Abstract
Background
There are conflicting reports on the results of several of the latest clinical trials related to the use of baricitinib in the management of COVID-19 patients. The aim of the current systematic review and meta-analysis was to evaluate the efficacy of baricitinib in COVID-19 patients.
Methods
Databases like ScienceDirect, PubMed/Medline, Publons, Google Scholar and other sources like ClinicalTrials.gov, Cochrane, medRxiv, Research Square and reference lists were thoroughly searched.
Results
Fifteen (15) articles which met the inclusion criteria were qualitatively and quantitatively analysed. Based on Cochrane and Newcastle-Ottawa Scale (NOS) risk of bias (RoB) analyses, 14/15 articles are grouped as high-quality. Meta-analyses revealed that randomised control trials (RCTs) and non-randomised control trials (nRCTs) statistically significantly reduced the mortality rate in COVID-19 patients, with a risk ratio (RR) in the fixed-effect model was RR = 0.64 [95% CI: 0.51 to 0.79; p < 0.0001] and RR = 0.58 [95% CI: 0.45 to 0.73; p < 0.00001], respectively, with insignificant heterogeneity and no publication bias found. For block/reduce disease progression (BDP), baricitinib did not statistically significantly reduce disease progression for RCTs. The RR in the random effect model was RR = 0.80 [95% CI: 0.58 to 1.10: p = 0.17], with significant heterogeneity, where I2 was 60%. On the other hand, baricitinib statistically significantly reduced disease progression in nRCTs, as the RR of the fixed effect model was RR = 0.54 [95% CI: 0.37 to 0.78; p = 0.001] with insignificant heterogeneity.
Conclusion
The current meta-analyses revealed that baricitinib statistically significantly reduced mortality rate and disease progression in COVID-19 patients.
Prospero registration number
CRD42021281556
Keywords: Baricitinib, Disease progression, Mortality, Meta-analysis, Systematic review, COVID-19
1. Introduction
As of 05th October 2021, approximately 236 million individuals were infected by the novel SARS-CoV-2 virus causing COVID-19 disease, resulting in more than 4.82 million deaths worldwide [1]. Older people with underlying diseases such as obesity, high blood pressure, diabetes, and kidney related diseases have been reported to have poorer recovery after infection by COVID-19 [2]. Even though infections and deaths due to COVID-19 are mainly concentrated in the population aged 60 and above, COVID-19 infection rates have increased over time in the younger population. This younger population group may act as a significant threat to the older population with underlying diseases [3,4]. The 11 months of evolution of the SARS-COV-2 virus from its emergence in late 2019 led to the birth of variants of concern. These mutations alter the characteristics of the virus, and some variants have been reported to have reduced sensitivity towards vaccines [5]. The SARS-CoV-2 B.1.617 lineage was originally detected in India in October 2020. This lineage became dominant in several regions in India and United Kingdom, and subsequently spread worldwide. There are three main subtypes, known as B1.617.1, B.1.617.2 and B.1.617.3, all of which have several spike protein mutations (T19R, Δ157–158, L452R, T478K, D614G, P681R, and D950 N) at the N-terminal domain and receptor binding domain. Several of the aforementioned mutations might alter immune responses targeting the key antigenic regions of receptor-binding protein (452 and 478), as well as the deletion of the region of N-terminal domain. Strains with mutations at the S1–S2 cleavage site (P618R) might have augmented replication. This replication would cause an elevation of viral load, leading to increased viral transmission. The subtype B.1.617.2, also known as variant Delta, is thought to have spread more rapidly than other variants [6,7]. The World Health Organization's (WHO) therapeutic and COVID-19: Living guideline, which was updated on 24th September 2021, contained two new conditional recommendations in the WHO's latest guideline for the treatment of COVID-19 patients [8]. With the addition of latest recommendation, there were nine recommendations available as of 24th September 2021. The latest two recommendations were a conditional recommendation to use a combination of neutralizing monoclonal antibodies (casirivimab and imdevimab) in severe and critically ill COVID-19 patients with seronegative status; and a conditional recommendation to use a combination of neutralizing monoclonal antibodies (casirivimab and imdevimab) in non-severe COVID-19 patients at the highest risk of severe disease. Another recommendation refers to the use of interleukin-6 (IL-6) receptor blockers, known as tocilizumab and sarilumab, to combat COVID-19. This update was made available in July 2021, following the publication of RECOVERY and REMAP-CAP trials, in which the researchers found that IL-6-receptor blockers may be a remedy for COVID-19 patients. The remaining six recommendations from earlier versions included the recommendation not to use ivermectin in patients with COVID-19 except in the context of a clinical trial (published 31 March 2021); a strong recommendation against hydroxychloroquine in patients with COVID-19 of any severity (published 17 December 2020); a strong recommendation against lopinavir/ritonavir in patients with COVID-19 of any severity (published 17 December 2020); a conditional recommendation against remdesivir in hospitalized patients with COVID-19 (published 20 November 2020); a strong recommendation for systemic corticosteroids in patients with severe and critical COVID-19 (published 2 September 2020); and a conditional recommendation against systemic corticosteroids in patients with non-severe COVID-19 (published 2 September 2020). These guidelines are reported directly without sentence modification from authors. It is worth mentioning that on 14 January 2022, WHO strongly recommended the use of baricitinib with corticosteroids in severe or critical COVID-19 patients [8].
Through artificial intelligence, baricitinib has been found to inhibit the SARS-CoV-2 virus. Richardson et al. [16] reported that using BenevolentAI’ knowledge graph to explore for approved drugs would help find a drug which can inhibit the viral infection process. Subsequent to this discovery, the researchers found that baricitinib has been projected to decrease the capability of the COVID-19 virus to infect lung cells. Receptor mediated endocytosis is a channel for viruses to enter cells. The COVID-19 virus possibly uses the ACE2 receptor to infect the lung cells. It is a cell surface protein which importantly is found on lung AT2 alveolar epithelial cells, which are prone to infection. AP2-associated protein kinase 1 (AAK1) is an endocytosis regulator. Blocking AAK1 might disturb the entrance of virus into the cells and disrupt the intracellular assembly of viral particles. Although several oncology drugs have been suggested to inhibit AAK1, the researchers did not consider to take them to next level due to safety concerns. The researchers found that baricitinib not only blocks AAK1, but also binds to cyclin-G associated kinase. Cyclin-G associated kinase is another regulator of endocytosis. The researchers suggested that it is worth using baricitinib in clinical trials. In general, the inhibition of AAK1 and cyclin-G associated kinase by baricitinib will lead to the prevention of endocytosis (taking in a substance from the outer to inner environment) and prevent the entry of the virus into cells, reducing lung inflammation by preventing lung AT2 alveolar epithelial cells from getting infected by the virus. According to the U.S Food and Drug Administration (FDA), one drug is currently approved for the treatment of COVID-19 disease, known as remdesivir [9]. Remdesivir received conditional recommendation from WHO. On 19 November 2020, the FDA issued emergency use authorization to use baricitinib together with remdesivir. As of 19 November 2020, baricitinib had not received approval nor authorization for use as a stand-alone drug to combat COVID-19. A recent report published in Science Advances confirmed the double actions of baricitinib, which include the ability to block viral entry in the direction of through to the primary human hepatocyte spheroids, as well as a decrease in inflammatory markers in COVID-19 sufferers. Furthermore, baricitinib averts the type-1 IFN–mediated surge in the expression of angiotensin converting enzyme 2 (ACE2), the receptor for COVID-19 virus [10]. Cantini et al. [11,12] reported promising effects of baricitinib in term of safety and improvement of clinical impact, as well as lessening of severity development in moderately affected COVID-19 patients. Goletti and Cantini [13] stated in the New England Journal of Medicine that based on the available evidence, remdesivir is expected to be most efficacious in early COVID-19 infection, while dexamethasone is expected to be most useful in later stages of disease progression. There is a need for additional treatment options for COVID-19 patients in order to decrease the chance of further advancement of disease to invasive procedures or death. The recent approval of baricitinib as a single drug treatment, instead of in combination with remdesivir, was due to recently-published patient-oriented clinical study reports. Baricitinib has been reported to reduce the cytokine storm; reduce adverse effect related to infection; improve recovery rate; shorten the length of hospital stays; and reduce the overall mortality rate in the COVID-19 virus infected patients [10,15,22,24]. Several clinical trials have been carried out using baricitinib and some of the data are conflicting. Does baricitinib have the ability to block COVID-19 disease progression to the next stage in patients and reduce mortality? To address these research questions, the current review was carried out systematically by examining the latest clinical trials related to baricitinib for COVID-19 treatment.
