Abstract
Background:
Bone marrow-derived mesenchymal stem cells (BMSCs) and bone morphogenetic protein-2 (BMP-2) have been studied for bone repair because they have regenerative potential to differentiate into osteoblasts. The development of injectable and in situ three-dimensional (3D) scaffolds to proliferate and differentiate BMSCs and deliver BMP-2 is a crucial technology in BMSC-based tissue engineering.
Methods:
The proliferation of mouse BMSCs (mBMSCs) in collagen/poly-γ-glutamic acid (Col/γ-PGA) hydrogel was evaluated using LIVE/DEAD and acridine orange and propidium iodide assays. In vitro osteogenic differentiation and the gene expression level of Col/γ-PGA(mBMSC/BMP-2) were assessed by alizarin red S staining and quantitative reverse-transcription polymerase chain reaction. The bone regeneration effect of Col/γ-PGA(mBMSC/BMP-2) was evaluated in a mouse calvarial bone defect model. The cranial bones of the mice were monitored by micro-computed tomography and histological analysis.
Results:
The developed Col/γ-PGA hydrogel showed low viscosity below ambient temperature, while it provided a high elastic modulus and viscous modulus at body temperature. After gelation, the Col/γ-PGA hydrogel showed a 3D and interconnected porous structure, which helped the effective proliferation of BMSCs with BMP-2. The Col/γ-PGA (mBMSC/BMP-2) expressed more osteogenic genes and showed effective orthotopic bone formation in a mouse model with a critical-sized bone defect in only 3–4 weeks.
Conclusion:
The Col/γ-PGA(mBMSC/BMP-2) hydrogel was suggested to be a promising platform by combining collagen as a major component of the extracellular matrix and γ-PGA as a viscosity reducer for easy handling at room temperature in BMSC-based bone tissue engineering scaffolds.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-022-00454-4.
Keywords: Hydrogel scaffold, Bone morphogenetic protein-2, Mesenchymal stem cell, Calvarial defect, Bone regeneration
Introduction
Bone has the ability to perform self-repair and most bone fractures can heal spontaneously without any surgical intervention. Nevertheless, critical-sized bone defects caused by trauma or malignant tumor resection require medical intervention. In clinical cases, a commonly used medical treatment is autogenous bone grafting, although this is associated with serious problems such as limitations in the amount of available bone, risk of infection, and pain at the harvest site. To resolve these problems, bone tissue engineering using mesenchymal stem cells has recently been studied as an alternative method of bone grafting [1–4]. Bone marrow-derived mesenchymal stem cells (BMSCs), which have no ethical issues, are the most practical adult stem cells and can be efficiently differentiated into osteoblasts [5–7]. To form bone tissue effectively from BMSCs, however, it is necessary to develop a biocompatible scaffold that can aid the proliferation of BMSCs in bone tissue regeneration [8–13]. Most studied or clinically evaluated scaffolds are pre-formed solid ones, which can lead to difficulties in handling and completely filling the damaged bone space [12, 14]. Bone morphogenetic protein-2 (BMP-2) is a promising tool because it is an osteoinductive growth factor for bone formation during bone regeneration and remodeling in bone therapy [15, 16]. Moreover, reports have described that BMP-2 can support the osteogenic differentiation of BMSCs [17, 18]. Various carrier delivery systems have been studied for the local delivery of BMP-2 to maintain retention time and sustained release, and then enhance effective bone regeneration in bone tissue engineering applications [19, 20].
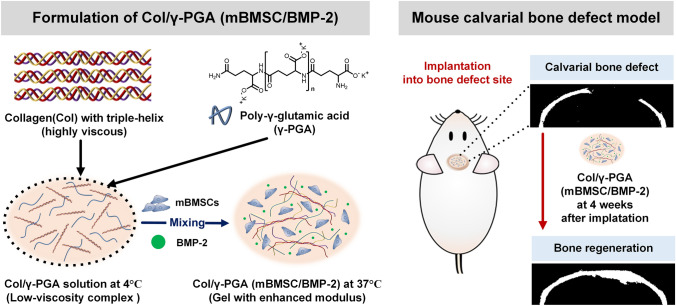
Here, collagen/poly-γ-glutamic acid (Col/γ-PGA) hydrogel is proposed as an injectable and three-dimensional (3D)-scaffold-forming platform for the delivery of mouse BMSCs (mBMSCs) and BMP-2 using the combination of collagen as a major component of the extracellular matrix and γ-PGA as a viscosity-reducing agent as well as helper materials for bone mineralization [21–23]. Because collagens contain integrin receptors (α1β1, α2β1), BMSCs can proliferate by integrin-mediated cell adhesion in the Col/γ-PGA scaffold [24–26]. Collagen scaffolds are commonly used in medical applications for tissue regeneration and wound healing [27, 28]. Collagen has also been extensively studied in research on tissue engineering and stem cell-based therapy [29, 30]. Collagen type I accounts for approximately 90% of the organic components in bone, and atelocollagen has low antigenicity and immunogenicity [23, 31]. However, high-concentration collagen solutions are very viscous with dense spaces, which limits their homogeneous mixing with stem cells and their molding into the complex shapes of bone defects at injury sites at the handling temperature. Furthermore, collagen has low moduli for bone tissue and dense pores, which can inhibit stem cell proliferation [14, 32, 33]. To overcome these limitations of applying collagen in scaffolds, γ-PGA was introduced. γ-PGA is an anionic biopolymer that is synthesized naturally by various bacilli and has biocompatibility and low immunogenicity [34, 35]. γ-PGA is also commonly used as a promising biomaterial in pharmaceutical and medical applications, cosmetics, and the food industry [36–38]. Moreover, in recent studies, γ-PGA has been shown to be helpful for osteogenesis and chondrogenesis [39–42]. Notably, when a low-viscosity γ-PGA solution is added to a high-viscosity collagen solution, the γ-PGA solution can serve as a viscosity-reducing agent. When the triple-helix structure of collagen is loosened at 37 °C, γ-PGA can become entangled with cationic collagen via electrostatic interactions. As a result, the moduli of Col/γ-PGA hydrogel can be increased even without any chemical cross-linking. In addition, the interconnected porous structure shown after the gelation of Col/γ-PGA hydrogel was found to confer a strong advantage for tissue regeneration using stem cells because it provides space for their proliferation [33].
In this study, Col/γ-PGA(mBMSC/BMP-2) hydrogel showed good performance for osteogenesis both in vitro and in vivo experiemnts in a short period (Scheme 1). Therefore, our study suggests that Col/γ-PGA hydrogel could be used as a promising and optimized hydrogel scaffold for BMSC-based bone tissue regeneration with BMP-2.
Scheme 1.
