Abstract
Tropane alkaloids (TAs) are among the most valued chemical compounds known since pre-historic times. Poisonous plants from Solanaceae family (Hyoscyamus niger, Datura, Atropa belladonna, Scopolia lurida, Mandragora officinarum, Duboisia) and Erythroxylaceae (Erythroxylum coca) are rich sources of tropane alkaloids. These compounds possess the anticholinergic properties as they could block the neurotransmitter acetylcholine action in the central and peripheral nervous system by binding at either muscarinic and/or nicotinic receptors. Hence, they are of great clinical importance and are used as antiemetics, anesthetics, antispasmodics, bronchodilator and mydriatics. They also serve as the lead compounds to generate more effective drugs. Due to the important pharmacological action they are listed in the WHO list of essential medicines and are available in market with FDA approval. However, being anticholinergic in action, TA medication are under the suspicion of causing dementia and cognitive decline like other medications with anticholinergic action, interestingly which is incorrect. There are published reviews on chemistry, biosynthesis, pharmacology, safety concerns, biotechnological aspects of TAs but the detailed information on anticholinergic mechanism of action, clinical pharmacology, FDA approval and anticholinergic burden is lacking. Hence the present review tries to fill this lacuna by critically summarizing and discussing the above mentioned aspects.
Graphical Abstract
Keywords: Tropane alkaloids, Poisonous plants, Anticholinergic action, Muscarinic and nicotinic receptors, Therapeutics, Anticholinergic burden
Introduction
Tropane alkaloids (TAs) belonged to a class of about 200 alkaloids with a distinctive bicyclic tropane ring in their chemical structure [1]. Occurrences and distributions of tropane alkaloids are reported in the plants from families like, Convolvulaceae, Erythroxylaceae, Solanaceae, Proteaceae, Euphorbiaceae, Rhizophoraceae, and Cruciferae [2]. Interestingly, the highest concentrations of TAs are present in Solanaceae (Hyoscyamus niger, Datura, Atropa belladonna, Scopolia lurida, Mandragora officinarum, Duboisia) and Erythroxylaceae (Erythroxylum coca) [3, 4]. The existing important bioactive compounds in these plants are atropine, hyoscyamine and hyoscine (scopolamine). These plants are poisonous upon ingestion and can have dire consequences. The high doses of their extracts and/or compounds could result in delirium, stupefaction and intense hallucinations [5]. The lethal dose of atropine is around 10 mg while that of scopolamine is much lower (2–4 mg) [6] Relatively very high concentration of atropine (0.1 mg/ seed) and scopolamine (3.85 mg/g leaves) is present in Datura stramonium (Jimsonweed) and Datura inoxia (Moon flower), respectively [6]. The scopolamine content of Scopolia lurida (1.5 mg/100 mg dry weight of leaves) is reported to be higher than that of A. belladonna [4, 7]. Scopolamine is listed among the indispensable medicines of the world by the World Health Organization (WHO) [8]. Both Atropa and Datura species accounts for roughly 0.2–0.8% of total alkaloids with a fairly low scopolamine (hyoscine) content. So they are not commercially used for the extraction of TAs. To meet the needs of pharmaceutical industry, another plant (Duboisia) is cultivated on a commercial scale for being a rich source (2–4%) of TAs (constituting about 60% scopolamine, and 30% hyoscyamine). The pharmaceutical industry need uninterrupted high supply of TAs (specially, scopolamine and atropine) which is a limiting factor in the field grown plants as various biotic and abiotic factors affect the production and concentration of TAs. Hence, to overcome this problem, unconventional methods, like metabolic engineering is used to increase the availability [9–12].
TAs are the secondary plant metabolites which have been used since ancient times in traditional medicine [13], poison [14–16], cosmetics [17], recreational purposes [18, 19] and, blood sport [16, 20]. The leaves of Erythroxylum coca (source of cocaine) were chewed to increase the stamina and are also consumed during religious sacrifices [21]. Deliriant poisoning was prevalent among American youth in 1960s by deliberate intake of ‘Asthmador’ (asthma medication containing extract of D. stramonium), for experiencing euphoria [22]. In fact, due to hallucinogenic and psychoactive properties TAs are associated with the magic/rituals by many ethnic groups all around the world. Under the influence of smoke from these plants, the priests were reported to deliver divinations after entering a trance [23]. In ancient times “flying salves” were made from these plant extracts (Atropa belladonna, Hyoscymaus niger and Mandragora officinarum) which when applied to the broom stick get absorbed through the skin of witches and imparted the power of flying to them [24–26] as mentioned in the ancient texts.
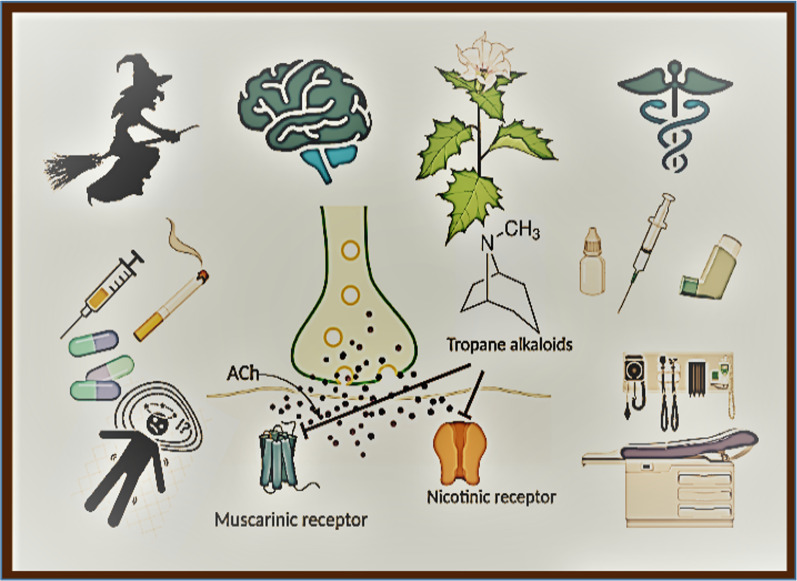
TAs display anticholinergic effect as they block neurotransmitter acetylcholine (ACh) action in the central and peripheral nervous system (CNS and PNS) by binding at either muscarinic receptors (mAChR) or nicotinic acetylcholine receptors (nAChR), to a lesser extent (Fig. 1).
Fig. 1.
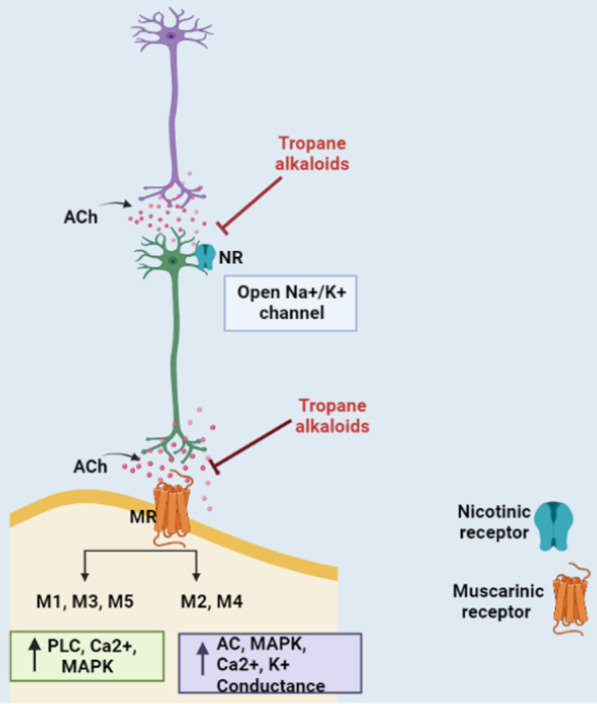
Anticholinergic Action of Tropane Alkaloids. Tropane alkaloids are anticholinergic in action. TAs competitively bind to muscarinic and/or nicotinic receptors and block ACh transmission. MR: Muscarinic receptor; NR: Nicotinic receptor; ACh: Acetylcholine; PLC: Phospholipase C; AC: Adenylyl cyclase; MAPK: Mitogen-activated protein kinase [Prepared using Biorender.com]
As a consequence of this anticholinergic action both CNS and PNS are affected and the symptoms are manifested by changes in heart rate & respiration, muscle contraction, hallucination etc. [27]. Even though all the TAs share structural similarity due to the presence of tropane ring in the scaffold, they exhibit considerably different pharmacological effects. Atropine, hyoscyamine, scopolamine and cocaine can cross the blood–brain barrier (BBB) to effect the CNS [28, 29], while the calystegine cannot, owing to its hydrophilic nature. As a result, calystegines are unable to show psychoactive results [3]. Cocaine blocks the reuptake of neurotransmitters like dopamine, noradrenaline and serotonin in the synaptic cleft [30]. As compared to atropine, scopolamine is more suitable in situations where decreased parasympathetic activity is required. In PNS it relaxes smooth muscles and decrease the body secretions. Additionally, in CNS scopolamine causes drowsiness, unlike atropine [31].
Hyoscine, the first drug to be called “truth serum” (a drug used to extract truth from the criminals), was accidently discovered by Dr. Robert House in early twentieth century [32]. Over twenty active pharmaceutical ingredients (API) having tropane moiety are being manufactured by various pharma companies [33]. The natural and synthetic TAs are anti-secretory [34] anti-spasmodic [35], antiemetic in action and are being used for treating allergies, asthma and chronic obstructive pulmonary disease (COPD) [36], urinary dysfunction, irritable bowel syndrome (IBS) [35], post-operative nausea and vomiting (PONV) [31], local anaesthetics [37], ophthalmological eye drops [38], antidote against organophosphorus compounds poisoning [39].
