Abstract
Abstract
Status epilepticus (SE) is an acute, life-threatening medical condition that requires immediate, effective therapy. Therefore, the acute care of prolonged seizures and SE is a constant challenge for healthcare professionals, in both the pre-hospital and the in-hospital settings. Benzodiazepines (BZDs) are the first-line treatment for SE worldwide due to their efficacy, tolerability, and rapid onset of action. Although all BZDs act as allosteric modulators at the inhibitory gamma-aminobutyric acid (GABA)A receptor, the individual agents have different efficacy profiles and pharmacokinetic and pharmacodynamic properties, some of which differ significantly. The conventional BZDs clonazepam, diazepam, lorazepam and midazolam differ mainly in their durations of action and available routes of administration. In addition to the common intravenous, intramuscular and rectal administrations that have long been established in the acute treatment of SE, other administration routes for BZDs—such as intranasal administration—have been developed in recent years, with some preparations already commercially available. Most recently, the intrapulmonary administration of BZDs via an inhaler has been investigated. This narrative review provides an overview of the current knowledge on the efficacy and tolerability of different BZDs, with a focus on different routes of administration and therapeutic specificities for different patient groups, and offers an outlook on potential future drug developments for the treatment of prolonged seizures and SE.
Graphical Abstract
Key Points
| Non-intravenous routes for the delivery of benzodiazepines are becoming increasingly important in pre-hospital and in-hospital settings. |
| Ready-to-use nasal sprays, syringes, rectioles or autoinjectors are particularly suitable for lay use by epilepsy patients or their caregivers. |
| Somnolence is frequently reported after benzodiazepine administration, while severe side effects, such as respiratory depression and hypoxia, are rare. |
| The choice of benzodiazepine depends amongst others on individual pharmacokinetic and pharmacodynamic characteristics and available routes of administration. |
Introduction
The emergency treatment of prolonged epileptic seizures, seizure clusters and status epilepticus (SE) is required to be rapid and efficient, as ongoing epileptic activity may lead to neuronal damage and result in increased morbidity and mortality [1–3]. The ideal anticonvulsant agent for this purpose should be safe, easy to administer, and exhibit a long-lasting anti-seizure effect without relevant side effects. The delineation between prolonged epileptic seizures, seizure clusters and SE is—to some extent—arbitrary and has evolved over the last few decades; however, any rescue medication should prevent seizure recurrence as well as the progression of a seizure or a series of seizures into SE [1, 4].
Benzodiazepines (BZDs), such as lorazepam (LZP), midazolam (MDZ), diazepam (DZP) and clonazepam (CZP), are established first-line drugs for the acute treatment of seizures [5]. BZDs are a family of drugs that exert their effects by allosterically modulating the activity of the ionotropic gamma-aminobutyric acid (GABA)-A receptor in the central nervous system (CNS). These drugs increase the probability that GABA binding to the receptor will open the associated Cl− channel. Thus, these drugs generally decrease neuronal excitation and exhibit antiseizure, sedative-hypnotic, anxiolytic, muscle relaxant and amnesic properties. As a side effect, BZDs can cause drug dependence, mostly due to recreational misuse or long-term intake against medical advice, cognitive impairment and—when administered in higher doses—can cause respiratory depression.
Available routes of delivery for these drugs include intravenous (i.v.), oral (p.o.), rectal (r.s.), intramuscular (i.m.), buccal, intranasal (i.n.), and even intraosseous [6–10]. Difficulties with achieving i.v. access may lead to a delay in drug administration, rendering the development of alternative suitable routes vital, as responsiveness to BZDs during seizures decays over time [11]. Jaw clenching, hypersalivation and uncontrollable swallowing are major limitations inherent to the p.o. and buccal routes [12], making it difficult to minimize variability in pharmacodynamics due to variable intake [13]. Intramuscular injections can also be challenging in patients with tonic–clonic or hypermotor seizures [7]. While r.s. administration might be hindered by generalized convulsions, it is also becoming less popular due to the social distress and sense of shame it imposes on both patients and caregivers [14].
In comparison, i.n. administration may be a more favorable option, as it can be administered in a significantly shorter amount of time without the need for an i.v. route, and may be preferred by caregivers compared to the r.s. route [15]. Recently approved commercial preparations of MDZ and DZP nasal sprays have become available for the treatment of seizure clusters, and there is growing evidence supporting the use of pharmacy-manufactured MDZ preparations for seizures and SE. However, the use in SE is not yet established.
In this review, we discuss the commonly used BZDs, with a focus on their pharmacodynamics, pharmacokinetics, metabolism and available formulations, and we summarize the published data on their efficacy, safety and routes of delivery in the clinical management of seizures, seizure clusters and SE.
Status Epilepticus and Seizure Clusters
In 2015, the International League Against Epilepsy published a report on the definition and classification of SE [16]. This SE definition sets two time points (t1, t2), where t1 defines the semiological transition of a seizure to SE and t2 marks the point in time when neurological injury is likely to occur. Typically, the start of SE treatment is based on t1, with the time limit being 5 min for generalized convulsive (tonic–clonic) SE, 10 min for complex focal SE (focal SE with impaired consciousness), and 10–15 min for absence SE [16]. This definition applies to ongoing seizures or a series of discrete seizures between which there is only incomplete recovery of the previous neurological status. SE-induced neuronal damage is assumed to occur later, after a time (t2) of 30 min with generalized convulsive SE (GCSE) and after 60 min with complex focal SE [16]. The 5-min time limit that usually marks the transition from a prolonged seizure to SE dates back to an operational definition proposed by Lowenstein in 1999, aimed at ensuring that patients receive treatment as soon as possible [17]. This approach—equaling seizures longer than 5 min and SE irrespective of seizure semiology—was amongst others adopted by the German clinical practice guidelines on SE [18].
Using a variety of different methodologies, regions and populations, the incidence of SE has been estimated to be 10–41 per 100,000. The overall mortality rate is 13%, but can reach up to 40% in a super-refractory course of SE [1, 19, 20]. Clinical and experimental data show a correlation between a delayed start of treatment and the reduced likelihood of successful SE termination with the first treatment attempt [11, 21, 22]. Potential underdosing might play a further role in this context [ 23, 24]. The urgency with which to begin treatment depends on the SE type and appears to be most pressing with GCSE [25].
The clustering of seizures in turn is a common clinical phenomenon and describes an increase in seizure frequency during a specific period of time, where the probability of seizure occurrence is affected by the occurrence of a previous seizure [26]. A recent review found the prevalence of seizure clusters to be between 13 and 76% for outpatient studies and 18% and 61% for inpatient video-EEG monitoring studies likely involving a high percentage of therapy-resistant epilepsy [26], while a cohort study using the United Kingdom General Practice Research Database estimated the age-adjusted prevalence at 2.5/10,000, with a peak in the 0–4 years age group (5.9/10,000) [27]. Despite being a common phenomenon, seizure clusters still lack a clear definition. Previous studies looking into acute treatment (with DZP) defined them as a characteristic episode of multiple complex partial or generalized convulsive seizures occurring within a 24-h period in adults or a 12-h period in children, with a pattern distinguishable from the patient’s usual seizure pattern, and with an onset readily recognizable by a caregiver that will predict further seizures [28, 29]. Other studies have used a minimum number of seizures as a criterion (e.g., at least three seizures within 24 h, or within 4 h during video-EEG monitoring) [30–32].
While the mechanisms leading to seizure clusters are not yet well understood, well-described catamenial phenomena (i.e., a worsening of seizures in relation to the menstrual cycle) may result in perimenstrual seizure clusters [33, 34]. A better understanding of the prediction, occurrence and clustering of seizures will likely result from big data approaches, which may help to better define the clinical course and dynamics of seizure clusters [35, 36].
Characteristics of Individual Benzodiazepines
In the following sections we discuss the characteristics of the most commonly used individual benzodiazepines—lorazepam, midazolam, diazepam and clonazepam.
Lorazepam
LZP is a BZD with a fast onset of anticonvulsant action: It begins to act about 1–3 min after i.v. injection and has a short-to-intermediate duration of action. Additionally, LZP has sedative-hypnotic and anti-anxiety effects. Common doses for the treatment of SE in adults are 2–4 mg in the pre-hospital phase [37]. The American Epilepsy Society recommends a LZP dose of 0.1 mg/kg (a maximum single dose of 4 mg), which may be repeated [38]. In adults and children, i.v. LZP is established as efficacious at stopping seizures lasting at least 5 min [38], but due to the faster time for administration, i.m. MDZ has superior effectiveness in adults with convulsive SE when an i.v. access is not established [38].
Pharmacodynamics, Pharmacokinetics and Metabolism
LZP is readily absorbed after sublingual, p.o. and i.m. administration, and the bioavailability for all routes of administration exceeds 94%. The preferential route of administration for seizures or SE, however, is i.v. injection, as peak plasma concentrations are reached significantly slower when using other routes (at least 1 h after p.o. or sublingual use) [39]. LZP is highly bound to plasma proteins (91%) [40] and only unbound drug fraction diffuses into the CNS. LZP remains mostly in the intravascular compartment, as its lipophilicity is lower than that of DZP and the volume of distribution (Vd) is thus comparatively low (1.3 L/kg body weight) [39, 40]. This leads to prolonged clinical effects of LZP after a single dose by a rebalancing of bound and free drug fractions, even though “paradoxically” the elimination half-life is shorter than that of DZP [41]. A slower redistribution from the CNS to other tissues due to its relatively lower lipophilicity than, for example, DZP might further contribute to the prolonged clinical effect. This also means that LZP does not significantly accumulate in body fat after repeated administrations. Hepatic one-step, non-oxidative conjugation at the 3-hydroxy group to LZP-glucuronide is rapid, after which this non-active metabolite is predominantly excreted renally [42]. The duration of action is about 10–20 h and the elimination half-life is 8–25 h [39, 40].
Available Formulations
LZP is available as a tablet (0.5 mg, 1 mg and 2 mg tablets) or a concentrate (2 mg/ml) for oral use, and as a solution for i.v. injection (2 mg/ml and 4 mg/ml). In Canada and Europe, a sublingual tablet is also available (0.5 mg, 1 mg and 2.5 mg tablets). In the setting of comfort care, LZP may be used subcutaneously or rectally, but this is off-label. There is currently no manufactured form of LZP for i.n. administration. There is some evidence that it might be efficacious in SE termination, at least in children [43]. However, nasal administration is off-label.
Clinical Efficacy
Before the US Food and Drug Administration (FDA) approved LZP on 30 September 1977, in 1975 a phase II trial of i.v. LZP showed promising results controlling seizures in 11 patients with EEG-confirmed SE [44]. Soon after the market introduction of LZP, Walker et al. [45] performed a non-randomized prospective trial in patients with SE, resulting in a seizure control rate of 88%. The first randomized, double-blind trial, which was designed to compare the efficacy of LZP and DZP, was published in 1983 [46] and demonstrated a higher percentage of seizure control with LZP compared to i.v. DZP. Pivotal results came from the first randomized and double-blind trial by Treiman et al. [47] in 1988, which demonstrated a superior efficacy of LZP over phenobarbital (PB) and at least equal efficacy to phenytoin (PHT ± DZP) as a first-line treatment for SE. Subsequently, further trials were designed to study the benefits of outpatient administration of LZP. Alldredge et al. [22] conducted a large placebo-controlled trial, showing that LZP at a dose of 2 mg led to seizure termination more frequently than placebo or DZP following i.v. administration by trained emergency medical technicians. However, in the largest preclinical trial to date, Silbergleit et al. [48] found that, in comparison to i.m. MDZ, the i.v. administration of LZP is more time-consuming due to venous access placement, resulting in inferiority despite high response rates in both groups. Another drawback of LZP is the need for refrigeration, limiting its use in ambulances. In the paediatric setting, LZP has been compared in controlled trials with DZP, demonstrating equal [49, 50] or higher efficacy [51]. Compared to i.m. MDZ, i.v. LZP showed a similar rate of seizure cessation [52]; albeit, this particular randomized trial was underpowered and was based on a re-analysis of [48].
In sum, LZP is frequently singled out among the BZDs for its rapid onset of action and effective seizure control when used as a first-line treatment for SE and given i.v. in adequate dose. It should be noted that, due to concerns for respiratory depression, the administration of insufficient doses with resulting limitations in efficacy is quite common. While the guidelines recommend a dose of 0.05–0.1 mg/kg [38], a prospective study of adult patients with SE demonstrated that a lower dose was administered in 84–95% of cases [53].
Tables 1 provide a detailed overview of the studies discussed in this section.
Table 1.
Summary of studies on the use of lorazepam for status epilepticus
| Delivery route | Study design | n | Age (years) | SE types | Dosage (mg) | Seizure control (%) | TEAE rate (%) | Frequent TEAE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Intravenous | |||||||||
| LZP | OLS, SC | 11 | ≥ 6 | SE | 2.5–5 | 100 | – | – | [44] |
| LZP | OLS, SC | 25 | 5–81 | SE, RS | 4–8 | 88 | 12 | Respiratory depression (4) | [45] |
| LZP | OLS, SC | 9 | 16–60 | SE | 4 | 88 | – | – | [191] |
| LZP | OLS, MC | 31 | 2–18 | SE | 0.05–0.1a | 81 | 0 | – | [192] |
| LZP | OLS, SC | 77 | < 12 | SE | 0.1a | 79 | – | – | [193] |
| LZP vs. i.v. CZP | OLS | 61 | ≥ 18 | SE | 4–10 | 51–63 | 100 | Drowsiness (100)psychomotor agitation (12) | [147] |
| LZP vs. i.v. DZP | RCT, DB, SC | 78 | ≥ 18 | SE | 4 | 89 | 13 | Respiratory depression | [46] |
| LZP vs. i.v. DZP | ReS, SC | 44 | 0–18 | SE, RS | 0.03–0.22a | 82 | 25 | Respiratory depression | [194] |
| LZP vs. i.v. DZP | RCT, DB, MC | 273 | 0.25–17 | CSE | 0.1a | 72.9 | – | Sedation (67)respiratory depression (37) | [50] |
| LZP vs. i.v. DZP | RCT, OLS, SC | 48 | 1–11 | CSE | 0.13a | 65 | 19 | Admission to ICU | [49] |
| LZP vs. i.v. LEV | RCT, OLS | 79 | 1–75 | CSE, NCSE, RS | 0.1a | 75.6 | – | Lethal course (43)respiratory depression (48)hypotension (8) | [195] |
| LZP vs. i.v. DZPvs. i.v. placebo | RCT, DB, SC | 205 | ≥ 18 | SE, RS | 2 (LZP) 5 (DZP) | 59 | 11 | Respiratory depression | [22] |
| LZP vs. i.v. DZP + PHT | RCT, OLS, SC | 178 | 1–12 | CSE | 0.1a | 100 | 4 | Respiratory depression | [121] |
| LZP vs. i.v. MDZ vs. i.v. CZP | PS, MC | 177 | ≥ 16 | SE, RSE | 0.1a | Inferior to CZP or MDZ | – | – | [53] |
| LZP vs. i.v. DZP + PHT vs. i.v. PHT vs. i.v. PB | RCT, DB, MC | 384 | 58.6 ± 15.6 (CSE) 62 ± 15.1 (NCSE) | CSE, NCSE | 0.1a | 65 | – | Hypotension (26) respiratory depression (10) cardiac arrhythmia (7) | [47] |
| LZP (i.v. and rectal) vs. i.v. DZP | PS, RCT, OLS, SC | 102 | < 18 | S, RS, SE | – | 76 | 3 | Respiratory depression | [51] |
| LZP vs. rectal DZP | PS, MC | 182 | 0.2–16 | CSE | 0.1a | Superior to DZP | – | – | [196] |
| LZP vs. i.m. MDZ | RCT, DB, MC | 893 | 1–94 | SE | 4 | 63,4 | – | Respiratory depression (14) hypotension (14) | [48] |
| LZP vs. i.m. MDZ | Secondary analysis | 120 | < 18 | SE | 4–10 | 71.6 | – | Respiratory depression (15) | [52] |
RCT randomized controlled trial, OLS open-label study, SC single-centre, MC multicentre, DB double-blind, PS prospective study, ReS retrospective study, CO cross-over, HV healthy volunteer, S seizure, RS repetitive seizure, SE status epilepticus, RSE refractory status epilepticus, TEAE treatment-emergent adverse event, i.v. intravenous, i.n. intranasal, i.m. intramuscular, p.o. oral, s.l. sublingual, MDZ midazolam, LZP lorazepam, CZP clonazepam, DZP diazepam, PHT phenytoin, CBZ carbamazepine
a Milligram per kilogram body weight (mg/kg BW)
Midazolam
MDZ has a relatively short half-life of only 1–4 h. This drug exhibits anticonvulsant, sedative-hypnotic, anxiolytic, muscle relaxant and amnesic properties [54]. Following its first FDA approval in 1985, MDZ was mainly used to promote preoperative sedation, anxiolysis and anesthesia induction. More recently, newer forms of administration have extended its use to treating prolonged seizures, seizure clusters and SE. In contrast to other BZDs, MDZ formulations are available with a wide range of administration routes including p.o. (tablet and syrup), i.v., i.m., r.s., buccal and i.n. However, officially approved indications vary across the administration routes.