Schematic illustration of in situ gel-forming collagen/poly-γ-glutamic acid hydrogel loaded with mouse bone marrow-derived mesenchymal stem cells (mBMSCs) and bone morphogenetic protein-2 (BMP-2) for bone tissue regeneration in a mouse calvarial bone defect model
Materials and methods
Materials
Collagen type I (atelocollagen form from porcine skin, Mw = 300 KDa) was purchased from SK Bioland (Cheonan, Republic of Korea). γ-PGA (K + form; Mw = 500 kDa) was a kind gift from BioLeaders Corporation (Daejeon, Republic of Korea). ProNectin® F Plus, dexamethasone, β-glycerophosphate, ascorbic acid, alizarin red S (ARS), cardiogreen, and fibronectin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Murine BMP-2 and BMP-2 Standard TME ELISA Development Kit were purchased from PeproTech (Rocky Hill, NJ, USA). Alkaline Phosphate (ALP) Live Stain was purchased from Thermo Fisher Scientific (Waltham, MA, USA). FITC rat anti-mouse antibodies (a surface marker of mBMSCs: Sca-1, CD44, CD29, CD11b, CD34, and CD45) were purchased from BD Pharmingen (San Diego, CA, USA). All other reagents were of molecular biology grade.
Isolation and culture of mBMSCs
mBMSCs were obtained from the bone marrow of the femur and tibia of mice (Balb/c, 6–8 weeks old; Orient Bio, Seongnam, Republic of Korea), in accordance with a previous report [43]. Briefly, the muscle tissue was removed from femurs and tibias and the bones were soaked in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Scientific, Waltham, MA, USA) containing 1% (v/v) penicillin/streptomycin (Thermo Scientific). Both ends of the bones were cut and the marrow was flushed with DMEM containing 1% (v/v) penicillin/streptomycin using a syringe. The bone marrow cell suspension was filtered through a 70-μm mesh cell strainer, and bone marrow cells were collected after centrifugation at 1500 rpm for 5 min. The collected bone marrow cells were seeded in six-well plates at a density of 5 × 106 cells per well (1.5 mL) with a complete culture medium [DMEM containing 15% (v/v) heat-inactivated fetal bovine serum (FBS; Thermo Scientific) and 1% (v/v) penicillin/streptomycin] and incubated under a humidified atmosphere with 5% CO2 at 37 °C. After 3 h, nonadherent cells were removed by replacing the medium with a fresh complete culture medium. After an additional 8 h of culture, the culture medium was repeatedly replaced with a fresh complete culture medium every 8 h for 3 days. The characterization (positive markers: Sca-1, CD29, CD44, negative markers: CD45, CD11b, CD34) of isolated mBMSCs was analyzed using FITC-conjugated antibodies with an Acurri™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The results indicated that mBMSCs were not contaminated by leukocytes, macrophages, or hematopoietic stem cells (Supplementary Fig. S1). All mBMSCs were cultured in 75 cm2 flasks with DMEM containing 15% (v/v) FBS and 1% (v/v) penicillin/streptomycin under a humidified atmosphere with 5% CO2 at 37 °C. mBMSCs between passages 4 and 6 were used for the experiments.
Preparation of Col/γ-PGA hydrogels and loading of mBMSCs with BMP-2 into Col/γ-PGA hydrogels
First, 133 mg of collagen was dissolved in 10 mL of DMEM at 4 °C by gentle stirring for 2 days. γ-PGA was dissolved in DMEM at a concentration of 20 mg mL−1. ProNectin® F Plus or fibronectin (only used for ALP Live Staining experiment) were dissolved in deionized water at a concentration of 1 mg mL−1. Col/γ-PGA hydrogels were prepared by mixing a collagen solution (1 mL) with a mixture of γ-PGA solution (222 μL) and ProNectin® F Plus or fibronectin solution (100 μL) with vortexing. To promote cell adhesion and proliferation, we used ProNectin® F Plus or fibronectin. Fibronectin has an RGD cell attachment epitope that binds to the integrin of the cell with high affinity. ProNectin® F Plus is a fibronectin-like engineered protein polymer that incorporates multiple copies of the RGD from fibronectin by fusing two amino acid motifs, RGD and (GAGAGS)9 [44]. Col/γ-PGA hydrogels were stored at 4 °C until used. To prepare Col/γ-PGA hydrogels containing BMP-2 [Col/γ-PGA(BMP-2)], BMP-2 reconstituted in PBS at a concentration of 1 μg μL−1 was mixed with Col/γ-PGA hydrogel (still in solution phase at 4 °C) by vortexing. For Col/γ-PGA(mBMSC), mBMSCs were mixed with Col/γ-PGA hydrogel after mBMSCs were harvested in the form of a pellet by centrifugation. Col/γ-PGA(mBMSC/BMP-2) was prepared by mixing the mBMSC pellet and Col/γ-PGA(BMP-2). For in vitro experiments, 100 ng of BMP-2 and 1 × 105 mBMSCs were added to 200 μL of Col/γ-PGA hydrogels. In addition, for in vivo experiments, 1 μg of BMP-2 and 1 × 106 mBMSCs were added to 20 μL of Col/γ-PGA hydrogels.
Rheological properties of Col/γ-PGA hydrogel
The rheological properties of Col/γ-PGA hydrogel were measured using a Bohlin Gemini II (Malvern Instruments Ltd., Malvern, UK) equipped with a 25 mm parallel plate. The prepared Col/γ-PGA hydrogel and collagen-only solution (13.3 mg mL−1 in DMEM) were placed on the plate of equipment, and the gap between the parallel plates was set to 1 mm. The viscoelastic properties of the samples were measured in oscillatory mode while maintaining a frequency of 1 rad s−1 (0.159 Hz) with a 2% constant strain value. The gelation time was determined by measuring the elastic modulus (G′) and viscous modulus (G″) at 37 °C over time. To determine the gelation temperature, G′ and G″ were measured at each temperature while increasing the temperature at a rate of 3 °C/min from 4 °C to 40 °C. In addition, to compare the moduli of only collagen and Col/γ-PGA hydrogel after gelation at 37 °C, G′ and G″ were measured by frequency-sweeping over a range from 0.1 rad/s to 70 rad/s at 37 °C after 10 min of thermal equilibrium. The viscosity was measured by the shear rate-sweeping over a range from 0.05 to 10 s−1 in non-oscillatory mode at 4 °C and 25 °C.
Injectable and gelation properties
To evaluate the injectable and moldable properties of Col/γ-PGA hydrogel in vitro, Col/γ-PGA as a low-viscosity liquid at low temperature was injected into the molds with complex shapes using a syringe. Then, the molds were removed after incubation at 37 °C for 30 min. To evaluate the injectable and gelation properties of Col/γ-PGA hydrogel in vivo, we used indocyanine green (cardiogreen) for visualizing Col/γ-PGA hydrogel. Indocyanine green was dissolved in deionized water at a concentration of 1 mg mL−1. Then, 1% (v/v) indocyanine green-loaded Col/γ-PGA hydrogel (100 μL) was injected into a male BALB/C nude mouse (6 weeks old) via subcutaneous injection. Finally, injected indocyanine green-loaded Col/γ-PGA hydrogel in mouse was dissected after 30 min.
Scanning electron microscopy (SEM)
To confirm the morphology of various hydrogels, each sample was rapidly frozen in liquid nitrogen and lyophilized. Following the lyophilization, a broken cross-section of the sample was sputter-coated with platinum and examined with field emission SEM (JMS-7000F; JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 5.0 kV.
