Abstract
We investigated inter-arm systolic blood pressure (sIAD) difference, reproducibility, and incident cardiovascular disease (CVD). We hypothesized that higher sIAD values have low prevalence and nonpersistence over years, but that CVD risk is higher starting from the time of first high absolute sIAD. In Multi-Ethnic Study of Atherosclerosis participants (n=6725, 53% female, 45–84 years old), Doppler systolic blood pressure (SBP) measurements were made in both arms (10-minute interval) thrice over 9.5 years. Proportional hazards for CVD (coronary heart disease, heart failure, stroke, peripheral arterial disease (PAD)) over 16.4 years were tested according to time-varying absolute inter-arm difference with covariates: 1) age, gender, race, and clinic; 2) model 1 plus height, heart rate, BP, antihypertensives, BMI, smoking status, lipids, lipid lowering medication, and diabetes. High sIAD was not persistent across exams. Maximum absolute sIAD ≥15 mmHg was found at least once in 815 persons. Maximum absolute sIAD had a graded relationship with incident stroke or PAD: 6.2% events; model 2 hazard ratio per 10 mmHg 1.34 (95% CI, 1.15–1.56) and this risk was approximately doubled for maximum absolute sIAD ≥15 mmHg vs 0–4 mmHg. Total CVD risk (18.4% events) was increased only for maximum absolute sIAD ≥25 mmHg. Associations with incident CVD did not differ for higher SBP in left vs right arm. A higher maximum absolute sIAD at any exam was associated with greater risk for stroke and PAD especially for values ≥15mmHg, and ≥25mmHg for other CVD. Measuring SBP between arms may help identify individuals at risk for CVD.
Keywords: inter-arm systolic blood pressure difference, MESA, cardiovascular disease, peripheral artery disease, stroke
Introduction
A large absolute inter-arm systolic blood pressure difference (sIAD) is relatively common in the general population [1–3]. As early as 1960, there was general acceptance that large inter-arm blood pressure (BP) difference was the result of significant stenosis in a major conduit artery in one of the upper extremities. The fact that this BP difference occurred somewhat commonly in healthy individuals rendered the stenosis explanation unlikely. As such, these early investigators warned that inappropriate interpretation of sIAD could lead to incorrect further evaluation and treatment [4].
The concept that a fixed stenosis is the cause of high sIAD has not received much study. If a large sIAD is caused by a fixed defect, such as subclavian artery stenosis, it would be expected to be reproducible between visits and the defect would be able to be visualized in people with high sIAD. Only a few studies have addressed reproducibility of large sIAD. In Kim et al. [5], where the prevalence of absolute sIAD ≥10 mmHg was 7.6% at baseline and 7.1% at a 3-month follow-up, only 21.8% of the patients had a repeat absolute sIAD ≥10 mmHg at 3 months. Moreover, Grossmann et al. [6] studied near term (2–7 days) reproducibility of absolute sIAD in 319 hospitalized patients of average age 85 years and found that sIAD > 10 mmHg occurred in 23%, but was repeated in only 38% of them. In 147 patients from a hypertension clinic, inter-arm difference was assessed simultaneously and repeatedly in left and right arms [7]. A study in patients with diabetes also found poor reproducibility. Kleefstra et al. [8] studied people with diabetes in repeat measures over 1 year. In 169 patients with either systolic or diastolic interarm blood pressure difference > 10 mmHg at baseline, the difference was <10 mmHg 1 year later in 79 % of these patients. The interarm difference was reproducible only in 2 patients who had known obstructive disease. However, visualization of the presumed fixed defect is rare. Huibers et al. [9] studied 182 patients with carotid artery stenosis and found that an absolute sIAD >15 mmHg occurred in 21% (n=39). Among those, only 18% (n=7) had a hemodynamically significant (>50%) ipsilateral stenosis in the subclavian or innominate artery. Thus, while a subset of people have both high sIAD and ipsilateral stenosis, a high sIAD may be rare and poorly reproducible in the general population, but more common in clinical patients. Nevertheless, the literature is clear that large sIAD predicts future CVD events. Clark et al. led a meta-analysis of absolute sIAD using 24 studies and 53,827 participants and found a continuous increase in CVD risk for higher absolute sIAD [10–18].
In the present paper, we used measurements at baseline and after median intervals of 3.2 and 9.5 years in the Multi-Ethnic Study of Atherosclerosis (MESA) to study reproducibility of high absolute sIAD at various cutoff values. We hypothesized that higher values of sIAD have low prevalence and do not persist over years, but that CVD risk is higher starting from the time at which high absolute sIAD occurred.
Materials and Methods
MESA investigates the prevalence, correlates, and progression of subclinical CVD in a multi-ethnic, population-based sample of 6814 men and women aged 45–84 years, free of clinical cardiovascular diagnoses at baseline [19]. Participants were enrolled and initially examined from 2000–2002 at six U.S. field centers: Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN. The study included 53% women and four ethnic groups: 38% non-Hispanic White, 28% African American, 22% Hispanic, 12% Chinese American. We included 6725 participants with sIAD ever assessed at exam 1, 3, or 5, of whom 6609 had a sIAD assessment at exam 1. The Institutional Review Boards at all centers approved the study and participants gave informed consent.
Brachial Systolic Blood Pressure Measurement
sIAD was computed from a sequence of measurements of arm and ankle blood pressures, taken over 12 minutes, as part of studies of peripheral artery disease (PAD), separately from the protocol for sitting, resting blood pressure. For detecting PAD, systolic blood pressure (SBP) was measured using a hand-held Doppler instrument with a 5-mHz probe in the following sequence: right side brachial, dorsalis pedis, and posterior tibial arteries and then left side dorsalis pedis, posterior tibial and brachial arteries. The cuff was slowly inflated to 20 mmHg above the pressure at which the pulse sound disappeared. The cuff was deflated slowly allowing the pressure to drop at a rate of 2 mmHg per second. The recorded pressure at which the first sustained (more than one beat) pulse reappeared was the systolic pressure at this location. The technician waited for 20 seconds between measurements, so that the left brachial artery pressure was assessed 10 minutes after the right side. The above measurements were performed at MESA study exam 1 (between 2000 and 2002), exam 3 (between 2003 and 2005, median 3.2 years after exam 1), and exam 5 (between 2010 and 2012, median 9.5 years after exam Median follow-up for surviving participants with no CVD event was 16.1 years.
