Abstract
An orchestrated cellular network, including adaptive lymphocytes and group 3 innate lymphoid cells (ILC3s), maintains intestinal barrier integrity and homeostasis. T cells can monitor environmental insults through constitutive circulation, scanning tissues and forming immunological contacts, a process named immunosurveillance. In contrast, the dynamics of intestinal ILC3s are unknown. Using intravital imaging, we observed that villus ILC3s were largely immotile at steady state but acquired migratory ‘patrolling’ attributes and enhanced cytokine expression in response to inflammation. We showed that T cells, the chemokine CCL25 and bacterial ligands regulated intestinal ILC3 behavior and that loss of patrolling behavior by interleukin-22 (IL-22)-producing ILC3s altered the intestinal barrier through increased epithelial cell death. Collectively, we identified notable differences between the behavior of ILC3s and T cells, with a prominent adaptation of intestinal ILC3s toward mucosal immunosurveillance after inflammation.
Subject terms: Innate lymphoid cells, Imaging the immune system, Cell migration, Mucosal immunology
Serafini and colleagues show that intestinal villus ILC3s, which are largely immotile at steady state, develop a patrolling behavior in response to inflammation.
Main
To support intestinal barrier function, both the innate and adaptive immune systems produce IL-22, which orchestrates an immune–epithelial cross talk1. An effective IL-22-dependent epithelial response involves the production of antimicrobial peptides that not only contain commensal communities but also restrict pathogenic infections2,3. In the intestine, ILC3srepresent an early dominant source of IL-22, which is critical for coordinating barrier maintenance at steady state and during bacterial infection4,5. While ILC3 activation signals are characterized4–7, the spatiotemporal regulation of intestinal ILC3 responses is poorly understood. While ILC3 localization should allow rapid responses to environmental and pathogenic signals8, little is known about ILC3 intratissue dynamics. In particular, whether ILC3s migrate and adapt their cellular behavior in response to environmental cues is to be characterized. In this study, we show that ILC3s in the intestinal villus are largely immotile under steady-state conditions. After activation, these cells acquire a patrolling behavior and IL-22 production, which contribute to maintaining the intestinal barrier integrity. Thus, our data reveal a prominent tissue adaptation of ILC3s to environmental signals, promoting protective immune responses in the gut.
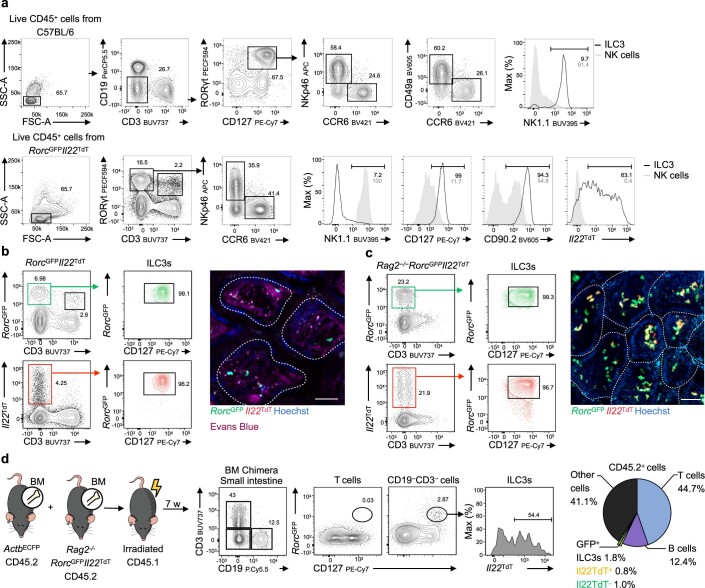
As ILC3s express the transcription factor RORγt and produce IL-22 (encoded by Rorc and Il22; Extended Data Fig. 1a), we applied intravital multiphoton imaging in RorcGFPIl22TdT reporter mice to identify RorcGFP+ ILC3s and RorcGFP+Il22TdT+ ILC3s in vivo (Extended Data Fig. 1b)9. Because RorcGFP+ helper T cells are also labeled using this approach, we created Rag2−/−RorcGFPIl22TdT reporter mice to selectively visualize and track Il22TdT+ ILC3s (Extended Data Fig. 1c). To study ILC3 responses in a lymphocyte-replete setting, we generated mixed bone marrow chimeras in which irradiated wild-type (WT) recipient mice received bone marrow nucleated cells from Rag2−/−RorcGFPIl22TdT and WT mice expressing cyan fluorescent protein (CFP) under the control of the actin promoter (ActbECFP). Seven weeks later, ActbECFP+ lymphocytes, as well as RorcGFP+ ILC3s, 54% of which were RorcGFP+Il22TdT+ ILC3s could be detected in the small intestine of the bone marrow (Extended Data Fig. 1d).
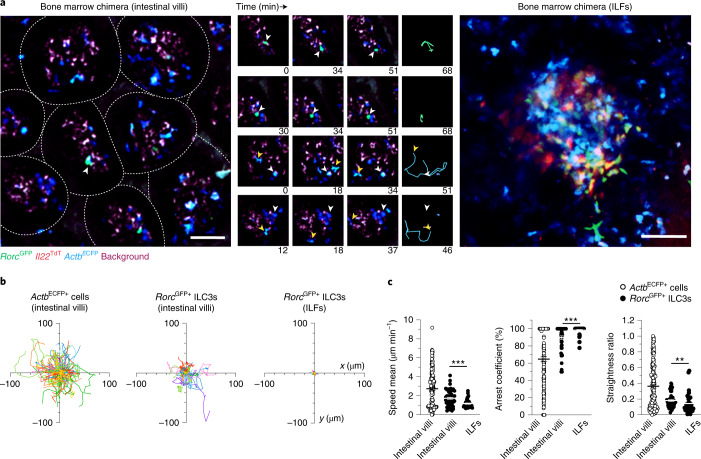
Because intestinal ILC3s are distributed in lamina propria villi and crypts8, we used intravital imaging to study ILC3 populations in the bone marrow chimera from both of these sites. We observed intestinal RorcGFP+ ILC3s and a larger population of ActbECFP+ lymphocytes within the intestinal villi (Fig. 1a and Supplementary Video 1). ActbECFP+ lymphocytes exhibited diverse in vivo dynamics, ranging from immobility to rapid migration (Fig. 1b,c), which likely reflected heterogeneity within the ActbECFP+ population, containing among other things B, T and myeloid cells. RorcGFP+ ILC3s in the intestinal villi were mostly nonmotile cells that lacked Il22TdT expression (Fig. 1a and Supplementary Video 1). RorcGFP+ ILC3s displayed low speed mean (<2 μm min−1) and were largely arrested (arrest coefficient approximately 90%) and confined (straightness ratio approximately 0.18) (Fig. 1b,c). In contrast, RorcGFP+ ILC3s in intestinal crypts were clustered in isolated lymphoid follicles (ILFs) and abundantly expressed Il22 transcripts (Fig. 1a and Supplementary Video 2), as reported previously10. ILC3s residing in ILFs exhibited even more restricted motility compared to villus ILC3s (Fig. 1b,c). These observations identified specific features of compartmentalized intestinal ILC3s with overall limited motility and stronger IL-22 expression in crypts at steady state.
Fig. 1. Intestinal ILC3s are compartmentalized and poorly motile at steady state.
a–c, Multiphoton microscopy of the small intestine of mixed bone marrow chimeras generated by injection of whole bone marrow CD45.2+ cells from ActbCFP and Rag2−/−RorcGFPIl22TdT mice into lethally irradiated congenic CD45.1+ C57BL/6J mice 7 weeks post-transfer. a, Representative image (left; scale bar, 50 µm) and time-lapse images (right; scale bar, 15 µm) of ILC3s and CFP+ cells in intestinal villi or ILF of bone marrow chimera. b, Individual tracks of intestinal ActbECFP+ cells and RorcGFP+ ILC3s in intestinal villi or ILF. c, Mean speed, arrest coefficient and straightness ratio of indicated populations in the intestine. Results in b,c are from two (ActbECFP+ cells and RorcGFP+ ILC3s in ILFs) or nine movies (RorcGFP+ ILC3s in intestinal villi) obtained in two independent experiments (n = 170 ActbECFP+ cells; n = 49 RorcGFP+ ILC3ss (intestinal villi); n = 38 RorcGFP+ ILC3s (ILFs)). Each line corresponds to the mean ± s.e.m. of the values obtained; only ILC3s were tested (**P < 0.002; ***P < 0.001; two-tailed Mann–Whitney U-test; exact P values are provided in the source data).