The common side effects of TAs are related to parasympathetic stimulation; decreased body secretion resulting in dry mouth and eyes, constipation, urine retention, tachycardia, depressive activity in CNS and delirium [38]. Due to the side effects caused by TA medications, their prescription may be considered unsuitable in some situations. Still, for some critical clinical conditions, like death rattle [40] the benefits of using TAs overrides their side effects.
The available reviews focused on TA occurrence and distribution [41], pharmacological outline [3, 42], biosynthesis [3, 43], biotechnology [44], poisoning [45–47], production techniques [48] and cytotoxicity [49]. However, the detailed information on anticholinergic mechanism of action, clinical pharmacology and anticholinergic burden is lacking. Therefore, in an effort to search the benefits of these so called venomous or dark compounds, a comprehensive information on therapeutic action mechanism and clinical pharmacology was collected using PubMed, Google Scholar, Scopus as online databases until May, 2022 with key words being tropane alkaloids, anticholinergic, clinical pharmacology, anticholinergic burden, therapeutics, FDA approval and toxicity. We included studies conducted on human and animals for clinical pharmacology. Additional information was extracted from the citations mentioned in the articles. However, the observational studies and any other unrelated pharmacological activity on the topic were excluded. Hence, this review not only summarized the literature but also discussed it critically. This information will be helpful in better understanding of the role of TAs in therapeutics and designing new pharmacophore with better therapeutic profile hence will contribute to new vistas for advanced research on TAs.
Tropane alkaloids
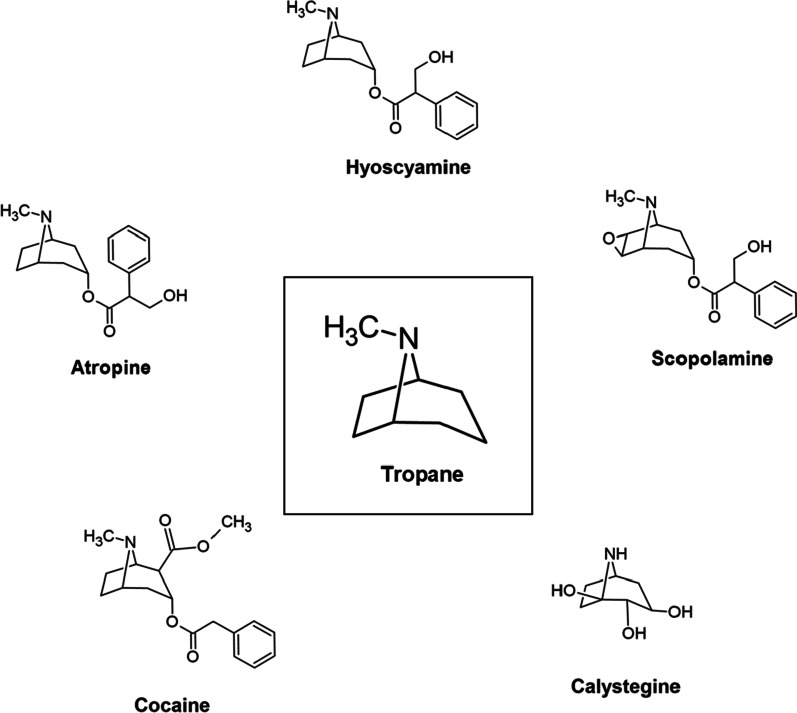
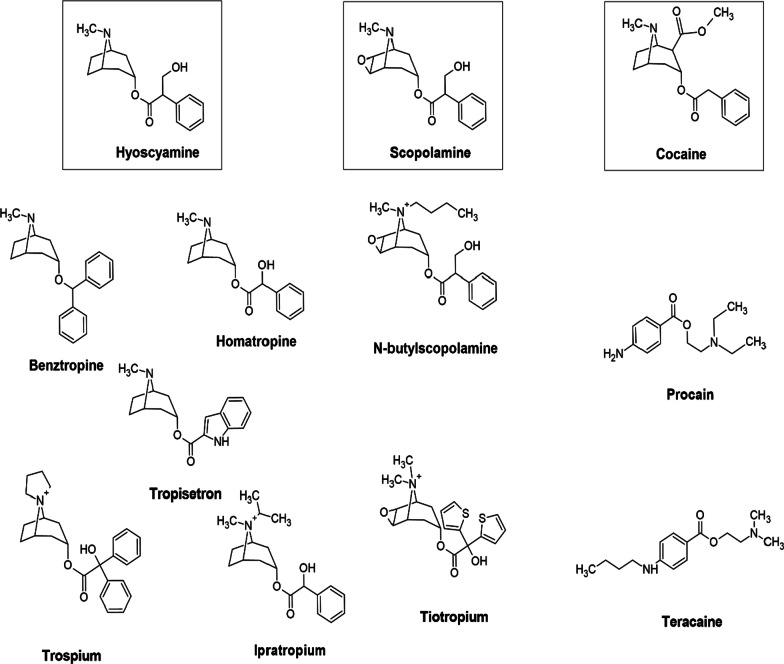
Tropane is the condensation product of pyrrolidine precursor (ornithine) and piperidine ring with a common nitrogen and two carbon atoms [1]. In nature, TAs occur as ester formed by the combination of organic acids (tropic acid) and alcoholic base (tropanol). The structure of natural TAs is given in Fig. 2. We will not discuss about calystegines as despite of structural similarity, they are nortropanes due to the absence of the nitrogen associated methyl group. Also, they do not share the bioactivity of TAs [50] and behave as glycosidase inhibitors [51].
Fig. 2.
Structure of natural tropane alkaloids
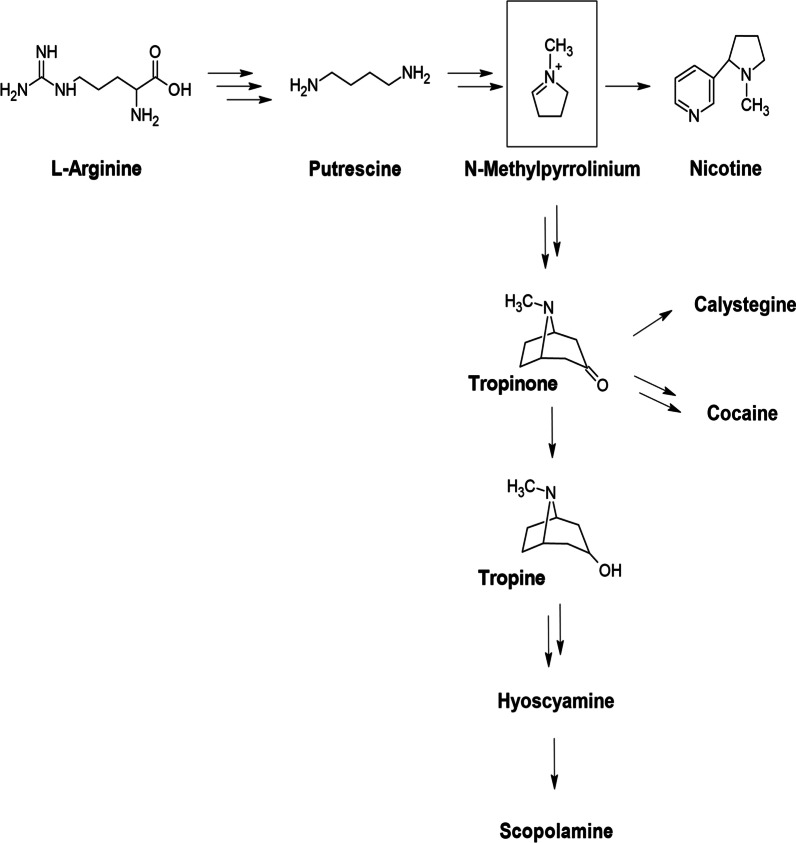
In plants, all the different types of TAs share a common biosynthetic pathway (Fig. 3) which starts with l-arginine (Arg). It undergoes a three-step decarboxylation and hydrolysis reactions to form Putrescine which gets methylated and undergo oxidative deamination to form N-methyl-Δ1-pyrrolinium, a branch point for the synthesis of TAs and nicotine.
Fig. 3.
Biosynthetic pathway of tropane alkaloids
History
Atropine and scopolamine are found in several plants of Solanaceae family and have been used in ancient system of medicine for treating mental problems, skin diseases and tumors [28]. Atropine was the first TA to be isolated from the leaves and roots of A. belladonna by Mein in 1832 but he did not publish his results. A year later, Geiger and Hesse published the isolation of atropine from Atropa belladonna and Hyoscyamus niger [52]. Fifty years later, scopolamine was isolated from Scopolia japonica [53]. In 1864, Kraut and Lossen revealed the stereochemistry and cleavage products (tropic acid and tropine) of atropine and hyoscyamine [54]. Cocaine is considered as a ‘wonder medicine’ in the texts as it has local anaesthetic action and has role in treating postnatal depression and morphine overdose [37]. It was isolated by Gaedcke in the year 1855, from the leaves of Erythroxylon coca [55]. His research was later extended by Neimann [56] who not only improved the isolation method of cocaine but also elucidated its mode of action.