Pharmacodynamics, Pharmacokinetics and Metabolism
MDZ is generally readily absorbed after administration. However, the exact time to peak plasma concentrations (Tmax) and bioavailability differ significantly between administration routes (p.o.: 1–2 h, 40–50%; i.v. < 5 min; i.m.: 45 min, > 90%; r.s.: 30 min, 50% (off-label); buccal: 30 min, 75%; i.n.: 7–15 min, 44%) [55–62]. When comparing to i.v. administration, i.n. administration reaches maximal plasma concentrations only slightly slower with lower maximal plasma levels. At a physiological pH, MDZ is bound to plasma proteins (97%) in part due to its lipophilia; however, at a lower pH, it becomes hydrophilic, allowing for water-based solutions. MDZ crosses the placenta and is also excreted into human milk [61]. Compared to other BZDs, MDZ has a rather short half-life of 1.5–3.5 h [63]. This drug is primarily metabolized in the liver by cytochrome P450 3A4 (CYP3A4), and its metabolites, mainly 1-hydroxymidazolam, are excreted in the urine [63]. MDZ, like other BZDs, may potentiate the action of other CNS depressants and alcohol.
Available Formulations
MDZ is available as a tablet (7.5 mg/tablet) or syrup (2 mg/ml) for p.o. use in adult and paediatric patients, respectively, mainly for sedation, anxiolysis and amnesia prior to diagnostic, therapeutic or endoscopic procedures, or before anesthesia induction. A solution for i.v. injection (1 mg/ml, 5 mg/ml) is available for promoting preoperative sedation, anxiolysis, anesthesia induction or amnesia. A solution for i.m. injection (5 mg/ml) is approved for the treatment of SE in adults. Rectal use has also been reported in several studies but is not officially approved. In 2019, MDZ was approved as a nasal spray (Nayzilam®) by the FDA for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy 12 years of age and older [64, 65]. Nayzilam® is licensed for administration by a non-healthcare professional in patients actively seizing when and where a seizure cluster occurs. Intranasal MDZ solutions can also be formulated by pharmacies [66–68].
In Europe, MDZ is also European Medicines Agency (EMA) approved for buccal use (Buccolam®, oromucosal solution: 2.5 mg/0.5 ml, 5 mg/1 ml, 7.5 mg/1.5 ml, 10 mg/2 ml) for the treatment of prolonged, convulsive seizures in infants, toddlers, children and adolescents (from 3 months to < 18 years). The reason for the exclusive paediatric-use marketing authorization is that it is a special approval procedure. Thus, only i.m., buccal and i.n. administrations are officially approved for the treatment of seizures.
Clinical Efficacy
Due to a long history of approval, the release of MDZ in 1985, and the different approval modalities for drugs at that time, there are no phase I or II clinical trials on the i.v. use of MDZ. There are, however, several studies that have assessed the efficacy of i.v. MDZ as a bolus dose followed by a continuous infusion to terminate refractory SE (RSE) [69–71]. More recent studies have focused on other routes of administration, including i.m., buccal and i.n. Regarding i.m. administration, the first evidence stems from 1992 when Chamberlain et al. [72] showed that children with prolonged motor seizures received medication sooner and had a faster cessation of seizures when administered i.m. MDZ compared to i.v. DZP. It was subsequently shown that MDZ is also effective in terminating SE (in combination with oral PHT or carbamazepine [CBZ]) [73]. More recently, it was reported that i.m. MDZ is not inferior to i.v. LZP with regard to prehospital SE [48], which was followed up by studies showing that i.m. MDZ can act faster than r.s. DZP [74] in terminating motor activity during SE in children, and that fewer children receiving i.m. MDZ had recurrent seizures, were intubated or required ICU care compared to i.v. LZP [52].
In 1999, it was also shown that the buccal administration of MDZ is at least as effective as r.s. DZP in terminating prolonged seizures in children and young adults [75]. In addition, buccal MDZ is effective in terminating prolonged seizures in children [76] and can terminate serial seizures or SE faster and with fewer adverse events than r.s. DZP in adults [77].
The latest MDZ formulation is a nasal spray. It has been shown that i.n. MDZ reaches dose-dependent maximal plasma concentrations after ~ 8–14 min, with sneezing and local irritation reported as the most common side effects [58, 78]. Two recent phase III studies have assessed the efficacy of 5–10 mg i.n. MDZ to terminate seizures and showed the drug to be effective in approximately 54% of seizure clusters within 10 min (placebo response rate 34%) [79, 80]. Intranasal administration of MDZ has also been compared to DZP and it was found that i.n. MDZ was equally as effective as i.v. DZP in controlling seizures in children [81]. While the treatment was initiated faster in the MDZ group, the seizures were controlled slightly faster with i.v. DZP when the time needed to establish an i.v. line was excluded. Subsequent studies showed that i.n. MDZ resulted in a faster onset of action and a faster termination of seizures compared to i.v. DZP in prolonged (febrile) seizures in children [15, 82]. Two additional studies showed no significant differences in efficacy between i.n. MDZ and r.s. DZP for terminating prolonged seizures in both adults [83] and children [14] when administered by caregivers. Importantly, i.n. MDZ was generally preferred by caregivers and patients over r.s. DZP in these settings due to the ease of administration. A retrospective study showed that i.n. MDZ was comparable to i.v. LZP for seizure termination and prevention of seizure clusters in the adult epilepsy monitoring unit [84]. Use of i.n. MDZ for the treatment of SE was demonstrated in a open-label pharmaco-EEG study showing SE termination in 57% of the cases at an average time of 5 min [66].
Open questions include whether i.n. MDZ can reduce the transformation of seizures into a status epilepticus, the number of applications without increasing adverse events, and whether the availability of MDZ spray increases the rate of BZD administrations by emergency medical services [85].
Tables 2 provide a detailed overview of the studies discussed in this section.
Table 2.
Summary of studies on the use of midazolam for status epilepticus
| Delivery route | Study design | n | Age (years) | SE types | Dosage (mg) | Seizure control (%) | TEAE rate (%) | Frequent TEAE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Intravenous | |||||||||
| MDZ | PS, OLS | 19 | 16–87 | RSE | 0.2a bolus then, 1 μg/kg/min | 94.7 | 21 | Pharyngeal hypersecretion | [69] |
|
MDZ vs. i.v. propofol |
ReS | 20 | 17–81 | RSE | 2–12 bolus, then 0.05–0.8a /h | 67 | – | – | [70] |
| Intramuscular | |||||||||
|
MDZ vs. i.v. LZP |
RCT, DB, MC | 102 | 0–102 | SE | 10 | 73.4 | – | Endotracheal intubation (14) hypotension (3) | [48] |
| MDZ vs. i.v. LZP | Secondary Analysis | 120 | 0–17 | SE | 5–10 | 68.3 | Inferior to LZP | Intubation hospitalization ICU care | [52] |
| MDZ vs. rectal DZP | RCT | 100 | 0–16 | SE | 0.3a | 96 | 0 | – | [74] |
| MDZ + p.o. PHT vs. p.o. CBZ | PS, OLS | 38 | – | SE | 15 | 84 | – | Drowsiness | [73] |
| Buccal | |||||||||
| MDZ | OLS, SC | 19 | 0–15 | S, SE | 0.3a | 100 (AS), 50 (SE) | – | [76] | |
| MDZ (s.l.) | OLS, SC | 10 | 23–47 | HV | 10 | – | – | – | [13] |
| MDZ vs. rectal DZP | PS | 22 | 25–68 | SE | 15.5 | 83.3 | 21 | Tiredness ataxia | [77] |
| MDZ vs. rectal DZP | RCT, OLS, SC | 18 | 5–19 | S | 10 | 75 | – | Mild decrease in blood pressure | [75] |
| Intranasal | |||||||||
| MDZ | OLS | 175 | 12–62 | RS | 5–10 | 55 | 40–57 | Nasal discomfort somnolence headache | [80] |
| MDZ vs. placebo | RCT, DB | 292 | 12–65 | RS | 5 | 54 | 2) | Nasal discomfort somnolence | [197] |
| MDZ vs. i.v. MDZ | OLS, CO, SC | 6 | 27–47 | HV | 5 | – | – | Temporary nasal irritation | [58] |
| MDZ vs. i.v. LZP | ReS, SC | 50 | 38.6 (SD 14.0) | S, RS | 3 MDZ or 1–2 LZP | No difference | 30.4 MDZ | Somnolence | [84] |
| MDZ vs. i.v. DZP | RCT | 70 | 0–15 | S | 0.2a | Inferior to DZP | 0 | – | [81] |
| MDZ vs. rectal DZP | PS, RCT, SC | 358 | < 18 | S | 0.2a | Not inferior to DZP | – | Respiratory insufficiency (6) intubation (2) | [14] |
| MDZ vs. rectal DZP | PS, OLS | 24 | 25–69 | RS, SE, RSE | 10 | 82 | Drowsiness (68) local irritation 29) | [83] | |
RCT randomized controlled trial, OLS open-label study, SC single-centre, MC multicentre, DB double-blind, PS prospective study, ReS retrospective study, CO cross-over, HV healthy volunteer, S seizure, RS repetitive seizure, SE status epilepticus, RSE refractory status epilepticus, TEAE treatment-emergent adverse event, i.v. intravenous, i.n. intranasal, i.m. intramuscular, p.o. oral, s.l. sublingual, MDZ midazolam, LZP lorazepam, CZP clonazepam, DZP diazepam, PHT phenytoin, CBZ carbamazepine
aMilligram per kilogram body weight (mg/kg BW)
Diazepam
DZP is a BZD with a relatively long half-life compared to other drugs in this class. DZP is approved by the FDA and EMA for the treatment of anxiety, acute alcohol withdrawal, skeletal muscle spasms and convulsive disorders, such as SE. In addition, it is commonly used off-label for numerous other conditions including insomnia, restless legs syndrome, and pre-/post-operative sedation [86]. DZP is available as an oral tablet, oral solution, as preparations for i.v. or i.m. use and, since 2020, as a ready-made solution with a one-way applicator for i.n. administration.
Pharmacodynamics, Pharmacokinetics and Metabolism
DZP interacts with alcohol and many different classes of drugs, including analgesics, antibiotics, anticonvulsants and antidepressants. In addition, oral contraceptives can inhibit the biotransformation of DZP, thereby increasing its effects and possibly increasing the incidence of break-through bleeding [87]. However, DZP should be avoided in pregnant women as there is evidence for an increased risk of harmful effects on the human fetus or neonate without causing malformations [88].
With a Vd of 0.95 to 2.01 L/kg, DZP is a widely distributed compound showing a bioavailability of 93–100% after p.o. administration, 90% after r.s. administration, and 97% after i.n. administration [89–92]. Peak plasma levels are reached after 30–90 min following p.o. administration, 30–60 min after i.m. injection, 10–45 min after r.s. administration, 45 min after i.n., administration, and < 5 min after i.v. delivery. With chronic dosing, steady-state levels are reached after 5–14 days [89–92]. The half-life of DZP is 24–48 h due to an extended redistribution into the muscle and fat tissue after initial adsorption [90]. However, the half-life and free fraction of DZP increases in aged populations due to reduced carrier albumin serum levels and a high plasma protein binding of > 98% [93, 94]. The metabolization of DZP is mediated by hepatic CYP450 enzymes and glucuronidation for the renal elimination of several metabolites; however, marginal amounts of unmetabolized DZP can be found in the urine [95].
Available Formulations
DZP is available as a tablet (2 mg, 5 mg and 10 mg tablets) or a concentrate (10 mg/ml) for oral use, as a rectal suppository (10 mg/suppository), as rectal enema (5 mg/vial, 10 mg/vial; also as a rectal gel in an administration device), and as solution for i.v. injection (5 mg/ml, available as a clear water solution and as a lipid emulsion). In the USA, an FDA-approved ready-to-use DZP nasal spray has been available since 2020 under the trade name VOLTOCO® (5 mg/0.1 ml, 7.5 mg/0.1 ml and 10 mg/0.1 ml) for seizure clusters or repetitive seizures in patients aged 6 years or older [89].
Clinical Efficacy
For i.n. use, there are four phase I studies in healthy volunteers showing a feasible pharmacokinetic profile for DZP [96–99]. Another study in heathy volunteers revealed that i.m. administration of DZP resulted in a more rapid and less variable drug absorption compared to r.s. delivery [100]. Overall, three phase II studies for DZP were identified that compared rectal gel to i.n. [92] or i.m. administration using an auto-injector [101, 102] for the treatment of repetitive seizures.
