Abstract
BACKGROUND:
Angiogenesis plays an important role in determining the fat graft survival. However, clinical preconditioning techniques that target angiogenesis during fat grafting have not been established so far. Adenosine has emerged as a regulator of angiogenesis under hypoxic conditions; therefore, the aim of this study was to investigate the effects and underlying mechanisms of adenosine prefabrication on fat graft survival.
METHODS:
In the first animal study, a total of 32 mice were transplanted with fat prefabricated with vehicle (Control, N = 16) or adenosine (Adenosine, N = 16). In the second animal study, 24 mice were divided into three groups based on the type of fat graft: Control (N = 8), Adenosine (N = 8), and Axitinib (cotreatment of adenosine with axitinib, N = 8). At 1- and 4-weeks post-transplantation, grafts were evaluated by histopathological and biochemical assessment. Adenosine-induced vascular endothelial growth factor (VEGF) production and angiogenesis were determined using cell cultures.
RESULTS:
The retention volumes of fat grafts in the adenosine group were significantly increased until 4 weeks. Fat grafts from the adenosine group exhibited greater structural integrity, reduced fibrosis, and increased blood vessels. The expression levels of angiogenesis-related genes, Vegfa, Vegfr1, Vegfr2, and Vwf, were elevated in the adenosine group. Furthermore, adenosine upregulated VEGF production in preadipocytes, thereby enhancing the migration of endothelial cells. Treatment with the axitinib, VEGF receptor inhibitor, abrogated the adenosine-induced angiogenesis in the fat grafts.
CONCLUSION:
Adenosine prefabrication in fat improved the graft survival by enhancing angiogenesis through the VEGF/VEGFR axis in the preadipocytes and endothelial cells. Therefore, this method may be used as a novel strategy to increase the retention rate in fat grafts.
Keywords: Adenosine, Vascular endothelial growth factor, Prefabrication, Fat graft
Introduction
Autologous fat is recognized as an ideal filler due to its biocompatibility, natural appearance, abundance, and ease of availability; therefore, it is generally used for soft tissue augmentation during aesthetic and plastic surgeries [1]. However, the major drawback of an autologous fat graft is its unpredictable survival rate, with a graft volume loss of 20–80% in the long term [2, 3]. Owing to the nonvascular nature of the procedure, the wide variation in the survival rate may be attributed to the differential exposure to hypoxia conditions, until neoangiogenesis occurs [4]. Several techniques have been proposed to reduce the duration of hypoxia exposure by modifying the four basic steps of grafting: donor site selection, adipose tissue harvesting, fat processing (centrifugation, standing, filtration, and washing), and fat transplantation [5, 6]. Yet the wide variations in the outcomes of fat grafting have not been completely resolved so far.
Recent studies showed that the injection of drugs (such as growth hormones, estrogen, and cytokines), referred to as drug-assistant transfer, increased the survival rate of the fat graft [7–10]. Furthermore, this technique increased neoangiogenesis, thereby implying its importance for the survival of the graft. However, repeated injections of the drug are required during and after fat grafting, which might cause safety concerns and prohibit approvals from the FDA. The identification of novel methods to increase angiogenesis without any safety concerns has gained popularity in recent years. Thus, the prefabrication of donor fat tissues could prove useful for this purpose.
Adenosine, an endogenous metabolite of adenosine triphosphate, plays an important role in various processes (such as inflammation, glucose and lipid homeostasis, and angiogenesis) via adenosine receptors, which are present in many types of cells [11]. Several studies have demonstrated that adenosine is produced in a hypoxic environment; additionally, it mediates the hypoxia-induced process by suppressing inflammation and promoting angiogenesis [12–17]. Furthermore, the regulatory role of adenosine in inflammation and angiogenesis has been reported in adipocytes and macrophages. These findings indicate that adenosine can be used during drug-assisted transfer to regulate inflammation and neoangiogenesis in the fat graft. However, to the best of our knowledge, the effects of adenosine on the survival rate of the fat graft has not been evaluated so far.
On the basis of the regulatory function of adenosine in inflammation and angiogenesis, we hypothesized that adenosine-prefabricated fat tissue transfer could increase the survival rate of the graft by modulating both inflammation and angiogenesis. Here, using the mice model and cell cultures, we show that adenosine prefabrication enhances the survival rate of the fat graft by promoting vascular endothelial growth factor (VEGF)-dependent angiogenesis.