For this study only the right and left brachial artery SBP were utilized. The difference of right minus left systolic blood pressure was then calculated to estimate sIAD, with positive values indicating that the right side pressure was higher than the left side. Unless otherwise explicitly stated, we analyzed the absolute (unsigned) value of sIAD (ignoring sidedness).
Events
Participants were followed for death and incident CVD events for a mean 16 years from the baseline examination. In addition to the in-person follow-up MESA study examinations a telephone interviewer contacted each participant every 9 to 12 months to inquire about all interim hospital admissions, CVD outpatient diagnoses, and deaths events and incidence dates were adjudicated by 2 blinded physicians from the MESA study events committee using pre-specified criteria. CVD events in MESA included myocardial infarction, resuscitated cardiac arrest, angina pectoris, stroke, transient ischemic attack, heart failure (HF), peripheral arterial disease (PAD), and CV death. All deaths were identified. For potential CVD deaths, cause was assigned committee review. Adjudication procedures in MESA have been previously published and through can be found in the MESA Protocol at https://www.mesa-nhlbi.org.
Statistical Analysis
Cross-sectional covariate characteristics according to exam 1 absolute sIAD in 6 categories were presented (n=6609). Reproducibility of high absolute sIAD was assessed among the subsets of participants with measurements at multiple exams by forming a cross table of exam 1 with exam 3 absolute sIAD in 5 mmHg categories (n=5816); then by tabulating the numbers of occasions at exams 3 and 5 in which absolute sIAD was at least 10, 15, 20, and 25 mmHg, according to absolute sIAD in 5 mmHg categories at exam 1 (n=4337). The entire Doppler measurement procedure was repeated on the same day at exam 3 (n=302). Using these data, correlations in sIAD within and between exams was assessed.
We also examined whether MESA sIAD data were consistent with the literature in finding that high absolute sIAD predicts CVD events. We examined unadjusted associations of absolute sIAD in 5 mmHg categories with incident events according to exam 1 category. Covariates in the baseline sIAD analysis in model 1 included exam 1 age, sex, race/ethnicity, and clinical site and in model 2 added height, heart rate and oscillometric systolic and diastolic blood pressures at sitting rest, antihypertensive medication use, body mass index, cigarette smoking, total cholesterol, HDL-C, triglycerides, lipid lowering statin medication, and diabetes assessed as previously described [20].
Although sIAD associations with each outcome were generally positive, numbers of events in the specific CVD subtypes were limited. Given the low level of reproducibility of sIAD, we further hypothesized that whenever high sIAD occurs, it is associated with future CVD. We formulated two approaches to prediction of future CVD from time-varying sIAD: replacing the previous sIAD value with the maximum observed to that time (time-varying maximum sIAD), or replacing the previous sIAD value with the mean to that time, in both cases, we carried forward the previous value when sIAD was missing (time-varying current sIAD). To illustrate, suppose absolute sIAD values at exams 1, 3, and 5 are 5, 20, and 8 mmHg. The individual would contribute to prediction of CVD events with absolute sIAD = 5 mmHg from exam 1 until exam 3, with a value of 20 mmHg from exam 3 until exam 5, and with a value of 20 mmHg from exam 5 until the end of followup. In the time-varying current sIAD approach, the individual with the same values would contribute to prediction with 5, 12.5 (mean of 5 and 20), 11 (mean of 5, 20, and 8) mmHg, respectively. Both of these analyses may suffer from bias of early disease that prevented clinic attendance for remeasurement. We evaluated this potential bias by examining a) the distribution of the characteristic “both exam 3 and exam 5 sIAD missing” according to maximum sIAD category and b) the event rates for those who had a measurement at either exam 3 or exam 5 compared to the event rates in those missing measurements at both exams.
In time-varying proportional hazards regression, time-varying continuous covariates were updated with the mean of the current and any prior values for the continuous variables, the two medication values were updated at exam 3 and at exam 5 to “ever user” at the first exam where use was indicated, and diabetes was updated to “ever diagnosed” at the first exam at which it was identified.
Given the suggestion in our data that the specific arm did not play an important role in our findings and that the sIAD over a 10 minute interval is a measure of BP variability, we also examined within person standard deviation between the second and third sitting rest oscillometric SBP values. Note that the standard deviation of two measures is the absolute difference of the measures, divided by √2. We examined prediction of CVD events using the time-varying maximum model. These measurements were taken in the same arm (usually the right) at a 1 minute interval. Our secondary hypothesis was that the time-varying maximum within person standard deviation of SBP would show a positive association with stroke, PAD, and extracoronary CVD, but weaker than for the sIAD performed with a 10 minute interval.
Analyses were performed in R and in SAS. P<0.05 (two-sided tests, without adjustment for multiple comparisons) was considered to be statistically significant.
Results
Distribution of sIAD
In each exam, the distributions of sIAD were similar, whether the higher SBP was on the left or the right side (data not shown). At exam 1, about 10–15% had absolute sIAD 10–14 mmHg, while about 6% had a sIAD ≥ 15 mmHg (Table 1) and similarly at each exam (data not shown). The maximum absolute sIAD across exams was substantially more variable than at any specific exam: 10–14 mmHg in about 25% of the participants, and ≥ 15 mmHg in about 12%.
Table 1.
Exam 1 characteristics by exam 1 absolute inter-arm systolic blood pressure difference (sIAD) category.