For phase III studies, two double-blinded, multicentre, randomized controlled trials and one single-centre open-label trial were found, which revealed significant increases in seizure-free intervals in patients with seizure clusters using a DZP auto-injector. The seizure termination rate in these studies was approximately 78% [103, 104]. The efficacy of p.o., i.v. or r.s. DZP at doses of 0.2–0.5 mg/kg, or up to 20 mg, for the termination of seizures, seizure clusters or SE in general has been extensively demonstrated [22, 28, 29, 105–115]. Subsequently, several studies have compared the efficacy of DZP and valproate (VPA) for terminating SE and showed a response rate of 56–85% for DZP and 50–80% for VPA [116, 117]. Additional studies comparing the i.v. use of DZP to LZP or MDZ revealed a SE cessation rate of 72–100% [46, 50, 51, 118, 119]. However, in some studies, multiple doses were required to finally gain seizure control [120]. Other studies comparing the combination of i.v. DZP plus PHT with i.v. LZP or PB treatment showed a cessation of seizures or SE in 56–100% of cases for DZP [40, 121, 122]. Further studies have compared the efficacy of DZP with i.m. MDZ, revealing a seizure control rate of 88–91% for i.m. and i.v. DZP, respectively [72, 123, 124], and a 94% rate for the r.s. delivery of DZP [74]. Comparative studies examining the differences between i.n. or buccal/sublingual MDZ or LZP and DZP showed a cessation rate of 65–93% for i.v. DZP [15, 125–130], and 45–100% for the r.s. administration of DZP [12, 75, 77, 83, 131–138]. In addition, several reports have revealed no differences in efficacy between i.n. MDZ or LZP and DZP [14, 139]. For a detailed overview of the mentioned studies please refer to Table 3.
Table 3.
Summary of studies on the use of diazepam for status epilepticus
| Delivery route | Study design | N | Age (years) | SE types | Dosage (mg) | Seizure control (%) | TEAE rate (%) | Frequent TEAE (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| Intravenous | |||||||||
| DZP | SC | 15 | 16–73 | RS, SE | 5–40 | 82 | – | – | [114] |
| DZP | RCT, MC | 21 | 0–2 | RS | – | 87 | – | Respiratory arrest | [111] |
| DZP infusion | ReS, SC | 62 | 1–12 | RSE | 0.017 mg/kg/min | 86 | Hypotension, respiratory depression | [113] | |
| DZP vs. i.v. VPA | RCT, SC | 20 | 0–12 | RSE | 10 | 85 | – | Respiratory depression | [117] |
| DZP vs. i.v. VPA | PS | 66 | 41d | SE | 2 × 0.2b in 10 min | 56 | – | – | [116] |
| DZPvs. i.v. LZP | RCT, DB, SC | 78 | – | S | 5 | 76 | – | Respiratory depression | [46] |
| DZP vs. i.v. LZP | RCT, DB, MC | 140 | 3–18 | SE | 0.2b | 72 | – | Respiratory depression | [50] |
| DZP vs. i.v. LZP | RCT, MC | 273 | 0–17 | SE | – | Not superior | – | Respiratory depression | [139] |
| DZP vs. i.v. LZP vs. i.v. placebo | RCT, DB, SC | 205 | ≥ 18 | SE | 5 | Superior to placebo | – | Respiratory complications, circulatory complications | [22] |
| DZP vs. i.v. LZP and rectal DZP vs. rectal LZP | RCT, SC | 53 | 3.3–6.6c | SE | 0.3–0.4b | 85 (i.v.) 37 (rectal) | 15 | Respiratory depression | [51] |
| DZP + PHT vs. i.v. LZP vs. i.v. PH | RCT, DB, MC | 570 | 58.6c | SE | 0.15b | 56 | – | Hypoventilation, cardiac arrhythmia | [47] |
| DZP + PHT vs. i.v. LZP | RCT, SC | 88 | 1–12 | SE | 0.2b (+ 18b PHT) | 100 | – | Respiratory depression | [121] |
| DZP vs. i.v. MDZ | RCT, SC | 19 | 2–12 | RSE | 0.5a | 90 | – | Respiratory depression | [118] |
| DZP vs. i.v. MDZ | RCT | 120 | Children | S | 0.3–0.5b | 100 | – | – | [119] |
| DZP vs. i.v. MDZ vs. i.v. LZP | RCT, SC | 120 | 0–14 | S | 0.3b | 73 | – | Somnolence, sedation | [198] |
| DZP + PHT vs. PB + PHT | RCT, SC | 36 | 43.8c | SE | 2/min | Inferior to PB | – | Respiratory failure | [122] |
| DZP vs. i.n. MDZ | RCT, SB, SC | 35 | 0–15 | S | 0.2b | Superior to MDZ | – | – | [81] |
| DZP vs. i.n. MDZ | RCT, SC | 60 | 0–15 | S | 0.3b | Inferior to MDZ | – | – | [127] |
| DZP vs. i.n. MDZ | RCT, SC | 50 | 1–12 | S | 0.3b | 65 | – | – | [15] |
| DZP vs. i.n. MDZ | RCT, SC | 125 | – | S | 0.3b | Inferior to MDZ | – | – | [130] |
| DZP vs. i.m. MDZ | RCT, SC | 115 | 0–12 | S | 0.2b | – | 11 | Thrombophlebitis | [123] |
| DZP vs. i.m. MDZ | RCT, SC | 32 | 0–14 | S | 0.5b | 88 | – | Vomiting, hyperactivity | [124] |
| DZP vs. i.m. MDZ | RCT, SC | 11 | 0–10 | SE | 0.3b | 91 | 9 | Respiratory depression | [72] |
| DZP vs. buccal MDZ | RCT, SC | 120 | 0–12 | S | 0.3b | 93.3 | – | – | [128] |
| DZP vs. buccal MDZ | RCT, SC | 92 | 0–14 | S | 0.3b | 70 | 42 | Respiratory failure | [129] |
| Intramuscular | |||||||||
| DZP | RCT, DB, MC | 234 | ≥ 2 | RS | 5, 10, 15, 20a | Reduction of time to next seizure | 42 | Injection site pain (17), injection site hemorrhage (5) | [103] |
| DZP | RCT, MC | 234 | 2–83 | RS | 5, 10, 15a | 78 | 75 | Injections site pain (11), injections site hemorrhage(6) | [104] |
| DZP vs. i.m. rectal gel | SC, OLS | 24 | 18–55 | HV | 10 | i.m. superior to rectal | – | Pain, discomfort, drowsiness | [100] |
| DZP vs. DZP rectal gel | RCT, DB, SC, CO | 48 | 18–40 | RS | 5, 10, 15a | – | 22 | Injection site discomfort | [101] |
| Rectal (gel or solution) | |||||||||
| DZP | ReS, SC | 50 | 34.7c | RS | 0.2b | 90 | Somnolence | [107] | |
| DZP | SC | 39 | 16–65 | RS | 20, 30 | 29–72 | Drowsiness | [108] | |
| DZP | SC | 17 | – | RS | 0.5b | 66 | Respiratory difficulties, dizziness | [115] | |
| DZP | OLS | 149 | 2–76 | RS | 0.2–0.5b | 77 | – | Sedation | [109] |
| DZP vs. placebo | RCT, DB, MC | 96 | ≥ 18 | RS | 0.2b | – | – | – | [105] |
| DZP vs. placebo | RCT, DB | 125 | 2–60 | RS | 0.2–0.5b | Superior to placebo | – | – | [28] |
| DZP (rectal or p.o.) vs. placebo | RCT, DB, SC | 40 | 18–60 | RS | 20 | Superior to placebo | – | – | [110] |
| DZP vs. placebo | RCT, DB, MC | 158 | > 2 | RS | 0.2–0.5b | 55 | – | Somnolence | [29] |
| DZP vs. placebo | PS, DB | 133 | 2–17 | RS | 5 | Superior to placebo | – | Somnolence | [112] |
| DZP vs. i.m. MDZ | RCT, SC | 100 | 0–16 | SE | 0.5b | 94 | – | – | [74] |
| DZP vs. buccal MDZ | RCT, MC | 110 | 0–15 | S | 2.5–10 | 45 | 6 | Respiratory depression | [12] |
| DZP vs. buccal MDZ | RCT, SB, SC | 165 | 0–12 | S | 2.5–10 | 57 | – | Respiratory depression | [131] |
| DZP vs. buccal MDZ | RCT, SC | 39 | 5–22 | S | 10 | 59 | – | – | [75] |
| DZP vs. buccal MDZ | RCT, MC | 98 | 0–12 | S | 0.5b | 82 | – | – | [132] |
| DZP vs. buccal MDZ | RCT, SC | 43 | 0–12 | S | 0.3–0.5b | 85 | – | – | [133] |
| DZP vs. buccal MDZ | RCT, SC | 22 | 25–82 | S | 5 | 83 | – | Tiredness, ataxia | [77] |
| DZP vs. buccal MDZ | RCT, SC | 34 | 0–18 | S | 0.5b | 100 | – | – | [136] |
| DZP vs. sublingual LZP | RCT, MC | 436 | 0–10 | S | 0.5b | 79 | – | – | [137] |
| DZP vs. i.n. MDZ | RCT, SC | 45 | 0–13 | S | 0.3b | 60 | – | – | [134] |
| DZP vs. i.n. MDZ | RCT, SC | 46 | 0–12 | S | 0.3b | 89 | – | Vomiting, drowsiness, hypoxia | [135] |
| DZP vs. i.n. MDZ | PS, SC | 21 | 25–69 | S | 10 | 89 | – | Drowsiness | [83] |
| DZP vs. i.n. MDZ | RCT, SB, MC | 358 | 3–11 | S | 0.3–0.5b | Not superior | – | Respiratory failure (1) | [14] |
| DZP vs. i.n. MDZ | RCT, MC | 358 | – | RS | 0.3–0.5b | – | – | – | [138] |
| Intranasal | |||||||||
| DZP | Pilot study | 24 | 18–45 | HV | 10 | – | – | – | [96] |
| DZP | Pilot study | 8 | 28.3c | HV | 5, 10 | – | – | – | [98] |
| DZP | MC | 78 | 18–65 | S | 0.2b | – | Nasopharyngeal signs | [92] | |
| DZP vs. i.v. MZD | Pilot study | 4 | 20–24 | HV | 5 | – | – | – | [99] |
| DZP vs. rectal gel DZP | OLS, CO | 24 | 18–50 | RS | 5, 20 a | – | 32–48 | Nasal redness, nasal discomfort | [102] |
| DZP vs. i.v. DZP | Pilot study | 9 | 20–30 | HV | 20 (i.n.) 2 (i.v.) | – | – | – | [97] |
RCT randomized controlled trial, OLS open-label study, SC single-centre, MC multicentre, DB double-blind, PS prospective study, ReS retrospective study, CO cross-over, HV healthy volunteer, S seizure, RS repetitive seizure, SE status epilepticus, RSE refractory status epilepticus, TEAE treatment-emergent adverse event, i.v. intravenous, i.n. intranasal, i.m. intramuscular, p.o. oral, MDZ midazolam, LZP lorazepam, DZP diazepam, VPA valproate, PHT phenytoin, PB phenobarbital
a Absolute
b Milligram per kilogram body weight (mg/kg BW)
c Average
With regard to the safety and tolerability of DZP, frequent substance-specific treatment-emergent adverse events (TEAEs) have been identified, including somnolence, sedation, drowsiness, vomiting, hyperactivity, tiredness, hypotension and ataxia [77, 83, 107, 113, 120, 124, 135]. Several severe TEAEs have also been reported, mostly due to respiratory depression that, in some cases, required mechanical ventilation [50, 72, 117, 118, 121]. In addition, different TEAEs have been reported that are specific to the individual routes of administration. For example, injection site pain for i.m. [103, 104], thrombophlebitis for i.v. [123], and nasal discomfort for i.n. administration [92, 101, 102].
Clonazepam
CZP has been used for over 40 years to treat seizures, paediatric and adult SE, chronic epilepsy (including severe childhood epilepsy, such as Lennox-Gastaut syndrome, absence or myoclonic seizures), and—in the USA—panic disorders. Evidence supporting the efficacy of CZP for SE is still scarce, mainly consisting of uncontrolled case series. Therefore, even if widely used in Latin America and in many European countries, CZP is still not recommended by the latest European or American SE guidelines [38, 140]. Various formulations and routes of delivery for CZP exist. However, their availability varies among countries. For example, i.v. formulations are not available in the USA, while disintegrating tablets are not accessible in Europe.
Most of the existing data regarding CZP efficacy for the treatment of SE or seizure clusters are based on i.v. administration studies [53, 141–147]. Anecdotal evidence exists regarding the wafer formulation (orally disintegrating tablets) [148], and only phase I and II trials have been conducted for i.n. [149], r.s. [150], i.m. [151], oral droplets [152] or subcutaneous [153] formulations.
Pharmacodynamics, Pharmacokinetics and Metabolism
CZP is more lipophilic than LZP, but less so than DZP, with consequently less redistribution compared to the latter drug. Its protein binding is 86%, which is somewhat lower than for other BZDs. After i.v. administration, CZP reaches the brain within 1 min, and its distribution follows a two-compartment model with a half-life from 0.7 to 3.4 h and a Vd from 1.5 to 4.4 L/kg [40]. After p.o. administration, CZP is completely absorbed with an absolute bioavailability of ~90% (Tmax is achieved within 1–4 h) [154].
In a preclinical study, i.n. CZP administration using nanocarriers was demonstrated to be safe and effective [149], and oral droplet administration (with CZP dissolved into droplets of propylene glycol) has been shown to achieve therapeutic levels within 10–15 min [152].
Rectal administration (11 healthy children with previous febrile convulsion, six aged 1.4–4.7 years at a dose of 0.05 mg/kg, and 5 aged 1.4–4.1 years at a dose of 0.1 mg/kg) showed plasma concentrations indicating a rapid absorption (Tmax 10 min–2 h, mean 30 min) [150]. One open-label study investigating the pharmacokinetics of i.v., i.m. and p.o. CZP (2 mg) reported a slower absorption rate after i.m. administration compared to p.o. administration (Tmax 3.1 h vs. 1.7 h) [151].
Subcutaneous injection of CZP (microspheres) demonstrated complete absorption with a slow-release pharmacokinetic profile [153]. CZP has a long half-life of 30–40 h and undergoes extensive metabolism, primarily by CYP3A4, with no formation of active metabolites. Due to its extensive CYP450 metabolism, several drug interactions exist. For example, CYP450 inducers, such as PHT, CBZ, lamotrigine (LTG) or PB, can cause a 38% decrease in CZP plasma levels. Similarly, CYP inhibitors, such as antifungal agents, may impair CZP metabolism [154, 155]. CZP can be kept at ambient temperature and can be administered as a rapid i.v. bolus (0.015 mg/kg, over ≤ 30 s).
Available Formulations
CZP is available as a tablet (0.5 mg and 2 mg tablets), orally disintegrating tablet and concentrate (2.5 mg/ml) for oral use and as solution for i.v. injection (1 mg/ml).
Clinical Efficacy
Since the first clinical publication by Gastaut in 1971 [142], few clinical trials have been conducted to evaluate the efficacy of CZP for SE. In addition to the trials described below, eight uncontrolled case series from the 1970s (a total of 385 patients) reported CZP to be effective in approximately 80–90% of patients [144]. In the initial publication, Gastaut and colleagues reported SE termination, without unfavorable side effects, in 38/39 SE episodes treated with i.v. CZP (1–8 mg) [142]. In children, an early study (17 children, age 2 weeks to 15 years) reported SE cessation after i.v. CZP (0.25–0.75 mg) in all cases without significant side effects [141].