Materials and methods
Animal studies
All animal procedures were conducted after obtaining ethical approval and were based on the guidelines of the Institutional Animal Care and Use Committee of Soonchunhyang University (IACUC Approval No. SCH20-U-104). 7-week-old C57BL/6 mice were purchased from Orient Bio (Sungnam, Korea) and housed at the SIMS Laboratory Animal Research Center, Soonchunhayng University (12:12-h light–dark cycle, maintained at 25°C). In the first animal study, a total of 32 mice were used as recipient and transplanted with fat prefabricated with vehicle (Control, N = 16) or adenosine (Adenosine, N = 16). For the fat tissue harvesting, mice at 9 weeks of age were euthanized and their epididymal adipose tissues were isolated, homogenized using blades, and treated with Krebs–Ringer bicarbonate–HEPES (KRBH buffer) containing vehicle or 500 nM adenosine. Subsequently, 0.2 mL of the prefabricated fat was injected into the subcutaneous layer between the ears using a Luer-Lok tip syringe (BD, Franklin Lakes, NJ, USA) and an 18-G needle. In the second animal study, 24 mice were divided into three groups based on the type of fat graft: Control (N = 8), Adenosine (N = 8), and Axitinib (cotreatment of adenosine with axitinib, N = 8). Donor fat tissues were isolated, homogenized, and treated with KRBH buffer containing vehicle, 500 nM adenosine, adenosine with 1 μM axitinib. For axitinib treatment, adipose tissues were pretreated with VEGFR inhibitor axitinib 5 min prior to adenosine treatment. Donor fat was injected to the recipient mice as previously described. One and four weeks after the surgical procedure, the recipients were euthanized, and the fat grafts were harvested and fixed in 10% neutral buffered formaline or frozen for further experiments.
Micro-computed tomography (micro-CT)
The volume of the fat graft was determined using high-resolution micro-CT, as described previously [18]. Briefly, at 1–4 weeks after fat transplantation, the mice were anesthetized via isoflurane inhalation and exposed to X-ray radiation. Images were taken at 90 kV and 88 μA using the Quantum GX2 micro-CT imaging system (PerkinElmer, Waltham, MA, USA) and analyzed using the Caliper Analyze 12.0 (AnalyzeDirect Inc., Overland Park, KS, USA) at the Soonchunhyang Biomedical Research Core Facility.
Histological analysis
The fat graft tissues were fixed, paraffinized, sectioned to a thickness of 0.5 μm, and placed on a slide. After deparaffinization, all the samples were dehydrated in ethanol and processed for hematoxylin and eosin (H&E) staining. For immunostaining, fixed tissues or slides were blocked with 5% BSA-PBST (0.1% Triton X-100/PBS) and incubated with one of the following primary antibodies: caveolin-1 (Santa Cruz Biotechnology, Dallas, TX, USA, SC-53564; 1:100), galectin 3 (Mac2) monoclonal antibody (eBioscience, San Diego, CA, USA, 14–5301-82; 1:100), or isolectin conjugated with fluorescence (Thermo Fisher Scientific, Waltham, MA, USA, I32450, 1:200). A secondary antibody conjugated with fluorescence dye (Thermo Fisher Scientific, 1:200) was also used. The stained samples were visualized using a fluorescent microscope (DMi8, Leica, Wetzlar, Germany) or a confocal microscope (LSM 710, Carl Zeiss, Oberkochen, Germany).
Quantitative reverse transcription polymerase chain reaction (qPCR)
Tissue and cell culture samples were homogenized with RiboEX (Geneall, Seoul, Korea), and RNA was isolated according to the manufacturer’s protocol. RNA quality was evaluated using NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific). cDNAs were synthesized using the High-Capacity Reverse Transcription kit (Applied Bio., Waltham, MA, USA). qPCR was performed using SYBR green PCR master mix (Toyobo, Osaka, Japan) and the Quant Studio 1 Real-Time PCR system (Applied Bio.). 18 s rRNA and Arbp genes were used as internal controls for normalization. All samples were tested in duplicate and analyzed for the relative gene expression using the 2−ΔΔCT method. The primers used in this study are presented in Table 1.
Table 1.
Primers for qPCR
| Target gene | Sequence (5′-3′) |
|---|---|
| Pparγ2 | For: TCGCTGATGCACTGCCTATG |
| Rev: GAGAGGTCCACAGAGCTGATT | |
| Fabp4 | For: AAGGTGAAGAGCATCATAACCCT |
