Abstract
Polyurethane (PU) has been widely examined and used for biomedical applications, such as catheters, blood oxygenators, stents, cardiac valves, drug delivery carriers, dialysis devices, wound dressings, adhesives, pacemaker, tissue engineering, and coatings for breast implants due to its mechanical flexibility, high tear strength, biocompatibility, and tailorable foams although bio-acceptability, biodegradability and controlled drug delivery to achieve the desired properties should be considered. Especially, during the last decade, the development of bio-based PUs has raised public awareness because of the concern with global plastic waste for creating more environmentally friended materials. Therefore, it is desirable to discuss polysaccharide (PS)-contained PU for the wound dressing and bone tissue engineering among bio-based PUs because PS has several advantages, such as biocompatibility, reproducibility from the natural resources, degradability, ease of incorporation of bioactive agents, ease of availability and cost-effectiveness, and structural feature of chemical modification to meet the desired needs to overcome the disadvantages of PU itself by containing the PS into the PU.
Keywords: Polyurethane, Polysaccharide, Wound dressing, Bone tissue engineering
Introduction
Polyurethane (PU) attracted the development of biomedical devices such as bone fixation [1], coatings [2], artificial heart [3], heart valves, and aortic grafts [4] due to mechanical flexibility, high tear strength, biocompatibility, and tailorable foam [5] up to the 1980s although the isocyanate toxicity, especially in the case of aromatic isocyanates, has been concerned as a major limitation. Nowadays, the one major diisocyanate used at an industrial scale is 4,4′-methylene diphenyl diisocyanate (MDI) as the aliphatic one due to its cost-efficiency, higher reactivity, mechanical strength, and abrasion resistance [6]. Therefore, the PU produced by the use of MDI has been applied for rigid and flexible foams, coatings, adhesives, sealants, and elastomers [7]. Current biomedical applications of PU are catheters, blood oxygenators, stents, cardiac valves, dialysis devices, dressings, adhesives, drug delivery devices, tissue engineering, pacemaker, and coatings for breast implants [8] although several requirements such as acceptability to the tissue, biodegradability, sustained drug delivery, and reconstruction of the proper organ to achieve the desired properties [4] should be considered. However, during the last decade, the development of bio-based PUs has raised concerns about environmental problems because everybody in the world is concerned with raising global plastic wastes to create more sustainable materials [6] although the issue of sustainability can be partially overcome using diols from natural biopolymers for PU synthesis. In this review, we want to discuss the relationship between structure and properties of PU, polysaccharide (PS)-contained PU for wound dressing and bone tissue engineering among biomedical applications because of advantages of PS such as acceptability to the tissue, reproducibility from the natural resources, biocompatibility, biodegradability, ease of availability and cost-effectiveness, the possibility of sustainability, and structural feature of chemical modification to meet the desired needs [9]. Among PS, chitosan (CS)-, alginate (AL)-, cellulose (CE)-, and hyaluronic acid (HA)-contained PU will be discussed.
Relationship between structure and properties of PU
The PU can be prepared by the nucleophilic addition polymerization between isocyanate compounds including aromatic, aliphatic, and alicyclic isocyanates and polyol compounds including polyester, polyether, and polybutadiene polyol compounds [7]. Additionally, chain extenders such as small molecule polyols and diamines, and catalysts such as amine and tin are used to cross-link the preformed linear macromolecules to form three-dimensional (3D) cross-linked networks [10]. Typical usual compounds of PUs are shown in Table 1. The PU properties can be broadly adjusted due to the variety of functional groups. The molecular structure of PU is different, from thermoplastic elastomers with linear and flexible chains to thermosetting PU with rigid cross-linked chains [11]. The molecular phase of thermoplastic PU consisting independent, linear molecules whereas that of thermosetting PU consisting covalently linked chain networks. Elastomeric PU consisting hard segments responsible for the increased mechanical strength and soft ones responsible for the elastomeric behavior. Soft segments corresponded to polyol compounds having lower glass transition temperature (Tg) provide the elastomeric properties while hard ones correspond to isocyanate and the chain extenders having higher Tg provide crystallinity and mechanical strength of Pus [12]. The PUs show microphase-separated structures owing to the thermodynamic incompatibility between the soft- and hard-segment domains [12]. This unique microphase-separated structure exhibits PUs with better possibilities for desired applications [13]. The effect of the chemical structure of the soft segments on the degradation rate of the PUs is related to the labile groups, hydrophilicity, and crystallinity whereas hard segments in the phase-separated PUs can control water diffusion. Polyether types in soft segments are frequently used due to their more flexibility and stability. Polyol types are used for resorbable Pus [14] while aromatic and aliphatic isocyanates are used to prepare biomedical PUs. It has been reported that the aliphatic isocyanates are used to reduce the potential toxicity of PUs although aromatic PUMs have excellent mechanical properties with concern about the carcinogenic property of the degraded diamine compounds [15]
Table 1.
Usual compounds for the synthesis of PUs (modified from Ref. 17)
| Compound | Type | Name of compound |
|---|---|---|
| Diisocyanate | Aromatic | 4,4′-Diphenylmethane diisocyanate (MDI) |
| 2,4-Tolulene diisocyanate (TDI) | ||
| Aliphatic | Hexamethylene diisocyanate (HDI) | |
| Alcyclic | 4,4′-Dicyclohexyl methane diisocyanate (HMDI) | |
| Polyol | Polyester | Polycaprolactone diol (PCLDL) |
| Polycarbonate diol (PCDL) | ||
| Polyether | Polytetrahydrofuran diol (PTHFDL) | |
| Polybutadiene | Polybutadiene diol (PBTDI) | |
| Chain extender | Diol | 1,4-Butanediol (BDL) |
| Diamine | 3,3′-Dichloro-4,4′-diamino-diphenylmethane (MOCA) | |
| Diethyltolune diamine (DETDA) | ||
| Catalyst | Amine | 1,4-Diazabicyclo-[2]-octane |
| Tin | Dibutyltindilaurate |
Biomedical applications of PS-contained PUs
Wound dressing
The PUs have been widely used for biomedical applications including wound dressing due to their excellent properties such as biocompatibility, versatile mechanical properties, processability, and unique chemistry [16] although acceptability to the wound tissue, biodegradability although the necessity of biodegradability depends on classified wound dressing, control of cellular behaviors such as cell adhesion, proliferation, and differentiation, regulation of release of bioactive agents from the wound dressing for rapid recovering wound tissue and raised global plastic wastes should be considered [17]. Generally, the wound dressing can be classified into three well-known groups: traditional dressings, skin substitutes, and dermal grafts. Examples of traditional dressings include bandages, gauze, films, gels, sprays although the traditional dressings are not necessary for degradability. Examples of skin substitutes composed of tissue-engineered structures include Apligraf, OrCel, TransCyte although polymeric scaffolds should be biodegradable in vivo when tissue engineering-based wound dressings are used for specific diseases such as traumatic wounds, defects after oncologic resection, burn reconstruction, scar contracture release, congenital skin deficiencies, and hair restoration [18]. Examples of dermal grafts are acellular xenografts, allografts, and autografts although they are not suitable for the treatment of complex injuries such as conditions with exposed bones and deep spaces [19]. In this review, we only discuss traditional dressings and skin substitutes due to page limitations.
The introduction of PS in PUs can enhance biodegradability, the resemblance with bodily macromolecules, biocompatibility, control of renewable resources, cost-effectiveness, and easy availability when PS-based PUs are used for biomedical applications because aliphatic polyesters such as poly (lactic acid) (PLA), poly(glycolic acid) (PGA), poly (lactic-co-lactic acid (PLGA) and poly (caprolactone) (PCL) [9] does not have acceptability to the tissue, control of cellular behaviors, and non-wettability compared with PS although they are fully bioabsorbable. Before starting this section, we want to discuss the general requirement of wound dressings that could make readers easy to follow. Wound healing with a complex and regulated physiological process activates various cell types via several processes, such as homeostasis, inflammation, proliferation, and tissue remodeling [20]. Therefore, well-designed wound dressings should be used to meet its requirement for optimal healing. First, a moist wound environment is very critical because it recruits immune cells for promoting wound healing through the elaboration of growth factors and it diminishes pain during wound dressing changes [21]. Second, absorption of excess exudates including blood at the wound place is important because the excess exudates contain enzymes that affect cellular behaviors with losing activity of growth factors and results in the delay in the healing process [22]. Third, the bacterial infection should be prevented because the infected wound delays extracellular matrix synthesis and prolongs the inflammation state [22]. Fourth, adequate oxygen and water exchange are necessary because oxygen is an essential component for cell metabolism and the water vapor can manage the permeability of exudates derived from the wound site [23]. Fifth, the wound dressing should have low adherent to the wound site to prevent trauma and pain because the high adherent wound dressing further damage the wound site [24]. Sixth, mechanical properties, and degradation of wound dressing should be taken into account for topical application to match the timeline with the healing process [22]. Seventh, the wound dressing should be non-toxic without an inflammatory response [22]. Finally, the cost of wound dressing should be taken into account including minimal change of the wound dressing from the wound site in terms of production cost [22 The requirement of wound dressing is summarized in Table 2. In this section, we discuss PS-contained PUs for wound dressing applications.
Table 2.
Requirement of wound dressings
| Requirement | References |
|---|---|
| Moistened wound environment for recruiting-related cells and diminishing pain | [27] |
| Absorption of exudates including blood at the wound place | [28] |
| Protection of bacterial infection for host repair | [28] |
| Adequate oxygen and water exchange for the metabolism of related cells | [29] |
| Low adherent to the wound site for preventing pain and trauma | [30] |
| Desirable mechanical and degradable properties for topical application | [28] |
| Should be non-toxic without an inflammatory response | [28] |
| Should be cost-effective for mass production | [28] |
CS-contained PUs
CS can be obtained from chitin as an important component of the animal exoskeleton and cell wall of fungi [25]. CS composed of glucosamine and N-acetyl glucosamine residues after deacetylation from the chitin has been applied for various biomedical applications such as wound dressing [26, 27], drug delivery carriers [28, 29], and tissue engineering [30, 31] because the CS has excellent biological properties such as nontoxicity, biocompatibility, biodegradability, anti-fungistatic, and antibacterial properties [32] as well as adjustable physicochemical properties due to the three kinds of reactive groups including amino group, primary and secondary hydroxyl groups [32]. In this part, we discuss CS-contained PUs for wound dressing applications.
Tan et al. prepared composite nanofibrous mats consisting of CS/AL as natural polymers to improve biological properties and shape memory PU as a synthetic polymer to get shape memory function and mechanical matrix by electrospinning technique and subsequent post-treatment using silver nitrate solution to prevent the bacteria infection [33]. The composite mats showed cytocompatibility to fibroblasts, good water vapor permeability, surface wettability, hemostatic property through a whole blood clotting test due to CS/AL, antibacterial activity against the common Gram-negative and Gram-positive bacteria due to silver, and shape memory effect due to shape memory PU, suggesting that they can be used as the wound healing materials having shape fixation-assisted easy processing and shape recovery-assisted closure of cracked wounds having biocompatibility and antibacterial activity although they did not check the possibility in vivo.
Wang et al. prepared a hybrid bilayer dermal substitute by integrating PU membrane as a temporary epidermal layer with cross-linked PLGA knitted mesh/collagen (CO)/CS blend for the repair of full-thickness skin wounds in rats to overcome the poor mechanical properties of commercially available CO-based dermal scaffolds [34]. The results indicated that the hybrid bilayer dermal substitute showed desirable porous microstructure for structural stability of dermal substitutes and mechanical properties, and significantly inhibited wound contracture, enhanced angiogenesis with facilitating ordered arrangement of neotissue in rats compared with the commercial production of PELNAC™ as shown in Fig. 1, demonstrating its potential use for full-thickness skin defect repair.
Fig. 1.