More recently, two retrospective studies suggested that i.v. CZP may not be inferior—and may even be superior—to other BZDs [53, 145]. A retrospective multicentre study including 177 adult patients reported the prescription of CZP as a first-line treatment in 72 (41%) SE patients, with 82 (46%) and 23 (13%) using LZP and MDZ, respectively. Interestingly, only 85% of the patients received BZDs as a first-line treatment and 59% were prescribed an insufficient dosage [53]. A single-centre retrospective assessment of 167 SE episodes described i.v. CZP as the most effective treatment (50% response rate) for terminating GCSE compared to i.v. DZP (18%), i.v. LZP (29%) or i.m. MDZ (12%) [145]. Even if not designed to evaluate CZP efficacy, a randomized double-blind trial (SAMUKeppra) evaluating i.v. levetiracetam (LEV; 2.5 g) plus i.v. CZP (1 mg) versusplacebo plus i.v. CZP in outpatient GCSE treatment offered good insights regarding CZP efficacy [143]. No advantage was found with the addition of LEV over CZP alone. Convulsions stopped after 15 min in 84% (57/68) of patients receiving CZP alone [143]. Regarding non-i.v. formulations, a retrospective study investigating the efficacy of CZP wafers for the acute treatment of prolonged seizures in children and young adults (2–25 years) reported seizure termination in 38/56 (68%) patients [148]. In 19/56 (34%) patients, seizure termination was reported within 1 min, perhaps partially corresponding to a spontaneous termination.
In conclusion, although class I evidence is currently lacking, i.v. CZP, which is already widely used, constitutes a good option for first-line SE treatment. Regarding alternative routes of administration, p.o. administration (CZP wafers) and i.m. administration is not recommended due to its slower absorption rate, and—despite promising phase I–II studies—i.n. and r.s. formulations are not yet available.
Tables 4 provide a detailed overview of the studies discussed in this section.
Table 4.
Summary of studies on the use of clonazepam for status epilepticus
| Delivery route | Study design | n | Age (years) | SE types | Dosage (mg) | Seizure control (%) | TEAE rate (%) | Frequent TEAE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Per os | |||||||||
| CZP | ReS, SC | 56 | 2–25 | RS, SE | 0.25, 0.5, 1 or 2 | 68 | – | – | [148] |
| Intravenous | |||||||||
| CZP | – | 16 | – | SE | 1 | 88 | – | – | [199, 200] |
| CZP | – | 17 | – | SE | 1–6 | 82 | – | – | [201] |
| CZP | – | 194 | – | SE | 0.5–10 | 81 (19 multiple doses) | – | Respiratory depression, somnolence | [202] |
| CZP | PS, OLS, SC | 8 | – | SE | 2 | 100 | – | Drowsiness, ataxia | [203] |
| CZP | PS, OLS, SC | 65 | – | SE | 1–4 | 83 | – | Respiratory depression, somnolence | [204] |
| CZP | PS, OLS, SC | 40 | < 18 | SE | 0.5–2 | 75 | – | – | [205] |
| CZP | – | 13 | 0.15–15 | SE | 0.4–3 | 77 | – | – | [206] |
| CZP | – | 32 | – | SE | 2 | 84 | – | – | [207] |
| CZP | ReS, OLS, SC | 17 | 0–15 | SE | 0.25–0.75 | 100 | – | – | [141] |
| CZP | OLS | 24 | SE, RS | 1–2 | 100 (focal aware), 66 (focal unaware) 50 (GTC) | 42 | Respiratory depression, drowsiness | [146] | |
| CZP (bolus vs. perfusion) | ReS, OLS, SC | 37 | – | SE | 1–8 | 97 | – | – | [142] |
| CZP vs. i.v. LZP vs. i.v. LZP + CZP | OLS | 61 | ≥ 18 | SE | 1 | 76 | – | Drowsiness, psychomotor agitation | [147] |
|
CZP vs. i.v. DZP vs. i.v. LCM vs. i.v. LEV vs. i.v. LZP vs. i.v. MDZ vs. i.v. PHT vs. i.v. VPA |
ReS, SC | 167 | 63.1 (SD 17.4) | S, SE, RSE | 1.2 (SD 1) –5.5 (SD 5.3) | 50 | – | – | [145] |
|
CZP vs. i.v. LZP vs. i.v. MDZ |
PS, OLS, MC | 177 | ≥ 16 | SE, RS | 0.015a | 56.96 (endpoint: not RSE) | – | – | [53] |
|
CZP + placebo vs. i.v. CZP + LEV |
RCT, DB | 205 | S, RS | 1 | 84 | 19 | Respiratory failure, cardiac failure | [143] | |
RCT randomized controlled trial, OLS open-label study, SC single-centre, MC multicentre, DB double-blind, PS prospective study, ReS retrospective study, S seizure, RS repetitive seizure, SE status epilepticus, RSE refractory status epilepticus, GTC generalized tonic clonic sizure, TEAE treatment-emergent adverse event, i.v. intravenous, p.o. oral, MDZ midazolam, LZP lorazepam, CZP clonazepam, VPA valproate, PHT phenytoin, LCM lacosamide, LEV levetiracetam
aMilligram per kilogram body weight (mg/kg BW)
General Considerations
We would like to point out that, although the previously discussed established pharmacokinetic and pharmacodynamic measures provide a good overview of how a drug acts, additional factors might contribute to complex drug behaviors. For instance, time of maximum concentration in plasma after IV administration does not necessarily correspond to the time of maximum pharmacologic effect, for example due to the delay in diffusion across the blood-brain barrier [156–158]. Furthermore, the half-life of a drug might not necessarily correspond to the duration of action (as mentioned above), which, for example, also depends on the size of the dose and the extent of peripheral tissue distribution during the initial distribution phase.
Group-Specific Side Effects
All benzodiazepines can—due to their action as a GABAA-receptor agonists—cause amnesia, sedation, respiratory depression and coma. Therefore, after administration, monitoring of the patient´s clinical status, especially vigilance breathing, and protective reflexes is mandatory. In case of a severe respiratory depression, protective intubation usually involving muscle relaxants can be necessary, which in turn hinders further evaluations of the patient’s clinical status, neurological assessment and seizure detection without EEG. Furthermore, consequent admission to the intensive care unit might increase the risk of ventilation-associated pneumonia, which in turn can promote refractory SE and lead to poorer outcome [159–161]. Though, it should be mentioned that SE per se can also cause severe respiratory depression.
As side effects depend on dose and time of action, choice of an adequate initial dose and of a short-acting drug are important factors (e.g., MDZ i.v. or the newer formulations). The benzodiazepine effect can further be counteracted by the BZD antagonist flumazenil.
Of the discussed benzodiazepines, all but LZP are mainly metabolized via CYP450 enzymes and glucuronidation for the renal elimination of metabolites. LZP in turn is metabolized by hepatic one-step, non-oxidative conjugation after which the non-active metabolite is predominantly excreted renally. This might make LZP a preferential choice in patients with liver disease. Dose adjustments should be made according to product labels.
Novel Administration Routes and Other Benzodiazepines
New routes of drug administration, such as the intrapulmonary route, are currently being investigated for the treatment of epilepsy and other disorders [162, 163]. Due to a large surface area, and high permeability and blood flow in the lung, rapid systemic drug effects are possible with inhalation therapies [164]. Therefore, intrapulmonary administration for the treatment of SE seems promising. In 1994, Xi et al. treated 120 patients with seizures in a single-blind trial with inhalable DZP and showed a significant effect on seizure control [165]. Dhir and colleagues also showed that MDZ and a propofol prodrug are highly effective antiseizure drugs when administered intrapulmonarily in a preclinical model [166, 167]. Currently, intrapulmonary administration of alprazolam delivered via the Staccato delivery system is being developed as a rapid epileptic seizure termination therapy. This drug delivery system vaporizes the drug through a heating package that is activated by breathing through the system. The drug vapor condenses into aerosol particles and is delivered into the lungs by normal breathing.
Alprazolam is a 1,4-benzodiazepine and also an allosteric modulator of GABAA‐receptors. This drug was approved by the FDA for the treatment of anxiety and panic disorders in 1981 and is available for p.o. administration [168]. Alprazolam does not have an indication for seizure therapy at present, but preclinical studies suggest that it does have a potent antiseizure effect [164]. After p.o. administration, peak concentration in the plasma is reached in 1–2 h and is proportionate to the dose. Single doses usually range from 0.5–3 mg and result in peak levels of 8–37 ng/ml. Alprazolam has an intermediate onset of action (function of rate of absorption) compared to other BZDs and mean plasma elimination half-life of ~6–15 h [169, 170]. In a phase 1 study, 1 mg of inhaled alprazolam reached a peak concentration of 48 ng/ml in smokers and 26.72 ng/ml in non-smokers after 1.8 min [171]. In vitro, alprazolam is mostly bound to serum albumin (80%). It is mainly metabolized by CYP3A4, which means that pharmacological interactions must be considered. The two main metabolites of alprazolam are 4-hydroxyalprazolam and α-hydroxyalprazolam, both of which have low plasma concentrations in comparison to alprazolam and do not contribute to the pharmacological effects of this drug. Alprazolam and its metabolites are excreted primarily in the urine [171]. In a phase IIa study, five patients with a diagnosis of photoparoxysmal response on EEG received alprazolam intrapulmonarily via the Staccato system in 0.5–2 mg doses after intermittent photic stimulation [164]. The number of photic stimulation frequencies that produced a photoepileptiform response was measured after inhalation. Staccato alprazolam reduced the number of photic stimulation frequencies at 2 min compared to placebo, and the effect stopped after 4 h for the 0.5 mg dose and after 6 h for the 1 and 2 mg doses. Alprazolam plasma concentrations were dose related and reached 31.5 ± 3.14 ng/ml within 2 mins after the inhalation of 2 mg [164].
Potential limitations include the patient’s compliance, insufficient spontaneous breathing during seizures, or reduced transpulmonary diffusion capacity. The results of a phase IIb randomized, double-blind, inpatient study and phase III studies exploring the efficacy and safety of Staccato alprazolam for the acute termination of a predictable seizure pattern are currently pending.
Special Therapeutic Aspects for Infants and Children
Particular Features of the Pharmacokinetics of Benzodiazepines (BZDs) in Children
As with many other drugs, the pharmacokinetics of BDZs vary with age. This is mainly due to the maturation of the hepatic microsomal oxidizing system over the 6 months after birth. Consequently, the pharmacokinetics of hepatic metabolized pharmaceuticals in children less than 6 months of age are significantly different from adults. Cytochrome P450-catalyzed metabolism tends to be low at birth but exceeds adult values by 2–3 years of age. Thereafter, CYP-catalyzed metabolism decreases again, reaching adult levels around puberty [172]. Metabolism via glucuronidation tends to be low in neonates, reaching adult levels by the age of 3–4 years. Overall, pharmacokinetics continue to differ from adults until around age 12 years. The dosages used and substances preferred therefore vary depending on the patient’s age. Currently, available data regarding the efficacy and safety of BZDs in neonates are still insufficient.
DZP has a long half-life in neonates and should be avoided until hepatic metabolic pathways have matured at the age of approximately 6 months [173]. Doses of BZDs given to children must be calculated on a mg/kg basis. For children 6 months to 5 years of age, the initial dose of MDZ should be 0.05–0.1 mg/kg. A total, slowly titrated dose up to 0.6 mg/kg may be necessary to achieve the desired endpoint. For children 6–12 years of age, the initial dose should be 0.025–0.05 mg/kg, with a total dose up to 0.4 mg/kg [174]. LZP pharmacokinetics in children are similar to the adult pharmacokinetic parameters except for increased clearance. Therefore, uniform paediatric dosing (0.1 mg/kg, to a maximum of 4 mg) can be used to achieve serum concentrations of 50–100 ng/ml in children with SE, which have been previously associated with effective seizure control [175, 176].
Rescue Medications in Paediatric Epilepsy Patients
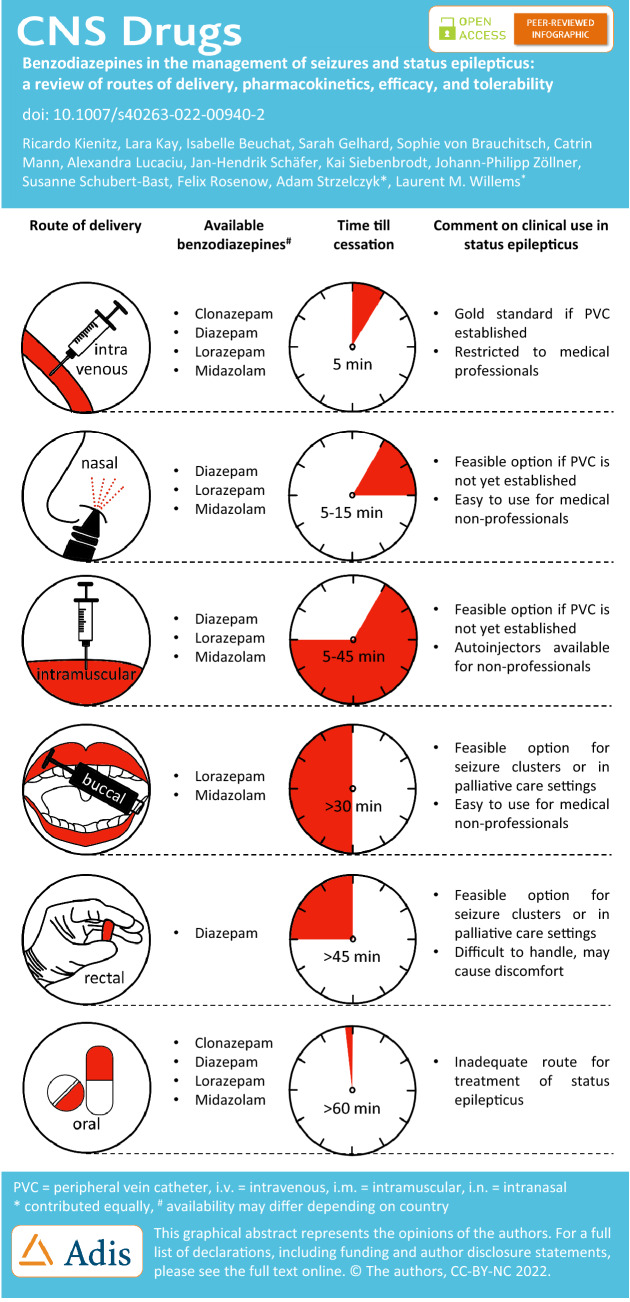
Rescue medications for lay use are frequently prescribed for paediatric epilepsy patients. The availability of such preparations differs between the USA and the EU. Oromucosal MDZ (Buccolam®) is a BZD approved by the EMA—but not by the FDA—for the treatment of paediatric patients aged 3 months to < 18 years with prolonged, convulsive seizures [177]. The ready-to-use, prefilled oral syringes containing a MDZ solution (in a concentration of 5 mg/ml with different volumes to provide an age-appropriate dose) are administered in fixed doses depending on the patient's age. The recommended doses are 2.5 mg (in patients aged 3 months to < 1 year), 5 mg (in patients aged 1 year to < 5 years), 7.5 mg (in patients aged 5 years to < 10 years), and 10 mg (in patients aged 10 years to < 18 years) [178]. Oromucosal MDZ (0.2–~0.5 mg/kg or 10 mg) is at least as effective as r.s. DZP (~0.5 mg/kg or 10 mg), and as effective as i.v. DZP (0.3 mg/kg) with regard to response rate (cessation of seizures) in paediatric patients in randomized, controlled trials [12, 75]. DZP rectal gel is approved by the EMA for epilepsy patients weighing a minimum of 10 kg (a patient age of approximately 1 year), and FDA approval exists for the treatment of occasional episodes of increased seizures that are different from a patient’s usual seizure pattern in patients with epilepsy that are 2 years of age and older [179]. The recommended dose for DZP rectal gel is 0.2–0.5 mg/kg depending on age. The ready-for-use rectal delivery system is provided in unit doses of 2.5, 5, 7.5, 10, 12.5, 15, 17.5 and 20 mg DZP, and the prescribed dose is obtained by rounding upward to the next available dose. DZP nasal spray (VOLTOCO®, FDA approved for epilepsy patients aged 6 years and older) and MDZ nasal spray (Nayzilam®, FDA approved for epilepsy patients aged 12 years and older) are not yet approved in the EU (Fig. 1).