| Rev: TCACGCCTTTCATAACACATTCC | |
| Lpl | For: GGGAGTTTGGCTCCAGAGTTT |
| Rev: TGTGTCTTCAGGGGTCCTTAG | |
| Tnf | For: CCCTCACACTCAGATCATCTTCT |
| Rev: GCTACGACGTGGGCTACAG | |
| Il6 | For: TAGTCCTTCCTACCCCAATTTCC |
| Rev: TTGGTCCTTAGCCACTCCTTC | |
| Il1β | For: GCAACTGTTCCTGAACTCAACT |
| Rev: ATCTTTTGGGGTCCGTCAACT | |
| Nos2 | For: CCAAGCCCTCACCTACTTCC |
| Rev: CTCTGAGGGCTGACACAAGG | |
| Arg1 | For: CTCCAAGCCAAAGTCCTTAGAG |
| Rev: AGGAGCTGTCATTAGGGACATC | |
| Mcp1 | For: TTAAAAACCTGGATCGGAACCAA |
| Rev: GCATTAGCTTCAGATTTACGGGT | |
| Mgl1 | For: TGAGAAAGGCTTTAAGAACTGGG |
| Rev: GACCACCTGTAGTGATGTGGG | |
| Cd11b | For: GGGAGGACAAAAACTGCCTCA |
| Rev: ACAACTAGGATCTTCGCAGCAT | |
| Emr1 | For: CCCCAGTGTCCTTACAGAGTG |
| Rev: GTGCCCAGAGTGGATGTCT | |
| Pecam1 | For: GAGCCCAATCACGTTTCAGTTT |
| Rev: TCCTTCCTGCTTCTTGCTAGCT | |
| Vegfa | For: GCACATAGAGAGAATGAGCTTCC |
| Rev: CTCCGCTCTGAACAAGGCT | |
| Vegfc | For: GAGGTCAAGGCTTTTGAAGGC |
| Rev: CTGTCCTGGTATTGAGGGTGG | |
| Vegfr1 | For: GTGAGCACTGCGGCAAAAAG |
| Rev: ACTCATTTTGGGAGGAGCGT | |
| Vegfr2 | For: CAGGCAACATCGGTCCACAT |
| Rev: TGTGCCAGCCTACTACAACA | |
| Vwf | For: CTTCTGTACGCCTCAGCTATG |
| Rev: GCCGTTGTAATTCCCACACAAG |
Pparγ2, Peroxisome proliferator-activated receptor gamma 2; Fabp4, Fatty acid-binding protein 4; Lpl, Lipoprotein lipase; Tnf, Tumor necrosis factor; Il6, Interleukin 6; Il1β, interleukin 1 beta; Nos2, Nitric oxide synthase 2; Arg1, Arginase 1; Mcp1, Monocyte chemoattracted protein 1; Mgl1, Macrophage galactose-type lectin 1; Cd11b, Integrin alpha M subunit; Emr1, Epidermal growth factor-like module containing mucin-like hormone receptor 1; Pecam1, Platelet and endothelial cell adhesion molecule 1; Vegfa, Vascular endothelial growth factor A; Vegfc, Vascular endothelial growth factor C; Vegfr1, Vascular endothelial growth factor receptor 1; Vegfr2, Vascular endothelial growth factor receptor 2; Vwf, Von Willebrand factor
Cell culture and enzyme-linked immunosorbent assay (ELISA)
Murine 3T3-L1 preadipocytes were cultured in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific) containing 10% fetal bovine serum in the absence or presence of adenosine for 24 h. The media were collected and used to measure the amounts of VEGF. VEGF amount was determined using the mouse VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA).
Cell migration assay
Human umbilical vein endothelial cells (HUVECs) were cultured in RPMI1640 media supplemented with 20% FBS and 1% LSGS with penicillin–streptomycin. For the cell migration assay, HUVECs were seeded (5 × 105/well) onto a 6-well plate. After the attachment of the cells, a sterile pipette tip was used to create a scratch on the bottom of the well. The scratched cells were incubated with 50% diluted CM from the vehicle or adenosine-treated preadipocytes for 16 h. An inverted microscope (DMi8, Leica) was used to capture images of the cell migration, and an ImageJ program (NIH) was used to quantify the extent of migration.
Statistical analysis
All values are presented as mean standard error of the mean. The statistical significance was determined using the unpaired two-tailed Student’s t-test for independent groups. All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). A p-value of < 0.05 was considered statistically significant.
Results
Adenosine prefabrication improved fat graft survival at 4 weeks
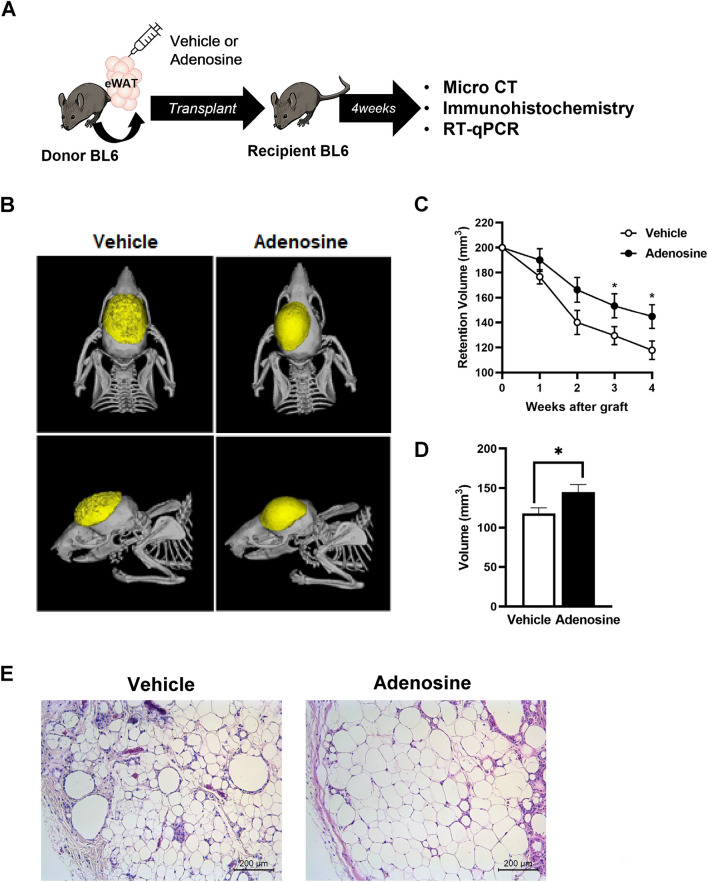
The donor fat tissue was prefabricated using KRBH buffer alone (control group) or in combination with 500-nM adenosine (adenosine group) before transplantation into the recipient mice (Fig. 1A). The retention volume of the implanted fat was measured every week using micro-CT. The shape of the fat tissues was substantially retained, whereas the volume retention was significantly increased at 3- and 4-weeks post-transplantation in the adenosine group than in the control group (Fig. 1B, C). Quantitative analysis revealed that fat graft volume was ~ 1.5-fold higher in the adenosine group than in the control group (Fig. 1D). Additionally, H&E staining demonstrated that the integrity of the structure of the adipose tissue was superior in the adenosine group, implying less shrinkage of adipocytes and fibrosis in this group than in the control group (Fig. 1E).