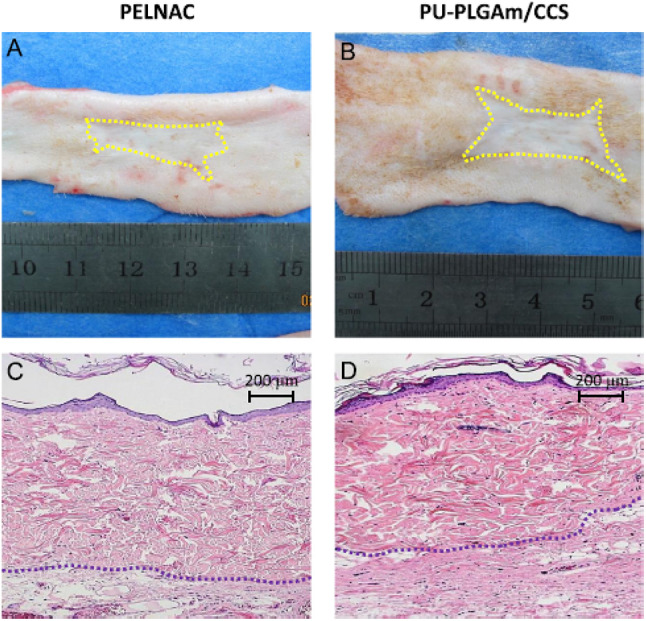
A-D Macroscopic observation of healed wounds and the related HE staining results in the PELNAC (A, C) and PU-PLGAm/CCs (B, D) groups for 12 weeks post-operation. Yellow lines indicate the margin of the healed wounds, and the blue lines mean the interface of dermal layer and hypodermal tissue (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) (Adapted from Wang et al. Journal of the Mechanical Behavior of Biomedical Materials 2016;56:120–133, with permission from Elsevier [34])
Klempaiova et al. prepared plasma-treated electrospun PU nanofiber membranes coated with CS/berberine-encapsulated β-cyclodextrin using diffuse coplanar surface barrier discharge for wound dressing application [35] because the PU has barrier properties with a good oxygen permeability, the CS has a potential to accelerate wound healing, and the berberine has antibacterial and anti-inflammation activities [35]. The results indicated that the nanofiber membranes exhibited good cell growth activity, cell viability, and mitochondrial activity in human dermal fibroblasts and 3T3 murine fibroblasts used as biological models, suggesting its potential use for temporary wound dressing with non-toxicity and biocompatibility although they did not perform in vivo experiment.
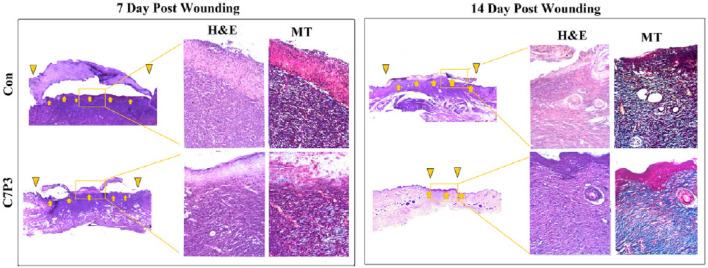
Bankoti et al. blended PU diol dispersion with CS to get self-organized macroporous hydrogel scaffolds having hydrophobic/hydrophilic balance which showed adequate swelling with reduction of bacterial adhesion for accelerated healing of full-thickness dermal wounds [36]. Scanning electron microscope (SEM) showed that the macroporosity on the top and fracture of hydrogel scaffolds having phase separation due to the intramolecular and intermolecular hydrogen bonding between the two polymers with good mechanical properties and in vitro degradation with a cytocompatibility in rat primary fibroblast cells by the proliferation. Furthermore, the study demonstrated an accelerated healing process with enhanced wound contraction, vascularization in the wound area as shown in Fig. 2, and higher collagen synthesis in Wistar rats compared with the commercial dressing of Tegaderm™, an indication of promising material for full-thickness wound healing dressing.
Fig. 2.
Representative images of Masson’s Trichrome and H&E stained histological sections of day 7 and day 14 after initial wounding: yellow arrow represents wound area, and yellow inverted triangles represent healing wound edges. (Adapted from Bankoti et al. Materials Science and Engineering C 2017;18:138–143, with permission from Elsevier [36])
Jafari et al. prepared PU nanocomposites consisting of PU membrane, CS nanoparticles (NPs) obtained by the ionic-gelation method as a hydrophilic organic filler, and titanium dioxide (TD) NPs as an inorganic filler using phase inversion technique to increase swelling degree and mechanical properties of PU membrane itself for application of a wound dressing [37]. The results indicated that the phase inversion technique manipulated by exchange of polymer–solvent to non-solvent induced a microstructure change from finger-like to sponge-like, which is more suitable for water uptake and consequently more potential for wound dressing applications. Also, the swelling percent and tensile strength were increased to 71.5 and 18.94%, respectively, compared to neat PU with the antibacterial activity of 69% against Staphylococcus aureus and non-toxicity to human fibroblast cells in vitro, the suggestion of potential wound dressing applications although they did not perform in vivo experiment.
Uscategui et al. prepared PU membranes by a reaction of polyol derived from castor oil and isophorone diisocyanate, and then PCL diol/CS were added to the above pre-polymer to enhance mechanical and biological properties of PU for application of wound dressing [38]. The results indicated that the mechanical properties of the prepared PU membranes were increased due to the polyols from castor oil without cytotoxicity in three kinds of fibroblast cell lines and pro-inflammatory cytokine stimulation in monocytic leukemia cell line compared to polypropylene (PP) as reference material, suggesting a potential candidate for wound dressing due to their enhanced mechanical properties and biocompatibility although they did not test in vivo.
Najafabadi et al. prepared PU nanocomposite film consisting of PU matrices and CS-modified graphene oxide (CS-GO) nanosheets to enhance thermo-mechanical properties, wettability, biocompatibility, and antibacterial properties of PU film for application of the wound dressing [39]. The results indicated that the PU/CS-GO exhibited higher Tg and storage modulus with good dispersion of CS-GO nanosheets in PU film, better biocompatibility, and antibacterial activity against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria than the PU/GO sample, the suggestion of a promising antibacterial wound dressing although they did not perform in vivo experiment.
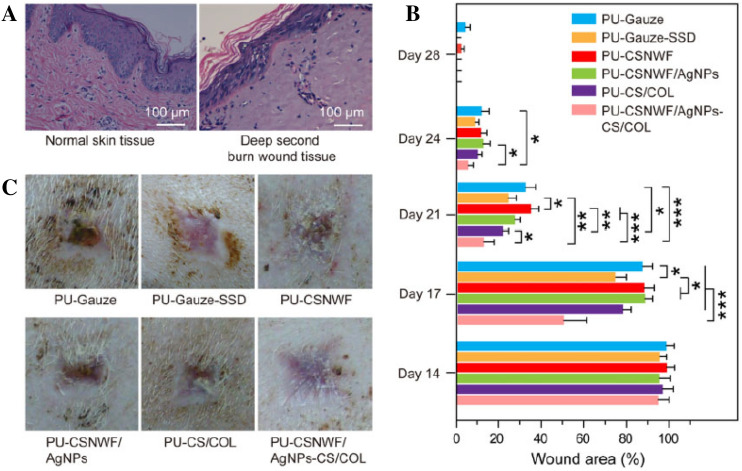
Yang et al. prepared a sandwich structure composite consisting of fibers of CS nonwoven fabric anchored with silver NPs as the interlayer and PU membrane as the outer layer to get biocompatibility and antibacterial activity for wound dressing applications [40]. The results indicated that the composite wound dressing maintained high antimicrobial activity against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus and minimal cytotoxicity by the firmly anchored silver NPs with a reduction of inflammatory response due to the sustained silver ions from silver NPs and used CS fibers. Also, the composites accelerated the wound healing process in a deep dermal burn porcine model with excellent angiogenesis and re-epithelialization through promoting vascular endothelial growth factor (VEGF) and inhibiting nitric oxide (NO) production as shown in Fig. 3, suggesting a potential application for severe wound care.
Fig. 3.
A Histological observation of porcine skin tissue structure on normal and the deep second burn wound area by H&E staining. B Percent change in wound area over a 4-week period. C Photographs of the wound healing effect in all groups on day 28 post-surgery (*p < 0.05, **p < 0.01 and ***p < 0.001) (Adapted from Yang et al. Regenerative Biomaterials 2021; 8:rbab037 [40])
Recently, Zhang et al. prepared carboxymethyl chitosan (CMCS)-based hydrogel film loaded with PU-gelatin (GE) hydrolysate synthesized by aqueous emulsion copolymerization to get thermal stability, good swelling content, and controllable biodegradability for applications of the wound dressing [41]. The results indicated that the maximum tensile strength and elongation at the break of the hydrogel film were obtained when the content of CMCS was loaded at 6 wt.-% in the hydrogel film with significant antibacterial activity against E. coli and S. aureus with good thermal stability, swelling behavior, and controllable biodegradability, the indication of potential antibacterial wound dressings although they did not perform in vivo experiments.
AL-contained PUs
AL can be obtained mostly from the cell wall of a brown seaweed among different sources of macroalgae [42]. The AL is a linear anionic polymer consisting of β-(1–4)-D-mannuronic (M-blocks) and α-L-guluronic acid (G-blocks) with the hetero-arrangement of uronic acids although the M/G block ratio affects the properties of the AL. [43] The AL has been used for biomedical applications such as drug delivery systems, tissue engineering, and wound healing because the AL can bind with divalent metal ions for cross-linking bulk AL, the AL is non-toxic, biodegradable, biocompatible, and biostable, and the AL can be easily processed into film, gel, hydrogel, foam, wafer, nanofiber, and gauze [42]. In this part, we discuss AL-contained PUs for wound dressing applications.
Xie et al. prepared CS-collagen (CO)-AL/PU composite dressing after attaching of CS-CO-AL mixture obtained by paint coat and freeze–drying to anti-seawater immersion PU membrane for application on wounded soldiers, seamen, and sea-fishers worked on sea [44]. The results indicated that the composite dressing had good water absorption and mechanical properties with no significant cytotoxicity and favorable hemocompatibility. Also, higher wound healing was obtained in rats with more fibroblast, intact re-epithelialization, and increased expressions of EGF (epidermal growth factor), bFGF (beta-fibroblast growth factor), TFG-β (transforming growth factor-beta), and CD31 than in gauze or CS-treated ones, suggesting that the composite dressing can be used for special persons who work on the sea with having biosecurity.
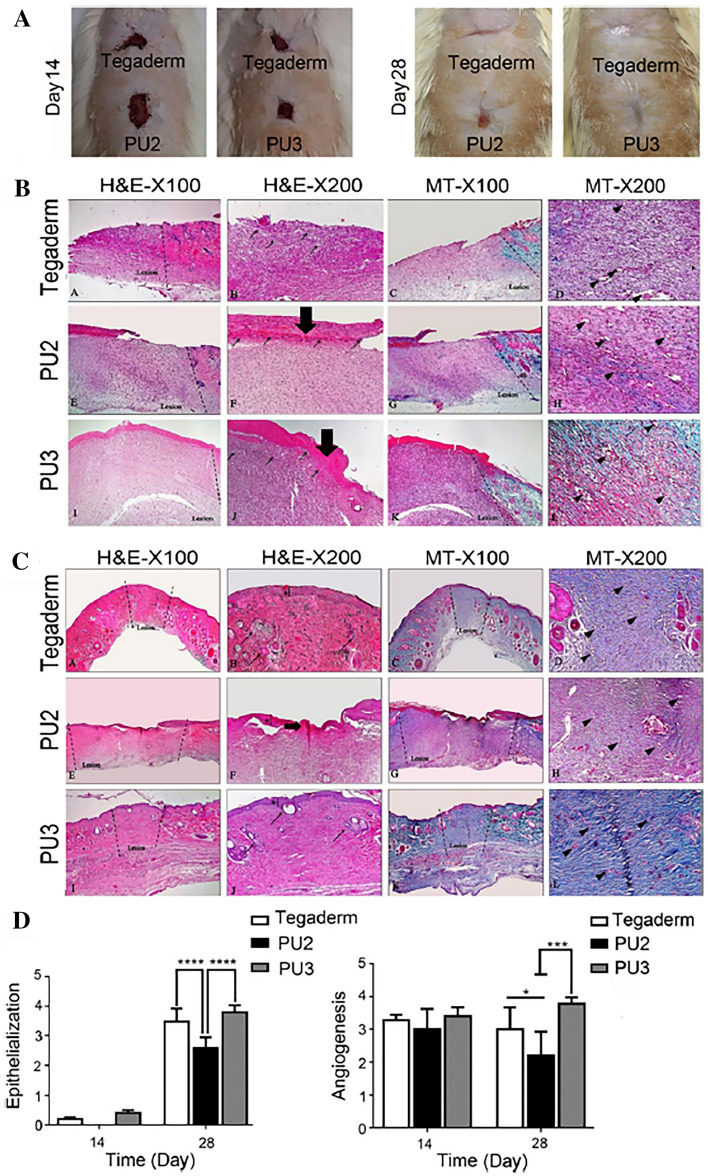
Daemi et al. prepared tributyl ammonium AL-coated cationic PU films for applications of full-thickness wounds to protect persistent bacterial infections and rapid onset of dehydration at wound sites [45]. The results indicated that the surface-modified PU increased hydrophilicity and tensile Young’s modulus ranging 1.5–3 MPa with cytocompatible and fibroblast migratory-promoting and antibacterial activity against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria. Also, they showed a faster healing rate due to the reduction of the persistent inflammatory phase, increased mature blood vessel formation, and collagen deposition when compared to cationic PU and commercial Tegaderm™ as shown in Fig. 4, suggestion of potential applications of the wound dressing.