| All | 0–4 mmHg | 5–9 mmHg | 10–14 mmHg | 15–19 mmHg | 20–24 mmHg | ≥25 mmHg | |
|---|---|---|---|---|---|---|---|
| Sample size | 6609 | 3962 | 1567 | 787 | 154 | 95 | 44 |
| Exam 1 characteristic | |||||||
| Age (yr) | 62.1±10.2 | 61.5±10.3 | 62.5±10.2 | 63.3±9.8 | 63.8±10.1 | 64.2±9.3 | 66.2±9.2 |
| Female (%) | 3465 (52.4) | 2048 (51.7) | 816 (52.1) | 428 (54.4) | 85 (55.2) | 63 (66.3) | 25 (56.8) |
| Race/Ethnicity (%) | |||||||
| Black | 1814 (27.4) | 1000 (25.2) | 430 (27.4) | 252 (32) | 68 (44.2) | 50 (52.6) | 14 (31.8) |
| Chinese | 793 (12) | 583 (14.7) | 158 (10.1) | 44 (5.6) | 6 (3.9) | 2 (2.1) | 0 (0) |
| Hispanic | 1471 (22.3) | 983 (24.8) | 329 (21) | 132 (16.8) | 13 (8.4) | 12 (12.6) | 2 (4.5) |
| White | 2531 (38.3) | 1396 (35.2) | 650 (41.5) | 359 (45.6) | 67 (43.5) | 31 (32.6) | 28 (63.6) |
| Clinic (%) | |||||||
| Columbia | 1075 (16.3) | 649 (16.4) | 243 (15.5) | 144 (18.3) | 25 (16.2) | 11 (11.6) | 3 (6.8) |
| JHU | 1020 (15.4) | 520 (13.1) | 280 (17.9) | 148 (18.8) | 31 (20.1) | 30 (31.6) | 11 (25) |
| Minnesota | 1035 (15.7) | 652 (16.5) | 271 (17.3) | 91 (11.6) | 13 (8.4) | 7 (7.4) | 1 (2.3) |
| NWU | 1134 (17.2) | 641 (16.2) | 281 (17.9) | 161 (20.5) | 27 (17.5) | 18 (18.9) | 6 (13.6) |
| UCLA | 1302 (19.7) | 1008 (25.4) | 257 (16.4) | 33 (4.2) | 1 (0.6) | 2 (2.1) | 1 (2.3) |
| WFU | 1043 (15.8) | 492 (12.4) | 235 (15) | 210 (26.7) | 57 (37) | 27 (28.4) | 22 (50) |
| Height (cm) | 166.4±10 | 166.3±10.1 | 166.5±9.7 | 167.1±10.1 | 167.2±10.6 | 164±9.5 | 167.9±9.8 |
| Heart Rate (Beats/Min) | 63.1±9.6 | 63.1±9.6 | 63.1±9.7 | 63.5±9.4 | 62.2±9.4 | 63±9.3 | 61±12.8 |
| Seated Systolic Blood Pressure (mmHg) | 126.6±21.5 | 125±21.1 | 127.4±21.6 | 130.3±21.5 | 132.9±23.7 | 134.6±19.9 | 134.3±26.1 |
| Seated Diastolic Blood Pressure (mmHg) | 72±10.3 | 71.7±10 | 72±10.2 | 72.8±10.9 | 73±11.6 | 72.8±12.5 | 71.3±10.2 |
| Antihypertensive use (%) | 2441 (36.9) | 1357 (34.3) | 590 (37.7) | 348 (44.2) | 75 (48.7) | 50 (52.6) | 21 (47.7) |
| Hypertension (140/90/Rx) (%) | 3184 (48.2) | 1762 (44.5) | 780 (49.8) | 450 (57.2) | 98 (63.6) | 65 (68.4) | 29 (65.9) |
| Body Mass Index (kg/m2) | 28.3±5.5 | 28±5.3 | 28.6±5.6 | 28.9±5.6 | 29.5±5.3 | 31.5±6.4 | 28.3±5.8 |
| Total Cholesterol (mg/dL) | 194.2±35.7 | 193.5±35.6 | 195.9±36.5 | 194±34.9 | 198.5±37.5 | 192.9±33.3 | 184.1±29.6 |
| HDL Cholesterol (mg/dL) | 51±14.8 | 50.9±14.8 | 51±14.8 | 51.3±15.3 | 52±14 | 50.7±12.1 | 48.8±13.3 |
| Triglycerides (mg/dL) | 131.5±89 | 131.7±92.4 | 133.8±88.4 | 127.5±77.4 | 128±80.3 | 127.7±64.4 | 125.2±64.9 |
| Statin Use (%) | 968 (14.6) | 550 (13.9) | 225 (14.4) | 129 (16.4) | 28 (18.2) | 23 (24.2) | 13 (29.5) |
| Cigarette smoking (%) | |||||||
| Former | 2421 (36.6) | 1426 (36) | 583 (37.2) | 298 (37.9) | 63 (40.9) | 32 (33.7) | 19 (43.2) |
| Current | 863 (13.1) | 499 (12.6) | 212 (13.5) | 110 (14) | 22 (14.3) | 14 (14.7) | 6 (13.6) |
| Never | 3325 (50.3) | 2037 (51.4) | 772 (49.3) | 379 (48.2) | 69 (44.8) | 49 (51.6) | 19 (43.2) |
| Diabetes (%) | 825 (12.5) | 471 (11.9) | 204 (13) | 100 (12.7) | 28 (18.2) | 15 (15.8) | 7 (15.9) |
Column percentages are given.
Correlates of sIAD
The primary correlates of high exam 1 absolute sIAD were older age, higher SBP, and higher BMI (Table 1). The only statistically significant difference in absolute sIAD by race/ethnicity was that White participants had higher sIAD than Chinese participants. High sIAD was most common in the Wake Forest site and least common in the UCLA site.
Reproducibility of high sIAD across years
Despite the frequent occurrence of high absolute sIAD at some point during the study, high values often did not persist. The exam 3 absolute sIAD value of 0–4 mmHg occurred in 57.6% of those who were 0–4 mmHg at exam 1; this is not very different from the 44.7% whose absolute sIAD was 0–4 mmHg at exam 3 among those who were ≥25 mmHg at exam 1 (Table 2). Among 38 people who had absolute sIAD ≥25 mmHg at exam 1, only 7.9% (n=3) repeated a value ≥25 mmHg at exam 3, while another 7.9% came close to their exam 1 value (an exam 3 value of 20–24 mmHg, 1 category lower). For each exam 1 level, few people had exam 3 absolute sIAD over 20 mmHg.
Table 2.
Frequency distribution of inter-arm systolic blood pressure difference (sIAD) categories at exam 1 and exam 3, N = 5816.