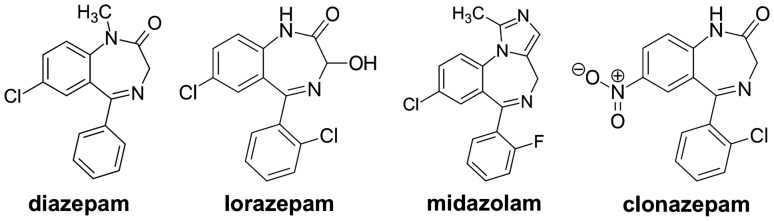
Fig. 1.
Overview of the structural forms of the benzodiazepines diazepam, lorazepam, midazolam and clonazepam, which are discussed in this review. Even though the benzodiazepines are structurally very similar and closely related, their different pharmacological and pharmacokinetic properties result in relevant differences that are of particular importance when considering various delivery routes. All structural forms displayed have been released into the public domain by their creators
Conclusion: Comparing Different BZDs and Delivery Routes for Status Epilepticus
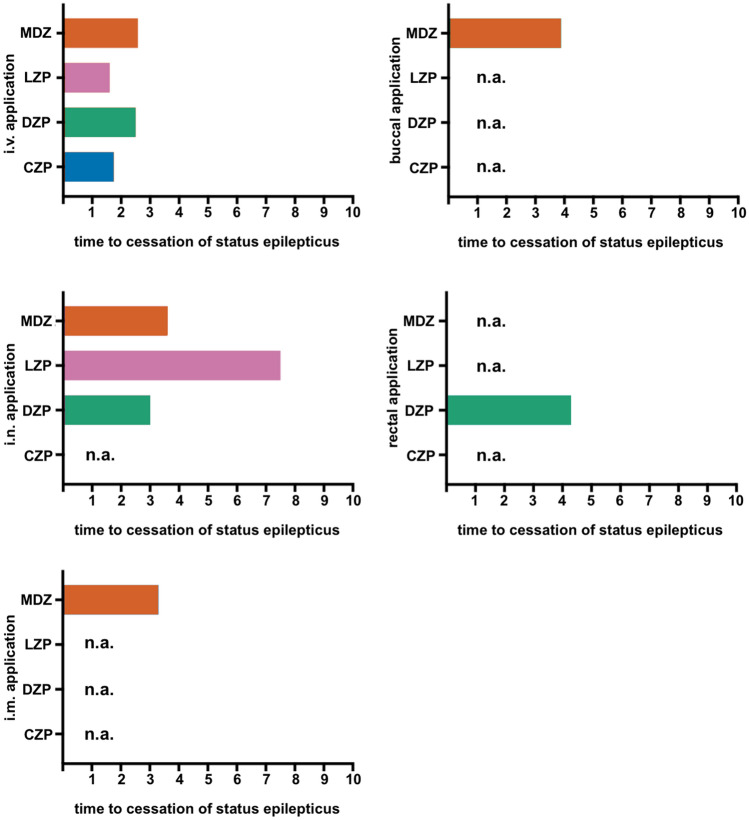
Over the last several decades, different BZDs have been established as first-line therapies for SE, each of which has its own pharmacological characteristics with advantages and disadvantages [180]. Here, the comparably long half-life of DZP especially stands out [181]. As it has been shown that interrupting SE as soon as possible reduces the chance of developing RSE and improves outcomes, ensuring rapid treatment in acute care is at least as important, if not more important, than the BZD choice [182]. Fortunately, several delivery routes for BZDs have now been established, which are suitable for both lay users and medical staff [9]. To provide an overview of the available treatment options and delivery routes, Fig. 2 displays this information according to the type of administration, and Fig. 3 displays this according to the duration of action, considering the time required to establish access. The relevant pharmacokinetic and pharmacodynamic properties of the different BZDs are presented in Table 5, including delivery route-specific dosing recommendations for adult patients.
Fig. 2.
Mean time from drug administration to cessation of status epilepticus (SE) in minutes is displayed for different delivery routes, based on literature research, as far as available (n.a. = not available). Intravenous (i.v.) administration showed the fastest onset of action with a latency of less than 3 min, followed by intranasal (i.n.) and intramuscular (i.m.) administration, as well as buccal and rectal delivery. Due to the heterogeneous patient populations and study settings, the displayed values should only serve as a basic guidance [48, 50, 51, 146, 186–190]. MDZ midazolam, LZP lorazepam, DZP diazepam, CZP clonazepam
Fig. 3.
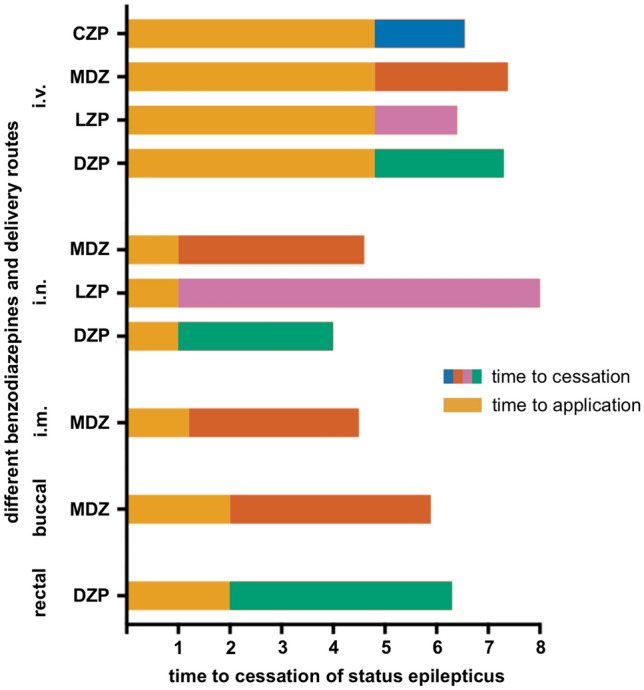
Time from therapy administration to cessation of status epilepticus (SE) is displayed to help decide on the clinical use of different delivery routes for benzodiazepines in SE, based on published preparations and process times for drug administration via intravenous injection (i.v.), intramuscular injection (i.m.), rectal, intranasal (i.n.) and buccal administration [48]. For i.v. administration, it was assumed that no peripheral indwelling venous catheter had yet been established analogous to Silbergleit et al. [48]. MDZ midazolam, LZP lorazepam, DZP diazepam, CZP clonazepam
Table 5.
Comparison and dosing of frequently used benzodiazepines for the treatment of status epilepticus
| Pharmacokinetics | Dosing and delivery route specific aspects | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Vdistribution (l/kg) | PB (%) | Half-life a (h) | Clearance (ml/min/kg) | Delivery routes | BA (%) |
Tmax
b (min) |
Initial dose (mg/kg BW) | Initial dose (mg) | Dose repetition (min) | Max. dose (mg) |
|
Lorazepam (LZP) |
0.8–1.3 | 90 | 8–20 | 0.7–1.2 |
i.v. i.n. i.m. s.l. |
100 77 100 94 |
10 30 80 120 |
0.1 – – – |
2–4 – – – |
5–10 – – – |
8 – – – |
|
Midazolam (MDZ) |
4.2–6.6 | 98 | 2–3 | 4–9 |
i.v. i.n. i.m. buccal |
100 78 91 75 |
4.5 10–15 20 15–90 |
0.2 – – – |
10 5–10 10 5–10 |
5–10 no no no |
10 – – – |
|
Diazepam (DZP) |
0.8–1.4 | 99 | 40–60 | 0.5 |
i.v. i.n. i.m. r.s. |
100 70–90 100 80–100 |
1 60–90 60 30–74 |
0.15–0.2 – 0.1–0.2 0.2–0.5 |
5–10 – 5–10 10–30 |
5 – – no |
20 – 30 20 |
|
Clonazepam (CZP) |
3 | 86 | 17–55 | 0.42 |
i.v. i.m. |
90 93 |
< 10 3.1 |
0.015 – |
1–2 – |
5–10 | 2 |
Due to a short Tmax of between 90 and 150 s and the reliability of correct administration, i.v. administration of MDZ, LZP or DZP remains the gold standard in the professional medical setting [9, 151, 181, 183–185]. The i.v. administration of CZP in SE care is still a additional alternative [185]. However, establishing a peripheral vein catheter in patients with poor venous conditions or during motor seizures can be challenging. A quick but risky alternative in patients with poor venous conditions could be an intraosseous approach, which—due to its invasive nature—carries the risk of infection, fracture or osteomyelitis [38]. Data from the RAMPART study showed that the time to successful administration of BZDs via the i.m. route was significantly shorter than by the i.v. route [48]. Even if seizure termination after i.v. administration was shorter compared to i.m. injection, the resulting overall time from the start of treatment to the termination of convulsions was similar [48]. Despite their almost complete bioavailability, the Tmax of 20, 60 and 80 min after i.m. injection described for MDZ, DZP and LZP injections are absorbed slowly, and might be therefore less, suitable for the acute treatment of SE [9]. To ensure a fast and safe administration of medication, even in difficult situations, i.n. administration has become widely accepted (MDZ, LZP and DZP). In addition to BZD administration via mucosal atomization devices or specifically formulated nasal sprays, commercially available applicators have been approved for DZP and MDZ in the USA for the treatment of seizure clusters or SE [64, 65, 89]. With a bioavailability of ~70–90% and a Tmax between 30 and 60 min, the nasal route seems inferior to i.v. and i.m. administration for LZP and DZP [183]. However, with a Tmax of < 15 min and a bioavailability of 44%, the i.n. administration of MDZ appears to be a feasible alternative, especially due to the comparably short and uncomplicated administration. In a pharmaco-EEG study, a relevant effect of MDZ after i.n. administration could already be measured after 4 min, which preceded the clinical end of the seizure by ~ 1 min [66]. For lay use, there are also BZD formulations available that can be administered either rectally or buccally. Buccal MDZ can easily be applied into the cheek pouches using prefabricated disposable syringes. With a bioavailability of 75% and a Tmax between 15 and 90 min, this delivery route is mainly used in the treatment of seizure series or clusters, but there is a risk of aspiration and the pharmacokinetics can vary greatly depending on mucosal absorption. Therefore, the comprehensive use of buccal MDZ in professional emergency care does not appear to be reasonable. In addition, DZP is available for rectal use as rectioles, which are mainly used in paediatrics. Here, again, a Tmax of 30–74 min limits this administration in the professional medical setting, despite almost complete bioavailability. Based on a disproportionately long Tmax of approximately 120 min, sublingual LZP is not an adequate therapeutic option for the acute treatment of SE. Other new delivery routes, such as intrapulmonary administration, appear promising but require further studies before clinical use. To ensure further therapeutic management including prevention of potential RSE and for safety reasons, the establishment of a peripheral venous catheter for the repeated administration of BZDs at the earliest possible time seems indispensable.
Declarations
Funding
The authors were supported via the Center for Personalized and Translational Epilepsy Research (CePTER) with a LOEWE grant from the State of Hessen. Open access funding enabled and organized by Projekt DEAL.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Competing Interests
RK, LW, IB, SG, SvB, CM, AL, JHS, KS and LMW declare that they have no conflicts of interest. JPZ reports speakers’ honoraria and travel grants from Eisai and Desitin Arzneimittel. SSB reports personal fees from Eisai, Desitin Pharma, GW Pharmaceuticals companies, LivaNova, UCB, and Zogenix. FR reports grants and personal fees from UCB Pharma, Arvelle Therapeutics, and Desitin Arzneimittel, personal fees from Eisai, GW Pharmaceuticals, Novartis, Medtronic, Cerbomed, Sandoz, BayerVital, and Shire, and grants from the European Union, Deutsche Forschungsgemeinschaft, the LOEWE Programm of the state of Hesse, and the Detlev-Wrobel-Fonds for Epilepsy Research. AS reports personal fees and grants from Angelini Pharma/Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals, Marinus Pharmaceuticals, UCB Pharma, UNEEG medical, and Zogenix. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this review apart from those disclosed.
Authors' contributions
AS developed the idea for this review. AS and RK drafted the concept. All authors performed the literature search and data analysis. AS, RK and LMW drafted the graphical abstract and the final manuscript. All authors contributed substantially to the final manuscript. All authors approved the final manuscript for submission. All authors agree to be accountable for the work.
Footnotes
Adam Strzelczyk and Laurent M. Willems contributed equally.