Fig. 4.
In vivo assessments of synthesized PUs: A wound contraction in the presence of three different treatment dressings on days 14 and 28. B Histological analysis with H&E and MT staining of the treated wounds was performed after 14 days (thick arrow and thin arrow showed the presence of a scab and inflammatory cells, respectively). C Histological analysis of the treated wounds, using H&E and MT staining, after 28 days. The asterisk, thin arrow, and arrowhead show epidermal layer formation, sebaceous glands, and vascularization, respectively, after 28 days. The PU3 sample had better healing properties in terms of epithelial layer formation, collagen deposition, and skin appendage rejuvenation. D Quantitative analysis of epithelialization and angiogenesis for the different treatment groups showed similar healing for the PU3 sample compared with Tegaderm and a better healing rate compared with PU2 (n = 5). *p < 0.05, **p < 0.01, and ***p < 0.001. (Adapted from Salekdeh et al. Acs Appl Mater Inter. 2020;12:3393–406 [45])
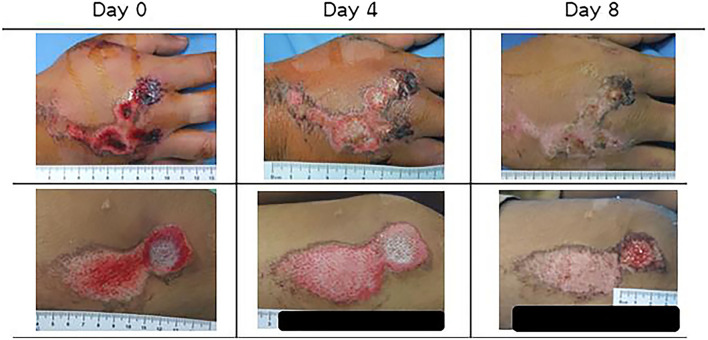
Namviriyachote et al. prepared PU foam dressing by reaction of diisocyanate and polypropylene (PP) glycol as the main diol individually mixed with hydroxypropyl methylcellulose, CS and AL as the natural polyols while silver as an antimicrobial agent and asiaticoside (AS) as an herbal wound healing agent were impregnated into the obtained PU foam dressing sheets for healing dermal wound [46]. The results indicated that the PU foam sheets obtained from AL showed the highest silver and AS release with the non-cytotoxicity to human fibroblast cells and antimicrobial activity against a few bacteria strains in vitro although foam sheets with AL showed the highest silver and AS release. Also, the PU foam dressing in 6 wt.-% AL, 1 mg/cm2 silver, and 5 wt.-% AS improved wound healing in both wound closure and histological parameter of the pig dermal wound without the dermatologic reactions, the suggestion of a potential candidate for wound dressing. Furthermore, they clinically tried on healthy volunteers with traumatic dermal wounds using PU-AL combined foam dressing absorbed with silver NPs and AS [47]. The results showed improved healing with shortened wound closure time, less pain score, and higher re-epithelialization without skin irritation and with retained moisture compared to gauze soaked with chlorhexidine as shown in Fig. 5, the indication of potential applications of the traumatic dermal wound dressing.
Fig. 5.
Comparison of wounds treated with selected PUC foam dressing (study group) (top row) and gauze dressing (standard group) (bottom row). (Adapted from Namuiriyachote et al. Asian J Pharm Sci. 2019;14:63–77, with permission from Elsevier [46])
Lu et al. developed AL/waterborne PU blend electrospun nanofibers for applications of wound dressings because the AL has a hydrophilic property with spinnability and the PU has good mechanical strength [48]. The blend nanofibers showed porous structure, good mechanical strength with water-stable after the addition of calcium chloride as the crosslinker although initial modulus and the elongation strength of the blend nanofibers increased with AL content, moisture-permeable, and water-absorbable properties, the suggestion of cost-effective wound dressings although they did not check the possibility in vitro and in vivo.
Recently, Claudio-Rizo et al. prepared highly absorbent hydrogels obtained from interpenetrated networks (IPNs) by the interpenetrating process with aqueous PU dispersion with PU crosslinker and AL with Ca2+ ion to improve the swelling, degradation rate, and mechanical properties of the wound dressings [49]. The results indicated that the crosslinking of AL with PU-generated IPNs hydrogels having high capacity of water absorption, controlling degradation rate and storage module as well as good biocompatibility, increased hemocompatibility, inhibition of E. coli growth, and no cytotoxicity for monocytes and fibroblasts for up to 72 h in vitro, an indication of a new design of wound dressing although they did not check in vivo.
HA-contained PUs
The HA consisted of N-acetyl-glucosamine and glucuronic acid as a glycosaminoglycan family has been widely used in biomedical applications including wound dressings because it stimulates cell migration, angiogenesis, and reduces inflammation with its high ability for water absorption and flexibility [50] although it has weak mechanical properties for wound dressing applications.
Reyes-Ortega et al. prepared bilayered wound dressings consisting of an internal layer obtained by a formation of semi-IPNs of HA/gelatin cross-linked by the genipin having highly hydrophilic and biodegradable properties with a loading of proadrenomedullin N-terminal 20 peptide (PAMP) and external layer obtained by a PU derived from PCL diol, Pluronic L61, and poly (tetramethylene ether) glycol as the chain extender with the loading of resorbable bemiparin (BE) NPs [51] as a fractionated low molecular weight heparin because the PAMP has proangiogenic, anti-inflammatory, and antibacterial properties, and the BE NPs promote the activation of growth factors of FGF and VEGF [52]. The results indicated that the bilayered wound dressings having good biomechanical stability and controlled permeability showed an improved cicatrization, favored epithelialization, and remarkably reduced the wound contraction and inflammation in both ischemic and non-ischemic defects of rabbit ear model, suggesting potential application for diabetic ulcer.
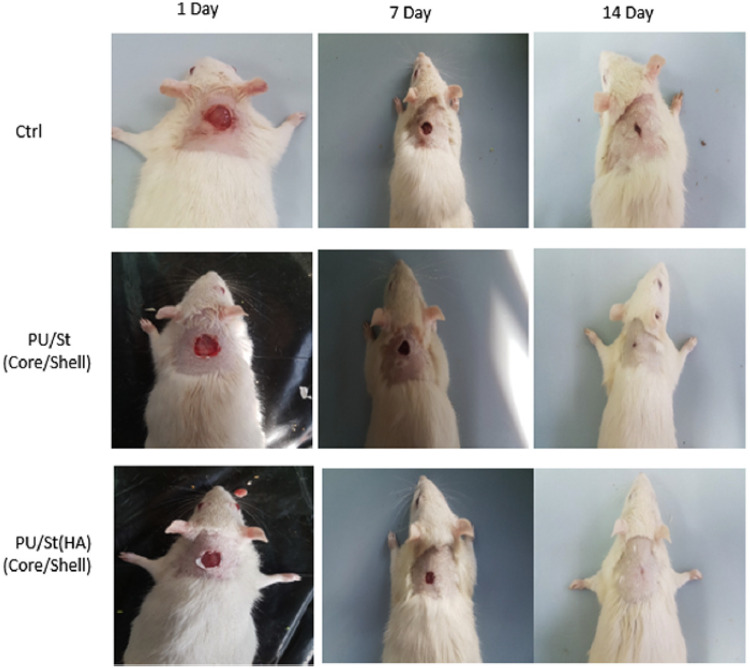
Movahedi et al. prepared core–shell structure of electrospun nanofibers consisting of PU, starch (ST), and HA through coaxial electrospinning technique for wound dressing applications because the PU has strong mechanical properties with good barrier properties and oxygen permeability, and the HA supplies a moist environment with the promotion of heal process [53]. The results indicated that the core–shell PU/ST/HA nanofibers confirmed by contact-angle measurement exhibited significant enhancement of proliferation and attachment of mouse fibroblasts in vitro, and showed fasten wound closure in rats compared to cotton gauze as control as shown in Fig. 6 due to the attribution to used HA in cellular repair and keeping the skin moist, the suggestion of a potential application of wound dressing.
Fig. 6.
Photographs of wounds treated with the dressings on days 1, 7, and 14 after full-thickness skin excision. Evaluation of the wounds area closure (1–14 days). The data are presented as mean ± SD (n = 8). *showsthesignificant difference between groups at p b .05. (Adapted from Movahedi et al. Int J Biol Macromol. 2020;146:627–37, with permission from Elsevier [53])
Eskandarinia et al. prepared propolis-loaded PU/HA nanofibrous wound dressing through electrospinning technique to get antibacterial and wound properties [54] because the propolis has antifungal and anti-inflammatory properties [55], and the HA promotes the healing process [53]. The results indicated that the propolis-loaded PU/HA nanofibers exhibited higher antibacterial activity against S. aureus and E. coli with higher biocompatibility in L929 fibroblast cells in vitro compared with PU or PU/HA. Also, the nanofibers significantly accelerated wound closure with the wound healing progression and improved dermis development with collagen deposition in rats as shown in Fig. 7, the indication of a promising wound dressing.
Fig. 7.
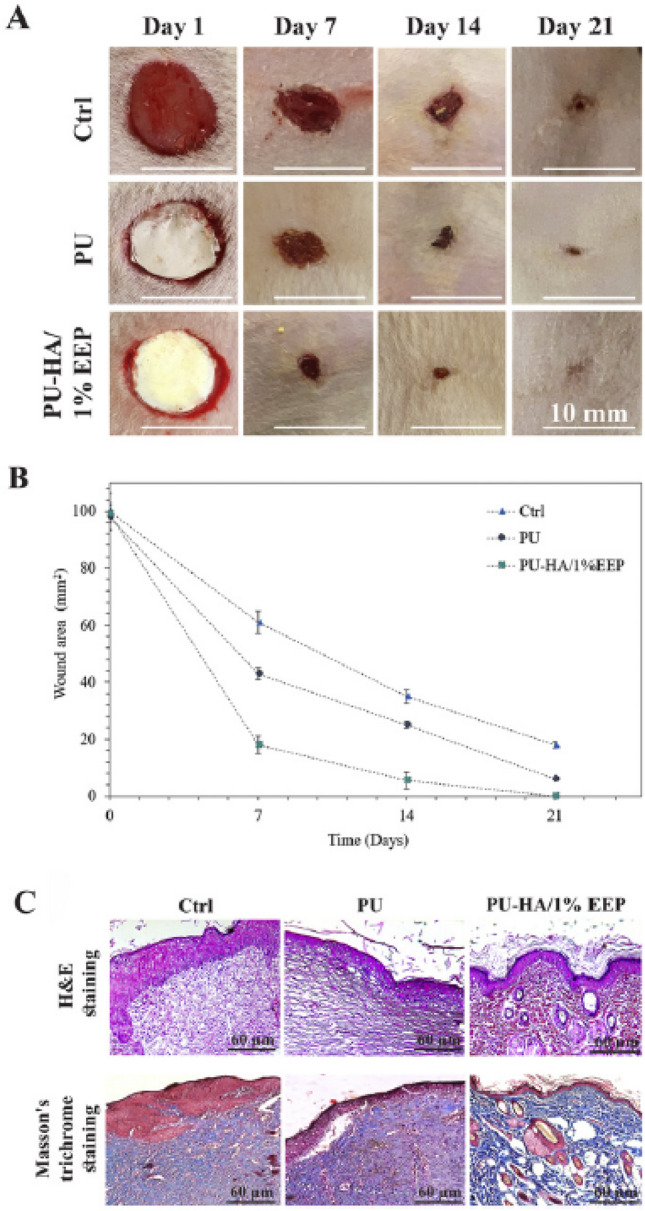
A Macroscopic photographs of wounds at 1st, 7th, 14th and 21st day after full-thickness skin wound creation. B Wound closure progression (1–21 days) at different groups (Ctrl, PU, and PU-HA/1% EEP). The data are presented as mean ± SD (n = 8). C Histology analyses of the wound area sections at different groups after 21 days from wounds creation (Ctrl, PU, and PU-HA/1% EEP). (Adapted from Eskandarinia et al. Int J Biol Macromol. 2020;149:467–76, with permission from Elsevier [54])
CE-contained PUs
The CE as a highly abundant PS and the main structural biomaterials of plant cell walls, a linear homopolymer of β-(1 → 4)-linked D-glucopyranosyl units, is widely used for biomedical applications due to the increase of the hydrophilicity and biodegradability of synthetic hydrophobic polymers while CE derivatives have been used due to its insolubility in water and organic solvents [56].
