Abstract
Various tissues, including the heart, cornea, bone, esophagus, bladder and liver, have been vascularized using the cell sheet technique. It overcomes the limitations of existing techniques by allowing small layers of the cell sheet to generate capillaries on their own, and it can also be used to vascularize tissue-engineered transplants. Cell sheets eliminate the need for traditional tissue engineering procedures such as isolated cell injections and scaffold-based technologies, which have limited applicability. While cell sheet engineering can eliminate many of the drawbacks, there are still a few challenges that need to be addressed. The number of cell sheets that can be layered without triggering core ischemia or hypoxia is limited. Even when scaffold-based technologies are disregarded, strategies to tackle this problem remain a substantial impediment to the efficient regeneration of thick, living three-dimensional cell sheets. In this review, we summarize the cell sheet technology in myocardial infarcted tissue regeneration.
Keywords: Myocardial infarction, Stem cells, Cell sheet, Regenerative medicine
Introduction
Myocardial infarction (MI, also called heart attack or silent killer disease), caused by major coronary artery occlusion or an imbalance between oxygen supply and demand, results in acute loss of non-self-regenerating cardiac muscle cells [1]. MI leads to the release of a multifunctional cytokines that triggers an inflammatory cascade [2]. If left untreated, the increased inflammatory cytokines contribute to apoptosis and scarring, leading to the development of heart failure due to the lack of proliferation and regenerative capacity of mature cardiomyocytes (CMs) [3–5]. In humans, apoptosis occurs mainly in the outer zones or even in the remote areas of ischemia [5]. Some researchers have reported that a few replicating CMs can be detected in the damaged area after myocardial injury, representing a possible attempt to regenerate the myocardium [6, 7]. However, the repair process typically proceeds via a scarring mechanism and the attempt showed little effect. Cell death can occur approximately 20 min after the onset of ischemia, therefore MI is a concerning condition as it can go undetected and lead to sudden death.
The most recent World Health Organization report states that cardiovascular disease (CVD) is the leading cause of death worldwide, claiming up to 17.9 million lives each year, and among CVD-related deaths, ischemic disease accounts for 42%, despite numerous MI medical and surgical treatment efforts [8]. Analysts predict that by 2030, annual global mortality from cardiovascular disease will be 23.3 million, with ischemic heart disease being the leading cause of death at 13.4% [9]. Therefore, myocardium remains one of the most researched topics in regenerative medicine [10, 11].
Due to the limited regenerative capacity of cardiac cells, heart transplantation has been considered the only ultimate solution at present, despite mechanical revascularization and pharmacological treatment [12, 13]. However, the overwhelming shortage of organ donors and the high rate of graft rejection remain an obstacle. New advances in the treatment of heart disease have been developed worldwide [14]. The most advanced treatments in regenerative medicine, such as cell transplantation with various stem cells like embryonic stem cells (ESCs) and induced pluripotent stem (iPSCs) have helped to solve some cardiovascular problems by rapidly restoring diseased organs. Chong et al. showed that transplanted human ESC cardiomyocytes (at a dose of 1 × 109 cells per heart) survive and contribute new myocardium to macaque hearts after being directly injected 2 weeks after myocardial infarction [15]. Cell transplantation strategies are easy to use because they do not require specific control or manipulation of tissues and can improve cardiac function [16]. However, cell transplantation to injury sites, critical cell loss due to essential hypoxia or cell washout, difficulty controlling the area of transplanted cells, and overall inadequate and inconclusive therapeutic outcomes continue to be issues, with numerous studies showing that the majority of injected cells were lost at the injection sites, followed by a low survival rate [17, 18].
In this manner, tissue-engineered therapies using biodegradable scaffold-seeded cell technology have been developed and have proven successful in regenerative medicine [19]. Many types of three-dimensional (3D) myocardial tissue are produced by seeding cardiomyocytes in gelatin, alginate, poly (glycolic acid), and collagen [20, 21]. Gaetani et al. seeded alginate with human cardiac cardiomyocyte progenitor cells and the construct showed high cell viability (92% and 89% after 1 and 7 days of cultivation, respectively), retained cardiac lineage, and showed increased gene expression of the early cardiac transcription factor, sarcomeric protein [22]. The study also suggests that the cells were able to migrate out of the construct matrix and form tube-like structures by settling on the matri-gel layer. Moreover, Zimmermann et al. fabricated 3D cardiac tissue by allowing the mixture of cardiomyocytes and collagen solution to gel instead of seeding cells into preformed scaffolds, which allowed direct measurement of isometric contraction force as a cardiac tissue model [23]. However, biodegradable scaffolds themselves weaken cell-to-cell connections, and scaffold degradation after transplantation results in fibrous tissue containing an excessive amount of extra cellular matrix (ECM), as evidenced by pathological conditions such as ischemic heart disease or dilated cardiomyopathy [24]. Along with achieving a balance between tissue formation by the cells and degradation of the scaffold; inadequate cell migration, a high rate of cell death due to various factors such as inflammation at the graft site and mechanical injury limit the use of scaffold-seeded cell technology [25].
As a result, numerous research groups have discovered a scaffold-free strategy to enhance heart regeneration efficacy [23, 26]. T. Okano and colleagues were the first to report cell sheet technology employing special culture vessels by grafting temperature responsive polymer like poly-N-isopropylacrylamide (PIPAAm), which are hydrophobic at 37 °C and hydrophilic below 32 °C. In this study, overlapping cell layers were collected as intact cell sheets by simply lowering the temperature, which improved uniform cell dispersion without the requirement for any proteolytic enzymatic treatments [27]. Also, Sekine et al. and Elloumi‐Hannachi et al. also demonstrated that the donor cells contained in the cell sheet are superior to cells generated in suspension using cell dissociation procedures such as trypsinization [17, 28]. These methods enable to utilize intact cells and secreted ECM in sheet form, as well as the construction of 3D structures without the use of biodegradable scaffolds. This 3D microenvironment mimics in vivo tissue conditions, which means that the corresponding cells have behaviors and functions that resemble those of native tissue, such as cell junctions and ECM proteins (which regulate cell proliferation, growth factor presentation, migration, lineage specification, and intercellular signaling) [29].
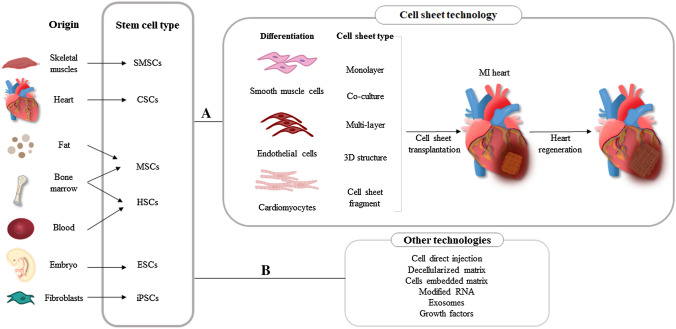
Despite the effective developments achieved, more technological advances are needed to establish a well-organized vascular network. This review compares the optimization of various cell sources, including Skeletal myoblasts-derived stem cell sheet (SMSCs), Mesenchymal derived stem cell sheet (MSCs), and Pluripotent stem cells (PSCs), for high-quality cell sheet fabrication and transplantation into the infarcted heart for regenerative purposes. Figure 1 describes the overall cell sheet technology applications.
Fig. 1.
Illustration of stem cell-derived cell sheet technology and other therapies in ischemic heart disease. A Cell sheet technology. Different responsive systems for cell sheet detachment are used after stem cell differentiation, including thermo-responsive systems, electro-responsive systems, photo-responsive systems, pH-responsive systems, magnetic systems, and mechanical systems. Growth factors such as adiponectin, hepatocyte growth factor (HGF), and ventricular endothelial growth factor (VEGF) play an important role in tissue regeneration after cell sheets are transplanted into the infarct area. Reduced fibrosis, higher left ventricular ejection fraction (LVEF), anti-inflammatory effects, angiogenesis, and ECM recovery are all essential paracrine effects of a regenerated heart. B Cardiac regeneration technologies other than cell sheet technology
Stem cell sources for myocardial tissue regeneration
Adult stem cells
Skeletal myoblasts-derived stem cells (SMSCs)
Skeletal myoblasts (SMs) are abundant in the human body, and are known to be among the first cell-based cardiac regenerative strategies and currently represent the pinnacle of cell sheet transplantation in the treatment of myocardial infarction [30]. SMs are derived from skeletal muscle progenitor cells (satellite cells) and contains immature (myoblasts) that retain the capacity to fuse with surrounding myoblasts or with damaged muscle fibers to regenerate functional skeletal muscle, therefore they are able to repair themselves after injury unlike other muscles such as heart muscle. Their advantages include the ability to resist ischemia, the ability to contract, the ability to proliferate and expand in vitro once isolated, differentiation into non-myocyte lineages, and possibility of autologous use [31, 32]. Fukushima et al. exhibited that injection of SMs via either the intra-myocardial or retrograde intracoronary route in the same way improved cardiac function and physical activity, accompanying with decreased cardiomyocyte-hypertrophy and fibrosis [33]. In another preclinical study, chronically infarcted Göttingen mini-pigs were separated into four groups that received either media control or one, two, or three doses of SMs every 6 weeks, and the pigs were followed for a total of 7 months [34]. A significantly greater increase in the left ventricular ejection fraction (LVEF), an increase in tissue vasculogenesis, and a decreased fibrosis was detected in animals that received three doses vs. a single dose and it was confirmed that repeated injection of skeletal myoblast in a model of chronic MI is feasible and safe and lead to a significant improvement in cardiac function. However, SMs do not appear to be functionally integrated with the host myocardium because they cannot beat synchronously with it either in vitro or in vivo [35]. In addition, deficiencies in main gap junction proteins like nexin-43 and N-cadherin led to the incapability of myoblasts to form gap junctions resulting in the lack of electrical integration with the myocardium [36]. Several investigations have found that myoblasts have low electrical potential, suggesting that if they survive in the myocardium, they may be able to cause arrhythmia [31, 32].