References
- 1.Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14(6):615–624. doi: 10.1016/S1474-4422(15)00042-3. [DOI] [PubMed] [Google Scholar]
- 2.Roberg LE, Monsson O, Kristensen SB, Dahl SM, Ulvin LB, Heuser K, et al. Prediction of long-term survival after status epilepticus using the ACD score. JAMA Neurol. 2022;79(6):604–613. doi: 10.1001/jamaneurol.2022.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schubert-Bast S, Zollner JP, Ansorge S, Hapfelmeier J, Bonthapally V, Eldar-Lissai A, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: a population-based study on German health insurance data. Epilepsia. 2019;60(5):911–920. doi: 10.1111/epi.14729. [DOI] [PubMed] [Google Scholar]
- 4.Poukas VS, Pollard JR, Anderson CT. Rescue therapies for seizures. Curr Neurol Neurosci Rep. 2011;11(4):418–422. doi: 10.1007/s11910-011-0207-x. [DOI] [PubMed] [Google Scholar]
- 5.Humphries LK, Eiland LS. Treatment of acute seizures: is intranasal midazolam a viable option? J Pediatr Pharmacol Ther. 2013;18(2):79–87. doi: 10.5863/1551-6776-18.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures Focus on delivery routes. Clin Pharmacokinet. 1999;36(6):409–424. doi: 10.2165/00003088-199936060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GD, Saneto RP. Current oral and non-oral routes of antiepileptic drug delivery. Adv Drug Deliv Rev. 2012;64(10):911–918. doi: 10.1016/j.addr.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Kadel J, Bauer S, Hermsen AM, Immisch I, Kay L, Klein KM, et al. Use of emergency medication in adult patients with epilepsy: a multicentre cohort study from Germany. CNS Drugs. 2018;32(8):771–781. doi: 10.1007/s40263-018-0544-2. [DOI] [PubMed] [Google Scholar]
- 9.Mula M. New non-intravenous routes for benzodiazepines in epilepsy: a clinician perspective. CNS Drugs. 2017;31(1):11–17. doi: 10.1007/s40263-016-0398-4. [DOI] [PubMed] [Google Scholar]
- 10.Lathers CM, Jim KF, Spivey WH. A comparison of intraosseous and intravenous routes of administration for antiseizure agents. Epilepsia. 1989;30(4):472–479. doi: 10.1111/j.1528-1157.1989.tb05328.x. [DOI] [PubMed] [Google Scholar]
- 11.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17(19):7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(9481):205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 13.Scott RC, Besag FM, Boyd SG, Berry D, Neville BG. Buccal absorption of midazolam: pharmacokinetics and EEG pharmacodynamics. Epilepsia. 1998;39(3):290–294. doi: 10.1111/j.1528-1157.1998.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 14.Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in paediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747–753. doi: 10.1001/archpediatrics.2010.130. [DOI] [PubMed] [Google Scholar]
- 15.Thakker A, Shanbag P. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J Neurol. 2013;260(2):470–474. doi: 10.1007/s00415-012-6659-3. [DOI] [PubMed] [Google Scholar]
- 16.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40(1):120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenow F. Status epilepticus im Erwachsenenalter. In: Diener HC, Weimar C, editors. Leitlinien für Diagnostik und Therapie in der Neurologie. Stuttgart: Thieme Verlag; 2012. [Google Scholar]
- 19.Strzelczyk A, Ansorge S, Hapfelmeier J, Bonthapally V, Erder MH, Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. 2017;58(9):1533–1541. doi: 10.1111/epi.13837. [DOI] [PubMed] [Google Scholar]
- 20.Neligan A, Noyce AJ, Gosavi TD, Shorvon SD, Kohler S, Walker MC. Change in mortality of generalized convulsive status epilepticus in high-income countries over time: a systematic review and meta-analysis. JAMA Neurol. 2019;76(8):897–905. doi: 10.1001/jamaneurol.2019.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43(3 Pt 1):483–488. doi: 10.1212/WNL.43.3_Part_1.483. [DOI] [PubMed] [Google Scholar]
- 22.Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345(9):631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 23.Sathe AG, Underwood E, Coles LD, Elm JJ, Silbergleit R, Chamberlain JM, et al. Patterns of benzodiazepine underdosing in the established status epilepticus treatment trial. Epilepsia. 2021;62(3):795–806. doi: 10.1111/epi.16825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellinghaus C, Rossetti AO, Trinka T, Lang N, May TW, Unterberger I, et al. Factors predicting cessation of status epilepticus in clinical practice: Data from a prospective observational registry (SENSE). Ann Neurol. 2019;85(3):421–32. [DOI] [PubMed]
- 25.Willems LM, Bauer S, Jahnke K, Voss M, Rosenow F, Strzelczyk A. Therapeutic options for patients with refractory status epilepticus in palliative settings or with a limitation of life-sustaining therapies: a systematic review. CNS Drugs. 2020;34(8):801–26. [DOI] [PMC free article] [PubMed]
- 26.Haut SR. Seizure clusters: characteristics and treatment. Curr Opin Neurol. 2015;28(2):143–150. doi: 10.1097/WCO.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 27.Martinez C, Sullivan T, Hauser WA. Prevalence of acute repetitive seizures (ARS) in the United Kingdom. Epilepsy Res. 2009;87(2–3):137–143. doi: 10.1016/j.eplepsyres.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM, Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338(26):1869–1875. doi: 10.1056/NEJM199806253382602. [DOI] [PubMed] [Google Scholar]
- 29.Cereghino JJ, Mitchell WG, Murphy J, Kriel RL, Rosenfeld WE, Trevathan E. Treating repetitive seizures with a rectal diazepam formulation: a randomized study. The North American Diastat Study Group. Neurology. 1998;51(5):1274–1282. doi: 10.1212/WNL.51.5.1274. [DOI] [PubMed] [Google Scholar]
- 30.Rose AB, McCabe PH, Gilliam FG, Smith BJ, Boggs JG, Ficker DM, et al. Occurrence of seizure clusters and status epilepticus during inpatient video-EEG monitoring. Neurology. 2003;60(6):975–978. doi: 10.1212/01.WNL.0000053748.83309.28. [DOI] [PubMed] [Google Scholar]
- 31.Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology. 2005;65(8):1313–1315. doi: 10.1212/01.wnl.0000180685.84547.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Gennaro G, Picardi A, Sparano A, Mascia A, Meldolesi GN, Grammaldo LG, et al. Seizure clusters and adverse events during pre-surgical video-EEG monitoring with a slow anti-epileptic drug (AED) taper. Clin Neurophysiol. 2012;123(3):486–488. doi: 10.1016/j.clinph.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Herzog AG, Frye CA. Progesterone Trial Study G. Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology. 2014;83(4):345–348. doi: 10.1212/WNL.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire MJ, Nevitt SJ. Treatments for seizures in catamenial (menstrual-related) epilepsy. Cochrane Database Syst Rev. 2019;10:13225. doi: 10.1002/14651858.CD013225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferastraoaru V, Goldenholz DM, Chiang S, Moss R, Theodore WH, Haut SR. Characteristics of large patient-reported outcomes: where can one million seizures get us? Epilepsia Open. 2018;3(3):364–373. doi: 10.1002/epi4.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldenholz DM, Rakesh K, Kapur K, Gainza-Lein M, Hodgeman R, Moss R, et al. Different as night and day: patterns of isolated seizures, clusters, and status epilepticus. Epilepsia. 2018;59(5):e73–e77. doi: 10.1111/epi.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Möddel G, Kellinghaus C, Strzelczyk A. Initial treatment of status epilepticus. Z Epileptol. 2018;31:245–9.
- 38.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: Report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Pharmacokinetic comparison of sublingual lorazepam with intravenous, intramuscular, and oral lorazepam. J Pharm Sci. 1982;71(2):248–252. doi: 10.1002/jps.2600710227. [DOI] [PubMed] [Google Scholar]
- 40.Treiman DM. Pharmacokinetics and clinical use of benzodiazepines in the management of status epilepticus. Epilepsia. 1989;30(Suppl 2):S4–10. doi: 10.1111/j.1528-1157.1989.tb05824.x. [DOI] [PubMed] [Google Scholar]
- 41.Ameer B, Greenblatt DJ. Lorazepam: a review of its clinical pharmacological properties and therapeutic uses. Drugs. 1981;21(3):162–200. doi: 10.2165/00003495-198121030-00001. [DOI] [PubMed] [Google Scholar]
- 42.Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89–105. doi: 10.2165/00003088-198106020-00001. [DOI] [PubMed] [Google Scholar]
- 43.Arya R, Gulati S, Kabra M, Sahu JK, Kalra V. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open-label study. Epilepsia. 2011;52(4):788–793. doi: 10.1111/j.1528-1167.2010.02949.x. [DOI] [PubMed] [Google Scholar]
- 44.Waltregny A, Dargent J. Preliminary study of parenteral lorazepam in status epilepticus. Acta Neurol Belg. 1975;75(5):219–229. [PubMed] [Google Scholar]
- 45.Walker JE, Homan RW, Vasko MR, Crawford IL, Bell RD, Tasker WG. Lorazepam in status epilepticus. Ann Neurol. 1979;6(3):207–213. doi: 10.1002/ana.410060305. [DOI] [PubMed] [Google Scholar]
- 46.Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA. 1983;249(11):1452–1454. doi: 10.1001/jama.1983.03330350028021. [DOI] [PubMed] [Google Scholar]
- 47.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339(12):792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 48.Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qureshi A, Wassmer E, Davies P, Berry K, Whitehouse WP. Comparative audit of intravenous lorazepam and diazepam in the emergency treatment of convulsive status epilepticus in children. Seizure. 2002;11(3):141–144. doi: 10.1053/seiz.2001.0635. [DOI] [PubMed] [Google Scholar]
- 50.Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for paediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311(16):1652–1660. doi: 10.1001/jama.2014.2625. [DOI] [PubMed] [Google Scholar]
- 51.Appleton R, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol. 1995;37(8):682–688. doi: 10.1111/j.1469-8749.1995.tb15014.x. [DOI] [PubMed] [Google Scholar]
- 52.Welch RD, Nicholas K, Durkalski-Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the paediatric population. Epilepsia. 2015;56(2):254–262. doi: 10.1111/epi.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez V, Lee JW, Drislane FW, Westover MB, Novy J, Dworetzky BA, et al. Practice variability and efficacy of clonazepam, lorazepam, and midazolam in status epilepticus: A multicentre comparison. Epilepsia. 2015;56(8):1275–1285. doi: 10.1111/epi.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15(3):357–365. doi: 10.1016/S0736-4679(97)00022-X. [DOI] [PubMed] [Google Scholar]
- 55.Agency EM. Buccolam: EPAR - Product Information. European Medicines Agency.
- 56.Drug Information. FDA Label & American Society of Health-System Pharmacists 2014. 2014.
- 57.Haschke M, Suter K, Hofmann S, Witschi R, Frohlich J, Imanidis G, et al. Pharmacokinetics and pharmacodynamics of nasally delivered midazolam. Br J Clin Pharmacol. 2010;69(6):607–616. doi: 10.1111/j.1365-2125.2010.03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knoester PD, Jonker DM, Van Der Hoeven RTM, Vermeij TAC, Edelbroek PM, Brekelmans GJ, et al. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol. 2002;53(5):501–507. doi: 10.1046/j.1365-2125.2002.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wermeling DP, Record KA, Archer SM, Rudy AC. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83(2–3):124–132. doi: 10.1016/j.eplepsyres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Wermeling DP, Record KA, Kelly TH, Archer SM, Clinch T, Rudy AC. Pharmacokinetics and pharmacodynamics of a new intranasal midazolam formulation in healthy volunteers. Anesth Analg. 2006;103(2):344–349. doi: 10.1213/01.ane.0000226150.90317.16. [DOI] [PubMed] [Google Scholar]
- 61.FDA Label Midazolam Injection. US Food and Drug Administration.
- 62.FDA Label NAYZILAM. US Food and Drug Administration.
- 63.Ulgey A, Aksu R, Bicer C. Nasal and buccal treatment of midazolam in epileptic seizures in paediatrics. Clin Med Insights Pediatr. 2012;6:51–60. doi: 10.4137/CMPed.S8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prescribing information for NAYZILAM. US Food and Drug Administration. 2019; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211321Orig1s000lbl.pdf Reference ID: 443479; accessed on 5 July 2020.
- 65.Freilich ER. Clinical Review NDA 211321 Nayzilam (Intranasal midazolam). Center for Drug Evaluation and Research, US Food and Drug Administration. 2017; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211321Orig1s000MedR.pdf; accessed on 28 June 2020.
- 66.Kay L, Merkel N, von Blomberg A, Willems LM, Bauer S, Reif PS, et al. Intranasal midazolam as first-line inhospital treatment for status epilepticus: a pharmaco-EEG cohort study. Ann Clin Transl Neurol. 2019;6(12):2413–2425. doi: 10.1002/acn3.50932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kay L, Reif PS, Belke M, Bauer S, Frund D, Knake S, et al. Intranasal midazolam during presurgical epilepsy monitoring is well tolerated, delays seizure recurrence, and protects from generalized tonic-clonic seizures. Epilepsia. 2015;56(9):1408–1414. doi: 10.1111/epi.13088. [DOI] [PubMed] [Google Scholar]
- 68.von Blomberg A, Kay L, Knake S, Fuest S, Zollner JP, Reif PS, et al. Efficacy, tolerability, and safety of concentrated intranasal midazolam spray as emergency medication in epilepsy patients during video-EEG monitoring. CNS Drugs. 2020;34(5):545–553. doi: 10.1007/s40263-020-00720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ulvi H, Yoldas T, Müngen B, Yigiter R. Continuous infusion of midazolam in the treatment of refractory geralized convulsive status epilepticus. Neurol Sci. 2002;23(4):177–182. doi: 10.1007/s100720200058. [DOI] [PubMed] [Google Scholar]
- 70.Prasad A, Worrall BB, Bertram EH, Bleck TP. Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia. 2001;42(3):380–386. doi: 10.1046/j.1528-1157.2001.27500.x. [DOI] [PubMed] [Google Scholar]
- 71.Singhi S, Murthy A, Singhi P, Jayashree. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17(2):106–10. [DOI] [PubMed]
- 72.Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13(2):92–94. doi: 10.1097/00006565-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Galdames-Contreras D, Carrasco-Poblete E, Aguilera-Olivares L, Fabres-Oyarzo L, Galdames-Poblete D. Intramuscular midazolam in the initial treatment of status epilepticus. Rev Neurologia. 2006;42(6):332–335. doi: 10.33588/rn.4206.2004365. [DOI] [PubMed] [Google Scholar]
- 74.Momen AA, Azizi Malamiri R, Nikkhah A, Jafari M, Fayezi A, Riahi K, et al. Efficacy and safety of intramuscular midazolam versus rectal diazepam in controlling status epilepticus in children. Eur J Paediatr Neurol. 2015;19(2):149–154. doi: 10.1016/j.ejpn.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Scott RC, Besag FMC, Neville BGR. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353(9153):623–626. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 76.Kutlu NO, Dogrul M, Yakinci C, Soylu H. Buccal midazolam for treatment of prolonged seizures in children. Brain Develop. 2003;25(4):275–278. doi: 10.1016/s0387-7604(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 77.Nakken KO, Lossius MI. Buccal midazolam or rectal diazepam for treatment of residential adult patients with serial seizures or status epilepticus. Acta Neurol Scand. 2011;124(2):99–103. doi: 10.1111/j.1600-0404.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 78.Hardmeier M, Zimmermann R, Rüegg S, Pflüger M, Deuster S, Suter K, et al. Intranasal midazolam: pharmacokinetics and pharmacodynamics assessed by quantitative EEG in healthy volunteers. Clin Pharmacol Ther. 2012;91(5):856–862. doi: 10.1038/clpt.2011.316. [DOI] [PubMed] [Google Scholar]
- 79.Detyniecki K, Van Ess PJ, Sequeira DJ, Wheless JW, Meng TC, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters—a randomized, double-blind, placebo-controlled trial. Epilepsia. 2019;60(9):1797–1808. doi: 10.1111/epi.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wheless JW, Meng TC, Van Ess PJ, Detyniecki K, Sequeira DJ, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters: An open-label extension trial. Epilepsia. 2019;60(9):1809–1819. doi: 10.1111/epi.16300. [DOI] [PubMed] [Google Scholar]
- 81.Mahmoudian T, Mohammad MZ. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5(2):253–255. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: Prospective randomised study. Brit Med J. 2000;321(7253):83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Haan GJ, Van Der Geest P, Doelman G, Bertram E, Edelbroek P. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia. 2010;51(3):478–482. doi: 10.1111/j.1528-1167.2009.02333.x. [DOI] [PubMed] [Google Scholar]
- 84.Owusu KA, Dhakar MB, Bautista C, McKimmy D, Cotugno S, Sukumar N, et al. Comparison of intranasal midazolam versus intravenous lorazepam for seizure termination and prevention of seizure clusters in the adult epilepsy monitoring unit. Epilepsy Behav. 2019;98(Pt A):161–167. doi: 10.1016/j.yebeh.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 85.Sutter R, Kaplan PW. Transnasal revolution? The promise of midazolam spray to prevent seizure clusters. CNS Drugs. 2020;34(5):555–557. doi: 10.1007/s40263-020-00724-6. [DOI] [PubMed] [Google Scholar]
- 86.Calcaterra NE, Barrow JC. Classics in chemical neuroscience: diazepam (valium) ACS Chem Neurosci. 2014;5(4):253–260. doi: 10.1021/cn5000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bojanic Z, Bojanic N, Bojanic V, Lazovic M. Drug interactions with diazepam. Acta Medica Medianae. 2011;Corpus ID: 51776045.
