Abstract
Background
Diabetic ketoacidosis (DKA) is a critical manifestation in patients with diabetes mellitus. DKA has been conventionally diagnosed by the presence of hyperglycemia (blood glucose levels > 250 mg/dl) and metabolic acidosis (blood gas bicarbonate [HCO3−] < 18 mmol/l and pH in blood gas < 7.30). However, quantitative evaluation of serum ketone bodies has not been established. The current study investigates serum ketone body levels in patients suspected for DKA.
Methods
We have retrospectively evaluated patients with hyperglycemia whose serum ketone body levels at the outpatient clinic and emergency department were measured simultaneously with blood gas analysis during 2011–2019. Clinical backgrounds, severity of diabetes, serum ketone bodies, and blood gas factors were analyzed.
Results
Seventy-two patients were enrolled in the study, after the exclusion of patients who had ketosis due to factors other than diabetes. Serum ketone body levels were negatively correlated with the levels of blood HCO3− and pH. By receiver-operating-characteristic curve analyses, optimal cut-off values for diagnosis of DKA were determined at 6.3 mmol/l of beta-hydroxybutyrate, 1.4 mmol/l of acetoacetate, and 8.0 mmol/l of total ketone body, respectively. Moreover, serum ketone bodies appeared to effectively differentiate between type 1 and type 2 diabetes mellitus. The cut-off values were higher than those in previous reports.
Conclusions
Serum ketone body levels were thought to be useful in the diagnosis of DKA. Further investigations with increased numbers of patients are required to establish the appropriate application of serum ketone bodies in patients with DKA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-022-00581-2.
Keywords: Type 1 diabetes mellitus, Type 2 diabetes mellitus, Diabetic ketoacidosis, Ketone bodies
Introduction
Diabetic ketoacidosis (DKA) is a severe acute complication of diabetes mellitus (DM) caused by insufficient insulin effect and characterized by the triad of hyperglycemia, metabolic acidosis and hyperketonemia [1–5].
DKA is comprehensively diagnosed by medical history, clinical manifestations, and laboratory findings. Diagnostic criteria of DKA proposed in the 2001 American Diabetes Association (ADA) guidelines were plasma glucose concentration > 250 mg/dl, blood gas bicarbonate (HCO3−) level < 18 mmol/l, and blood gas pH < 7.30 [6]. Meanwhile, in the criteria of the International Society of Pediatric and Adolescent Diabetes (ISPAD), the cut-off values for DKA are plasma glucose > 200 mg/dl and blood gas HCO3− < 15 mmol/l [7]. Hyperketonemia is mentioned as a characteristic of DKA in the diagnostic criterion above [6, 7]. But the definition of ‘hyperketonemia’ is not clear; the guideline of ADA recommends the measurement of serum beta-hydroxybutyrate (βOHB) without cut-off values [6]. Moreover, the diagnostic cut-off value of serum or capillary βOHB in DKA varied in reports [7–9].
Harano, et al. previously reported serum ketone body levels in Japanese patients with type 1 DM (T1DM) and type 2 DM (T2DM) [10]. To our knowledge, there have been no investigations of ketone bodies for diagnosis of DKA in Japan. The purpose of the study was to evaluate serum ketone bodies in Japanese patients with T1DM and T2DM who had exhibited DKA.
Materials and methods
Patients
This single-center retrospective study was based in an outpatient setting (at the outpatient clinic or emergency room) at the Department of Diabetes and Endocrinology at the Japanese Red Cross Wakayama Medical Center between April 2011 and April 2019. Patients with DM (including both T1DM and T2DM) who were suspected to have DKA were enrolled in the study [6] (Supplemental Fig. 1). Briefly, (1) hyperglycemic signs include polyuria with polydipsia, weight loss, fatigue, vomiting, illness, abdominal pain, (2) symptoms of dehydration such as tachycardia, poor skin turgor, dry mucous membranes, hypotension, Kussmaul respirations, confusions, and consciousness loss, and (3) nonadherence or interruption of therapy.