Cell sheets may not induce arrhythmia, owing to their attachment to the epicardium [24, 37]. Since the 2000s, Cell sheet technology has been practiced for the reconstruction of myocardial tissues and was developed to deliver cells efficiently without damaging the myocardium and, thus, effectively improve cardiac function more than the needle injection method, and other therapies. It has been proposed that implantation of a SMs sheet reverses left ventricular (LV) remodeling by paracrine effects, in which angiogenic substances produced continuously from the implanted cell sheets cause neo-angiogenesis, increased vascular density, and blood flow, reversing hibernating myocardium [38]. Studies showed that SMs enhanced heart function by suppressing fibrosis, increasing systolic function, increasing wall thickness, and improving neovascularization, demonstrating the sufficient, practicality, and safety of cell sheet-based therapy in clinic MI [39, 40]. Autologous myoblast cell sheet transplantation may positively contribute to the improvement of the clinical condition of patients with MI [41]. Cytokine release, hematopoietic stem cells (HPSCs) recruitment, angiogenesis and the recovery of diastolic function are major components of the regenerative mechanism in myoblast sheet implantation. Memon et al. used non-ligature implantation of a skeletal myoblast sheet into a rat cardiac ligation model (to induce regeneration and angiogenesis) that hence attenuated cardiac remodeling via HPSCs attraction to the infract area and growth-factor release with better restoration of the implanted cells [42].
Cardiac-derived stem cells (CSCs)
Bearzi et al. reported that the identification of cardiac progenitor cells in mammals suggests that the human heart contains a population of stem cells that can differentiate into cardiomyocytes [43]. They reported human cardiac stem cells (hCSCs) that produced a chimeric heart with human myocardium composed of myocytes, coronary resistance arterioles, and independent of cell fusion when injected into the infarcted myocardium of immunodeficient mice and immunosuppressed rats. Sekine et al. contrasted transplantation of cardiac cell sheets with direct injection of cells in a rat MI model to elucidate the difference in their survival efficiency and found that twenty-four hours after cell transplantation, the cell sheet had a higher cell survival rate with a significantly higher number of mature capillaries, mature blood vessels composed of endothelial cells surrounded by pericytes, and a lower number of apoptotic cells, and inhibition of LV dilation, which contributed to an improved cardiac function four weeks after transplantation [17]. However successfully isolating hCSCs is challenging and the immunological challenges of allogeneic cells lead to the use of immunosuppression [44]. Therefore, methods that isolate adult mammalian CSCs based on cell surface markers should be thoroughly investigated.
Mesenchymal stem cells (MSCs)
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can expand in culture and differentiate into multiple mesenchymal cell phenotypes, including bone, cartilage, and fat [45]. In addition, MSCs are capable of differentiating into non-mesenchymal phenotypes skeletal muscle genitor cells, neurons, vascular endothelial cells, and CMs [46]. Research on adult stem cells has led to several important breakthroughs and since, the homing ability of MSCs has been proven to be an effective method for tissue regeneration [47, 48]. MSCs are applicable either through their direct contribution to the formation of vasculatures or via the indirect paracrine secretion of proangiogenic and cardio-protective factors. It may be possible to repair small ischemic areas by injecting MSCs into the tissue, but repair of large damaged areas requires laboratory grown cell sheets [30, 49].
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have a multipotent capacity, differentiating not only into osteoblasts and chondrocytes, but also into vascular endothelial cells or CMs in vitro, and have been used for both allogeneic and autologous purposes with improved ejection fractions and cardiac improvement over a 12-week period [50, 51]. It has been proven that other bone marrow cells also have the ability to differentiate into many cell types, such as cardiomyocytes and vascular endothelial cells, and can be autologously transplanted without causing cell rejection problems [52]. The first phase I clinical trials with bone marrow-derived stem cells for MI were conducted in 2004 [53]. Bone marrow-derived mononuclear cells (BM-MNCs) were the first stem cells to be incorporated into treatment for heart disease, and pre-clinical studies indicated that bone BM-MNCs could contribute to revascularization of ischemic regions in infarcted myocardium [54]. Intramyocardial or intravenous injection of BM-MNCs resulted in improved LVEF through angiogenesis, decreased apoptosis, and remodeling. Several clinical studies have demonstrated the benefit of this therapy [54, 55]. A florescence-activated cell sorting technique was used by Du et al. to select Lin-C-kit + bone marrow cells from mice and inject them directly into the infarct area, where they formed new cardiomyocytes (CMs) and a de novo myocardium with living tissue 9 days after cell transplantation [56]. The results were promising, as the regenerated myocardium occupied 68% of the infarct area and the mice that received cell transplantation survived in greater numbers than the mice that did not receive cell transplantation. Table 1 describes the results of various clinical trials on MI regeneration utilizing various stem cell sources. Despite numerous reports and studies such as the TIME study, the POSEIDON study, and the BAMI study (the largest study investigating the use of autologous Bone marrow-derived mononuclear BM-MNC at AMI), the efficacy of BMSCs have been disappointing [70–72].
Table 1.
Stem cell therapy for myocardial infarction: clinical trials results
| Cell type | Injection | Effectiveness | Clinical trial name |
|---|---|---|---|
|
Autologous BMNCs |
25 × 106 cells 4–8 days after Percutaneous coronary intervention |
LVEF increased BMNC group | BOOST [53] |
| Effective if EF < 48.9% on baseline | BOOST [57] | ||
|
68 × 106 cells 5–8 days |
LVEF increased 8.1% 6 months after treatment Infarct size decreased 11% |
ASTAMI [58] | |
|
236 × 106 3–6 days after AMI |
Contractile-function improvement BMNC group Maximal-vascular conductance capacity was improved |
REPAIR-AMI [59] | |
|
236 × 106 cells, 3–7 days after AMI |
Improvement of infarct size and regional contractility in BMNC treated group | REPAIR-AMI [60] | |
| 25–500 × 106 cells 2–8 days | Non- specific | BAMI [61] | |
| 236 × 106 cells, 3–7 days after AMI |
LVEF increased 1.2%, Decreased infarct size Increased RWM |
CELLWAVE [62] | |
| CD34 + from BMCs | 10–20 × 106 cells, 4–11 days | Improved LVEF relative to cell dose | Pre-SERVE-AMI [63] |
| hESC-derived cardiovascular progenitors | 8.2 × 106 cells |
No ventricular arrhythmias No cardiac teratoma LVEF increased 12.5% at 1 year |
ESCORT [64] |
| Autologous hMSCs | 7.2 × 107 cells | LVEF increased 5.9% 6 months after treatment | NCT01392105 [65] |
| Allogeneic hMSCs | Injected on 1–10 days after AMI |
Less premature ventricular contraction Increased LVEF and reverse remodeling of the heart |
PROCYMAL [66] |
| Autologous cardiac stem cells |
Injected 10 × 106 cells into anterior wall infarcts 0.5 × 106 cells injected in the left circumflex or right coronary artery |
LVEF increased 8.2% at 4 months after infusion | SCIPIO [67] |
| Skeletal Myoblasts | 400 × 106 or 800 × 106 cells | Abundant, contractile abilities, withstand ischemic insult | MAGIC [31] |
| Human ADSCs | 42 × 106 cells ADRCs in 3 ml volume | Global WMSI improved at 6 months | PRECISE [68] |
| Human ADSCs | 20 × 106 cells |
Improved LEVF Reduction of left ventricle infarcted size Improvement of the perfusion defect, Reduction of myocardial scar formation |
APOLLO [69] |
AMI, acute myocardial infarction; ADRC, Adipose-derived regenerative cells, ADSCs, Adipose-derived stem cells; BMNCs, Bone marrow-derived mononuclear stem cells; hMSCs, Human mesenchymal stem cells; EF, ejection fraction; LVEF, left ventricular ejection fraction; RWM, Regional Wall Motion; SMs, Skeletal myoblasts
As a result, BM-MSCs have gained popularity and are now the most commonly used cells in the treatment of CVD [73]. The time course of cell sheet adhesion to porcine heart tissue after transplantation was studied by a research group led by Dehua Chang at the Institute of Advanced Biomedical Engineering and Science in 2015. The purpose of the study was to examine how long transplanted cell sheets took to adhere to heart tissue. They discovered that MSCs made up the majority of bone marrow adherent cells. BM-MSCs were cultivated at 37 °C for 6–7 days on temperature-responsive dishes to reach approximately 100% confluence. MSCs that were confluent detached into a monolayer cell sheet with intact cell–cell connections. Only a small portion of the cell sheets was in contact with the epicardium of the heart 15 min after transplantation. This research provided clinical proof and guidelines for the successful application of MSC sheets to the heart, paving the way for cell sheet technology to be used in clinical cell therapy for MI. Kawamura et al. created ten BM-MSCs sheets using temperature-responsive culture dishes and successfully transplanted a total cell number of 1 × 108 MSCs over the infarcted myocardium of porcine ICM models with no mortality and no detected arrhythmia, with attenuated LV remodeling, increased neovascularization in the infarct border area, 4 weeks after transplantation [74]. The study demonstrated the MSC sheets feasibility, safety, and effectiveness in the treatment of HF. Tanaka et al. used mid-size animals to determine whether implantation of autologous preconditioned cell sheets improves the left ventricular function of chronically infarcted hearts [75]. They undertook 48 h of hypoxic preconditioning to improve VEGF secretion of autologous BM-MSCs sheets, which showed higher therapeutic efficacy than standard cultured sheets in a rabbit MI model. The enhancement of anti-fibrotic activity further promotes the use of hypoxic preconditioning of cell sheets; autologous implantation of preconditioned BM-MSCs sheets resulted in enhanced therapeutic angiogenesis, reduced fibrosis, and improvement of left ventricular function of rabbit infarcted hearts. BM-MSCs sheets were also transplanted to ischemic cardiomyopathy (ICM), and showed good curative results in an ICM rat model, with an improvement in left ventricular ejection fraction (LVEF) of 6% at 28 days after the transplant [76]. However, limitations such as cell number and age-related changes such as reduced growth and differentiation ability pose many limitations, especially in autologous use [55, 70, 77, 78].