In vitro release study of BMP-2 from Col/γ-PGA Hydrogel
The release study of BMP-2 from Col/γ-PGA hydrogel was performed by migration through a polycarbonate membrane of 6.5 mm diameter with 3 µm pore size in a 24-well transwell chamber (Corning, Cambridge, MA, USA). The Col/γ-PGA hydrogel containing BMP-2 (100 µL) was added to the upper chamber, all transwell plate wells in the lower compartment were filled with 1 mL of PBS supplemented with collagenase type I (100 μg mL−1 or 1 mg mL−1). The transwell plate was incubated at 37 °C with shaking at 60 rpm. The sample released from the inside of the transwell inserts was collected at 1, 3, 5, 7, 9, 24, 48, 72, 96, and 168 h after the incubation. After samples were collected, each well in the transwell plate was filled with fresh PBS supplemented with collagenase type I (100 μg mL−1 or 1 mg mL−1). The released BMP-2 was quantified using Murine BMP-2 Standard TMB ELISA Development Kit (PeproTech, Rocky Hill, NJ, USA).
The proliferation of mBMSCs in Col/γ-PGA hydrogel scaffolds
To evaluate the proliferation of mBMSCs in Col/γ-PGA hydrogel, a LIVE/DEAD assay was conducted. mBMSCs were embedded into Col/γ-PGA hydrogels at a density of 1 × 106 cells per 100 μL of hydrogel. The Col/γ-PGA(mBMSC) samples were placed on μ-slide eight-well chambers (ibidi GmbH, Martinsried, Germany), and gelation was induced at 37 °C for 20 min. Then, these chambers were incubated with complete mBMSC media for 3, 7, 11, and 15 days under a humidified atmosphere with 5% CO2 at 37 °C. The Col/γ-PGA(mBMSC) samples were then washed in PBS and stained with a LIVE/DEAD assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA), in accordance with the manufacturer’s instructions. Fluorescence images were obtained using a DeltaVision PD (GE Healthcare Life Sciences, Marlborough, MA, USA) with a filter set (fluorescein isothiocyanate: excitation 490/20, emission 525/36; tetramethylrhodamine isothiocyanate: excitation 555/25, emission 605/52; Omega Optical, Inc., Brattleboro, VT, USA). The proliferation of mBMSCs in Col/γ-PGA hydrogel was also quantitatively assessed after culture under osteogenic conditions. mBMSCs were embedded into Col/γ-PGA hydrogels at a density of 1 × 105 cells per 100 μL of hydrogel. The Col/γ-PGA(mBMSC) samples were placed on a 24-well plate and cultured with osteogenic differentiation medium (α-MEM supplemented with 10% FBS, 0.1 μM dexamethasone, 10 mM β-glycerophosphate, and 50 μM ascorbic acid) for 3, 7, 11, and 15 days under a humidified atmosphere with 5% CO2 at 37 °C. On the indicated day, the Col/γ-PGA(mBMSC) samples were treated with 10 mg of collagenase type I (Worthington Biochemical Corp., Freehold, NJ, USA) to digest the hydrogel for 5 min at 37 °C. Then, live mBMSCs were counted using a LUNA-FL Dual Fluorescence cell counter (Logos Biosystems, Annandale, VA, USA) after staining with acridine orange and propidium iodide (AO/PI).
In vitro osteogenic differentiation of mBMSCs with BMP-2 in Col/γ-PGA hydrogel scaffolds
To confirm the osteogenic differentiation of mBMSCs, calcium content and expression of osteogenic genes were evaluated by staining with ARS, ALP Live Stain, and quantitative reverse-transcription polymerase chain reaction (qRT-PCR). For ARS staining and qRT-PCR, mBMSCs (1 × 105), mBMSC/BMP-2 (1 × 105/100 ng), and Col/γ-PGA (mBMSC/BMP-2) (1 × 105/200 μL) were each placed on 24-well plates (Corning Life Sciences, Tewksbury, MA, USA). Then, osteogenic differentiation medium (DMEM supplemented with 15% FBS, 1% penicillin/streptomycin, 0.1 μM dexamethasone, 10 mM β-glycerophosphate, 50 μM ascorbic acid) was added to the mBMSCs in each experimental group on 24-well plates to induce osteogenic differentiation. The culture medium was changed to a fresh complete osteogenic differentiation medium every 3 days. mBMSCs (1 × 105) cultured with normal culture medium on 24-well plates were used as a control group, and all expression levels of osteogenic genes were estimated relative to those of the control group. The formation of calcium phosphate in each experimental group was visualized by staining with ARS. For ARS staining, each sample was washed twice with 1 mL of PBS at 7 days after culturing in osteogenic differentiation medium and then incubated with 1 mL of 4% (w/v) paraformaldehyde phosphate buffer solution for 30 min at room temperature for fixation. The fixed samples were washed again with deionized water and incubated with 1 mL of the 2% ARS staining solution at pH 4.3 for 10 min at room temperature. After the staining solution had been removed, the samples were washed again with deionized water. Finally, calcium deposition was observed by visualizing the red color. qRT-PCR was also performed to evaluate the expression of osteogenic genes. The total RNA was extracted from mBMSCs with BMP-2 in Col/γ-PGA hydrogels by treating with TRIzol (Invitrogen, Grand Island, NY, USA), and the extracted RNA samples were reverse-transcribed into cDNA with the Tetro cDNA Synthesis Kit (Bioline, London, UK), in accordance with the manufacturer’s instructions. The internal control for the expression analysis was GUSB (beta-D-glucuronidase). The primer sequences used in the experiment were as follows: GUSB FW, 5′-GCGTCCCACCTAGAATCTGC-3′ and RV, 5′-CATACGGAGCCCCCTTGTC-3′; ALP (alkaline phosphatase) FW, 5′-GGACAGGACACACACACACA-3′, and RV, 5′-CAAACAGGAGAGCCACTTCA-3′; BSP (bone sialoprotein) FW, 5′-ACAATCCGTGCCACTCACT-3′, and RV, 5′-TTTCATCGAGAAAGCACAGG-3′; and OCN (osteocalcin) FW, 5′-CTCACTCTGCTGGCCCTG-3′, and RV, 5′-CCGTAGATGCGTTTGTAGGC-3′. qRT-PCR was performed using a SensiFAST SYBR Lo-ROX mix (Bioline, London, UK) on a Rotor-Gene Q Instrument (Qiagen, Valencia, CA, USA). Data analysis was performed using the delta delta Ct method. For ALP Live Staining, mBMSCs (5 × 105), mBMSC/BMP-2 (5 × 105/25 ng), and Col/γ-PGA (mBMSC/BMP-2) (5 × 105/50 μL) were each placed on μ-slide eight-well chambers (ibidi GmbH, Martinsried, Germany), and gelation was induced at 37 °C for 20 min. Then, these chambers were incubated for 3 and 7 days under a humidified atmosphere with 5% CO2 at 37 °C. The culture medium was changed to a fresh complete osteogenic differentiation medium every 3 days. The Col/γ-PGA (mBMSC) samples were then washed and stained with ALP Live Stain (Thermo Fisher Scientific Inc., Waltham, MA, USA), following the manufacturer’s instructions. Fluorescence images were obtained using a Leica TSC SP8 (Leica Microsystems, Wetzlar, Germany).