Patients suspected DKA underwent measurement of serum and urine ketone bodies, then patients without simultaneous measurement of blood gas, and those who had any insulin treatment within 24 h were excluded from the study. Patients with malignancy, liver cirrhosis, severe malnutrition, hypoglycemia or alcohol abuse were excluded from the study, as were users of sodium-glucose-cotransporter-2 inhibitors. The study protocol was approved by the Japanese Red Cross Wakayama Medical Center Ethics Committee (No. 680, April 30. 2019), and all participants had the opportunity to opt-out of the research. The necessity for written informed consent from each patient was waived in this retrospective study. We used clinical information obtained in routine clinical practice, and no patients refused to participate in the study. All procedures were in accordance with the institutional ethical standards regarding human experimentation and with the Declaration of Helsinki, as revised in Fortaleza, Brazil in October 2013.
Diagnosis of DKA and DM
Diagnosis of DKA was made according to criteria from ADA: plasma glucose concentration > 250 mg/dl, blood gas bicarbonate (HCO3−) level < 18 mmol/l, and blood gas pH < 7.30 [6], and T1DM was diagnosed by Japan Diabetes Society criteria [11].
Briefly, this comprised rapid development of ketosis or ketoacidosis within 3 months of the appearance of hyperglycemic symptoms, the permanent need for insulin therapy, and positive pancreatic autoantibody or absolute insulin deficiency: serum fasting C-peptide (CPR) levels < 0.6 ng/ml. Patients with T2DM were further divided into 2 groups. We have defined patients with decreased fasting CPR levels: < 0.6 ng/ml were considered to be insulin-dependent T2DM (T2DM-IDDM), and patients with CPR levels ≥ 0.6 ng/ml were considered to be non-insulin-dependent T2DM (T2DM-NIDDM).
Clinical profiles of the patients and measurement of serum ketone bodies
Clinical backgrounds, duration of DM, glycemic controls, and severity of diabetes mellitus were analyzed. Serum levels of total ketone body (TKB), acetoacetate (AcAc), and βOHB were measured by an enzymatic cycling method (Kainos Laboratories, Inc. Tokyo, Japan). The levels of serum ketone bodies were compared with the factors in blood gas analysis, such as HCO3− and pH. The measurement methods of serum ketone bodies and blood gas were unchanged between 2011 and 2019.
Statistical analysis
Clinical data among the three groups were compared by Kruskal–Wallis test (continuous variable) or chi-square test (nominal variable). To determine the ketone body values that corresponded to a HCO3− level of 18 mmol/l, serum ketone body levels from each group were compared with simultaneously taken HCO3− levels by using regression analysis. The levels of serum ketone bodies were compared with simultaneously blood levels of HCO3− or pH by Spearman’s rank correlation coefficient test. Receiver-operating-characteristic (ROC) curve analyses were used to determine cut-off values of serum or urine ketone bodies for diagnosis of DKA. P values less than 0.05 were considered to be statistically significant. Statistical analyses were performed using JMP, version15 (SAS Institute Inc., Cary, NC, USA).
Results
Among consecutive outpatients with DM, a total of 202 patients were recruited for the study. Based on the inclusion and exclusion procedures as indicated, 72 patients were enrolled in the study: 21 patients with T1DM, 29 patients with T2DM-IDDM (8 of whom could discontinue insulin therapy within a half-year), and 22 patients with T2DM-NIDDM were analyzed (Supplemental Fig. 1) (Table 1).
Table 1.