Adipose-derived mesenchymal stem cells (AD-MSCs); among various stem cells, AD-MSCs remain an attractive multipotent stem cell resource compared to their competitors, BM-MSCs and umbilical cord-derived mesenchymal stem cells (UCMSCs), because they are abundant, have potent paracrine effects, have less painful during collection and can be easily derived in large quantities from adipose tissue for longer periods of time [79, 80]. AD-MSCs secrete many different types of growth factors and immunomodulatory cytokines that may contribute to the stimulation of angiogenesis, anti-apoptosis, prevention of exaggerated inflammatory response, reduction of scarring and prevention of adverse cardiac remodeling after ischemic injury, reduction of scarring, prevention of adverse cardiac remodeling, and overall improved myocardial function and reduced scarring [81, 82]. Bai et al. demonstrated that human AD-MSCs expressing connexin 43 and troponin I, are detectable in the mouse model of AMI, suggesting in vivo CMs differentiation of AD-MSCs by a fusion-independent mechanism [83]. Using cell sheet technology, Miyahara et al. evaluated the therapeutic potential of monolayered MSCs generated from adipose tissue [84]. They demonstrated that monolayered mesenchymal stem cells have multipotent and self-propagating capabilities following transplantation into infarcted rat hearts utilizing cell sheet technology. The monolayered MSCs reversed wall thinning in the scar area and enhanced heart function in rats with MI, unlike a fibroblast cell sheet. They found that AD-monolayered MSCs can easily engraft to damaged myocardium, develop progressively in situ, and produce a thick stratum of newly formed vasculature, cardiomyocytes, and undifferentiated MSCs. As a result, monolayered MSC transplantation could be a new therapeutic method for heart tissue regeneration. However, several studies have shown that the efficacy of AD-MSCs in generating CMs is unpromising [85].
UC-MSCs have demonstrated the ability to self-renew more rapidly and differentiate into three germ layers, accumulate in damaged tissue or inflamed regions, promote tissue repair, and modulate the immune response [86]. Nakao et al. compared the cytokine secretion and proliferation abilities of BM-MSCs, AD-MSCs, and UC-MSCs [47]. The results demonstrated that UC-MSCs sheets had a higher proliferation rate and secreted more interleukin (IL)-6 than the other two kinds of cell sheets. Guo et al. are the first were the first to investigate the safety and efficacy of human UC-MSC (hUC-MSCs) sheets in an AMI mice model. The cell sheet technique improved cardiac function and reduced harmful ventricular remodeling while increasing hUC-MSC retention, regulating the inflammatory response, stimulating angiogenesis in the AMI border, resulting in a more effective therapeutic impact [87].
Pluripotent stem cells (PSCs)
Since the discovery of human embryonic stem cells (hESCs) in 1998 by Thomson and his colleagues, several groups have shown that hESCs can differentiate into CMs in culture [88, 89]. hESCs have the ability to differentiate into any cell in the adult organism, and the potential to fully regenerate cardiac muscle, making genetic manipulation of these cells relatively easy [90]. Unlike adult or neonatal CMs, hESC-derived CMs retain the ability to proliferate and functionally connect to the host myocardium in culture and after transplantation. hESCs are initially considered as the best source of stem cells for regenerative therapy [91]. Menasché et al. reported improved cardiac differentiation and LV by producing hESC-derived cardiac progenitor cells that formed a cardiac lineage under safe and cost-effective conditions [64]. Tohyama et al. isolated CMs populations with up to 99% purity from hESC progenitor cells by using a technique for sorting cells based on their differences in glucose and lactate metabolism [92]. Park, M., and Yoon, Y.S. also showed that transplanted CMs derived from hESCs were able to integrate electrically, structurally, and mechanically with rat cardiac cells in vitro and able to survive in a porcine in vivo model, implying that these cells could be useful as a biological pacemaker and in regenerative cardiac medicine in general [93].
Masumoto et al. reported that the coexistence of and vascular cell lines within the hESC-engineered cell sheet structure in mice improved cell sheet function [94]. Stevens et al. produced scaffold-free patches composed of more than 75% CMs that synchronously transferred intracellular calcium transients with the human host myocardium and were thus electromechanically coupled [95]. However, many reports suggest that ethical concerns such as immunological rejection, technical difficulties in harvesting immature cells and the likelihood of teratoma formation in vivo prevent the use of hESCs for clinical cardiac regeneration [96]. Although various methods have been developed to limit teratoma formation, such as genetic selection of differentiated hESCs, several obstacles, including generating large numbers of pure CMs cultures, preventing immune rejection, and demonstrating that grafts survive, function, and improve myocardial performance in diseased hearts, should be considered in future research [97]. Despite numerous research efforts suggesting that cell sheets derived from fetal or neonatal CMs could be used in cell therapy for MI in animal studies, the cell sheets derived from such sources pose an ethical dilemma in humans, and the limited number of CMs for clinical application of such methods is another major issue to be addressed [93, 98]. Despite that somatic stem cells have been used to produce cell sheets for cardiac repair in animal models due to their ability to differentiate CMs and their paracrine action, their differentiation capacity has always been controversial and generally considered too low to produce enough functional CMs for clinical application, and the MI repair effect of their paracrine action should still be investigated in animal models [97, 99]. The low cardiogenic capacity, the insufficient survival rate, and the unestablished strategy to produce a large number of CMs limit the clinical application of adult stem cells.
Fortunately, the pluripotency of human induced pluripotent stem cells (iPSCs) means that these cells can be differentiated indefinitely with high efficiency into multiple disease-relevant cell types, particularly those that are otherwise difficult to access, such as CMs, making iPSCs a superior cell source for cell shifts for use in MI [100]. Technologies to differentiate iPSCs into cardiac cells with high efficacy have already been developed [101, 102]. The iPSCs derived-CMs (iPSCs-CMs) have become the preferred option because they are available and can be generated from the patient's own somatic cells, avoiding ethical issues for personalized and because they are superior to other somatic cell sources in terms of improving regional contractile function and cardiac bio-energetic efficiency, suggesting greater clinical benefit in a severely damaged myocardium. Several research groups have produced functional CMs in vitro from murine and human iPSCs. In 2006, Takashi and Yamanaka used nuclear reprogramming of mouse fibroblasts as the first strategy to generate induced pluripotent stem cells [103]. They discovered a technique of retroviral transduction of cells by ectopic expression of four transcription factors (Yamanaka factors), Octamer-binding transcription factor 4 (Oct4), Kruppel-like factor (Klf4), Sex determining region Y-box 2 (Sox2) and myelocytomatosis (c-MYC), to reprogram embryonic and mature mouse fibroblasts for induction (establishment) of PSCs without ethical constraints, providing the possibility of producing autologous target tissues.
The iPSCs sheet technique can deliver a large number of cells into the damaged myocardium without losing the grafted cells or injuring the host myocardium [104]. To promote differentiation, myofibril maturation, and cell–cell interactions with clear gap junctions of (iPSCs-CMs), Yoshida et al. co-cultured the iPSCs with MSCs that secrete soluble factors and release exosomes, and formed a cell sheet with longer survival and improved therapeutic effect in a 2-week MI model [105]. Several studies have shown that co-culture with an adequate number of non-CMs, such as cardiac fibroblasts, vascular endothelial cells, and vascular smooth muscle cells, promotes the functional maturation of CMs and artificial cardiac tissues through paracrine effects and cell–cell interactions [105, 106]. Kawamura et al. reported that a hiPSCs-CMs sheet with a purity of approximately 90% and in combination with an omental flap attenuated LV remodeling, inhibited fibrosis, increased neovascularization, induced angiogenesis, and induced cardio-myogenesis 1, 4, and 8 weeks after transplantation in a 12-week MI porcine model [107]. The availability of pluripotent cells from individual patients makes it possible to study pathogenesis and carry out experiments on the therapy of inherited diseases, the development of which is associated with distinct cell types that are hard to obtain by biopsy, so the use of iPSCs provides almost an unlimited resource for these investigations. Achieving long-term stability and integration into the myocardium is a crucial during cardio-genesis with iPSCs, as many cell types derived from iPSCs are incompletely differentiated compared to the mature cell. Component (e.g., limited understanding of the mechanism of action of various device components; device components do not comply with regulatory frameworks; toxicity issues) and process (e.g., too complex to allow for large-scale efficient and reproducible manufacturing; too long to be profitable) limitations have been attributed to this limited technology transfer from laboratory benchtop to clinical applicability [108].
Advantages of cell sheet technology over cell injection
Direct cell transplantation has long been the most popular method of cell transplantation. Cell transplantation by injection, on the other hand, has a number of drawbacks, including the loss of transplanted cells, arrhythmogenicity, grafted cell aggregation, and necrosis, with only around 15% of transplanted CMs surviving. These issues can be solved by cell sheet technology [109]. With improved heart wall thickness, decreased apoptosis, decreased inflammation, and neovascularization, cell sheets can attach to host tissues and even wound sites, covering the surface with minimum cell loss, thus ameliorating MI in both the acute and chronic phases (Table 2).
Table 2.