Unnithan et al. prepared streptomycin-loaded PU/cellulose acetate (CEA)/zein electrospun nanofibrous mats for wound dressing application because the CEA has good water absorption abilities and good biocompatibility, and the zein as a plant protein exhibits resistance to microbial attack and antioxidant activity [57]. The results indicated that the streptomycin-loaded PU/CEA/zein showed better cell viability, proliferation, and adhesion of 3T3-LI fibroblasts with enhanced clotting ability, improved hydrophilicity, and antibacterial activity against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria compared to PU itself due to the used zein although they did not test in vivo. Also, they similarly prepared paclitaxel-loaded PU/CEA electrospun nanofibrous mats for wound dressing applications because the CEA has excellent biocompatibility with high water uptake and the PU has excellent mechanical properties [58]. The results indicated that the proliferation of NIH 3T3 fibroblast cells attached on the nanofibrous mats was increased as the content of CEA in the PU/CEA mats and the drug release increased proportionally with increasing swelling rate of the composite nanofibrous mats was increased, the suggestion of a promising candidate for wound dressing applications although it is hard to understand why paclitaxel as one of breast cancer drugs was used in wound dressing.
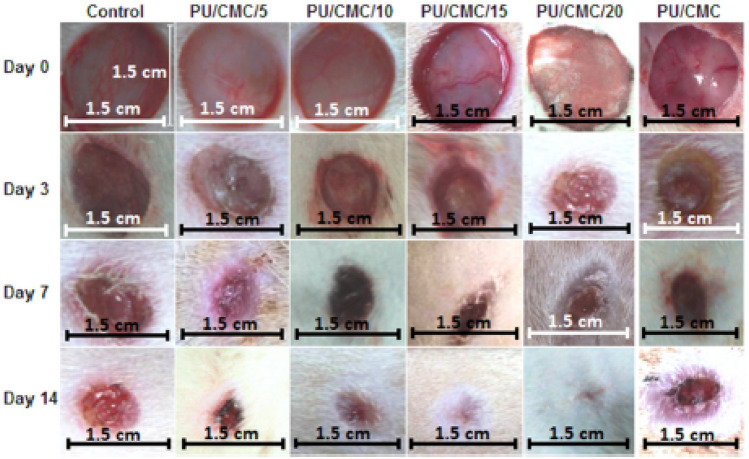
Almasian et al. prepared extracted Malva sylvestris (MS)-loaded PU/carboxymethylcellulose(CMC) nanofibers via an electrospinning method for healing of diabetic wounds with the idea that the CMC can improve the absorption ability of exudates in the wound site and the extracted MS as one of the herbal compounds has antibacterial and anti-inflammatory activities [56]. The results indicated that the extracted MS-loaded nanofibers with an average diameter of 386.5 nm showed higher antibacterial activities against S. aureus and E. coli pathogens than gauze bandages in vitro after the release of loaded MS. Also, the average healing rate of the extract containing PU/CMC nanofibers was increased with lowering acute and chronic inflammations in wounds of rats compared to the gauze bandage nanofibers after histological performance observation. Furthermore, herbal extract-loaded nanofibers showed higher collagen deposition and neovascularization in wounds of diabetic rats compared to wounds treated with a gauze bandage as a control group as shown in Fig. 8, suggesting that the herbal extract-loaded nanofibers are a potential dual antimicrobial and anti-inflammatory wound dressing for using diabetic wound healing.
Fig. 8.
Representative wounds on an animal in each group on days zero, three, seven and 14 after treatment. (Adapted from Almasian et al. Mat Sci Eng C-Mater. 2020;114:111039, with permission from Elsevier [56])
The characteristics of PS-contained PUs for wound dressing were summarized in Table 3.
Table 3.
Characteristics of PS-contained PUs for wound dressing
| Kind of PS | Processing type | Used cells | In vivo model | Outcomes | References |
|---|---|---|---|---|---|
| CS | Nanofiber | Fibroblasts | – | Cytocompatibility, hemostatic property, antibacterial activity, shape memory effect | [33] |
| Membrane | – | Rats | Good porous structure and mechanical properties, inhibition of wound contracture enhanced angiogenesis | [34] | |
| Nanofiber | Fibroblasts | – | Good cell growth, good mitochondrial activity | [35] | |
| Hydrogel | Fibroblasts | Rats | Phase separation structure, cytocompatibility, accelerated healing, enhanced vascularization | [36] | |
| Membrane | Fibroblasts | – | Sponge-like structure, increased tensile strength, antibacterial activity | [37] | |
| Membrane | Fibroblasts | – | Increased mechanical properties, non-cytotoxicity, stimulation of pro-inflammatory cytokine | [38] | |
| Film | Fibroblasts | – | High storage modulus, good biocompatibility, antibacterial activity | [39] | |
| Membrane | Fibroblasts | – | High antimicrobial activity, minimal cytotoxicity with a reduction of inflammation, accelerated wound healing | [40] | |
|
Hydrogel Film |
– | – | High mechanical strength and high antibacterial activity | [41] | |
| AL | Membrane | – | Rats | Good water absorption and mechanical properties, higher wound healing, increased expression of EGF, bFGF, TAG-ß, and CD31 | [44] |
| Film | Fibroblasts | – | Increased hydrophilicity and tensile modulus, faster wound healing, increased blood vessel formation and collagen deposition | [45] | |
| Foam | Fibroblasts |
Pig Human |
Non-cytotoxicity, antimicrobial activity, improved wound healing, higher re-epithelialization | [46, 47] | |
| Nanofiber | – | – | Porous structure, good mechanical strength moisture-permeable | [48] | |
| Hydrogel | Fibroblasts | – | High capacity of water absorption, controlling degradation rate, good biocompatibility, antibacterial properties | [49] | |
| HA | Membrane | – | Rabbit | Improved cicatrization, favored epithelialization, reduced wound contraction and inflammation | [52] |
| Nanofiber | Fibroblasts | Rats | Enhanced attachment and proliferation of cells, faster wound closure in rats | [53] | |
| Nanofiber | Fibroblasts | Rats | Higher antibacterial activity and biocompatibility, accelerated wound closure, improved dermis development | [54] | |
| CE | Nanofiber | Fibroblasts | – | Good cell viability, proliferation, and adhesion enhanced clotting ability, improved hydrophilicity | [57] |
| Nanofiber | – | Rats | High antibacterial activities, increased wound healing rate, lowered inflammation, higher collagen deposition, and neovascularization | [56] |
Bone tissue engineering
Tissue engineering and regenerative medicine as a multidisciplinary science and advanced technology, including material science, molecular biology, and devices required in the clinical sector has recently become important therapeutic strategies to develop biological substitutes for regeneration of defective organs or tissues resulting from degenerative diseases or trauma [59]. Among defective organs, treatments for bone defects are one of the most urgent issues in orthopedic surgery. Indeed, autograft, allograft, and xenograft are currently performed despite several limitations, such as insufficient availability, chronic pair, transplant rejection, the transmission of disease, immunogenicity, and graft rejection [59]. As an alternative method besides traditional transplantation, tissue-engineered implants consisting of the scaffold, biologically active cells, and growth factors will provide a new therapeutic method to solve the above-mentioned limitations [59]. PU scaffolds have been attracted to bone tissue engineering because they have shown good performance on their excellent properties such as versatile mechanical properties, processability, injectability, and unique chemistry. However, they have still several limitations: there are no binding sites of bioactive agents because the bone growth takes place due to stimulation by proteins and osteogenic cytokines, and also they should be anti-bacteriostatic, control of their biodegradability is not easy because they gradually degrade and are replaced by new bone formation although the necessity of degradability and absorbability of PU scaffolds depends on hard tissue such as bone or soft one such as cartilage because it is not necessary to get degradability in the bone as hard tissue, and there are less hydrophilic and no binding sites of the cells because cellular behaviors should be regulated, and raised global plastic wastes should be considered. To overcome the limitation of PUs for bone tissue engineering, the introduction of PS in PUs can enhance hydrophilicity, biodegradability, the resemblance with bodily macromolecules, cost-effectiveness, and easy availability because ideally, bone graft substitutes should be biocompatible, biodegradable according to the used bone sites, osteoconductive, osteoinductive, and show a minimal fibrotic reaction, support new bone formation, and a natural extracellular matrix [60]. Especially, the PS can be used to control the release of bioactive agents from PU scaffolds because the PS does not induce inflammatory or allergic reactions by biodegradation products although aliphatic polyesters such as PLA, PGA, PLGA, and PCL induce inflammatory or allergic reactions due to acid pH by biodegradation products. In this section, we discuss PS-contained PUs for ideal bone tissue engineering applications in the order of CS, AL, CE, and HA (Table 4).
Table 4.
Characteristics of PS-contained PUs for bone tissue engineering
| Kind of PS | Processing type | Used Cells | In vivo model | Outcomes | References |
|---|---|---|---|---|---|
| CS | Membrane | ASC/AC | Rabbit | Increase of tensile modulus and degradation rate by CS addition, good meniscus regeneration | [61] |
| Nanofiber | Fibroblasts | – | Macropore structure, fast cell adhesion with spindle-like shapes | [65] | |
| Membrane | Osteoblasts | – | Increased mechanical properties, interconnected porous structure, increased cellular behaviors and excretion of ECM | [66] | |
| Membrane | HAMSCs | – | Excellent shape memory property, self-healing ability, promotion of HAMSCs and matrix mineralization, enhanced gene expression of COL-1, ALP, and OCN | [68] | |
| Nanofiber | Pre-osteoblasts | – | Improved mechanical strength, hydrophilicity, and antibacterial, efficacy, expression of osteogenic protein marker | [70] | |
| AL | Hydrogel | MSCs | Rabbits | Synergistic chondrogenesis of MSCs by KGIN and TGF-β3, promotion of MSCs migration and cartilage regeneration with superior mechanical properties | [73] |
| Hydrogel | ADMSCs | – | Acceleration of differentiation of ADMSCs into osteoblasts, increased collagen, osteopontin, osteocalcin expression, and alkaline phosphatase activity | [76] | |
| HA | 3D Printed Scaffold | MSCs | Rabbits | Induction of chondrogenic differentiation of MSCs, production of ECM, improved cartilage regeneration | [78] |
| 3D Printed Scaffold | WJMSCs | – | High cytocompatibility, excellent chondrogenic differentiation | [81] | |
| 3D Scaffold | hBMSCs | – | Enhanced production of sulfated glycosaminoglycans, decreased upregulation of collagen | [82] | |
| 3D Scaffold | AMSCs | Rabbits | Excellent macroporous structure, strong mechanical properties, high expression of chondrocyte marker genes, significant meniscus tissue regeneration | [83] | |
| CE | 3D Scaffold | MG63 | – | Interconnected pore network with porosity of 83%, good cytocompatibility, anti-bacterial properties | [86] |
| Hydrogel | BMSC | – | Enhanced chondrogenic gene expression, production of sulfated glycosaminoglycans and collagen II | [88] |
CS-contained PUs
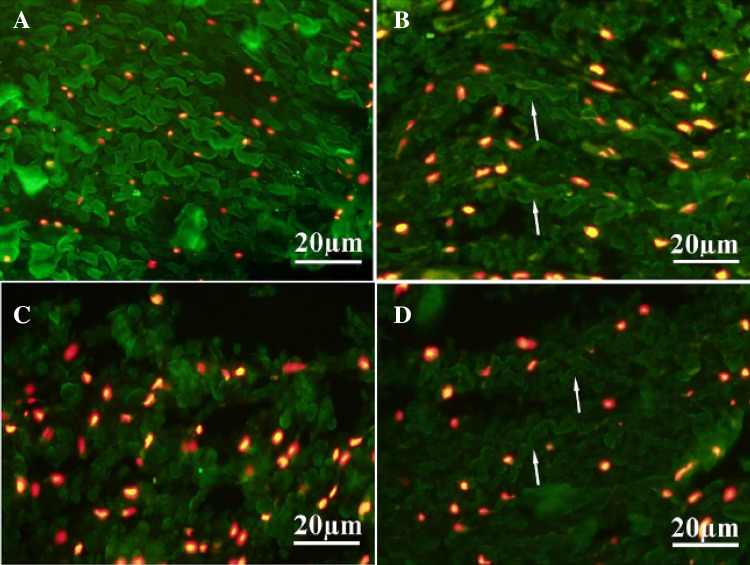
Moradi et al. prepared adipose-derived mesenchymal stem cells (AMSC)/articular chondrocytes (ACs)-seeded hybrid scaffolds consisting of poly (vinyl alcohol) (PVA)/CS cross-linked by pre-PU chains for the regeneration of meniscus tissue [61] because the PVA has been used in biomedical applications due to its biocompatibility and mechanical strength [62], and at the same time, the CS showed chondrogenic differentiation potential of mesenchymal stem cells (MSCs) [63] while the PU promoted fibrocartilage formation [64]. The results indicated that the weight ratio of PVA/CS/PU (1:4:1) hybrid scaffold seeded by AC showed the highest expression of collagen II and aggrecan, and tensile modulus, swelling ratio, and degradation rate increased by CS addition in a dose-dependent manner while toughness showed a decrease by increasing amount of CS in the hybrid scaffolds at day 21. Also, the PVA/CS/PU (1:4:1) hybrid scaffold revealed better unilateral total meniscus regeneration in rabbits after 7 months post-implantation when ACs were seeded in hybrid scaffold compared to cell-free scaffold group, indicating that AC-seeded hybrid scaffolds can successfully regenerate meniscus tissue as shown in Fig. 9, a suggestion of a potential meniscus tissue engineering application.
