24,25 with positive ΔEST (refs. 26,27). Furthermore, the recent computational screening by Pollice et al. has demonstrated that appropriate chemical modifications of heptazine recover f while retaining negative ΔEST (ref. 19). As such, we introduced 186 different substituents to heptazine to generate 34,596 candidate molecules for computational screening. The structures of all substituents are available in Supplementary Fig. 2. To ensure the synthetic feasibility, at most two distinct types of substituents were introduced to the heptazine core as R1 and R2 (Fig. 1b). We used standard linear-response time-dependent density functional theory (TDDFT) to calculate ΔEST and f, which are more affordable in computational cost than those calculated by correlated wave function theories. Although the commonly used adiabatic approximation in TDDFT does not account for double-excitation character16,28, the properties calculated by TDDFT are still useful to prescreening for narrowing the list of the candidate molecules before the high-cost calculations and experimental evaluation, as both S1 and T1 of heptazine are almost dominated by the single-excitation configuration between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO)15.
Figure 1c shows the statistics of the screened molecules as a function of ΔEST and f calculated by TDDFT. A well-known trade-off between small ΔEST and large f is evident from this particular dataset of heptazine analogues. Although balancing such a trade-off is a key concern in recent synthetic efforts on TADF materials, Fig. 1c demonstrates the optimal combinations of ΔEST and f for which one parameter can no longer be improved without sacrificing the other. Figure 1d further visualizes the trade-off between ΔEST and f for each fluorescence colour. The screening data suggest 5,264 promising candidates to show fluorescence across the entire visible spectrum, with ΔEST < 0.35 eV and f > 0.01. Setting the range of the vertical S1–S0 energy gap to 2.70–2.85 eV for blue fluorescence further narrows down the candidates to 1,028 molecules, corresponding to 2.97% of all the screened molecules. We then assessed their synthetic feasibility and selected two heptazine analogues HzTFEX2 and HzPipX2 (Fig. 2a) for further evaluation. We note that these molecules recover f while retaining small ΔEST (f = 0.010 and 0.015 and ΔEST = 210 and 334 meV for HzTFEX2 and HzPipX2, respectively). This trend is consistent with the recent computational screening on heptazine analogues with asymmetrical substitutions19.
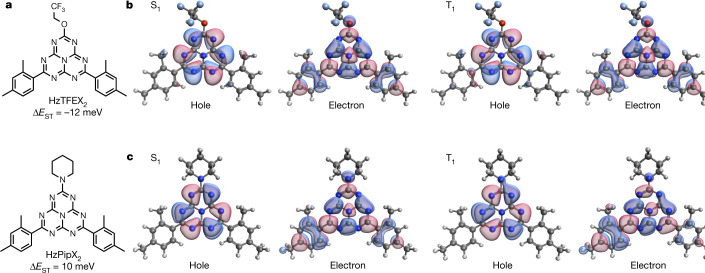
Fig. 2. Lead candidate molecules HzTFEX2 and HzPipX2.
a, Molecular structures of HzTFEX2 and HzPipX2 with ΔEST calculated by EOM-CCSD. b,c, Dominant pair of NTOs of S1 and T1 of HzTFEX2 (b) and HzPipX2 (c) for the EOM-CCSD calculations.
To examine whether HzTFEX2 and HzPipX2 could have negative ΔEST, we computed their S1 and T1 by correlated wave function theories. Equation-of-motion coupled cluster with single and double excitation (EOM-CCSD)29 calculations predict HzTFEX2 to possess negative ΔEST of −12 meV, affirming its potential for exhibiting DFIST. In comparison, ΔEST calculated for HzPipX2 remains at a positive value of 10 meV, which is comparable with those of the current state-of-the-art TADF materials30–38. Figure 2b,c shows the dominant pair of natural transition orbitals (NTOs)39 for S1 and T1. In both molecules, the hole orbitals are exclusively localized on the peripherical six nitrogen atoms of the heptazine core, whereas the electron orbitals are localized on the central nitrogen atom and the carbon atoms of the core, as well as on the substituents. The spatial separation of these orbitals indicates that the exchange interaction is weak, resulting in nearly degenerate S1 and T1 in the single-excitation picture. Similar spatial separations of NTOs have also been found in the multi-resonant TADF materials, such as DABNA-1 (refs. 22,23). In this situation, the stabilization of S1 by including the double-excitation configurations becomes more dominant to determine the sign of ΔEST. Indeed, S1 of both molecules comprise double-excitation configurations with weights of around 1% described as the sum of the squares of the doubles amplitudes in EOM-CCSD, which are slightly higher than those of T1. Two other wave-function-based calculations using second-order algebraic diagrammatic construction (ADC(2))40 and complete active space with second-order perturbation theory (CASPT2)41 further validate the inversion of S1 and T1 in HzTFEX2 with calculated ΔEST of −34 meV and −184 meV, respectively. However, for HzPipX2, the two methods also invert ΔEST (−12 meV with ADC(2) and −171 meV with CASPT2) as compared with the positive value of 10 meV predicted with EOM-CCSD (Supplementary Table 1). This variation in estimates of ΔEST highlights the current limitations of excited-state calculations and demands conclusive experimental evaluation. We note that ΔEST calculated by other second-order methods are given in the Supplementary Information, as well as the dependence of the choice of the guess orbitals and the size of the active space on the CASPT2 results.