Fig. 1.
Adenosine treatment increased the survival rate of the fat graft at 4 weeks after surgery. A Schematic design of the fat grafting process and further experiments. B Representative micro-computed tomography (micro-CT) image. C The volumes of the grafts were measured during the 4 weeks after grafting. D The final volumes of the fat grafts in each group. Data are presented as mean ± standard error of the mean (SEM) *p < 0.05. E Representative hematoxylin and eosin (H&E) staining of grafted fat from the control and adenosine-treated groups 4 weeks after surgery
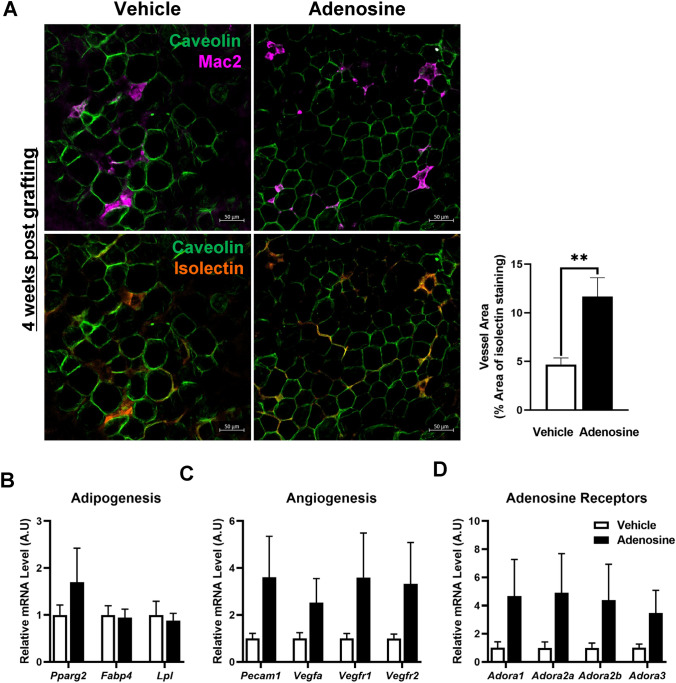
Immunostaining with caveolin and Mac2 showed that the adenosine group had a greater number of viable adipocytes and fewer pro-inflammatory macrophages compared with the control group. Isolectin immunofluorescent images, which represent the blood vessel, showed that the blood vessel area in the adenosine group was ~ 2.5-fold higher than that in the control group (Fig. 2A).
Fig. 2.
Adenosine treatment enhanced adipocyte viability in the fat grafts 4 weeks after surgery. A Representative immunofluorescence image of the grafted fat stained with caveolin (green), Mac2 (magenta), and isolectin (orange). The vessel area was calculated from the percentage of the total area using ImageJ. B–D mRNA was isolated from the grafted fat, and the gene expression levels were analyzed using qRT-PCR. B Adipogenesis-related genes, C angiogenesis-related genes, and D adenosine receptor genes. Data are presented as means ± SEM; **p < 0.01
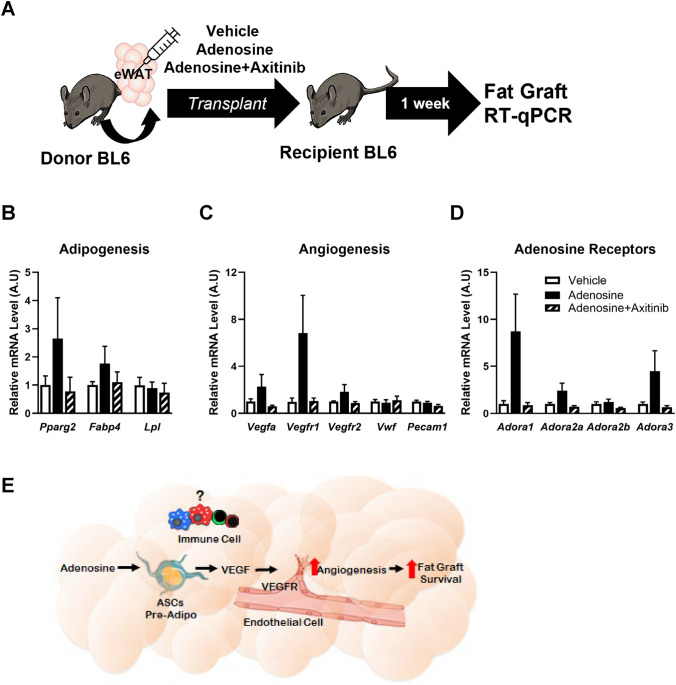
Next, we examined the adipogenic and angiogenic gene expressions in fat grafts 4 weeks post-transplantation. The mRNA expression levels of Fabp4 and Lpl were incomparable between the two groups; however, the expression level of Pparg2, a master regulator of adipogenesis, was ~ 50% higher in the adenosine group compared with the control group (Fig. 2B). Additionally, all the angiogenesis-related genes, Pecam1, Vega, Vegfr1, and Vegfr2, and the adenosine receptor genes, Adora1, Adora2a, Adora2b, and Adora3, showed increasing trends in the adenosine group (Fig. 2C, D). Collectively, the results of the histological, and biochemical analyses indicated that adenosine prefabrication improved the survival of the fat graft.