Stem cell-derived cell sheet transplantation experiments in MI
| Cell source/ origin | Effectiveness | Species | References |
|---|---|---|---|
| Myoblast |
Improved EF Increased neovascularization Decreased fibrosis |
Rat | Sekiya et al. [109] |
| Myoblast |
EF Improved Decreased fibrosis |
Porcine | Miyagawa et al. [110] |
| Myoblast with omentopexy |
EF Improved Decreased fibrosis |
Porcine | Shudo et al. [111] |
| Muscle-derived stem cell sheets |
EF Improved Inhibition of LV dilatation Decreased fibrosis |
Mice | Yang et al. [112] |
| Human iPS cells-derived cardiomyocytes |
EF Improved Inhibition of LV dilatation Decreased fibrosis |
Porcine | Kawamura et al. [113] |
| Adipose tissue-derived MSC sheets | EF Improved -Decreased fibrosis | Rats | Fabian et al. [114] |
| Human adipose tissue-derived MSC sheets | EF Improved | Nude rats | Kanzaki et al. [115] |
| Autologous muscle derived stem cell sheet |
LVEDD decreased from 66.5 ± 15.4% to 64.9 ± 15.7% (p < 0.05) LVEF increased from 26.74 ± 8.0% to 30.7 ± 10.0% (p < 0.01) 1 year after the treatment Improved pulmonary hypertension |
Human | Miyagawa et al. [116] |
| Mouse adipose tissue-derived stromal cells |
EF Improved Decreased fibrosis |
Rat | Kim et al. [117] |
EF, ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic dimension
Previous study demonstrated that transplanted MSCs were not converted to structurally and functionally integrated CMs in vivo, although MSCs have shown potential for cardiomyogenic differentiation in vitro [118]. Furthermore, because no artificial scaffold is employed, the resulting constructions are more biocompatible and have a lower risk of foreign body reaction than any other method to date [119]. Cell fate and function are determined by effective cell–cell and cell–ECM communications in this densely populated microenvironment. Because ECM proteins adhere to biological surfaces and remain on the surface of cell sheets, they can be transplanted to wounded tissues without the use of sutures or external fixation. The deposited ECM also serves as a store for a variety of bioactive and trophic molecules, as well as protecting and orienting the transplanted cells at the implant site.
Three-layer AD-MSCs sheets were implanted on the epicardium of a chronic rat MI model [120]. The AD-MSCs alone group had no cells, whereas the cell sheet group had a 25.3% engraftment rate at one week and one month after MI. The same group tested cell engraftment in a rat MI model, comparing conventional injection, bilayer myoblast cell sheet deposition, and myoblast cells seeded in collagen sponge deposition. Standard needle injection is outperformed by both cell constructs. In terms of engraftment cells and fibrosis reduction, the myoblast-seeded collagen sponge group had the best outcome.
Strategies to improve the efficacy of cell sheet transplantation
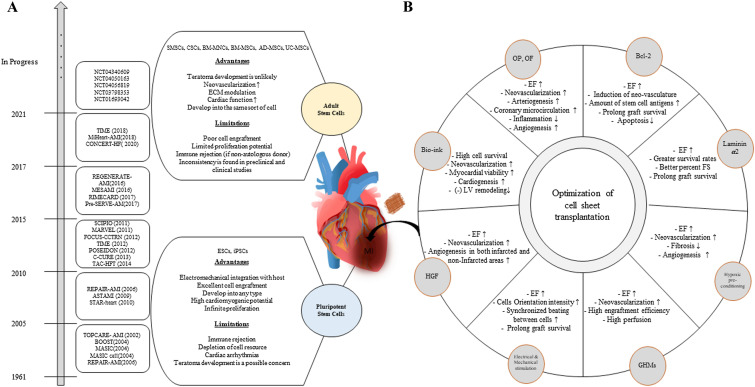
It is well known that heart tissue is difficult to repair itself and transplantation of cell sheets can repair damaged heart. Regardless of the cell type used, however, low cell survival and post-transplantation grafting rates have been recurring barriers in various studies (Fig. 2).
Fig. 2.
The progress of stem cell therapy in myocardial infarcted heart regeneration: Cell sheet technology. A Development of stem cell therapy for MI. Early clinical trials have suggested that stem cells could be used to prevent and reverse myocardial damage, as well as promote heart regeneration. The benefits and drawbacks of employing adult stem cells and pluripotent stem cells for MI regeneration are outlined. SMSCs skeletal myoblast stem cells; CSCs cardiac stem cells; BM-MSCs bone marrow-derived mesenchymal stem cells; BM-MNCs bone marrow-derived mononuclear cells; AD-MSCs, adipose-derived mesenchymal stem cells; UC-MSCs, umbilical cord-derived mesenchymal stem cells; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells. B Strategies to improve the efficacy of cell sheet transplantation. Mimicking the physiological environment is a key strategy for engineering cultured cells. To simulate physiological circumstances in vitro, co-culturing cells with other cell types, electrical and mechanical stimulation of the cells, and 3D structures creation can all be employed. MI, myocardial infarction; OP, omentopexy; OF, omental flap; HGF, hepatocyte growth factor; GHMs, gelatin hydrogel microspheres; EF, ejection fraction
Biochemical-culturing strategies
Memon et al. compared the efficacy of epicardial implantation of monolayer cell sheets in a rat AMI model [42]. In terms of ejection fraction, LV dimensions, fibrosis, and capillary density, animals in the cell sheet group demonstrated more significant attenuation of remodeling. Matsuura et al. employed the same technique to administer clonally expanded cardiac stem cell sheets to mice with similar beneficial effects on LV function after MI [121].
Since classical methods of obtaining suspended embryoid bodies (EBs) for hESCs may not be suitable for obtaining sufficient quantities of CMs for bioengineered myocardium preparation, several methods have been developed to overcome this limitation, including multi-well plates, microtiter plates, and agitated suspension cultures [89, 122, 123]. Retinoic acid, dimethyl sulfoxide (DMSO) plus oxytocin in P19 embryonic carcinoma (EC) and ascorbic acid all have been proven to enhance and promote cardiac differentiation [124, 125].
Siltanen et al. combined cell sheet technology with gene therapy using the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) to improve the therapeutic effects of myoblast cell sheet transplantation in a rat model of chronic heart failure [126]. Together with many researchers, they proved that the combination therapy improves cardiac function, reduces apoptosis, increases the amount of stem cell antigen, c-kit positive cells, promotes cell proliferation and myocyte survival, and prolongs graft survival in the myocardium [127, 128]. They compared a group that only received myoblast sheets to a group that received myoblast sheets that expressed the Bcl-2. Both groups exhibited some improvement in heart function after two weeks, but only the Bcl-2 gene-expressing myoblast sheets demonstrated a consistent improvement after four weeks. The Bcl-2 group had a higher number of Von Willebrand Factor (vWF)-positive cells in the infarct and heart lining areas than the other groups, implying that the Bcl-2 sheets were more efficient at inducing neo-vasculature production.
Shudo et al. implanted mini-pig autologous SMs-derived 30 cell sheets per transplant containing 1.5 × 107 cells per sheet by placing 6 sheets (5-layered) wrapped with omentum and covered with omental flap on different areas, immediately adjacent to but not overlapping one another to cover all regions of infarcted area [111]. Compared to SM sheets-only implantation, the use of omentum therapy permitted high numbers of SMs in the infarcted area with reduced scarring, increased vascularization, production of angiogenic growth factors such as VEGF that stimulates blood flow, and enhanced cardiac function.
Overexpression of the pleiotropic, proangiogenic, and cardioprotective hepatocyte growth factor (HGF) in myoblast sheet gene improves cardiac function and induces therapeutic angiogenesis via a paracrine mechanism in MI, according to Siltanen et al. [128]. Although the HGF sheet caused cardiac endothelial and smooth muscle cells to migrate but not CMs, and did not improve heart function further, the therapy efficiently encouraged angiogenesis in both infarcted and non-infarcted locations in vivo.
Multilayered sheets of fibroblasts expressing laminin G-module (LG) of α2 and skeletal myoblasts (SMs) were transplanted into ischemia cardiomyopathy model rats to assess the therapeutic effects [129]. The transplanted myoblasts in the skeletal myoblast sheet (SMB) + laminin Fb group had considerably greater survival rates than those in the other groups, according to quantitative measurement of nebulin mRNA levels. In line with these findings, SMB + laminin Fb myoblasts expressed higher levels of growth factors, and SMB + laminin Fb rats had significantly better percent fractional shortening (percent FS) and LV remodeling than the other groups. In the presence of culture medium taken from laminin G-module (LG4)-5-secreting fibroblasts and a solution containing 5 mg/mL mouse laminin protein, adult rat CMs demonstrated equal levels of adherence. Cell sheets do not migrate within the myocardium since they are implanted on the epicardium of host hearts. The cardio-protective benefits of myoblast transplantation are mediated by autocrine or paracrine pathways. Myoblasts placed on the epicardium secrete growth factors and promote growth factor secretion by surrounding cells, thus they do not need to be transplanted within the myocardium.
Dergilev et al. also found that after epicardial transplantation, a combination of c-kit + cardiac progenitor cell (CPC) and endothelial progenitor cell-derived cell sheets with a mean cell proliferation of 548.9 ± 103 per mm2 of cell sheet area improved endocardial scar tissue function more effectively than a single CPC-derived cell sheet by differentiating into multiple cell lineages and replacing vascular cells and CMs improved endocardial scar tissue function more using thermo-responsive systems [130]. They extracted c-kit + CPC from male Wistar rats, forming a cell sheet with a diameter of 0.98 ± 0.36 cm2 and an average thickness of 111.8 ± 24 µm that expressed gap junctions, connexin-43, and active caspase-3. Between 7 and 14 days, they discovered a significant migration of CPC from the cell sheet into the injured myocardium. The area of CPC spreading was threefold larger than the original cell sheet on the 14th day, and the scar size was reduced by nearly twofold compared to the control group. Epicardial transplantation of cell sheets from c-kit + CPC onto infarcted heart in rats considerably increased animal survival rate within 8-week follow-up period most probably due to reduction of left ventricle remodeling related to stimulation of angiogenesis and fibrosis decrease. Cell sheet survived and even proliferated after transplantation. Within an 8-week follow-up period, the epicardial transplantation of cell sheets from c-kit + CPC onto infarcted hearts in rats significantly improved animal survival rate, most likely due to reduced left ventricular remodeling due to stimulation of angiogenesis and fibrosis reduction. After transplantation, the cell sheet survived and even proliferated.