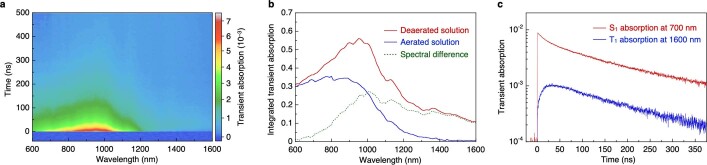
HzTFEX2 and HzPipX2 were synthesized by nucleophilic aromatic substitution of 2,5,8-trichloroheptazine with corresponding alcohol or amine, followed by Friedel–Crafts reactions with m-xylene. The details of the synthesis and characterization are given in the Supplementary Information. The photophysical properties of the two molecules were evaluated in deaerated toluene solutions (Fig. 3a and Extended Data Table 1). The steady-state absorption spectra of HzTFEX2 and HzPipX2 comprise the lowest-energy absorption band centred at 441 nm and 429 nm, respectively, with small molar absorption coefficients on the order of 103 M−1 cm−1, reflecting the spatial separation between the hole and electron NTOs computed for S1 of each molecule. On photoexcitation, HzTFEX2 exhibits blue emission with a peak wavelength (λPL) of 449 nm and a photoluminescence (PL) quantum yield (ΦPL) of 74%, whereas slightly blue-shifted λPL of 442 nm and similar ΦPL of 67% are observed for HzPipX2. These energy differences in absorption and emission are also predicted by TDDFT calculations and are attributed to the stronger electron-donating effect of the piperidyl group in HzPipX2 than that of 2,2,2-trifluoroethoxy group in HzTFEX2. In aerated toluene solutions, ΦPL of HzTFEX2 and HzPipX2 decrease to 54% and 37%, respectively. Because atmospheric O2 can quench molecular triplet excited states and the change in ΦPL is reversible, we ascribe the blue emissions of the two molecules, at least partially, to delayed fluorescence through forward intersystem crossing (ISC) and RISC between S1 and T1. This assumption is supported by transient absorption decay measurements on HzTFEX2, which scrutinized ISC from S1 to T1 as the signal decay of S1 at 700 nm and the signal growth of T1 at 1,600 nm, followed by the persistent signal decays of both S1 and T1 (Extended Data Fig. 1). We also note that both decays have similar time constants (223 ns for S1 and 210 ns for T1), indicating the steady-state condition with the constant population ratio maintained by ISC and RISC.
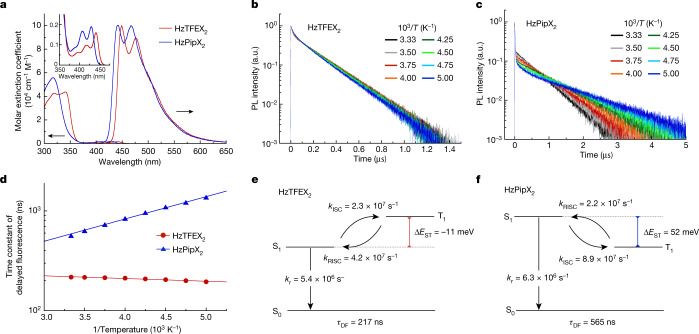
Fig. 3. Photophysical properties of HzTFEX2 and HzPipX2 in deaerated toluene solutions.
a, Steady-state absorption and PL spectra of HzTFEX2 and HzPipX2. The inset is the magnified view of the absorption spectra. b,c, Transient PL decays of HzTFEX2 (b) and HzPipX2 (c) at varying temperatures. d, Temperature dependence of τDF of HzTFEX2 and HzPipX2; the solid lines in d represent the fits of τDF to a single exponential in inverse temperature. e,f, Schematic diagram of the excited states and the associated transitions of HzTFEX2 (e) and HzPipX2 (f).
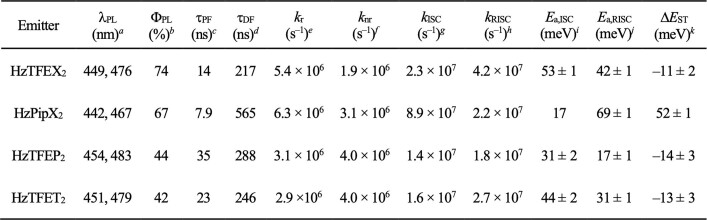
Extended Data Table 1.
Photophysical properties of HzTFEX2 and HzPipX2 in deaerated toluene solutions
aPhotoluminescence (PL) peak wavelength. bPL quantum yield. cTime constant of prompt fluorescence. dTime constant of delayed fluorescence. eRate constant of radiative decay of the lowest-energy excited state (S1) to the ground state (S0). fRate constant of non-radiative decay of S1 to S0. gRate constant of intersystem crossing (ISC) of S1 to the lowest-energy triplet excited state (T1). hRate constant of reverse intersystem crossing (RISC) of T1 to S1. iActivation energy of ISC. jActivation energy of RISC. kEnergy gap between S1 and T1.