| All | Absolute sIAD (mmHg), exam 3 (row %) | ||||||
|---|---|---|---|---|---|---|---|
| Absolute sIAD (mmHg), exam 1 | 0–4 | 5–9 | 10–14 | 15–19 | 20–24 | ≥25 | |
| 0–4 | 3464 | 1995 (57.6) | 859 (24.8) | 450 (13) | 94 (2.7) | 50 (1.4) | 16 (0.5) |
| 5–9 | 1380 | 751 (54.4) | 346 (25.1) | 201 (14.6) | 42 (3) | 27 (2) | 13 (0.9) |
| 10–14 | 712 | 370 (52) | 182 (25.6) | 112 (15.7) | 27 (3.8) | 15 (2.1) | 6 (0.8) |
| 15–19 | 140 | 65 (46.4) | 36 (25.7) | 27 (19.3) | 7 (5) | 2 (1.4) | 3 (2.1) |
| 20–24 | 82 | 41 (50) | 21 (25.6) | 8 (9.8) | 5 (6.1) | 5 (6.1) | 2 (2.4) |
| ≥25 | 38 | 17 (44.7) | 9 (23.7) | 3 (7.9) | 3 (7.9) | 3 (7.9) | 3 (7.9) |
| All | 5816 | 3239 | 1453 | 801 | 178 | 102 | 43 |
Repeated high absolute sIAD values across all 3 exams among 4337 participants who attended all 3 exams was rare. Among 25 people with exam 1 absolute sIAD ≥25 mmHg, that level was repeated 1 time (at either exam 3 or exam 5, Figure 1) in 8% and twice (at both exam 3 and exam 5, not shown) in 4%. Absolute sIAD ≥ 10 mmHg ever during followup among 2613 participants with exam 1 absolute sIAD between 0 and 4 mmHg was 29%, almost as common in those with exam 1 absolute sIAD ≥25 mmHg (44%). Among the few people whose high sIAD was repeated between exams, it appeared on the contralateral side of the body in about 1/3 of people (data not shown).
Figure 1.
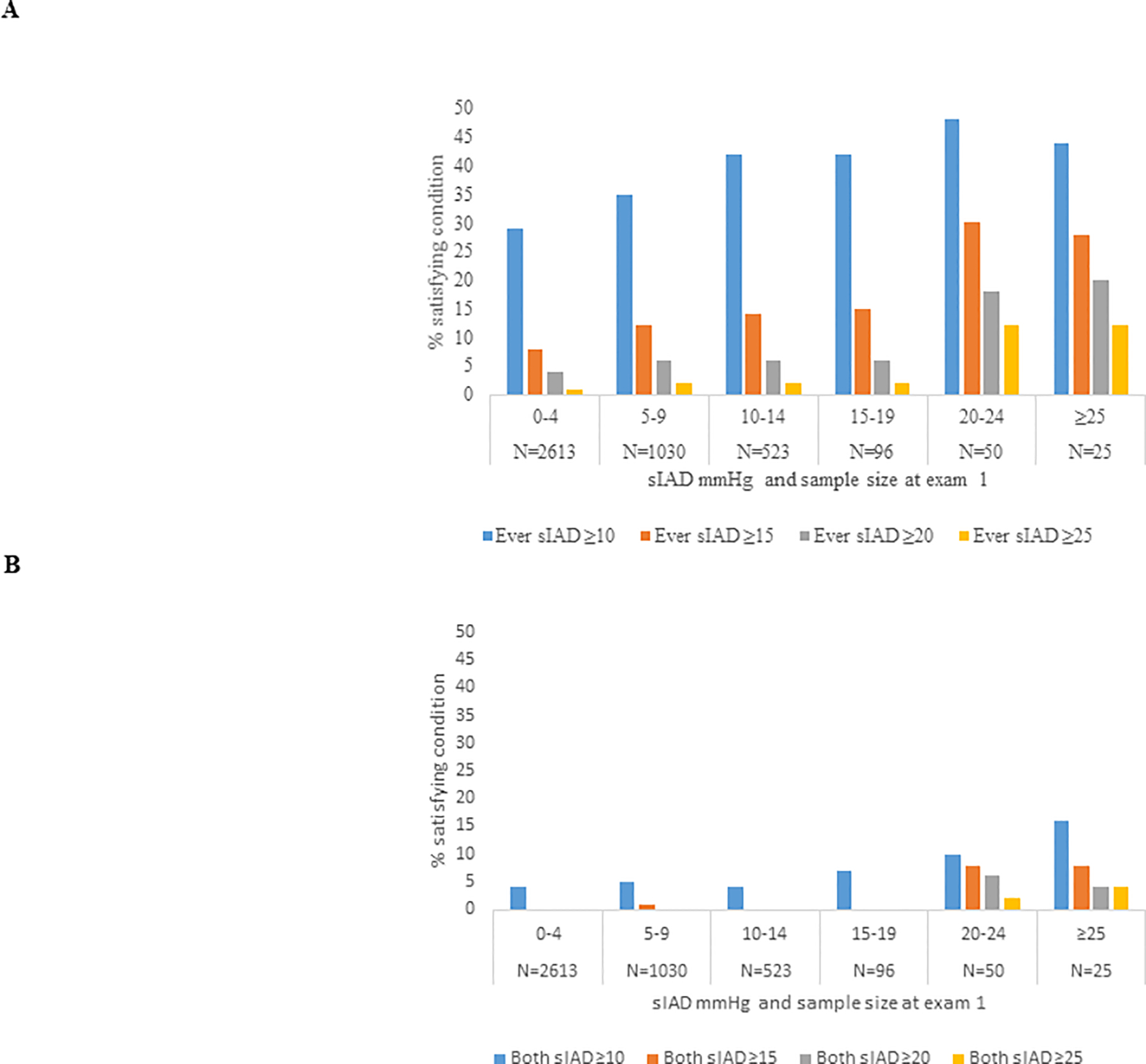
Percent of people with high absolute sIAD (for 4 definitions of high). N=4337 who attended exams 1, 3 and 5. A) high sIAD at either exam 3 or exam 5 or both, by sIAD at exam 1. B) high sIAD at both exam 3 and exam 5, by sIAD at exam 1. Note that the % satisfying the condition in Figure 1B is a subset of the % satisfying the condition in Figure 1A.
In each exam, the both right and left Doppler SBP measures had correlation of about 0.8 with seated rest oscillometric measure on the same day; the Doppler measure tended to be a few mmHg higher than the seated rest oscillometric measure. The Doppler SBP correlations in the same day (n=302) at exam 3 were over 0.9 for first vs second SBP, whether on the left or right side. Whether in the same day repeatability sample or the full sample, correlations between exams were lower, about 0.6 for SBP at one exam vs at another, whether on the left or right side. We also examined sIAD correlations within and between exams. Within exam the correlation of repeated sIAD was about 0.45, but only about 0.1 between exams. Thus concordance of sIAD was imperfect but substantial within the same day, but was very low over years.
Absolute sIAD and incident CVD
Point estimates for the associations of exam 1 sIAD with incident CVD were generally positive but not statistically significant (Table 3). Total death occurred in 22.5% (1489/6609) of participants, with hazard ratio 1.11 (1.00–1.22) and p = 0.05 in model 1, and 1.09 (0.99–1.21), p = 0.09 in model 2 (not shown in Table 3).