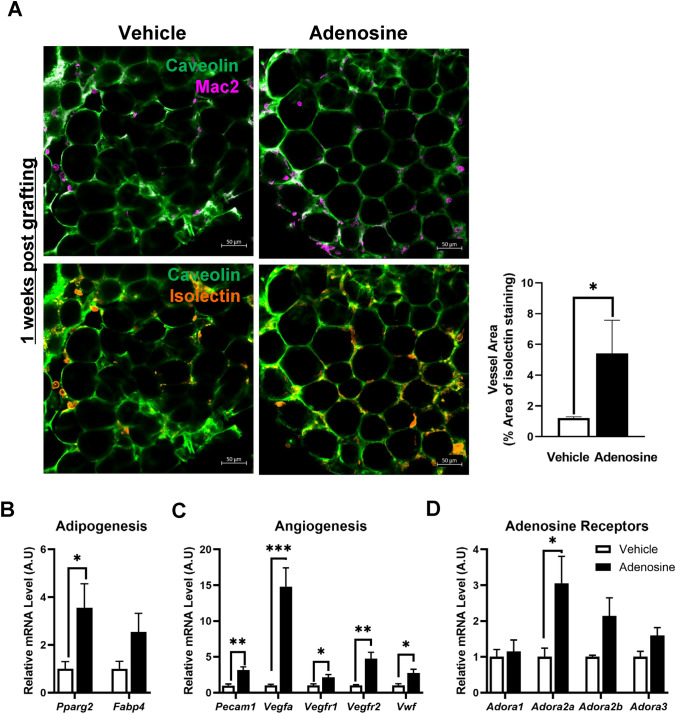
Adenosine prefabrication increased angiogenesis 1 week after the fat graft
Since angiogenesis in the early stage of graft is the critical factor in the fat survival, we determined the histological and transcriptional changes in fat graft at 1 week post transplantation. Immunofluorescent staining analysis showed that fat graft from the adenosine group had significantly increased vessel areas whereas they had comparable amounts of macrophages (Fig. 3A). In the adenosine- group, the expression levels of both Pparg2 and Fabp4 were up-regulated; the increase in Pparg2 was significantly higher than that in the control group (Fig. 3B). All the angiogenesis-related genes, including Pecam1, Vegfa, Vegfr1, Vegfr2, and Vwf, were significantly increased in the adenosine group; in particular, the expression of the key angiogenesis regulator Vegfa was increased by 15-fold (Fig. 3C). Additionally, adenosine prefabrication resulted in the markedly enhanced expression of the adenosine receptor gene Adora2a (Fig. 3D). Taken together, these findings indicated that adenosine prefabrication increased angiogenesis in the fat graft 1-week post-transplantation, which led to improved survival of the fat graft.
Fig. 3.
Adenosine treatment enhanced adipocyte viability in the fat graft 1 week after surgery. A Immunofluorescence staining, with caveolin (green), Mac2 (magenta), and isolectin (orange) in grafted fat from each group 1 week after fat grafting. B–D mRNA was isolated from the grafted fat 1-week and analyzed using qRT-PCR. B Adipogenesis-related genes, C angiogenesis-related genes, and D adenosine receptor genes. Data are presented as means ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001
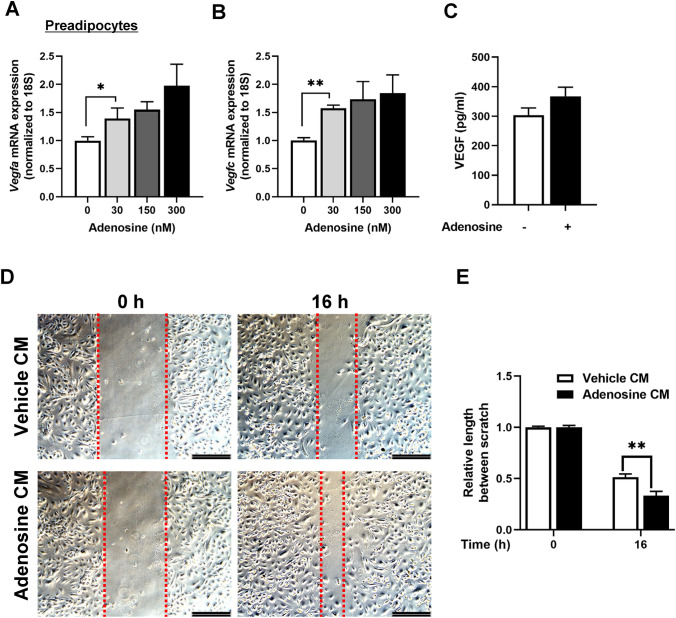
Adenosine-induced VEGF secretion from preadipocytes
On the basis of the increase in Vegfa in the adenosine group, the role of adenosine on VEGF production in adipose stem cells (preadipocytes) was evaluated. As shown in Fig. 4A–C, adenosine up-regulated the expression levels of the isoforms of VEGF, Vegfa and Vegfc, in the preadipocytes in a dose-dependent manner; additionally, the amount of VEGF in the supernatant was increased in the adenosine-treated preadipocytes. Furthermore, CM from adenosine-treated preadipocytes significantly increased the migration capacity of the endothelial cells (Fig. 4D, E). These results indicated that adenosine induced VEGF secretion in preadipocytes and promoted endothelial cell migration.