Extended Data Fig. 1. Transient absorption data of HzTFEX2.
a, Transient absorption of HzTFEX2 as a function of wavelength and time in a deaerated toluene solution. b, Integrated transient absorption spectra of HzTFEX2 over 0–500 ns in deaerated and aerated toluene solutions; the dashed green dashed line represents their spectral difference and mainly corresponds to the transient absorption of T1. c, Transient absorption decays of S1 and T1 monitored at 700 nm and 1,600 nm, respectively.
To show the excited-state kinetics of the two molecules in detail, we performed transient PL decay measurements at varying temperatures (Fig. 3b,c and Supplementary Fig. 3 for the log–log representation). Both molecules exhibit biexponential transient PL decays, which comprise nanosecond-order prompt fluorescence followed by sub-microsecond delayed fluorescence with temperature-dependent time constants. Remarkably, the time constant of delayed fluorescence (τDF) of HzTFEX2 gradually decreases from 217 ns to 195 ns with decreasing temperature from 300 K to 200 K (Fig. 3d). This anomalous temperature dependence of τDF indicates that S1 lies energetically below T1, for which lowering the temperature shifts the steady-state population towards emissive S1 relative to dark T1 and thus accelerates the delayed fluorescence (that is, decreases τDF). In comparison, τDF of HzPipX2 increases from 565 ns to 1,372 ns by the same temperature decrease, as has been similarly observed in conventional TADF materials5–7. It is worth noting that τDF of HzTFEX2 is much shorter than emission time constants ever reported for TADF materials30–38 and phosphorescent materials2–4 used for efficient OLEDs, which are typically in the microsecond range.
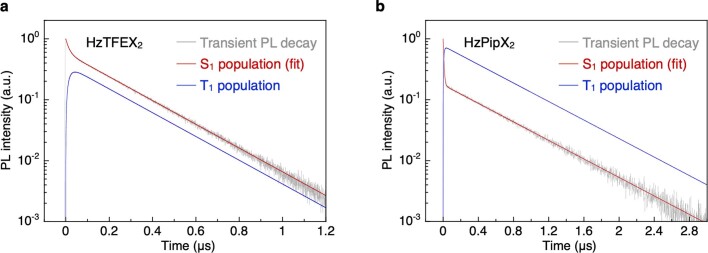
We further analysed the temperature-dependent PL decay kinetics with the underlying rate equation. In the absence of phosphorescence and non-radiative decay of T1 to S0, the rate equation for the populations of S1 and T1 is given by
| 1 |
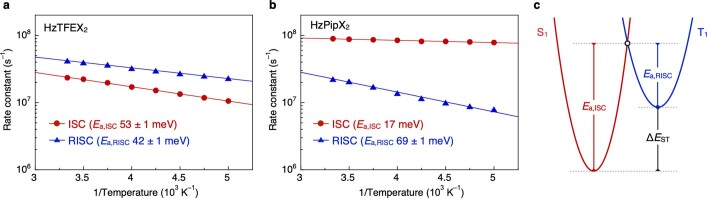
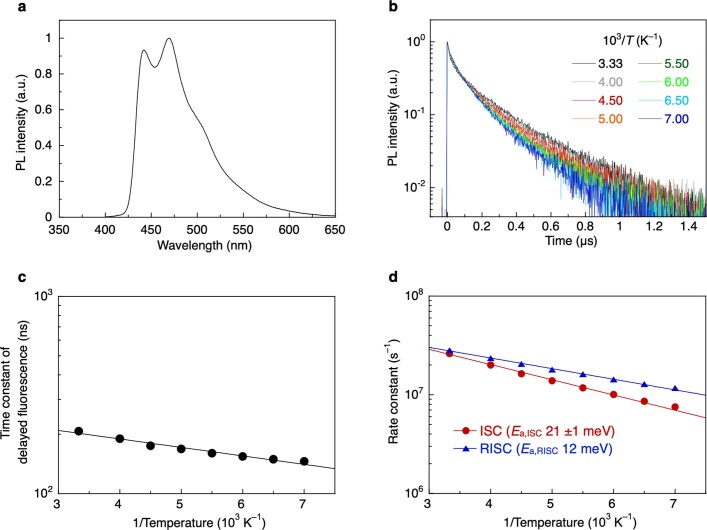
in which kr, knr, kISC and kRISC are the rate constants of radiative decay of S1 to S0, non-radiative decay of S1 to S0, ISC of S1 to T1 and RISC of T1 to S1, respectively. By numerically fitting equation (1) to the PL decay data at 300 K, we found that RISC is faster than ISC in HzTFEX2 (kRISC = 4.2 × 107 s−1 versus kISC = 2.3 × 107 s−1), whereas RISC is slower than ISC in HzPipX2 (kRISC = 2.2 × 107 s−1 versus kISC = 8.9 × 107 s−1) (Fig. 3e,f). These parameters simulate that the population of T1 is lower than that of S1 in HzTFEX2 under the steady-state condition, indicating that S1 lies energetically below T1 (Extended Data Fig. 2). Furthermore, the temperature dependence of kISC and kRISC follows the Arrhenius equation, k = Aexp(−Ea/kBT), in which k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, kB is the Boltzmann constant and T is the absolute temperature (Extended Data Fig. 3). The best-fit parameters of the Arrhenius equation yield the activation energies of ISC and RISC (Ea,ISC and Ea,RISC) (Extended Data Table 1). Subtracting Ea,ISC from Ea,RISC, we determined ΔEST of HzTFEX2 to be −11 ± 2 meV, which is in marked contrast to positive ΔEST ever observed in numerous molecules, as well as in HzPipX2 (ΔEST = 52 ±1 meV). We note that the change in kr + knr at varying temperatures is negligible compared with those in kISC and kRISC (Supplementary Fig. 4) and thus the decreasing trend of τDF of HzTFEX2 is more reasonably attributed to the inverted S1 and T1. The negative ΔEST of HzTFEX2 is retained in a solid-state host matrix (see Extended Data Fig. 4 and the Supplementary Information for details).