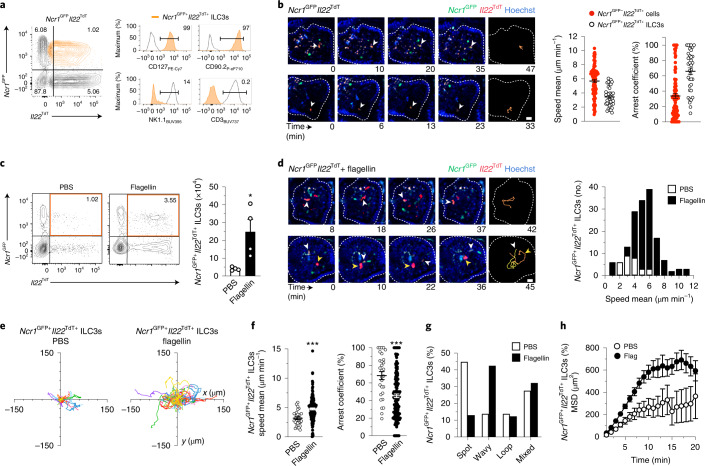
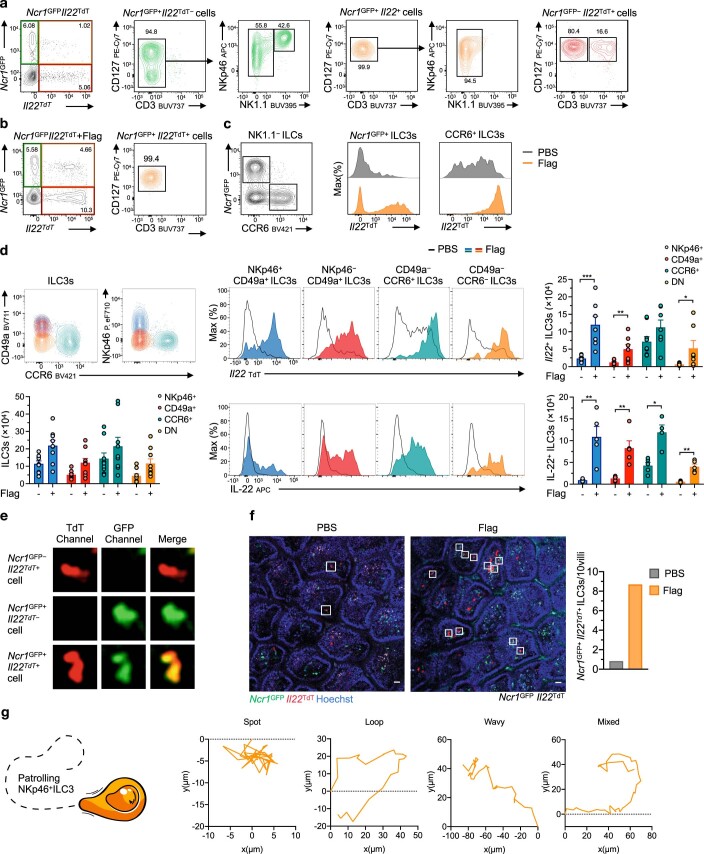
NKp46+ ILC3s selectively localize to the intestinal villus lamina propria8. To understand the cellular dynamic of this ILC3 subset, we imaged Ncr1GFPIl22TdT reporter mice at steady state. Because Ncr1GFP is expressed by both group 1 ILCs and NKp46+ ILC3s, we focused our analysis on Ncr1GFP+Il22TdT+ cells, which represent Il22-expressing NKp46+ ILC3s (Fig. 2a,b). The migration of Ncr1GFP+Il22TdT+ ILC3s was limited (average speed of approximately 3.4 μm min−1) with most cells (>60%) arrested over the track duration (Fig. 2b and Supplementary Video 3). To characterize the impact of acute inflammation on ILC3 homeostasis, function and behavior, Ncr1GFPIl22TdT mice were injected with bacterial flagellin, which can activate ILC3s indirectly through the stimulation of Toll-like receptor 5+ myeloid cells, thereby mimicking bacterial infection-induced inflammation11,12. Absolute numbers of gut Ncr1GFP+Il22TdT+ ILC3s were increased fivefold 5 h post-flagellin stimulation (Fig. 2c and Extended Data Fig. 2b,c); analysis of NKp46+/−CD49a+/−CCR6+/− ILC3s, which include all ILC3 subsets, showed higher frequencies and absolute numbers of IL-22 and Il22TdT-expressing ILC3s compared to PBS-treated mice (Extended Data Fig. 2c,d). Intravital imaging indicated a marked eightfold increase of Ncr1GFP+Il22TdT+ ILC3s in intestinal villi, which also displayed increased motility in mice treated with flagellin challenge compared to PBS-treated mice (Fig. 2d, Supplementary Video 4 and Extended Data Fig. 2e,f). Ncr1GFP+Il22TdT+ ILC3s in flagellin-treated mice migrated within the intestinal villi with enhanced velocity compared to steady-state Ncr1GFP+Il22TdT+ ILC3s (Fig. 2d–f). Moreover, activated Ncr1GFP+Il22TdT+ ILC3s exhibited unique movements in the intestinal lamina propria 5 h post-flagellin challenge, shifting from ‘spot’ migration patterns corresponding to restricted motility to ‘wavy’ migration patterns (Fig. 2g and Extended Data Fig. 2g). As a result, tissue scanning by Ncr1GFP+Il22TdT+ ILC3s was increased (Fig. 2h). Together, these data indicate that environmental signals, such as acute generalized inflammation caused by systemic bacterial flagellin administration, impacted ILC3 behavior and induced a patrolling function that was associated with enhanced expression of IL-22.
Fig. 2. Monitoring of intestinal tissue during inflammation by patrolling ILC3s .
a, Representative flow cytometry analysis for the identification of Ncr1GFP+Il22TdT+ ILC3s in intestinal CD45+ cells from Ncr1GFPIl22TdT reporter mice. Data are representative of three independent experiments. b, Time-lapse images (scale bar, 15 µm) of Ncr1GFP+Il22TdT+ cells in intestinal villi. Mean speed and arrest coefficient of Ncr1GFP+Il22TdT+ ILC3s (n = 36) compared to Ncr1GFP−Il22TdT+ cells (n = 95) c, Representative flow cytometry analysis of intestinal Ncr1GFP+Il22TdT+ ILC3s, pregated on CD45+ cells from one of two independent experiments and absolute numbers of Ncr1GFP+Il22TdT+ ILC3s (n = 4 mice per condition) in Ncr1GFPIl22TdT mice treated or not with flagellin 5 h before. d, Time-lapse images (scale bar, 15 µm) of Ncr1GFP+Il22TdT+ ILC3s in intestinal villi 5 h post-flagellin stimulation as in c. Mean speed distribution of intestinal Ncr1GFP+Il22TdT+ ILC3s, with or without flagellin. e–h, Individual tracks (e), mean speed and arrest coefficient (f), distribution of ILC3 patrolling behaviors (g) and MSD (h) of Ncr1GFP+Il22TdT+ ILC3s as in c (n = 29 and 156). Results are from at least nine movies per condition obtained in three independent experiments. Each bar corresponds to the mean ± s.e.m. of the values obtained; only Ncr1GFP+Il22TdT+ ILC3s were tested (*P < 0.05; ***P < 0.001; two-tailed Mann–Whitney U-test; exact P values are provided in the source data).
Extended Data Fig. 1. BM chimera, a novel model to investigate ILC3 dynamics in vivo.
a, Representative gating strategy for identification of ILC3s from small intestine lamina propria of C57BL6 and RorcGFPIl22TdT mice from one of three independent experiments (ILC3, CD45+ CD3− CD19− CD90+ CD127+ NK1.1− RORγt+ cells; NK cells, CD45+ CD3− CD19− NKp46+ NK1.1+ RORγt− cells). b, Representative flow cytometry analysis of small intestine from RorcGFPIl22TdT from one of three independent experiments. Representative intravital image of ILC3s (scale bar, 50 µm) in intestinal villi (V) of RorcGFP Il22TdT mice. Mice were injected with Hoechst and Evans blue before imaging to visualize all nuclei (blue) and blood vessels (magenta). Data are representative of three independent experiments. c, Representative intravital image (scale bar 50 µm) and flow cytometry analysis of small intestine from Rag2−/−RorcGFPIl22TdT reporter mice from one of three independent experiments. c, Bone marrow (BM) chimeric mice were created by cell transfer. Mixed bone marrow cells from Rag2−/−RorcGFPIl22TdT (95%) and ActbECFP (5%) mice was injected into lethally irradiated congenic C57BL/6 J mice and later analyzed for reconstitution. Representative flow cytometry analysis of reconstitution by donor cells (CD45.2+) in the intestinal lamina propria from one of two independent experiments.
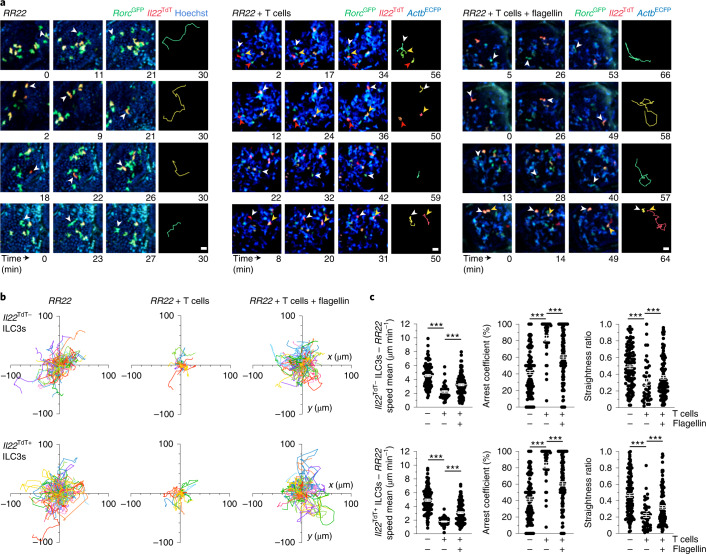
Intestinal ILC3s are ideally positioned to recognize and process a wide range of external and host-derived signals, allowing them to adapt their effector responses13. Increased numbers of intestinal ILC3 and production of IL-22 has been reported in Rag2−/− mice compared to WT13,14. Intravital imaging indicated that large numbers of patrolling ILC3s were present in the intestinal villi of Rag2−/−RorcGFPIl22TdT mice at steady state (Fig. 3a and Supplementary Video 5). Villus ILC3s in Rag2−/−RorcGFPIl22TdT mice constantly scanned intestinal tissue and showed low confinement, reduced arrest coefficient and enhanced velocity compared to WT mice (Fig. 3a–c). Consistent with previous findings13,14, ILC3s in Rag2−/−RorcGFPIl22TdT mice had high expression of Il22TdT compared to WT mice (Extended Data Fig. 1); their enhanced patrolling behavior appeared independent of Il22 expression (Fig. 3c). These results indicate that dynamic behavior of villus ILC3s is strongly upregulated in the absence of adaptive immune cells.