In vivo bone formation in a mouse calvarial bone defect model
The male BALB/c nude mice (6 weeks old) were purchased from ORIENT BIO (Seongnam, Republic of Korea) and maintained on a standard diet with freely available water in a specific-pathogen-free facility. To evaluate in vivo bone formation in the mouse model, the mice underwent calvarial defect procedures. During the surgery, the mice were maintained under anesthesia with 2% isoflurane in oxygen, and the surgical site was disinfected. For the critical-sized calvarial defect model, one 4-mm-diameter calvarial defect was created on a parietal bone in each mouse using a trephine bur. Meticulous care was taken to protect the underlying dura mater and neighboring cranial sutures. Following the generation of the calvarial bone defects, mBMSCs (1 × 106) (n = 3), mBMSC/BMP-2 (1 × 106/1 μg) (n = 2), Col/γ-PGA (BMP-2) (1 μg/20 μL) (n = 3), Col/γ-PGA (mBMSC) (1 × 106/20 μL) (n = 3), or Col/γ-PGA(mBMSC/BMP-2) (1 × 106/1 μg/20 μL) (n = 3) was implanted into the defect site. The skin incision was then closed using sutures and treated with povidone-iodine (Green Pharmaceutical Co., Ltd., Seoul, Republic of Korea).
Micro-computed tomography (μ-CT)
μ-CT imaging was performed using a volumetric CT scanner (NFR-MXSCAN-G90; NanoFocusRay, Iksan, Republic of Korea) designed to minimize motion artifacts, and respiratory anesthesia (2% isoflurane) was maintained during the scanning to obtain CT images of live mice. Images were acquired at 60 kVp, 60 mA, and 800 ms per frame, with 400 views. Images were reconstructed using the Feldkamp cone-beam reconstruction algorithm. The reconstruction image size was 1024 × 1024 pixels, and 512 slices were acquired. The final reconstructed data were converted to the Digital Imaging and Communications in Medicine format to produce 3D-rendered images using 3D-rendering software (Lucion; MeviSYS, Seoul, Republic of Korea). The percentage of the recovered area was analyzed using the National Institutes of Health ImageJ software.
Histology
At 4 weeks post-implantation, the harvested specimens were fixed in 4% paraformaldehyde for 48 h and decalcified in 19% EDTA for 1 week at 4 °C. The samples were dehydrated through an alcohol gradient and embedded in paraffin blocks. Eight micron-thick histological sections were cut from the center of the embedded specimens, followed by staining with hematoxylin and eosin (H&E) to assess bone formation. Images were obtained under a bright field microscope (Olympus, Tokyo, Japan) and combined using Adobe Photoshop (Adobe® Systems, San Jose, CA, USA).
Statistical analysis
The significance of differences between groups was determined by analysis of variance (ANOVA). All values are expressed as mean ± standard deviation. GraphPad Prism software (version 8) was used for all statistical analyses.
Results and discussion
Characterization of Col/γ-PGA hydrogels
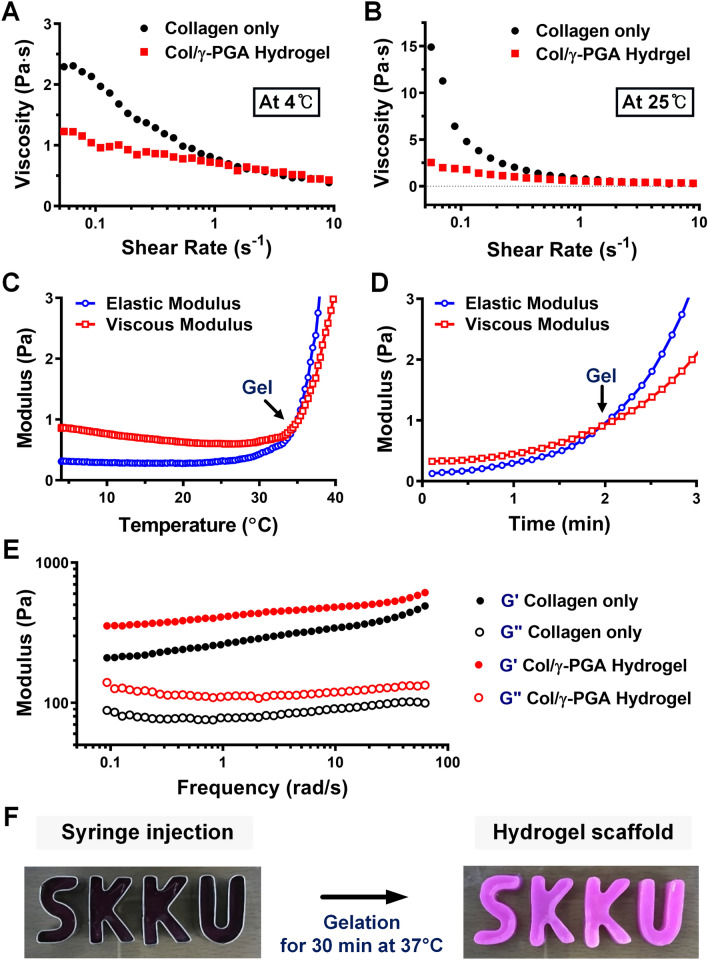
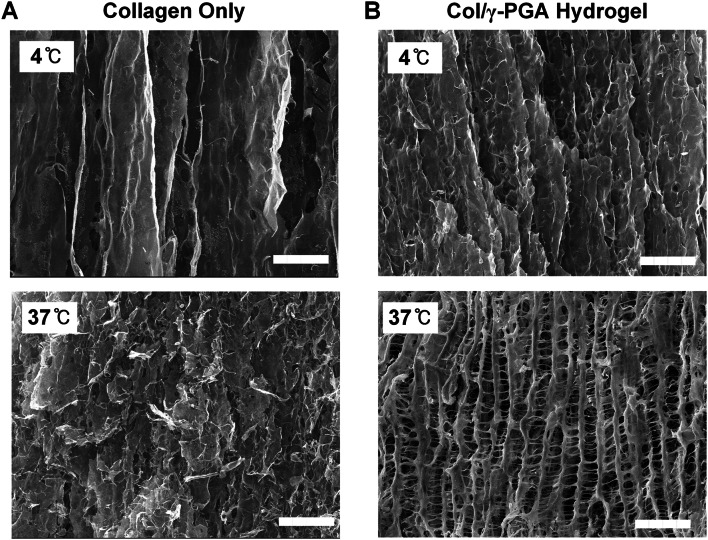
The Col/γ-PGA hydrogel is a free-flowing liquid at 4 °C, but it turns into in situ gel-forming non-flowing hydrogel at body temperature [45, 46]. The sol–gel phase transition of Col/γ-PGA hydrogel was confirmed by analyzing its rheological properties, such as viscosity, moduli, gelation temperature (Tgel), and gelation time. The intersection of elastic modulus (G′) and viscous modulus (G″) commonly indicates the gel formation point when G′ is greater than G″ [47]. When the viscosities of collagen only and Col/γ-PGA hydrogel were measured, the viscosity of Col/γ-PGA hydrogel was reduced by 1.9-fold at 4 °C (Fig. 1A) and by 5.8-fold at 25 °C (Fig. 1B). When the low-viscosity γ-PGA solution was added to the high-viscosity collagen solution, the γ-PGA solution might act to decrease viscosity. The gelation temperature of Col/γ-PGA hydrogel was measured at approximately 35 °C (Fig. 1C). The gelation occurred 2 min after the hydrogel had been exposed to isothermal conditions at 37 °C (Fig. 1D). Interestingly, however, the G′ and G″ of Col/γ-PGA hydrogel at 37 °C were increased by 1.7-fold and 1.6-fold, respectively, compared with those of collagen alone (Fig. 1E). It should be noted that the increases in both G′ and G″ of Col/γ-PGA hydrogel were accomplished by simply mixing collagen and γ-PGA, even without any chemical cross-linking at 37 °C after 10 min of stabilization. After the triple-helix structure of collagen had been loosened at 37 °C, γ-PGA