An innovative strategy for inducing CMs and vascular endothelial cells/vascular mural cells (VECs/VMCs) simultaneously and producing completely hiPSC-engineered cardiovascular cell sheets with beneficial therapeutic effects in infarcted hearts [131]. The approach extends a prior differentiation protocol for exclusive CMs by inducing vascular cells utilizing stage-specific supplementation of VEGF. In vitro, three hiPSC-CTSs (cardiac tissue sheets) were layered to create a three-sheet structure. All transplanted and sham-operated rats (n = 19 each) survived the 8-week post-transplant observation period without developing tumors. Physically integrated cardiac tissue sheets were successfully created using this cell sheet technology. The iPSC-CTSs transplantation to iPSCs sheets with CMs and vascular cells for cardiac regeneration significantly improved cardiac function (restored anterior wall contraction, LV systolic function parameters, fractional shortening (FS) and fractional area change (FAC) and recovered infarct wall systolic thickening). They found that engrafted human cells primarily consisted of CMs in > 40% of transplanted rats four weeks after transplantation, in addition to neovascularization. The engrafted area accounted for 24.76% of the infarcted area (ranged from 5.5% to 44%).
There has been research on the effects of cytokines, chemokines, and growth factors on MSCs chemotaxis and site-specific migration [132]. MSCs have been associated with early cellular aging, loss of chemokine indicators during ex vivo expansion, and hyper-immunogenicity to xeno-contaminated MSCs, among other things. Ex vivo multiplication of MSCs in hypoxic culture conditions using a well-defined or xeno-free medium such as growth factor-supplemented media, human serum, or platelet lysate can alleviate these issues. The potential for transitory adaptation of expanded MSCs in an autologous serum-supplemented medium before transplantation for long-term restorative advantages has been re-evaluated. MSCs have recently been reported to be derived from hESCs and iPSCs. MSCs derived from these sources can also be employed for tissue engineering and cell-based treatment. As a result, iPSCs may be able to alleviate patient-specific MSCs scarcity. Ex vivo multiplication of MSCs prior to transplantation is essential, regardless of the source, to provide enough MSCs for cell-based therapy. Position emission tomography (PET) tracking of MSCs given by catheter-based trans-endocardial injection revealed that roughly 6% of injected cells were retained in swine ischemic myocardium 10 days after injection.
Electrical and mechanical stimuli strategies
Patients undergoing open heart bypass surgery—a method in which cell sheet therapy is possible are more likely to have multiple infarctions, and their myocardial fibrosis and remodeling have already occurred. Regenerative paracrine stimulation or tissue replacement would be required for a therapy to be effective at this level. In comparison to intramyocardial injections of cells or cytokines, using cell sheets as vehicles for therapeutic stimulatory paracrine effectors allows for higher concentration and longer duration of therapy [17].
Roberts et al. fabricated a bio‐MEMS device designed to evaluate contractile force and conduction velocity of cell sheets in response to patient-based mechanical and electrical stimulation of the cell source as it grows to form a cellular sheet. The device that can be used to monitor the growth and contraction of cardiac cell sheets in vitro and during implantation in patients. At the end of characterization and or conditioning, ideally, the device is able to release the cell sheet for implantation [133].
Key biophysical features of mature mammalian ventricular myocardium include a positive force-preload relationship (Frank-Starling mechanism) and a positive force frequency relationship (FFR) [134]. The lack of a positive FFR in mammalian tissue created myocardium, on the other hand, has perplexed the research for nearly two decades. By combining mechanical and electrical stimulation to enable auxotonic contraction at a developmental stage-adapted beating frequency of 4 Hz, Godier-Furnémont et al. discovered for the first time a clearly positive FFR in tissue engineered myocardium and succeeded in achieving this missing physiological property, concluding that electrical stimulation at physiological frequency is critical for functional maturation [135].
Tsuruyama et al. reported that tubular cardiac tissue can pulsate in response to electrical stimulation [136]. The researchers wanted to use hiPSc-derived cardiomyocytes and cell sheet-based tissue engineering to create human tubular cardiac tissues. In response to surface electrical potential, the layered tubular tissues pulsated spontaneously and elicited considerable inside pressure. They showed partial maturation in gene expression after external electrical stimulation. Using human induced pluripotent stem cells-derived cardiomyocytes, this work created the first functioning heart tissue model for monitoring interior pressure. The researchers anticipate that the constructed pulsatile cardiac tube will help with medication screening and regenerative therapy for heart diseases in the future.
Homma et al. were the first to report a novel method for producing a cardiac tissue-like construct with aligned cells based on unidirectional stretching of hiPS-CM sheets utilizing single mechanical stretching that transformed the cell sheet shape from square to rectangle [137]. They harvested a square cell sheet from a temperature-responsive cell growth dish, which was then placed on a silicone surface and stretched in one direction using an extending force on the silicone. They created a cell sheet by co-culturing hiPS-CMs and human-ADSCs in order to assess cardiomyocyte morphology in vitro. A stretched double-layered cell sheet made entirely of hiPS-CMs was transplanted into the muscle of an athymic rat in separate trials, and its characteristics were compared to those of a non-stretched (control) cell sheet. They found that the stretched cell sheet was substantially longer than the control cell sheet right after stretching. The cardiomyocytes showed unidirectional alignment in the stretched cell sheet but random directionality in that of the control. The stretched cell sheet had kept the unidirectionality of its cardiac fibers and had a higher orientation intensity two weeks after transplantation than the control cell sheet or the stretched cell sheet before transplantation, according to the study.
State-of-the-art: Cell sheet fabrication
Cell membrane proteins and ECM, which are present in serum or released by cells, help cells adhere to hydrophobic culture surfaces. The membrane and ECM proteins are both destroyed when an enzymatic digesting agent is introduced, resulting in cell detachment [138]. When the cells are cultured on temperature-dependent culture surfaces, the bond between the ECM and the hydrophilic culture surfaces is broken by only lowering the temperature, allowing the cells to detach along with the intact proteins. The cells are joined to each other by cell-to-cell connection proteins when they are confluently cultured. The cell-to-cell connections are destroyed, and the cells detach independently when the cells are treated with proteases during harvest. PIPAAm-grafted surfaces are used by many researchers to repair cell-to-cell connections, maintain higher differentiation, sustain albumin secretion, and release a contiguous layer of cells containing ECM as an adhesive [139].
In addition, direct manipulation of cell sheets with forceps or pipettes after the sheets have been fully harvested results in proportionally shrunken and thicker constructs due to active reorganization of the cytoskeleton and preservation of cell–cell junctions. The use of a hydrophilic modified Poly-vinylidene difluoride (PVDF) membrane to maintain cell leaf morphology without shrinkage was also investigated. Support membranes are placed over the confluent cells before the cell sheets are released. Then, the cell sheets physiologically adhering to the support membranes are harvested with preserved cell–cell junctions and deposited ECM from the grafted surfaces at lower critical temperature (LCST) of above 32 °C and transferred to other surfaces via the remaining adhesive proteins by incubating them at 37 °C. It is reported that the interaction between adhesive proteins and culture surfaces depends on the wettability of the surface, therefore, PIPAAm is also immobilized (grafted) covalently on ordinary tissue culture polystyrene (TCPS) [140, 141].
To study the pulsation and in vivo survival of implanted graft constructs, Shimizu et al. Cultured cells on a temperature-dependent culture surface and obtained cell plates from neonatal rat CMs, four of which they superimposed to form heart grafts with morphologic, electrical communication via connexin43 between plates, gap junctions, sarcomeres, desmosomes, and with a 12-week long-term in vitro pulse rate of 13 to 96 bpm after subcutaneous tissue transplantation [142, 143]. In another study, Wang et al. developed a method for fabricating cell sheet fragments of MSCs without utilizing proteolytic enzymes by using a thermo-responsive methylcellulose hydrogel system [144]. Zhang et al. also successfully exploited fibrin gel-enhanced administration of iPSCs-CMs sheet to minimize MI via vascularity, decrease apoptosis, and increase engraftment rates in a mouse model 4 weeks after MI [145].
Electro-responsive systems, which enable cell attachment and release upon an electrical trigger the peptide Cys-Gly-Arg-Gly-Asp-Ser (CGRGDS) containing arginyl-gly-cylaspartic acid (Arg-glyv-asp) as a cell adhesive ligand was tethered to monolayers of alkane-thiolates via electroactive o-silyl hydroquinone [146]. Fibroblast cells were cultured on the RGD-presenting monolayers. When an electrical potential of 550 mV was applied to the monolayers for 5 min, the o-silyl hydroquinone oxidized to benzoquinone and the silyl ether was hydrolyzed, releasing the RGD containing peptide from the monolayer with the attached cells. This approach has been used to control cell adhesion, pattern cells and activate cell migration. Furthermore, employing an electro-responsive cell-sheet detaching device for direct transplantation, a technique for synthesizing cell sheets with custom-made 3D shapes has been developed [147]. The cell sheet detaching surface was constructed on the surface of 3D-printed objects to achieve custom-made 3D shapes, exhibiting the creation of a 3D cell sheet. This type of cell sheet could be useful in the regeneration of complex organs. In addition, nude mice were implanted with human neonatal skin fibroblast sheets to test the biocompatibility of the cell sheet removed using this procedure. Furthermore, human neonatal skin fibroblast sheets were transplanted into nude mice, to confirm the biocompatibility of the cell sheet detached by using this method, confirming the technique's benefit.