Fig. 3. Local environmental stimuli regulate ILC3 patrolling.
a, Representative time-lapse images (scale bar, 15 µm) of ILC3s within intestinal villi of the indicated mice. Data are representative of three independent experiments. b, Individual tracks of Il22TdT− or Il22TdT+ RorcGFP+ ILC3s in noted mice. c, Mean speed, arrest coefficient and straightness ratio of Il22TdT− and Il22TdT+ ILC3s as in a. Results are from 1–3 movies per condition from 1 of 3 independent experiments (n = 109 and n = 120 for Il22TdT− and Il22TdT+RorcGFP+ ILC3s in RR22 mice, respectively and n = 39 and 120 Il22TdT−RorcGFP+ ILC3s; n = 45 and 109 Il22TdT+RorcGFP+ ILC3s for RR22 + ActbCFP+ T cell mice without or with flagellin, respectively). Each bar corresponds to the mean ± s.e.m. of the values obtained (***P < 0.001; one-way analysis of variance (ANOVA); exact P values are provided in the source data).
Reciprocal interactions between intestinal T cells and ILC3s have been reported15. To test whether T cells could regulate ILC3 dynamics, we transferred ActbECFP+ T cells in Rag2−/−RorcGFPIl22TdT mice and imaged their impact on intestinal ILC3s (Extended Data Fig. 3). Compared to ILC3s in Rag2−/−RorcGFPIl22TdT mice, ILC3s in T cell-reconstituted Rag2−/−RorcGFPIl22TdT mice showed reduced exploration of the intestinal tissue (straightness ratio approximately 0.25), strongly reduced velocity (<2.2 μm min−1) and were mainly arrested (arrest coefficient approximately 85%), regardless of Il22 expression (Fig. 3a–c and Supplementary Video 6). Thus, the presence of T cells appeared to suppress ILC3 patrolling behavior under steady-state conditions. Because T cells can restrict ILC3 activation in part by limiting the expansion of specific microbial species16–18, we studied the impact of the microbiota on ILC3 migratory behavior. We treated Rag2−/−RorcGFPIl22TdT mice for two weeks with antibiotics (including ampicillin, streptomycin and colistin) which strongly deplete microbiota in adult mice19. Motility of all ILC3s was decreased after antibiotic treatment compared to water-treated mice (Extended Data Fig. 4). However, ILC3 exploration of the intestinal tissue, based on the straightness ratio, was not changed compared to controls (Extended Data Fig. 4). Hence, while altered microbiota may partially promote ILC3 patrolling in Rag2−/− mice, T cells are the main regulators of this process and do not rely on cues from the microbiota.
Extended Data Fig. 2. NKp46+ ILC3 responses upon bacterial challenge.
a, b, Representative flow cytometry analysis of small intestine leukocytes from Ncr1GFPIl22TdT mice at indicated conditions from one of three independent experiments. c, Representative flow cytometry analysis of Il22TdT expression in NKp46+ and CCR6+ ILC3s (CD45+ CD3− CD5− CD90+ CD127+ cells) from Ncr1GFPIl22TdT mice from one of two independent experiments. d, Representative flow cytometry analysis of small intestine ILC3 subsets (pre-gated on CD45+ CD3− CD5− NK1.1− CD127+ RORγt+ cells) from PBS or flagellin (Flag)-treated wild-type mice from one of five experiments. Absolute numbers of total, Il22TdT+ and IL-22+ ILC3s (n = 9). Each bar corresponds to the mean ± s.e.m of the values obtained (*P < 0.05; **P < 0.01; ***P < 0.001; two-tailed Mann-Whitney test; exact P values are provided in the source data). e, Representative images of observable cells in Ncr1GFPIl22TdT mice by intravital imaging rom one of three independent experiments. f, Representative images and absolute numbers of Ncr1GFP+Il22TdT+ ILC3s in intestinal villi of from PBS or Flag-treated Ncr1GFPIl22TdT mice from one of three independent experiments (scale bar, 30 µm). g, Sample tracks of Ncr1GFP+Il22TdT+ ILC3s patrolling behaviors used in Fig. 2g.
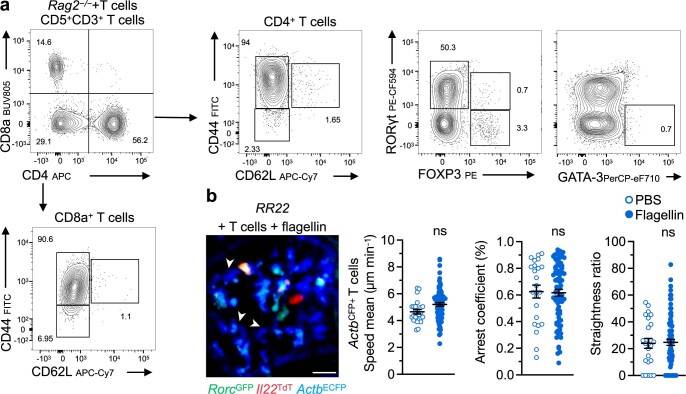
Extended Data Fig. 3. Phenotype and dynamics of adoptively transferred intestinal T cells.
a, Representative flow cytometry analysis of T cells in indicated mice. b, Representative intravital image (scale bar 20 µm) of ActbECFP+ T cells (white arrowhead) in intestinal villi of indicate mice from one of three independent experiments (scale bar, 40 µm). c, Mean speed, arrest coefficient and straightness ratio of adoptively transferred ActbECFP+ T cells in the intestine from treated mice. Results are from at least one movie per condition from one of three independent experiments (n = 24 PBS and 80 Flag). Each bar corresponds to the mean ± s.e.m of the values obtained (ns, not significant; two-tailed Mann-Whitney test; exact P values are provided in the source data). RR22, Rag2−/−RorcGFPIl22TdT. Flag, Flagellin.
To assess the balance between T cell-mediated suppression and inflammation-mediated activation of ILC3 patrolling, we challenged T cell-reconstituted Rag2−/−RorcGFPIl22TdT mice with flagellin (Fig. 3c). In this setting, despite the presence of T cells, both Il22TdT− and Il22TdT+ intestinal ILC3s exhibited enhanced patrolling behavior compared to PBS-treated mice, with increased velocity and reduced arrest and confinement (Fig. 3c–e and Supplementary Video 7). Considering that T cells patrol the intestine under physiological and inflammatory conditions, these data suggested that regulation of intestinal T cells and ILC3 patrolling behavior could be linked.
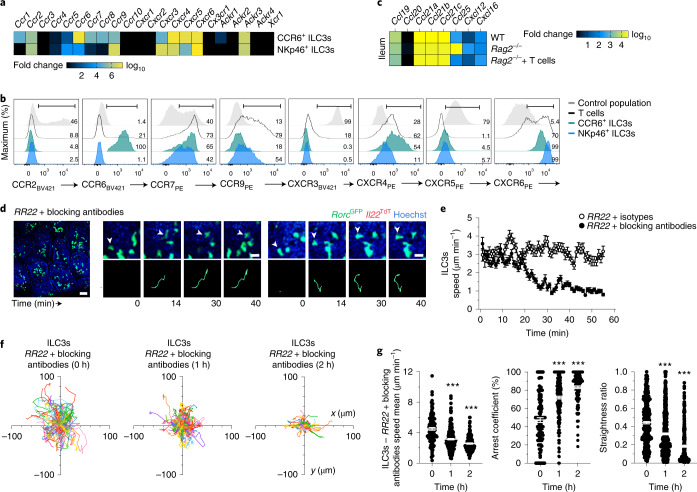
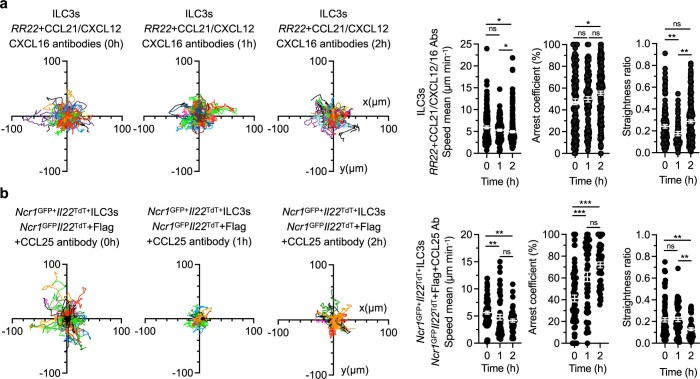
We next investigated the molecular mechanisms driving the ILC3 migration patterns in intestinal villi. Because chemokines play a fundamental role in lymphocyte migration and function20 and are involved in ILC3 homing and trafficking8,17,18, we tested whether chemokine–chemokine receptors regulated intestinal ILC3 dynamic behavior. Ccr6, Ccr9 and Cxcr6 were expressed on intestinal NKp46+ ILC3s and CCR6+ ILC3s8,18,21. We further identified the expression of Ccr7 and Cxcr4 transcripts and CCR7, CXCR4 and CXCR6 protein expression on both ILC3 subsets—albeit in various amounts—and CCR9 on NKp46+ ILC3s (Fig. 4a,b). Among the relevant chemokines (Ccl19 and Ccl21 for CCR7, Cxcl12 for CXCR4, Cxcl16 for CXCR6, Ccl25 for CCR9 and Ccl20 for CCR6), Ccl25, Cxcl12 and Cxcl16 were upregulated in whole ileal tissue extracts from Rag2−/− mice compared to WT and T cell-reconstituted Rag2−/− mice; Ccl25 was the most upregulated and abundant chemokine (Fig. 4c). To assess the role for CXCL12, CXCL16, CCL25 and CCL21 on ILC3 patrolling in vivo, we blocked their activity with neutralizing antibodies (Fig. 4d). Imaging of intestinal ILC3s in Rag2−/−RorcGFPIl22TdT mice before and immediately after injection with antibodies against CXCL12, CXCL16, CCL25 and CCL21 indicated that the velocity of intestinal ILC3s strongly decreased 20 min after neutralization (Fig. 4e, Extended Data Fig. 5a–c and Supplementary Videos 8 and 9). Chemokine blockade impaired ILC3 patrolling and tissue scanning, which was reflected by a reduction in villus ILC3 trajectories, average speed and straightness ratios compared to isotype-injected mice (Fig. 4f,g). Patrolling ILC3s lost motility with a high arrest coefficient (over 70%) (Fig. 4f,g). The effects of combined CXCL12, CXCL16, CCL25 and CCL21 blockade were observed within the first hour postinjection and intensified with time (30–60 min later; Fig. 4g).
