Abstract
Background
Coronavirus disease 2019- (COVID-19-) associated cytotoxic lesions of the corpus callosum (CLOCCs) have been reported as a rare neurological abnormality in severe cases. Here, a case of CLOCCs in the early stages of mild COVID-19 infection during the Omicron BA.1 epidemic is reported along with a literature review.
Case report
A Japanese woman with COVID-19 presented to the emergency department with altered consciousness and cerebellar symptoms a day after fever onset. Magnetic resonance imaging (MRI) revealed a lesion with restricted diffusion in the corpus callosum. She exhibited no complications of pneumonia, her neurological symptoms resolved after two days, and after 10 days, the brain lesion was not detected on MRI.
Literature review
The PubMed database was searched for case reports that met the CLOCC definition proposed by Starkey et al. The search yielded 15 COVID-19-associated cases reported as CLOCCs and 13 cases described under former terms, including mild encephalitis/encephalopathy with a reversible splenial lesion. Adult cases with a documented course were accompanied by pneumonia or hypoxemia, whereas pediatric cases were mostly accompanied by a multisystem inflammatory syndrome.
Conclusion
COVID-19-associated CLOCCs can occur, even at an early, non-severe stage. Therefore, this condition may be underdiagnosed if MRI is not performed.
Keywords: COVID-19, CLOCCs, MERS, RESLES, Corpus callosum, Severity
1. Introduction
COVID-19 (novel COronaVIrus Disease-2019) is a respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has been reported to be complicated by various neurological abnormalities at various times (Chou et al., 2021; Moriguchi et al., 2020; Travi et al., 2021). COVID-19-associated cytotoxic lesions of the corpus callosum (CLOCCs) have been reported as a rare neurological abnormality in severe cases due to pneumonia (Elkhaled et al., 2020; Hayashi et al., 2020). Retrospective studies of patients with COVID-19 who underwent neuroimaging showed CLOCC-like findings on magnetic resonance imaging (MRI), three out of 73 adults in France and one out of 47 adults in Sweden. However, these studies did not provide details on the severity of the disease (Chougar et al., 2020; Klironomos et al., 2020).
CLOCCs are nonspecific secondary lesions of the corpus callosum that occur as a result of many triggers and are characterized by the following neuroradiological feature: reduced diffusion (low apparent diffusion coefficient [ADC] value) on MRI (Starkey et al., 2017). Previously referred to as “mild encephalopathy with reversible splenic lesions (MERS)” (Tada et al., 2004) and “reversible splenic lesion syndrome (RESLES)” (Garcia-Monco et al., 2011), this condition has been recently termed “CLOCCs” (Supplementary Table 1). CLOCCs are associated with various conditions including infection, seizures/status epilepticus, drug therapy, alcohol, metabolic disturbance, subarachnoid hemorrhage, trauma, and malignancy, among others. This clinical entity was frequently reported as MERS in Asian children during the pre-COVID-19 era; Hoshino et al. reported that MERS was the second most common cause of acute encephalopathy in children (15.6% of 983 cases, median age 5 years, 90.2% fully recovered), while preceding causes were influenza virus in 53 cases (34.4%) as well as rotavirus, mumps virus, HHV-6, and bacterial infections in other patients (Hoshino et al., 2012).
The clinical characteristics and severity of CLOCCs in cases of coronavirus infection, including infection with mild COVID-19, are not fully understood. Therefore, we report a new uncomplicated case of CLOCC in a patient with mild COVID-19 with no concurrent pneumonia and reviewed previous cases of CLOCCs associated with coronaviruses.
2. Case report
A 61-year-old Japanese woman presented to the emergency department with acute stroke-like symptoms in January 2022 (Day 1), the predominant phase of SARS-CoV-2 Omicron sublineage BA.1.1 (Omicron BA 1.1 or Nextstrain clade 21K: 91%; Delta or Nextstrain clade 21J: 9% in Japan(Nextstrain/Ncov/Gisaid/Global/6m, n.d.)). She had a history of asthma with no treatment except during attacks. She was allergic to pyrine. She had received two doses of Comirnaty® (COVID-19 vaccine, mRNA) six months earlier. She experienced generalized pain, and her family physician performed a PCR test for SARS-CoV-2. She had fever at night on Day 1, vomited in the morning on Day 2, and was lying in bed unable to move. In the afternoon, she was informed that the PCR test was positive, at which time she experienced dysarthria and was transported to our ER. Upon arrival, she had a Glasgow Coma Scale score of E3V5M6, a pulse of 80 beats/min, a respiratory rate of 24 breaths/min, an SpO2 of 98% (room air), and a body temperature of 37.5°C. She complained of general weakness, dysarthria, and numbness in her hands and fingers but denied headache and dizziness. Physical examination revealed tetany-like stiffness of fingers and intention tremor in both hands. On neurological examination, she exhibited dysarthria with slight drooping of the left side of the mouth but no obvious facial palsy. She experienced difficulty remaining in a seated position and gait disturbance, but no obvious paralysis was noted. Further detailed neurological examination was not possible due to her altered state of consciousness. Laboratory tests revealed a white blood cell count of 3,800/µL and a C-reactive protein level of 75.9 mg/L.