Table 3.
Associations of CVD Events with continuous absolute inter-arm systolic blood pressure (sIAD) at baseline and with continuous maximum absolute sIAD as a time-varying variable.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Event type (n/N) | HR (CI) | p | HR (CI) | p | |
| Baseline | Any CVD (1220/6609) | 1.17 (1.04–1.30) | 0.006 | 1.09 (0.97–1.21) | 0.15 |
| Time-varying maximum | Any CVD (1236/6725) | 1.23 (1.12–1.36) | <.0001 | 1.14 (1.04–1.26) | 0.008 |
| Time-varying current | Any CVD (1236/6725) | 1.23 (1.11–1.36) | <.0001 | 1.16 (1.05–1.29) | 0.003 |
| Baseline | CHD (479/6609) | 1.07 (0.89–1.28) | 0.48 | 0.99 (0.82–1.19) | 0.87 |
| Time-varying maximum | CHD (485/6725) | 1.10 (0.94–1.29) | 0.23 | 1.03 (0.87–1.21) | 0.75 |
| Time-varying current | CHD (485/6725) | 1.11 (0.94–1.31) | 0.20 | 1.06 (0.9–1.25) | 0.49 |
| Baseline | Heart Failure (375/6609) | 1.11 (0.91–1.35) | 0.32 | 1.01 (0.83–1.24) | 0.92 |
| Time-varying maximum | Heart Failure (379/6725) | 1.13 (0.94–1.34) | 0.19 | 1.03 (0.86–1.23) | 0.72 |
| Time-varying current | Heart Failure (379/6725) | 1.15 (0.97–1.38) | 0.11 | 1.08 (0.91–1.3) | 0.38 |
| Baseline | Stroke (299/6609) | 1.18 (0.95–1.48) | 0.14 | 1.11 (0.88–1.39) | 0.37 |
| Time-varying maximum | Stroke (306/6725) | 1.42 (1.19–1.70) | <.0001 | 1.35 (1.13–1.61) | 0.001 |
| Time-varying current | Stroke (306/6725) | 1.39 (1.16–1.67) | 0.0004 | 1.34 (1.11–1.61) | 0.002 |
| Baseline | PAD (121/6609) | 1.16 (0.81–1.65) | 0.41 | 1.00 (0.69–1.45) | 0.99 |
| Time-varying maximum | PAD (121/6725) | 1.49 (1.12–1.97) | 0.006 | 1.31 (0.99–1.74) | 0.06 |
| Time-varying current | PAD (121/6725) | 1.52 (1.15–2.01) | 0.004 | 1.43 (1.07–1.9) | 0.01 |
| Baseline | Extracor. CVD (Stroke or PAD) (412/6609) | 1.17 (0.96–1.41) | 0.11 | 1.07 (0.88–1.30) | 0.49 |
| Time-varying maximum | Extracor. CVD (Stroke or PAD) (419/6725) | 1.45 (1.25–1.69) | <.0001 | 1.34 (1.15–1.56) | 0.0002 |
| Time-varying current | Extracor. CVD (Stroke or PAD) (419/6725) | 1.45 (1.25–1.69) | <.0001 | 1.34 (1.15–1.56) | 0.0002 |
| Baseline | CVD death (349/6609) | 1.16 (0.95–1.42) | 0.15 | 1.12 (0.92–1.38) | 0.26 |
| Time-varying maximum | CVD death (354/6725) | 1.25 (1.06–1.48) | 0.009 | 1.19 (1.01–1.40) | 0.04 |
| Time-varying current | CVD death (354/6725) | 1.20 (1.00–1.44) | 0.05 | 1.17 (0.98–1.40) | 0.09 |
Hazard ratios are per 10 mmHg of sIAD. Model 1 adjustment: age, race/ethnicity, sex, and clinic at exam 1. Model 2 adjustment : Model 1 + additionally adjusts for height, heart rate, systolic blood pressure, diastolic systolic blood pressure, use of antihypertensive medication, body mass index, smoking (current, former, never), cholesterol, HDL-cholesterol, triglycerides, use of statin medication, diabetes. Covariates are time- varying in the time-varying models.
Extracor. = extracoronary.
Interpretation of the time-varying analyses requires examination of a potential bias due to missing later exams. Missing both exam 3 and exam 5 was rare: 6% (293/4796) among those with maximum absolute sIAD ≥5 mmHg. However, a maximum absolute sIAD of 0 mmHg tended to occur only at exam 1, because a higher value at any subsequent exam would increase the maximum value. In fact 74% (93/126) of those with maximum absolute sIAD = 0 mmHg were examined only at exam 1. Although there was a similar bias for maximum sIAD 1–4 mmHg, it was smaller: 20% (366/1805) of them were missing both exam 3 and exam 5. Independently of maximum absolute sIAD category, 48% (361/752) of participants died among those missing both exams 3 and 5, compared to 19% (1152/5973) who died among those assessed at either exam 3 or exam 5 or both. The bias was much less for any CVD (22% events in those missing both exams versus 18% events in those seen at either or both exams) and for extracoronary CVD (8.5% events in those missing both exams versus 5.9% events in those seen at either or both exams). Therefore we examined incident CVD events in time-varying models (Table 4), but total mortality only in the exam 1 model.
Table 4.
Associations of CVD Events with continuous within person standard deviation of the second and third sitting rest oscillometric systolic blood pressure, as a time-varying variable.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Event type (n/N) | HR (CI) | p | HR (CI) | P |
| Any CVD (1236/6725) | 1.08 (1.03–1.13) | 0.0007 | 1.03 (0.98–1.08) | 0.17 |
| CHD (485/6725) | 1.06 (0.99–1.14) | 0.11 | 1.03 (0.95–1.11) | 0.51 |
| Heart Failure (379/6725) | 1.03 (0.94–1.12) | 0.54 | 0.98 (0.9–1.08) | 0.73 |
| Stroke (306/6725) | 1.09 (1.01–1.19) | 0.04 | 1.06 (0.97–1.15) | 0.24 |
| PAD (121/6725) | 1.18 (1.04–1.33) | 0.008 | 1.13 (0.98–1.29) | 0.08 |
| Extracor. CVD (Stroke or PAD) (419/6725) | 1.12 (1.05–1.2) | 0.0007 | 1.08 (1.01–1.17) | 0.03 |
| CVD death (354/6725) | 1.05 (0.97–1.13) | 0.25 | 1.03 (0.95–1.12) | 0.50 |
Higher time-varying absolute sIAD was significantly associated with increased stroke and PAD risk (time-varying maximum categories are shown in Figure 2). Approximate doubling of risk for extracoronary CVD (stroke or PAD) was seen for maximum absolute sIAD ≥15 mmHg, compared to the 0–4 mmHg category (Figure 3). Estimated HR were generally concordant with those for stroke and PAD analyzed separately.