2. Methodology
In the current systematic review and meta-analysis, Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were used to develop this review. These guidelines were followed accordingly. The current systematic review and meta-analysis is registered in PROSPERO (registration number: CRD42021281556). During improvement of the manuscript, some deviations from the protocol took place and listed at the end of manuscript.
2.1. Research questions
In the current review, the authors have critically addressed the main questions, while the outcomes of the supplementary questions are tabulated in Table 1 , and analysed in Fig. 2.
Table 1.
Characteristics of the included articles and the outcomes.
| Study | Study design | Period of study | Country | Population and age (B vs C) | Intervention | Comparator | Outcomes | Px follow-up |
|---|---|---|---|---|---|---|---|---|
| Stebbing [10] | Observational | Mar–Apr 2020 | Italy & Spain | 2 centres/hospitals & 66 vs 65-Italy 80.9 vs 80.6-Spain |
|
Concomitant antiviral therapy with HCQ and lopinavir/ritonavir, antibiotics, corticosteroids, and low–molecular weight heparin (LMWH) |
|
Not clear but based on Kaplan-Meier analysis, the observation was made for 40 days. |
|
|
|||||||
| ||||||||
| ||||||||
| Bronte [15] | Observational & longitudinal | Mar–Apr 2020 | Italy | 2 centres/hospitals & 68 vs 77.5 | Baricitinib 4 mg twice daily for 2 days then 4 mg/day for 7 days | Treated with HCQ or antiviral ((lopinavir/ritonavir) or combination |
|
Not clear but the authors mentioned as short follow up time |
| ||||||||
| ||||||||
| ||||||||
| Hasan [17] | Prospective | July–Oct 2020 | Bangladesh | 1 centre/hospital & 63 vs 59 |
|
Comparison between 4 mg & 8 mg groups only. No group without baricitinib designed for actual comparison |
|
Not mentioned |
|
|
|||||||
| ||||||||
| ||||||||
| Hasan [18] | Prospective case control | May–June 2020 | Bangladesh | 1 centre/hospital & 59 vs 52 |
|
Comparison between 4 mg & 8/4 mg groups only. No group without baricitinib designed for actual comparison |
|
Not mentioned |
|
|
|||||||
| ||||||||
| ||||||||
| Rodriguez-Garcia [19] | Observational | Mar–Apr 2020 | Spain | 1 centre/hospital & 63 vs 64 |
|
Comparison between baricitinib + CS & CS group |
|
1 month |
|
|
|||||||
| ||||||||
| ||||||||
| Kalil [22] | RCT | May–July 2020 | 8 countries | 67 trial sites & 55 vs 55.8 |
|
Remdesivir |
|
28 days |
|
|
|||||||
| ||||||||
| ||||||||
| Marconi [24] | RCT | Jun 2020–Jan 2021 | 12 countries | 101 centres & 57.8 vs 57.5 |
|
Remdesivir, systemic corticosteroid and dexamethasone was used |
|
28 days |
|
|
|||||||
| ||||||||
| ||||||||
| Abizanda [26] | Retrospective | Mar–July 2020 | Spain | 1 centre/hospital & 58.6 vs 59.2 (<70-year-old); 79.2 vs 79.1 (>70-year-old) | Mean total dose of baricitinib was 17.6 mg for mean treatment day of 5.9 days. | Anakinra, tocilizumab, or corticosteroids (mostly used) Also lopinavir/ritonavir, HCQ and LWMH |
|
On average the follow up was 2 weeks |
| ||||||||
| ||||||||
| ||||||||
| Cantini [11] | Clinical trial/pilot study | Mar–Mar 2020 | Italy | 1 centre/hospital & 63.5 vs 63 | Baricitinib 4 mg/day with lopinavir/ritonavir for 2 weeks | Hydroxychloroquine with lopinavir/ritonavir. Mainly was SOC |
|
Planned for 1.5 months of follow-up |
| ||||||||
| ||||||||
| ||||||||
| Cantini [12] | Observational, retrospective, longitudinal multicentre-study | Feb–Mar 2020-Control arm Mar–May 2020- Baricitinib arm | Italy | 7 centres/hospitals & 68 vs 63 | Baricitinib 4 mg/day with lopinavir/ritonavir for 2 weeks | Hydroxychloroquine with lopinavir/ritonavir. Mainly was SOC |
|
Not mentioned |
| ||||||||
| ||||||||
| ||||||||
| Rosas [27] | Retrospective observational | Mar–Apr 2020 | Spain | 1 centre/hospital & 67.8 vs 73.8 | Baricitinib monotherapy 2mg/4 mg |
|
|
1 month |
|
|
|||||||
|
|
|||||||
| ||||||||
| Perez-Alba [20] | Retrospective | Mar–Nov 2020 | Mexico | 1 centre/hospital & 60.7 vs 58.5 | Baricitinib + dexamethasone | Dexamethasone monotherapy |
|
No follow-up |
| ||||||||
| ||||||||
| ||||||||
| García-García [23] | Retrospective | Sept–Nov 2020 | Spain | 2 centres/hospitals & 71 vs 73 | Baricitinib 4 mg/day for up to 10 days + SOC | Anakinra 200 mg on first day then 100 mg for up to 10 days |
|
Not clear but mentioned about follow up in the article |
| ||||||||
| ||||||||
| ||||||||
| Wesley-Ely [30] | RCT | Dec 2020–April 2021 | 4 countries (American continent) | 18 centres/hospitals & 58.4 vs 58.8 | Baricitinib 4 mg + SOC | Placebo + SOC |
|
28-days |
| ||||||||
| ||||||||
| ||||||||
| Falcone [31] | Observational | Mar–Apr 2020 | Italy | 1 hospital & Unclear | Unclear | Unclear |
|
Until death or 1 month |
| ||||||||
| ||||||||
|
BDP: Block Disease Progression; MRR: Mortality Rate Reduction; IPC: Improved Patient Condition; SLHS: Shorten Length of Hospital Stay; LD: Loading Dose; ICU: Intensive Care Unit; AMV: Assisted Mechanical Ventilation; IMV: Invasive mechanical ventilation; ECMO: Extracorporeal membrane oxygenation; Px: Patient; SOC: Standard of Care; HCQ: Hydroxychloroquine; B: Baricitinib; C: Control.