Fig. 4. Chemokines are involved in the control of ILC3 motility.
a, Heatmap of relative expression of chemokine receptors in sorted NKp46+ ILC3s (n = 6) and CCR6+ ILC3s (n = 6) from WT mice detected by bulk multiplex Biomark assay. b, Representative flow cytometry analysis of chemokine receptors profiles of intestinal NKp46+ ILC3s, CCR6+ ILC3s and T cells from one of three independent experiments in WT mice. A negative or positive population is shown as a control for each chemokine receptor. c, Heatmap of relative expression of chemokines in whole ileum of WT (n = 10), Rag2−/− (n = 10) and Rag2−/− adoptively transferred with T cells (n = 8) detected by bulk multiplex Biomark assay. d–g, Intravital imaging of intestinal ILC3s in RR22 mice with combination of isotype controls (mouse IgG1, rat IgG2a and rat IgG2b) or blocking monoclonal antibodies (anti-CXCL12, anti-CXCL16, anti-CCL21 and anti-CCL25). Data are representative of three independent experiments. Representative image (left; scale bar, 50 µm), time-lapse images (d) (right; scale bar, 15 µm), speed over time (e) (n = 216 and 248), individual tracks (f), mean speed, arrest coefficient and straightness ratio (g) of intestinal ILC3s as in d at the indicated time points. Results in f,g are from 3 movies per condition obtained in 3 independent experiments (n = 364, 0 h; n = 617, 1 h; n = 310, 2 h). Each bar corresponds to the mean ± s.e.m. of the values obtained (***P < 0.001; one-way ANOVA; exact P values are provided in the source data).
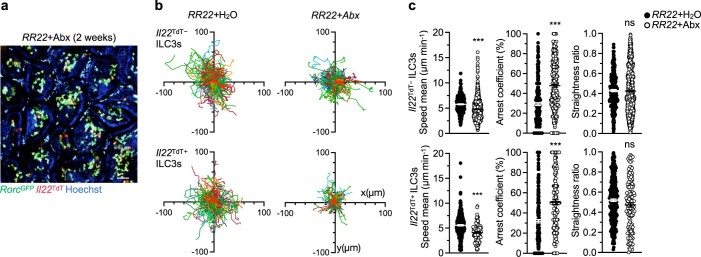
Extended Data Fig. 4. Impact of the microbiota on ILC3 patrolling.
a, Representative intravital images of ILC3s (scale bar 50 µm) in intestinal villi Abx-treated RR22 mice (2 weeks after treatment) from one of three independent experiments. b, Individuals tracks of intestinal Il22− and Il22+ ILC3s in treated RR22 mice. c, Graphs show mean speed, arrest coefficient and straightness ratio of indicated populations in RR22 and RR22 + Abx mice. Results are from one to three movies per condition from one of three independent experiments (n = 257 and n = 341 for Il22− and Il22+ ILC3s in RR22 mice, respectively; n = 124 and n = 134 Il22− and Il22+ ILC3s in RR22 + Abx mice, respectively). Each bar corresponds to the mean ± s.e.m of the values obtained (ns, not significant; ***P < 0.001; one-way ANOVA; exact P values are provided in the source data). RR22, Rag2−/−RorcGFPIl22TdT; Abx, antibiotics.
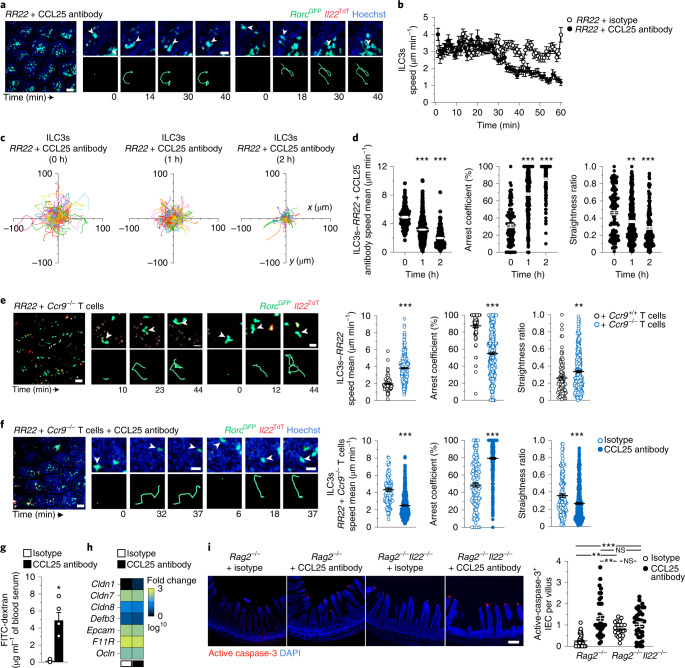
Next, we analyzed the specific role of CCL25 in controlling the patrolling behavior of ILC3s. Administration of CCL25-specific antibodies to Rag2−/−RorcGFPIl22TdT mice reduced villus ILC3 patrolling compared to isotype-treated mice, with a progressive decrease in ILC3 speed 30 min after injection (Fig. 5a,b, Extended Data Fig. 5d–f and Supplementary Videos 10 and 11). CCL25 blockade limited ILC3 migration, with reduced trajectories, speed mean (2 μm min−1), augmented arrest coefficient (approximately 85%) and confinement (approximately 0.3) compared to isotype-injected mice (Fig. 5c,d). Combined neutralization of CXCL12, CXCL16 and CCL21 was less effective than CCL25 blockade in inhibiting ILC3 motility, with modest reduction of ILC3 velocity and time arrested at 1 and 2 h postinjection (Extended Data Fig. 6a), suggesting that CCR9-CCL25 signaling was a critical regulator of ILC3 patrolling in the intestine. To evaluate whether CCL25 controlled inflammation-induced intratissular migration of ILC3s in WT mice, we imaged intestinal Ncr1GFP+Il22TdT+ ILC3s in flagellin-treated mice before, 1 h and 2 h after injection with CCL25-blocking antibodies. Neutralization of CCL25 reduced Ncr1GFP+Il22TdT+ ILC3 patrolling trajectories and dynamics (Extended Data Fig. 6b), suggesting that CCL25 was an important signal involved in the ILC3 migratory behavior in response to generalized inflammation.
Fig. 5. IEC death is prevented by ILC3 patrolling, which is regulated by CCL25 and T cells.
a–f, Intravital imaging of intestinal ILC3s in RR22 mice injected with isotype or CCL25 antibody (a–d,f), with or without Ccr9+/+ (e) or Ccr9−/− T cells (e,f) showing representative images (left; scale bar, 50 µm), time-lapse images (right; scale bar, 15 µm) of villus ILC3s (a,e,f). Speed over time of intestinal ILC3s in RR22, after either isotype or CCL25 antibody injection at time 0 (b), individual tracks (c), mean speed, arrest coefficient and straightness ratio (d–f) of intestinal ILC3s at 1 h (d–f) and 2 h (d) or 1 h with isotype or CCL25 antibody (b–d), Ccr9+/+ or Ccr9−/− T cells (e), isotype or CCL25 antibody + Ccr9−/− T cells (f). Data are representative of three independent experiments (a,c,e,f). Results in b–f are from at least 2 movies per condition obtained in 2 independent experiments (b–d: n = 121, 0 h; n = 400, 1 h; n = 179, 2 h; e: n = 84, RR22+ Ccr9+/+ T cells and n = 338, RR22+ Ccr9−/− T cells; f: n = 152, isotype and n = 516, CCL25 antibody). g, Intestinal permeability assay in Rag2−/− mice injected intravenously with either isotype or CCL25 antibody 18 h and 4 h before analysis. h, Heatmap of tight junctions and adhesion molecule expression in whole ileum (n = 5) in Rag2−/− mice as in g, detected by Biomark assay. i, Representative immunofluorescence analysis (ileum; scale bar, 50 µm) and absolute numbers of active caspase-3+ IECs in isotype or CCL25 antibody-treated Rag2−/− and Rag2−/−Il22−/−mice. Results in i are from two independent experiments (n = 4 mice Rag2−/− and n = 43 out of 48 fields for each condition; n = 3 Rag2−/−Il22−/− mice and n = 27 out of 37 fields for each condition). Each bar corresponds to the mean ± s.e.m. of the values obtained (NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001; one-way ANOVA in d–f and two-tailed Mann–Whitney U-test in g–i; exact P values are provided in the source data).