- 88.Leppee M, Culig J, Eric M, Sijanovic S. The effects of benzodiazepines in pregnancy. Acta Neurol Belg. 2010;110(2):163–167. [PubMed] [Google Scholar]
- 89.Full Prescribing Information VALTOCO (diazepam nasal spray): Food and Drug Agency (FDA); 2020.
- 90.Mandelli M, Tognoni G, Garattini S. Clinical pharmacokinetics of diazepam. Clin Pharmacokinet. 1978;3(1):72–91. doi: 10.2165/00003088-197803010-00005. [DOI] [PubMed] [Google Scholar]
- 91.Klotz U, Antonin KH, Bieck PR. Pharmacokinetics and plasma binding of diazepam in man, dog, rabbit, guinea pig and rat. J Pharmacol Exp Ther. 1976;199(1):67–73. [PubMed] [Google Scholar]
- 92.Sperling MR, Haas KF, Krauss G, Seif Eddeine H, Henney HR, 3rd, Rabinowicz AL, et al. Dosing feasibility and tolerability of intranasal diazepam in adults with epilepsy. Epilepsia. 2014;55(10):1544–1550. doi: 10.1111/epi.12755. [DOI] [PubMed] [Google Scholar]
- 93.Thiessen JJ, Sellers EM, Denbeigh P, Dolman L. Plasma protein binding of diazepam and tolbutamide in chronic alcoholics. J Clin Pharmacol. 1976;16(7):345–351. doi: 10.1002/j.1552-4604.1976.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 94.Vozeh S. Pharmacokinetic of benzodiazepines in old age. Schweiz Med Wochenschr. 1981;111(47):1789–1793. [PubMed] [Google Scholar]
- 95.Hooper WD, Dickinson RG, Dunstan PR, Pendlebury SC, Eadie MJ. Oxcarbazepine: preliminary clinical and pharmacokinetic studies on a new anticonvulsant. Clin Exp Neurol. 1987;24:105–112. [PubMed] [Google Scholar]
- 96.Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105(3):362–367. doi: 10.1016/j.eplepsyres.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Gizurarson S, Gudbrandsson FK, Jonsson H, Bechgaard E. Intranasal administration of diazepam aiming at the treatment of acute seizures: clinical trials in healthy volunteers. Biol Pharm Bull. 1999;22(4):425–427. doi: 10.1248/bpb.22.425. [DOI] [PubMed] [Google Scholar]
- 98.Ivaturi VD, Riss JR, Kriel RL, Siegel RA, Cloyd JC. Bioavailability and tolerability of intranasal diazepam in healthy adult volunteers. Epilepsy Res. 2009;84(2–3):120–126. doi: 10.1016/j.eplepsyres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Ivaturi VD, Riss JR, Kriel RL, Cloyd JC. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol Scand. 2009;120(5):353–357. doi: 10.1111/j.1600-0404.2009.01170.x. [DOI] [PubMed] [Google Scholar]
- 100.Garnett WR, Barr WH, Edinboro LE, Karnes HT, Mesa M, Wannarka GL. Diazepam autoinjector intramuscular delivery system versus diazepam rectal gel: a pharmacokinetic comparison. Epilepsy Res. 2011;93(1):11–16. doi: 10.1016/j.eplepsyres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Lamson MJ, Sitki-Green D, Wannarka GL, Mesa M, Andrews P, Pellock J. Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin Drug Investig. 2011;31(8):585–597. doi: 10.2165/11590250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 102.Henney HR, 3rd, Sperling MR, Rabinowicz AL, Bream G, Carrazana EJ. Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults. Epilepsy Res. 2014;108(7):1204–1211. doi: 10.1016/j.eplepsyres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Abou-Khalil B, Wheless J, Rogin J, Wolter KD, Pixton GC, Shukla RB, et al. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia. 2013;54(11):1968–1976. doi: 10.1111/epi.12373. [DOI] [PubMed] [Google Scholar]
- 104.Rogin J, Wheless J, Abou-Khalil B, Wolter KD, Pixton GC, Sherman NA, et al. Safety and effectiveness of long-term treatment with diazepam auto-injector administered by caregivers in an outpatient setting for the treatment of acute repetitive seizures. Epilepsia. 2014;55(9):1444–1451. doi: 10.1111/epi.12685. [DOI] [PubMed] [Google Scholar]
- 105.Cereghino JJ, Cloyd JC, Kuzniecky RI. North American Diastat Study G Rectal diazepam gel for treatment of acute repetitive seizures in adults. Arch Neurol. 2002;59(12):1915–1920. doi: 10.1001/archneur.59.12.1915. [DOI] [PubMed] [Google Scholar]
- 106.Pellock JM. Treatment of seizures and epilepsy in children and adolescents. Neurology. 1998;51(5 Suppl 4):S8–14. doi: 10.1212/WNL.51.5_Suppl_4.S8. [DOI] [PubMed] [Google Scholar]
- 107.Fakhoury T, Chumley A, Bensalem-Owen M. Effectiveness of diazepam rectal gel in adults with acute repetitive seizures and prolonged seizures: a single-centre experience. Epilepsy Behav. 2007;11(3):357–360. doi: 10.1016/j.yebeh.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Remy C, Jourdil N, Villemain D, Favel P, Genton P. Intrarectal diazepam in epileptic adults. Epilepsia. 1992;33(2):353–358. doi: 10.1111/j.1528-1157.1992.tb02327.x. [DOI] [PubMed] [Google Scholar]
- 109.Mitchell WG, Conry JA, Crumrine PK, Kriel RL, Cereghino JJ, Groves L, et al. An open-label study of repeated use of diazepam rectal gel (Diastat) for episodes of acute breakthrough seizures and clusters: safety, efficacy, and tolerance North American Diastat Group. Epilepsia. 1999;40(11):1610–1617. doi: 10.1111/j.1528-1157.1999.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 110.Milligan NM, Dhillon S, Griffiths A, Oxley J, Richens A. A clinical trial of single dose rectal and oral administration of diazepam for the prevention of serial seizures in adult epileptic patients. J Neurol Neurosurg Psychiatry. 1984;47(3):235–240. doi: 10.1136/jnnp.47.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith BT, Masotti RE. Intravenous diazepam in the treatment of prolonged seizure activity in neonates and infants. Dev Med Child Neurol. 1971;13(5):630–634. doi: 10.1111/j.1469-8749.1971.tb08328.x. [DOI] [PubMed] [Google Scholar]
- 112.Kriel RL, Cloyd JC, Pellock JM, Mitchell WG, Cereghino JJ, Rosman NP. Rectal diazepam gel for treatment of acute repetitive seizures. The North American Diastat Study Group. Pediatr Neurol. 1999;20(4):282–288. doi: 10.1016/S0887-8994(98)00156-8. [DOI] [PubMed] [Google Scholar]
- 113.Singhi S, Banerjee S, Singhi P. Refractory status epilepticus in children: role of continuous diazepam infusion. J Child Neurol. 1998;13(1):23–26. doi: 10.1177/088307389801300104. [DOI] [PubMed] [Google Scholar]
- 114.Henriksen PB. Acute treatment of epileptic seizures with diazepamum (Valium) Acta Neurol Scand. 1967;43(S31):168–169. doi: 10.1111/j.1600-0404.1967.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 115.Hoppu K, Santavuori P. Diazepam rectal solution for home treatment of acute seizures in children. Acta Paediatr Scand. 1981;70(3):369–372. doi: 10.1111/j.1651-2227.1981.tb16565.x. [DOI] [PubMed] [Google Scholar]
- 116.Chen WB, Gao R, Su YY, Zhao JW, Zhang YZ, Wang L, et al. Valproate versus diazepam for generalized convulsive status epilepticus: a pilot study. Eur J Neurol. 2011;18(12):1391–1396. doi: 10.1111/j.1468-1331.2011.03420.x. [DOI] [PubMed] [Google Scholar]
- 117.Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: a randomized controlled trial. J Child Neurol. 2007;22(10):1191–1197. doi: 10.1177/0883073807306248. [DOI] [PubMed] [Google Scholar]
- 118.Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17(2):106–110. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 119.Tang RH, Zhou JB. A control study on the treatment of acute seizures with midazolam and diazepam in children. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12(7):530–532. [PubMed] [Google Scholar]
- 120.Gathwala G, Goel M, Singh J, Mittal K. Intravenous diazepam, midazolam and lorazepam in acute seizure control. Indian J Pediatr. 2012;79(3):327–332. doi: 10.1007/s12098-011-0505-y. [DOI] [PubMed] [Google Scholar]
- 121.Sreenath TG, Gupta P, Sharma KK, Krishnamurthy S. Lorazepam versus diazepam-phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. Eur J Paediatr Neurol. 2010;14(2):162–168. doi: 10.1016/j.ejpn.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 122.Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38(2):202–207. doi: 10.1212/WNL.38.2.202. [DOI] [PubMed] [Google Scholar]
- 123.Shah I, Deshmukh CT. Intramuscular midazolam vs intravenous diazepam for acute seizures. Indian J Pediatr. 2005;72(8):667–670. doi: 10.1007/BF02724074. [DOI] [PubMed] [Google Scholar]
- 124.Portela JL, Garcia PC, Piva JP, Barcelos A, Bruno F, Branco R, et al. Intramuscular midazolam versus intravenous diazepam for treatment of seizures in the paediatric emergency department: a randomized clinical trial. Med Intensiva. 2015;39(3):160–166. doi: 10.1016/j.medin.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5(2):253–255. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. 2012;22(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 128.Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev. 2009;31(10):744–749. doi: 10.1016/j.braindev.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 129.Tonekaboni SH, Shamsabadi FM, Anvari SS, Mazrooei A, Ghofrani M. A comparison of buccal midazolam and intravenous diazepam for the acute treatment of seizures in children. Iran J Pediatr. 2012;22(3):303–308. [PMC free article] [PubMed] [Google Scholar]
- 130.Mittal P, Manohar R, Rawat AK. Comparative study of intranasal midazolam and intravenous diazepam sedation for procedures and seizures. Indian J Pediatr. 2006;73(11):975–978. doi: 10.1007/BF02758299. [DOI] [PubMed] [Google Scholar]
- 131.Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121(1):e58–64. doi: 10.1542/peds.2007-0930. [DOI] [PubMed] [Google Scholar]
- 132.Ashrafi MR, Khosroshahi N, Karimi P, Malamiri RA, Bavarian B, Zarch AV, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. 2010;14(5):434–438. doi: 10.1016/j.ejpn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 133.Baysun S, Aydin OF, Atmaca E, Gurer YK. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr (Phila). 2005;44(9):771–776. doi: 10.1177/000992280504400904. [DOI] [PubMed] [Google Scholar]
- 134.Fisgin T, Gurer Y, Tezic T, Senbil N, Zorlu P, Okuyaz C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17(2):123–126. doi: 10.1177/088307380201700206. [DOI] [PubMed] [Google Scholar]
- 135.Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34(5):355–359. doi: 10.1016/j.pediatrneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 136.Trajano M, Sanchez B, Lukban M, Salonga A. PO18WE40 Buccal midazolam compared to rectal diazepam and intravenous midazolam as emergent treatment of acute seizures in children: preliminary study. J Neurol Sci. 2009;S255.
- 137.Malu CK, Kahamba DM, Walker TD, Mukampunga C, Musalu EM, Kokolomani J, et al. Efficacy of sublingual lorazepam versus intrarectal diazepam for prolonged convulsions in Sub-Saharan Africa. J Child Neurol. 2014;29(7):895–902. doi: 10.1177/0883073813493501. [DOI] [PubMed] [Google Scholar]
- 138.Sirsi D. Is intranasal midazolam better than rectal diazepam for home management of acute seizures? Arch Neurol. 2011;68(1):120–121. doi: 10.1001/archneurol.2010.337. [DOI] [PubMed] [Google Scholar]
- 139.Chamberlain DB, Chamberlain JM. Making sense of a negative clinical trial result: a bayesian analysis of a clinical trial of lorazepam and diazepam for pediatric status epilepticus. Ann Emerg Med. 2017;69(1):117–124. doi: 10.1016/j.annemergmed.2016.08.449. [DOI] [PubMed] [Google Scholar]
- 140.Meierkord H, Boon P, Engelsen B, Gocke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 141.Congdon PJ, Forsythe WI. Intravenous clonazepam in the treatment of status epilepticus in children. Epilepsia. 1980;21(1):97–102. doi: 10.1111/j.1528-1157.1980.tb04049.x. [DOI] [PubMed] [Google Scholar]
- 142.Gastaut H, Courjon J, Poire R, Weber M. Treatment of status epilepticus with a new benzodiazepine more active than diazepam. Epilepsia. 1971;12(3):197–214. doi: 10.1111/j.1528-1157.1971.tb04928.x. [DOI] [PubMed] [Google Scholar]
- 143.Navarro V, Dagron C, Elie C, Lamhaut L, Demeret S, Urien S, et al. Prehospital treatment with levetiracetam plus clonazepam or placebo plus clonazepam in status epilepticus (SAMUKeppra): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2016;15(1):47–55. doi: 10.1016/S1474-4422(15)00296-3. [DOI] [PubMed] [Google Scholar]
- 144.Pinder RM, Brogden RN, Speight TM, Avery GS. Clonazepam: a review of its pharmacological properties and therapeutic efficacy in epilepsy. Drugs. 1976;12(5):321–361. doi: 10.2165/00003495-197612050-00001. [DOI] [PubMed] [Google Scholar]
- 145.Rantsch K, Walter U, Wittstock M, Benecke R, Rosche J. Treatment and course of different subtypes of status epilepticus. Epilepsy Res. 2013;107(1–2):156–162. doi: 10.1016/j.eplepsyres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 146.Singh AN, Le Morvan P. Treatment of status epilepticus with intravenous clonazepam. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6(4–6):539–542. doi: 10.1016/S0278-5846(82)80146-2. [DOI] [PubMed] [Google Scholar]
- 147.Sorel L, Mechler L, Harmant J. Comparative trial of intravenous lorazepam and clonazepam im status epilepticus. Clin Ther. 1981;4(4):326–336. [PubMed] [Google Scholar]
- 148.Troester MM, Hastriter EV, Ng YT. Dissolving oral clonazepam wafers in the acute treatment of prolonged seizures. J Child Neurol. 2010;25(12):1468–1472. doi: 10.1177/0883073810368312. [DOI] [PubMed] [Google Scholar]
- 149.Abdel-Bar HM, Abdel-Reheem AY, Awad GA, Mortada ND. Evaluation of brain targeting and mucosal integrity of nasally administrated nanostructured carriers of a CNS active drug, clonazepam. J Pharm Pharm Sci. 2013;16(3):456–469. doi: 10.18433/J30S31. [DOI] [PubMed] [Google Scholar]
- 150.Rylance GW, Poulton J, Cherry RC, Cullen RE. Plasma concentrations of clonazepam after single rectal administration. Arch Dis Child. 1986;61(2):186–188. doi: 10.1136/adc.61.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Crevoisier C, Delisle MC, Joseph I, Foletti G. Comparative single-dose pharmacokinetics of clonazepam following intravenous, intramuscular and oral administration to healthy volunteers. Eur Neurol. 2003;49(3):173–177. doi: 10.1159/000069089. [DOI] [PubMed] [Google Scholar]
- 152.Sakata O, Onishi H, Machida Y. Clonazepam oral droplets for the treatment of acute epileptic seizures. Drug Dev Ind Pharm. 2008;34(12):1376–1380. doi: 10.1080/03639040802122977. [DOI] [PubMed] [Google Scholar]
- 153.Greenblatt DJ, Blaskovich PD, Nuwayser ES, Harmatz JS, Chen G, Zinny MA. Clonazepam pharmacokinetics: comparison of subcutaneous microsphere injection with multiple-dose oral administration. J Clin Pharmacol. 2005;45(11):1288–1293. doi: 10.1177/0091270005280861. [DOI] [PubMed] [Google Scholar]
- 154.Roche. KLONOPIN TABLETS (clonazepam), KLONOPIN WAFERS (clonazepam orally desintegrating tablets). https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/017533s045,020813s005lbl.pdf.