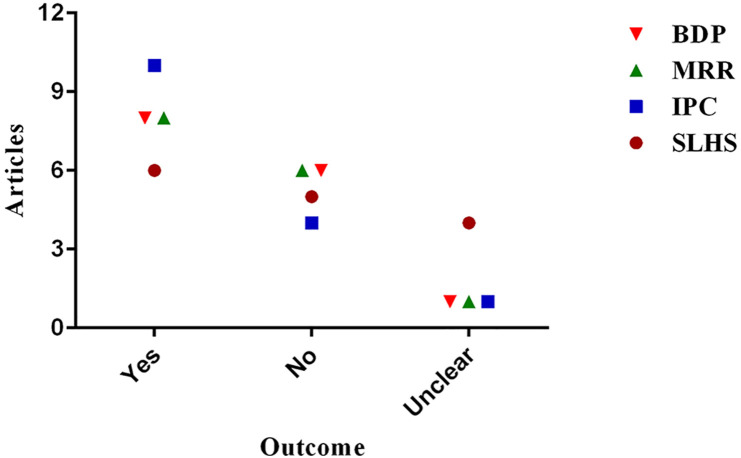
Fig. 2.
Summary of outcomes based on 15 literatures documented in Table 1. The image was prepared with GraphPad Prism 6.0.
The primary questions include the following:
-
•
Is baricitinib able to reduce the mortality rate in COVID-19 patients?
-
•
Is baricitinib able to reduce or prevent COVID-19 disease stage progression?
Supplementary questions:
-
•
Is baricitinib able to improve COVID-19 patients' conditions?
-
•
Is baricitinib able to shorten the length of hospital stays?
2.2. Search strategies and article eligibility criteria
A mixture of different types of articles was searched as described in abstract. The keywords used were ‘Baricitinib COVID-19; Baricitinib SARS-CoV-2 virus; Baricitinib Pneumonia’. The searched year was between 2020-early September 2021. Each article was screened and articles related to non-human studies (in vitro, in vivo, in silico, case study, poster, review articles) were excluded from the main analysis. Randomised control trials (RCTs), observational, retrospective and prospective cohort studies were included. Only English language articles were included in the search. By implementing language restriction, the authors were aware that some valuable articles would be missed, but it would be risky to do a direct translation of such articles, because the quality of translation and the message may vary. Additional searches on the references list of included articles were carried out based on the keywords. Since ‘baricitinib related to COVID-19 management’ is a very new topic, a search for grey literature was not thoroughly performed. However, searches were performed in preprint related repositories known as medRxiv and Research Square. Two days after the completion of the current review, one valuable RCT article [30] was found published as a medRxiv preprint (12 October 2021) and included in RCTs analysis. A list of excluded studies with explanations for each exclusion is provided in supplementary file (S1).
2.3. Data charting process
The data charting process, including screening of titles, abstract and text, was carried out independently by two different individuals.
2.4. Risk of bias assessment (RoB) and grading the evidence with GRADEpro Guideline Development Tool
The Cochrane risk-of-bias tool was used to assess the quality of RCTs, while Newcastle-Ottawa scale (NOS) was used for nRCTs. Review Manager 5.4.1 [28] was used for RoB related to RCTs. Seven tools were used in order to assess the risk of biases present in all included articles: (i) random sequence generation; (ii) allocation concealment; (iii) blinding participants and personnel; (iv) blinding of outcome assessment; (v) incomplete outcome data; (vi) selective reporting; and (vii) other bias. The outcomes were categorised as (i) low risk; (ii) high risk; and (iii) unclear. For NOS, the nRCTs articles were evaluated according to their selection, comparability and outcomes. Articles with scores of 6 or above were regarded as high-quality articles [32]. Additionally, the authors used GRADEpro Guideline Development Tool software [29] to grade article quality.
2.5. Meta-analysis
Meta-analysis was carried out to answer the two primary research questions listed in section 2.1. Analysis was carried out using Review Manager 5.4.1. Dichotomous data type was used. The data were pooled, and relative risk, confidence interval (CI) and Mantel-Haenszel statistical method were used. The degree of heterogeneity was evaluated based on p value, the I2 test and the I2 measure was used to assess statistical heterogeneity. Heterogeneity was defined as significant when p < 0.1 or I2>50% [38]. A fixed-effect model was used when the data were homogeneous, while a random-effects model was used if the data were heterogeneous. With the presence of at least 10 articles in single analysis, publication bias was interpreted using a funnel plot. The analysis was carried out separately to figure out the outcome of individual type of studies (example: RCTs vs nRCTs). Furthermore, different types of study have different levels of RoB. The authors decided to avoid pulling the different levels of RoB in the analysis. Due to this, the exact outcomes of RCTs and nRCTs were revealed.
3. Results
3.1. Study inclusion
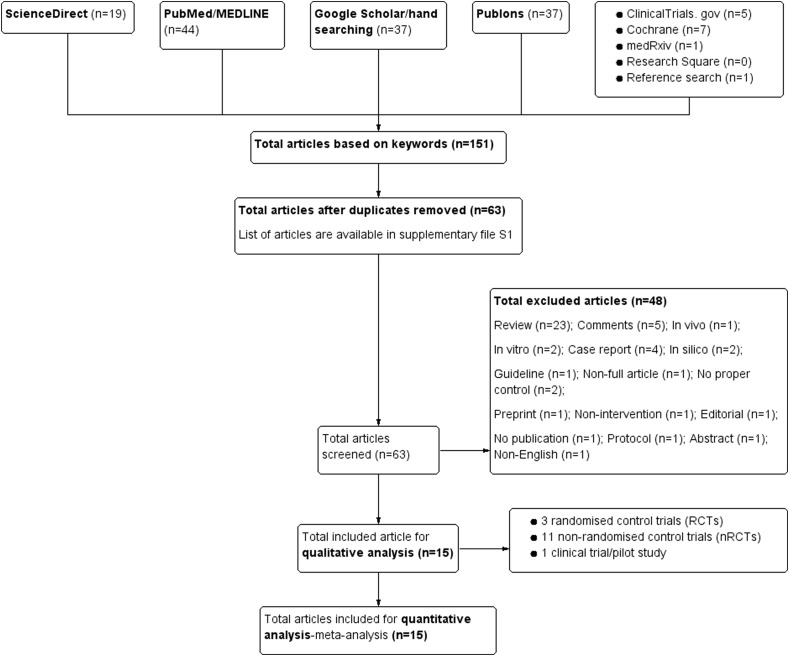
Based on the literature search, a total of 151 articles were found using the keywords. The same keywords were used in all databases and repositories. Fifteen (15) articles which met the inclusion criteria have been included in this review. The inclusion criteria are: (1) patients with COVID-19; (2) use of a baricitinib inhibitor as the intervention; (3) presence of control/s; (4) RCT design or other types of human related studies-nRCTs; (5) clinical efficacy reported in study outcomes; and (6) published in English between 2020–September 2021. Fig. 1 summarises the process of literature inclusion for this study. RCT is a gold standard in clinical trial. Three available RCTs which met the inclusion criteria were included in current analysis. Due to very limited availability of RCTs, eleven non-RCTs (nRCTs) studies were included. The authors were aware that RCTs and nRCTs are different in term of study design, RoB and the quality of outcomes.