Fig. 9.
A-D IFC staining of the native and regenerated meniscus tissue at 7 months post-transplantation shows accumulation of Collagen II (green colour) in the inner zone of experimental groups: Native meniscus (A), AC/scaffold (B), ASC/scaffold (C) and AC-ASC/scaffold (D) groups. Collagen II fibers are more organized in AC/scaffold (B, arrows) and AC-ASC/scaffold (co-culture) groups (D, arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.). (Adapted from Moradi et al. Biomaterials. 2017;126:18–30, with permission from Elsevier [61])
Topsakal et al. prepared amoxicillin-loaded CS/β-tricalcium phosphate (β-TCP)/PU hybrid nanofibers via electrospinning process for bone tissue engineering application [65] because the CS has biodegradable, bioactive, biocompatible, and antibacterial properties, the β-TCP has been widely used to fabricate scaffold due to structural similarity to mineral components of human bone [65], and the PU has good mechanical property due to the microphase separation structure between soft and hard parts [65]. The results indicated that the tensile strength of the hybrid nanofibers increased in a PU concentration-dependent manner while that of the hybrid nanofibers decreased when the β-TCP concentration increased. Also, the morphologies of the nanofibrous scaffolds revealed well-distributed macropores through the scaffolds with some micropores. Furthermore, L929 fibroblasts were attached faster to the hybrid nanofibers with spindle-like shapes than polystyrene tissue culture (PTC) well as a control due to the 3D nonwoven porous/fibrous network in comparison to flat 2D surface of PTC wells, suggestion of a potential bone tissue engineering applications although they did not perform in vivo study.
Zo et al. prepared carboxymethyl (CM) CS 3D porous scaffolds grafted with waterborne PU via the freeze–drying method for bone tissue engineering applications [66] because the CMCS allows water solubility of CS itself and causes processability of preparing scaffolds [66], and the waterborne PU formed by dispersing of PU pre-polymer containing isocyanate groups in water has several advantages, such as versatility, low-temperature flexibility, no use of organic solvent, and nontoxic, thereby easy integration of cells into the scaffolds [67]. The results indicated that the mechanical properties such as strain, and compression strength of the CMCS/waterborne PU scaffolds were better increased with a highly interconnected porous structure compared to those of CMCS or waterborne PU due to the grafting of waterborne PU into CMCS. Also, cellular behaviors such as adhesion, proliferation and excretion of extracellular matrix (ECM) cultured in osteoblast cells using the 3D scaffolds were more increased than them of the 2D general plate without scaffolds due to the 3D microporous network structure having a large surface area and osteoconductive environments, a suggestion of potentials for bone tissue engineering applications although they did not check in vivo study.
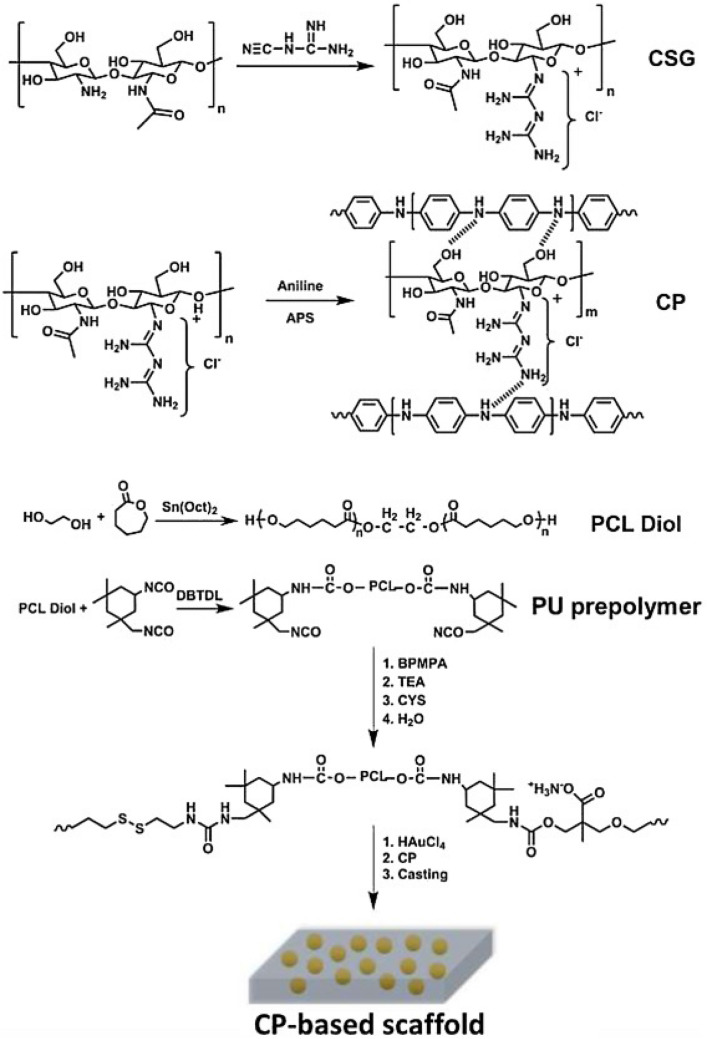
Shaabani et al. prepared HAMSCs-seeded electrically conducting self-healing hybrid scaffolds consisting of chitosan biguanidine (CSG)/polyaniline (PAN) SIPNs obtained by the polymerization of aniline (AN) in the presence of CSG and PU prepolymer obtained by the reaction of PCL diol and isophorone diisocyanate (IPDI) as shown in Fig. 10 for bone tissue engineering [68] because the CSG allows surface hydrophilicity, hydrogen interactions with PAN and biological properties, the PAN allows high conductivity and tunable electrical properties, and the PU allows strong mechanical properties, non-cytotoxic decomposition products, and good biocompatibility [69]. The prepared scaffolds showed excellent shape memory property with shape fixity (> 97%) and shape recovery ratio (> 98%) with self-healing ability (> 93%) at a temperature close to the body temperature. Also, the scaffolds promoted proliferation of HAMSCs and matrix mineralization and enhanced gene expression of COL-1, ALP, RUN2, and OCN, the indication of repairing bone defects owing to due to the multifunctional engineered scaffolds although they did not perform in vivo study and it is not easy to apply for clinical trials due to the complicated construction of the scaffolds.
Fig. 10.
The schematic represented the synthesis of the CP-contained scaffolds. (Adapted from Shaaban et al. Carbohydr Polym. 2021; 264:118,045, with permission from Elsevier [68])
Recently, Shrestha et al. prepared biomimetic hybrid nanofibrous scaffolds consisting of zein/CS/multiwalled carbon nanotube (MWCN)/PU through the electrospinning method for bone cell regeneration [70] with the hypothesis where the zein promotes bone cell differentiation [71], the MWCN allows stimulation of biological molecules for bone cell growth and prevention of bone resorption [72]. The results indicated that the hybrid nanofibrous scaffolds showed improved mechanical strength, hydrophilicity, and antibacterial efficacy with the ability of the rapid cell to cell communication through a bio-interface and a great promotion of regenerative effect of pre-osteoblast in vitro having cell growth, proliferation, and differentiation. Also, the scaffolds showed the nucleation of hydroxyapatite (HY) nanocrystals and the expression of osteogenic differentiation markers such as osteopontin and osteocalcin, suggesting a potential scaffold for bone tissue engineering although they did not perform in vivo study.
AL-contained PUs
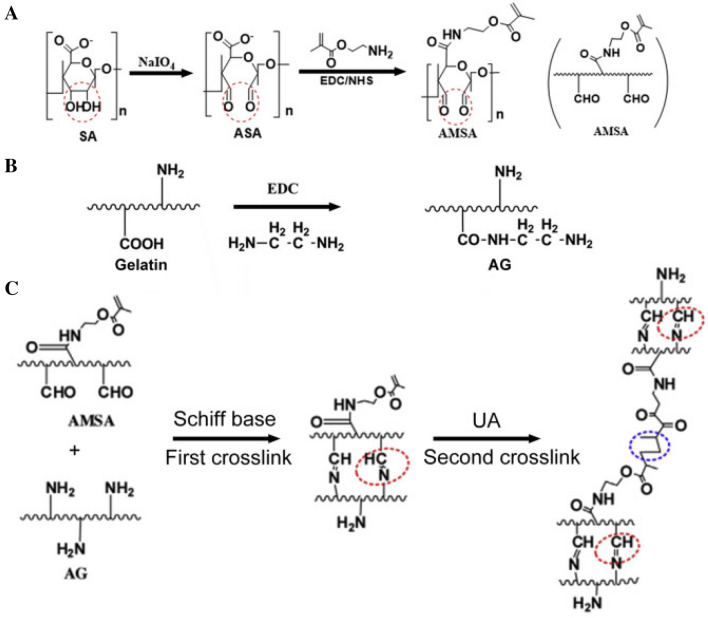
Fan et al. prepared injectable double cross-linked hydrogels by the reaction of aldehyde methylene sodium alginate (AMAL) and amino gelatin (AGE) via Schiff base reaction for the first crosslink and UV irradiation for the second crosslink, and then kartogenin (KGN)-conjugated PU NPs and TGF-β3 were loaded into the double-crosslinked hydrogels for cartilage regeneration as shown in Fig. 11 [73] with an idea that the KGN has regenerative and protective effects on the cartilage [74], the TGF-β3 induces chondrogenesis and promotes MSC migration [75], and the injectable hydrogels facilitate surgical procedures in the clinic. The results indicated that synergistic effects of KGN and TGF-β3 on the chondrogenesis of MSCs through attenuating the degradation of Runx1 were obtained in vitro. Also, KGN-conjugated PUNPs/TGF-β3-loaded injectable hydrogels promoted the MSC migration and cartilage regeneration with superior mechanical property in rabbits with osteochondral defect when compared to pure hydrogels only, the suggestion of providing a promising method for cartilage repair although optimal conditions should be further examined for the cartilage regeneration using injectable hydrogels.
Fig. 11.