Extended Data Fig. 2. Analysis of the transient PL decays of HzTFEX2 and HzPipX2.
a,b, Fit (solid red line) of the S1 population in equation (1) to the transient PL decay of HzTFEX2 (a) and HzPipX2 (b) in deaerated toluene solutions at 300 K. The solid blue line represents the T1 population simulated by the best-fit parameters kr + knr, kISC and kRISC in equation (1).
Extended Data Fig. 3. kISC and kRISC of HzTFEX2 and HzPipX2.
a,b, Temperature dependence of kISC and kRISC of HzTFEX2 (a) and HzPipX2 (b) in deaerated toluene. The solid lines in a and b represent the fits of the plots to the Arrhenius equation. The error bars of the plots in a and b are smaller than the plot size. c, Schematic diagram of the potential energy surfaces of S1 and T1 and the activation energies of ISC and RISC.
Extended Data Fig. 4. Photophysical properties of HzTFEX2 in a solid-state host matrix.
a, Steady-state PL spectra of a thin film of bis(diphenylphosphoryl)dibenzo[b,d]furan (PPF):1 wt% HzTFEX2. b, Transient PL decays of PPF:1 wt% HzTFEX2 at varying temperatures under a N2 atmosphere. c, Temperature dependence of τDF of PPF:1 wt% HzTFEX2. d, Temperature dependence of kISC and kRISC of PPF:1 wt% HzTFEX2. The solid lines in d represent the fits of the plots to the Arrhenius equation.
Having experimentally determined negative ΔEST, we conclude that HzTFEX2 exhibits DFIST. Further synthetic efforts replacing the xylyl groups in HzTFEX2 with either phenyl or tolyl groups led to HzTFEP2 and HzTFET2, which similarly show DFIST with measured ΔEST of −14 ± 3 meV and −13 ± 3 meV, respectively (see Extended Data Table 1 and Supplementary Fig. 5 for details), indicating the potential of heptazines for further developing efficient DFIST materials. In common with the three materials, ISC from S1 to T1 competes with the inherently slow radiative decay of heptazines, followed by faster RISC, leading to a significant S1 population relative to T1 and sub-microsecond DFIST. Thus, we propose to refer to the present type of emissions as ‘H (heptazine)-type delayed fluorescence’ by analogy with ‘E (eosin)-type delayed fluorescence’ referred to as TADF42 and ‘P (pyren)-type delayed fluorescence’ involving triplet–triplet annihilation43.
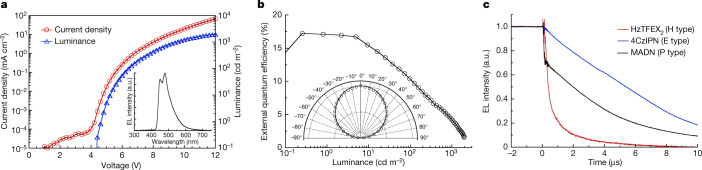
Finally, we evaluated the electroluminescence (EL) properties of HzTFEX2 in OLEDs fabricated by thermal evaporation. The details of the fabrication procedures and the device structures are given in the Supplementary Information. Figure 4a,b shows the EL spectra, current density–voltage–luminance characteristics and external quantum efficiency–luminance characteristics of the OLED. Intense blue EL originating from HzTFEX2 was observed with spectral peak wavelengths (λEL) at 450 nm and 479 nm and Commission internationale de l’éclairage (CIE) coordinates of (0.17, 0.24). The maximum external quantum efficiency reached 17%, corresponding to the internal quantum efficiency of 80% for a bottom-emission OLED with a typical light-outcoupling efficiency of 20% 44. We note that the viewing-angle dependence of the luminance followed the Lambertian distribution (Fig. 4b inset), ensuring accurate estimation of the external quantum efficiency from the forward emission. Remarkably, HzTFEX2 exhibited fast transient EL decay, reflecting the sub-microsecond H-type delayed fluorescence (Fig. 4c). In comparison, much slower transient EL decays were observed for E-type delayed fluorescence of 2,4,5,6-tetra(carbazol-9-yl)isophthalonitrile (4CzIPN)6 and P-type delayed fluorescence of 2-methyl-9,10-bis(naphthalen-2-yl)anthracene (MADN)45, although the EL of MADN initially decayed faster by the prompt fluorescence solely from S1 (ref. 46). It is thus evident that the fast triplet harvesting of HzTFEX2 with negative ΔEST can be retained even in actual OLEDs. Although the efficiency roll-off is still marked in this preliminary device concerning the large hole injection barrier caused by the high ionization potential of HzTFEX2 (6.3 eV), we anticipate that further optimization of molecular design will address this issue and allow a conclusive exploration of the effects of negative ΔEST on efficiency roll-off and device stability.