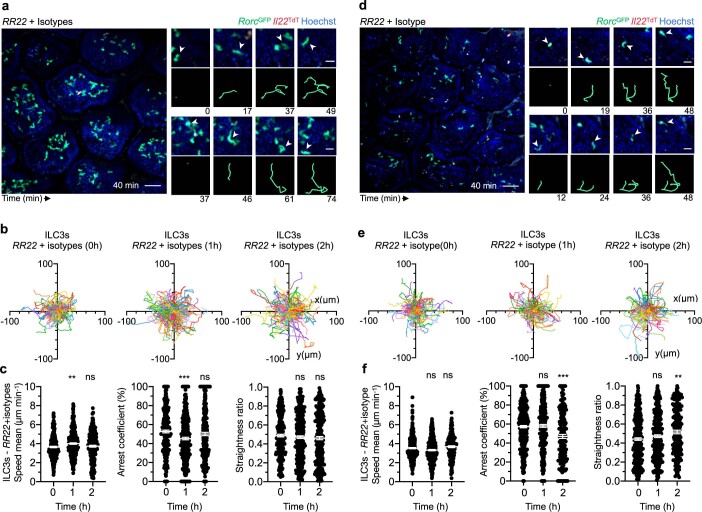
Extended Data Fig. 5. Isotypes control have little effect on ILC3 migration.
a-f, RR22 mice were injected i.v. with combination of isotypes in (a-c): mouse IgG1, rat IgG2a and IgG2b, or in (d-f) rat IgG2b only. Representative image (left; scale bar 50 µm) and time-lapse images (right; scale bar 15 µm) of ILC3 in intestinal villi after isotype controls injection at 0 min (combination in c or rat IgG2b alone in f). Data are representative of three independent experiments. Individual tracks of intestinal ILC3s before (-60-0 min), 1 h (0–60 min) and 2 h (60–120 min) after isotype controls injection (combination in d or rat IgG2b alone in g) of RR22 mice. Mean speed, arrest coefficient and straightness ratio of intestinal ILC3s at indicated time points after isotype controls (combination in e or rat IgG2b alone in h) injection. Results in (d-e) are from two to four movies per condition obtained in two independent experiments (n = 266, 0 h; n = 340, 1 h; n = 259, 2 h). Results in (g-h) are from three to four movies per condition obtained in two independent experiments (n = 349, 0 h; n = 307, 1 h; n = 178, 2 h). Each bar corresponds to the mean ± s.e.m of the values obtained (ns, not significant; **P < 0.002, ***P < 0.0001; one-way ANOVA; exact P values are provided in the source data).
Since a subset of intestinal T cells express CCR9 (refs. 22), we assessed whether T cells may regulate ILC3 patrolling by competing for CCL25 that drive ILC3 migration. To investigate the role of CCR9+ T cells in ILC3 motility, we adoptively transferred T cells from WT or Ccr9−/− mice into Rag2−/−RorcGFPIl22TdT recipients. While villus ILC3 patrolling was ablated in mice transferred with WT T cells, ILC3s exhibited a patrolling behavior in mice that received Ccr9−/− T cells (Fig. 5e, Extended Data Fig. 7 and Supplementary Video 12), suggesting that T cell regulation of ILC3 patrolling occurred, at least in part, through the regulation of CCL25 availability by CCR9+ T cells. To assess whether T cells regulated ILC3 patrolling through buffering CCL25, we treated Rag2−/−RorcGFPIl22TdT mice that had been adoptively transferred with Ccr9−/− T cells with CCL25 blocking antibodies. One hour post-CCL25 neutralization, ILC3 patrolling was reduced, with ILC3s arresting and displaying reduced speed mean as well as straightness ratio compared to isotype-treated mice (Fig. 5f and Supplementary Video 13). These findings indicated that regulation of CCL25 availability by CCR9+ T cells represented one mechanism by which T cells regulate ILC3 patrolling.
Extended Data Fig. 6. Identification of CCL25 as an essential chemokine controlling ILC3 motility.
a, Intestinal ILC3s were imaged in RR22 mice using intravital imaging for one hour. Then, mice were injected i.v. with a combination of blocking monoclonal antibodies (anti-CXCL12, anti-CXCL16, and anti-CCL21) and subsequently imaged for 2 hours. Individual tracks of intestinal ILC3s before (−60–0 min), 1 h (0–60 min) and 2 h (60–120 min) after blocking antibodies injection. Mean speed, arrest coefficient and straightness ratio of intestinal ILC3s at indicated timepoints after blocking antibodies injection. Results in (a) are from at least five movies per condition obtained in two independent experiments (n = 129, n = 100, and n = 184 ILC3s for 0 h, 1 h and 2 h, respectively). b, Individual tracks of intestinal NKp46+Il22+ ILC3s before (−60–0 min), 1 h (0–60 min) and 2 h (60–120 min) after CCL25 Ab injection and 5 h after Flag injection. Mean speed, arrest coefficient and straightness ratio of intestinal NKp46+ Il22+ ILC3s at indicated timepoints after CCL25 Ab injection. Results in (b) are from at least five movies per condition obtained in two independent experiments (n = 78, 0 h; n = 50, 1 h; n = 49, 2 h). Each bar corresponds to the mean ± s.e.m of the values obtained (ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA; exact P values are provided in the source data). RR22, Rag2−/−RorcGFPIl22TdT; CCL25 Ab, CCL25 blocking antibody; Flag, Flagellin.
To investigate whether ILC3 patrolling had an impact on the intestinal barrier, we treated Rag2−/− mice with CCL25 blocking antibodies and used oral gavage of fluorescein isothiocyanate (FITC)-dextran to measure intestinal permeability. Rag2−/− mice treated with CCL25 blocking antibodies exhibited a significant increase in FITC-dextran in the serum compared to isotype-treated mice (Fig. 5g). Because IL-22–IL-22 receptor interactions and ILC3s are responsible for homeostatic signaling and activation of intestinal epithelial cells (IECs)16,23,24, we investigated the influence of ILC3 patrolling on mechanisms involved in preserving barrier integrity, including epithelial expression of tight junction proteins and cell death. Expression of tight junctions and adhesion proteins was similar in isotype and CCL25 antibody-treated Rag2−/− mice (Fig. 5h). However villus IEC death—as detected by immunofluorescence staining of cleaved caspase-3—was increased in Rag2−/− mice after CCL25 neutralization (Fig. 5i), suggesting that patrolling ILC3s have a role in epithelial homeostasis. To investigate whether this protection is mediated by IL-22, we quantified IEC death in isotype and CCL25 antibody-treated Rag2−/−Il22−/− mice. Ileal IEC death was higher in Rag2−/−Il22−/− mice compared to Rag2−/− mice and at levels comparable to that seen in Rag2−/− mice treated with CCL25 blocking antibodies (Fig. 5i); CCL25 antibody neutralization in Rag2−/−Il22−/− mice did not further increase IEC death compared to isotype-treated Rag2−/−Il22−/− mice (Fig. 5i). These observations suggested that patrolling ILC3s delivered homeostatic signals, including IL-22, that maintained the intestinal barrier (Extended Data Fig. 8).
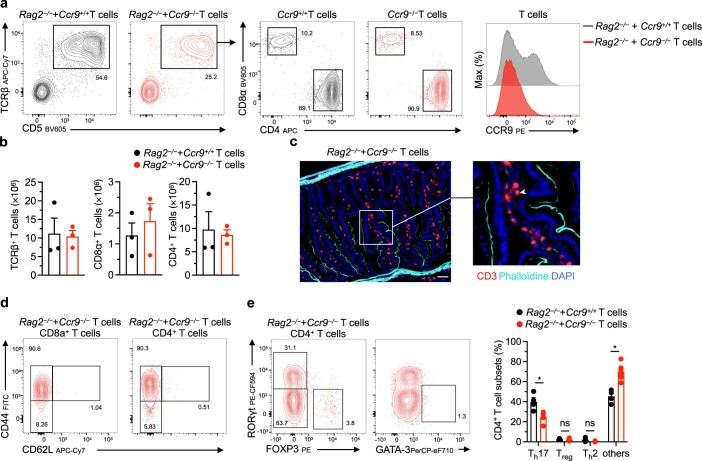
Extended Data Fig. 7. Ccr9−/− T cells home into the intestinal tissue after adoptive transfer.
a, Representative flow cytometry analysis of intestinal T cells in Rag2−/− mice two weeks post-adoptive transfer of either Ccr9+/+ or Ccr9−/− T cells from one of two independent experiments. b, Absolute numbers of total TCRβ+ T cells, as well as CD8α+ and CD4+ T cells subsets, in the small intestine of Rag2−/− mice two weeks post-adoptive transfer of either control Ccr9+/+ (n = 3) or Ccr9−/− (n = 3) T cells. c, Immunofluorescence of ileum from Rag2−/− mice adoptively transferred with Ccr9−/− T cells (scale bar 50 µm). Data are representative of two independent experiments. d-e, Representative flow cytometry analysis of intestinal CD8a+ (d) and CD4+ (e) T cell subsets in Rag2−/− mice two weeks post adoptive transfer of Ccr9−/− T cells from one of two independent experiments. Frequencies of CD4+ T cells in the small intestine of Rag2−/− mice two weeks post-adoptive transfer of either control Ccr9+/+ (n = 4) or Ccr9−/− (n = 6) T cells. Results in were obtained in three independent experiments. Each bar corresponds to the mean ± s.e.m of the values obtained (ns, not significant; *P < 0.05; two-tailed Mann-Whitney test; exact P values are provided in the source data).