Figure 2.
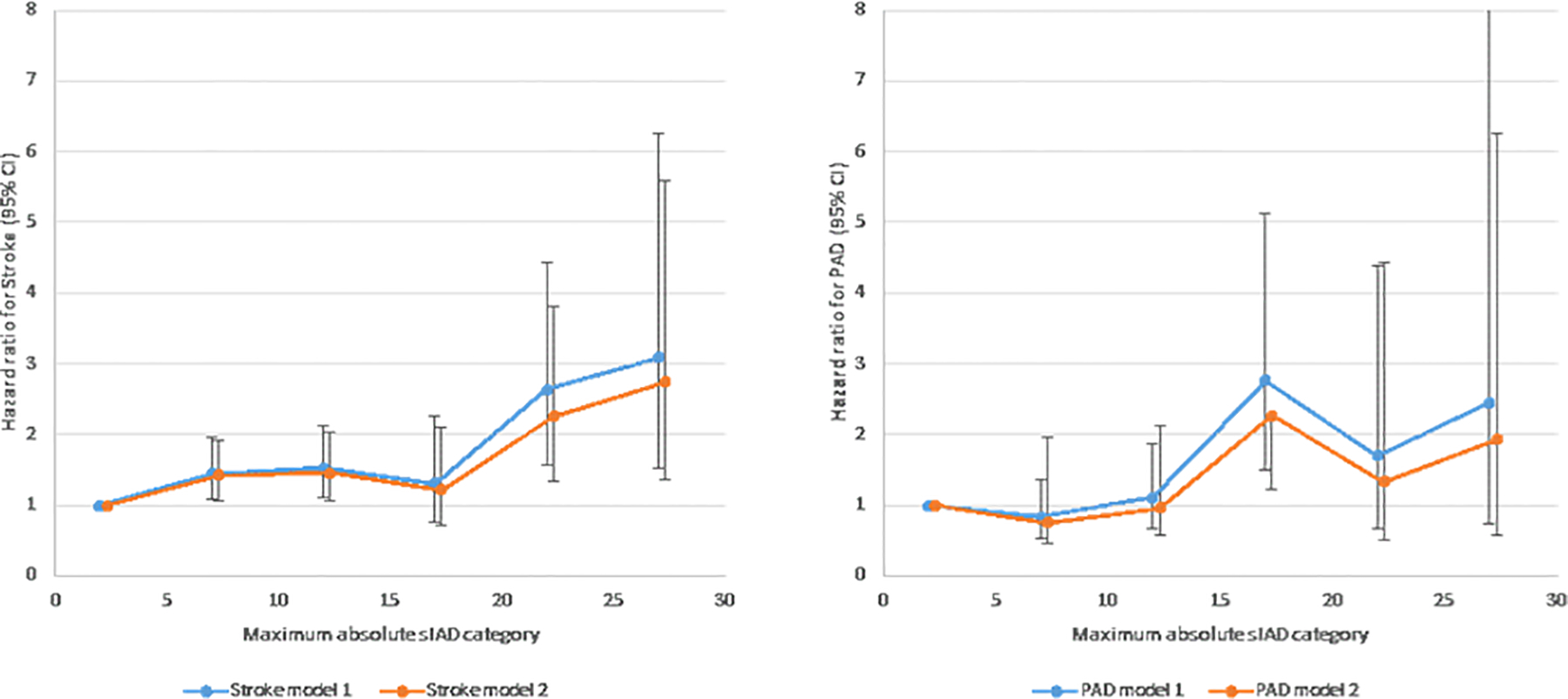
Hazard ratios (95% confidence interval) for incident A) stroke, B) peripheral artery disease (PAD) by maximum absolute inter-arm systolic blood pressure difference (sIAD) category. Covariates are as in Table 3.
Figure 3.

Hazard ratios (95% confidence interval) for summaries of cardiovascular disease subtypes. A Incident Extracoronary CVD (Stroke + PAD) and B) total Cardiovascular Disease (CVD) by maximum absolute inter-arm systolic blood pressure difference (sIAD) category. Covariates are as in Table 3.
Findings were weaker for the 1236 any CVD events, than for stroke and PAD. Specifically, the HR per 10 mmHg of continuous maximum absolute sIAD for any CVD was 1.23, p < 0.01 in model 1 and 1.14, p < 0.01 in model 2. In the analysis of categories, only the ≥25 mmHg category was statistically significant with HR 2.11 (p < 0.01) in model 1 and 1.81 (p < 0.01) in model 2. Separate analysis of CHD events and of heart failure both showed an elevated risk among those with maximum absolute sIAD ≥25 mmHg (data not shown), similar to the risk for any CVD.
In a sensitivity analysis, within person standard deviation of the second and third sitting rest oscillometric SBP was 3.6±3.2 mmHg at each of exams 1, 3, and 5. These standard deviations had correlation of about 0.15 with single sitting rest SBP measures and about 0.06 with each other across pairs of exams. The associations of CVD events with this continuous within person standard deviation as a time-varying maximum variable is shown in Table 4. The associations were positive and significant for any CVD, stroke, PAD, and extracoronary CVD in model 1, reaching statistical significance in model 2 only for extracoronary CVD (p=0.03). As is customary in analysis of sitting rest SBP, we omitted the first sitting rest measure. Inclusion of the first sitting rest SBP in the within person standard deviation weakened the findings slightly (data not shown).
Discussion
Our first novel finding is large variability in sIAD over longer time periods than have previously been assessed, namely 3.2 and 9.5 years, and even with repeat assessment in the same day. This non-persistence of sIAD is not consistent with the interpretation that a culprit lesion leading to subclavian stenosis or other obstructive disease is the primary source of large sIAD. Nevertheless, in agreement with a large literature [10], sIAD as a continuous variable or as 6 categories at baseline showed a tendency to predict incident CVD and total death. Furthermore, time-varying absolute sIAD during the follow-up duration was a strong and significant predictor of incident CVD. Most prior studies [10] have relied on a fixed baseline, whereas our findings are that sequential measures of sIAD identified additional persons at risk. A second novel finding is that this association with time-varying sIAD was particularly strong with incident extracoronary CVD (including stroke and PAD).