Fig. 4. OLED performance.
a,b, Current density–voltage–luminance characteristics (a) and external quantum efficiency–luminance characteristics (b) of the fabricated OLED using HzTFEX2; the inset in a shows the EL spectra measured at 1.0 mA and the inset in b represents the viewing-angle dependence of the luminance, which is almost consistent with the Lambertian distribution. c, Transient EL decays of the OLEDs using HzTFEX2, 4CzIPN and MADN, respectively, measured in pulse operation with square-wave voltages of 8 V and −4 V.
In conclusion, we have demonstrated fluorescent heptazine molecules that possess negative ΔEST. We observed their blue delayed fluorescence in both PL and EL with anomalous features: (1) the very short decay time constants (τDF ≈ 0.2 μs), (2) the decreasing trend of τDF with decreasing temperature and (3) the rate inversion of RISC and ISC (kRISC > kISC). These features indeed arise from negative ΔEST and led to the terminology ‘delayed fluorescence from inverted singlet and triplet excited states (DFIST)’ or ‘H (heptazine)-type delayed fluorescence’. We predict that further development of DFIST materials will offer stable and efficient OLEDs based on the fast triplet-to-singlet downconversion, with great implications for displays, lighting and lasers.
Methods
Quantum-chemical calculations
For the 34,596 heptazine molecules, the T1 geometries were optimized using spin-unrestricted DFT with the LC-BLYP functional and the 6-31G basis set. Vibrational frequency analysis for HzTFEX2, HzPipX2, HzTFEP2 and HzTFET2 gave no imaginary frequencies at the same level of theory. The vertical excitation energies of S1 and T1 were calculated using liner-response TDDFT with the LC-BLYP functional and the 6-31G(d) basis set within the Tamm–Dancoff approximation. The range-separation parameter of the LC-BLYP functional was non-empirically optimized to 0.18 bohr−1 to minimize the difference between the energy of the HOMO and the ionization potential of the neutral system and the difference between the energy of the HOMO of the radical anion system and the electron affinity of the neutral system47 of 2,5,8-triphenylheptazine. The T1 geometries of HzTFEX2 and HzPipX2 were also optimized using spin-unrestricted second-order Møller–Plesset perturbation theory (MP2) with the correlation consistent cc-pVDZ basis set. At the MP2 geometries of HzTFEX2 and HzPipX2, the vertical excitation energies of S1 and T1 were calculated using EOM-CCSD29, ADC(2)40 and CASPT241 with the cc-pVDZ basis set. The CASPT2 calculations were performed with the fully internally contracted scheme over the state-averaged complete active space self-consistent field (CASSCF) wavefunctions with the active space of 12 electrons and 12 orbitals using the resolution of identity approximation with the auxiliary fitting basis set. The DFT, TDDFT, MP2 and EOM-CCSD calculations were performed using the Gaussian 16 Rev C.01 program. The ADC(2) calculations were performed using the Q-Chem 5.3.0 program. The CASSCF and CASPT2 calculations were performed using the Orca 4.2.1 program.
Materials and synthesis
Commercially available reagents and solvents were used without further purification unless otherwise noted. 4CzIPN and MADN were purchased from Luminescence Technology Corporation and e-Ray Optoelectronics Technology, respectively. The synthetic procedures and characterization data of the heptazine molecules are detailed in the Supplementary Information.
Photophysical measurements
Steady-state ultraviolet–visible absorption spectra were recorded on a Shimadzu UV-3600i Plus spectrophotometer. Steady-state PL spectra were acquired on a HORIBA FL3 spectrofluorometer with 370-nm photoexcitation from a Xe arc lamp. The absolute PL quantum yields were determined using a Hamamatsu Photonics C9920 integrated sphere system with 370-nm excitation from a Xe arc lamp. Transient absorption decay measurements were performed by a randomly interleaved plus train method48 on a UNISOKU picoTAS system with a 355-nm Q-switched laser pump source (pulse width <350 ps) and a supercontinuum white probe source (pulse width <100 ps). Transient PL decay measurements were performed by time-correlated single-photon counting on a HORIBA FL3 spectrofluorometer with a 370-nm LED pump source (pulse width <1.2 ns) and a UNISOKU CoolSpek cryostat using liquid nitrogen as the coolant. Ionization potentials were determined using a RIKEN KEIKI AC-3 ultraviolet photoelectron yield spectrometer.