could become entangled with the helix structure of collagen via electrostatic interaction between the cationic collagen and anionic γ-PGA (Fig. S2). In addition, hydrogen bonds could form between the NH groups of collagen and the CO groups of γ-PGA. These interactions between collagen and γ-PGA might contribute to the increases in both G′ and G″ values of Col/γ-PGA hydrogel at 37 °C. Owing to the in situ gelling properties, mBMSCs could be easily embedded in Col/γ-PGA hydrogel at 4 °C or ambient temperature, and then the hydrogel could be transformed into a specific shape with gelation properties in vitro and in vivo at 37 °C, as shown in Fig. 1F and Fig. S3. Through SEM imaging (Fig. 2), we also observed that an interconnected porous structure was successfully formed in Col/γ-PGA hydrogel. As shown in Fig. 1, while the cross-section of pristine collagen had a plain sheet-like structure at both 4 °C and 37 °C (Fig. 2A), the Col/γ-PGA hydrogel at 37 °C showed an open-porous 3D interconnected structure cross-section. The interconnected porous structure was not observed in Col/γ-PGA at 4 °C (Fig. 2B). These results suggested that γ-PGA contributed to the formation of the interconnected porous structure by interacting with the loosened structure of collagen at 37 °C. In fact, the highly porous gel structure of Col/γ-PGA hydrogels, which was not observed in conventional collagen hydrogels, suggests that strong interactions occur between the abundant carboxylate groups of γ-PGA and water molecules [48]. The pores in the hydrogels are assumed to indicate the location of water molecules before freeze-drying of the hydrogel solutions. From the experimental data, it should also be noted that the hard-to-handle character of highly concentrated collagen solutions could be modified to exhibit an easy-to-handle, low-viscosity sol state by introducing γ-PGA while keeping the intrinsic non-flowing gel state at body temperature. The experimental data also suggested that we could successfully develop an optimized hydrogel system with in situ gelling properties, increased moduli, and an interconnected porous structure for the proliferation of stem cells using biocompatible polymers (i.e., collagen and γ-PGA), even without any chemical cross-linking. The release profile of BMP-2 from Col/γ-PGA hydrogel was performed with collagenase at 37 °C to mimic the biological condition as shown in Fig. S4. This result showed that the remaining BMP-2 in Col/γ-PGA hydrogel as it is released slowly would promote the osteogenic differentiation of mBMSCs.
Fig. 1.
Rheological properties of collagen only and Col/γ-PGA hydrogels. Viscosities of collagen only and Col/γ-PGA hydrogel at A 4 °C and B 25 °C. C Gelation behavior of Col/γ-PGA hydrogel with increasing temperature. D Gelation time of Col/γ-PGA hydrogel at 37 °C. E Viscoelastic properties of collagen only and Col/γ-PGA hydrogels in frequency-sweeping mode at 37 °C after 10 min of stabilization. F Col/γ-PGA hydrogel is moldable and can be injected to form complex shapes that are maintained after gelation for 30 min at 37 °C
Fig. 2.
Representative SEM images of A collagen only and B Col/γ-PGA hydrogels at 4 °C and 37 °C. Scale bar = 10 μm
Proliferation of mBMSCs in Col/γ-PGA hydrogels
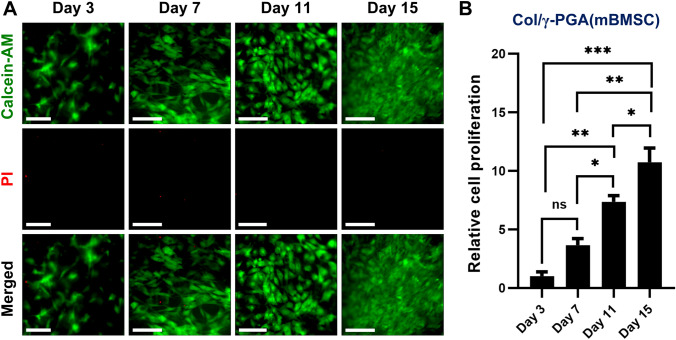
The survival and proliferation of stem cells in hydrogel scaffold are very important issues for effective tissue regeneration after the implantation of stem cells. To confirm the survival and proliferation capability of mBMSCs in Col/γ-PGA hydrogels, LIVE/DEAD assays with calcein-AM and PI were performed at 3, 7, 11, and 15 days after mBMSCs were cultured in Col/γ-PGA hydrogels. The results showed that mBMSCs proliferated gradually in Col/γ-PGA hydrogels over time and few dead mBMSCs were observed (Fig. 3A). The spread of mBMSCs after adhesion on Col/γ-PGA hydrogel was observed in fluorescence images in LIVE/DEAD assays. To quantitatively evaluate the proliferation of mBMSCs in Col/γ-PGA hydrogel, the number of live mBMSCs was counted by staining with AO/PI at 3, 7, 11, and 15 days after mBMSC culture in Col/γ-PGA hydrogel. As shown in Fig. 3B, the number of mBMSCs at 15 days after culture was increased about 10.7-fold compared with that of mBMSCs at 3 days. These results indicate that Col/γ-PGA hydrogel composed of generally recognized as safe materials could be used for clinical applications for the proliferation of mBMSCs.
Fig. 3.