Clinical characteristics of the patients
| T1DM | T2DM | Total | 1* p value | |||
|---|---|---|---|---|---|---|
| IDDM | NIDDM | Subtotal | ||||
| n (%) | 21 (29.1) | 29 (40.2) | 22 (30.5) | 51 (70.8) | 72 | |
| Age (years) | 48 (42–69) | 61 (49–75) | 50 (41–58) | 54 (44–69) | 54 (44–68) | 0.0842 |
| Male (%) | 10 (47.6) | 15 (51.7) | 16 (72.7) | 31 (60.8) | 41 (56.9) | 0.1918 |
| BMI | 19 (16–23) | 22 (19–25) | 28 (26–32)‡,§ | 25 (20–30) | 23 (19–26) | < 0.0001 |
| Duration (years) | 0 (0–16) | 5 (1–16) | 3 (0–15) | 5 (0–16) | 5 (0–15) | 0.3731 |
| Glucose (mg/dl) | 547 (439–824) | 437 (360–624) | 341 (265–536)‡ | 418 (302–287) | 445 (341–617) | 0.0032 |
| HbA1c (%) | 10.2 (8.2–11.9) | 12.7 (9.4–13.5) | 11.8 (9.5–14.3) | 12.0 (9.6–13.5) | 11.3 (9.1–13.4) | 0.0505 |
| TKB (mmol/l) | 7.1 (5.3–12.6) | 3.4 (0.7–8.8)† | 2.9 (0.7–4.9)‡ | 2.9 (0.8–6.6) | 4.4 (1.5–9.6) | 0.0015 |
| βOHB (mmol/l) | 5.3 (3.9–9.2) | 2.4 (0.5–6.4)† | 2.2 (0.5–4.0)‡ | 2.4 (0.6–5.0) | 3.3 (1.1–7.2) | 0.0008 |
| AcAc (mmol/l) | 1.5 (1.0–3.1) | 1.0 (0.2–2.0)† | 0.6 (0.2–1.1)‡ | 0.6 (0.2–1.7) | 1.0 (0.4–2.1) | 0.0034 |
| pH | 7.27 (7.15–7.37) | 7.38 (7.30–7.41)† | 7.38 (7.34–7.42)† | 7.38 (7.31–7.42) | 7.35 (7.24–7.40) | 0.0168 |
| Bicarbonate (mmol/l) | 17 (8–20) | 22 (11–26) | 21 (16–25) | 22 (14–26) | 20 (11–25) | 0.1027 |
| Lactate (mmol/l) | 2.0 (1.4–3.1) | 1.6 (1.1–2.8) | 2.0 (1.4–3.0) | 2.0 (1.4–3.1) | 1.9 (1.3–2.9) | 0.5782 |
| Urine ketone (%) | 0.2590 | |||||
| – | 1 (4.8) | 5 (17.2) | 4 (18.2) | 9 (17.6) | 10 (13.9) | |
| 1 + | 0 (0.0) | 3 (10.3) | 1 (4.6) | 4 (7.8) | 4 (5.6) | |
| 2 + | 5 (23.8) | 7 (24.1) | 5 (22.7) | 12 (23.5) | 17 (23.6) | |
| 3 + | 14 (66.7) | 13 (44.8) | 8 (36.4) | 21 (41.1) | 35 (48.6) | |
| 4 + | 1 (4.8) | 1 (3.5) | 4 (18.2) | 5 (9.8) | 6 (8.3) | |
| DKA (%)a | 11 (52.4) | 10 (34.5) | 6 (27.3) | 16 (31.4) | 27 (37.5) | 0.2145 |
Data are shown as median (lower quartile-upper quartile), or number (proportion)
1* Comparisons between the 3 groups (T1DM, T2D-IDDM, and T2D-NIDDM) were made by Kruskal–Wallis test (continuous variable) or Chi-square test (nominal variable), and p-values less than 0.05 are shown in bold
For comparison of the 2 groups, Steel–Dwass test was performed. Values shown in bold text are statistically significant. †p < 0.05 vs T1D; ‡p < 0.01 vs T1D; §p < 0.01 vs T2D-IDDM
IDDM, insulin-dependent diabetes mellitus; NIDDM, non-insulin-dependent diabetes mellitus
aNumber of patients with DKA and its proportion was shown. DKA has been diagnosed by the presence of hyperglycemia (blood glucose levels > 250 mg/dl) and metabolic acidosis (blood gas bicarbonate [HCO3-] < 18 mmol/l and pH in blood gas < 7.30)
Patients with T2DM-NIDDM had greater BMI compared with patients with T1DM, and compared with patients with T2DM-IDDM. Patients with T1DM had higher glucose levels than those with T2DM-NIDDM. No significant differences were seen in glycated hemoglobin (HbA1c) levels among the 3 groups. The levels of TKB, βOHB, and AcAc in patients with T1DM were significantly higher than those who were T2DM-IDDM, and also in those who were T2DM-NIDDM. In ROC analysis, 2.5 mmol/l of βOHB had 95.2% of sensitivity and 56.9% of specificity for differentiating T1DM from T2DM (Supplemental Fig. 2).