Analysis of transient PL decay kinetics
The time constants of prompt and delayed fluorescence (τPF and τDF) were determined by biexponential decay fitting and deconvolution with the instrument response function. It is common when determining the rate constants of the transitions involved in TADF to assume kISC >> kRISC such that the contribution of RISC to the prompt fluorescence is negligible49. However, this assumption does not hold true for DFIST materials with kRISC > kISC. Thus, kr + knr, kISC and kRISC were determined without assuming kISC >> kRISC by fitting the S1 population in equation (1) to the transient PL decay data using the scipy.integrate.odeint and scipy.optimize.curve_fit functions in Python 3.750. kr and knr were determined from ΦPL = kr/(kr + knr) assuming negligible non-radiative decay of T1 to S0. Activation energies of ISC and RISC (Ea,ISC and Ea,RISC) were determined by fitting the Arrhenius equation to the temperature dependence of kISC and kRISC, respectively. ΔEST was determined by subtracting Ea,ISC from Ea,RISC.
OLED fabrication and evaluation
The fabrication procedures of OLEDs are detailed in the Supplementary Information. EL spectra were recorded using a Hamamatsu Photonics PMA-12 photonic multichannel analyser. Current density–voltage–luminance characteristics were measured using a Konica Minolta CS-200 luminance meter and a Keithley 2400 source meter. The viewing-angle dependence of luminance was measured using a home-build spectro-goniometer with a Konica Minolta CS-2000 spectroradiometer. Transient EL decays measurements were performed using a home-build set-up with a Hamamatsu Photonics H7826 silicon photomultiplier tube (time response = 1.5 ns) and an Agilent 33220A function generator for pulse OLED operation (square-wave voltages = 8, −4 V and frequency = 2 kHz).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-05132-y.
Supplementary information
This file contains Supplementary Figs. 1–12, Supplementary Materials and Methods and Supplementary Tables 1–12: see contents page for details.
Acknowledgements
This work was supported in part by an Industrial Technology Research Grant for Young Researchers from the New Energy and Industrial Technology Development Organization (NEDO) (grant no. 09151455 to Y.-J.P.), JST PRESTO (grant nos. 13417316 to Y.-J.P. and JPMJPR17N1 to N.A.) and JSPS KAKENHI (grant nos. 24685029, 17H03103, 20H02554 to Y.-J.P. and 20K15252, 21H05413, 22H02051 to N.A.). The authors thank M. Kim at RIKEN CEMS, T. Chiba, and M. Hirasawa at Yamagata University for their support with the OLED fabrication and evaluation. The computations were partially performed using the HOKUSAI Big Waterfall system at RIKEN.
Extended data figures and tables
Author contributions
N.A. and D.M. conceived the project. N.A., Y.H. and S.M. conceived the procedures of the computational calculations. N.A., Y.-J.P., Y.H., K.N. and S.M. performed the computational calculations. N.A., H.I. and Y.-J.P. performed the photophysical measurements. N.A., Y.K. and Y.-J.P. fabricated and evaluated the OLEDs. B.D., R.I., H.I. and A.N. performed synthetic experiments and assisted the characterization of the synthesized compounds and analysis of data. N.A., Y.-J.P., F.A. and D.M. designed the experiments and analysed the data. N.A., Y.-J.P. and D.M. wrote the manuscript.
Peer review
Peer review information
Nature thanks Pierre Audebert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data underlying this article are available at 10.6084/m9.figshare.20058977.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naoya Aizawa, Email: aizawa@chem.eng.osaka-u.ac.jp.
Yong-Jin Pu, Email: yongjin.pu@riken.jp.
Daigo Miyajima, Email: daigo.miyajima@riken.jp.
Extended data
is available for this paper at 10.1038/s41586-022-05132-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-05132-y.