Fig. 4.
Adenosine stimulated VEGF secretion in preadipocytes and enhanced the migration of endothelial cells. A mRNA expression levels of Vegfa in preadipocytes based on the concentration of adenosine used (0, 30, 150, and 300 nM). B mRNA expression levels of Vegfc in the preadipocytes based on the concentration of adenosine (0, 30, 150, and 300 nM). C The amount of VEGF in media from preadipocytes treated with vehicle or 50-nM adenosine for 24 h, measured using ELISA. D Migration assay of HUVECs incubated with 50% diluted conditioned media (CM) from the control and adenosine treatment groups. E Quantitation of the relative length between scratch
VEGF receptor inhibitor blocked adenosine prefabrication-induced angiogenesis in the fat graft
Last, axitinib, a VEGFR inhibitor, was used to determine whether the increase in VEGF expression played a role in improving the survival of the fat graft in the adenosine group [19–22] (Fig. 5A). The expression levels of adipogenic genes, such as Pparg2 and Fabp4, were higher in the adenosine group than the control group; however, the expression levels of the genes in the adenosine group following cotreatment with axitinib were similar to those in the control group (Fig. 5B). The expression levels of angiogenesis-related genes were up-regulated in the adenosine group, but treatment with the VEGFR inhibitor abolished this effect (Fig. 5C). Furthermore, alterations in the expression levels of the adenosine receptor genes in the adenosine group were restored following treatment with axitinib (Fig. 5D). Thus, the VEGF/VEGFR axis blocker completely inhibited the adenosine prefabrication-induced alterations in the fat graft.
Fig. 5.
VEGF/VEGFR axis mediated the survival of the adenosine-induced fat graft. A Schematic diagram of fat grafting with the VEGFR inhibitor neutralizing the expression of genes induced by adenosine treatment. B–D mRNA was isolated from grafted fat in each group 1 week after surgery, and the relative gene expression levels were analyzed using RT-qPCR. B Adipogenesis-related genes, C angiogenesis-related genes, and D adenosine receptor genes. Data are presented as means ± SEM. E Proposed mechanism for improvement in the survival of the fat graft via the enhancement of angiogenesis through the VEGF/VEGFR axis using adenosine prefabrication
Discussion
Although autologous fat grafting has several advantages in terms of its ease of use, cost-effectiveness, and effect on immunology [23–26], the maintenance of the fat volume postoperatively remains erratic, thus presenting a considerable obstacle to the fat transplantation process. The retention of the grafted fat tends to vary widely depending on the recipient area and the processing technique used [27]. Fat grafting is a nonvascular procedure; hence, the transferred fat tissue is initially hypoxic, which can lead to inflammation and lipolysis. Under these unfavorable conditions, angiogenesis in the graft might prove beneficial for the survival of the transplanted adipose tissue. However, the intervention methods used to increase angiogenesis and survival need to be explored.
In this study, prefabrication of the fat graft with adenosine improved its survival by promoting angiogenesis. Adenosine increased angiogenesis through the VEGF/VEGFR axis in the adipose stem cells (preadipocytes) and endothelial cells within the fat graft (Fig. 5E). The improvement in the survival of the fat graft was achieved via the one-time treatment of adenosine during the fat processing step in fat transplantation.
The regulatory role of adenosine in angiogenesis is reported to be involved in the production of pro- and anti-angiogenic factors [21]. Adenosine is generated in tissues under ischemic/hypoxic conditions and serves as a potent vasodilator and stimulator of angiogenesis to maintain tissue oxygenation [28, 29]. Additionally, adenosine has been shown to stimulate the migration and proliferation of endothelial cells and tube formation in cell culture systems [28–31]. Consistent with these findings, we observed that adenosine prefabrication increased angiogenesis in the fat graft, thus implying that the fat graft was in a hypoxic condition. Furthermore, adenosine prefabrication improved the survival of the fat graft by increasing the retention volume, the number of viable adipocytes, and expression levels of the adipogenic genes at 4 weeks post-transplantation (Figs. 1 and 2). Moreover, the vessel areas and expression levels of angiogenic genes were significantly increased 1 week post-transplantation. These results indicate that the angiogenesis of the graft might prove critical for the transplanted adipose tissue; additionally, adenosine might be considered a novel factor that can improve the survival of the fat graft by forming new capillary networks.