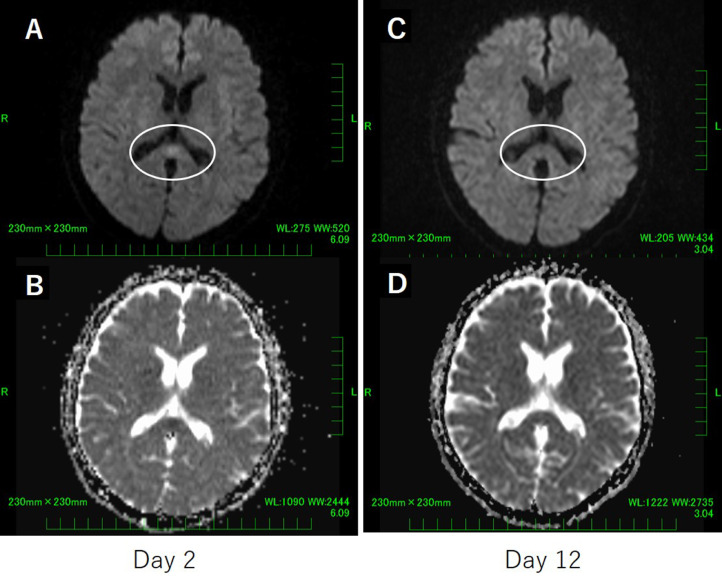
Brain MRI performed at admission revealed a round diffusion-restricted lesion in the splenium of the corpus callosum on diffusion-weighted imaging and a slight decrease in ADC value in the same lesion on an ADC map (Figure 1 ). As no other intracranial signal changes were observed, CLOCCs were suspected, and additional investigative tests, such as cerebrospinal fluid examination, were withheld and follow-up was selected. A chest-computed tomography (CT) scan revealed no pneumonia or other abnormal findings.
Figure 1.
Brain magnetic resonance imaging on day 2 (A and B) and day 12 (C and D)
A: Diffusion-weighted imaging revealed hyperintensity in the splenium of the corpus callosum on day 2.
B: Apparent diffusion coefficient map showed a decrease in the apparent diffusion coefficient value in the splenium of the corpus callosum on day 2.
C,D: Disappearance by day 12 of the lesion in the splenium of the corpus callosum seen on day 2.
After admission, the patient was treated with 1000 mL/day of intravenous fluids and 800 mg of molnupiravir orally twice a day for 5 days, and the fever resolved after the third day. On Day 4, her neurological symptoms resolved, she was able to walk, and oral ingestion became possible. After admission, she developed cough, but her oxygenation capability did not deteriorate, and she was discharged after a 10-day isolation. On Day 12, the patient was reexamined, and a brain MRI revealed that the lesion in the splenium of the corpus callosum had completely disappeared (Figure 1). Based on the clinical course and imaging changes, a diagnosis of CLOCCs associated with COVID-19 was confirmed.
3. Literature review
This case shows that CLOCCs can occur even in non-severe COVID-19 cases in the very early phase. An article that reviewed nine case reports of COVID-19-associated CLOCCs found no similar cases of this type (Sriwastava et al., 2021). The clinical characteristics and severity of CLOCCs in cases of coronavirus infection, including COVID-19, are not fully understood. Therefore, a literature review was performed using PubMed. This is currently the most complete review of case reports and case series on CLOCCs associated with coronaviruses.
3.1. Methods
We searched the PubMed electronic database with no language restrictions from inception through March 2022 to identify cases of patients with coronavirus infection complicated by isolated CLOCCs. The inclusion criteria were cases of solitary lesions that met the definition proposed by Starky et al., regardless of which terms, such as CLOCCs/MERS/RESLES/transient splenic lesions, were used to describe the lesions (Starkey et al., 2017). Cases of intracranial lesions in addition to corpus callosum lesions were excluded. The detailed search terms are shown in Supplementary Table 2.
We included case reports, case series, and other descriptive studies in which patient background and/or clinical course could be extracted, even partially, in addition to MRI findings. Reviews of previously published cases were excluded to avoid duplication of cases. Letters to the editor were included. In addition, the references cited in previously published reports were also reviewed.
3.2. Search results
No reports associated with the previously known four human coronaviruses (229E, OC43, NL63, and HKU1), severe acute respiratory syndrome coronavirus (SARS-CoV or SARS-CoV-1), or Middle East respiratory syndrome coronavirus (MERS-CoV) were found through the literature search. In contrast, we identified 29 cases of CLOCCs (15 case reports and 11 case series) associated with COVID-19 (Supplementary Table 3). Details of the 30 cases, including our report, are summarized in Table 1 and are described below.
Table 1.