Significantly lower blood gas pH levels were observed in patients with T1DM among the 3 groups, whereas no significant differences were observed in blood gas HCO3− and lactate levels. The proportion of DKA in patients with T1DM was higher among the 3 groups, but without significance (T1DM 52.4%; T2DM-IDDM 34.5%; T2DM-NIDDM 27.5%, P = 0.2145) (Table 1).
Note that 2 patients with T2DM-NIDDM who developed DKA had no obvious episode of soft drink abuse, and showed spontaneous recovery from insulin therapy, and 12 cases of ketosis and 4 cases of ketoacidosis in T2DM-NIDDM patients were seemed to be due to excessive soft drink intake.
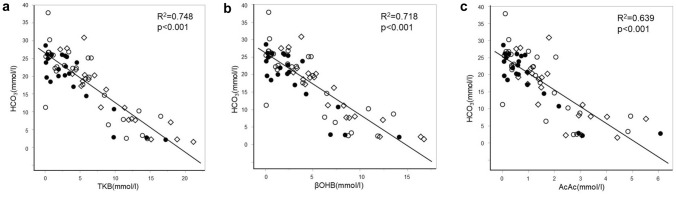
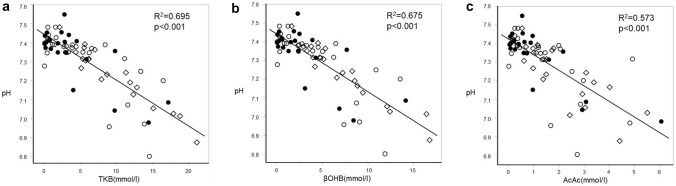
The levels of serum TKB, AcAc, and βOHB were all negatively correlated with the levels of blood gas HCO3− and also those of pH (Figs. 1, 2). HCO3− levels of 18 mmol/l corresponded to serum βOHB levels of 4.6 mmol/l, AcAc of 1.5 mmol/l, TKB of 6.1 mmol/l, respectively. Similarly, pH of 7.30 corresponded to βOHB of 4.6 mmol/l, AcAc of 1.5 mmol/l, TKB of 6.0 mmol/l, respectively.
Fig. 1.
Comparisons between HCO3− and blood ketone body levels. TKB (a), βOHB (b), and AcAc (c) levels were compared with blood levels of HCO3− by Spearman’s rank correlation coefficient test. Patients with T1DM (white quadrangle), T2DM-IDDM (white circle), and T2DM-NIDDM (filled circle) were analyzed separately. TKB total ketone body, βOHB beta-hydroxybutyrate, AcAc acetoacetate, HCO3− bicarbonate, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, IDDM insulin dependent diabetes mellitus, NIDDM non-insulin dependent diabetes mellitus
Fig. 2.
Comparisons between pH and blood ketone body levels. TKB (a), βOHB (b), and AcAc (c) levels were compared with blood levels of pH by Spearman’s rank correlation coefficient test. Patients with T1DM (white quadrangle), T2DM-IDDM (white circle), and T2DM-NIDDM (filled circle) were analyzed independently. TKB total ketone body, βOHB beta-hydroxybutyrate, AcAc acetoacetate, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, IDDM insulin dependent diabetes mellitus, NIDDM non-insulin dependent diabetes mellitus
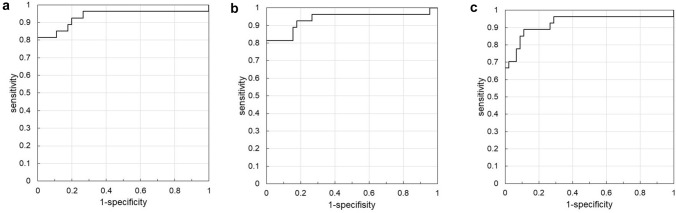
In ROC analysis, 8.0 mmol/l of TKB had 81.5% of sensitivity and 100% of specificity for the diagnosis of DKA, the area under the curve (AUC) was 0.93498. Similarly, 6.3 mmol/l of βOHB had 81.5% of sensitivity and 100% of specificity, AUC was 0.93663, 1.4 mmol/l of AcAc had 88.9% of sensitivity and 88.9% of specificity, AUC was 0.92593, respectively (Fig. 3).