Fig. 1.
PRISM study flow diagram which was constructed with Review Manager 5.4.1 software, a Cochrane's software.
3.2. Characteristics of sources of evidence and outcome(s) of the studies
The characteristics of the articles, study design, population and age, interventions, comparators, the main outcome/s and patients' follow-up are listed in Table 1. The outcomes listed in Table 1 are presented to address the four research questions raised in this review, including block disease progression (BDP), mortality rate reduction (MRR), improve patients' condition (IPC) and shorten length of hospital stay (SLHS). Based on Table 1 and Fig. 2, baricitinib blocked the disease progression in 8/15 articles (53.3%); reduced mortality rate in 8/15 articles (53.3%); improved patients’ condition in 10/15 articles (66.7%); and shortened length of hospital stay in 6/15 articles (40%). Based on these crude findings, only 40% of the articles stated that baricitinib treatment may shorten the length of hospital stay.
3.3. Cochrane bias risk assessment and grading the evidence with GRADEpro Guideline Development Tool
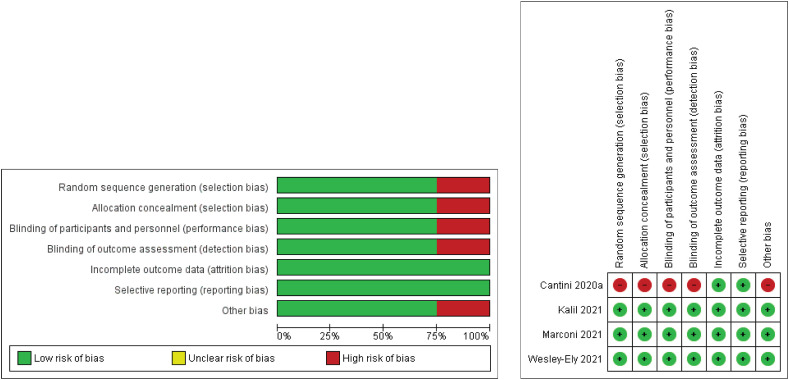
Cochrane bias risk assessment analyses were carried out using Review Manager 5.4.1 software for RCTs while NOS guideline for nRCTs. A total of four articles (3 RCTs and 1 clinical trial) were subjected to Cochrane bias risk assessment and the reviewers' judgements were translated as Fig. 3 . Except the pilot study article, all 3 RCTs are found to be in low risk of bias category. For nRCTs, all 11 articles were regarded as high-quality. No serious presence of confounding factor was identified. The presence of a confounding factor is related to the unequal number of patients in the control and intervention groups in several articles. Although the number of patients in each group is the confounding factor for several articles, the ages of the participants did not significantly differ. When looking at individual studies, we found age as a confounding factor in Stebbing et al. [10], but this was balanced due to presence of two centres' populations (mean age). For clinical trials, Cantini et al. [11] reported a pilot study of non-randomised, non-blinded, non-properly controlled clinical trials. Only data related to ‘block disease progression’ were used from Cantini et al. [11], since mortality data was unavailable. The evidence was graded with GRADEpro Guideline Development Tool and the outcomes are tabulated in Table 3 . In general, based on Table 3, baricitinib reduces mortality rate and disease progression. Although the certainty assessment was not serious for observational studies, the overall certainty outcome was low. Due to the study design itself (observational study), the overall certainty output was low, but this needed to be matched with NOS scale of RoB before reaching any conclusion. When comparing RoB in Fig. 3, Table 2 and Table 3, the overall quality of the included articles (14/15 articles) is high, except for a non-randomised, non-blinded, non-properly controlled clinical pilot trial by Cantini et al. [11], which falls under high RoB. Based on the identified RoB, most of the subsequent meta-analyses were based on 14 high quality articles. The overall high quality of the articles included in the current systematic review and meta-analysis will lead to concrete outcomes and conclusions.
Fig. 3.
Cochrane risk assessment analyses of four clinical trials. Of these, three were RCTs and one was clinical trial-pilot study without randomization, blinding and control. The image was prepared using Review Manager 5.4.1 software. For the pilot study, the authors were unclear about blinding for data analysis. The doubt was cleared through email conversations. An email was sent to Dr Cantini on 31st January 2022 and a reply was received on the same day. Dr Fabrizio Cantini wrote ‘no blinding method was employed for data analysis in our work’.
Table 3.
Grading the evidence with GRADEpro Guideline Development Tool.
| Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Baricitinib | Control | Relative (95% CI) | Absolute (95% CI) | ||
| Mortality | ||||||||||||
| 3 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 106/1330 (8.0%) | 166/1329 (12.5%) | RR 0.64 (0.51–0.79) | 45 fewer per 1000 (from 61 fewer to 26 fewer) |
⨁⨁⨁⨁ High |
CRITICAL |
| Mortality | ||||||||||||
| 11 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | 102/967 (10.5%) | 157/975 (16.1%) | RR 0.58 (0.45–0.73) | 68 fewer per 1000 (from 89 fewer to 43 fewer) |
⨁⨁◯◯ Low |
CRITICAL |
| Disease progression | ||||||||||||
| 2 | Randomised trials | Not serious | Seriousa | Not serious | Seriousb | None | 171/1225 (14.0%) | 206/1222 (16.9%) | RR 0.80 (0.58–1.10)< | 34 fewer per 1000 (from 71 fewer to 17 more) |
⨁⨁◯◯ Low |
CRITICAL |
| Disease progression without 2 PB articles | ||||||||||||
| 8 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | 44/689 (6.4%) | 79/733 (10.8%) | RR 0.54 (0.37–0.78) | 50 fewer per 1000 (from 68 fewer to 24 fewer) |
⨁⨁◯◯ Low |
CRITICAL |
CI: confidence interval; RR: risk ratio; PB: publication bias.
Explanation.
No agreement between 2 studies where one said yes and one said no to BDP.
Based on optimal information size (OIS) calculation; the OIS did not achieve.
Table 2.