References
- 1.Hund F. Zur Deutung verwickelter Spektren, insbesondere der Elemente Scandium bis Nickel. Z. Phys. 1925;33:345–371. [Google Scholar]
- 2.Baldo MA, et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature. 1998;395:151–154. [Google Scholar]
- 3.Baldo MA, Lamansky S, Burrows P, Thompson ME, Forrest SR. Very high-efficiency green organic light-emitting devices based on electrophosphorescence. Appl. Phys. Lett. 1999;75:4–6. [Google Scholar]
- 4.Reineke S, et al. White organic light-emitting diodes with fluorescent tube efficiency. Nature. 2009;459:234–238. doi: 10.1038/nature08003. [DOI] [PubMed] [Google Scholar]
- 5.Endo A, et al. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 2011;98:083302. [Google Scholar]
- 6.Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature. 2012;492:234–238. doi: 10.1038/nature11687. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Li C, Ren Z, Yan S, Bryce MR. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018;3:18020. [Google Scholar]
- 8.Baldo MA, Adachi C, Forrest SR. Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys. Rev. B. 2000;62:10967. [Google Scholar]
- 9.Murawski C, Leo K, Gather MC. Efficiency roll-off in organic light-emitting diodes. Adv. Mater. 2013;25:6801–6827. doi: 10.1002/adma.201301603. [DOI] [PubMed] [Google Scholar]
- 10.Schmidbauer S, Hohenleutner A, König B. Chemical degradation in organic light-emitting devices: mechanisms and implications for the design of new materials. Adv. Mater. 2013;25:2114–2129. doi: 10.1002/adma.201205022. [DOI] [PubMed] [Google Scholar]
- 11.Slater JC. The theory of complex spectra. Phys. Rev. 1929;34:1293. [Google Scholar]
- 12.Ziegler T, Rauk A, Baerends EJ. On the calculation of multiplet energies by the Hartree–Fock–Slater method. Theor. Chem. Acc. 1977;43:261–271. [Google Scholar]
- 13.Leupin W, Wirz J. Low-lying electronically excited states of cycl[3.3.3]azine, a bridged 12π-perimeter. J. Am. Chem. Soc. 1980;102:6068–6075. [Google Scholar]
- 14.Leupin W, Magde D, Persy G, Wirz J. 1,4,7-Triazacycl[3.3.3]azine: basicity, photoelectron spectrum, photophysical properties. J. Am. Chem. Soc. 1986;108:17–22. [Google Scholar]
- 15.Ehrmaier J, et al. Singlet–triplet inversion in heptazine and in polymeric carbon nitrides. J. Phys. Chem. A. 2019;123:8099–8108. doi: 10.1021/acs.jpca.9b06215. [DOI] [PubMed] [Google Scholar]
- 16.de Silva P. Inverted singlet–triplet gaps and their relevance to thermally activated delayed fluorescence. J. Phys. Chem. Lett. 2019;10:5674–5679. doi: 10.1021/acs.jpclett.9b02333. [DOI] [PubMed] [Google Scholar]
- 17.Sanz-Rodrigo J, Ricci G, Olivier Y, Sancho-Garcia J-C. Negative singlet–triplet excitation energy gap in triangle-shaped molecular emitters for efficient triplet harvesting. J. Phys. Chem. A. 2021;125:513–522. doi: 10.1021/acs.jpca.0c08029. [DOI] [PubMed] [Google Scholar]
- 18.Ricci G, San-Fabián E, Olivier Y, Sancho-García J-C. Singlet-triplet excited‐state inversion in heptazine and related molecules: assessment of TD‐DFT and ab initio methods. ChemPhysChem. 2021;22:553–560. doi: 10.1002/cphc.202000926. [DOI] [PubMed] [Google Scholar]
- 19.Pollice R, Friederich P, Lavigne C, dos Passos Gomes G, Aspuru-Guzik A. Organic molecules with inverted gaps between first excited singlet and triplet states and appreciable fluorescence rates. Matter. 2021;4:1654–1682. [Google Scholar]
- 20.Sobolewski AL, Domcke W. Are heptazine-based organic light-emitting diode chromophores thermally activated delayed fluorescence or inverted singlet–triplet systems? J. Phys. Chem. Lett. 2021;12:6852–6860. doi: 10.1021/acs.jpclett.1c01926. [DOI] [PubMed] [Google Scholar]
- 21.Audebert P, Kroke E, Posern C, Lee S-H. State of the art in the preparation and properties of molecular monomeric s‐heptazines: syntheses, characteristics, and functional applications. Chem. Rev. 2021;121:2515–2544. doi: 10.1021/acs.chemrev.0c00955. [DOI] [PubMed] [Google Scholar]
- 22.Hatakeyama T, et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 2016;28:2777–2781. doi: 10.1002/adma.201505491. [DOI] [PubMed] [Google Scholar]
- 23.Pershin A, et al. Highly emissive excitons with reduced exchange energy in thermally activated delayed fluorescent molecules. Nat. Commun. 2019;10:597. doi: 10.1038/s41467-019-08495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galmiche L, Allain C, Le T, Guillot R, Audebert P. Renewing accessible heptazine chemistry: 2,5,8-tris(3,5-diethyl-pyrazolyl)-heptazine, a new highly soluble heptazine derivative with exchangeable groups, and examples of newly derived heptazines and their physical chemistry. Chem. Sci. 2019;10:5513–5518. doi: 10.1039/c9sc00665f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le T, Galmiche L, Masson G, Allain C, Audebert P. A straightforward synthesis of a new family of molecules: 2,5,8-trialkoxyheptazines. Application to photoredox catalyzed transformations. Chem. Commun. 2020;56:10742–10745. doi: 10.1039/d0cc05118g. [DOI] [PubMed] [Google Scholar]