In this study, we provided an in-depth characterization of intestinal ILC3 behavior. We showed that ILC3s were compartmentalized in the intestine and largely immotile under basal conditions, in both the intestinal villus and crypts. We showed that environmental signals provided tissue-resident ILC3s with new migratory attributes. On inflammation, villus ILC3s rapidly increased their motility to become patrolling cells, resulting in enhanced tissue scanning. While most immune cells behaved as ‘explorers’ that exhibit robust cellular migration under basal conditions and reduced motility after activation25, our study detected a unique behavior for ILC3 and suggested a new ‘gatekeeper’ model, in which tissue protection operates through distinct T and ILC3 migration patterns. Our study showed an important role of T cells in regulating intratissular ILC3 migration through competition for the chemokine CCL25 and identified the CCL25–CCR9 axis as one of the signals controlling patrolling ILC3s. Thus, we propose that microenvironmental signals promote tissue adaptation of intestinal ILC3 responses, including patrolling behavior and IL-22 production, which contributes to maintaining the balance between regulatory and immune protective ILC3 functions. As such, we suggest that patrolling ILC3s help to diffuse IL-22 within the villi to prevent epithelial cell death and support intestinal barrier integrity. Dysregulated ILC3 responses have been associated with the development of intestinal pathology, such as inflammatory bowel diseases26. Therefore, a better understanding of ILC3 behavior and associated regulatory mechanisms may lead to future therapeutic approaches for these debilitating diseases.
Methods
Mice
All mice used were on a C57BL/6 background and bred in dedicated animal facilities of the Institut Pasteur. ActbECFP, Ccr9−/−, RorcGFP, Il22TdT and Ncr1GFP mice were provided by P. Bousso, I. Prinz, G. Eberl, S. Durum and O. Mandelboim, respectively. C57BL/6 and C57BL/6 Ly5.1 mice were purchased from the Charles River Laboratories. All experiments were performed on 8–14-week-old male and female animals, except for experiments on bone marrow chimeric mice which involved 16-week-old males at the time of the analysis. All experiments involving mice were performed according to guidelines issued by the Institut Pasteur Ethics Committee and were approved by the French Ministry of Research (project nos. DHA170001, CETEA 2013-033 and CETEA 17500).
Adoptive transfer of T cells
Cells from the spleen and lymph nodes of ActbECFP reporter or Ccr9−/− mice were isolated by being passed through a steel wire mesh, filtered on a 40-μm cell strainer and after red blood cells lysis using ACK buffer. To enrich for T cells, single-cell suspensions were first incubated with biotin-conjugated anti-B220 (clone RA3-6B2; eBioscience), anti-CD19 (clone 1D3; eBioscience), anti-CD11b (clone M1/70; BD Biosciences), anti-CD11c (clone N418; eBioscience), anti-Gr1 (clone RB6-8C5; eBioscience); anti-NK1.1 (clone PK136; BD Biosciences) and anti-Ter119 (clone TER-119; BD Biosciences) antibodies followed by antibiotin microbeads (Miltenyi Biotec) and negatively selected by magnetic cell separation with magnetic-activated cell sorting technology (Miltenyi Biotec); 5 × 106 enriched T cells were intravenously transferred to Rag2−/−RorcGFPIl22TdT (RR22) mice; recipient mice were analyzed at day 14 post-transfer.
Generation of bone marrow chimeric mice
Cells from the bone marrow of ActbECFP and RR22 mice were isolated by crushing the bones with a mortar and pestle, being filtered on a 40-μm cell strainer and after red blood cells lysis using the ACK buffer. Bone marrow cells were mixed at a 1:19 ratio—0.5 × 106 and 10 × 106 cells from ActbECFP and RR22 mice, respectively. Mixed bone marrow cells were transferred intravenously into lethally irradiated (9 Gy X-ray irradiation 1 d before injection) congenic C57BL/6J mice. Bone marrow chimeric mice were analyzed seven weeks later for reconstitution (donor cells in the small intestine lamina propria) and imaged by multiphoton microscopy.
Isolation of intestinal cells
Small intestine was collected from euthanized mice and placed into cold complete medium: Roswell Park Memorial Institute (RPMI) 1640 GlutaMAX (Gibco) supplemented with 5% fetal calf serum (FCS) (Eurobio) and 10 mM HEPES (Sigma-Aldrich). The mesenteric adipose tissue and Peyer’s patches were first pulled out before cutting the small intestine longitudinally and removing feces. Intestinal tissue was washed in PBS (Gibco) to eliminate mucus, cut into 1–2-cm pieces and IECs were eliminated by shaking incubation in complete medium containing 5 mM EDTA (Invitrogen) for 20 min at 37 °C. Subsequently, intestinal tissue was minced and incubated twice in a digestion solution of complete medium containing Collagenase VII (0.5 mg ml−1; Sigma-Aldrich) for 15 min at 37 °C in a shaking incubator to isolate the lamina propria lymphocytes. Lamina propria lymphocytes were filtered through a 40-μm cell strainer and kept in complete medium for downstream analysis.
Flow cytometry
Cells were first blocked with FcR Blocking Reagent (Miltenyi Biotec) and stained with Flexible Viability Dye (eFluor 506 or 780; eBioscience) for 15 min, followed by 30 min of surface antibody staining on ice except for chemokine receptors (at 37 °C for 30 min then at 4 °C for 15 min). Cells were generally fixed in 4% paraformaldehyde (PFA) (Sigma-Aldrich). Only for experiments involving intranuclear transcription factor staining, cells were fixed, permeabilized and stained using Foxp3/Transcription Factor Staining Buffer Kit (eBioscience). Intranuclear and cell surface staining were performed with the following antibodies from BD Biosciences, eBioscience and BioLegend: anti-CCR2 (clone SA203G11); anti-CCR6 (clone 140706); anti-CCR7 (clone 4B12); anti-CCR9 (clone CW-1.2); anti-CD11b (clone M1/70); anti-CD19 (clone 1D3); anti-CD127 (clone A7R34); anti-CD3 (clone 145-2C11); anti-CD3 (clone 500A2 or 17A2); CD44 (clone IM7); anti-CD45 (clone 30-F11); anti-CD45.1 (clone A20); anti-CD45.2 (clone 104); anti-CD5 (clone 53-7.3); CD62L (clone MEL-14); anti-CD8a (clone 53-6.7); anti-CD4 (clone GK1.5); anti-CD90.2 (clone 30-H12); anti-CD90.2 (clone 53-2.1); anti-CXCR3 (clone CXCR3-173); anti-CXCR4 (clone QA16A08); anti-CXCR5 (clone L138D7); anti-CXCR6 (clone SA051D1); Foxp3 (clone FJK-16s); Gata-3 (clone TWAJ); anti-KLRG1 (clone 2F1); anti-NK1.1 (clone PK136); anti-NKp46 (clone 29A1.4); anti-RORγt (clone Q31-378); and anti-TCRβ (clone H57-597). All samples were acquired on a custom-configured LSR Fortessa or sorted using a FACSAria III (BD Biosciences). To sort the ILC3 subsets, we applied the following gating strategies: live CD45.2+CD3−NK1.1−KLRG1−CD90.2+CD127+ and subgated on NKp46+ or CCR6+ cells. Data were analyzed with the FlowJo 10 software (FlowJo LLC).
Intravital two-photon imaging
Mice were first anesthetized with a mixture of ketamine (62.5 mg kg−1), xylazine (12.5 mg kg−1) and acepromazine (3.1 mg kg−1) and the abdomen hair was shaved. The abdominal skin and wall musculature were incised along the linea alba to expose the intestine. A loop of the terminal ileum (2-cm upstream the cecum) was exposed and cut open on the opposite side of the mesentery using an electrical cautery. Feces were gently removed using PBS and mice were placed on a heated (37 °C) steel plate. Mice were immobilized by placing polyvinyl siloxane-based paste (3M) on both sides of the abdomen and a SuperFrost Plus slide (Menzel Gläser; VWR) was placed across the paste with a PBS-soaked tissue paper (Kimtech; Kimberly-Clark Corporation) on top. The intestinal tissue was positioned on the slide, immobilized with a coverslip (Menzel Gläser; VWR) on top held by paper clips and covered with Supragel ultrasound gel (LCH). During imaging, mice were supplied with oxygen and their temperature was controlled and maintained at 37 °C using a heating pad, heating cover and objective heater for the tissue. When indicated, intestine and blood vessels labeling was performed by intravenous injection of Hoechst 33342 (40 μl of 10 mM solution, prepared in PBS; Thermo Fisher Scientific) and Evans Blue (25 μl of 2 μg μl−1 solution, prepared in PBS; Sigma-Aldrich), respectively. Two-photon imaging was performed with an upright microscope FVMPE-RS (Olympus Lifescience) and a ×25, 1.05-numerical aperture, water-dipping objective (Olympus Lifescience). Excitation was provided by an InSight DeepSee dual laser (Spectra-Physics) tuned at 920. The following filters were used for fluorescence detection: CFP or Hoechst (483/32), GFP (520/35), TdT (593/40) and background (624/40). To create time-lapse sequences, we typically scanned a 40–60-μm-thick volume of tissue at 7-μm Z-steps and 60-s intervals.