A Summary of individual cases reporting COVID-19-associated CLOCCs/MERS/RESLES in order of patient age.
| First author | Article type | Country | Patient age/gender | Risk | Diagnosis | SARS-CoV-2 PCR | SARS-CoV-2 serology | Time between reported COVID-19 symptoms and onset of CLOCCs (days) | Hypoxemia (oxygen saturation ≤ 94% on room air) | Pneumonia | CRP (mg/L) | Na (mmol/L) | Overall outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID 1 | Hayashi M | CR | Japan | 75/M | Alzheimer's disease | C | Positive for oropharyngeal swab | NR | A few | + | + | 53.2 | Normal | Died due to respiratory failure |
| ID 2 | Kakadia B | CR | United States | 69/M | Hypertension | C | Negative for nasopharyngeal swab | Positive IgA/IgG | NR | NR | NR | 503.2 | NR | Resloved |
| (our case) | Kubo M | CR | Japan | 61/F | Asthma | C | Positive for nasopharyngeal swab | NR | 1 | - | - | 75.9 | 129 | Resloved, discharged |
| ID 3 | El Aoud S | CR | France | 60/M | Dyslipidemia | C | Negative for oropharyngeal swab | Positive IgG | 9 | - | + | 50 | NR | Improved |
| ID 4 | Usta NC | CS | Turkey | 57/M | Hypertension, diabetes | C | NR | NR | NR | NR | + | NR | NR | Recovered |
| ID 5 | Forestier G | CR | France | 55/M | None | C | Positive for nasopharyngeal swab | NR | NR | NR | + | 8.1 | Normal | NR |
| ID 6 | Edjlali M | CS | France | 51/M | NR | C | Positive for nasopharyngeal swab | NR | NR | NR | NR | NR | NR | NR |
| ID 7 | Esra Demir | CR | Turkey | 50/M | None | C | Positive for nasopharyngeal and oropharyngeal swab | NR | 0 | - | + | 170 | NR | Resolved, discharged |
| ID 8 | Klironomos S | CS | Sweden | late 40s/F | NR | C | NR | NR | NR | NR | NR | NR | NR | NR |
| ID 9 | Edjlali M | CS | France | 49/M | NR | C | Positive for nasopharyngeal swab | NR | NR | NR | NR | NR | NR | NR |
| ID 10 | Eren F | CR | Turkey | 47/M | None | C | Positive for nasopharyngeal swab | NR | 5 | - | + | 14.2 | NR | Recovered completely, discharged |
| ID 11 | Chauffier J | CR | France | 47/M | None | C | Positive for nasopharyngeal swab | NR | 13 | + | + | 171 | Hyponatremia | Improved, discharged |
| ID 12 | Micci L | CR | United States | 45/M | None | C | Positive | NR | 4 | + | + | NR | NR | Discharged |
| ID 13 | Chevalier K | CR | France | 45/M | None | C | NR | NR | 3 | NR | NR | NR | NR | Recovered, discharged |
| ID 14 | Arıkan FA | CR | Turkey | 43/M | None | C | Positive for nasopharyngeal swab | NR | 5 | NR | + | 94 | NR | Recovered |
| ID 15 | DE Oliveira FAA | CR | Brazil | 40/M | NR | C | Positive for nasal swab | NR | 4 | NR | NR | NR | NR | Improved |
| ID 16 | Usta NC | CS | Turkey | 38/M | None | C | Positive | NR | 3≦ | NR | + | NR | NR | NR |
| ID 17 | Benameur K | CS | United States | 34/M | Hypertension | C | NR | Positive IgM/IgG | 8 | + | + | NR | NR | NR |
| ID 18 | Sen M | CR | Turkey | 33/F | NR | C | Negative | Positive IgM | NR | NR | + | 123 | Normal | Improved, discharged |
| ID 19 | Moreau A | CR | Belgium | 26/M | None | C | Negative for nasopharyngeal swab | Positive IgG | 2 | - | + | 200 | NR | Improved |
| ID 20 | Elkhaled W | CR | Qatar | 23/M | None | C | Positive for nasopharyngeal swab | NR | 0 | + | ARDS | 379.8 | 137 | Died due to multiple organ failure |
| ID 21 | Aksu Uzunhan T | CS | Turkey | 16/M | None | C | Positive | NR | NR | NR | NR | 45 | 138 | NR |
| ID 22 | Elmas B | CS | Brazil | 15/M | NR | C | Positive | Positive IgM/IgG | 2 | NR | - | 7.64 | NR | Recovered |
| ID 23 | Abdel-Mannan O | CS | United Kingdom | 15/F | NR | MIS-C | Positive for nasopharyngeal swab | Positive IgG | 5 | NR | NR | 328 | Normal | Resolved, fully ambulant |
| ID 24 | Çetin H | CS | Turkey | 14/M | NR | C | Positive | NR | NR | NR | NR | NR | NR | Recovered |
| ID 25 | Ucan B | CS | Turkey | 14/M | None | MIS-C | NR | NR | NR | NR | NR | NR | NR | NR |
| ID 26 | Lin J | CR | United States | 13/M | None | MIS-C | Positive | NR | 3 | - | - | 109 | 128 | Recovering |
| ID 27 | Gaur P | CS | United Kingdom | 12/M | NR | MIS-C | Negative for 2 nasopharyngeal swabs | Positive IgG | 5 | + | NR | Elevated | NR | Recovered, discharged |
| ID 28 | Çelebi Y | CR | Turkey | 11/M | None | MIS-C | Negative for 2 nasal swab | Positive IgG | 2 | NR | - | Elevated | Hyponatremia | Recovered |
| ID 29 | Bektaş G | CS | Turkey | 10/M | None | MIS-C | Negative | Positive IgM/IgG | 2 | - | - | 392 | 133 | Recovered completely, discharged |
| First author | Neurological symptoms | Olfactory and taste dysfunction | CT | MRI DWI Findings (days since first neurological symptoms) | CSF | Neurological symptoms (days since CLOCCs occurred) | MRI (days since first imaging) | Neuroradiological terms | |
|---|---|---|---|---|---|---|---|---|---|