Anticholinergic action
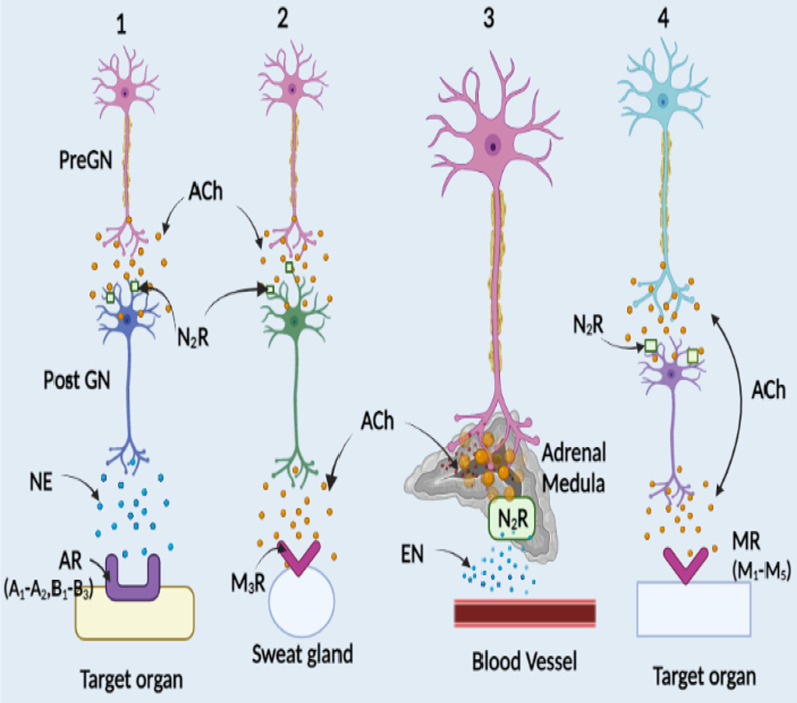
TAs are anticholinergic in action i.e. they inhibit ACh mediated response by competitively binding to muscarinic and/or nicotinic receptors in the CNS and PNS (Fig. 1). There is almost no structural or physiological similarity between muscarinic and nicotinic receptors except for the fact that they both bind to ACh. Under the normal conditions, these receptors allow the binding of neurotransmitter ACh and help in signal transduction (Fig. 4).
Fig. 4.
Autonomic nervous system and location of receptors. Nerves of autonomic nervous system (ANS) extend from central nervous system (CNS) to cardiac muscle, smooth muscle, organs and glands by preganglionic (Pre GN) and postganglionic (Post GN) neurons. PreGN releases acetylcholine (ACh) into the synaptic cleft and get bind to nicotine receptors (NR) in Post GN. Depolarization of membrane results in action potential which on arriving at the axon terminal releases neurotransmitter: ACh, norepinephrine (NE) or epinephrine (EN) in the synaptic cleft. The binding of neurotransmitter to the receptors: Androgenic (AR) or muscarinic (M) on the target organ results in excitation or inhibition. (1–3) Sympathetic system, (4) Parasympathetic system. These two systems differ in the type of neurotransmitter released, type of receptors and secondary messengers expressed [Prepared using Biorender.com]
Muscarinic receptors are G-protein-coupled receptors (GPCRs), more precisely rhodopsin-like (class A) receptors. Receptors M1, M3, and M5 activate phospholipase C by Gαq group whereas receptors M2 and M4 inhibit adenylate cyclase by Gαi group of G proteins [57]. The muscarinic acetylcholine receptors (mAChRs) are of five types (M1-M5) and dispersed all over the body [58]. In brain, they are predominantly located in the hippocampus, cerebral cortex, neostriatum and substantia nigra [59]. Receptor M1 is the most abundant receptor in the cerebral cortical region and is associated with memory and learning processes [58]. The non CNS locations of mAChRs are mainly, smooth muscle, cardiac muscle, ciliary muscle, salivary glands and sympathetic ganglia. Antagonism at M1 and M2 receptors have negative impact on memory and cognition [60]. In addition, pre-synaptic release of ACh is regulated by M2 receptor which is essential for the ACh homeostasis [61]. The regulation of other neurotransmitters (like dopamine) is altered by antagonism at M4 and M5 receptors. Interestingly, blocking of M3 receptor has apparently no effect on the cognition [60].
Neuronal nicotinic acetylcholine receptors (nAChRs) are cation-selective, ligand-gated ion channels, widely present in CNS and PNS. Seventeen different types of nAChRs have been identified including, ten α (α1–α10), four β (β1–β4), γ, δ, and ε subunits [62]. In brain, homomeric α7 nAChRs and heteromeric α4β2.∗ nAChRs are predominantly present [63, 64] and thought to have important role in the neurodegenerative diseases [65–67]. The nAChRs are also known to regulate dopamine release in the brain striatum. Cocaine reduces dopamine release in dopaminergic neurons by antagonizing α4β2-containing (α4β2*) nAChRs (IC50 4–15 μM) [68, 69]. At high concentrations (40 µM), cocaine also inhibits voltage-gated Na channels (INa) in dopanergic neurons [68]. Binding of nicotine and cocaine to nAChRs has rewarding effect on the brain. Thus, people are easily addicted to these compounds. Researchers confirmed the role of α6β2* and α4β2* in nicotine and α6β2* nAChRs in cocaine addiction [70]
Atropine and scopolamine are non-selective competitive antagonist of muscarinic receptors. Atropine has the highest affinity for subtype M1, followed by M2 and M3 and weak affinity for M4 and M5 [71]. On the other hand, scopolamine has strong affinity for M1-M4 compared to M5 [72] while hyoscyamine binds to M2 only [73]. By stabilizing the receptors in inactive conformation TAs increase the intracellular levels of 3′,5′-cyclic adenosine monophosphate (cAMP) [74, 75]. Additionally, atropine inhibited phosphodiesterase (PDE4), causing increased heart contraction after β-adrenergic stimulus. This perspective might be helpful to elucidate cardiovascular adverse effects of atropine in part [76].
Both atropine and scopolamine displayed a high affinity towards mAChR in porcine brain (IC50 4.7 nM and 2.2 nM, respectively). N-methylation of hyoscyamine and scopolamine increased the affinity (IC50 0.1–0.3 nM) whereas removal of N-methyl group of atropine reduced the affinity considerably [77]. Scopolamine and hyoscyamine are known to bind to mAChRs exclusively. However, binding assay results clearly indicated that TAs can bind to both mAChR and nAChR. For example, atropine (mAChR: IC50 4.7 nM; nAChR: IC50 284 µM); scopolamine (mAChR: IC50 2 nM; nAChR: IC50 928 µM); cocaine (mAChR: IC50 57 µM; nAChR: IC50 371 µM) [77] bind to both the receptors but with different affinity. At higher concentration scopolamine also increased α7-nAChR expression [78]. The micromolar scale of activities might not be suitable for medical application of but certainly they enhance their toxicity under intoxication/overdose state, as with high concentration they can block both the receptors.
Clinical pharmacology
For the pharmacological action, stereo-selectivity is the most important requirement since it effects the binding affinity of molecule to the receptor. It was observed that the S-(–)-hyoscyamine is around 30–300 times more potent than the R-( +)-isomer. However, being unstable, S-( −)-isomer quickly forms + hyoscyamine (+ atropine). As being a racemic mixture atropine is more stable hence is frequently used for clinical practice instead of its isomers [79].
TAs (atropine, scopolamine, and hyoscyamine) can be administered by multiple routes viz. oral, intravenous, intramuscular, endotracheal and transdermal [80]. Oral administration is more patient friendly, compared to transdermal application, and assures quick absorption and pharmacological action. Both atropine and scopolamine are easily absorbed from the gastrointestinal tract but the pharmacokinetics might vary in the patients [81]. However, the transdermal delivery is preferred for scopolamine as it reduces drastic drug variabilities and side effects, and more patient-friendly [82].
Atropine
Atropine or atropine sulfate is a US Food & Drug administration (FDA) approved drug. It is first line therapy for bradycardia, organophosphate poisoning and anti-sialagogue/anti-vagal action [83].
Depending on the route of administration the peak plasma levels vary from few min (Intramascular:13 min) to hours (Oral:1 h; Aerosol:1.5–4 h) [84]. Sex based variances in the pharmacokinetics of atropine was also observed as the AUC (Area under the curve) and Cmax (peak plasma concentration) being 15% higher in females compared to the males. Additionally, female have shorter half-life of atropine (~ 20 min) than the males. Atropine binds to 14–22% plasma proteins [85]. Atropine is metabolised in the liver and almost 90% of the drug is cleared from the body through urine within 24 h [86]. In a clinical study, atropine (1 mg, intravenous) was administered to healthy volunteers and its plasma levels were analysed by radioimmunoassay. The maximum plasma atropine concentration correlated with the increased pulse rate between 12 and 16 min. However, no correlation between time of maximum response and atropine plasma concentration was perceived. Age is also an important factor in determining kinetics of atropine as both children and elderly are more sensitive to the atropine. In earlier times, rectal administration (0.01 mg/kg) was a preferred method of atropine delivery in children to slow down systemic absorption rate of atropine and this displayed better pre-operative sedation with lesser side effects [87]. Delayed time and lower peak plasma concentration of atropine was observed for rectal (15 min, 0.7 ng/ml) compared to intramuscular (5 min, 2.4 ng/ml) administration [88]. In conditions requiring high sedation, the use of 8 mg/kg ketamine, 0.5 mg/kg midazolam and 0.02 mg/kg atropine was found more effective in children [89]. The concentration of atropine should be decided very carefully as 0.3 mg/L of it is lethal in the peripheral blood [90].
Atropine metabolism significantly differs amid species on the basis of glucuronidation and N-demethylation reactions [91]. In rabbits, atropine was reported to be cleaved by serum carboxylesterase to tropic acid and tropine but such activity was not observed in serum from humans and monkeys [92]and the major metabolite (29%) was found to be tropine in human [93]. On the contrary, in another study, metabolism of radiolabelled atropine resulted in recovery of 57% radioactivity in urine as (+)-hyoscyamine followed by nortropine (24%), atropine oxide (15%), tropic acid (3%), and tropine (2%). However, no conjugated products of atropine with glucuronides or sulfates were observed [94].
Scopolamine
Scopolamine is an intravenous, oral, ophthalmic or topical drug having several uses including the prevention of motion sickness and postoperative nausea. It competitively blocks 5-hydroxytryptamine (HT3) receptors, with IC50 2.09 µM [95]. Scopolamine is often administered to induce cognitive dysfunction in animal models. Apart from being a non-selective mAChR antagonist, scopolamine also inhibits G-protein coupled post-ganglionic muscarinic receptors. For this reason, it affects both CNS and PNS [96].