VEGF is a well-known regulator of angiogenesis [22]. Numerous studies have shown that VEGF supplementation leads to neoangiogenesis, and the inhibition of VEGFRs blocks the formation of new vessels. In agreement with the findings of these studies, increased expression of Vegfa was observed in the adenosine group (Figs. 2 and 3), and this expression was hindered following the addition of the VEGFR inhibitor axitinib (Fig. 5). These observations suggest that adenosine prefabrication induces angiogenesis through the VEGF/VEGFR axis, thus highlighting the importance of this axis in the regulation of angiogenesis. Among the various types of cells used in this study, including preadipocytes, macrophages, endothelial cells, and adipocytes, preadipocytes participated in the VEGF/VEGFR axis-mediated angiogenesis in the fat grafts. In vitro cell culture experiments demonstrated that VEGF expression in preadipocytes was significantly increased using 30-nM adenosine, which represents the concentration observed during hypoxic conditions (Fig. 4). Although preadipocytes are known to express adenosine receptors [32, 33], there are no reports on the secretion of VEGF in response to adenosine in these cells. Notably, the angiogenetic property of preadipocyte-derived VEGF was validated by the enhanced migration of endothelial cells (Fig. 4). In line with our observation, a recent study reported that the interaction between endothelial and adipose stem cells is necessary for the promotion of angiogenesis [34]. Thus, the results of the current study suggest that crosstalk between preadipocytes and endothelial cells via the VEGF/VEGFR axis could mediate adenosine-induced angiogenesis in the fat graft.
Adenosine prefabrication during fat grafting has several clinical advantages. First, patient safety is guaranteed because adenosine is not directly injected into the recipient but is processed in vitro via fat processing. A one-time treatment of adenosine during fat processing is sufficient to improve the survival of the fat graft. Therefore, this procedure does not require repeated injections of adenosine, which is a major concern in the drug-assisted transfer procedure. Second, this procedure can be easily applied to several other fat grafting procedures, such as micro-fat or nano-fat injections. However, because the experimental analysis in this study was performed in animal models, additional studies are required to determine the effects in humans. Moreover, the long-term effects on the survival rate need to be assessed in the future.
In conclusion, adenosine prefabrication improved the fat graft survival rate by enhancing angiogenesis via the VEGF/VEGFR axis in preadipocytes and endothelial cells. To the best of our knowledge, this is the first study to identify a new intervention method to increase fat graft survival and elucidate the underlying mechanisms, particularly at the cellular level. On the basis of these findings, adenosine prefabrication can be utilized as a novel strategy to increase the fat retention rate in autologous fat grafts.
Acknowledgements
This study was supported by the Soonchunhyang University Research Fund and the National Research Foundation of Korea (NRF) grant funded by the Korean government (2019R1A2C1084684).
Author’s contributions
J.C., W.J.S, C.Y.C, and K.W.C. conceived the study and developed the study design; J.C., S.S., and S.S. generated data; W.J.S., Y.J.K., C.Y.C, and K.W.C interpreted the data; W.J.S., C.Y.C., and K.W.C. wrote the manuscript.
Declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal studies were performed after receiving approval from the Institutional Animal Care and Use Committee (IACUC) at Soonchunhyang University (IACUC Approval No. SCH20-U-104).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiyeon Chang and Woo Jin Song have been contributed equally to this study.
Contributor Information
Chang Yong Choi, Email: 73120@schmc.ac.kr.
Kae Won Cho, Email: kwcho@sch.ac.kr.