Fig. 3.
ROC analysis of serum ketone bodies in the diagnosis of DKA. Optimal cut-off levels of TKB (a), βOHB (b), and AcAc (c) for diagnosis of DKA were estimated by ROC analysis. a 8.0 mmol/l of TKB had 81.5% of sensitivity and 100% of specificity, area under the curve (AUC) was 0.93498. b 6.3 mmol/l of βOHB had 81.5% of sensitivity and 100% of specificity, AUC was 0.93663. c 1.4 mmol/l of AcAc had 88.9% of sensitivity and 88.9% of specificity, AUC was 0.92593. TKB total ketone body, Βohb beta-hydroxybutyrate, AcAc acetoacetate, ROC receiver-operating-characteristic
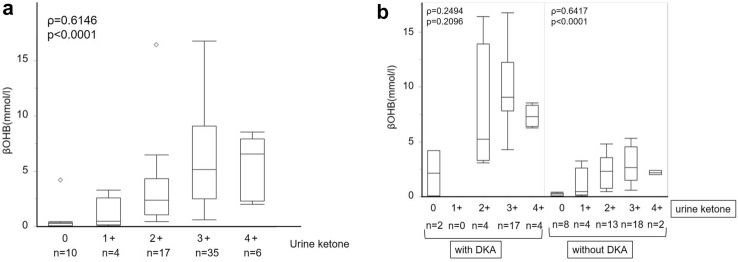
Serum βOHB levels were positively correlated with urine ketone body levels (ρ = 0.6146, p < 0.0001) (Fig. 4a) Notably, serum βOHB levels were higher in patients with DKA than in those without DKA, even at the same urinary ketone excretion level (Fig. 4b). No significant difference was observed between serum βOHB levels and urine ketone in patients with DKA (ρ = 0.2494, p = 0.2096), and 2 cases of DKA exhibited no urinary ketone excretion (Fig. 4b left). Serum βOHB levels were positively correlated with urine ketone levels in patients without DKA (ρ = 0.6417, p < 0.0001) (Fig. 4b right).
Fig. 4.
Serum βOHB levels in comparison to the levels of urine ketone body. In box-and-whisker plots, minimum, lower quartile, median, upper quartile, and maximum levels are shown as indicated. Serum βOHB levels were compared with urine ketone levels by Spearman’s rank correlation coefficient tests in all cases (a), and patients with and without DKA (b), respectively. In all cases (a), serum βOHB levels (mmol/l) in median (interquartile ranges: IQR) were 0.3 (0.1–0.4) at urine ketone (−), 0.5 (0.2–2.6) at urine ketone (+), 2.4 (1.1–4.3) at urine ketone (2 +), 5.2 (2.5–9.1) at urine ketone (3 +), 6.6 (2.3–7.9) at urine ketone (4 +). In patients with DKA (b left), serum βOHB levels (mmol/l) in median (IQL) were 2.1 (0.1–4.2) at urine ketone (−), 5.2 (3.3–13.9) at urine ketone (2 +), 9.1 (7.8–12.3) at urine ketone (3 +), 7.3 (6.4–8.3) at urine ketone (4 +). In patients without DKA (b right), serum βOHB levels (mmol/l) in median (IQL) were 0.3 (0.1–0.3) at urine ketone (−), 0.5 (0.2–2.6) at urine ketone ( +), 2.3 (0.8–3.6) at urine ketone (2 +), 2.7 (1.5–4.5) at urine ketone (3 +), 2.2 (2.0–2.4) at urine ketone (4 +). βOHB beta-hydroxybutyrate, DKA diabetic ketoacidosis
Discussion
The current study has three principal findings, (1) an association of serum ketone body levels with blood gas HCO3−, and that with blood gas pH were shown in our Japanese cohort, (2) diagnostic utilities of these ketone body levels in DKA were shown with high sensitivity and specificity, (3) serum ketone bodies seemed to effectively differentiate between T1DM and T2DM. Taken together, blood ketone body levels seem to be useful for the diagnosis of DKA.