Illustration of procedures to synthesize AMSA/AG hydrogels. A Preparation of AMSA. B Modification of AG. C Formation of injectable double-crosslinked hydrogels. (Adapted from Fan et al. Mat Sci Eng C-Mater. 2020;110:110,705, with permission from Elsevier [73])
Rahmani-Moghadam et al. prepared HY NPs through the fabrication of casting into PU foam and sintering at 800 and 1250 °C, and then the obtained HY NPs were impregnated into the thymoquinone (TQ)-loaded AL hydrogels prepared by adding CaCl2 for bone tissue engineering [76] based on the idea that the TQ accelerates bone formation by inducing bone morphogenic protein 2 (BMP-2) through ERK signaling pathway [77]. The results indicated that the impregnation of HY NPs into the TQ-loaded AL hydrogels showed significantly accelerated the differentiation of the ADMSCs into the osteoblasts with the significant increase of collagen, osteopontin, osteocalcin expression, and alkaline phosphatase activity in vitro with decelerated degradation rate and reinforcement of mechanical strength, the suggestion of a synergic effect on the differentiation of MSC due to the HY and TQ although they neither perform in vivo study and nor mention why PU foam was used in the preparation of HY NPs.
HA-contained PUs
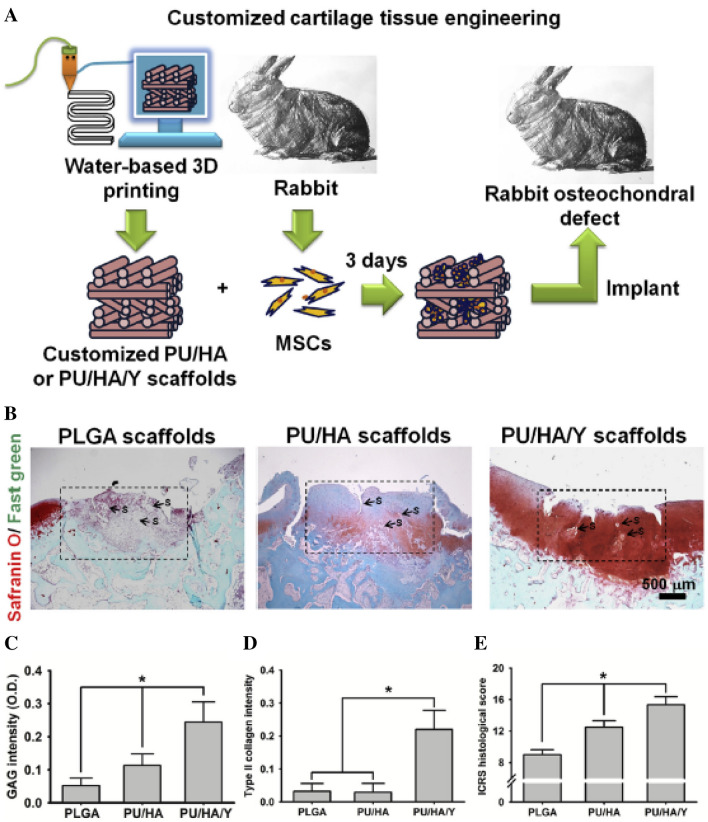
Hung et al. developed water-based PU 3D printed scaffolds consisting of a water dispersion of PU elastic NPs, HA, and TGFβ3 by the 3D printing technique for cartilage tissue engineering [78] with an idea that the aqueous dispersion of PU elastomer as a 3D printing material mimics cartilage tissue without using toxic organic solvents, HA promotes cartilage repair, and TGFβ3 induces chondrogenic differentiation although it is very expensive and causes the hypertrophy due to quick release from the scaffolds [79]. The results indicated that the compliant scaffolds printed from the ink at low temperature promoted the self-aggregation of MSCs, induced the chondrogenic differentiation of MSCs, and produced ECM for cartilage repair with the timely release of TGFβ3. Also, the cartilage regeneration was much improved by transplantation of the MSC-seeded PU/HA/Y27632 (a small molecule drug) scaffold in rabbit chondral defects than MSC-seeded PU/HA or PLGA scaffolds with significantly high expression of type II collagen as shown in Fig. 12 because Y27632 increases the differentiation of chondroprogenitors [80], suggestion of potential 3D printing composite scaffolds with controlled release of bioactive ingredients for customized cartilage tissue engineering.
Fig. 12.
Histological examination of regenerated cartilage. A Experimental protocol for regenerating rabbit cartilage defect. Customized scaffolds may be fabricated from the aqueous PU/HA/Y or PU/HA feed by 3D printing. Rabbit MSCs were then seeded in the scaffolds and cultured for 3 days before implantation into the osteochondral defect of rabbit knee joints. PLGA scaffolds used for comparison were made from 1,4-dioxane solution by a similar 3D printing procedure. B Histological images based on safranin O/fast green stained sections from the regenerated cartilage in defects implanted with the constructs after one month. The box indicates the repair region. S: scaffold debris. C The intensities of GAG stain (safranin O stain) and D type II collagen (immunofluorescence) quantified by image analysis. E The ICRS histological scores for cartilage evaluation based on the sections. *p < 0.05 among the indicated groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.). (Adapted from Hung et al. Biometerials 2016;83:156–168, with permission from Elsevier [78])
Similarly, Shie et al. developed water-based 3D printed- and light-cured scaffolds consisting of a mixture of water-based PU and water-based thermoplastic PU (TPU), hydroxyethyl methacrylate (HEMA), photoinitiator for light curing, and HA by the 3D printing technique for the cartilage tissue engineering application [81] based on the idea that the TPU allows biodegradation due to the aliphatic polyesters in the TPU, light-sensitive materials allow water-based 3D printing, and the HA promoted cartilage repair [81]. According to the results, the 3D printed scaffolds had a high cytocompatibility with mimicking the mechanical properties of articular cartilages and showed excellent chondrogenic differentiation capacity when human Wharton’s jelly mesenchymal stem cells (WJMSCs) were seeded during the 3D printing process, the suggestion of promising potential in the customized cartilage tissue engineering although they did not perform in vivo study.
Monaco et al. studied whether HA-supplemented culture medium has an ability of chondrogenic differentiation of human bone marrow stem cells (hBMSCs) using the fibrin-PU scaffolds for in vitro/ex vivo screening of potential cartilage repair therapies and making better predictions for in vivo outcomes because the HA plays a critical role on the protection of opposing articular cartilage sites in the synovial joint [82]. The results indicated that the addition of HA with TGFβ to the culture media enhanced the production of sulfated glycosaminoglycans at the early stage of chondrogenesis, and decreased the upregulation of collagen X as the hypertrophic cartilage marker while HA was added inside the fibrin gel led to the best matrix deposition, suggesting HA as the one key medium component to reduce in vitro artifacts although in vivo studies are required for accurate screening of potential cartilage repair therapies.
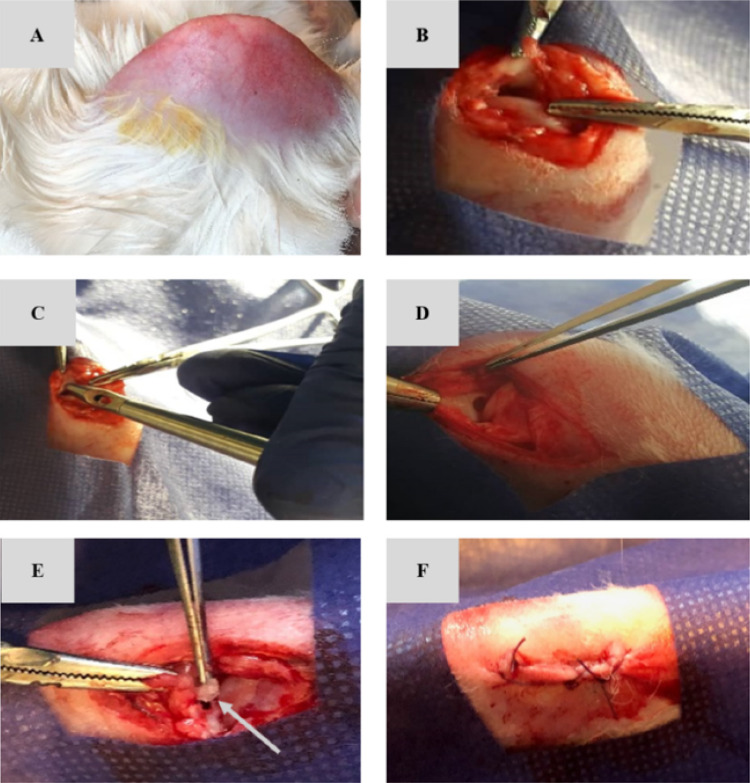
Recently, Abpeikas et al. prepared hybrid scaffolds consisting of PCL/PU surface modified by GE, CS, and HA with adding piroxicam-loaded GE nanofibers and crosslinking the surface of hybrid scaffolds for meniscus cartilage repair [83] since GE, CS, and HA as the biocompatible and biodegradable biopolymers enhance cellular behaviors such as cell adhesion, proliferation, and differentiation [84], and piroxicam as the non-steroidal drug reduces joint inflammation and pain [85]. The results indicated that the hybrid scaffolds exhibited excellent macroporous structure, strong mechanical properties with tensile and compressive Young’s modulus being near to human native meniscus tissue, and functional characteristics for meniscus regeneration with providing a suitable condition for retention, proliferation, and ECM deposition with the high expression of chondrocyte marker genes after seeding of rabbit AMSCs in the scaffolds in vitro. Also, the AMSCs-seeded and drug-loaded hybrid scaffolds exhibited a significant regeneration in rabbits with a partial meniscus injury after 3 months as shown in Fig. 13, the suggestion of promising potential for meniscus tissue regeneration in the clinical trials although there are several biological biopolymers with the anti-inflammation drug which should be optimized before clinical trials.
Fig. 13.
Surgical process steps, including shaving (A), opening the medial knee joint compartment and exposing the medial meniscus (B), creating partial meniscectomy with 2 mm diameter using a biopsy punch (C and D), implantation of the scaffold at the lesion site (E) and suturing the surgical site (F). Arrow shows the scaffold. (Adapted from Abpeikar et al. Int J Biol Macromol. 2021;183:1327–45, with permission from Elsevier [83])
CE-contained PUs
Liu et al. prepared HY/PU scaffold incorporated with ceftazidime (CEF)-loaded ethyl CE microparticles using a two-step in situ polymerization by the reaction of PEG and MDI with adding 1,4-butanediol as the chain extender for the bone regeneration [86] with a hypothesis that the high local antibiotic drug of CEF can prevent bacterial infections during prosthesis implantation [87]. Collectively, HY/PU composite scaffold had an interconnected pore network with an average porosity of 83% and the uniform distribution of the CEF-loaded ethyl CE microparticles in the scaffold when observed by SEM. Also, the composite scaffold showed good cytocompatibility in MG63 cells and anti-bacterial properties with sustained release of CEF as the antibacterial drug for up to 60 days without changing the pore structure of the scaffold and with reduction of the initial burst release by incorporation of microparticles into scaffolds, the suggestion of a promising composite scaffold for controlled drug delivery in bone regeneration although they did not study in vivo.
Cochis et al. studied the possibility of thermo-reversible methyl CE-derived hydrogel formulation as a 3D injectable scaffold for bioreactor-guided BMSC chondrogenesis in combination with a PU scaffold for cartilage repair[88] based on the idea that the mechanical loading of BMSC can differentiate into chondrocytes without the use of exogenous factors. According to the results, PU/methyl CE composite scaffolds showed a solution-gelation transition between 34 and 37 °C with a low bulk degradation after 1 month. And the composite scaffolds seeded with BMSC led to a significant enhancement of chondrogenic gene expression and, the production of sulfated glycosaminoglycans and collagen II due to the mechanical stimulation of 3D bioreactor by the combination of shear forces and compression for 21 days in vitro, suggesting an effective technique for the BMSC chondrogenesis by the mechanical stimulation although they did not check in vivo.