The half-life of scopolamine depends on the method of administration. For example, for oral (63.7 ± 1.3 min), intravenous (68.7 ± 1.0 min), intramuscular (69.1 ± 8.0 min) and subcutaneous administration (213 min) [97]. Similarly, the pharmacokinetics of scopolamine also depend considerably on the route of administration. The intravenous dose (0.5 mg) showed better results (Cmax 5.00 ± 0.43 ng/ml, tmax 5.0 min, AUC 369.4 ± 2.2 ng min/ml) compared to the oral (Cmax 0.54 ± 0.1 ng/ml, tmax 23.5 ± 8.2 min, AUC 50.8 ± 1.76 ng min/ml). However, rapid absorption (Cmax 1.68 ± 0.23 ng/ml, tmax 2.2 ± 3 min, AUC 167 ± 20 ng min/ml) was observed after intranasal administration [97].
FDA has approved scopolamine transdermal therapy system patch (TTS-patch) [98] to overcome the dose-dependent adverse effects of the drug and to obtain therapeutic plasma concentrations over a longer period of time. The scopolamine in TTS-patch reaches protective levels in 6–8 h with optimum efficacy up to 72 h consequently a constant high plasm concentration (56–245 pg/ml) is reached. Whereas, oral or intravenous dose display quick effectiveness (0.5 h) but lasted for a short time (6 h). With a combined transdermal and oral administration, a peak at ∼0.37 ng/ml was reported after an hour [99]. Though scopolamine metabolizing enzymes are unknown, it is thought to be metabolised through oxidative methylation via cytochrome P450 (CYP450) and after Phase-II conjugation (gluronidation and sulphation) around 2.6% scopolamine is eliminate in the urine [97]. Metabolism of scopolamine is highly species-specific as metabolites p-hydroxy-, m-hydroxy- and p-hydroxy-m-methoxy-scopolamine are reported in rats while tropic acid was the major metabolite in rabbits and guinea pigs, but not in the mice [100].
In toxicity studies, oral dose of scopolamine displayed LD50 (Lethal dose for 50% deaths) of 1880 and 1270 mg/kg in mice and rats, respectively while the subcutaneous administration had much lower LD50 (1650 mg/kg and 296 mg/kg in mice and rats, respectively) [101].
Cocaine
After Phase 3 trials and Phase 1 pharmacokinetic study, FDA had approved Numbrino (cocaine hydrochloride) nasal spray as a topical solution for the nasal cavity mucus membrane [102]. Before the approval also the cocaine was in use as local anaesthetic agent, but later it was replaced by safer drugs.
After cocaine administration (intravenous/pulmonary), instantaneously high peak plasma levels are attained [103] and is cleared from the body within 4–6 h [104]. Pharmacokinetics of cocaine was studied in human subjects, after intravenous (32 mg, 1 ml/min) and intranasal (64 and 96 mg, inhaled with 5 cm straw/min) administration. Based on the one-compartment model data, intravenous administration displayed first-order elimination while intranasal exhibited both first-order absorption and elimination. The mean ± SEM half-life of cocaine through intravenous was 41.4 ± 8.2 min. AUC was significantly different in both type of administration [105]. In a similar study, oral (100 and 200 mg) and intravenous dose (40 mg) was given to volunteers and the cocaine metabolites were analysed by gas chromatography-mass spectrometry (GC–MS) [106] and identified as major (benzoylnorecgonine, ecgonine methyl ester) and minor (m- and p-hydroxybenzoylecgonine, m- and p-hydroxycocaine and norcocaine) metabolites [107]. Non-compartmental analysis and a two-factor model was used to evaluate the pharmacokinetic parameters. The bioavailability of cocaine was 0.32 ± 0.04, and 0.45 ± 0.06 after 100 and 200 mg oral dose, respectively. Both volume of distribution (Vd) and clearance (CL) are found lowest for intravenous dose (Vd: 1.3 L/kg; CL: 32.7 ml/min.kg) and highest for 100 mg oral dose (Vd: 4.2 L/kg; CL: 116.2 ml/min.kg) compared to 200 mg oral (Vd: 2.9 L/kg; CL: 87.5 ml/min.kg). The oral administration displayed a distinctive metabolic profile, with superior concentrations of major and minor metabolites as compared to the intravenous dose [106].
Tropane alkaloid derivatives in therapeutics
TAs serve as important lead molecules in the pharmacology. Various derivatives are synthesised and most of them have been approved by FDA. We will discuss about few important TA derivatives here (Fig. 5).
Fig. 5.
Pharmaceutically important tropane alkaloid derivatives
Scopolamine is prescribed to treat nausea and slobbering in the patients suffering from Alzheimer and Parkinson diseases (AD and PD) [108]. Motion sickness, postoperative nausea and vomiting can be treated by transdermal application of scopolamine [109, 110]. Additionally, being anti-muscarinic it produces antiemetic and sedative effect. Hyoscine is also used during endoscopic retrograde cholangiopancreatography (ERCP) for relaxing the smooth muscles [111]. Atropine is effective in treating nocturnal asthma by improving mucociliatory function of the lungs [112]. It also influences the vagal tone of diabetic patients [113] and controlled the tremors in monkey with PD [114].
USL Pharma developed benzotropine as a tropane-based dopamine inhibitor which was approved by FDA as adjunctive therapy for PD [115]. Benzotropine is a selective M1 mAChR antagonist which also inhibits dopamine uptake and decreases serotonin and norepinephrine inhibition. As a result, it is used in the symptomatic treatment for PD [116] and cocaine addiction [117]. Another tropane derivative, homatropine was launched as a mydriatic drug (dilate pupil) by Merck Darmstadt in 1883 [118]. Homatropine bind to muscarinic receptors in stomach and atria with similar affinity [119]. Homatropine is not FDA approved but a mixture of homatropine methylbromide and hydrocodone bitartrate has been approved by FDA as antitussive agent (inhibit cough) [120]. A quaternary ammonium derivative, trospium chloride, is a FDA approved drug, manufactured by Indevus Pharmaceutical Inc. [121]. Trospium is anti-spasmodic agent which is helpful in relaxing smooth muscles thus used to treat over active bladder [122]. Tropisetron is another tropane derivative which functions serotonin receptor antagonist. It is used as antiemetic and as analgesic in fibromyalgia [123].
N-Butylation of scopolamine yields N-butylscopolamine which is on the WHO “List of Essential Medicines”[124] and is used as anti-spasmodic and used to treat abdominal cramping, colic pain and bladder spasms [125]. Its use in animals has been approved by FDA [126] as Buscopan® (Boehringer Ingelheim's animal-health US Inc.).
Tiotropium is a FDA approved inhalation spray, Spiriva® [127]. It is a bronchodilator used in the management of chronic obstructive pulmonary disease (COPD). The drug bind well with M1-M3 receptors, more potent than ipratropium and is considered safe [128].
The first derivative of cocaine was procaine which was used in dentistry as anaesthetic and pain killer. Tetracaine [129] (STERI-UNIT®, FDA approved), a potent derivative of procaine is also used in local anaesthesia in minor surgeries [130]. Tetracaine mixed with oxymetazoline is better topical aesthetic for nasal procedures compared to cocaine [131]. It is used as local ophthalmic anaesthetic [132]. Another derivative, lidocaine (Xylocaine, FDA approved) is used on the skin to comfort itching and pain due to minor burns, eczema, insect bites. Lidocaine is a sodium channel blocker which ultimately decrease muscle contraction and result in vasodilation, hypotension and irregular heartbeat. Hence it is classified as class 1b anti-arrhythmic agent and is on the WHO List of Essential Medicines [8]. The mechanism of action (MOA) and the minimum effective dose of some important tropane alkaloids and derivatives have been summarized in Table 1.
Table 1.
Important tropane alkaloids and derivatives used in therapeutics
| Drug | MOA | Role | Minimum effective daily dose (mg) in adult | Refs. |
|---|---|---|---|---|
| Atropine products | Sodium channel antagonist; mAChR antagonist | Gastrointestinal antispasmodic | 0.0582 | [80, 133] |
| Benzotropine | M1 mAChR antagonist | Anti-parkinson | 0.5 | [133] |
| Cocaine hydrochloride | Adrenergic receptor agonist; Sodium channel antagonist | Topical anaesthetics | 40.0 | [134, 135] |
| Homatropine | mAChR antagonist | Gastrointestinal antispasmodic | 6.0 | [133] |
| Hyoscyamine | mAChR antagonist | Gastrointestinal antispasmodic | 0.31 | [133] |
| Lidocaine | Adrenergic receptor agonist; Sodium channel antagonist | Skin anaesthetics; Antiarrhythmic |
< 300 (anaesthesia) 50 (IV bolus) followed by 1 mg/min continuous IV (arrhythmia) |
[136] [137] |
| Scopolamine | mAChR antagonist | Antispasmodic |
0.33 (Patch); 0.0195(Oral) |
[133] |
| Tetracaine | Rynodine receptor; nAChR antagonist | Ophthalmic anaesthetics | 0.5% (1–2 drops) | [132, 138, 139] |
| Tiotropium | M1-M3 mAChR receptors agonist | Bronchodilator |
0.018 (Oral) 0.0025 (Inhalation) |
[140, 141] |
| Triprolidine | Downregulation of histamine H1 receptor (H1R) by M3 mAChR | Antihistamine | 10.0 | [133, 142] |
| Tropisetron | Serotonin 5HT3-receptor antagonist | Antiemetic | 2.0 | [143] |
| Trospium | mAChR antagonist | Bladder antispasmodic | 20.0 | [133] |
Tropane alkaloid toxicity
The Solanaceae family plants (Atropa, Datura, Hyoscyamus species) which are rich in TAs are usually found growing in fields as weeds and pose the threat of accidental contamination in food and feed during harvesting or processing. The highest TAs levels have been reported in herbal teas which are found to be mostly contaminated with leaves and berries of A. belladonna [144] with extremely high atropine (> 30 mg/g) affirmed in one of the cases [145]. The unprocessed cereals (like buckwheat, maize, millet, sorghum, wheat) and the cereal-based foods prepared from them are often adulterated (~ 7%–27%) with TAs (atropine, scopolamine) [144]. Surprisingly, presence of TAs (11.5 µg/kg of atropine; 2.8 µg/kg of scopolamine) was also detected in baby food samples which was well above the EU regulatory limit (1 µg/kg for each alkaloid) [146]. Seeds of D. stramonium are the most common contaminants in the food, followed by H. niger seeds, and berries of A. belladonna [46, 147, 148]. They are the main culprits of numerous cases of food poisoning worldwide [45, 149–151]. TA toxicity in livestock and other grazing animals is exceptional as the plants containing TAs are generally inedible and animals avoid feeding on them. However, consuming feed contaminated with TAs (like millet, corn etc.) can lead to toxicity [148].