Harano, et al. investigated serum ketone body levels in Japanese patients with T1DM (n = 117), and with T2DM (n = 281), and this included 4 patients with T1DM and DKA [10]. They finally concluded that serum levels of TKB did not exceed 2.0 mmol/l in patients with T2DM, that insulin therapy would reduce serum ketone bodies in patients with T1DM, and that serum levels of ketone bodies are useful for the diagnosis of T1DM (those with TKB > 2 mmol/l) and for detecting insufficient insulin therapy. The current study further suggests the novel beneficial impact of serum ketone bodies on the diagnosis of DKA in both T1DM and T2DM.
‘Ketone body’ consists of acetone, AcAc, and βOHB, which derives from lipolysis and are utilized as fuels [2]. Ketone body production is suppressed by insulin [3]. Physiological hyperketonemia reflects the acceleration of lipolysis and ketogenesis due to insulin insufficiency, and it can also be seen in healthy individuals such as in fasting [2–4]. When AcAc and βOHB are overproduced and accumulated, metabolic acidosis is induced [2, 5]. Therefore, the ketone body is widely known as a marker of insulin action on adipose tissue, and may reflect the severity of DKA.
In comparison with previous reports on serum ketone bodies in DKA [7–9], the cut-off value of βOHB, 4.6 mmol/l in DKA in the current study is considered to be higher than that of previous studies. In the guideline of ISPAD, ketonemia is defined as blood βOHB ≥ 3mmol/l [7]. Sheikh-Ali, et al. showed a strong negative association between serum HCO3- and βOHB and suggested a cut-off value of βOHB ≥ 3.0mmol/l in children, and ≥ 3.8mmol/l in adults for diagnosis of DKA [8]. Arora, et al. finally concluded the cut-off value of capillary βOHB ≥ 1.5mmol/l for diagnosis of DKA [9].
The reason for this discrepancy is unclear, but possible explanations might be a difference in ethnicity, prevalence of T1DM, etiology of DKA, capacity of insulin secretion, or body composition. Importantly, higher cut-off values of ketone bodies in this study might be due to the different inclusion criteria among the studies. Moreover, the differences in the measurements of methods for both serum ketone bodies and blood gas (venous, arterial, or capillary) may affect the absolute values. Sheikh-Ali, et al. used ‘P Module of a Roche Modular Analytics System to measure βOHB’ [8], whereas Arora S, et al. used a ‘point-of-care βOHB measurement’ (Precision Xtra meter; Medisense/Abbot Laboratories) [9].
Serum ketone body levels were surely and negatively correlated with the levels of blood gas HCO3−, and those of blood gas pH. ROC analyses revealed that optimal cut-off values for diagnosis of DKA were determined at 6.3 mmol/l of βOHB, 1.4 mmol/l of AcAc, and 8.0 mmol/l of total ketone body, respectively. Therefore, from a practical point of view, these cut-off levels may be used in the diagnosis of DKA.
Significantly higher levels of TKB, βOHB, and AcAc, and lower blood gas pH were observed in patients with T1DM than in patients with T2DM-IDDM and T2DM-NIDDM. Therefore, serum ketone bodies appeared to effectively differentiate between T1DM and T2DM. No significant differences were observed in blood gas HCO3− levels among the three groups, although the reasons for this remain unknown.
Diagnostic power in DKA based on the conventional criteria for DKA and the current study which includes ketone body-based diagnosis appeared to be similar [7]. In addition to the diagnosis of DKA, the current study might have advantages in the diagnosis and therapeutic strategy for certain subtypes of DM other than typical T1DM or T1DM. First, regarding atypical T2DM along with ketosis or ketoacidosis onset, known as ketosis-prone T2DM (KPD) [12, 13]. These patients are characterized by higher BMI, being negative for islet-cell autoimmunity, spontaneous recovery from β-cell dysfunction, and a predominance among African–American individuals [12]. Aizawa et al. reported 5 Japanese patients with KPD, and the duration of insulin dependency was shorter than 4 weeks [13]. Two T2DM-NIDDM patients with DKA in the current study had no obvious episode of soft drink abuse, required insulin therapy, and subsided. Therefore, they might be diagnosed with KPD. However, future studies are required to diagnose them definitely as KPD by definition of appropriate cut-off levels of serum ketone bodies.