The RoB analysis using Newcastle-Ottawa Scale (NOS) for nRCTs studies.
| Study | S (/4) | C (/2) | O (/3) | NOS score | Confounding factor | Mortality (B vs Ct) |
|---|---|---|---|---|---|---|
| Stebbing [10] | 4 | 2 | 2 | 8 | – | 1/37 vs 4/37 |
| Bronte [15] | 4 | 2 | 2 | 8 | + | 1/20 vs 25/56 |
| Hasan [17] | 4 | 1 | 1 | 6 | – | 4/122 vs 7/116 |
| Hasan [18] | 4 | 1 | 1 | 6 | – | 1/21 vs 1/17 |
| Rodriguez-Garcia [19] | 4 | 1 | 2 | 7 | + | 5/117 vs 11/270 |
| Abizanda [26] | 4 | 2 | 1 | 7 | – | 22/164 vs 43/164 |
| Cantini [12] | 4 | 2 | 3 | 9 | + | 0/113 vs 5/78 |
| Rosas [27] | 4 | 2 | 2 | 8 | – | 2/12 vs 6/17 |
| Perez-Alba [20] | 4 | 1 | 2 | 7 | + | 25/123 vs 30/74 |
| García-García [23] | 4 | 1 | 2 | 7 | + | 36/217 vs 22/125 |
| Falcone [31] | 4 | 2 | 1 | 7 | + | 5/21 vs 3/21 |
S = selection; C = comparability; O = outcomes; + = present; - = absent; vs = versus; B = baricitinib; Ct = control.
3.4. Meta-analysis
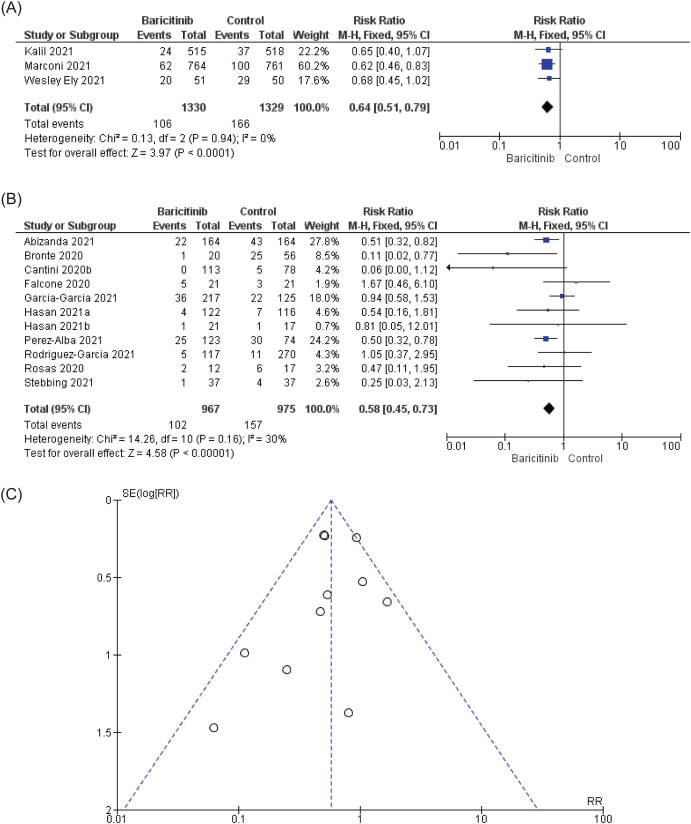
Two main research questions were addressed: (i) Does baricitinib have the ability to block the COVID-19 disease progression from entering the next stage? (ii) Does baricitinib have the ability to reduce the mortality rate in COVID-19 patients? To answer these questions precisely, the meta-analysis was carried out for each question. Based on Fig. 4 A for mortality in RCTs, heterogeneity was insignificant, with p = 0.94 and I2 was 0%. Overall, baricitinib treatment significantly reduced the mortality rate in admitted COVID-19 patients with RR in fixed-effect model was RR = 0.64 [95% CI = 0.51, 0.79; p < 0.0001]. Based on Fig. 4B, baricitinib statistically significantly reduced the mortality rate in nRCTs, with an RR in the fixed effect model of RR = 0.58 [96% CI: 0.45 to 0.73; p < 0.00001] with insignificant heterogeneity, where p = 0.16 and I2 was 30%. Based on the funnel plot (Fig. 4C), no publication bias was detected. Both RCTs and nRCTs analyses were comparable, and it was demonstrated that baricitinib statistically significantly reduced the mortality rate in COVID-19 patients.
Fig. 4.
The effect of baricitinib on mortality. (A) and (B) Based on the forest plots for RCTs and nRCTs, respectively, baricitinib does reduce mortality significantly in COVID-19 patients without significant heterogeneity. (C) Based on the funnel plot, no publication bias was spotted in Fig. 4B (nRCTs). The authors had contacted Falcone et al. [31] on 4th February 2022 for additional information and received the information on 7th February 2022 from Dr. Giusy Tiseo, a co-author. Falcone et al. [31] supplied information related to mortality and BDP for Propensity score (PS)-matched analysis.
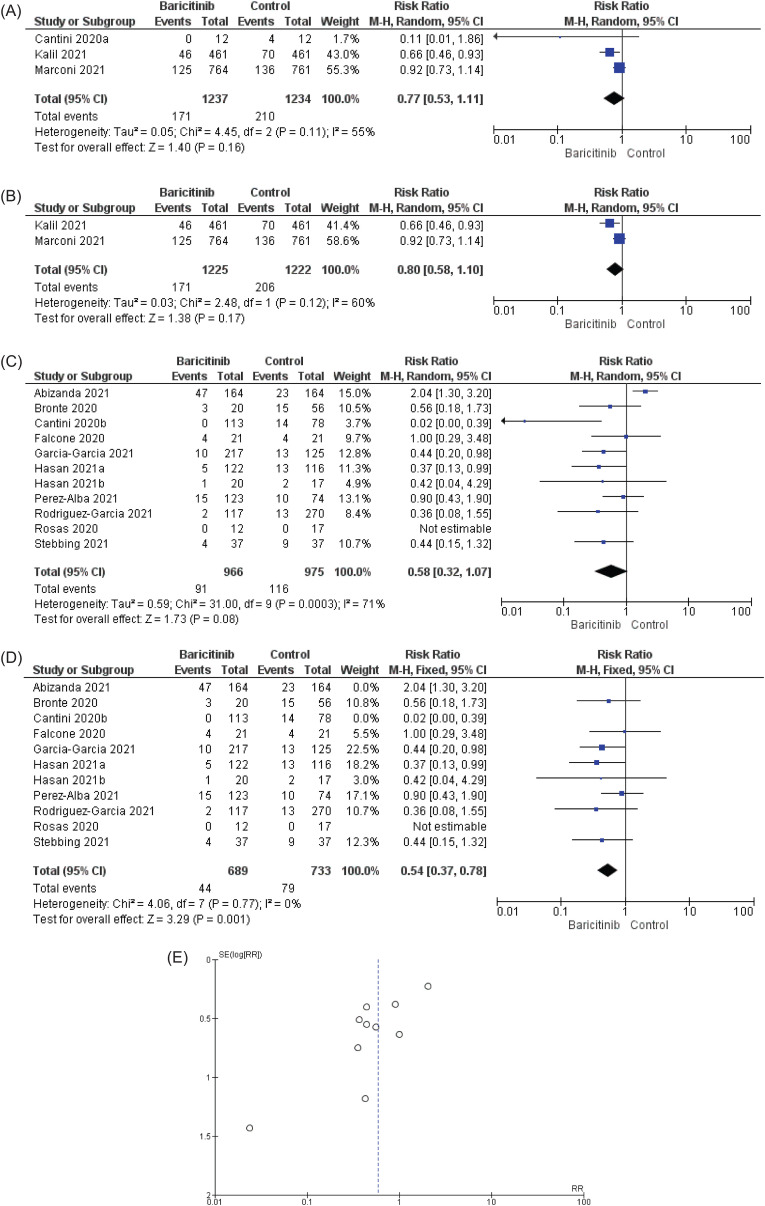
In case of blocking or reducing COVID-19 disease progression from entering the next stage, based on Fig. 5 A, the heterogeneity found from the combination of 2 RCTs and 1 clinical trial was significant, where I2 was 55%. The RR in random-effect model was RR = 0.77 [95% CI: 0.53 to 1.11: p = 0.16]. Due to high risk of bias found in Cantini et al. [11], the meta-analysis was carried out with 2 RCTs (Fig. 5B). Based on 2 RCTs, the drug did not block/reduce disease progression in COVID-19 patients with RR in random effect model was RR = 0.80 [95% CI: 0.59 to 1.10; p = 0.17] with considerably high heterogeneity which was I2 = 60%. We speculate that the disagreement found between these two studies is the reason behind the high heterogeneity and inconsistency. Similar to RCTs, nRCTs statistically significantly did not reduce or block disease progression. The RR in random effect model was RR = 0.58 [95% CI: 0.32 to 1.07; p = 0.08] with high heterogeneity, where p = 0.0003 and I2 = 71%. When comparing the individual data for nRCTs with the total RR [95% CI], we found out that the RR [95% CI] for Abizanda et al. [26] did not overlap the total RR [95% CI]. The authors speculated that Abizanda et al. [26] could be the reason behind the high heterogeneity found in Fig. 5C. Together, based on the funnel plot in Fig. 2, Fig. 5 publication bias articles were found. The authors removed Abizanda et al. [26] and Cantini et al. [12] and performed the analysis. After the removal of the above-mentioned articles, baricitinib statistically significantly reduced or blocked disease progression, with RR in fixed effect model was RR = 0.54 [95% CI: 0.37 to 0.78; p < 0.001] with insignificant heterogeneity, where p = 0.77 and the I2 measure was 0% (Fig. 5D). The results of RCTs and nRCTs for reducing or blocking disease progression are conflicting.
Fig. 5.