| ID 1 | Hayashi M | Alerted consciousness, ataxia, kinetic tremor in his hands, walking instability, urinary incontinence | Normal | NR | (a) (a few days) | Not performed | Partially improved (neurological deficit and cerebellar ataxia had resolved on admission day 2), but died due to respiratory failure | NR | MERS |
| ID 2 | Kakadia B | Disorientation, inattention, and bradyphrenia without focal deficits. | NR | NR | (a) | Normal cell, protein, glucose | Resolved over the course of two weeks | Completely disappeared (14 days) | MERS |
| (our case) | Kubo M | Aletered consiousness (drowsiness), truncal ataxia, dysarthria, intension tremor | Normal | Normal | (a) (0 day) | Not performed | Resloved (2 days) | Completely disappeared (10 days) | CLOCC/MERS/RESLES |
| ID 3 | El Aoud S | Psychomotor slowing, vertigo, headaches, intermittent disturbance of consciousness | NR | Normal | (a) (0 day) | Normal cell, protein, glucose | Psychomotor impairment gradually improved, vertigo and headaches completely recovered | Completely disappeared (1 month) | MERS |
| ID 4 | Usta NC | Dizziness | NR | NR | (a) | NR | Resolved | Not performed | diffusion restriction in the splenium of the corpus callosum (DRCC) |
| ID 5 | Forestier G | Fainting sensations on standing with dizziness and impaired consciousness, headache | NR | NR | (a) | Normal cell, protein, glucose | No interval modification of the neurological symptoms | Completely disappeared (24 days) | CLOCC/MERS |
| ID 6 | Edjlali M | Acute encephalopathy | NR | NR | (a) | NR | NR | NR | CLOCC |
| ID 7 | Esra Demir | Impaired consciousness, slow messy thinking, Romberg sign +, impaired finger-to-nose testing | NR | Normal | (a) (3 days) | Normal cell, protein, glucose | Resolved (7 days) | Completely disappeared (4 days) | CLOCC/MERS |
| ID 8 | Klironomos S | Consciousness @disturbance, paretic extremities |
NR | NR | (a) (14 days after the ICU adimission) | NR | NR | NR | CLOCC |
| ID 9 | Edjlali M | Acute encephalopathy | NR | NR | (a) | NR | NR | NR | CLOCC |
| ID 10 | Eren F | Personality @changes, confusion, and aggression |
NR | NR | (a) (0 day) | Not performed due to high probability of MERS | Regressed (4 days) | Completely disappeared (15 days) | MERS |
| ID 11 | Chauffier J | Confusion, behavioral abnormalities, dysexecutive and memory disorder | Normal | NR | (a) (2 days) | Normal cell, protein, glucose | Improved (7 days) | NR | MERS |
| ID 12 | Micci L | Intermittent blurred vision | NR | NR | (a) (1 day) | NR | NR | NR | CLOCC |
| ID 13 | Chevalier K | Left hemiparesis and psychomotor retardation (significant slowing in movements and speech) | NR | NR | (b) (0 day) | NR | Improved | Completely disappeared (7 days) | CLOCC |
| ID 14 | Arıkan FA | Dysarthria, ataxia | NR | NR | (a) | Elevated protein only (143 mg/dL) | Resolved | Completely disappeared (after discharge on day 40) | CLOCC/Isolated corpus callosum lesion |
| ID 15 | DE Oliveira FAA | Progressive paresthesias in the extremities, mild headache, and visual turbidity. A decrease in visual acuity in the left eye. | NR | NR | (a) | NR | Improved (7 days) | Completely disappeared (1 month) | Others (Transient lesion in the splenium of the corpus callosum) |
| ID 16 | Usta NC | Short-term loss of @consciousness, urinary incontinence |
NR | NR | (a) | NR | NR | Not performed | diffusion restriction in the splenium of the corpus callosum (DRCC) |
| ID 17 | Benameur K | Consciousness disturbance, multifocal myoclonus involving both arms, absent corneal and gag reflexes, absent withdrawal to painful stimuli | NR | NR | (a) (6 days) | Normal cell, protein, glucose | NR | NR | Others (Lesion within the splenium of the corpus callosum) |
| ID 18 | Sen M | Paranoid delusions symptoms, insomnia and irritability | NR | NR | (a) | NR | Improved | Completely disappeared (4 days) | CLOCC |
| ID 19 | Moreau A | Confusion (agitated and disoriented), violent behavior, inappropriate speech | NR | NR | (a) (2 days) | Normal cell, protein, negative for neurotropic viruses PCR | Improved (2 days) | Completely disappeared (21 days) | CLOCC |
| ID 20 | Elkhaled W | Auditory hallucinations, restlessness, suicidal ideations | NR | Normal | (a) (2 days) | Normal cell, chemistry | Not assessable | NR | CLOCC/MERS |
| ID 21 | Aksu Uzunhan T | Normal | NR | NR | (a) | Normal cell, protein, glucose. Negative for viral and bacterial panel. | NR | Completely disappeared | CLOCC/MERS/RESLES |
| ID 22 | Elmas B | Headache, decreased taste | Decreased taste | NR | (a) | NR | Resloved (14 days) | Completely disappeared (14 days) | Others (Diffusion restriction in the corpus callosum splenium section) |