The strategy of looking at time-varying maximum absolute sIAD in effect replicates the exam 1 finding by greatly expanding the number of participants who ever experienced high absolute sIAD and showing that the baseline tendencies for higher risk with high sIAD were found, no matter when the high sIAD occurred. A clinical cutpoint suggested for approximate doubling of risk of stroke or PAD was absolute sIAD ≥15 mmHg, but for other forms of CVD doubling of risk was seen only for absolute sIAD ≥25 mmHg. These findings were true both in the simple adjusted model and in the more extended adjusted model.
Therefore, we considered the interpretation that sIAD may be a manifestation of BP variability, expressed over a 10 minute interval. Intermittently large sIAD may be regarded as episodic hypertension or an occasional BP spike. BP oscillates over the short and long periods, whether over minutes, days, months, or years [21]. To some extent, variations in sympathetic tone may mediate these oscillations. Rothwell et al. [22] emphasized the importance of both variability and maximum blood pressure reached, particularly at older ages when most vascular events occur [23]. Stevens et al. [24] reviewed the substantial literature that has found that long-term (across clinic visits), mid-term (home), and short-term (ambulatory) BP variability predict CVD events and mortality. Their systematic review and meta-analysis was based on 19 observational cohort studies and 17 clinical trials and showed that higher SBP long term variability was associated with a higher risk of all-cause mortality (hazard ratio 1.15, 95% confidence interval 1.09 to 1.22) and similarly with CVD mortality, CVD events, coronary heart disease, and stroke. Notably, the associations were stronger in cerebral rather than coronary events. Generally concordant results were seen for increased mid- and short-term variability, though the literature for these phenomena was sparse. This meta-analysis provides us evidence that both long-term and shorter-term BP variability are associated with cardiovascular morbidity and mortality over and above the effect of mean BP.
There is little information about prediction of PAD from long-term BP variability. However, Yeh et al. [25] did show an increased risk for incident PAD in those with greater BP variability over 10 years among patients seen frequently after diabetes diagnosis. More recently, Yano et al. [26] found in the CARDIA study sample free of overt CVD at baseline that long-term visit-to-visit BP variability predicted future CVD events. Subclinical cardiovascular phenotype is also related to BP variability. Specifically, a CARDIA study about long-term BP variability focused on myocardial structure and function in later life [27] showed that BP variability across 8 visits over 25 years was associated with a higher left ventricular mass index and worse systolic and diastolic function, independent of the mean BP levels. In the same study cohort, both SBP and DBP variability across 20 years were positively associated with greater carotid intima-media thickness but not with coronary artery calcium at the end of the follow-up period [28]. Finally, Tedla et al. [29] found that higher long-term BP variability correlated with greater 10-year progression of Young’s Elastic Modulus in the carotid artery within the MESA study.
On the other hand, Muntner et al. have reported that within visit BP variability (3 assessments over several minutes) did not predict total mortality [30]. It may be that our short-term measure of sIAD is predictive because it was assessed over a somewhat longer interval (10 minutes rather than 3 minutes), it involved the differential anatomy between arms, and a person had 3 opportunities over 10 years to exhibit high sIAD. Indeed, in our data the within person standard deviation of the second and third sitting rest oscillometric SBP, assessed in 1 arm 1 minute apart, was positively associated with CVD events, but more weakly than was sIAD at a 10 minute interval.
Our study findings may be influenced by the particular ankle-brachial index protocol within which the two arms were assessed. The protocol involved only single BP measurements, obtained by Doppler ultrasound, which tend to be a little higher than oscillometric measurements. While this particular protocol is not similar to sIAD assessed in a typical physician office visit, therefore limiting generalizability, this protocol is also a strength. sIAD values were obtained in all study participants in the same sequence during the 3 MESA visits spaced over 10 years. While we did study interarm difference, sidedness was not important in prediction of future events. Remeasurement of the same arm after 10 minutes might have produced the same findings as we observed for sIAD, but we do not have same arm measurement, except in the sensitivity analysis of same arm repeated oscillometric SBP 1 minute apart. Moreover, there could have been some loss of reproducibility given a 10 minute interval (as opposed to simultaneous or sequential measurement) and intervening measurement of leg pressures. Another limitation is that estimates based on the time-varying maximum absolute sIAD models may be biased due to missing sIAD values, especially at MESA exam 3 or exam 5. Our analysis of this potential bias suggested that it primarily affected total mortality; there we did not present time-varying models for prediction of total mortality. Additionally, in our time-varying analyses, we carried forward the most recent sIAD value, meaning that any effect of high sIAD is assumed to persist long after it is observed. Finally, numbers of stroke and PAD events were relatively low, which may have limited statistical power.
Our findings suggest that sIAD is primarily a measure of BP variability, more specifically a substantial transient increase in BP. Any observed instance of high absolute sIAD predicted incident CVD for absolute sIAD ≥25 mmHg and predicted stroke and PAD at absolute sIAD ≥15 mmHg. Given its seeming relevance to incident morbidity and mortality, sIAD should be further investigated because measuring BP in both arms is inexpensive and simple to perform.
Summary Table.
What is known about topic
A large absolute inter-arm systolic blood pressure difference (sIAD) is relatively common in the general population.
Inter-arm blood pressure is often not reproducible.
What this study adds
There is a large variability in sIAD with 10 minutes between measurements over a long period of time, but also with repeat assessment in the same day.
Any observed instance of absolute sIAD ≥25 mmHg predicts incident CVD and any absolute sIAD ≥15 mmHg predicts stroke and PAD.
Source of Funding:
This research project was supported by R01 HL142283-01A1. The Multi-ethnic Study of Atherosclerosis was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC- 95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung and Blood Institute(NHLBI) and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources.
Nonstandard Abbreviations and Acronyms
- CARDIA
Coronary Artery Risk Development in Young Adults
- MESA
Multi-Ethnic Study of Atherosclerosis
- sIAD
inter-arm systolic blood pressure difference
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest
Data Availability:
Data are available on reasonable request to the MESA Coordinating Center (https://www.mesa-nhlbi.org/).