The effect of baricitinib on block/reduce disease progression (BDP) in COVID-19 patients. (A) Data of two RCTs and 1 clinical trial were pooled and found out baricitinib did not block/reduce disease progression. The authors contacted Marconi et al. [24] for additional information regarding IMV. We received an official reply from Eli Lilly. An official letter from Eli Lilly can be found as supplementary file 2. Due to high risk of bias found in Cantini et al. [11], the meta-analysis was carried out with 2 RCTs (Fig. 5B). Based on two RCTs, the drug did not block/reduce disease progression in COVID-19 patients with RR in random effect model was 0.80 [95% CI: 0.59 to 1.10; p = 0.17] with considerably high heterogeneity (I2 = 60%). We speculate that the disagreement found in between two studies is the reason behind the high heterogeneity. In (C), the pooled data of nRCTs did not produce statistical significance (p = 0.08) with high heterogeneity (I2 = 71%). The heterogeneity in 5C could be explained by comparing the risk ratio and range of 95% CI of individual result to the total risk ratio and 95% CI. The risk ratio and 95% CI of Abizanda et al. [26] did not overlap with the total risk ratio and 95% CI. Furthermore, 2 publication bias articles found from the funnel plot in Fig. 5E. In (D) based on funnel plots, the data of Abizanda et al. [26] and Cantini et al. [12] were removed and it was found that baricitinib statistically significantly (p = 0.001) blocked/reduced disease progression with insignificant heterogeneity (I2 = 0%). For BDP, due to unavailability of single parameter (Example: IMV) in all studies to define BDP, data on IMV were mostly sought after and followed by ICU admission (Related information are available in Table 1).
4. Discussions
Several clinical studies related to baricitinib and its role in combatting COVID-19 infections have emerged and received widespread attention. A cytokine storm is a major problem in COVID-19 patients. There are several components of cytokine, including interleukin (IL), interferon (IFN), tumour necrosis factor (TNF), colony stimulating factor (CSF), chemokine and growth factor. When microorganisms like viruses enter the human body, it will activate the immune system and release a large number of cytokines. The uncontrolled release of such large amount of pro-inflammatory cytokines may lead to an event known as cytokine storm. This situation is applicable for the COVID-19 virus, and can lead to patient death due to multiple organ failures. Cytokine storms are frequently seen in severe cases of COVID-19. Numerous cytokines like IL-1β, IL-2, IL- 6, IL-8, IL-10, TNF-α and IFN-γ have been observed to be significantly augmented in the patients infected with COVID-19 virus which linked to cytokines storm, mainly IL-6. The usage of anti-inflammatory drugs including JAK inhibitors may prevent disease progression [14]. Bronte et al. [15] stated that in general, COVID-19 patients develop pneumonia due to reduction of lymphocyte, a type of white blood cell and severe response to the inflammation due to uncontrolled release of cytokines. This is due to JAK/STAT signalling pathways which can be blocked by small molecules such as baricitinib. In a clinical trial (ClinicalTrials.gov NCT04438629) conducted by Bronte et al. [15], 20 patients were treated with baricitinib in an off-label use of the drug. For the first two days, each patient was treated with 4 mg of baricitinib twice a day, followed by 4 mg once daily dosage for the remaining 7 days. The authors reported that patients treated with baricitinib showed a significant decrease in serum levels of IL-6, IL-1β, and TNF-α; improvement in circulating frequencies of T and B cells; and improvement in antibody secretion against COVID-19 spike protein. These factors led to a decrease in the requirement of oxygen therapy and continuous rise in the P/F (oxygenation index) ratio. The data from this study indicate that baricitinib may be useful to prevent COVID-19 disease progression to a severe or extreme form. Previously, with RCT by Kalil et al. [22] the reduction in mortality rate and disease progression was clear-cut, and baricitinib did statistically reduce disease progression, while some degree of reduction was found in mortality rate but not statistically significant. With the addition of the latest RCT by Marconi et al. [24], the overall results conflict with those of Kalil et al. [22], as Marconi et al. [24] stated that mortality rate was reduced significantly while disease progression was not. In this review, 3 RCTs, 1 pilot study and 11 non-RCTs were included and analysed to derive the conclusion. Based on analysis done for systematic review, baricitinib does reduce mortality rate and disease progression in SARS-CoV-2 virus infected patients. Despite the low certainty of evidence for observational studies in Table 3, based on NOS scale for RoB, most articles appeared to be high-quality. Meta-analysis was carried out to validate the answers for the primary questions obtained from systematic review. As shown in Fig. 4A and B, baricitinib statistically significantly reduced the mortality rate in COVID-19 patients. The RoB of all articles for mortality was low and the quality of articles is high, thus making the conclusion for mortality concrete. The data were homogenous and a direct clear-cut conclusion was reached that baricitinib reduced mortality rate in COVID-19 patients significantly. These results validate the analysis performed for the systematic review.