There is another type of T2DM so-called ‘soft-drink ketosis’, where acute deterioration of hyperglycemia is driven by excessive intake of soft drinks containing sugar, which can give rise to ketosis or ketoacidosis [14]. Patients with T2DM-NIDDM and high BMI are often diagnosed as T2DM due to ‘soft-drink ketosis’. Our data had 12 cases of ketosis and 4 cases of DKA due to excessive soft drink intake. The serum ketone body levels in the cases of ‘soft-drink ketosis’ remain unknown, and further investigations are needed to clarify the role of serum ketone bodies in ‘soft-drink ketosis’.
Our data indicated a practical limit of urine ketone in the diagnosis of DKA, which is in concordance with previous reports [2, 9, 15]. In addition to the low specificity of urinary ketone measurements, serum ketone bodies are more reliable than urine ketone bodies for the detection of DKA.
Our study has some limitations. First, sampling bias is inevitable because of the retrospective study design. Second, counter-regulatory hormones were not measured. Third, the results of serum ketone bodies cannot be obtained immediately in most of the hospitals, unfortunately. Fourth, meal statuses were unclear, although most patients had a loss of appetite. Fifth, SGLT2i user was excluded from this study because the number of those was limited. Taken together, DKA should be managed comprehensively based on complex examinations including usual laboratory examination, serum ketone bodies, and blood gas tests. In addition to the diagnosis of DKA, an indication of intensive insulin therapy needs optimal cut-off value of serum ketone body levels. Future studies of serum ketone body levels in relation to therapy (with or without SGLT2i) and prognosis for DM should be considered.
In conclusion, serum ketone body might be useful in the diagnosis of DKA, as well as serum HCO3− and pH. Further investigations with a greater number of patients are required to establish the cut-off levels of serum ketone body levels in the management of DKA.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplemental Fig. 1 Patient enrollment procedures. Detailed patient enrollment procedures were described thoroughly in the method. Among consecutive outpatients with DM at our department and emergency room during the 2011- 2019 year, a total of 202 patients were recruited for the study. Based on the inclusion and exclusion procedures as indicated, patients with T1DM (n=21), patients with T2DM-IDDM (n=29), and patients with T2DM-NIDDM (n=22) during the follow-up period were analyzed. *T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; IDDM, insulin dependent diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus (JPG 256 KB)
Supplementary file2 Supplemental Fig. 2 ROC analysis of serum βOHB levels for diagnosis of T1DM from T2DM. Optimal cut-off value of βOHB for diagnosis of T1DM was estimated by ROC analysis. 2.5 mmol/l of βOHB had 95.2% of sensitivity and 56.9% of specificity, area under the curve (AUC) was 0.77871. *βOHB, beta-hydroxybutyrate; ROC, receiver-operating-characteristic; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus (JPG 66 KB)
Acknowledgements
We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center, Wakayama Medical University.
Abbreviations
- AcAc
Acetoacetate
- CPR
C-peptide
- DM
Diabetes mellitus
- DKA
Diabetic ketoacidosis
- GADAb
Glutamic acid decarboxylase autoantibody
- HCO3−
Bicarbonate
- ICAs
Autoantibodies to islet cells
- IDDM
Insulin-dependent diabetes mellitus
- KPD
Ketosis-prone type 2 diabetes mellitus
- NIDDM
Non-insulin-dependent diabetes mellitus
- ROC
Receiver-operating-characteristic
- TKB
Total ketone body
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- βOHB
Beta-hydroxybutyrate
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The datasets in the manuscript are available from the corresponding authors on reasonable request.
Declarations
Conflict of interest
The authors have no competing interests to disclose.
Compliance with ethical standards
The study protocol was approved by the Japanese Red Cross Wakayama Medical Center Ethics Committee (No. 680, April 30. 2019), and all participants had the opportunity to opt-out of research. The necessity for written informed consent from each patient was waived in this retrospective study. We used clinical information obtained in routine clinical practice, and no patients refused to participate in the study. All procedures were in accordance with the institutional ethical standards regarding human experimentation and with the Declaration of Helsinki, as revised in Fortaleza, Brazil in October 2013.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tomonao Hirobata, Email: thirobata8@icloud.com.
Hidefumi Inaba, Email: inaba@wakayama-med.ac.jp.