TAs poisoning is common in children following consumption of attractive and colourful berries of Solanaceae family plants (A. belladonna, H. niger) [152] The symptoms of TA toxicity appear within 2 h of oral intake [153]. The classic signature of TAs poisoning can be identified from the mnemonic “red as a beet (flushing), dry as a bone (dry mouth), blind as a bat (mydriasis), mad as a hatter (mental confusion), hot as a hare (fever), full as a flask (urinary retention) [154]. Absence of sweating makes anticholinergic toxicity different from sympathomimetic toxicity [155]. The lasting effects of TAs on CNS is much longer (over 8 h) as compared to the cardiovascular system [156].
The treatment of TA poisoning including gastric emptying, use of activated charcoal (0.5 to 1 g/kg in children or 25 to 100 g in adults) to absorb the drug and benzodiazepines for managing agitation [157, 158]. Physostigmine (an AChE inhibitor) is recommended in the case when both PNS and CNS are affected by anticholinergic poisoning [159, 160]. In such cases, intravenous dose of physostigmine (0.02 mg/kg for children and 0.5 to 2 mg/kg for adults) is recommended [159]. Physostigmine is helpful in restoring the level of consciousness to its baseline [157] which is different from sedative action of benzodiazepines.
Anticholinergic burden
The continuous use of medications with anticholinergic action (MACs) for a long time might cause cognitive decline, which is linked to the cholinergic changes [161] and increased the deposition of amyloid-beta (Aβ) peptide in several regions of brain including amygdala, cortex and hippocampus [162] A study reported increased frequency of neurofibrillary tangles (NFTs) in PD patients treated with MACs for a long time compared to short-term or no treatment [163]. However, these results were not proven in a related study [164]. Various contradictory assumptions are available in the literature regarding the reversibility of cognitive effects after discontinuation of MACs. As per some studies, withdrawal of MACs resulted in the improvement in cognitive function [165, 166] while such changes were not reported by another study [133, 167]. Additionally, discontinuation of treatment might worsen the disease state in some cases of psychotic or bipolar disorders [168].
The collective effect of anticholinergic medicines, also known as anticholinergic burden (AB), has been linked with detrimental effect on mental health of elderly which include cognitive debility [169–171], delirium [172, 173] and falls [174] especially in elderly. Recently, a study conducted by Reinold et. al. (2021) included a very large population size (~ 16 million) to accurately evaluate the predominance of AB on the basis of gender and age. A list of MACs, based on the German health care system, were evaluated in this study to assess AB using the Acetylcholinergic Cognitive Burden (ACB) scale. Total ACB score ≥ 1 signifies AB while ACB ≥ 3 suggests clinically substantial ACB [175] Interestingly, the highest share (39–86%) of total cumulated AB came from the MAC prescribed by general physicians. The most frequently prescribed MACs were for psychiatry (42.8%) and urinary diseases (40.2%) [176]. In addition, the study results revealed that AB is prevalent not only in younger population [177] but almost in all age groups [176]. Moreover, the clinically relevant AB was found higher in female compared to male population [177] and AB was highest in the elderly (43.2%), followed somewhat equally by the adults (25.8%) and children (20.7%).
The ACB score of various MACs including TA was also evaluated. Interestingly, least ACB scores (ACB score 1 and 2 = 0; ACB score 3 = 0.6%) are observed for TAs in the studied population, as compared to the other MACs. While, the maximum score was observed for the antidepressants (ACB = 1: 8.8%; ACB = 2: 22%; ACB = 3: 45.3%). The contribution of TAs to cumulative ACB in males (M) and females (F) was also calculated in various age groups: age ≤ 19 (2.0% M; 1.9% F), age 20–34 (0.1% M; 0.1% F), for all other age groups (age 35–49, 50–64, 65–79 and 80–94 and ≥ 95) cumulative ACB was 0.1% M and 0.0% F [177] This study negates the role of TAs in contributing to AB. Unfortunately, in the absence of additional such population based studies on TAs it is not possible to compare these results and come out with a conclusion.
Conclusion
TAs represent a big cluster of secondary metabolites, predominantly present in the Solanaceae family. Naturally occurring TAs include atropine, hyoscyamine, scopolamine, cocaine and calystegine. The concentration of TAs in the plant is dependent on many biotic and abiotic factors. Therefore, alternative production system like genetically engineered plants, climate independent production using plant and microbial cultures are more desirable than the field grown system. Accidental contamination of cereals by Solanaceae family plants (seeds/berries) is common during harvesting and results in TA toxicity, both in food and feed. Thus, to avoid cross-contamination, good agricultural and collection practices emphasized by the World health organisation (WHO) should be strictly followed. Additionally, deoxyribonucleic acid (DNA) barcoding is a popular tool to detect and eliminate the contaminants.
TAs are anticholinergic in action and block ACh transmission by binding to muscarinic and/or nicotinic receptors. Except for calystegine, all others can cross BBB and exert effect on CNS. They are clinically used to minimize salivation and respiratory secretions, treat overactive bladder, and as antipsychotics. The anticholinergic activity affects both PNS (dry mouth, constipation, blurry vision, tachycardia, urinary retention) and CNS (drowsiness, confusion, vertigo). However, in spite of the side effects TA treatment work wonders in the clinically critical situations such as death rattle and organosulphate poisoning cases. Additionally, the derivatives of TAs with better efficacy and lesser side effects are already in therapeutic use after FDA approval.
Being anticholinergic in action, TA medication are under the suspicion of causing dementia and cognitive decline like other MACs. However, a recent study [177] was conducted on over 16 million populations to describe the incidence and classes of medicines contributing to AB. Surprisingly, TA medication was at the bottom of the MAC list with a nominal ABC score, suggesting an insignificant contribution to AB. Yet, as this study lack evidence on medications used during hospitalization and over-the-counter (OTC) medicines, more studies are required to access the participation of TAs in AB.
Author contributions
Conceptualization, NS and SSAA; writing-original draft preparation, NS; Chemical structures: KHS; writing-review and editing, NS, MJK and SSAA; funding acquisition, SSAA. All authors read and approved the final manuscript.
Funding
This research was funded by the National Research Foundation of Korea and by the Korean Government (2020R1A2B5B01002463 and 2021R1A6A1A03038996).
Declarations
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Niti Sharma, Email: nitisharma@gachon.ac.kr.
Seong Soo A. An, Email: seongaan@gachon.ac.kr
References
- 1.Lounasmaa M, Tamminen T. The tropane alkaloids. Alkaloids. 1993;44:1–114. [Google Scholar]
- 2.Griffin WJ, Lin GD. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 2000;53(6):623–637. doi: 10.1016/S0031-9422(99)00475-6. [DOI] [PubMed] [Google Scholar]
- 3.Kohnen-Johannsen KL, Kayser O. Tropane alkaloids: chemistry, pharmacology, biosynthesis and production. Molecules. 2019;24(4):796. doi: 10.3390/molecules24040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J, et al. Analyzing the contents of tropane alkaloids in Scopolia lurida, a resource plant species of Tibetan medicines. Sci Technol Tibet. 2016;279:60–62. [Google Scholar]
- 5.Diaz JL. Sacred plants and visionary consciousness. Phenomenol Cogn Sci. 2010;9(2):159–170. doi: 10.1007/s11097-010-9157-z. [DOI] [Google Scholar]
- 6.Minn A. Anticholinergic plants. California poison control system. 2008; https://calpoison.org/news/anticholinergic-plants.
- 7.Mills D, Jackson B. Scopolia lurida. 1972, Dunal. [DOI] [PubMed]
- 8.WHO. List of essential medicines. 2019.
- 9.Cardillo AB, et al. Scopolamine, anisodamine and hyoscyamine production by Brugmansia candida hairy root cultures in bioreactors. Process Biochem. 2010;45(9):1577–1581. doi: 10.1016/j.procbio.2010.06.002. [DOI] [Google Scholar]
- 10.Palazón J, et al. Application of metabolic engineering to the production of scopolamine. Molecules. 2008;13(8):1722–1742. doi: 10.3390/molecules13081722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, et al. Enhancing the scopolamine production in transgenic plants of Atropa belladonna by overexpressing pmt and h6h genes. Physiol Plant. 2011;143(4):309–315. doi: 10.1111/j.1399-3054.2011.01506.x. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan P, Smolke CD. Biosynthesis of medicinal tropane alkaloids in yeast. Nature. 2020;585(7826):614–619. doi: 10.1038/s41586-020-2650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hocking GM. Henbane—healing herb of hercules and of apollo. Econ Bot. 1947;1(3):306–316. doi: 10.1007/BF02858575. [DOI] [Google Scholar]
- 14.Dioscorides P. De materia medica. Vol. 1. 1829: Knobloch.
- 15.Mayor A. Toxicology in Antiquity. Elsevier; 2019. Chemical and biological warfare in antiquity; pp. 243–255. [Google Scholar]
- 16.Waniakowa J. “Mandragora" and" Belladonna": the names of two magic plants. Studia Linguistica Universitatis Iagellonicae Cracoviensis, 2007. p. 124.