References
- 1.Weinberg I, Gona P, O’Donnell CJ, Jaff MR, Murabito JM. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham Heart Study. Am J Med. 2014; 127:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnett DK, Tang W, Province MA, Oberman A, Ellison RC, Morgan D et al. Interarm differences in seated systolic and diastolic blood pressure: the Hypertension Genetic Epidemiology Network study. J Hypertens. 2005; 23:1141–47. [DOI] [PubMed] [Google Scholar]
- 3.Clark CE, Taylor RS, Shore AC, Campbell JL. Prevalence of systolic inter-arm differences in blood pressure for different primary care populations: systematic review and meta-analysis. Br J Gen Pract. 2016; 66:e838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison EG Jr, Roth GM. Hines EA Jr. Bilateral indirect and direct arterial pressures. Circulation 1960; 22:419–36. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Kim EJ, Namgung J, Cho BR, Nam CW, Kim YK et al. Between-visit reproducibility of inter-arm systolic blood pressure differences in treated hypertensive patients: the coconet study. Hypertens Res. 2017; 40:483–86. [DOI] [PubMed] [Google Scholar]
- 6.Grossman A, Weiss A, Beloosesky Y, Morag-Koren N, Green H, Grossman E. Inter- arm blood pressure difference in hospitalized elderly patients--is it consistent? J Clin Hypertens (Greenwich). 2014; 16:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguchi K, Yacoub M, Jhalani J, Gerin W, Schwartz JE, Pickering TG. Consistency of blood pressure differences between the left and right arms. Arch Intern Med. 2007; 167:388–93. [DOI] [PubMed] [Google Scholar]
- 8.Kleefstra N, Houweling ST, Meyboom-de Jong B, Bilo HJ. Measuring the blood pressure in both arms is of little use; longitudinal study into blood pressure differences between both arms and its reproducibility in patients with diabetes mellitus type 2.Ned Tijdschr Geneeskd. 2007;151:1509–14. (in Dutch, English abstract) [PubMed] [Google Scholar]
- 9.Huibers A, Hendrikse J, Brown MM, Pegge SA, Arnold M, Moll FL et al. Upper Extremity Blood Pressure Difference in Patients Undergoing Carotid Revascularisation. Eur J Vasc Endovasc Surg. 2017; 53:153–57. [DOI] [PubMed] [Google Scholar]
- 10.Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012; 379:905–14. [DOI] [PubMed] [Google Scholar]
- 11.Clark CE, Campbell JL, Powell RJ, Thompson JF. The inter-arm blood pressure difference and peripheral vascular disease: cross-sectional study. Fam Pract. 2007; 24:420–26. [DOI] [PubMed] [Google Scholar]
- 12.Clark CE, Steele AM, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Interarm blood pressure difference in people with diabetes: measurement and vascular and mortality implications: a cohort study. Diabetes Care. 2014; 37:1613–20. [DOI] [PubMed] [Google Scholar]
- 13.Kranenburg G, Spiering W, de Jong PA, Kappelle LJ, de Borst GJ, Cramer MJ et al. Inter-arm systolic blood pressure differences, relations with future vascular events and mortality in patients with and without manifest vascular disease. Int J Cardiol. 2017; 244:271–76. [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama H, Ohkuma T, Ninomiya T, Mastumoto C, Kario K, Hoshide S et al. Simultaneously Measured Interarm Blood Pressure Difference and Stroke: An Individual Participants Data Meta-Analysis. Hypertension. 2018; 71:1030–38. [DOI] [PubMed] [Google Scholar]
- 15.Clark CE, Warren FC, Boddy K, McDonagh STJ, Moore SF, Goddard J et al. Associations Between Systolic Interarm Differences in Blood Pressure and Cardiovascular Disease Outcomes and Mortality: Individual Participant Data Meta-Analysis, Development and Validation of a Prognostic Algorithm: The INTERPRESS-IPD (Inter-arm Blood Pressure Difference- individual Participant Data) Collaboration. Hypertension. 2021; 77:650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004; 44:618–23. [DOI] [PubMed] [Google Scholar]
- 17.Aboyans V, Criqui MH, McDermott MM, Allison MA, Denenberg JO, Shadman R et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007; 49:1540–45. [DOI] [PubMed] [Google Scholar]
- 18.Aboyans V, Kamineni A, Allison MA, McDermott MM, Crouse JR, Ni H et al. The epidemiology of subclavian stenosis and its association with markers of subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2010; 211:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871–81. [DOI] [PubMed] [Google Scholar]
- 20.Duprez DA, Jacobs DR Jr, Lutsey PL, Bluemke DA, Brumback LC, Polak JF et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011; 174:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell’oro R, Mancia G. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012; 14:333–8. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010; 375:938–48. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010; 375:895–905. [DOI] [PubMed] [Google Scholar]
- 24.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ et al. Blood pressure variability and cardiovascular disease: systematic review and meta- analysis. BMJ. 2016; 354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh CH, Yu HC, Huang TY, Huang PF, Wang YC, Chen TP et al. High Systolic and Diastolic Blood Pressure Variability Is Correlated with the Occurrence of Peripheral Arterial Disease in the First Decade following a Diagnosis of Type 2 Diabetes Mellitus: A New Biomarker from Old Measurement. Biomed Res Int. 2016:9872945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano Y, Reis JP, Lewis CE, Sidney S, Pletcher MJ, Bibbins-Domingo K et al. Association of Blood Pressure Patterns in Young Adulthood With Cardiovascular Disease and Mortality in Middle Age. JAMA Cardiol. 2020; 5:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nwabuo CC, Yano Y, Moreira HT, Appiah D, Vasconcellos HD, Aghaji QN et al. Between Visit-to-Visit Blood Pressure Variability in Early Adulthood and Myocardial Structure and Function in Later Life. JAMA Cardiol. 2020; 5:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nwabuo CC, Yano Y, Moreira HT, Appiah D, Vasconcellos HD, Aghaji QN. Long-Term Blood Pressure Variability in Young Adulthood and Coronary Artery Calcium and Carotid Intima-Media Thickness in Midlife: The CARDIA Study. Hypertension 2020; 76:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedla YG, Yano Y, Carnethon M, Greenland P. Association Between Long-Term Blood Pressure Variability and 10-Year Progression in Arterial Stiffness: The Multiethnic Study of Atherosclerosis. Hypertension. 2017; 69:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muntner P, Levitan EB, Reynolds K, Mann DM, Tonelli M, Oparil S et al. Within-visit variability of blood pressure and all-cause and cardiovascular mortality among US adults. J Clin Hypertens (Greenwich). 2012; 14:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request to the MESA Coordinating Center (https://www.mesa-nhlbi.org/).