- 155.Seree EJ, Pisano PJ, Placidi M, Rahmani R, Barra YA. Identification of the human and animal hepatic cytochromes P450 involved in clonazepam metabolism. Fundam Clin Pharmacol. 1993;7(2):69–75. doi: 10.1111/j.1472-8206.1993.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 156.Greenblatt DJ, Ehrenberg BL, Gunderman J, Locniskar A, Scavone JM, Harmatz JS, et al. Pharmacokinetic and electroencephalographic study of intravenous diazepam, midazolam, and placebo. Clin Pharmacol Ther. 1989;45(4):356–365. doi: 10.1038/clpt.1989.41. [DOI] [PubMed] [Google Scholar]
- 157.Greenblatt DJ, Ehrenberg BL, Gunderman J, Scavone JM, Tai NT, Harmatz JS, et al. Kinetic and dynamic study of intravenous lorazepam: comparison with intravenous diazepam. J Pharmacol Exp Ther. 1989;250(1):134–140. [PubMed] [Google Scholar]
- 158.Greenblatt DJ, Sethy VH. Benzodiazepine concentrations in brain directly reflect receptor occupancy: studies of diazepam, lorazepam, and oxazepam. Psychopharmacology. 1990;102(3):373–378. doi: 10.1007/BF02244106. [DOI] [PubMed] [Google Scholar]
- 159.Semmlack S, Tschudin-Sutter S, Widmer AF, Valenca M, Ruegg S, Marsch S, et al. Independent impact of infections on the course and outcome of status epilepticus: a 10-year cohort study. J Neurol. 2016;263(7):1303–1313. doi: 10.1007/s00415-016-8140-1. [DOI] [PubMed] [Google Scholar]
- 160.Sutter R, Tschudin-Sutter S, Grize L, Fuhr P, Bonten MJ, Widmer AF, et al. Associations between infections and clinical outcome parameters in status epilepticus: a retrospective 5-year cohort study. Epilepsia. 2012;53(9):1489–1497. doi: 10.1111/j.1528-1167.2012.03576.x. [DOI] [PubMed] [Google Scholar]
- 161.Guterman EL, Betjemann JP, Aimetti A, Li JW, Wang Z, Yin D, et al. Association between treatment progression, disease refractoriness, and burden of illness among hospitalized patients with status epilepticus. JAMA Neurol. 2021;78(5):588–595. doi: 10.1001/jamaneurol.2021.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479–489. doi: 10.1007/s40263-013-0075-9. [DOI] [PubMed] [Google Scholar]
- 163.Grosset KA, Malek N, Morgan F, Grosset DG. Inhaled apomorphine in patients with 'on-off' fluctuations: a randomized, double-blind, placebo-controlled, clinic and home based, parallel-group study. J Parkinsons Dis. 2013;3(1):31–37. doi: 10.3233/JPD-120142. [DOI] [PubMed] [Google Scholar]
- 164.French JA, Wechsler R, Gelfand MA, Pollard JR, Vazquez B, Friedman D, et al. Inhaled alprazolam rapidly suppresses epileptic activity in photosensitive participants. Epilepsia. 2019;60(8):1602–1609. doi: 10.1111/epi.16279. [DOI] [PubMed] [Google Scholar]
- 165.Xi LY, Zheng WM, Zhen SM, Xian NS. Rapid arrest of seizures with an inhalation aerosol containing diazepam. Epilepsia. 1994;35(2):356–358. doi: 10.1111/j.1528-1157.1994.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 166.Dhir A, Zolkowska D, Murphy RB, Rogawski MA. Seizure protection by intrapulmonary delivery of propofol hemisuccinate. J Pharmacol Exp Ther. 2011;336(1):215–222. doi: 10.1124/jpet.110.173591. [DOI] [PubMed] [Google Scholar]
- 167.Dhir A, Zolkowska D, Rogawski MA. Seizure protection by intrapulmonary delivery of midazolam in mice. Neuropharmacology. 2013;73:425–431. doi: 10.1016/j.neuropharm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 168.Ait-Daoud N, Hamby AS, Sharma S, Blevins D. A review of alprazolam use, misuse, and withdrawal. J Addict Med. 2018;12(1):4–10. doi: 10.1097/ADM.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith RB, Kroboth PD, Vanderlugt JT, Phillips JP, Juhl RP. Pharmacokinetics and pharmacodynamics of alprazolam after oral and IV administration. Psychopharmacology. 1984;84(4):452–456. doi: 10.1007/BF00431449. [DOI] [PubMed] [Google Scholar]
- 170.Huybrechts I. The pharmacology of alprazolam: a review. Clin Ther. 1991;13(1):100–117. [PubMed] [Google Scholar]
- 171.FDA. XANAX® - FDA label. Reference ID: 3004871: Food And Drug Administration (FDA); 2011.
- 172.Anderson GD. Children versus adults: pharmacokinetic and adverse-effect differences. Epilepsia. 2002;43(Suppl 3):53–59. doi: 10.1046/j.1528-1157.43.s.3.5.x. [DOI] [PubMed] [Google Scholar]
- 173.Morselli PL, Principi N, Tognoni G, Reali E, Belvedere G, Standen SM, et al. Diazepam elimination in premature and full term infants, and children. J Perinat Med. 1973;1(2):133–141. doi: 10.1515/jpme.1973.1.2.133. [DOI] [PubMed] [Google Scholar]
- 174.Blumer JL. Clinical pharmacology of midazolam in infants and children. Clin Pharmacokinet. 1998;35(1):37–47. doi: 10.2165/00003088-199835010-00003. [DOI] [PubMed] [Google Scholar]
- 175.Gonzalez D, Chamberlain JM, Guptill JT, Cohen-Wolkowiez M, Harper B, Zhao J, et al. Population pharmacokinetics and exploratory pharmacodynamics of lorazepam in pediatric status epilepticus. Clin Pharmacokinet. 2017;56(8):941–951. doi: 10.1007/s40262-016-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chamberlain JM, Capparelli EV, Brown KM, Vance CW, Lillis K, Mahajan P, et al. Pharmacokinetics of intravenous lorazepam in paediatric patients with and without status epilepticus. J Pediatr. 2012;160(4):667–672. doi: 10.1016/j.jpeds.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.European Medicines Agency. European Medicines Agency Committee for Medicinal Products for Human Use assessment report for Buccolam® (buccal midazolam); 2011.
- 178.Garnock-Jones KP. Oromucosal midazolam: a review of its use in paediatric patients with prolonged acute convulsive seizures. Paediatr Drugs. 2012;14(4):251–261. doi: 10.2165/11209320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 179.U.S. Food & Drug Administration. FDA-approved drugs - diazepam. 2021 [cited; Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=020648
- 180.Rogalski R, Rogalski A. Benzodiazepine selection in the management of status epilepticus: a review. Adv Emerg Nurs J. 2015;37(2):83–94. doi: 10.1097/TME.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 181.Trinka E, Hofler J, Leitinger M, Brigo F. Pharmacotherapy for status epilepticus. Drugs. 2015;75(13):1499–1521. doi: 10.1007/s40265-015-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Der-Nigoghossian C, Rubinos C, Alkhachroum A, Claassen J. Status epilepticus—time is brain and treatment considerations. Curr Opin Crit Care. 2019;25(6):638–646. doi: 10.1097/MCC.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41(11):1225–1231. doi: 10.1177/00912700122012779. [DOI] [PubMed] [Google Scholar]
- 184.Lau CE, Ma F, Wang Y, Smith C. Pharmacokinetics and bioavailability of midazolam after intravenous, subcutaneous, intraperitoneal and oral administration under a chronic food-limited regimen: relating DRL performance to pharmacokinetics. Psychopharmacology. 1996;126(3):241–248. doi: 10.1007/BF02246454. [DOI] [PubMed] [Google Scholar]
- 185.Shangguan Y, Liao H, Wang X. Clonazepam in the treatment of status epilepticus. Expert Rev Neurother. 2015;15(7):733–740. doi: 10.1586/14737175.2015.1056781. [DOI] [PubMed] [Google Scholar]
- 186.Hogan RE, Gidal BE, Koplowitz B, Koplowitz LP, Lowenthal RE, Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455–464. doi: 10.1111/epi.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet. 2006;367(9522):1591–1597. doi: 10.1016/S0140-6736(06)68696-0. [DOI] [PubMed] [Google Scholar]
- 188.Jeannet PY, Roulet E, Maeder-Ingvar M, Gehri M, Jutzi A, Deonna T. Home and hospital treatment of acute seizures in children with nasal midazolam. Eur J Paediatr Neurol. 1999;3(2):73–77. doi: 10.1016/S1090-3798(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 189.Kazmi A, Abbas G, Khurshid A, Sahr S, Malhi T, Hanif M, et al. A comparison of intravenous midazolam and diazepam in management of status epilepticus in children. J Pak Med Assoc. 2020;2:1–13. doi: 10.47391/JPMA.843. [DOI] [PubMed] [Google Scholar]
- 190.Kutlu NO, Dogrul M, Yakinci C, Soylu H. Buccal midazolam for treatment of prolonged seizures in children. Brain Dev. 2003;25(4):275–278. doi: 10.1016/s0387-7604(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 191.Griffith PA, Karp HR. Lorazepam in therapy for status epilepticus. Ann Neurol. 1980;7(5):493. doi: 10.1002/ana.410070521. [DOI] [PubMed] [Google Scholar]
- 192.Lacey DJ, Singer WD, Horwitz SJ, Gilmore H. Lorazepam therapy of status epilepticus in children and adolescents. J Pediatr. 1986;108(5 Pt 1):771–774. doi: 10.1016/S0022-3476(86)81065-4. [DOI] [PubMed] [Google Scholar]
- 193.Crawford TO, Mitchell WG, Snodgrass SR. Lorazepam in childhood status epilepticus and serial seizures: effectiveness and tachyphylaxis. Neurology. 1987;37(2):190–195. doi: 10.1212/WNL.37.2.190. [DOI] [PubMed] [Google Scholar]
- 194.Giang DW, McBride MC. Lorazepam versus diazepam for the treatment of status epilepticus. Pediatr Neurol. 1988;4(6):358–361. doi: 10.1016/0887-8994(88)90083-5. [DOI] [PubMed] [Google Scholar]
- 195.Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;259(4):645–648. doi: 10.1007/s00415-011-6227-2. [DOI] [PubMed] [Google Scholar]
- 196.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7(8):696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Detyniecki K, Van Ess PJ, Sequeira DJ, Wheless JW, Meng TC, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters-a randomized, double-blind, placebo-controlled trial. Epilepsia. 2019;60(9):1797–1808. doi: 10.1111/epi.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Acharya S, Adamova D, Adler A, Adolfsson J, Aglieri Rinella G, Agnello M, et al. Measurement of the cross sections of Xi_{c}^{0} and Xi_{c}^{+} baryons and of the branching-fraction ratio BR(Xi_{c}^{0}–>Xi^{-}e^{+}nu_{e})/BR(Xi_{c}^{0}–>Xi^{-}pi^{+}) in pp collisions at sqrt[s]=13 TeV. Phys Rev Lett. 2021;127(27):272001. doi: 10.1103/PhysRevLett.127.272001. [DOI] [PubMed] [Google Scholar]
- 199.Bergamini L, Mutani R, Fariello R, Liboni W, Quattrocolo GL. Action du Ro 5 ⁄ 4023 dans le traitement des differentes formes d epilepsie. Paper given at the Rivotril Symposium, Marseilles, France. 1972.
- 200.Bergamini L, Mutani R, Liboni W. Elektroenzephalographische und klinische Bewertung des neuen Benzodiazepin Ro 5 ⁄ 4023. EEG EMG. 1970;1:182–188. [Google Scholar]
- 201.Gimenez-Roldan S, Lopez Agreda J, Martin F. Un nuevo medicamento eficaz en el trataimento del status epilepticus - Ro 5-4023. Med Clin (Barc). 1972;58(133).
- 202.Beck H, Tousche C. Traitement des etats de mal epileptiques par le clonazepam Sem Hop Paris. 1973;49(21).
- 203.Bladin PF. The use of clonazepam as an anticonvulsant–clinical evaluation. Med J Aust. 1973;1(14):683–688. doi: 10.5694/j.1326-5377.1973.tb110623.x. [DOI] [PubMed] [Google Scholar]
- 204.Ketz E, Bernoulli C, Siegfried J. Clinical and electroencephalographic trial with clonazepam (Ro 5–4023) with special regard to status epilepticus. Acta Neurol Scand Suppl. 1973;53:47–53. [PubMed] [Google Scholar]
- 205.Kruse R, Blankenhorn V. Clinical use and effect of Ro 5–4023 (clonazepam) in different forms of epileptic seizures. Acta Neurol Scand Suppl. 1973;53:60–71. [PubMed] [Google Scholar]
- 206.Martin D, Hirt H. Clinical experience with clonazepam (Rivotril) in the treatment of epilepsies in infancy and childhood. Neuropaediatrics. 1973;4:245–266. doi: 10.1055/s-0028-1091744. [DOI] [PubMed] [Google Scholar]
- 207.Tridon P, Weber M. Conduite du traitement des etats de mal epileptiques par le Ro. Sem Hop Paris. 1973;49:29. [Google Scholar]