- 17.Forbes TR. Why is it called'beautiful lady'? A note on belladonna. Bull N Y Acad Med. 1977;53(4):403. [PMC free article] [PubMed] [Google Scholar]
- 18.Carruthers DM. Lines of flight of the deadly nightshade: An enquiry into the properties of the magical plant, its literature and history. Mosaic. 2015;48(2):119–132. doi: 10.1353/mos.2015.0025. [DOI] [Google Scholar]
- 19.Wexler P. History of Toxicology and Environmental Health: Toxicology in Antiquity II. New York: Academic Press; 2014. [Google Scholar]
- 20.Gunda B. Fish poisoning in the Carpathian area and in the Balkan Peninsula. California: University of California; 1967. [Google Scholar]
- 21.Naranjo P. Social function of coca in pre-Columbian America. J Ethnopharmacol. 1981;3(2–3):161–172. doi: 10.1016/0378-8741(81)90052-0. [DOI] [PubMed] [Google Scholar]
- 22.Lakstygal AM, et al. Dark classics in chemical neuroscience: atropine, scopolamine, and other anticholinergic deliriant hallucinogens. ACS Chem Neurosci. 2018;10(5):2144–2159. doi: 10.1021/acschemneuro.8b00615. [DOI] [PubMed] [Google Scholar]
- 23.Rätsch C. Rauch von Delphi: Eine ethnopharmakologische Annäherung. Curare. 1987;10(4):215–228. [Google Scholar]
- 24.Fatur K. “Hexing herbs” in ethnobotanical perspective: A historical review of the uses of anticholinergic Solanaceae plants in Europe. Econ Bot. 2020;74(2):140–158. doi: 10.1007/s12231-020-09498-w. [DOI] [Google Scholar]
- 25.Mann J. Murder, magic, and medicine. Oxford: Oxford University Press; 2000. [Google Scholar]
- 26.Wiart C. Ethnopharmacology of medicinal plants: Asia and the Pacific. Berlin: Springer; 2007. [Google Scholar]
- 27.Müller J, Wanke K. Toxic psychoses from atropine and scopolamine. Fortschr Neurol Psychiatr. 1998;66(7):289–295. doi: 10.1055/s-2007-995265. [DOI] [PubMed] [Google Scholar]
- 28.Wink M. Alkaloids. Springer; 1998. A short history of alkaloids; pp. 11–44. [Google Scholar]
- 29.Yang Y-C, Song Y-K, Kim T-Y. Comparative Study between Glycopyrrolate-Neostigmine and Atropine-Neostigmine in Postanesthetic Arousal. Korean J Anesthesiol. 1986;9:66–70. doi: 10.4097/kjae.1986.19.1.66. [DOI] [Google Scholar]
- 30.Verma V. Classic studies on the interaction of cocaine and the dopamine transporter. Clin Psychopharmacol Neurosci. 2015;13(3):227. doi: 10.9758/cpn.2015.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pergolizzi JV, Jr, et al. Perspectives on transdermal scopolamine for the treatment of postoperative nausea and vomiting. J Clin Anesth. 2012;24(4):334–345. doi: 10.1016/j.jclinane.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Bradbury N. Taste of Poison, ed. I.K. paperwhite. 2022.
- 33.Grynkiewicz G, Gadzikowska M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol Rep. 2008;60(4):439. [PubMed] [Google Scholar]
- 34.Dureng G, et al. Comparative affinity of several standard anti-secretory agents for the intestinal cholinergic receptors of of rats and dogs. C R Seances Soc Biol Fil. 1977;171(4):771–777. [PubMed] [Google Scholar]
- 35.del Valle-Laisequilla C, et al. Ketorolac tromethamine improves the analgesic effect of hyoscine butylbromide in patients with intense cramping pain from gastrointestinal or genitourinary origin. Arzneimittelforschung. 2012;62(12):603–608. doi: 10.1055/s-0032-1327678. [DOI] [PubMed] [Google Scholar]
- 36.Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533(1–3):36–39. doi: 10.1016/j.ejphar.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 37.Freud S. Über Coca (1884) Psyche. 1973;27(5):487–511. [Google Scholar]
- 38.Kukula-Koch, W. and J. Widelski, Pharmacognosy. Fundamentals, Applications and Strategies . Academic Press: Cambridge. USA: MA; 2017. [Google Scholar]
- 39.Worek F, Thiermann H, Wille T. Organophosphorus compounds and oximes: a critical review. Arch Toxicol. 2020;94(7):2275–2292. doi: 10.1007/s00204-020-02797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildiers H, et al. Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124–133. doi: 10.1016/j.jpainsymman.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Pigatto AG, et al. Tropane alkaloids and calystegines as chemotaxonomic markers in the Solanaceae. An Acad Bras Ciênc. 2015;87:2139–2149. doi: 10.1590/0001-3765201520140231. [DOI] [PubMed] [Google Scholar]
- 42.Sweta V, Lakshmi T. Pharmacological profile of tropane alkaloids. J Chem Pharm Res. 2015;7(5):117–119. [Google Scholar]
- 43.Huang J-P, et al. Tropane alkaloid biosynthesis: a centennial review. Nat Prod Rep. 2021;38(9):1634–1658. doi: 10.1039/D0NP00076K. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y, Tabata M. Plant biotechnology of tropane alkaloids. Plant Biotechnol. 1997;14(1):1–10. doi: 10.5511/plantbiotechnology.14.1. [DOI] [Google Scholar]
- 45.Abia WA, et al. Tropane alkaloid contamination of agricultural commodities and food products in relation to consumer health: learnings from the 2019 Uganda food aid outbreak. Compre Rev Food Sci Food Saf. 2021;20(1):501–525. doi: 10.1111/1541-4337.12664. [DOI] [PubMed] [Google Scholar]
- 46.Adamse P, et al. Tropane alkaloids in food: poisoning incidents. Quality Assur Saf Crops Foods. 2014;6(1):15–24. doi: 10.3920/QAS2013.0314. [DOI] [Google Scholar]
- 47.Jank B, Rath J. Emerging tropane alkaloid contaminations under climate change. Trends Plant Sci. 2021;26(11):1101–1103. doi: 10.1016/j.tplants.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Dehghan E, et al. Review on new techniques in tropane alkaloids production. J Med Plants. 2010;9(33):149–164. [Google Scholar]
- 49.Araújo Neto JF, et al. Cytotoxic activity of tropane alkaloides of species of Erythroxylum. Mini Rev Med Chem. 2021;21(17):2458–2480. doi: 10.2174/1389557521666210119123409. [DOI] [PubMed] [Google Scholar]
- 50.Stegelmeier BL, et al. Haschek and Rousseaux's Handbook of Toxicologic Pathology. Elsevier; 2013. Selected poisonous plants affecting animal and human health; pp. 1259–1314. [Google Scholar]
- 51.Kaliappan KP, et al. A versatile access to calystegine analogues as potential glycosidases inhibitors. J Org Chem. 2009;74(16):6266–6274. doi: 10.1021/jo901342w. [DOI] [PubMed] [Google Scholar]
- 52.Geiger PL, Hesse K. Darstellung des atropins. Ann Pharm. 1833;5(1):43–81. doi: 10.1002/jlac.18330050108. [DOI] [Google Scholar]
- 53.Schmidt E, Henschke H. Über die Alkaloide der Wurzel von Scopolia japonica. Arch Pharm. 1888;226(5):185–203. doi: 10.1002/ardp.18882260502. [DOI] [Google Scholar]
- 54.Lossen W, et al. Ueber das Atropin. Annalen der Chemie und Pharmacie. 1864;131(1):43–49. doi: 10.1002/jlac.18641310103. [DOI] [Google Scholar]
- 55.Gaedcke F. Ueber das Erythroxylin, dargestellt aus den Blättern des in Süd-Amerika kultivierten Strauches Erythroxylon Coca Lam. 1854.
- 56.Niemann A, Über eine neue organische Base in den Cocablättern: Inaug.-Diss. von Göttingen. Druck d. Buchdr. von EA Huth: Univers; 1860. [Google Scholar]
- 57.Knapp R, et al. Neurotransmitter Receptors. 2003.
- 58.Aronstam R, Patil P. Muscarinic receptors: autonomic neurons. 2009.