Summary and future perspectives
PUs have been attracted for the applications in the biomedical sector, such as bone fixation [1], coating of implanted materials [2], artificial heart [3], heart valves, and aortic grafts [4] due to the mechanical and thermal flexibility [5] coming from (1) soft segments responsible for the elastomeric behavior and (2) hard ones responsible for the thermosetting one [4], and (3) tailorable foams produced during the polycondensation reaction although the aromatic isocyanate toxicity has been concerned as a major limitation in biomaterials. Current biomedical applications of PUs produced by usage of aliphatic isocyanates are catheters, blood oxygenators, stents, cardiac valves, dialysis devices, wound dressings, adhesives, drug delivery devices, tissue engineering, pacemakers, and coating for implanted materials [8] although bioacceptability, biodegradability, regulation of cellular behaviors, controlled release of bioactive agents, and reconstruction of the proper organ to achieve the desired properties should be further considered in depth [4]. Also, the development of biobased PUs has raised attraction and public awareness during the last decades for concerns about plastic wastes worldwide although the issue of sustainability can be partially overcome and biodegradable PUs have been widely developed [6]. Therefore, we discussed PS-contained PUs for wound dressings and bone tissue engineering among bio-based PUs as the PS has several advantages, including reproducibility from the natural resources, biocompatibility, biodegradability, easy incorporation of bioactive agents, ease of availability with the cost-effectiveness, and structural characteristics of chemical modification to meet the desired needs [9]. Among the PS, CS, AL, HA, and CE were selected in the present article for the following reasons. The CS has been widely used for biomedical applications for its excellent biological properties such as nontoxicity, biocompatibility, biodegradability, anti-bacterial and anti-fungistatic properties [32] including adjustable physicochemical properties owing to the three kinds of reactive groups. The AL has been applied for biomedical applications because the AL is non-toxic, biodegradable, and biocompatible as well as easy processing into gel, film, foam, water, nanofiber and gauze [42]. The HA has been widely applied for its capacity to enhance cell migration, angiogenesis and decrease inflammation with high water absorption and flexibility [50]. The CE has been widely used for its characteristics to increase hydrophilicity and biodegradability of synthetic hydrophobic polymers [56].
The PUs have been widely used for the wound dressing applications due to the versatile mechanical properties, process-ability for the tailorable foams and elastomeric properties although moist wound environment, absorption of excess exudates at the wound site, antibacterial properties, non-toxicity without inflammatory response, biodegradability, incorporation of bioactive ingredients, and the capacity to create more sustainable materials should be advanced. To overcome the aforementioned limitations of the PUs, the PS-contained PUs were used for the wound dressing applications.
The PU scaffolds have been attracted to bone tissue engineering because they have biocompatibility and injectability although bone graft substitutes should be biodegradable according to the used bone organ, osteoconductive, osteoinductive with minimal fibrotic reaction, having antibacterial properties, and supporting a new bone formation [60]. To overcome the aforementioned limitations of the PUs, the PS-contained PUs are used for bone tissue engineering.
The limitation and challenge at the moment in this area are how to produce multi-functional wound dressings for simultaneously having several therapeutic properties including mechanical strength, cell adhesion, antibacterial property, and a moist wound environment using PS-contained PUs although the advancement of tissue engineering 3D bioprinting and organoid technologies can aid such limitation. Most of all, the development of novel PS-contained PUs to mimic the skin environment and structure would be essential and very critical for the clinical applications of various wound types. And also, bioactive agents-loaded dressings should be considered for specific diseases such as traumatic wounds, defects after oncologic resection, burn reconstruction, scar contracture release, congenital skin deficiencies, hair restoration, and diabetic injuries.
The multi-functional scaffolds including injectability, hydrogel, micro-/nano-particles to deliver cells, and growth factors for minimal surgical intervention are important for bone regeneration using PS-contained PUs because traditional tissue-engineered scaffolds must overcome painful procedures and longer healing times. Also, the biodegradability of the scaffolds is critical for harmony with bone regeneration although the selection of appropriate biodegradable scaffolds is dependent on the particular site of application.
This review discusses the recent status of PS-contained PUs in wound dressing and bone tissue engineering applications with current challenges and perspectives. We expect that this review will be helpful for the scientists in the field of PS-contained PUs-related approaches.
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R111A1A01053275).
Declarations
Conflict of interest
The authors have no financial conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chong-Su Cho, Email: chocs@snu.ac.kr.
Hyun-Joong Kim, Email: hjokim@snu.ac.kr.
References
- 1.Mandarino MP, Salvatore JE. Polyurethane polymer—its use in fractured and diseased bones. Am J Surg. 1959;97:442–446. doi: 10.1016/0002-9610(59)90011-x. [DOI] [PubMed] [Google Scholar]
- 2.Boretos JW, Pierce WS. Segmented polyurethane—a new elastomer for biomedical applications. Science. 1967;158:1481–1482. doi: 10.1126/science.158.3807.1481. [DOI] [PubMed] [Google Scholar]
- 3.Kolff WJ, Akutsu T, Norton H. Artificial heart in the chest and use of polyurethane for making hearts, valves, and aortas. Circulation. 1959;20:722–822. [Google Scholar]
- 4.LsIc C, Melo JA. Polyurethane: properties, structure and applications. New York: Nova Science Publishers; 2012. [Google Scholar]
- 5.Rusu LC, Ardelean LC, Jitariu AA, Miu CA, Streian CG. An insight into the structural diversity and clinical applicability of polyurethanes in biomedicine. Polymers (Basel) 2020;12:1197. doi: 10.3390/polym12051197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendels S, Averous L. Biobased polyurethanes for biomedical applications. Bioact Mater. 2021;6:1083–1106. doi: 10.1016/j.bioactmat.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akindoyo JO, Beg MDH, Ghazali S, Islam MR, Jeyaratnam N, Yuvaraj AR. Polyurethane types, synthesis and applications—a review. Rsc Adv. 2016;6:114453–114482. [Google Scholar]
- 8.Venkateshaiah A, Padil VVT, Nagalakshmaiah M, Waclawek S, Cernik M, Varma RS. Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers-Basel. 2020;12:512. doi: 10.3390/polym12030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solanki A, Das M, Thakore S. A review on carbohydrate embedded polyurethanes: An emerging area in the scope of biomedical applications. Carbohydr Polym. 2018;181:1003–1016. doi: 10.1016/j.carbpol.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Chen Z, Yang X. State of the art of small-diameter vessel-polyurethane substitutes. Macromol Biosci. 2019;19:e1800482. doi: 10.1002/mabi.201800482. [DOI] [PubMed] [Google Scholar]
- 11.Bellis M. The history of polyurethane. Otto Bayer. ThoughCo. 2020.
- 12.Cooper SL, Guan J. Advances in polyurethane biomaterials. Elsevier, Duxford: Woodhead Publishing; 2016. [Google Scholar]
- 13.Bonart R, Müller EH. Phase separation in urethane elastomers as judged by low-angle X-ray-scattering. II. Experimental results. J Macromol Sci Phys. 1974;10:345–57.
- 14.Cohn D, Stern T, Gonzalez MF, Epstein J. Biodegradable poly(ethylene oxide)/poly(epsilon-caprolactone) multiblock copolymers. J Biomed Mater Res. 2002;59:273–281. doi: 10.1002/jbm.1242. [DOI] [PubMed] [Google Scholar]
- 15.D'Arlas BF, Rueda L, De la Caba K, Mondragon I, Eceiza A. Microdomain composition and properties differences of biodegradable polyurethanes based on MDI and HDI. Polym Eng Sci. 2008;48:519–529. [Google Scholar]
- 16.Zuber M, Zia F, Zia KM, Tabasum S, Salman M, Sultan N. Collagen based polyurethanes—a review of recent advances and perspective. Int J Biol Macromol. 2015;80:366–374. doi: 10.1016/j.ijbiomac.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Wendels S, Averous L. Biobased polyurethanes for biomedical applications. Bioactive Materials. 2021;6:1083–1106. doi: 10.1016/j.bioactmat.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alven S, Peter S, Mbese Z, Aderibig BA. Polymer-based wound dressing materials loaded with bioactive agents. potential materials for the treatment of diabetic wounds. Polymers (Basel). 2022;14:724. [DOI] [PMC free article] [PubMed]
- 19.Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. [DOI] [PMC free article] [PubMed]
- 20.Suarato G, Bertorelli R, Athanassiou A. Borrowing from nature: biopolymers and biocomposites as smart wound care materials. Front Bioeng Biotech. 2018;6:137. [DOI] [PMC free article] [PubMed]
- 21.Parsons D, Bowler PG, Myles V, Jones S. Silver antimicrobial dressings in wound management: A comparison of antibacterial, physical, and chemical characteristics. Wounds. 2005;17:222–232. [Google Scholar]
- 22.Mayet N, Choonara YE, Kumar P, Tomar LK, Tyagi C, Du Toit LC, et al. A comprehensive review of advanced biopolymeric wound healing systems. J Pharm Sci-Us. 2014;103:2211–2230. doi: 10.1002/jps.24068. [DOI] [PubMed] [Google Scholar]
- 23.Kalliainen LKGG, Schlanger R, Sen CK. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology. 2003;2:81–87. doi: 10.1016/s0928-4680(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd AW. Interfacial bioengineering to enhance surface biocompatibility. Med Device Technol. 2002;13:18–21. [PubMed] [Google Scholar]
- 25.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–2349. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 26.Muzzarelli RAA. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohyd Polym. 2009;76:167–182. [Google Scholar]
- 27.Kumar PTS, Abhilash S, Manzoor K, Nair SV, Tamura H, Jayakumar R. Preparation and characterization of novel beta-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohyd Polym. 2010;80:761–767. [Google Scholar]
- 28.Dev A, Binulal NS, Anitha A, Nair SV, Furuike T, Tamura H, et al. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohyd Polym. 2010;80:833–838. [Google Scholar]
- 29.Yi HM, Wu LQ, Bentley WE, Ghodssi R, Rubloff GW, Culver JN, et al. Biofabrication with chitosan. Biomacromol. 2005;6:2881–2894. doi: 10.1021/bm050410l. [DOI] [PubMed] [Google Scholar]
- 30.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–576. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krajewska B. Membrane-based processes performed with use of chitin/chitosan materials. Sep Purif Technol. 2005;41:305–312. [Google Scholar]
- 32.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Tan L, Hu JL, Huang HH, Han JP, Hu HW. Study of multi-functional electrospun composite nanofibrous mats for smart wound healing. Int J Biol Macromol. 2015;79:469–476. doi: 10.1016/j.ijbiomac.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Wang XG, Wu P, Hu XY, You CG, Guo R, Shi HF, et al. Polyurethane membrane/knitted mesh-reinforced collagen-chitosan bilayer dermal substitute for the repair of full-thickness skin defects via a two-step procedure. J Mech Behav Biomed. 2016;56:120–133. doi: 10.1016/j.jmbbm.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Klempaiova M, Dragunova J, Kabat P, Hnatova M, Koller J, Bakos D. Cytotoxicity testing of a polyurethane nanofiber membrane modified with chitosan/beta-cyclodextrin/berberine suitable for wound dressing application: evaluation of biocompatibility. Cell Tissue Bank. 2016;17:665–675. doi: 10.1007/s10561-016-9585-2. [DOI] [PubMed] [Google Scholar]