References
- 1.Fredman R, Katz AJ, Hultman CS. Fat Grafting for Burn, Traumatic, and Surgical Scars. Clin Plast Surg. 2017;44:781–791. doi: 10.1016/j.cps.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: latest insights. Ann Med Surg (Lond) 2019;37:47–53. doi: 10.1016/j.amsu.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 4.Kerfant N, Albacete G, Guernec A, Inizan M, Amerand A, Hu W, et al. Fat grafting: early hypoxia, oxidative stress, and inflammation developing prior to injection. J Plast Reconstr Aesthet Surg. 2020;73:1775–1784. doi: 10.1016/j.bjps.2020.05.088. [DOI] [PubMed] [Google Scholar]
- 5.Shim YH, Zhang RH. Literature review to optimize the autologous fat transplantation procedure and recent technologies to improve graft viability and overall outcome: a systematic and retrospective analytic approach. Aesthetic Plast Surg. 2017;41:815–831. doi: 10.1007/s00266-017-0793-3. [DOI] [PubMed] [Google Scholar]
- 6.Vyas KS, Vasconez HC, Morrison S, Mogni B, Linton S, Hockensmith L, et al. Fat graft enrichment strategies: a systematic review. Plast Reconstr Surg. 2020;145:827–841. doi: 10.1097/PRS.0000000000006557. [DOI] [PubMed] [Google Scholar]
- 7.Luo S, Hao L, Li X, Yu D, Diao Z, Ren L, et al. Adipose tissue-derived stem cells treated with estradiol enhance survival of autologous fat transplants. Tohoku J Exp Med. 2013;231:101–110. doi: 10.1620/tjem.231.101. [DOI] [PubMed] [Google Scholar]
- 8.Park B, Kong JS, Kang S, Kim YW. The effect of epidermal growth factor on autogenous fat graft. Aesthetic Plast Surg. 2011;35:738–744. doi: 10.1007/s00266-011-9679-y. [DOI] [PubMed] [Google Scholar]
- 9.Shoshani O, Livne E, Armoni M, Shupak A, Berger J, Ramon Y, et al. The effect of interleukin-8 on the viability of injected adipose tissue in nude mice. Plast Reconstr Surg. 2005;115:853–859. doi: 10.1097/01.PRS.0000153036.71928.30. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Dai J, Xu Y, Yu L, Wang X. Liquid phase concentrated growth factor improves autologous fat graft survival in vivo in nude mice. Aesthetic Plast Surg. 2021;45:2417–2422. doi: 10.1007/s00266-021-02336-x. [DOI] [PubMed] [Google Scholar]
- 11.Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth ZH, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol. 2016;7:109. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 14.Ohisalo JJ. Effects of adenosine on lipolysis in human subcutaneous fat cells. J Clin Endocrinol Metab. 1981;52:359–363. doi: 10.1210/jcem-52-2-359. [DOI] [PubMed] [Google Scholar]
- 15.Turpin BP, Duckworth WC, Solomon SS. Perifusion of isolated rat adipose cells. Modulation of lipolysis by adenosine. J Clin Invest. 1977;60:442–8. doi: 10.1172/JCI108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merino M, Briones L, Palma V, Herlitz K, Escudero C. Role of adenosine receptors in the adipocyte-macrophage interaction during obesity. Endocrinol Diabetes Nutr. 2017;64:317–327. doi: 10.1016/j.endinu.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha HG, Kim DG, Chang J, Song Y, Jeong S, Nam SM, et al. Fasting: an effective preconditioning method to increase fat graft survival. Aesthetic Plast Surg. 2021 doi: 10.1007/s00266-021-02630-8. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Witman N, Yan D, Zhang S, Zhou M, Yan Y, et al. Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cell Res Ther. 2020;11:490. doi: 10.1186/s13287-020-02008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du X, Ou X, Song T, Zhang W, Cong F, Zhang S, et al. Adenosine A2B receptor stimulates angiogenesis by inducing VEGF and eNOS in human microvascular endothelial cells. Exp Biol Med (Maywood) 2015;240:1472–1479. doi: 10.1177/1535370215584939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auchampach JA. Adenosine receptors and angiogenesis. Circ Res. 2007;101:1075–1077. doi: 10.1161/CIRCRESAHA.107.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kling RE, Mehrara BJ, Pusic AL, Young VL, Hume KM, Crotty CA, et al. Trends in autologous fat grafting to the breast: a national survey of the American society of plastic surgeons. Plast Reconstr Surg. 2013;132:35–46. doi: 10.1097/PRS.0b013e318290fad1. [DOI] [PubMed] [Google Scholar]
- 24.Choi M, Small K, Levovitz C, Lee C, Fadl A, Karp NS. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 25.Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28:111–119. doi: 10.1016/S0094-1298(20)32343-9. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman MR, Miller TA, Huang C, Roostaien J, Wasson KL, Ashley RK, et al. Autologous fat transfer for facial recontouring: is there science behind the art? Plast Reconstr Surg. 2007;119:2287–2296. doi: 10.1097/01.prs.0000260712.44089.e7. [DOI] [PubMed] [Google Scholar]
- 27.Report on autologous fat transplantation ASPRS Ad-Hoc Committee on New Procedures, September 30, 1987. Plast Surg Nurs. 1987;7:140–141. doi: 10.1097/00006527-198700740-00027. [DOI] [PubMed] [Google Scholar]
- 28.Cronstein BN. Adenosine receptors and wound healing. Sci World J. 2004;4:1–8. doi: 10.1100/tsw.2004.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- 30.Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 31.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, et al. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90:531–538. doi: 10.1161/01.RES.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 32.Tozzi M, Novak I. Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Front Pharmacol. 2017;8:878. doi: 10.3389/fphar.2017.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gharibi B, Abraham AA, Ham J, Evans BA. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26:2112–2124. doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- 34.Kocherova I, Bryja A, Mozdziak P, Angelova Volponi A, Dyszkiewicz-Konwinska M, Piotrowska-Kempisty H, et al. Human Umbilical Vein Endothelial Cells (HUVECs) Co-Culture with Osteogenic cells: from molecular communication to engineering prevascularised bone grafts. J Clin Med. 2019;8:1602. doi: 10.3390/jcm8101602. [DOI] [PMC free article] [PubMed] [Google Scholar]