- 59.Fisher SK, Wonnacott S. Basic neurochemistry. Elsevier; 2012. Acetylcholine; pp. 258–282. [Google Scholar]
- 60.Abrams P, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148(5):565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scarr E. Muscarinic receptors: their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci Ther. 2012;18(5):369–379. doi: 10.1111/j.1755-5949.2011.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho TN, Abraham N, Lewis RJ. Structure-function of neuronal nicotinic acetylcholine receptor inhibitors derived from natural toxins. Front Neurosci. 2020;89:1209. doi: 10.3389/fnins.2020.609005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colombo SF, et al. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol. 2013;86(8):1063–1073. doi: 10.1016/j.bcp.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 64.Millar NS, Harkness PC. Assembly and trafficking of nicotinic acetylcholine receptors. Mol Membr Biol. 2008;25(4):279–292. doi: 10.1080/09687680802035675. [DOI] [PubMed] [Google Scholar]
- 65.D'Andrea MR, Nagele RG. Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer's disease pyramidal neurons. Curr Pharm Des. 2006;12(6):677–684. doi: 10.2174/138161206775474224. [DOI] [PubMed] [Google Scholar]
- 66.Freedman R, et al. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiat. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 67.Wang HY, et al. Amyloid peptide Aβ1-42 binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. J Neurochem. 2000;75(3):1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 68.Acevedo-Rodriguez A, et al. Cocaine inhibition of nicotinic acetylcholine receptors influences dopamine release. Front Synaptic Neurosci. 2014;6:19. doi: 10.3389/fnsyn.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Francis MM, et al. Subtype-selective inhibition of neuronal nicotinic acetylcholine receptors by cocaine is determined by the α4 and β4 subunits. Mol Pharmacol. 2000;58(1):109–119. doi: 10.1124/mol.58.1.109. [DOI] [PubMed] [Google Scholar]
- 70.Sanjakdar SS, et al. Differential roles of α6β2* and α4β2* neuronal nicotinic receptors in nicotine-and cocaine-conditioned reward in mice. Neuropsychopharmacology. 2015;40(2):350–360. doi: 10.1038/npp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berizzi AE, et al. Molecular mechanisms of action of M5 muscarinic acetylcholine receptor allosteric modulators. Mol Pharmacol. 2016;90(4):427–436. doi: 10.1124/mol.116.104182. [DOI] [PubMed] [Google Scholar]
- 72.Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694–709. doi: 10.1038/s41380-018-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Říčný J, Gualtieri F, Tuček S. Constitutive inhibitory action of muscarinic receptors on adenylyl cyclase in cardiac membranes and its stereospecific suppression by hyoscyamine. Physiol Res. 2002;51:131–137. [PubMed] [Google Scholar]
- 74.Jakubík J, et al. Constitutive activity of the M1–M4 subtypes of muscarinic receptors in transfected CHO cells and of muscarinic receptors in the heart cells revealed by negative antagonists. FEBS Lett. 1995;377(2):275–279. doi: 10.1016/0014-5793(95)01360-1. [DOI] [PubMed] [Google Scholar]
- 75.Vogel WK, et al. Porcine m2 muscarinic acetylcholine receptor-effector coupling in chinese hamster ovary cells (∗) J Biol Chem. 1995;270(26):15485–15493. doi: 10.1074/jbc.270.26.15485. [DOI] [PubMed] [Google Scholar]
- 76.Perera RK, et al. Atropine augments cardiac contractility by inhibiting cAMP-specific phosphodiesterase type 4. Sci Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-15632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmeller T, et al. Binding of tropane alcaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie. 1995;50(7):493–495. [PubMed] [Google Scholar]
- 78.Falsafi SK, et al. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS ONE. 2012;7(2):e32082. doi: 10.1371/journal.pone.0032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gyermek L. Structure-activity relationships among derivatives of dicarboxylic acid esters of tropine. Pharmacol Ther. 2002;96(1):1–21. doi: 10.1016/S0163-7258(02)00296-6. [DOI] [PubMed] [Google Scholar]
- 80.McLendon K, Preuss CV. Atropine, in StatPearls. 2021, StatPearls Publishing. [PubMed]
- 81.Mirakhur R. Comparative study of the effects of oral and im atropine and hyoscine in volunteers. Br J Anaesth. 1978;50(6):591–598. doi: 10.1093/bja/50.6.591. [DOI] [PubMed] [Google Scholar]
- 82.Shaoul E, et al. Transdermal delivery of scopolamine by natural submicron injectors: in-vivo study in pig. PLoS ONE. 2012;7(2):e31922. doi: 10.1371/journal.pone.0031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.More SR, Dabhade SS, Ghongane BB. Drug audit of intravenous anaesthetic agents in tertiary care hospital. J Clin Diagn Res. 2015;9(11):25. doi: 10.7860/JCDR/2015/14159.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown J, Taylor P. Agonists and antagonists of mucarinic receptors. In: Pharmacological basis of therapeutic. 10th ed. New York: McGraw-Hill; 2003. p. 162–81.
- 85.FDA, Atropine. 2018.
- 86.John H, et al. Simultaneous quantification of atropine and scopolamine in infusions of herbal tea and Solanaceae plant material by matrix-assisted laser desorption/ionization time-of-flight (tandem) mass spectrometry. Rapid Commun Mass Spectrom. 2018;32(22):1911–1921. doi: 10.1002/rcm.8264. [DOI] [PubMed] [Google Scholar]
- 87.Fuchs C, Schwabe M. Rectal premedication using ketamine-dehydrobenzperidol-atropine in childhood. Anaesthesiol Reanim. 1990;15(5):322–326. [PubMed] [Google Scholar]
- 88.Olsson G, et al. Plasma concentrations of atropine after rectal administration. Anaesthesia. 1983;38(12):1179–1182. doi: 10.1111/j.1365-2044.1983.tb12521.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, et al. A comparison of two different doses of rectal ketamine added to 0.5 mg kg-1 midazolam and 0.02 mg kg-1 atropine in infants and young children. Anaesthesia Intens Care. 2010;38(5):900–904. doi: 10.1177/0310057X1003800515. [DOI] [PubMed] [Google Scholar]
- 90.Ricard F, et al. Measurement of atropine and scopolamine in hair by LC–MS/MS after Datura stramonium chronic exposure. Forensic Sci Int. 2012;223(1–3):256–260. doi: 10.1016/j.forsciint.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Kalser SC. The fate of atropine in man. Ann N Y Acad Sci. 1971;179(1):667–683. doi: 10.1111/j.1749-6632.1971.tb46943.x. [DOI] [PubMed] [Google Scholar]
- 92.Harrison PK, Tattersall JE, Gosden E. The presence of atropinesterase activity in animal plasma. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(3):230–236. doi: 10.1007/s00210-006-0054-5. [DOI] [PubMed] [Google Scholar]
- 93.Hinderling PH, Gundert-Remy U, Schmidlin O. Integrated pharmacokinetics and pharmacodynamics of atropine in healthy humans I: Pharmacokinetics. J Pharm Sci. 1985;74(7):703–710. doi: 10.1002/jps.2600740702. [DOI] [PubMed] [Google Scholar]
- 94.Van der Meer M, Hundt H, Müller F. The metabolism or atropine in man. J Pharm Pharmacol. 1986;38(10):781–784. doi: 10.1111/j.2042-7158.1986.tb04494.x. [DOI] [PubMed] [Google Scholar]
- 95.Lochner M, Thompson AJ. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacology. 2016;108:220–228. doi: 10.1016/j.neuropharm.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang XC, et al. Postoperative anticholinergic poisoning: concealed complications of a commonly used medication. J Emerg Med. 2017;53(4):520–523. doi: 10.1016/j.jemermed.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005;27(5):655–665. doi: 10.1097/01.ftd.0000168293.48226.57. [DOI] [PubMed] [Google Scholar]
- 98.FDA. Transderm scop. 2013.
- 99.Nachum Z, et al. Scopolamine bioavailability in combined oral and transdermal delivery. J Pharmacol Exp Ther. 2001;296(1):121–123. [PubMed] [Google Scholar]
- 100.Wada S, et al. Metabolism in vivo of the tropane alkaloid, scopolamine, in several mammalian species. Xenobiotica. 1991;21(10):1289–1300. doi: 10.3109/00498259109043204. [DOI] [PubMed] [Google Scholar]
- 101.Cayman. 2022; Available from: https://cdn.caymanchem.com/cdn/msds/14108m.pdf.
- 102.FDA, Cocaine. 2017.
- 103.Paly D, et al. Plasma cocaine concentrations during cocaine paste smoking. Life Sci. 1982;30(9):731–738. doi: 10.1016/0024-3205(82)90606-3. [DOI] [PubMed] [Google Scholar]
- 104.Jatlow P. Cocaine: analysis, pharmacokinetics, and metabolic disposition. Yale J Biol Med. 1988;61(2):105. [PMC free article] [PubMed] [Google Scholar]
- 105.Javaid J, et al. Kinetics of cocaine in humans after intravenous and intranasal administration. Biopharm Drug Dispos. 1983;4(1):9–18. doi: 10.1002/bdd.2510040104. [DOI] [PubMed] [Google Scholar]
- 106.Coe MA, et al. Bioavailability and pharmacokinetics of oral cocaine in humans. J Anal Toxicol. 2018;42(5):285–292. doi: 10.1093/jat/bky007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jufer RA, et al. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24(7):467–477. doi: 10.1093/jat/24.7.467. [DOI] [PubMed] [Google Scholar]
- 108.Dreyfuss P, Vogel D, Walsh N. The use of transdermal scopolamine to control drooling: A case report. Am J Phys Med Rehab. 1991;70(4):220–222. doi: 10.1097/00002060-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 109.Attias J, et al. Efficacy of transdermal scopolamine against seasickness: a 3-day study at sea. Aviat Space Environ Med. 1987;58(1):60–62. [PubMed] [Google Scholar]
- 110.Spinks A, Wasiak J. Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Systema Rev. 2011;657:6. doi: 10.1002/14651858.CD002851.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salmanroghani H, et al. The efficacy and safety of low dose versus usual dose of hyoscine during endoscopic retrograde cholangiopancreatography: a randomized clinical trial. Clin Pharmacol. 2020;12:123. doi: 10.2147/CPAA.S263531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Groth ML, Langenback EG, Foster WM. Influence of inhaled atropine on lung mucociliary function in humans. Am Rev Respir Dis. 1991;144(5):1042–1047. doi: 10.1164/ajrccm/144.5.1042. [DOI] [PubMed] [Google Scholar]
- 113.Julu P, Adler J, Hondo R. Vagal tone in healthy-volunteers given atropine and in diabetic-patients. In Journal Of Physiology-London. 1991. Cambridge Univ Press 40 West 20th Street, New York, Ny 10011–4211.
- 114.Gomez-Mancilla B, Boucher R, Bedard PJ. Effect of clonidine and atropine on rest tremor in the MPTP monkey model of parkinsonism. Clin Neuropharmacol. 1991;14(4):359–366. doi: 10.1097/00002826-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 115.FDA. Benztropine. 1996.
- 116.Schlagmann C, Remien J. Treatment of Parkinson disease. Klin Wochenschr. 1986;64(19):939–942. doi: 10.1007/BF01728622. [DOI] [PubMed] [Google Scholar]
- 117.Rothman RB, et al. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008;75(1):2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sneader W. Drug discovery: a history. New York: Wiley; 2005. [Google Scholar]
- 119.Gilani S, Cobbin L. Interaction of himbacine with carbachol at muscarinic receptors of heart and smooth muscle. Arch Int Pharmacodyn Ther. 1987;290(1):46–53. [PubMed] [Google Scholar]
- 120.FDA. Hydrocodane. 2017.