| ID 23 | Abdel-Mannan O | Confused, disoriented, headache, weakness | NR | NR | (a) (4 days) | Not performed | Resloved | NR | Others (Signal changes in the splenium of the corpus callosum) |
| ID 24 | Çetin H | Headache | Hyposomia, hypogeusia | NR | (a) | NR | Resolved | Completely disappeared (after the odor symptom disappeared on the 17th day) | MERS |
| ID 25 | Ucan B | Cerebellar ataxia | NR | NR | (a) | NR | NR | Completely disappeared | MERS |
| ID 26 | Lin J | Delirium, auditory hallucinations, extremities weakness | Normal | NR | (a) (0 day) | Normal cell, protein, glucose | Improved gradually | Completely disappeared | CLOCC |
| ID 27 | Gaur P | Lethargy, severe headache | NR | NR | (a) | NR | Improved rapidly | Not performed due to clinical resolution | CLOCC |
| ID 28 | Çelebi Y | Auditory and visual hallucinations | NR | NR | (a) | NR | Resolved (3 days) | NR | CLOCC |
| ID 29 | Bektaş G | Personality changes, Hallucinations | NR | NR | (b) (0 day) | Normal cell, protein, glucose | Resloved (10 days) | Completely disappeared (6 days) | MERS/RESLES |
Abbreviations: C= COVID-19, CLOCC= cytotoxic lesion of the corpus callosum, CR= case report, CRP= C-reactive protein, CS= case series, CSF= cerebrospinal fluid, CT= computed tomography, DWI= diffusion-weighted imaging, F= female, IgM/IgG= immunoglobulin M/G, M= male, MERS= mild encephalopathy with reversible splenic lesions, MIS-C= multisystem inflammatory syndrome in children, MRI= magnetic resonance imaging, Na= serum natrium level, NR= not reported, RESLES= reversible splenic lesion syndrome.
3.3. Geographic location, age, sex, and underlying conditions
The reporting countries were in Europe and the Middle East (22 cases), North and South America (6 cases), and Asia (2 cases), with the largest number of cases in Turkey (11 cases). Of interest, a literature search of MERS case reports from the pre-COVID-19 era showed that most reports originated in Asia, while fewer were from Western countries, but the reasons for this difference are unclear (Yuan et al., 2017).
The median age of the patients was 40 (range, 10-75) years: of 26 males and 4 females, 22 were ≥16 years and 8 were <16 years. Fifteen of the 21 cases in which past medical or psychiatric history was mentioned were healthy subjects with no specific history.
3.4. Neuroradiological characteristics
Neurological symptoms (including duplication) in 30 cases included altered consciousness (including cognitive impairment, confusion, delirium, lethargy, coma, or personality changes) in 20 cases, ataxia in 5 cases, hallucinations (auditory or visual, etc.) in 3 cases, dysarthria in 2 cases, and suicidal ideation in 1 case; none had seizures and one case was asymptomatic. A case was reported as a psychiatric emergency in which the initial diagnosis was mania, which was later found to be COVID-19-associated CLOCCs (Sen et al., 2021). Brain CT findings were reported in four cases and were normal. Cerebrospinal fluid analyses were reported in 12 cases and were normal in 11 cases.
MRI findings revealed a small round or oval lesion in the middle of the corpus callosum (image pattern (a) from the article by Starkey et al.) in 28 of 30 patients, and a lesion extending laterally via callosal fibers into the adjacent white matter in addition to the above-mentioned lesion (image pattern (b) from the article by Starkey et al.) in two patients (Starkey et al., 2017).
The course of neurological symptoms improved in 18 cases and partially improved in two cases (one died of respiratory failure), but the symptom course was not described for eight cases. In most cases that experienced improvement, symptoms resolved within a few days up to a week. Another patient died of multi-organ failure before an assessment was performed, while the other had symptoms that were unchanged. A repeat MRI revealed that the lesion completely disappeared in 17 cases. These findings are consistent with reports that most patients improved within a week in the pre-COVID-19 era, although reversibility is not necessary for a diagnosis of CLOCCs (Hoshino et al., 2012; Starkey et al., 2017; Yuan et al., 2017).
The neuroradiological diagnosis terms (with duplicates) referred to in the 30 case reports varied from CLOCCs in 16 cases to MERS in 13 cases, RESLES in 3 cases, and other in 7 cases. Only one case other than ours mentioned all three terms (Aksu Uzunhan et al., 2021). This requires caution when reviewing previous publications. Sriwastava et al. reviewed nine cases of CLOCCs in their literature search up to March 2021, but we found eight more cases reported under terms other than CLOCCs published during the same period (Sriwastava et al., 2021).