Light is an ideal stimulus for responsive surfaces because it can be controlled with high spatial controllability and temporal resolution [148]. The electrical charge of culture surfaces can be controlled by ultraviolet (UV) or even via visible light exposure, and cell sheets can be harvested because of the changed electrical charge. One strategy for developing photosensitive surfaces is to modify the wettability of a surface by light. Metal oxides, especially zinc oxide (ZnO) and titanium dioxide (TiO2), have been most studied for this application because their wettability can be switched between hydrophilicity and hydrophobicity by light. By attaching cells to a PNIPAAm-spiropyran surface at 37 °C, Edahiro et al. utilized this concept [149]. Cells in the UV-irradiated region remained attached, whereas cells in the unirradiated region separated. The spiropyran isomerizes back into the non-ionic structure when exposed to visible light (400–440 nm), allowing the cells to be separated by cooling the surface. Using a photomask, the researchers demonstrated the capacity to spatially manipulate cell adhesion. This technology can be used to control where a specific cell type in a co-culture is located and harvest the cell layer in the appropriate arrangement.
Koo et al. reported a new ROS-induced cell detachment strategy based upon a photo-functional hematoporphyrin-incorporated polyketone films (Hp-PK films) that facilitates transfer of the cell sheet directly at the target area without the need for a harvesting process. the method can transfer the cell sheets via a one-step process by bringing the cultured film into contact with the target site, irradiating the light, and removing only the film. Unlike other methods, when the cell sheet is attached to the target, the adhesive surface is limited to the top side, so that the polarity of the cell sheet is turned upside down. The advantage of this method is the ease of transplantation [150].
Cell manipulation, including cell detachment, has been described using ultrasound [151, 152]. Researchers in Tokyo devised a method for forming large disc-like 3D aggregates in a clinically relevant cell culture dish using kilohertz-order ultrasound standing wave trapping (USWT), the thickness of which was controlled by the number of seeded cells. The method's largest aggregate had a diameter of 8 mm and a thickness of 2.7 mm, making it larger than any prior aggregates created by other USWT approaches. The quantity of injected cells can be adjusted to tailor the size of the aggregate, and the viability of the cells that make up the aggregate as measured by the lactate dehydrogenase assay (LDH) was > 94% for 9 h. The technique had no effect on cell protein expression, according to the researchers and might be used to create highly functioning, scaffold-free aggregates that help enhance cell biology research [153].
A 3D-printed heart patch has shown a significant reduction in adverse remodeling and preservation of cardiac performance in a mouse model of myocardial infarction [154, 155]. Gaetani et al. used 3D cell printing technology to print human fetal cardiac myocardial progenitor cells encapsulated in a hyaluronic acid/gelatin-based bio-ink to construct porous heart patches, which they successfully implanted into a mouse MI model. When compared to the group with a non-porous structure, the porous patch showed higher cell survival by maintaining its cardiogenic phenotype for up to 1 month after printing. By minimizing negative LV remodeling and enhancing myocardial viability, the cardiogenic 3D printed patch preserved heart function.
Polyacrylamide (PAAm), poly(acrylic acid) (PAA), poly(methacrylic acid) (PMAA), poly(2-diethylaminoethyl methacrylate) (PDEAEMA), and poly(N,N-dimethylaminoethyl methacrylate) are some of the main polymers utilized in pH-responsive systems (PDMAEMA) [156, 157]. Due to the limited pH range of 6.8–7.4 for typical cell functioning, only a few studies have been undertaken using pH-sensitive devices for cell-based applications, however reducing pH locally or globally can limit cell sheet detachment [158].
Ishii et al. successfully created multi-layered ADRC sheets by combining a tissue engineering model modality, magnetite tissue engineering technology termed Mag-TE system, and the ECM precursor embedding system and transplantation of ADRC sheets protected against adverse cardiac-remodeling following MI [159]. The favorable effects of ADRC sheets are associated with maintenance of capillary density via the angiogenic paracrine actions of cytokines.
Conclusion
In this review, we focus on different stem cell sources used in cell sheet preparation and transplantation into the infarcted heart for regenerative purposes.
This approach is more advantageous since it provides for a more protected distribution of cells to the infarcted area in contrast to single cell injections. The cell sheets are able to be directly layered on top of each other with gap junctions through the whole cell sheet and directly bind to the host, which cannot occur in the application of a scaffold. However, because transplanted cells and cell sheets are exposed to the same harsh environment as cells in the damaged area due to the MI myocardium's low activity, the cell sheet graft should be able to restore blood flow that was chronically deprived of nutrient oxygen during MI. The focus of cell sheet engineering is to be able to fully vascularize cell sheets in implantation, establish cell-to-cell interactions, and deliver large quantities of cells with minimal cell loss. The challenge in this approach is that the cell sheet is completely reliant on the capillaries that form in cell sheet tissue, therefore further attempts to control the cell distribution for a more organized tissue should be extensively studied to enhance cell sheet function and increase therapeutic effects after transplantation.
Acknowledgement
This research was supported by the National Research Foundation Grant (NRF- 2019M3E5D1A02070861).
Declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yurttas T, Hidvegi R, Filipovic M. Biomarker-based preoperative risk stratification for patients undergoing non-cardiac surgery. J Clin Med. 2020;9:351–360. doi: 10.3390/jcm9020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther. 2020;34:849–863. doi: 10.1007/s10557-020-07071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao MT, Ye S, Su J, Garg V. Cardiomyocyte proliferation and maturation: two sides of the same coin for heart regeneration. Front Cell Dev Biol. 2020;8:594226. [DOI] [PMC free article] [PubMed]
- 4.Lafuse WP, Wozniak DJ, Rajaram MV. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells. 2020;10:51–78. doi: 10.3390/cells10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teringova E, Tousek P. Apoptosis in ischemic heart disease. J Transl Med. 2017;15:87–94. doi: 10.1186/s12967-017-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham E, Bergmann O. Dating the heart: exploring cardiomyocyte renewal in humans. Physiology (Bethesda) 2017;32:33–41. doi: 10.1152/physiol.00015.2016. [DOI] [PubMed] [Google Scholar]
- 7.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 8.Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, Stevens G, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7:e1332–e1345. doi: 10.1016/S2214-109X(19)30318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442–e462. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 12.Maltês S, Rocha M, Cunha GJ, Brízido C, Strong C, Tralhão A, et al. Challenges of organ shortage for heart transplant: surviving amidst the chaos of long waiting times. Transplantat Direct. 2021;7:e671–e674. doi: 10.1097/TXD.0000000000001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saidi R, Kenari SH. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med. 2014;5:87–97. [PMC free article] [PubMed] [Google Scholar]
- 14.Sadek H, Olson EN. Toward the goal of human heart regeneration. Cell Stem Cell. 2020;26:7–16. doi: 10.1016/j.stem.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terashvili M, Bosnjak ZJ. Stem cell therapies in cardiovascular disease. J Cardiothorac Vasc Anesth. 2019;33:209–222. doi: 10.1053/j.jvca.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17:2973–2980. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 2008;1:1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Li M, Li M, Zhang Z, Ma H, Zhao L, et al. Adipose-derived mesenchymal stem cell seeded Atelocollagen scaffolds for cardiac tissue engineering. J Mater Sci Mater Med. 2020;31:83–92. doi: 10.1007/s10856-020-06425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam SY, Park SH. ECM based bioink for tissue mimetic 3D bioprinting. Adv Exp Med Biol. 2018;1064:335–353. doi: 10.1007/978-981-13-0445-3_20. [DOI] [PubMed] [Google Scholar]
- 22.Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach J, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 25.Shokrani H, Shokrani A, Sajadi SM, Seidi F, Mashhadzadeh AH, Rabiee N, et al. Cell-seeded biomaterial scaffolds: the urgent need for unanswered accelerated angiogenesis. Int J Nanomedicine. 2022;17:1035–1069. doi: 10.2147/IJN.S353062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KM, Shin YM, Kim K, Shin H. Tissue engineering and regenerative medicine 2017: a year in review. Tissue Eng Part B Rev. 2018;24:327–344. doi: 10.1089/ten.TEB.2018.0027. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi J, Okano T. Design of temperature-responsive polymer-grafted surfaces for cell sheet preparation and manipulation. Bull Chem Soc Jpn. 2019;92:817–824. [Google Scholar]
- 28.Elloumi-Hannachi I, Yamato M, Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim K, Bou-Ghannam S, Okano T. Cell sheet tissue engineering for scaffold-free three-dimensional (3D) tissue reconstruction. Methods Cell Biol. 2020:143–167. [DOI] [PubMed]
- 30.Menasché P, Hagège AA, Vilquin J-T, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 31.Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 32.Durrani S, Konoplyannikov M, Ashraf M, Haider KH. Skeletal myoblasts for cardiac repair. Regen Med. 2010;5:919–932. doi: 10.2217/rme.10.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukushima S, Coppen SR, Lee J, Yamahara K, Felkin LE, Terracciano CM, et al. Choice of cell-delivery route for skeletal myoblast transplantation for treating post-infarction chronic heart failure in rat. PLoS ONE. 2008;3:e3071–e3082. doi: 10.1371/journal.pone.0003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavira JJ, Nasarre E, Abizanda G, Perez-Ilzarbe M, De Martino Rodriguez A, García de Jalón JA, et al. Repeated implantation of skeletal myoblast in a swine model of chronic myocardial infarction. Eur Heart J. 2010;31:1013–1021. doi: 10.1093/eurheartj/ehp342. [DOI] [PubMed] [Google Scholar]