For the analysis of disease progression in Fig. 5, a clear-cut conclusion cannot be drawn. This is because the analysis of two RCTs revealed that baricitinib did not block or reduce disease progression in COVID-19 patients. In reality, Kalil et al. [22] reported that baricitinib reduce disease progression, while the latter RCT by Marconi et al. [24] revealed the opposite. This inconsistency leads to the highly heterogenous outcome. For nRCTs, data from Abizanda et al. [26] supports Marconi et al. [24]. It has been mentioned that observational studies are complementary to RCTs [34]. This is because the results found from meta-analysis of observational studies is less strong compared to the one from RCTs [34]. In current BDP analysis, the result of RCTs is opposite of nRCTs. The number of studies included for RCT in current analysis for BDP is small. More RCT studies are needed in order to derive a solid conclusion. Although no agreement was found for RCTs and nRCTs for BDP, with inclusion of all high quality of articles for nRCTs, the authors decided to acknowledge that baricitinib statistically significantly reduced or blocked the disease progression in COVID-19 patients. According to Goletti and Cantini. [13], while remdesivir is suitable for early stage COVID-19 treatment and corticosteroid is suitable for the later stage of the COVID-19 treatment, baricitinib can be used in between of these two drugs to prevent moderately severe COVID-19 patients from entering the later stage. Based on current systematic review and meta-analysis, it is clear that baricitinib could aid in the blockage or reduction of disease progression and mortality. Based on the literature search, baricitinib plus remdesivir [22] and baricitinib plus corticosteroid yielded better effects, respectively, in terms of outcome in COVID-19 patients, as compared to remdesivir and corticosteroid, respectively. Additionally, D-dimer was significantly reduced in COVID-19 patients treated with baricitinib. Rodriguez-Garcia et al. [19] reported that baricitinib in combination with corticosteroids significantly improved the pulmonary function in the patients compared to corticosteroids treatment alone. The authors further added that treatment at 4 mg of baricitinib yielded a better result compared to 2 mg. Based on the boxplot data presentations of SpO2/FiO2 and D-dimer found that SpO2/FiO2 was improved with the combination treatment of baricitinib and corticosteroids over corticosteroids alone. Perez-Alba et al. [20] too found the similar finding of the beneficial effect of combining baricitinib and corticosteroids than corticosteroids alone. On the other hand, the D-dimer was reduced in the patients receiving baricitinib and corticosteroids compared to corticosteroids alone [19]. An increase of D-dimer in the patients infected with COVID-19 virus indicates a hyper-coagulable state and thus a high blood clotting risk. The development of acute respiratory distress syndrome is predicted by the increase of D-dimer, which requires admission to the intensive care unit or may even lead to death in serious cases. This co-incidental discovery could reflect the protective outcome of baricitinib on lung endothelium and enhance or ameliorate respiratory function in baricitinib treated patients. Regardless of the dose of baricitinib (2 mg vs 4 mg), the concentration of baricitinib in the plasma is enough to block AAK1 [19]. Based on this evidence for D-dimer, it may act as a guide for the prognosis of COVID-19 infection [21], and the reduction of mortality and disease progression found in this systematic review and meta-analysis could be attributed to the improvement of D-dimer. On 28 July 2021, FDA revised the Emergency Use Authorization (EUA) for baricitinib, authorizing the use of baricitinib alone for the treatment of COVID-19 [25]. Under this revised EUA, baricitinib no longer needed to be given in combination with remdesivir. Baricitinib is not approved by the FDA, but is authorized for the COVID-19 treatment. The FDA reached this decision following the outcomes from the COV-BARRIER trial. This trial provided some important information to the FDA which was unavailable previously. In the COV-BARRIER trial, not all patients who received baricitinib received remdesivir but the mortality in baricitinib treated patients was reduced by 38.2% and was statistically significant.
Meta-analysis must be updated when new information becomes available in order to provide up-to-date information for clinicians and policy-makers. There are a several meta-analysis articles published on this topic [32,33,[35], [36], [37]]. The current meta-analysis has its own advantages compared to the available meta-analyses. First, the data of RCTs and nRCTs were not pooled together and were analysed individually. This is the first article to report in such a way. The RCTs and nRCTs have different level of risk of bias and we did not combine the different level of biases together. Second, we did not analyse other JAK inhibitors together. Only baricitinib was included in order to give a clear-cut benefit of baricitinib for the management of COVID-19 disease. Third, the analysis was carried out for mortality and disease progression. The authors tried the level best to gather as many articles as possible under these categories in order to provide concrete findings. Fourth, the authors contacted Marconi et al. [24] and Falcone et al. [31] to obtain more information which could not be found in other articles except the article belongs to the current authors [39]. For Falcone et al. [31], we used propensity-score (PS) matched data (for mortality and disease progression) instead of the data available in the article (data only available for mortality in the article). PS matched data have fewer confounding factors. These data were never presented before in any published systematic review and meta-analysis articles. Finally, we have included GRADEpro Guideline Development Tool for grading the evidence. This approach is lacking in most published articles.
This review has a few limitations. First, for BDP, due to unavailability of similar parameters in all articles, respiratory related outcome (IMV) was most often used, followed by ICU and 1 ARDS outcomes. In the case of COVID-19, admission to the ICU is typically related to respiratory problems, and ARDS is a respiratory problem. These data were pooled. The authors are aware that this step could be a confounding factor, but the authors tried the best to match the data within the same category (respiratory function). Second, for Hasan et al. [17,18], no proper controls were available where the authors compared the interventions between high and low doses of baricitinib. The information is available in Table 1. We attempted meta-analyses for mortality and BDP without these two articles and found out that the outcomes (data not shown) did not alter the current conclusions. Third, in comparison to the study protocol registered with PROSPERO, some changes can be found in this article. During the article development process, some valuable suggestions were received from the internal reviewer. Based on these suggestions, some changes were made. First, the data for RCTs and nRCTs were not pooled. Second, the analysis for publication bias was only carried out when at least 10 articles were available. Third, NOS guideline was used for nRCTs instead of Cochrane RoB. Fourth, all the databases used for article searching were listed out instead of 3 main databases. Fifth, analysis for Wesley-Ely et al. [30] was not carried out separately, but combined with main data because it is a valuable RCT. Sixth, p-value was included to measure heterogeneity instead of I2 measurement alone. This step gave us more confidence to determine the heterogeneity. In conclusion, baricitinib statistically significantly reduces the mortality rate and disease progression in patients infected with the SARS-CoV-2 virus.