Lesions of the corpus callosum associated with COVID-19 have been reported to be caused by acute disseminated encephalomyelitis, reversible posterior leukoencephalopathy syndrome, parts of other cerebral infarction lesions, and micro-bleeding (Fitsiori et al., 2020; Harapan & Yoo, 2021; Sawlani et al., 2021). The first three are usually accompanied by CNS lesions other than those in the corpus callosum, while the latter microhemorrhages could occur in critically-ill patients who undergo mechanical ventilation and/or extracorporeal membrane oxygenation (Thurnher et al., 2021); however, all these lesions can be differentiated by integrated clinical judgment in context and imaging findings.
Ischemic stroke is a common complication of COVID-19, which raises the issue of differential diagnosis of ischemic stroke in patients presenting with CLOCC-like MRI patterns. As life-long antiplatelet therapy is considered in ischemic stroke, it is important to recognize CLOCCs from the clinical management point of view. While it is difficult to differentiate between hyperacute stroke and CLOCCs based solely on MRI findings of the corpus callosum within the first 24 hours of onset, it can be helpful to focus on whether there are lesions other than the corpus callosum that are suggestive of cerebral infarction. This is due to the anatomical characteristics that the corpus callosum has multiple perfusion systems and a solitary lesion in the corpus callosum is exceedingly rarely caused by ischemia (Dhillon & Lenthall, 2020; Sparr & Bieri, 2020). The blood supply of the corpus callosum comes from both the anterior and posterior circulation and has developed collateral circulation. If a corpus callosum lesion is caused by ischemia, ischemic lesions are often present in other parts of the brain. It is also important to note that cerebral infarction of the corpus callosum is a condition that occurs in the elderly with vascular risk factors. A study of 127 cases of cerebral infarction of the corpus callosum, including 21 cases of pure corpus callosum infarction, found that >90% of patients had stenosis or occlusion of large cerebral arteries and most of them had lesions in more than two vessels (Sun et al., 2019). In our case, the clinical decision was made not to commence antiplatelet therapy at the time of the initial presentation, as cerebral infarction was not suspected based on the patient's little vascular risk factors and the distribution of lesions. Furthermore, if it is difficult to differentiate in the early stages of onset, a repeat MRI is useful to detect changes over time consistent with cerebral infarction. Some reports have suggested that CLOCCs were suspected based on an observed low density in the corpus callosum on brain CT, but we do not believe that this finding is sufficient or characteristic enough to confirm a diagnosis of CLOCCs (A. Agarwal et al., 2020; Dhillon & Lenthall, 2020). In contrast, the four cases reported as ischemic infarction of the corpus callosum with COVID-19 in the early phase of the pandemic were subsequently followed by multiple letters raising questions as to whether they were truly ischemic strokes (Dhillon & Lenthall, 2020; Sparr & Bieri, 2020). Therefore, caution is advised when diagnosing a solitary lesion of the corpus callosum associated with COVID-19 as cerebral infarction.
In our review, we sought to clarify the typical clinico-radiological features of COVID-19-associated CLOCCs by excluding reported cases in which the diagnosis was uncertain or that did not meet Starkey's definition even if the authors had reported the case as CLOCCs (A. Agarwal et al., 2020; N. Agarwal et al., 2020; Gaur et al., 2020; Hacohen et al., 2020; Rasmussen et al., 2020). Abdel-Mannan et al. reported a case series of four children with COVID-19 and multisystem inflammatory syndrome in children (MIS-C); these patients showed lesions in the splenium of the corpus callosum, three of whom did not exhibit findings consistent with CLOCCs, as the lesions were either not diffusion-restricted on MRI or they were present in areas in addition to the corpus callosum (Hacohen et al., 2020). We are unsure whether these cases are of similar or different pathophysiology to CLOCCs, but they may provide clues for further research.
3.5. COVID-19 status/severity and CLOCCs
COVID-19 diagnosis was based on PCR positive result in 18 cases and serodiagnosis in 8 cases but was unreported in 4 cases.
Of the 30 cases, 22 patients aged 16 years and older had developed CLOCCs during the course of COVID-19. In the 15 cases where the course was reported, the median time between the onset of COVID-19 and that of CLOCCs was 3 days (range, 0–13 days). Of the 15 cases, 14 were complicated by pneumonia or hypoxemia, and five received ventilation or critical care. Only our case was not complicated by pneumonia or hypoxemia.
Moreover, of eight cases under 16 years of age, six were complicated by COVID-19-associated MIS-C. The median time between the onset of MIS-C and the onset of CLOCCs was 3 days (range, 2–5 days). CLOCCs developed during the course of COVID-19 in the remaining two cases.
Thus, CLOCCs associated with COVID-19 were primarily reported in adults with pneumonia and in children with MIS-C, which occurred at a relatively early phase of the disease (median, 3 days). This is consistent with the assumption that the pathogenesis of CLOCCs involves nonspecific cytokines independent of triggers such as influenza (Zhu et al., 2016).
Our case did not have pneumonia but presented with typical CLOCCs only one day after the onset of COVID-19. To our knowledge, this is the first report of CLOCCs in an adult with mild COVID-19. This suggests that COVID-19-associated CLOCCs can occur even at an early, non-severe stage.