Image analysis
Movies were processed with the Imaris 7.4.2 (Bitplane) or Fiji 2 (ImageJ2) software and analyzed as two-dimensional (2D) projections of three-dimensional (3D) data to avoid wrong tracking related to motion (intestinal villi or diaphragmatic movement). As such, the represented speeds of motile cells are likely to be slightly underestimated. When necessary, drifting correction was also applied using the ‘Correct Drift’ function in Imaris to minimize XY tissue drift. DiPer27 was used to calculate mean square displacement (MSD) and tracking behaviors. Movies and figures based on two-photon microscopy are shown as 2D projections of 3D data. For optimal contrast rendering, background was pseudocolored in magenta in some images. The arrest coefficient was defined as the percentage of time where instantaneous velocity was <3 µm min−1. The straightness ratio was defined as the ratio track displacement/track path length that reflects directionality.
In vivo treatments
To induce intestinal inflammation, mice were injected intravenously with purified flagellin from Salmonella typhimurium (FLA-ST Ultrapure, 5 μg; Invivogen) 5 h before analysis. For microbiota depletion, mice were orally treated for 2 weeks with a combination of antibiotics, administered in their drinking water, including ampicillin (1 mg ml−1), streptomycin (5 mg ml−1), colistin (1 mg ml−1) and sucrose (25 mg l−1), all from Sigma-Aldrich. For chemokine neutralization, mice were injected intravenously during imaging with a mixture of Hoechst 33342 (see above) to control intravenous injection and the following monoclonal blocking antibodies (50 µg): anti-CXCL12 (catalog no. MAB310); anti-CXCL16 (catalog no. MAB503); anti-CCL21 (catalog no. MAB4572) and anti-CCL25 (catalog no. MAB481), all from R&D Systems. Control mice were injected intravenously in accordance with the following isotype controls (50 µg): mouse IgG1 (catalog no. MAB002); rat IgG2a (catalog no. MAB006); and rat IgG2b (catalog no. MAB0061), all from R&D Systems.
Immunofluorescence staining and confocal imaging
The ileal portion of the small intestine was cut longitudinally, washed in PBS and prepared using the Swiss-rolling technique. Intestinal rolls were fixed overnight in 4% PFA followed by dehydration in 30% sucrose (Sigma-Aldrich) before embedding in Tissue-Tek OCT compound (Sakura Finetek). Samples were frozen in an isopentane bath cooled with liquid nitrogen and stocked at −80 °C; 8-μm sections were cut on a CM3050 S cryostat (Leica Biosystems) and adhered to SuperFrost Plus slides. Frozen sections were first hydrated with PBS-TS (PBS supplemented with 0.1% Triton X-100, 1% FCS, 1% bovine serum albumin (Sigma-Aldrich) and filtered) and blocked for 1 h at room temperature with PBS-TS 10% FCS. Slides were then incubated overnight at 4 °C with the following antibodies diluted in PBS-TS: anti-red fluorescent protein (Rockland); phalloidin (Invitrogen); anti-CD3 (clone 500A2; BD Biosciences); and anti-active caspase-3 (clone C92-605; BD Biosciences). The next day, slides were washed, incubated for 1 h at room temperature with secondary conjugated antibodies, washed again and incubated for 2 min at room temperature with 4,6-diamidino-2-phenylindole (DAPI) (for nuclei, 1 μg ml−1; Sigma-Aldrich). After staining, slides were mounted with Fluoromount-G (SouthernBiotech) and examined on an Axio Imager Z.2 microscope (ZEISS).
In vivo intestinal permeability assay
Mice received FITC-dextran (4 kDa, 12 mg g−1 of body weight, Sigma-Aldrich) by oral gavage. One hour later, sera were collected and analyzed for fluorescence intensity using the FLUOstar OPTIMA (BMG Labtech).
Bulk RNA isolation and multiplex quantitative PCR
For ILC3, 3.3–7.4 × 103 and 2.2–4.5 × 103 NKp46+ and CCR6+ ILC3 bulks were sorted directly into RLT buffer (QIAGEN). For whole ileal tissue, dissected ileum was snap-frozen in liquid nitrogen, crushed with a mortar and pestle and homogenized into RLT buffer. Samples were stored at −80 °C until messenger RNA purification using RNeasy Micro and Minikit with a DNase digestion step using the RNase-Free DNase Set (QIAGEN) for ILC3s and ilea, respectively. mRNA quantity, quality and integrity were checked on a Bioanalyzer system (Agilent) or on a Nanodrop Spectrophotometer (Thermo Fisher Scientific) for ILC3s and ilea, respectively. To synthesize complementary DNA, PCR with reverse transcription was performed using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche). From the cDNA, we followed the protocol ‘Fast Gene Expression Analysis Using Evagreen on the Biomark’ from Fluidigm. Briefly, preamplified cDNA was obtained after a preamplification step with Delta Genes Assays (Fluidigm) using the TaqMan PreAmp Master Mix (Applied Biosystems) and a subsequent purification step using exonuclease I (New England Biolabs) and was then diluted 1:5 in Tris EDTA buffer (Invitrogen). The sample mix was prepared as follows: diluted preamplified cDNA (3.6 μl), DNA Binding Dye Sample Loading Reagent (4 μl; Fluidigm) and SsoFast EvaGreen Supermix with low ROX (0.4 μl; Bio-Rad Laboratories). The assay mix was prepared as follows: primers (4 μl, 10 μM; Fluidigm) and assay loading reagent (4 μl; Fluidigm). A 96.96 Dynamic Array Integrated Fluidic Circuit (Fluidigm) was primed with control line fluid and the chip was loaded with assays and samples using an HX Integrated Fluidic Circuit controller (Fluidigm). The experiments were run on a Biomark HD (Fluidigm) for amplification and detection (70 °C, 2,400 s; 60 °C, 30 s; 95 °C, 60 s; 96 °C 5 s, 60 °C, 20 s) 30 cycles; melting curve: 60 °C, 3 s; 60–95 °C 1 °C 3 s−1). Real-time PCR analysis 4.5.2 software (Fluidigm) was used to view Ct data and amplification curves for the run and export results. The relative abundance of mRNA was normalized to Ppia.
Statistical analysis
All statistical tests were performed using Prism 8 (GraphPad Software). Points in graphs indicate individual cells (except for speed over time analysis, where points indicate the mean of individual cells), lines indicate the means and error bars indicate the s.e.m. of individual cells. For fluorescence-activated cell sorting analysis, points represent samples, and bar graphs and error bars indicate sample means and s.e.m., respectively. For the intravital imaging experiments, measurements were made on multiple samples (animals) whenever possible (limited motion of the tissue) or necessary (small number of events per sample) and the results were pooled. Otherwise, quantification was performed on a representative sample (animal) and representative results are shown (as indicated in the figure legends). Individual tracks are presented from only one representative experiment for the sake of readability, except for Figs. 1 and 2 and Extended Data Fig. 5 where tracks were pooled from multiple experiments because of the small number of events per sample. The statistical tests employed are detailed in the figure legends. Briefly, for small individual cell numbers or biological samples (<30), a normal distribution was not assumed and nonparametric tests were used systematically. When >30 individual cells were analyzed, the normal distribution was tested and, according to the results, parametric or nonparametric tests were performed.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-022-01284-1.
Supplementary information
Supplementary Videos 1–13
Time-lapse video (Z-stack) of intravital imaging of ileum villi in bone marrow chimeric mice. ActbECFP+ cells, square 1; RorcGFP+ ILC3s, square 2 and 3. Scale bar, 50 μm. The Z-stack video is representative of nine different movies obtained in two independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum-isolated lymphoid follicle in bone marrow chimeric mice. Scale bar, 30 μm. The Z-stack video is representative of four different movies obtained in two independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ncr1GFPIl22TdT mice. Nuclei were stained with Hoechst before imaging. Ncr1GFP+Il22TdT+ ILC3, square 1; Ncr1GFP−Il22TdT+ cell, square 2. Scale bar, 50 μm. The Z-stack video is representative of nine different movies obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ncr1GFPIl22TdT mice 5 h after flagellin injection. Scale bar, 50 μm. The Z-stack video is representative of 13 different movies obtained in 3 independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice. Nuclei were stained with Hoechst before imaging. ILC3S are shown in the green (GFP) channel, Il22 transcripts are shown in the red (TdT) channel. Scale bar, 50 μm. The Z-stack video is representative of nine different movies obtained in three independent experiments.
Two weeks before intravital imaging of the intestine, Rag2−/−RorcGFPIl22TdT mice were adoptively transferred with ActbECFP+ T cells. Time-lapse video (Z-stack) of intravital imaging of ileum villi in T cell reconstituted Rag2−/−RorcGFPIl22TdT mice. ILC3s are shown in the green (GFP) channel, Il22 transcripts are shown in the red (TdT) channel, T cells are shown in the blue (CFP) channel. Squares number 1 and 2 highlight ILC3s. Scale bar, 50 μm. The Z-stack video is representative of 13 different movies obtained in 3 independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in flagellin-stimulated T cell reconstituted Rag2−/−RorcGFPIl22TdT mice (5 h after injection). The square highlights ILC3. Scale bar, 50 μm. The Z-stack video is representative of ten different movies obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after the blocking antibody anti-CXCL12, anti-CXCL16, anti-CCL21, anti-CCL25 injection with Hoechst. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after isotype (mouse IgG1 50 µg, rat IgG2a 100 µg, rat IgG2b) injection with Hoechst. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after Hoechst and anti-CCL25 injection. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after Hoechst and isotype (rat IgG2b) injection. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ccr9−/− T cell-reconstituted Rag2−/−RorcGFPIl22TdT mice. Scale bar, 50 μm. The Z-stack video is representative of 13 different movies obtained in 3 independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ccr9−/− T cell-reconstituted Rag2−/−RorcGFPIl22TdT mice before and after Hoechst and anti-CCL25 injection. Scale bar, 50 μm. The Z-stack video is representative of four different movies per condition obtained in three independent experiments.