- 35.Léobon B, Garcin I, Menasché P, Vilquin J-T, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Levin MD, Xiong Y, Petrenko N, Patel VV, Radice GL. N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J Mol Cell Cardiol. 2008;44:597–606. doi: 10.1016/j.yjmcc.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Serpooshan V, Zhang J. Engineering human cardiac muscle patch constructs for prevention of post-infarction LV remodeling. Front Cardiovas Med. 2021;8:111–119. doi: 10.3389/fcvm.2021.621781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shudo Y, Miyagawa S, Nakatani S, Fukushima S, Sakaguchi T, Saito A, et al. Myocardial layer-specific effect of myoblast cell-sheet implantation evaluated by tissue strain imaging. Circ J. 2013;77:1063–1072. doi: 10.1253/circj.cj-12-0615. [DOI] [PubMed] [Google Scholar]
- 39.Ott HC, Kroess R, Bonaros N, Marksteiner R, Margreiter E, Schachner T, et al. Intramyocardial microdepot injection increases the efficacy of skeletal myoblast transplantation. Eur J Cardiothorac Surg. 2005;27:1017–1021. doi: 10.1016/j.ejcts.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 40.Yoon DM, Curtiss S, Reddi AH, Fisher JP. Addition of hyaluronic acid to alginate embedded chondrocytes interferes with insulin-like growth factor-1 signaling in vitro and in vivo. Tissue Eng Part A. 2009;15:3449–3459. doi: 10.1089/ten.TEA.2009.0069. [DOI] [PubMed] [Google Scholar]
- 41.Terajima Y, Shimizu T, Tsuruyama S, Sekine H, Ishii H, Yamazaki K, et al. Autologous skeletal myoblast sheet therapy for porcine myocardial infarction without increasing risk of arrhythmia. Cell Med. 2014;6:99–109. doi: 10.3727/215517913X672254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, et al. Human cardiac stem cells. Proc Acad Sci. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zakrzewski JL, Van Den Brink MR, Hubbell JA. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol. 2014;32:786–794. doi: 10.1038/nbt.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nardi NB, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. In: Wobus AM, Boheler KR. editors. Stem cells. Handbook of experimental Pharmacology, vol 174. Springer, Berlin, Heidelberg. 2008. p. 249–82. [PubMed]
- 46.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakao M, Inanaga D, Nagase K, Kanazawa H. Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins. Regen Ther. 2019;11:34–40. doi: 10.1016/j.reth.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez-Vilchis RA, Piedra-Ramirez A, Patiño-Morales CC, Sanchez-Gomez C, Beltran-Vargas NE. Sources, characteristics, and therapeutic applications of mesenchymal cells in tissue engineering. Tissue Eng Regen Med. 2022;19:325–61. [DOI] [PMC free article] [PubMed]
- 49.Van Nguyen T-T, Vu NB, Van Pham P. Mesenchymal stem cell transplantation for ischemic diseases: mechanisms and challenges. Tissue Eng Regen Med. 2021;18:587–611. doi: 10.1007/s13770-021-00334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbau GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wollert KC, Meyer GP, Lotz J, Lichtenberg SR, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 54.Kocher A, Schuster M, Szabolcs M, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow–derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 55.Stamm C, Westphal B, Kleine H-D, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 56.Du M, Schmull S, Zhang W, Wang C, Lian F, Chen Y, et al. c-kit+ AT2R+ bone marrow mononuclear cell subset is a superior subset for cardiac protection after myocardial infarction. Stem Cells Int. 2016;2016:4913515–4913530. doi: 10.1155/2016/4913515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 58.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K, et al. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J. 2005;39:150–158. doi: 10.1080/14017430510009131. [DOI] [PubMed] [Google Scholar]
- 59.Dill T, Schächinger V, Rolf A, Möllmann S, Thiele H, Tillmanns H, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–547. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow–derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 61.Mathur A, Fernández-Avilés F, Bartunek J, Belmans A, Crea F, Dowlut S, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41:3702–3710. doi: 10.1093/eurheartj/ehaa651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, et al. Effect of shock wave–facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309:1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 63.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ Res. 2017;120:324–331. doi: 10.1161/CIRCRESAHA.115.308165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 65.Lee J-W, Lee S-H, Youn Y-J, Ahn M-S, Kim J-Y, Yoo B-S, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29:23–31. doi: 10.3346/jkms.2014.29.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lancet T. Retraction—Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2019;393:1084. doi: 10.1016/S0140-6736(19)30542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perin EC, Sanz-Ruiz R, Sánchez PL, Lasso J, Pérez-Cano R, Alonso-Farto JC, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE Trial. Am Heart J. 2014;168(88–95):e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 69.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, et al. First experience in humans using adipose tissue–derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 70.Hare JM, Fishman JE, Gerstenblith G, Velazquez DLD, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathur A, Arnold R, Assmus B, Bartunek J, Belmans A, Bönig H, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: rationale and design of the BAMI trial. Eur J Heart Fail. 2017;19:1545–1550. doi: 10.1002/ejhf.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang D, Shimizu T, Haraguchi Y, Gao S, Sakaguchi K, Umezu M, et al. Time course of cell sheet adhesion to porcine heart tissue after transplantation. PLoS One. 2015;10:e0137494–e0137508. doi: 10.1371/journal.pone.0137494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawamura M, Miyagawa S, Fukushima S, Saito A, Toda K, Daimon T, et al. Xenotransplantation of bone marrow-derived human mesenchymal stem cell sheets attenuates left ventricular remodeling in a porcine ischemic cardiomyopathy model. Tissue Eng Part A. 2015;21:2272–2280. doi: 10.1089/ten.tea.2014.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka Y, Shirasawa B, Takeuchi Y, Kawamura D, Nakamura T, Samura M, et al. Autologous preconditioned mesenchymal stem cell sheets improve left ventricular function in a rabbit old myocardial infarction model. Am J Transl Res. 2016;8:2222–2233. [PMC free article] [PubMed] [Google Scholar]
- 76.Tano N, Narita T, Kaneko M, Ikebe C, Coppen SR, Campbell NG, et al. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol Ther. 2014;22:1864–1871. doi: 10.1038/mt.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012; 2:CD006536.pub3. [DOI] [PubMed]
- 78.Hauskeller C, Baur N. Travelling cells: harmonized European regulation and the BAMI stem cell trial. In: Phuc VP, Achim R, editors. Safety, ethics and regulations. Champa: Springer; 2017. pp. 201–216. [Google Scholar]
- 79.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 80.Nakagami H, Morishit R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 81.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 82.Yang D, Wang W, Li L, Peng Y, Chen P, Huang H, et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS ONE. 2013;8:e59020–e59031. doi: 10.1371/journal.pone.0059020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 84.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 85.Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, Le Coz O, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–824. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo R, Wan F, Morimatsu M, Xu Q, Feng T, Yang H, et al. Cell sheet formation enhances the therapeutic effects of human umbilical cord mesenchymal stem cells on myocardial infarction as a bioactive material. Bioactive Mater. 2021;6:2999–3012. doi: 10.1016/j.bioactmat.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Sci. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 89.Zandstra P, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, et al. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767–778. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 90.Schroeder M, Niebruegge S, Werner A, Willbold E, Burg M, Ruediger M, et al. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920–933. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 91.Zhu W-Z, Hauch KD, Xu C, Laflamme MA. Human embryonic stem cells and cardiac repair. Transplant Rev. 2009;23:53–68. doi: 10.1016/j.trre.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tohyama S, Fujita J, Fujita C, Yamaguchi M, Kanaami S, Ohno R, et al. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Reports. 2017;9:1406–1414. doi: 10.1016/j.stemcr.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park M, Yoon YS. Cardiac regeneration with human pluripotent stem cell-derived cardiomyocytes. Korean Circ J. 2018;48:974–988. doi: 10.4070/kcj.2018.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–1205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
- 95.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moyzis AG, Sadoshima J, Gustafsson ÅB. Mending a broken heart: the role of mitophagy in cardioprotection. Am J Physiol Heart Circ Physiol. 2015;308:H183–H192. doi: 10.1152/ajpheart.00708.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hata H, Matsumiya G, Miyagawa S, Kondoh H, Kawaguchi N, Matsuura N, et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J Thorac Cardiovasc Surg. 2006;132:918–924. doi: 10.1016/j.jtcvs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 99.Pawani H, Bhartiya D. Pluripotent stem cells for cardiac regeneration: overview of recent advances & emerging trends. Indian J Med Res. 2013;137:270–282. [PMC free article] [PubMed] [Google Scholar]
- 100.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsuura K, Haraguchi Y, Shimizu T, Okano T. Cell sheet transplantation for heart tissue repair. J Controlled Release. 2013;169:336–340. doi: 10.1016/j.jconrel.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 104.Krencik R, Weick JP, Liu Y, Zhang Z-J, Zhang S-C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, et al. Maturation of human induced pluripotent stem cell-derived cardiomyocytes by soluble factors from human mesenchymal stem cells. Mol Ther. 2018;26:2681–2695. doi: 10.1016/j.ymthe.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, et al. Enhanced survival of transplanted human induced pluripotent stem cell–derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. 2013;128:S87–S94. doi: 10.1161/CIRCULATIONAHA.112.000366. [DOI] [PubMed] [Google Scholar]