- 36.Bankoti K, Rameshbabu AP, Datta S, Maity PP, Goswami P, Datta P, et al. Accelerated healing of full thickness dermal wounds by macroporous waterborne polyurethane-chitosan hydrogel scaffolds. Mat Sci Eng C-Mater. 2017;81:133–143. doi: 10.1016/j.msec.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Jafari A, Hassanajili S, Karimi MB, Emami A, Ghaffari F, Azarpira N. Effect of organic/inorganic nanoparticles on performance of polyurethane nanocomposites for potential wound dressing applications. J Mech Behav Biomed. 2018;88:395–405. doi: 10.1016/j.jmbbm.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Uscategui YL, Diaz LE, Gomez-Tejedor JA, Valles-Lluch A, Vilarino-Feltrer G, Serrano MA, et al. Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications. Molecules. 2019;24:237. doi: 10.3390/molecules24020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najafabadi SAA, Mohammadi A, Kharazi AZ. Polyurethane nanocomposite impregnated with chitosan-modified graphene oxide as a potential antibacterial wound dressing. Mat Sci Eng C-Mater. 2020;115:110899. doi: 10.1016/j.msec.2020.110899. [DOI] [PubMed] [Google Scholar]
- 40.Yang JM, Huang YF, Dai JJ, Shi XA and Zheng YQ. A sandwich structure composite wound dressing with firmly anchored silver nanoparticles for severe burn wound healing in a porcine model. Regen Biomater. 2021;8:rbab037. [DOI] [PMC free article] [PubMed]
- 41.Zhang M, Yang M, Woo MW, Li YC, Han WJ, Dang XG. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohyd Polym. 2021;256:117590. doi: 10.1016/j.carbpol.2020.117590. [DOI] [PubMed] [Google Scholar]
- 42.Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: A mini review. Carbohyd Polym. 2020;236:116025. doi: 10.1016/j.carbpol.2020.116025. [DOI] [PubMed] [Google Scholar]
- 43.Torres MR, Sousa APA, Filho EATS, Dirce FMBB, Feitosa JPA, de Paula RCM, et al. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohyd Res. 2007;342:2067–2074. doi: 10.1016/j.carres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Xie HX, Chen XL, Shen XR, He Y, Chen W, Luo Q, et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int J Biol Macromol. 2018;107:93–104. doi: 10.1016/j.ijbiomac.2017.08.142. [DOI] [PubMed] [Google Scholar]
- 45.Salekdeh SSH, Daemi H, Zare-Gachi M, Rajabi S, Bazgir F, Aghdami N, et al. Assessment of the efficacy of tributylammonium alginate surface-modified polyurethane as an antibacterial elastomeric wound dressing for both noninfected and infected full-thickness wounds. Acs Appl Mater Inter. 2020;12:3393–3406. doi: 10.1021/acsami.9b18437. [DOI] [PubMed] [Google Scholar]
- 46.Namuiriyachote N, Lipipun V, Althhatuattananglzul Y, Charoonrut P, Ritthidej GC. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J Pharm Sci. 2019;14:63–77. doi: 10.1016/j.ajps.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Namviriyachote N, Muangman P, Chinaroonchai K, Chuntrasakul C, Ritthidej GC. Polyurethane-biomacromolecule combined foam dressing containing asiaticoside: fabrication, characterization and clinical efficacy for traumatic dermal wound treatment. Int J Biol Macromol. 2020;143:510–520. doi: 10.1016/j.ijbiomac.2019.10.166. [DOI] [PubMed] [Google Scholar]
- 48.Lu WC, Chuang FS, Venkatesan M, Cho CJ, Chen PY, Tzeng YR, et al. Synthesis of water resistance and moisture-permeable nanofiber using sodium alginate-functionalized waterborne polyurethane. Polymers-Basel. 2020;12:2882. doi: 10.3390/polym12122882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claudio-Rizo JA, Escobedo-Estrada N, Carrillo-Cortes SL, Cabrera-Munguia DA, Flores-Guia TE, Becerra-Rodriguez JJ. Highly absorbent hydrogels comprised from interpenetrated networks of alginate-polyurethane for biomedical applications. J Mater Sci-Mater M. 2021;32:70. doi: 10.1007/s10856-021-06544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qu J, Zhao X, Liang Y, Xu Y, Ma PX, Guo B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J. 2019;362:548–560. [Google Scholar]
- 51.Reyes-Ortega F, Cifuentes A, Rodriguez G, Aguilar MR, Gonzalez-Gomez A, Solis R, et al. Bioactive bilayered dressing for compromised epidermal tissue regeneration with sequential activity of complementary agents. Acta Biomater. 2015;23:103–115. doi: 10.1016/j.actbio.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Movahedi M, Asefnejad A, Rafienia M, Khorasani MT. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int J Biol Macromol. 2020;146:627–637. doi: 10.1016/j.ijbiomac.2019.11.233. [DOI] [PubMed] [Google Scholar]
- 54.Eskandarinia A, Kefayat A, Gharakhloo M, Agheb M, Khodabakhshi D, Khorshidi M, et al. A propolis enriched polyurethane-hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int J Biol Macromol. 2020;149:467–476. doi: 10.1016/j.ijbiomac.2020.01.255. [DOI] [PubMed] [Google Scholar]
- 55.Khodabakhshi D, Eskandarinia A, Kefayat A, Rafienia M, Navid S, Karbasi S, et al. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloid Surface B. 2019;178:177–184. doi: 10.1016/j.colsurfb.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Almasian A, Najafi F, Eftekhari M, Ardekani MRS, Sharifzadeh M, Khanavi M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mat Sci Eng C-Mater. 2020;114:111039. doi: 10.1016/j.msec.2020.111039. [DOI] [PubMed] [Google Scholar]
- 57.Unnithan AR, Gnanasekaran G, Sathishkumar Y, Lee YS, Kim CS. Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing. Carbohyd Polym. 2014;102:884–892. doi: 10.1016/j.carbpol.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 58.Ko SW, Lee JY, Lee J, Son BC, Jang SR, Aguilar LE, et al. Analysis of Drug Release Behavior Utilizing the Swelling Characteristics of Cellulosic Nanofibers. Polymers-Basel. 2019;11:1376. doi: 10.3390/polym11091376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szczepańczyk P, Szlachta M, Zlocista-Szewczyk N, Chlopek J, Pielichowska K. Recent developments in polyurethane-based materials for bone tissue engineering. Polymers (Basel). 2021;13:946. [DOI] [PMC free article] [PubMed]
- 60.Laurencin CT, Nair LS. Nanotechnology and tissue engineering: the scaffold. Boca Raton: CRC Press; 2008. [Google Scholar]
- 61.Moradi L, Vasei M, Dehghan MM, Majidi M, Mohajeri SF, Bonakdar S. Regeneration of meniscus tissue using adipose mesenchymal stem cells-chondrocytes co-culture on a hybrid scaffold: In vivo study. Biomaterials. 2017;126:18–30. doi: 10.1016/j.biomaterials.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 62.Yu QA, Song YN, Shi XM, Xu CY, Bin YZ. Preparation and properties of chitosan derivative/poly(vinyl alcohol) blend film crosslinked with glutaraldehyde. Carbohyd Polym. 2011;84:465–470. [Google Scholar]
- 63.Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohyd Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 64.Rongen JJ, van Tienen TG, van Bochove B, Grijpma DW, Buma P. Biomaterials in search of a meniscus substitute. Biomaterials. 2014;35:3527–3540. doi: 10.1016/j.biomaterials.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Topsakal A, Uzun M, Ugar G, Ozcan A, Altun E, Oktar FN, et al. Development of amoxicillin-loaded electrospun polyurethane/chitosan/ beta-tricalcium phosphate scaffold for bone tissue regeneration. IEEE T Nanobiosci. 2018;17:321–328. doi: 10.1109/TNB.2018.2844870. [DOI] [PubMed] [Google Scholar]
- 66.Zo S, Choi S, Kim H, Shin E, Han S. Synthesis and characterization of carboxymethyl chitosan scaffolds grafted with waterborne polyurethane. J Nanosci Nanotechnol. 2020;20:5014–5018. doi: 10.1166/jnn.2020.17844. [DOI] [PubMed] [Google Scholar]
- 67.Zo SM, Choi SM, Han SS. Use of water-borne polyurethane as a crosslinker on gelatin three-dimensional constructs. Chem Sci J. 2018;9:81.
- 68.Shaabani A, Sedghi R. Preparation of chitosan biguanidine/PANI-containing self-healing semi-conductive waterborne scaffolds for bone tissue engineering. Carbohydr Polym. 2021;264:118045. doi: 10.1016/j.carbpol.2021.118045. [DOI] [PubMed] [Google Scholar]
- 69.Shalumon KT, Anulekha KH, Girish CM, Prasanth R, Nair SV, Jayakumar R. Single step electrospinning of chitosan/poly(caprolactone) nanofibers using formic acid/acetone solvent mixture. Carbohyd Polym. 2010;80:413–419. [Google Scholar]
- 70.Shrestha S, Shrestha BK, Ko SW, Kandel R, Park CH, Kim CS. Engineered cellular microenvironments from functionalized multiwalled carbon nanotubes integrating Zein/Chitosan @Polyurethane for bone cell regeneration. Carbohydr Polym. 2021;251:117035. [DOI] [PubMed]
- 71.Zhang Y, Li WY, Lan R, Wang JY. Quality monitoring of porous Zein scaffolds: a novel biomaterial. Eng-Prc. 2017;3:130–135. [Google Scholar]
- 72.Hirata E, Uo M, Takita H, Akasaka T, Watari F, Yokoyama A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon. 2011;49:3284–3291. [Google Scholar]
- 73.Fan WS, Yuan L, Li JH, Wang Z, Chen JF, Gun CG, et al. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor beta 3 for in-situ cartilage regeneration. Mat Sci Eng C-Mater. 2020;110:110705. doi: 10.1016/j.msec.2020.110705. [DOI] [PubMed] [Google Scholar]
- 74.Johnson KA. A stem cell-based approach to cartilage repair. Osteoarthr Cartilage. 2013;21:S4–S4. [Google Scholar]
- 75.Deng MY, Mei TN, Hou TY, Luo KY, Luo F, Yang AJ, et al. TGF beta 3 recruits endogenous mesenchymal stem cells to initiate bone regeneration. Stem Cell Res Ther. 2017;8:258. doi: 10.1186/s13287-017-0693-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Rahmani-Moghadam E, Talaei-Khozani T, Zarrin V, Vojdani Z. Thymoquinone loading into hydroxyapatite/alginate scaffolds accelerated the osteogenic differentiation of the mesenchymal stem cells. Biomed Eng Online. 2021;20:76. doi: 10.1186/s12938-021-00916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thummuri D, Jeengar MK, Shrivastava S, Nemani H, Ramavat RN, Chaudhari P, et al. Thymoquinone prevents RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-KB and MAPK Signalling. Pharmacol Res. 2015;99:63–73. doi: 10.1016/j.phrs.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Hung KC, Tseng CS, Dai LG, Hsu SH. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials. 2016;83:156–168. doi: 10.1016/j.biomaterials.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Matsiko A, Levingstone TJ, O'Brien FJ. Advanced strategies for articular cartilage defect repair. Materials. 2013;6:637–668. doi: 10.3390/ma6020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chou HC, Huang LT, Yeh TF, Chen CM. Rho-kinase inhibitor Y-27632 attenuates pulmonary hypertension in hyperoxia-exposed newborn rats. Acta Pharmacol Sin. 2013;34:1310–1316. doi: 10.1038/aps.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shie MY, Chang WC, Wei LJ, Huang YH, Chen CH, Shih CT, et al. 3D Printing of cytocompatible water-based light-cured polyurethane with hyaluronic acid for cartilage tissue engineering applications. Materials. 2017;10:136. doi: 10.3390/ma10020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monaco G, El Haj AJ, Alini M, Stoddart MJ. Sodium hyaluronate supplemented culture media as a new hMSC chondrogenic differentiation media-model for in vitro/ex vivo screening of potential cartilage repair therapies. Front Bioeng Biotechnol. 2020;8:243. [DOI] [PMC free article] [PubMed]
- 83.Abpeikar Z, Javdani M, Mirzaei SA, Alizadeh A, Moradi L, Soleimannejad M, et al. Macroporous scaffold surface modified with biological macromolecules and piroxicam-loaded gelatin nanofibers toward meniscus cartilage repair. Int J Biol Macromol. 2021;183:1327–1345. doi: 10.1016/j.ijbiomac.2021.04.151. [DOI] [PubMed] [Google Scholar]
- 84.Asadpour S, Yeganeh H, Ai J, Ghanbari H. A novel polyurethane modified with biomacromolecules for small-diameter vascular graft applications. J Mater Sci. 2018;53:9913–9927. [Google Scholar]
- 85.Swanepoel E, Liebenberg W, de Villiers MM, Dekker TG. Dissolution properties of piroxicam powders and capsules as a function of particle size and the agglomeration of powders. Drug Dev Ind Pharm. 2000;26:1067–1076. doi: 10.1081/ddc-100100270. [DOI] [PubMed] [Google Scholar]
- 86.Liu HH, Zhang L, Shi PJ, Zou Q, Zuo Y, Li YB. Hydroxyapatite/polyurethane scaffold incorporated with drug-loaded ethyl cellulose microspheres for bone regeneration. J Biomed Mater Res B Appl Biomater. 2010;95:36–46. [DOI] [PubMed]
- 87.Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–2467. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 88.Cochis A, Grad S, Stoddart MJ, Fare S, Altomare L, Azzimonti B, et al. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Sci Rep-Uk. 2017;7:45018. doi: 10.1038/srep45018. [DOI] [PMC free article] [PubMed] [Google Scholar]