- 26.Li J, et al. Highly efficient organic light-emitting diode based on a hidden thermally activated delayed fluorescence channel in a heptazine derivative. Adv. Mater. 2013;25:3319–3323. doi: 10.1002/adma.201300575. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Zhang Q, Nomura H, Miyazaki H, Adachi C. Thermally activated delayed fluorescence from 3nπ* to 1nπ* up-conversion and its application to organic light-emitting diodes. Appl. Phys. Lett. 2014;105:013301. [Google Scholar]
- 28.Elliott P, Goldson S, Canahui C, Maitra NT. Perspectives on double-excitations in TDDFT. Chem. Phys. 2011;391:110–119. [Google Scholar]
- 29.Stanton JF, Bartlett RJ. The equation of motion coupled-cluster method. A systematic biorthogonal approach to molecular excitation energies, transition probabilities, and excited state properties. J. Chem. Phys. 1993;98:7029–7039. [Google Scholar]
- 30.Di D, et al. High-performance light-emitting diodes based on carbene-metal-amides. Science. 2017;356:159–163. doi: 10.1126/science.aah4345. [DOI] [PubMed] [Google Scholar]
- 31.Zeng W, et al. Achieving nearly 30% external quantum efficiency for orange–red organic light emitting diodes by employing thermally activated delayed fluorescence emitters composed of 1,8‐naphthalimide‐acridine hybrids. Adv. Mater. 2018;30:1704961. doi: 10.1002/adma.201704961. [DOI] [PubMed] [Google Scholar]
- 32.Wu T-L, et al. Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off. Nat. Photonics. 2018;12:235–240. [Google Scholar]
- 33.Dos Santos PL, et al. Triazatruxene: a rigid central donor unit for a D–A3 thermally activated delayed fluorescence material exhibiting sub-microsecond reverse intersystem crossing and unity quantum yield via multiple singlet–triplet state pairs. Adv. Sci. 2018;5:1700989. doi: 10.1002/advs.201700989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo Y, et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photonics. 2019;13:678–682. [Google Scholar]
- 35.Hamze R, et al. Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime. Science. 2019;363:601–606. doi: 10.1126/science.aav2865. [DOI] [PubMed] [Google Scholar]
- 36.Kim JU, et al. Nanosecond-time-scale delayed fluorescence molecule for deep-blue OLEDs with small efficiency rolloff. Nat. Commun. 2020;11:1765. doi: 10.1038/s41467-020-15558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui L-S, et al. Fast spin-flip enables efficient and stable organic electroluminescence from charge-transfer states. Nat. Photonics. 2020;14:636–642. [Google Scholar]
- 38.Wada Y, Nakagawa H, Matsumoto S, Wakisaka Y, Kaji H. Organic light emitters exhibiting very fast reverse intersystem crossing. Nat. Photonics. 2020;14:643–649. [Google Scholar]
- 39.Martin RL. Natural transition orbitals. J. Chem. Phys. 2003;118:4775–4777. [Google Scholar]
- 40.Schirmer J. Beyond the random-phase approximation: a new approximation scheme for the polarization propagator. Phys. Rev. A. 1982;26:2395. [Google Scholar]
- 41.Andersson K, Malmqvist PÅ, Roos BO. Second-order perturbation theory with a complete active space self‐consistent field reference function. J. Chem. Phys. 1992;96:1218–1226. [Google Scholar]
- 42.Parker C, Hatchard C. Triplet-singlet emission in fluid solutions. Phosphorescence of eosin. Trans. Faraday Soc. 1961;57:1894–1904. [Google Scholar]
- 43.Parker C. Sensitized P-type delayed fluorescence. Proc. R. Soc. Lond. A Math. Phys. Sci. 1963;276:125–135. [Google Scholar]
- 44.Nowy S, Krummacher BC, Frischeisen J, Reinke NA, Brütting W. Light extraction and optical loss mechanisms in organic light-emitting diodes: influence of the emitter quantum efficiency. J. Appl. Phys. 2008;104:123109. [Google Scholar]
- 45.Lee M-T, Chen H-H, Liao C-H, Tsai C-H, Chen CH. Stable styrylamine-doped blue organic electroluminescent device based on 2-methyl-9,10-di(2-naphthyl)anthracene. Appl. Phys. Lett. 2004;85:3301–3303. [Google Scholar]
- 46.Pu Y-J, et al. Absence of delayed fluorescence and triplet–triplet annihilation in organic light emitting diodes with spatially orthogonal bianthracenes. J. Mater. Chem. C. 2019;7:2541–2547. [Google Scholar]
- 47.Baer R, Livshits E, Salzner U. Tuned range-separated hybrids in density functional theory. Annu. Rev. Phys. Chem. 2010;61:85–109. doi: 10.1146/annurev.physchem.012809.103321. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa T, Okamoto K, Hanada H, Katoh R. Probing with randomly interleaved pulse train bridges the gap between ultrafast pump-probe and nanosecond flash photolysis. Opt. Lett. 2016;41:1498–1501. doi: 10.1364/OL.41.001498. [DOI] [PubMed] [Google Scholar]
- 49.Goushi K, Yoshida K, Sato K, Adachi C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photonics. 2012;6:253–258. [Google Scholar]
- 50.Haase N, et al. Kinetic modeling of transient photoluminescence from thermally activated delayed fluorescence. J. Phys. Chem. C. 2018;122:29173–29179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Figs. 1–12, Supplementary Materials and Methods and Supplementary Tables 1–12: see contents page for details.
Data Availability Statement
The data underlying this article are available at 10.6084/m9.figshare.20058977.