- 108.De Pieri A, Rochev Y, Zeugolis DI. Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. NPJ Regen Med. 2021;6:1–15. doi: 10.1038/s41536-021-00133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sekiya N, Matsumiya G, Miyagawa S, Saito A, Shimizu T, Okano T, et al. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J Thorac Cardiovasc Surg. 2009;138:985–993. doi: 10.1016/j.jtcvs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 110.Miyagawa S, Saito A, Sakaguchi T, Yoshikawa Y, Yamauchi T, Imanishi Y, et al. Impaired myocardium regeneration with skeletal cell sheets—a preclinical trial for tissue-engineered regeneration therapy. Transplantation. 2010;90:364–372. doi: 10.1097/TP.0b013e3181e6f201. [DOI] [PubMed] [Google Scholar]
- 111.Shudo Y, Miyagawa S, Fukushima S, Saito A, Shimizu T, Okano T, et al. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg. 2011;142:1188–1196. doi: 10.1016/j.jtcvs.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 113.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 114.Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg. 2003;75:1587–1592. doi: 10.1016/s0003-4975(02)04994-9. [DOI] [PubMed] [Google Scholar]
- 115.Kanzaki M, Yamato M, Yang J, Sekine H, Takagi R, Isaka T, et al. Functional closure of visceral pleural defects by autologous tissue engineered cell sheets. Eur J Cardiothorac Surg. 2008;34:864–869. doi: 10.1016/j.ejcts.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 116.Miyagawa S, Domae K, Yoshikawa Y, Fukushima S, Nakamura T, Saito A, et al. Phase I clinical trial of autologous stem cell–sheet transplantation therapy for treating cardiomyopathy. Am Heart J. 2017;6:e003918–e003929. doi: 10.1161/JAHA.116.003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim J-H, Joo HJ, Kim M, Choi S-C, Lee JI, Hong SJ, et al. Transplantation of adipose-derived stem cell sheet attenuates adverse cardiac remodeling in acute myocardial infarction. Tissue Eng Part A. 2017;23:1–11. doi: 10.1089/ten.TEA.2016.0023. [DOI] [PubMed] [Google Scholar]
- 118.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 119.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta Gen Subj. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Araña M, Gavira JJ, Peña E, González A, Abizanda G, Cilla M, et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials. 2014;35:143–151. doi: 10.1016/j.biomaterials.2013.09.083. [DOI] [PubMed] [Google Scholar]
- 121.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 123.Khademhosseini A, Ferreira L, Blumling J, III, Yeh J, Karp JM, Fukuda J, et al. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 124.Wobus AM, Kaomei G, Shan J, Wellner M-C, Rohwedel J, Guanju J, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol. 1997;29:1525–1539. doi: 10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, et al. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 126.Siltanen A, Kitabayashi K, Pätilä T, Ono M, Tikkanen I, Sawa Y, et al. Bcl-2 improves myoblast sheet therapy in rat chronic heart failure. Tissue Eng Part A. 2011;17:115–125. doi: 10.1089/ten.TEA.2010.0205. [DOI] [PubMed] [Google Scholar]
- 127.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, et al. Bcl‐2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–27. [DOI] [PubMed]
- 128.Siltanen A, Kitabayashi K, Lakkisto P, Mäkelä J, Pätilä T, Ono M, et al. hHGF overexpression in myoblast sheets enhances their angiogenic potential in rat chronic heart failure. PLoS ONE. 2011;6:e19161–e19171. doi: 10.1371/journal.pone.0019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uchinaka A, Tasaka K, Mizuno Y, Maeno Y, Ban T, Mori S, et al. Laminin α 2-secreting fibroblasts enhance the therapeutic effect of skeletal myoblast sheets. Eur J Cardiothorac Surg. 2017;51:457–464. doi: 10.1093/ejcts/ezw296. [DOI] [PubMed] [Google Scholar]
- 130.Dergilev K, Tsokolaeva Z, Makarevich P, Beloglazova I, Zubkova E, Boldyreva M, et al. C-kit cardiac progenitor cell based cell sheet improves vascularization and attenuates cardiac remodeling following myocardial infarction in rats. BioMed Res Int. 2018;2018:3536854–3536867. doi: 10.1155/2018/3536854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Masumoto H, Ikuno T, Takeda M, Fukushima H, Marui A, Katayama S, et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4:1–7. doi: 10.1038/srep06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haque N, Kasim NHA, Rahman MT. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int J Biol Sci. 2015;11:324–334. doi: 10.7150/ijbs.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roberts EG, Kleptsyn VF, Roberts GD, Mossburg KJ, Feng B, Domian IJ, et al. Development of a bio-MEMS device for electrical and mechanical conditioning and characterization of cell sheets for myocardial repair. Biotechnol Bioeng. 2019;116:3098–3111. doi: 10.1002/bit.27123. [DOI] [PubMed] [Google Scholar]
- 134.Endoh M. Force–frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 135.Godier-Furnémont AF, Tiburcy M, Wagner E, Dewenter M, Lämmle S, El-Armouche A, et al. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 2015;60:82–91. doi: 10.1016/j.biomaterials.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsuruyama S, Matsuura K, Sakaguchi K, Shimizu T. Pulsatile tubular cardiac tissues fabricated by wrapping human iPS cells-derived cardiomyocyte sheets. Regen Ther. 2019;11:297–305. doi: 10.1016/j.reth.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Homma J, Shimizu S, Sekine H, Matsuura K, Shimizu T. A novel method to align cells in a cardiac tissue-like construct fabricated by cell sheet-based tissue engineering. J Tissue Eng Regen Med. 2020;14:944–954. doi: 10.1002/term.3074. [DOI] [PubMed] [Google Scholar]
- 138.Kim H, Witt H, Oswald TA, Tarantola M. Adhesion of epithelial cells to pnipam treated surfaces for temperature-controlled cell-sheet harvesting. ACS Appl Mater. 2020;12:33516–33529. doi: 10.1021/acsami.0c09166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamato M, Konno C, Kushida A, Hirose M, Utsumi M, Kikuchi A, et al. Release of adsorbed fibronectin from temperature-responsive culture surfaces requires cellular activity. Biomaterials. 2000;21:981–986. doi: 10.1016/s0142-9612(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 140.Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Macromol Rapid Commun. 1990;11:571–576. [Google Scholar]
- 141.Tekin H, Sanchez JG, Tsinman T, Langer R, Khademhosseini A. Thermoresponsive platforms for tissue engineering and regenerative medicine. AIChE J. 2011;57:3249–3258. doi: 10.1002/aic.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimizu T, Yamato M, Kikuchi A, Okano T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng. 2001;7:141–151. doi: 10.1089/107632701300062732. [DOI] [PubMed] [Google Scholar]
- 143.Shimizu T, Yamato M, Akutsu T, Shibata T, Isoi Y, Kikuchi A, et al. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res. 2002;60:110–117. doi: 10.1002/jbm.1284. [DOI] [PubMed] [Google Scholar]
- 144.Wang C-C, Chen C-H, Lin W-W, Hwang S-M, Hsieh PC, Lai P-H, et al. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res. 2008;77:515–524. doi: 10.1093/cvr/cvm046. [DOI] [PubMed] [Google Scholar]
- 145.Zhang L, Guo J, Zhang P, Xiong Q, Wu SC, Xia L, et al. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ Heart Fail. 2015;8:156–166. doi: 10.1161/CIRCHEARTFAILURE.114.001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeo WS, Hodneland CD, Mrksich M. Electroactive monolayer substrates that selectively release adherent cells. ChemBio Chem. 2001;2:590–593. doi: 10.1002/1439-7633(20010803)2:7/8<590::AID-CBIC590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 147.Kobayashi Y, Cordonier CE, Noda Y, Nagase F, Enomoto J, Kageyama T, et al. Tailored cell sheet engineering using microstereolithography and electrochemical cell transfer. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-46801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Na J, Heo JS, Han M, Lim H, Ki HO, Kim E. Harvesting of living cell sheets by the dynamic generation of diffractive photothermal pattern on PEDOT. Adv Funct Mater. 2017;27:1604260–1604268. [Google Scholar]
- 149.Edahiro J-I, Sumaru K, Tada Y, Ohi K, Takagi T, Kameda M, et al. In situ control of cell adhesion using photoresponsive culture surface. Biomacromol. 2005;6:970–974. doi: 10.1021/bm0493382. [DOI] [PubMed] [Google Scholar]
- 150.Koo M-A, Lee MH, Kwon B-J, Seon GM, Kim MS, Kim DH, et al. Exogenous ROS-induced cell sheet transfer based on hematoporphyrin-polyketone film via a one-step process. Biomaterials. 2018;161:47–56. doi: 10.1016/j.biomaterials.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 151.Kang B, Shin J, Park H-J, Rhyou C, Kang D, Lee S-J, et al. High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nat Commun. 2018;9:1–13. doi: 10.1038/s41467-018-07823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kurashina Y, Imashiro C, Hirano M, Kuribara T, Totani K, Ohnuma K, et al. Enzyme-free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves. Commun Biol. 2019;2:1–11. doi: 10.1038/s42003-019-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakao M, Imashiro C, Kuribara T, Kurashina Y, Totani K, Takemura K. Formation of large scaffold-free 3-D aggregates in a cell culture dish by ultrasound standing wave trapping. Ultrasound Med Biol. 2019;45:1306–1315. doi: 10.1016/j.ultrasmedbio.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 154.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 155.Jang J, Park H-J, Kim S-W, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 156.De las Heras Alarcón C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. ChSRv. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 157.Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release. 2002;78:295–303. doi: 10.1016/s0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 158.Guillaume-Gentil O, Semenov OV, Zisch AH, Zimmermann R, Vörös J, Ehrbar M. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials. 2011;32:4376–4384. doi: 10.1016/j.biomaterials.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 159.Ishii M, Shibata R, Shimizu Y, Yamamoto T, Kondo K, Inoue Y, et al. Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. Int J Cardiol. 2014;175:545–553. doi: 10.1016/j.ijcard.2014.06.034. [DOI] [PubMed] [Google Scholar]