This case was reported during the Omicron predominance period. The severity of COVID-19 has been reported to be much lower during this period (Iuliano et al., 2022). Thus, this case might reflect Omicron features. Meanwhile, sufficient information on the SARS-CoV-2 sublineage was unavailable from our literature review, with 28 cases submitted or published online by December 2021 and one published online in February 2022. As the first case of Omicron was reported from South Africa to the World Health Organisation on November 24, 2021, it is presumed that these cases were reported before its emergence.
This study has some limitations. This is a single-case report, and we could not examine the sublineage in this case. Furthermore, the literature review of case reports and case series inevitably contains reporting biases and missing information. Therefore, it is unclear whether this case represented a rare phenotype of the broad spectrum of COVID-19-associated CLOCCs or whether it could be more common in the relatively less severe Omicron or future prevalent strains. Thus, further studies are needed to fully understand the pathophysiology and severity of COVID-19-associated CLOCCs, including those of mild cases.
4. Summary
We reported a case in which the diagnosis of CLOCCs was reached by MRI performed to differentiate cerebellar infarction when the patient presented to the emergency department the day after a fever. In patients with COVID-19, CLOCCs may easily be underdiagnosed if MRI scans are not performed, given that most mild cases are treated at home, the need for infection control raises the threshold for MRI scans, and CLOCCs have the characteristic of neurological symptoms that improve within several days. Appropriate diagnosis could prevent unnecessary further invasive testing, such as cerebrospinal fluid testing, and lead to the initiation of infection control measures in undiagnosed COVID-19 cases. During the COVID-19 pandemic, clinicians should be mindful of COVID-19-associated CLOCCs as a differential diagnosis in patients presenting with altered consciousness/personality or ataxia, even in cases of mild illness.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
Acknowledgments
The authors thank ENAGO (https://www.enago.jp/) for the English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Approval
This study adhered to the Declaration of Helsinki. No approval was required. Informed consent was obtained from the patient in the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.09.009.
Appendix. Supplementary materials
References
- Agarwal A., Pinho M., Raj K., Yu F.F., Bathla G., Achilleos M., ONeill T., Still M., Maldjian J. Neurological emergencies associated with COVID-19: stroke and beyond. Emergency Radiology. 2020;27(6) doi: 10.1007/s10140-020-01837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N., Martini R., Pedrotti G., della Sala S.W. Unusual lesion in the splenium of the corpus callosum and coronavirus infectious disease-19. BJR|case Reports. 2020;6(3) doi: 10.1259/bjrcr.20200068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu Uzunhan T., Maraş Genç H., Kutlubay B., Kalın S., Bektaş G., Yapıcı Ö., Çıracı S., Sözen H.G., Şevketoğlu E., Palabıyık F., Gör Z., Çakar N.E., Kara B. Cytotoxic lesions of the corpus callosum in children: Etiology, clinical and radiological features, and prognosis. Brain and Development. 2021;43(9) doi: 10.1016/j.braindev.2021.05.001. [DOI] [PubMed] [Google Scholar]
- Chou S.H.Y., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., Mainali S., Bassetti C., Suarez J.I., McNett M. Global Incidence of Neurological Manifestations among Patients Hospitalized with COVID-19 - A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Network Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.12131. –e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougar L., Shor N., Weiss N., Galanaud D., Leclercq D., Mathon B., Belkacem S., Ströer S., Burrel S., Boutolleau D., Demoule A., Rosso C., Delorme C., Seilhean D., Dormont D., Morawiec E., Raux M., Demeret S., Gerber S.…Pyatigorskaya N. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. 2020;297(3):E313–E323. doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon, P. S., & Lenthall, R. (2020). Letter by Dhillon and Lenthall Regarding Article, “infarction of the Splenium of the Corpus Callosum in the Age of COVID-19: A Snapshot in Time.” In Stroke. https://doi.org/10.1161/STROKEAHA.120.032156 [DOI] [PubMed]
- Elkhaled W., Abid F.ben, Akhtar N., Abukamar M.R., Ibrahim W.H. A 23-year-old man with SARS-CoV-2 infection who presented with auditory hallucinations and imaging findings of cytotoxic lesions of the corpus callosum (CLOCC) American Journal of Case Reports. 2020;21 doi: 10.12659/AJCR.928798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. COVID-19 is Associated with an Unusual Pattern of Brain Microbleeds in Critically Ill Patients. Journal of Neuroimaging. 2020;30(5) doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Monco J.C., Cortina I.E., Ferreira E., Martínez A., Ruiz L., Cabrera A., Beldarrain M.G. Reversible Splenial Lesion Syndrome (RESLES): What's in a Name? In Journal of Neuroimaging. 2011;21(2) doi: 10.1111/j.1552-6569.2008.00279.x. IssueJ Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Gaur P., Dixon L., Jones B., Lyall H., Jan W. COVID-19-associated cytotoxic lesions of the corpus callosum. American Journal of Neuroradiology. 2020;41(10) doi: 10.3174/ajnr.A6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y., Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B., Hemingway C. Neurologic and Radiographic Findings Associated with COVID-19 Infection in Children. JAMA Neurology. 