- 121.FDA. Trospium Chloride. 2007.
- 122.Rudy D, et al. Multicenter phase III trial studying trospium chloride in patients with overactive bladder. Urology. 2006;67(2):275–280. doi: 10.1016/j.urology.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 123.Sorbe B, et al. Tropisetron (Navoban) in the prevention of chemotherapy-induced nausea and vomiting—the Nordic experience. Support Care Cancer. 1994;2(6):393–399. doi: 10.1007/BF00344055. [DOI] [PubMed] [Google Scholar]
- 124.WHO. Hyoscine butylbromide-EML. 2013.
- 125.Tytgat GN. Hyoscine butylbromide. Drugs. 2007;67(9):1343–1357. doi: 10.2165/00003495-200767090-00007. [DOI] [PubMed] [Google Scholar]
- 126.FDA. N-buscopan 2004.
- 127.FDA. Spiriva. 2009.
- 128.Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(2):63S–66S. doi: 10.1378/chest.117.2_suppl.63S. [DOI] [PubMed] [Google Scholar]
- 129.FDA. Tetracaine. 2016.
- 130.Dewick PM. Medicinal natural products: a biosynthetic approach. New York: Wiley; 2002. [Google Scholar]
- 131.Noorily AD, Noorily SH, Otto RA. Cocaine, lidocaine, tetracaine: which is best for topical nasal anesthesia? Anesth Analg. 1995;81(4):724–727. doi: 10.1097/00000539-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 132.Drugs. Tetracaine hydrochloride. 2022.
- 133.Gray SL, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drugs. Numbrino. 2020.
- 135.Springer. Cocaine intranasal. 2020.
- 136.Drugs. Lidocaine Anesthesia. 2020.
- 137.Drugs. Lidocaine Dosage. 2020.
- 138.Cobo R, et al. Mechanisms underlying the strong inhibition of muscle-type nicotinic receptors by tetracaine. Front Mol Neurosci. 2018;11:193. doi: 10.3389/fnmol.2018.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shoshan-Barmatz V, Zchut S. The interaction of local anesthetics with the ryanodine receptor of the sarcoplasmic reticulum. J Membr Biol. 1993;133(2):171–181. doi: 10.1007/BF00233797. [DOI] [PubMed] [Google Scholar]
- 140.Drugs. Tiotropium Dosage. 2022.
- 141.Barnes PJ, et al. Asthma and COPD: basic mechanisms and clinical management. 2009.
- 142.Miyoshi K, et al. Histamine H1 receptor down-regulation mediated by M3 muscarinic acetylcholine receptor subtype. J Pharmacol Sci. 2004;95(4):426–434. doi: 10.1254/jphs.FPJ04012X. [DOI] [PubMed] [Google Scholar]
- 143.Medsafe. Tropisetron.
- 144.Chan TY. Worldwide occurrence and investigations of contamination of herbal medicines by tropane alkaloids. Toxins. 2017;9(9):284. doi: 10.3390/toxins9090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bryson PD, et al. Burdock root tea poisoning: Case report involving a commercial preparation. JAMA. 1978;239(20):2157–2157. doi: 10.1001/jama.1978.03280470069028. [DOI] [PubMed] [Google Scholar]
- 146.Marín-Sáez J, Romero-González R, Frenich AG. Reliable determination of tropane alkaloids in cereal based baby foods coupling on-line spe to mass spectrometry avoiding chromatographic step. Food Chem. 2019;275:746–753. doi: 10.1016/j.foodchem.2018.09.137. [DOI] [PubMed] [Google Scholar]
- 147.Adams RG, et al. Plasma pharmacokinetics of intravenously administered atropine in normal human subjects. J Clin Pharmacol. 1982;22(10):477–481. doi: 10.1002/j.1552-4604.1982.tb02638.x. [DOI] [PubMed] [Google Scholar]
- 148.Beuerle T, et al. Scientific Opinion on Tropane alkaloids in food and feed. EFSA J. 2013;11(10):1–113. [Google Scholar]
- 149.González-Gómez L, et al. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods. 2022;11(3):407. doi: 10.3390/foods11030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haughey SA, et al. Laboratory investigations into the cause of multiple serious and fatal food poisoning incidents in Uganda during 2019. Food Control. 2021;121:107648. doi: 10.1016/j.foodcont.2020.107648. [DOI] [Google Scholar]
- 151.Mulder PP, et al. Occurrence of tropane alkaloids in food. EFSA Supporting Publications. 2016;13(12):1140E. doi: 10.2903/sp.efsa.2016.EN-1140. [DOI] [Google Scholar]
- 152.O'Shaughnessy KM. Clinical Pharmacology. Elsevier; 2012. Cholinergic and antimuscarinic (anticholinergic) mechanisms and drugs; pp. 372–381. [Google Scholar]
- 153.Scavone JM, et al. Pharmacokinetics and pharmacodynamics of diphenhydramine 25 mg in young and elderly volunteers. J Clin Pharmacol. 1998;38(7):603–609. doi: 10.1002/j.1552-4604.1998.tb04466.x. [DOI] [PubMed] [Google Scholar]
- 154.Broderick ED, Metheny H, Crosby B. Anticholinergic toxicity. StatPearls. 2020. [PubMed]
- 155.Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479–498. doi: 10.1016/j.ccc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 156.Ali-melkkilä T, Kanto J, Iisalo E. Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scand. 1993;37(7):633–642. doi: 10.1111/j.1399-6576.1993.tb03780.x. [DOI] [PubMed] [Google Scholar]
- 157.Furbee B. Clinical neurotoxicology. Elsevier; 2009. Neurotoxic plants; pp. 523–542. [Google Scholar]
- 158.Isbister GK, Kumar VVP. Indications for single-dose activated charcoal administration in acute overdose. Curr Opin Crit Care. 2011;17(4):351–357. doi: 10.1097/MCC.0b013e328348bf59. [DOI] [PubMed] [Google Scholar]
- 159.Arens AM, et al. Safety and effectiveness of physostigmine: a 10-year retrospective review. Clin Toxicol. 2018;56(2):101–107. doi: 10.1080/15563650.2017.1342828. [DOI] [PubMed] [Google Scholar]
- 160.Derinoz O, Emeksiz HC. Use of physostigmine for cyclopentolate overdose in an infant. Pediatrics. 2012;130(3):e703–e705. doi: 10.1542/peds.2011-3038. [DOI] [PubMed] [Google Scholar]
- 161.Swami S, et al. Anticholinergic drug use and risk to cognitive performance in older adults with questionable cognitive impairment: a cross-sectional analysis. Drugs Aging. 2016;33(11):809–818. doi: 10.1007/s40266-016-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Caccamo A, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 163.Perry EK, et al. Increased Alzheimer pathology in Parkinson's disease related to antimuscarinic drugs. Ann Neurol. 2003;54(2):235–238. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- 164.Gray SL, et al. Exposure to strong anticholinergic medications and dementia-related neuropathology in a community-based autopsy cohort. J Alzheimers Dis. 2018;65(2):607–616. doi: 10.3233/JAD-171174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Richardson K, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:456. doi: 10.1136/bmj.k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salahudeen MS, Duffull SB, Nishtala PS. Impact of anticholinergic discontinuation on cognitive outcomes in older people: a systematic review. Drugs Aging. 2014;31(3):185–192. doi: 10.1007/s40266-014-0158-4. [DOI] [PubMed] [Google Scholar]
- 167.Limback-Stokin MM, et al. Anticholinergic medications and cognitive function in late mid-life. Alzheimer Dis Assoc Disord. 2018;32(3):262. doi: 10.1097/WAD.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lupu AM, et al. Reducing anticholinergic medication burden in patients with psychotic or bipolar disorders. J Clin Psychiatry. 2017;78(9):17141. doi: 10.4088/JCP.16m11269. [DOI] [PubMed] [Google Scholar]
- 169.Campbell NL, et al. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy. 2016;36(11):1123–1131. doi: 10.1002/phar.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chatterjee S, et al. Anticholinergic burden and risk of cognitive impairment in elderly nursing home residents with depression. Res Social Adm Pharm. 2020;16(3):329–335. doi: 10.1016/j.sapharm.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 171.Wouters H, et al. Long-term exposure to anticholinergic and sedative medications and cognitive and physical function in later life. J Gerontol A. 2020;75(2):357–365. doi: 10.1093/geronb/gbx180. [DOI] [PubMed] [Google Scholar]
- 172.Mueller A, et al. Anticholinergic burden of long-term medication is an independent risk factor for the development of postoperative delirium: a clinical trial. J Clin Anesth. 2020;61:109632. doi: 10.1016/j.jclinane.2019.109632. [DOI] [PubMed] [Google Scholar]
- 173.Rigor J, et al. Prehospital anticholinergic burden is associated with delirium but not with mortality in a population of acutely Ill medical patients. J Am Med Dir Assoc. 2020;21(4):481–485. doi: 10.1016/j.jamda.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 174.Jamieson HA, et al. Drug burden and its association with falls among older adults in New Zealand: a national population cross-sectional study. Drugs Aging. 2018;35(1):73–81. doi: 10.1007/s40266-017-0511-5. [DOI] [PubMed] [Google Scholar]
- 175.Boustani M, et al. Impact of anticholinergics on the aging brain: a review and practical application. 2008.
- 176.Cebron-Lipovec N, Jazbar J, Kos M. Anticholinergic burden in children, adults and older adults in Slovenia: A Nationwide database study. Sci Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-65989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reinold J, et al. Anticholinergic burden: First comprehensive analysis using claims data shows large variation by age and sex. PLoS ONE. 2021;16(6):e0253336. doi: 10.1371/journal.pone.0253336. [DOI] [PMC free article] [PubMed] [Google Scholar]