The proliferation of mBMSCs embedded in Col/γ-PGA hydrogel. A Representative fluorescence microscopy image of mBMSCs cultured in Col/γ-PGA hydrogel stained by LIVE/DEAD assay with calcein-AM (green color, live cells) and PI (red color, dead cells) at 3, 7, 11, and 15 days. Scale bar = 40 μm (at 3 days) and 90 μm (at 7, 11, and 15 days). B Relative proliferation of mBMSCs embedded in Col/γ-PGA hydrogel determined by cell counting after staining with AO/PI at 3, 7, 11, and 15 days. Data are presented as the mean ± SD (*p < 0.05, **p < 0.005, ***p < 0.001; ns not significant)
In vitro osteogenic differentiation of mBMSCs with BMP-2 in Col/γ-PGA hydrogels
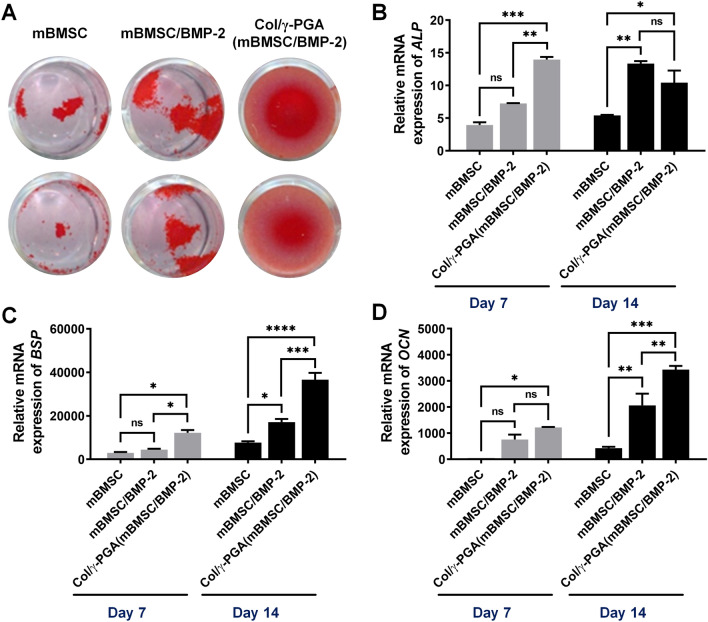
The osteogenic differentiation of mBMSCs in Col/γ-PGA hydrogels was assessed by staining with ARS, ALP Live Stain, and osteogenic gene expression levels were determined by qRT-PCR. ARS staining revealed calcium deposition as a result of osteogenesis at 7 days after culture of mBMSCs with osteogenic differentiation medium on 24-well plates (Fig. 4A). The largest red-stained area was observed in Col/γ-PGA (mBMSC/BMP-2) while mBMSCs cultured with BMP-2 (mBMSC/BMP-2) on the plate showed larger red-stained are than mBMSCs cultured without BMP-2. Also, ALP Live Staining showed ALP activity as a result of osteogenesis at 3 and 7 days after culture of mBMSCs with osteogenic differentiation medium. A higher green fluorescent signal was observed in Col/γ-PGA (mBMSC/BMP-2) than mBMSC and mBMSC/BMP-2 (Fig. S5). To quantitatively evaluate the level of osteogenic differentiation, the gene expressions of osteogenic markers such as alkaline phosphatase (ALP), bone sialoprotein (BSP), and osteocalcin (OCN) were analyzed by qRT-PCR at 7 and 14 days after culture of mBMSCs under osteogenic conditions. The results showed that the expression levels of all genes (ALP, BSP, and OCN) were higher in the Col/γ-PGA (mBMSC/BMP-2) than mBMSC/BMP-2 and mBMSC groups (Fig. 4B–D). Specifically, the results showed significant differences in the expression levels of osteogenic markers within only 7 or 14 days. It has been reported that ALP is most highly expressed in the mature osteoblast stage and tends to show decreased expression in the late osteoblast stage [49]. Accordingly, it indicated that the Col/γ-PGA (mBMSC/BMP-2) group was in the late osteoblast stage at 14 days past the mature osteoblast stage at 7 days, based on the result that ALP increased at 7 days and decreased at 14 days (Fig. 4B). BSP is particularly abundant in the region of bone formation and is a potent mineralization nucleator [50]. The expression level of BSP in the Col/γ-PGA (mBMSC/BMP-2) group increased 2.1-fold compared with that in the mBMSC/BMP2 group and 4.7-fold compared with that in the mBMSC group at 14 days (Fig. 4C). OCN is a bone-specific protein and a well-known osteogenic marker associated with bone turnover for osteogenic maturation [51]. The expression level of OCN in the Col/γ-PGA (mBMSC/BMP-2) group increased 1.7-fold compared with that in the mBMSC/BMP2 group and eightfold compared with that in the mBMSC group at 14 days (Fig. 4D).
Fig. 4.
In vitro osteogenesis of mBMSCs and BMP-2 embedded in Col/γ-PGA hydrogel under osteogenesis conditions. A ARS staining at 7 days after culturing in differentiation medium on 24-well plates. Relative mRNA expression of B ALP, C BSP, and D OCN measured by qRT-PCR at 7 and 14 days after culturing in differentiation medium. Relative mRNA expression was normalized to the control (mBMSC culture in normal medium). Data are presented as the mean ± SD (*p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001; ns not significant)
In vivo mBMSC-based bone regeneration in mouse calvarial bone defect model
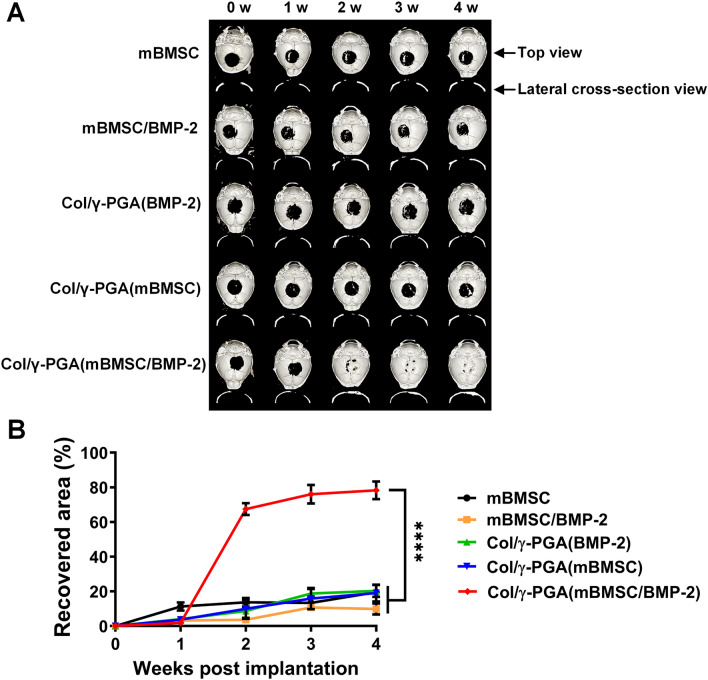
The efficacy of mBMSC-based bone regeneration using Col/γ-PGA hydrogel was evaluated in a mouse model with a critical-sized and full-thickness bone defect. The cranial bones of the mice were monitored by μ-CT at 0, 1, 2, 3, and 4 weeks after the implantation of each experimental sample. The results showed that the area of bone defect in Col/γ-PGA (mBMSC/BMP-2)-implanted mice almost completely recovered in only 3 weeks, while the recoveries in all other experimental groups were less than 20%, even at 4 weeks after implantation (Fig. 5A). In the quantitative analysis of the recovery rate against calvarial bone defect, bone defect in Col/γ-PGA (mBMSC/BMP-2)-implanted mice quickly recovered from 1 to 2 weeks (Fig. 5B). These results indicate that mBMSCs, BMP-2, and Col/γ-PGA hydrogel were essential for effective bone regeneration and that Col/γ-PGA hydrogel was an effective scaffold for the proliferation and osteogenic differentiation of mBMSCs as well as the delivery of BMP-2.