Acknowledgements
We thank G. Eberl, S. K. Durum and O. Mandelboim for the RorcGFP, Il22TdT and Ncr1GFP mice, respectively. At Institut Pasteur, we are grateful to the Central Animal Facility and especially to the animal caretakers, the CB UTechS platform for cytometry support and L. Guenin-Macé (Immunobiology and Therapy Unit) for her help with the permeability assay. We thank all the members of the Innate Immunity and Dynamics of Immune Responses Units for support and helpful discussions, with specific contributions from A. Schiavo, A. Bohineust, R. Thibaut, J. Postat and C. Grandjean. This work is supported by grants from Institut Pasteur, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Agence National pour le Recherche (ANR-15-CE15-0004–ILC3_MEMORY) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (695467–ILC_REACTIVITY). A.J. is supported by INSERM under an ERC grant and the Fondation pour la Recherche Médicale through the ‘Espoirs de la Recherche’ program.
Extended data
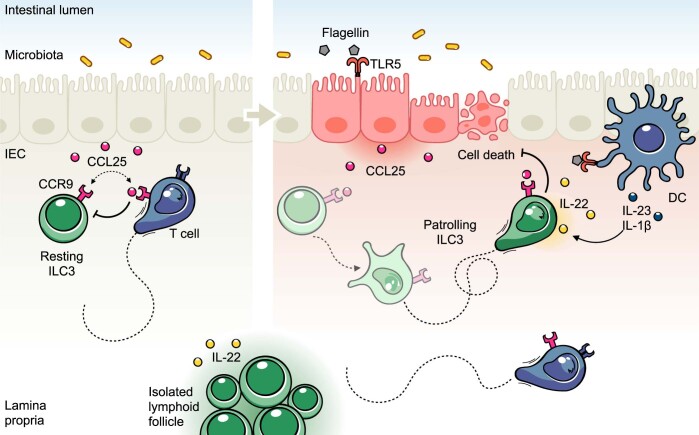
Extended Data Fig. 8. Monitoring of intestinal tissue by patrolling ILC3s.
The intestinal mucosa is continuously exposed to environmental stimuli that can induce immune responses through adaptation of local immune cells, including ILC3s. Under basal conditions, villus T cells control ILC3 patrolling though competition for the chemokine CCL25, and consequently villus ILC3s are largely immotile. Disruption of intestinal homeostasis, notably during TLR5-mediated inflammation, leads to the activation of ILC3s and to CCL25-mediated ILC3 patrolling of the intestinal barrier. Intestinal ILC3s produce IL-22 which is critical to prevent IEC death and to maintain the integrity of the intestinal barrier. Tissue scanning of patrolling ILC3s within intestinal villi could be important to support elevated IL-22 concentrations close to IECs and for optimal induction of their functional programs in order to maintain intestinal integrity. Collectively, tissue environmental signals shape intestinal ILC3 activity, including patrolling behavior and IL-22 production, to promote appropriate immune responses and intestinal barrier function. ILC3, group 3 innate lymphoid cell; DC, dendritic cell. CCL25, chemokine ligand 25; TLR-5, Toll-like receptor 5; IL-22, interleukin 22; IEC, intestinal epithelial cell.
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Author contributions
A.J., J.P.D. and N.S. conceived the study. A.J. and N.S. designed and performed the experiments, together with Z.G. for all intravital imaging experiments. S.M. performed the Biomark experiments and analyzed the data. A.D. and I.P. provided the Ccr9−/− lymphocytes for the adoptive transfer experiments. A.J. and N.S. analyzed the data and performed the statistical analysis. J.P.D. and P.B. provided key intellectual expertise, resources and acquired the funding. A.J., J.P.D. and N.S. wrote the manuscript. N.S. supervised the study.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Ioana Visan, in collaboration with the Nature Immunology team.
Data availability
All datasets generated and/or analyzed during the current study are included in this published article. Source data are provided with this paper. The accompanying source data or supplementary information are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41590-022-01284-1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-022-01284-1.
References
- 1.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 3.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Longman RS, et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 2014;211:1571–1583. doi: 10.1084/jem.20140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manta C, et al. CX3CR1+ macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013;6:177–188. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh-Takayama N, et al. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 2014;41:776–788. doi: 10.1016/j.immuni.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Serafini N, et al. Trained ILC3 responses promote intestinal defense. Science. 2022;375:859–863. doi: 10.1126/science.aaz8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage AK, Liang H-E, Locksley RM. The development of steady-state activation hubs between adult LTi ILC3s and primed macrophages in small intestine. J. Immunol. 2017;199:1912–1922. doi: 10.4049/jimmunol.1700155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Maele L, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3negCD127+ immune cells in spleen and mucosa. J. Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinnebrew MA, et al. Interleukin 23 production by intestinal CD103+CD11b+ dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao K, et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature. 2018;554:255–259. doi: 10.1038/nature25437. [DOI] [PubMed] [Google Scholar]
- 14.Sawa S, et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg GF, Hepworth MR. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 2019;19:599–613. doi: 10.1038/s41577-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano T, et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell. 2015;163:381–393. doi: 10.1016/j.cell.2015.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackley EC, et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat. Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MH, Taparowsky EJ, Kim CH. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity. 2015;43:107–119. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lochner M, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J. Exp. Med. 2011;208:125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krummel MF, Bartumeus F, Gérard A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 2016;16:193–201. doi: 10.1038/nri.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawa S, et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 22.Johansson‐Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindemans CA, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelik R, Gautreau A. Quantitative and unbiased analysis of directional persistence in cell migration. Nat. Protoc. 2014;9:1931–1943. doi: 10.1038/nprot.2014.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Videos 1–13
Time-lapse video (Z-stack) of intravital imaging of ileum villi in bone marrow chimeric mice. ActbECFP+ cells, square 1; RorcGFP+ ILC3s, square 2 and 3. Scale bar, 50 μm. The Z-stack video is representative of nine different movies obtained in two independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum-isolated lymphoid follicle in bone marrow chimeric mice. Scale bar, 30 μm. The Z-stack video is representative of four different movies obtained in two independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ncr1GFPIl22TdT mice. Nuclei were stained with Hoechst before imaging. Ncr1GFP+Il22TdT+ ILC3, square 1; Ncr1GFP−Il22TdT+ cell, square 2. Scale bar, 50 μm. The Z-stack video is representative of nine different movies obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ncr1GFPIl22TdT mice 5 h after flagellin injection. Scale bar, 50 μm. The Z-stack video is representative of 13 different movies obtained in 3 independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice. Nuclei were stained with Hoechst before imaging. ILC3S are shown in the green (GFP) channel, Il22 transcripts are shown in the red (TdT) channel. Scale bar, 50 μm. The Z-stack video is representative of nine different movies obtained in three independent experiments.
Two weeks before intravital imaging of the intestine, Rag2−/−RorcGFPIl22TdT mice were adoptively transferred with ActbECFP+ T cells. Time-lapse video (Z-stack) of intravital imaging of ileum villi in T cell reconstituted Rag2−/−RorcGFPIl22TdT mice. ILC3s are shown in the green (GFP) channel, Il22 transcripts are shown in the red (TdT) channel, T cells are shown in the blue (CFP) channel. Squares number 1 and 2 highlight ILC3s. Scale bar, 50 μm. The Z-stack video is representative of 13 different movies obtained in 3 independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in flagellin-stimulated T cell reconstituted Rag2−/−RorcGFPIl22TdT mice (5 h after injection). The square highlights ILC3. Scale bar, 50 μm. The Z-stack video is representative of ten different movies obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after the blocking antibody anti-CXCL12, anti-CXCL16, anti-CCL21, anti-CCL25 injection with Hoechst. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after isotype (mouse IgG1 50 µg, rat IgG2a 100 µg, rat IgG2b) injection with Hoechst. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after Hoechst and anti-CCL25 injection. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Rag2−/−RorcGFPIl22TdT mice before and after Hoechst and isotype (rat IgG2b) injection. Scale bar, 50 μm. The Z-stack video is representative of three different movies per condition obtained in three independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ccr9−/− T cell-reconstituted Rag2−/−RorcGFPIl22TdT mice. Scale bar, 50 μm. The Z-stack video is representative of 13 different movies obtained in 3 independent experiments.
Time-lapse video (Z-stack) of intravital imaging of ileum villi in Ccr9−/− T cell-reconstituted Rag2−/−RorcGFPIl22TdT mice before and after Hoechst and anti-CCL25 injection. Scale bar, 50 μm. The Z-stack video is representative of four different movies per condition obtained in three independent experiments.
Data Availability Statement
All datasets generated and/or analyzed during the current study are included in this published article. Source data are provided with this paper. The accompanying source data or supplementary information are available from the corresponding author upon reasonable request.