2020;77(11) doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan B.N., Yoo H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) Journal of Neurology. 2021;268(9) doi: 10.1007/s00415-021-10406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Sahashi Y., Baba Y., Okura H., Shimohata T. Vol. 415. Elsevier B.V; 2020. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. (Journal of the Neurological Sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Saitoh M., Oka A., Okumura A., Kubota M., Saito Y., Takanashi J., Hirose S., Yamagata T., Yamanouchi H., Mizuguchi M. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain and Development. 2012;34(5) doi: 10.1016/j.braindev.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Iuliano A.D., Brunkard J.M., Boehmer T.K., Peterson E., Adjei S., Binder A.M., Cobb S., Graff P., Hidalgo P., Panaggio M.J., Rainey J.J., Rao P., Soetebier K., Wacaster S., Ai C.E., Gupta V., Molinari N.A.M., Ritchey M.D. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods — United States, December 2020–January 2022. MMWR Recommendations and Reports. 2022;71(4):146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klironomos S., Tzortzakakis A., Kits A., Öhberg C., Kollia E., Ahoromazdae A., Almqvist H., Aspelin Å., Martin H., Ouellette R., Al-Saadi J., Hasselberg M., Haghgou M., Pedersen M., Petersson S., Finnsson J., Lundberg J., Delgado A.F., Granberg T. Nervous system involvement in coronavirus disease 2019: Results from a retrospective consecutive neuroimaging cohort. Radiology. 2020;297(3):E324–E334. doi: 10.1148/radiol.2020202791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H.…Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. International Journal of Infectious Diseases. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nextstrain /ncov / gisaid / global / 6m. (n.d.). Retrieved June 19, 2022, from https://nextstrain.org/ncov/gisaid/global/6m?f_country=Japan
- Rasmussen C., Niculescu I., Patel S., Krishnan A. COVID-19 and involvement of the corpus callosum: Potential effect of the cytokine storm? American Journal of Neuroradiology. 2020;41(9) doi: 10.3174/ajnr.A6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawlani V., Scotton S., Nader K., Jen J.P., Patel M., Gokani K., Denno P., Thaller M., Englezou C., Janjua U., Bowen M., Hoskote C., Veenith T., Hassan-Smith G., Jacob S. COVID-19-related intracranial imaging findings: a large single-centre experience. Clinical Radiology. 2021;76(2) doi: 10.1016/j.crad.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Yesilkaya U.H., Balcioglu Y.H. SARS-CoV-2-associated first episode of acute mania with psychotic features. Journal of Clinical Neuroscience. 2021;87 doi: 10.1016/j.jocn.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparr, S. A., & Bieri, P. (2020). Response by Sparr and Bieri to Letter Regarding Article, “infarction of the Splenium of the Corpus Callosum in the Age of COVID-19: A Snapshot in Time.” In Stroke. https://doi.org/10.1161/STROKEAHA.120.032521 [DOI] [PubMed]
- Sriwastava S., Tandon M., Podury S., Prasad A., Wen S., Guthrie G., Kakara M., Jaiswal S., Subedi R., Elkhooly M., Lisak R.P. COVID-19 and neuroinflammation: a literature review of relevant neuroimaging and CSF markers in central nervous system inflammatory disorders from SARS-COV2. Journal of Neurology. 2021;268(12) doi: 10.1007/s00415-021-10611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey J., Kobayashi N., Numaguchi Y., Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: Mechanisms, causes, and manifestations. Radiographics. 2017;37(2) doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
- Sun X., Li J., Fan C., Zhang H., Si Y., Fang X., Guo Y., Zhang J.H., Wu T., Ding S., Bi X. Clinical, neuroimaging and prognostic study of 127 cases with infarction of the corpus callosum. European Journal of Neurology. 2019;26(8):1075–1081. doi: 10.1111/ene.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Takanashi J.I., Barkovich A.J., Oba H., Maeda M., Tsukahara H., Suzuki M., Yamamoto T., Shimono T., Ichiyama T., Taoka T., Sohma O., Yoshikawa H., Kohno Y. Vol. 63. Lippincott Williams and Wilkins; 2004. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion; pp. 1854–1858. (Neurology). Issue. [DOI] [PubMed] [Google Scholar]
- Thurnher M.M., Boban J., Röggla M., Staudinger T. Distinct pattern of microsusceptibility changes on brain magnetic resonance imaging (MRI) in critically ill patients on mechanical ventilation/oxygenation. Neuroradiology. 2021;63(10) doi: 10.1007/s00234-021-02663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travi G., Rossotti R., Merli M., D'Amico F., Chiappetta S., Giussani G., Panariello A., Corradin M., Vecchi M., Raimondi A., Baiguera C., Nocita B., Epis O.M., Tarsia P., Galbiati F., Colombo F., Fumagalli R., Scaglione F., Moreno M.…Puoti M. Neurological manifestations in patients hospitalized with COVID-19: A retrospective analysis from a large cohort in Northern Italy. European Journal of Neuroscience. 2021;53(8):2912–2922. doi: 10.1111/ejn.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Yang S., Wang S., Qin W., Yang L., Hu W. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults-a case report and literature review. BMC Neurology. 2017;17(1) doi: 10.1186/s12883-017-0875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Zheng J., Zhang L., Zeng Z., Zhu M., Li X., Lou X., Wan H., Hong D. Reversible splenial lesion syndrome associated with encephalitis/encephalopathy presenting with great clinical heterogeneity. BMC Neurology. 2016;16(1) doi: 10.1186/s12883-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.