Funding
No funding received for this work.
Authors contribution
SM:LYY contributed 80:20 to develop this manuscript. Both authors are agreed on this ratio.
Declaration of competing interest
No conflict of interest.
Acknowledgement
The author would like to thank the Director General of Health Malaysia for his permission to publish this article, and the Director of the Institute for Medical Research for his support. The authors would like to thank Low Chui Thean, a registered Pharmacist from Institute for Medical Research, National Institutes of Health (NIH) for her contribution in reviewing the manuscript before submission.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmed.2022.106986.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Worldometer . 2021. Covid-19 Coronavirus Pandemic.https://www.worldometers.info/coronavirus/ [Google Scholar]
- 2.Piscoya A., Ng-Sueng L., Parra del Riego A., Cerna-Viacava R., Pasupuleti V., Roman Y., et al. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmgren J., Guo B., Kaplan H. Continued proportional age shift of confirmed positive COVID-19 incidence over time to children and young adults: Washington State March-August 2020. PLoS One. 2021;16 doi: 10.1371/journal.pone.0243042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn M.R., DeJonckheere M., Schuiteman S., Strome A., Herbert K., Waselewski M., et al. Stay home so this can be over: a national study of youth perspectives on social distancing during the COVID-19 pandemic. Prevent Med Reports. 2021;22 doi: 10.1016/j.pmedr.2021.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey W., Carabelli A., Jackson B., Gupta R., Thomson E., Harrison E., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2021. Therapeutics and COVID-19: Living Guideline.https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.3 [PubMed] [Google Scholar]
- 9.U.S Food Drug Administration . 2021. Know Your Treatment Options for COVID-19.https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19 [Google Scholar]
- 10.Stebbing J., Sánchez Nievas G., Falcone M., Youhanna S., Richardson P., Ottaviani S., et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci. Adv. 2020;7 doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantini F., Niccoli L., Nannini C., Matarrese D., Natale M., Lotti P., et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goletti D., Cantini F. Baricitinib therapy in covid-19 pneumonia - an unmet need fulfilled. N. Engl. J. Med. 2021;384:867–869. doi: 10.1056/NEJMe2034982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Zhang Y., Qiao W., Zhang J., Qi Z. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int. Immunopharm. 2020;86 doi: 10.1016/j.intimp.2020.106749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Canè S., et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Invest. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan M.J., Rabbani R., Anam A.M., Huq S., Polash M., Nessa S., et al. Impact of high dose of baricitinib in severe COVID-19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect. Dis. 2020;21:427. doi: 10.1186/s12879-021-06119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan M.J., Rabbani R., Anam A.M., Huq S. Additional baricitinib loading dose improves clinical outcome in COVID-19. Open Med. 2020;16:41–46. doi: 10.1515/med-2021-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatism. 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Alba E., Nuzzolo-Shihadeh L., Aguirre-García G.M., Espinosa-Mora J., Lecona-Garcia J.D., Flores-Pérez R.O., et al. Baricitinib plus dexamethasone compared to dexamethasone for the treatment of severe COVID-19 pneumonia: a retrospective analysis. J Microbiol Immunol Infect = Wei mian yu gan ran za zhi. 2021;(21) doi: 10.1016/j.jmii.2021.05.009. S1684-1182. 00133-X: Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidali S., Morosetti D., Cossu E., Luisi M., Pancani S., Semeraro V., et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00260-2020. 00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-García J.A., Pérez-Quintana M., Ramos-Giráldez C., Cebrián-González I., Martín-Ponce M.L., del Valle-Villagrán J., et al. Anakinra versus baricitinib: different strategies for patients hospitalized with COVID-19. J. Clin. Med. 2021;10:4019. doi: 10.3390/jcm10174019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marconi V.C., Ramanan A.V., de Bono S., Kartman C.E., Krishnan V., Liaoet R., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S Food and Drug Administration . 2021. Baricitinib Letter of Authorization Revised July 28 2021.https://www.fda.gov/media/143822/download [Google Scholar]
- 26.Abizanda P., Mayo J.M.C., Mas Romero M., Zamora E.B.C., Sahuquillo M.T.T., Rizos L.R., et al. Baricitinib reduces 30-day mortality in older adults with moderate-to-severe COVID-19 pneumonia. J. Am. Geriatr. Soc. 2021;69:2752–2758. doi: 10.1111/jgs.17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas J., Liaño F.P., Cantó M.L., Barea J., Beser A.R., Rabasa J., et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus covid-19: a real-world study. Reumatol. Clínica. 2020;18:150–156. doi: 10.1016/j.reuma.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Review Manager (RevMan) The Cochrane Collaboration; 2020. [Computer Program]. Version 5.4. [Google Scholar]
- 29.GRADEpro GDT . McMaster University; 2020. GRADEpro Guideline Development Tool [Software] [Google Scholar]
- 30.Wesley Ely E., Ramanan A.V., Kartman C.E., de Bono S., Liao R., Piruzeli M.L.B., et al. Baricitinib plus standard of care for hospitalised adults with covid-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: results of a randomised, placebo-controlled trial. medRxiv. 2021 doi: 10.1101/2021.10.11.21263897. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falcone M., Tiseo G., Barbieri G., Galfo V., Russo A., Virdis A., et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa563. ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C.X., Wang J.J., Li H., Yuan L.T., Gale R.P., Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35:2616–2620. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C.Y., Chen W.C., Hsu C.K., Chao C.M., Lai C.C. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Int. Immunopharm. 2021;99 doi: 10.1016/j.intimp.2021.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grootendorst D.C., Jager K.J., Zoccali C., Dekker F.W. Observational studies are complementary to randomized controlled trials. Nephron Clin. Pract. 2010;114:c173–c177. doi: 10.1159/000262299. [DOI] [PubMed] [Google Scholar]
- 35.Limen R.Y., Sedono R., Sugiarto A., Hariyanto T.I. Janus kinase (JAK)-inhibitors and coronavirus disease 2019 (Covid-19) outcomes: a systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2021;20:425–434. doi: 10.1080/14787210.2021.1982695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijaya I., Andhika R., Huang I., Purwiga A., Budiman K.Y., Bashari M.H., et al. The use of Janus Kinase inhibitors in hospitalized patients with COVID-19: systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;11 doi: 10.1016/j.cegh.2021.100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Z., Niu J., Xu Y., Qin L., Ding J., Zhou L. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis. J. Med. Virol. 2022;94:1523–1534. doi: 10.1002/jmv.27482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deeks J.J., Higgins J.P., Altman D.G., Group C.S.M. In: Cochrane Handbook for Systematic Reviews of Interventions. second ed. T Higgins J.P., Thomas J., Chandler J., et al., editors. John Wiley & Sons; Chichester, UK: 2019. Analysing data and undertaking meta-analyses; pp. 241–284. [Google Scholar]
- 39.Manoharan S., Ying L.Y. Baricitinib for the Management of SARS-CoV-2-Infected Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Canadian. J. Infect. Dis. Med. Microb. 2022;2022 doi: 10.1155/2022/8332819. Article ID 8332819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.