Fig. 5.
In vivo bone regeneration by mBMSCs and BMP-2 embedded in Col/γ-PGA hydrogel after implantation in a critical-sized calvarial bone defect model. A Representative images of μ-CT analysis and the calvarial bones for the indicated groups, and B quantitative analysis of the recovered area at 1, 2, 3, and 4 weeks after implantation. The recovered area, which represents the healing of defects, was evaluated using μ-CT images. Data are presented as the mean ± SD (****p < 0.0001)
Histological analysis of the orthotopic bone formation
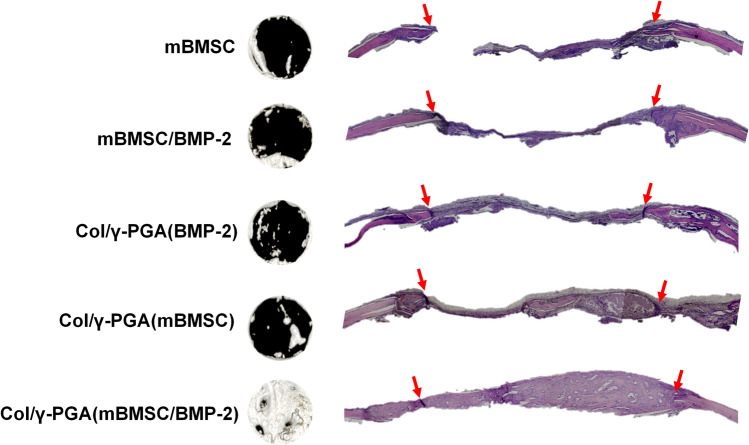
Histological analysis showed that the Col/γ-PGA (mBMSC/BMP-2) group had formed complete bony bridging in the defect areas at 4 weeks after implantation (Fig. 6), while bony bridging was not observed in the mBMSC and mBMSC/BMP-2 groups. Although the bone formation represented partial regeneration in the Col/γ-PGA (mBMSC) group, more bone tissue was produced in the Col/γ-PGA (mBMSC/BMP-2) group, which suggested the synergistic effect of mBMSC with BMP2. These results suggested that Col/γ-PGA hydrogel is extremely promising for the efficient culture of BMSCs as well as the delivery of BMP2 for bone regeneration.
Fig. 6.
H&E-stained histological sections and analysis of the recovered area at 4 weeks by mBMSCs and BMP-2 embedded in Col/γ-PGA hydrogel after implantation in a critical-sized calvarial bone defect model. The region between the two red arrows indicates the defect area
In this study, we developed an optimized Col/γ-PGA hydrogel system for BMSC-based bone regeneration to treat calvarial bone defects. The Col/γ-PGA hydrogel was shown to be a free-flowing sol at 4 °C and ambient temperature, but it turned into a non-flowing gel at body temperature. While the viscosity of Col/γ-PGA hydrogel was lower than that of collagen only at both 4 °C and 25 °C, the moduli (G′ and G″) of Col/γ-PGA gel at body temperature increased compared with those of collagen only. Because of these properties, the Col/γ-PGA hydrogel could be injected directly into tissues and molded into specific shapes. Furthermore, the Col/γ-PGA hydrogel at 37 °C showed an interconnected porous structure cross-section, which would provide space for the effective binding and subsequent proliferation of stem cells inside them. The Col/γ-PGA (mBMSC/BMP-2) group in both in vitro and in vivo experiments showed excellent osteogenesis performance in a short period. Taking these findings together, the Col/γ-PGA hydrogel scaffold could be a powerful platform in tissue engineering and regeneration to treat bone defects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Grant Nos. 2020R1A2C3006888, 2021R1C1C2003091), Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program, and an “R&D Convergence Program” of National Research Council of Science and Technology (NST) grant from the Korea government (MSIT) (Grant No. CAP-18-02-KRIBB).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
All animal experiments were performed following protocols approved by the Animal Care and Use Committee of the Korean Research Institute of Bioscience and Biotechnology (KRIBB) (Approval No. KRIBB-AEC-14154).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sun-Hee Cho, Keun Koo Shin, and Sun-Young Kim have contributed equally to this study (as co-first authors).
Doo-Byoung Oh and Yong Taik Lim have contributed equally to this study (as co-corresponding authors).
Contributor Information
Doo-Byoung Oh, Email: dboh@kribb.re.kr.
Yong Taik Lim, Email: yongtaik@skku.edu.
References
- 1.Gao X, Usas A, Tang Y, Lu A, Tan J, Schneppendahl J, et al. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials. 2014;35:6859–6870. doi: 10.1016/j.biomaterials.2014.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon JS, Kim SW, Kwon DY, Park SH, Son AR, Kim JH, et al. In vivo osteogenic differentiation of human turbinate mesenchymal stem cells in an injectable in situ-forming hydrogel. Biomaterials. 2014;35:5337–5346. doi: 10.1016/j.biomaterials.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Park HJ, Yu SJ, Yang K, Jin Y, Cho AN, Kim J, et al. Paper-based bioactive scaffolds for stem cell-mediated bone tissue engineering. Biomaterials. 2014;35:9811–9823. doi: 10.1016/j.biomaterials.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Singh J, Onimowo JO, Khan WS. Bone marrow derived stem cells in trauma and orthopaedics: a review of the current trend. Curr Stem Cell Res Ther. 2015;10:37–42. doi: 10.2174/1574888x09666140710105141. [DOI] [PubMed] [Google Scholar]
- 5.Xu XL, Yi F, Pan HZ, Duan SL, Ding ZC, Yuan GH, et al. Progress and prospects in stem cell therapy. Acta Pharmacol Sin. 2013;34:741–746. doi: 10.1038/aps.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10:77–89. doi: 10.1021/mp3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 8.Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15:718–746. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen X, Zhang Y, Gu Y, Xu Y, Liu Y, Li B, et al. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials. 2016;106:205–216. doi: 10.1016/j.biomaterials.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Armiento AR, Hatt LP, Sanchez Rosenberg G, Thompson K, Stoddart MJ. Functional biomaterials for bone regeneration: a lesson in complex biology. Adv Funct Mater. 2020;30:1909874. [Google Scholar]
- 11.Li L, Zhou G, Wang Y, Yang G, Ding S, Zhou S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218–229. doi: 10.1016/j.biomaterials.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Wang Z, Jiang Y, Liu H, Song S, Wang C, et al. Biomimetic composite scaffolds to manipulate stem cells for aiding rheumatoid arthritis management. Adv Func Mater. 2019;29:1807860. [Google Scholar]
- 13.Yun HM, Ahn SJ, Park KR, Kim MJ, Kim JJ, Jin GZ, et al. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials. 2016;85:88–98. doi: 10.1016/j.biomaterials.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 14.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aryal R, Chen XP, Fang C, Hu YC. Bone morphogenetic protein-2 and vascular endothelial growth factor in bone tissue regeneration: new insight and perspectives. Orthop Surg. 2014;6:171–178. doi: 10.1111/os.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poon B, Kha T, Tran S, Dass CR. Bone morphogenetic protein-2 and bone therapy: successes and pitfalls. J Pharm Pharmacol. 2016;68:139–147. doi: 10.1111/jphp.12506. [DOI] [PubMed] [Google Scholar]