Yosuke Kaido, Email: yosuke24_92@yahoo.co.jp.
Daisuke Kosugi, Email: daisuke.kosugi.0908@gmail.com.
Saya Itoh, Email: i_saya523@yahoo.co.jp.
Takaaki Matsuoka, Email: matsuoka@wakayama-med.ac.jp.
Gen Inoue, Email: inoue_gen@me.com.
References
- 1.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. doi: 10.1038/s41572-020-0165-1. [DOI] [PubMed] [Google Scholar]
- 2.Dhatariya KK. Blood ketones: measurement, interpretation, limitations, and utility in the management of diabetic ketoacidosis. Rev Diabet Stud. 2016;13(4):217–225. doi: 10.1900/RDS.2016.13.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304(8):H1060–H1076. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Féry F, Balasse EO. Ketone body production and disposal in diabetic ketosis: a comparison with fasting ketosis. Diabetes. 1985;34(4):326–332. doi: 10.2337/diab.34.4.326. [DOI] [PubMed] [Google Scholar]
- 5.Fulop M, Murthy V, Michilli A, Nalamati J, Qian Q, Saitowitz A. Serum β-hydroxybutyrate measurement in patients with uncontrolled diabetes mellitus. Arch Intern Med. 1999;159(4):381–384. doi: 10.1001/archinte.159.4.381. [DOI] [PubMed] [Google Scholar]
- 6.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Diabetes. 2018;19(Suppl 27):155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh-Ali M, Karon BS, Basu A, Kudva YC, Muller LA, Xu J, et al. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647. doi: 10.2337/dc07-1683. [DOI] [PubMed] [Google Scholar]
- 9.Arora S, Henderson SO, Long T, Menchine M, et al. Diagnostic accuracy of point-of care testing for diabetic ketoacidosis at emergency-department triage. Diabetes Care. 2011;34(4):852–854. doi: 10.2337/dc10-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harano Y, Kosugi K, Hyosu T, Suzuki M, Hidaka A, Kashiwagi A, et al. Ketone bodies as markers for type 1 (insulin-dependent) diabetes and their value in the monitoring of diabetic control. Diabetologia. 1984;26(5):343–348. doi: 10.1007/BF00266034. [DOI] [PubMed] [Google Scholar]
- 11.Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical guideline for diabetes 2019. Diabetol Int. 2020;11(3):165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umpierrez GE. Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care. 2006;29(12):2755–2757. doi: 10.2337/dc06-1870. [DOI] [PubMed] [Google Scholar]
- 13.Aizawa T, Katakura M, Taguchi N, Kobayashi H, Aoyagi E, Hashizume K, Yoshizawa K. Ketoacidosis-onset noninsulin dependent diabetes in Japanese subjects. Am J Med Sci. 1995;310(5):198–201. doi: 10.1097/00000441-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Matsui J, Tamasawa N, Tanabe J, Kasai N, Murakami H, Matsuki K, et al. Clinical characteristics of Japanese youth-onset type 2 diabetes with ketonuria. Diabetes Res Clin Pract. 2005;70(3):235–238. doi: 10.1016/j.diabres.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplemental Fig. 1 Patient enrollment procedures. Detailed patient enrollment procedures were described thoroughly in the method. Among consecutive outpatients with DM at our department and emergency room during the 2011- 2019 year, a total of 202 patients were recruited for the study. Based on the inclusion and exclusion procedures as indicated, patients with T1DM (n=21), patients with T2DM-IDDM (n=29), and patients with T2DM-NIDDM (n=22) during the follow-up period were analyzed. *T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; IDDM, insulin dependent diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus (JPG 256 KB)
Supplementary file2 Supplemental Fig. 2 ROC analysis of serum βOHB levels for diagnosis of T1DM from T2DM. Optimal cut-off value of βOHB for diagnosis of T1DM was estimated by ROC analysis. 2.5 mmol/l of βOHB had 95.2% of sensitivity and 56.9% of specificity, area under the curve (AUC) was 0.77871. *βOHB, beta-hydroxybutyrate; ROC, receiver-operating-characteristic; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus (JPG 66 KB)
Data Availability Statement
The datasets in the manuscript are available from the corresponding authors on reasonable request.