- 17.Jung T, Lee JH, Park S, Kim YJ, Seo J, Shim HE, et al. Effect of BMP-2 delivery mode on osteogenic differentiation of stem cells. Stem Cells Int. 2017;2017:7859184. doi: 10.1155/2017/7859184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Li J, Li C, Yu Y. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol Med Rep. 2015;12:4230–4237. doi: 10.3892/mmr.2015.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Bialy I, Jiskoot W, Reza Nejadnik M. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm Res. 2017;34:1152–1170. doi: 10.1007/s11095-017-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh Z, Javaid MA, Hamdan N, Hashmi R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials (Basel) 2015;8:1778–1816. doi: 10.3390/ma8041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013;2:447. doi: 10.1038/bonekey.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 24.Jokinen J, Dadu E, Nykvist P, Käpylä J, White DJ, Ivaska J, et al. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279:31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 25.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gullberg D, Gehlsen KR, Turner DC, Ahlén K, Zijenah LS, Barnes MJ, et al. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell–collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J. 1992;11:3865–3873. doi: 10.1002/j.1460-2075.1992.tb05479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoof H, Apel J, Heschel I, Rau G. Control of pore structure and size in freeze-dried collagen sponges. J Biomed Mater Res. 2001;58:352–357. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 28.Wang YK, Chen CS. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J Cell Mol Med. 2013;17:823–832. doi: 10.1111/jcmm.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Hesse E, Hefferan TE, Tarara JE, Haasper C, Meller R, Krettek C, et al. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J Biomed Mater Res A. 2010;94:442–449. doi: 10.1002/jbm.a.32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano A, Hojo T, Maeda M, Fujioka K. Protein release from collagen matrices. Adv Drug Deliv Rev. 1998;31:247–266. doi: 10.1016/s0169-409x(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 32.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14:1269–1277. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 34.Seo JH, Kim CS, Lee SP. Physicochemical properties of poly-γ-glutamic acid produced by a novel bacillus subtilis HA isolated from Cheonggukjang. Prev Nutr Food Sci. 2008;13:354–361. [Google Scholar]
- 35.Lee EH, Kamigaito Y, Tsujimoto T, Uyama H, Sung MH. Synthesis of an amphiphilic poly(gamma-glutamic acid)-cholesterol conjugate and its application as an artificial chaperone. J Microbiol Biotechnol. 2010;20:1424–1429. doi: 10.4014/jmb.1006.06004. [DOI] [PubMed] [Google Scholar]
- 36.Shin EJ, Sung MJ, Park JH, Yang HJ, Kim MS, Hur HJ, et al. Poly-gamma-glutamic acid induces apoptosis via reduction of COX-2 expression in TPA-induced HT-29 human colorectal cancer cells. Int J Mol Sci. 2015;16:7577–7586. doi: 10.3390/ijms16047577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee EH, Son WC, Lee SE, Kim BH. Anti-obesity effects of poly-γ-glutamic acid with or without isoflavones on high-fat diet induced obese mice. Biosci Biotechnol Biochem. 2013;77:1694–1702. doi: 10.1271/bbb.130253. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Gao FP, Tang HB, Bai YG, Li RF, Li XM, et al. Self-assembled nanoparticles of cholesterol-conjugated carboxymethyl curdlan as a novel carrier of epirubicin. Nanotechnology. 2010;21:265601. doi: 10.1088/0957-4484/21/26/265601. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki T, Kuramoto A, Hirakawa A, Shirosaki Y, Ohtsuki C. Biomineralization on chemically synthesized collagen containing immobilized poly-gamma-glutamic acid. Dent Mater J. 2013;32:544–549. doi: 10.4012/dmj.2012-324. [DOI] [PubMed] [Google Scholar]
- 40.Antunes JC, Tsaryk R, Goncalves RM, Pereira CL, Landes C, Brochhausen C, et al. Poly(gamma-glutamic acid) as an exogenous promoter of chondrogenic differentiation of human mesenchymal stem/stromal cells. Tissue Eng Part A. 2015;21:1869–1885. doi: 10.1089/ten.tea.2014.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh CY, Tsai SP, Wang DM, Chang YN, Hsieh HJ. Preparation of gamma-PGA/chitosan composite tissue engineering matrices. Biomaterials. 2005;26:5617–5623. doi: 10.1016/j.biomaterials.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Kuo YC, Chung CY. TATVHL peptide-grafted alginate/poly(γ-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials. 2012;33:8955–8966. doi: 10.1016/j.biomaterials.2012.08.073. [DOI] [PubMed] [Google Scholar]
- 43.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 44.Nishishita N, Shikamura M, Takenaka C, Takada N, Fusak N, Kawamata S. Generation of virus-free induced pluripotent stem cell clones on a synthetic matrix via a single cell subcloning in the naïve state. PLoS One. 2012;7:e38389. doi: 10.1371/journal.pone.0038389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho SH, Kim A, Shin W, Heo MB, Noh HJ, Hong KS, et al. Photothermal-modulated drug delivery and magnetic relaxation based on collagen/poly(gamma-glutamic acid) hydrogel. Int J Nanomedicine. 2017;12:2607–2620. doi: 10.2147/IJN.S133078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho SH, Noh JR, Cho MY, Go MJ, Kim YH, Kang ES, et al. An injectable collagen/poly(gamma-glutamic acid) hydrogel as a scaffold of stem cells and alpha-lipoic acid for enhanced protection against renal dysfunction. Biomater Sci. 2017;5:285–294. doi: 10.1039/c6bm00711b. [DOI] [PubMed] [Google Scholar]
- 47.Dang JM, Sun DD, Shin-Ya Y, Sieber AN, Kostuik JP, Leong KW. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials. 2006;27:406–418. doi: 10.1016/j.biomaterials.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Zohuriaan-Mehr MJ, Pourjavadi A, Salimi H, Kurdtabar M. Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties. Polym Adv Technol. 2009;20:655–671. [Google Scholar]
- 49.Id Boufker H, Lagneaux L, Najar M, Piccart M, Ghanem G, Body JJ, et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer. 2010;10:298. doi: 10.1186/1471-2407-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouet G, Bouleftour W, Juignet L, Linossier MT, Thomas M, Vanden-Bossche A, et al. The impairment of osteogenesis in bone sialoprotein (BSP) knockout calvaria cell cultures is cell density dependent. PLoS One. 2015;10:e0117402. doi: 10.1371/journal.pone.0117402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, et al. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15:169–180. doi: 10.1089/ten.tec.2007.0334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.