Abstract
Despite the poor prognosis of unresectable colorectal cancer (CRC), some patients survive after intensive chemotherapy followed by complete resection of the primary and metastatic tumors. The pretreatment C-reactive protein/albumin ratio (CAR) is a significant prognostic indicator in various carcinomas. Therefore, in this retrospective study, we evaluated the prognostic significance of pretreatment CAR in patients with unresectable CRC. The 61 patients were diagnosed as having initially unresectable disease between January 2004 and December 2013. We analyzed the clinical courses of these patients. Blood samples were taken routinely at their first visit to our hospital. C-reactive protein (CRP), albumin (ALB), and carcinoembryonic antigen (CEA) were analyzed. The median survival time (MST) and 2-year overall survival (OS) of the patients was 9 months (range, 1–96 months) and 23%, respectively. The median CRP, ALB, CAR, and CEA levels of the patients were 2.2 mg/dL, 3.5 g/dL, 0.65, and 20.6 ng/mL, respectively. There was no correlation between CEA levels and the CAR. Patients were divided into two sub-groups using the median CAR level as the cut-off: high CAR (>0.65) and low CAR (≤0.65). Both MST and 2-year OS were significantly lower in the 30 high-CAR patients (4 months, 6.7%) than in the 31 low-CAR patients (13 months, 38.7%, p < 0.001). The primary tumors of three low-CAR patients could be removed after intensive chemotherapy. Thus, low-CAR patients with locally advanced CRC with or without distant metastasis may survive following intensive treatment, even if their tumors were previously deemed to be unresectable.
Keywords: Chemotherapy, Colorectal cancer, Conversion therapy, Inflammation, Prognosis
Introduction
The current first-line and standard treatment of unresectable colorectal cancer (CRC) is 5-fluorouracil in combination with leucovorin and oxaliplatin (FOLFOX) or irinotecan (FOLFIRI), with or without molecularly targeted drugs. These and other anti-cancer agents have improved the prognosis of patients with many forms of cancer [1, 2] by allowing complete resection of both the primary tumor and its metastases after intensive chemotherapy. However, intensive chemotherapy induces severe adverse events, resulting in a significant reduction in the quality of life of treated patients. The identification of markers to identify patients likely to survive after intensive chemotherapy would spare non-treatable patients unnecessary suffering.
Two markers of the systemic inflammatory response, C-reactive protein (CRP) and albumin (ALB), have been used in combination to diagnose not only chronic inflammation but also the nutritional status of cancer patients. In addition, the pretreatment CRP/ALB ratio (CAR) was shown to be a significant prognostic indicator in various carcinomas [3–5]. Thus, in the present study, we evaluated the pretreatment CARs of consecutive patients with locally advanced CRC diagnosed as unresectable. Our aim was to determine whether pretreatment CAR predicted the response to chemotherapy and the long-term survival of these patients.
Methods
Patients
Between January 2004 and December 2013, 615 patients with primary CRC were treated at the Department of Surgery, Tottori University Hospital. The 61 patients with locally advanced CRC invading neighboring important organs, with or without synchronous distant metastasis, were diagnosed as having initially unresectable disease. In this study, we analyzed the clinical courses of these 61 patients. Local invasion by the tumor of the pelvic cavity, including the sacral bone, was confirmed in 19 patients. Other targets of tumor invasion included the liver, duodenum, and pancreas (11 patients), the uterus or bladder (18), and the kidney or ureter (13). The clinicopathological characteristics of the 61 patients are shown in Table 1. Obstruction was the most commonly detected symptom related to the primary tumor (obstruction, 30; bleeding or anemia, 5; obstruction and bleeding, 6; and local pain, 4). In cases of intestinal obstruction due to unresectable colorectal cancer, bypass operation or artificial anus construction is mainly performed. Especially, in unresectable cases with rectal cancer located at lower rectum, it is difficult to perform the bypass operation, so in such cases, we reconstructed artificial anus at oral site from obstruction. The 36 patients (59%) with obstruction caused by the primary tumor underwent bypass surgery (n = 8), artificial anus reconstruction (n = 26), or both (n = 2). Synchronous metastases were identified in 46 patients (75.4%), with liver metastasis detected most frequently. The remaining 15 patients had no distant metastases. Initial chemotherapy was performed in 40 patients (65.6%, uracil-tegafur and oral leucovorin, n = 6; FOLFOX, n = 32; and FOLFIRI, n = 2). Seven patients with advanced age or poor performance status received best supportive care. The 61 patients were followed until December 2015; the median follow-up time was 9 months. Informed consent concerning medical treatment and use of the clinical data from the medical records was obtained from all patients.
Table 1.
Clinicopathological characteristics of the 61 study patients
| Age (mean, median, and range) | 67.8, 68, 35–94 |
| Sex (male/female) | 35/26 |
| Eastern Cooperative Oncology Group Performance Status, 0/1/2/3 | 33/13/12/3 |
| Tumor location (colon/rectum) | 32/29 |
| Primary tumor-related obstruction (yes/no) | 36/25 |
| Distant metastasis | |
| Liver only | 20 |
| Liver and lung | 10 |
| Liver and peritoneum | 5 |
| Extended lymph node only | 4 |
| Peritoneum only | 4 |
| Lung only | 1 |
| Bone only | 1 |
| Lung and bone | 1 |
Blood Samples
Blood samples were taken from the patients routinely at their first visit to our hospital. CRP, serum ALB, and carcinoembryonic antigen (CEA) levels were measured for each patient. Levels of CEA and CRP were determined by standard electrochemiluminescence assay method by means of Roche (CEA Kit No. 11731629322 and CRP Kit No. 11761428622).
Statistical Analysis
Differences between two parameters were compared using χ2 tests for independence, Fisher’s exact probability test, and the Mann-Whitney U test. Spearman’s rank correlation coefficient was used to assess the correlation between two parameters. Survival rates were estimated by the Kaplan-Meier method. The significance of differences between survival curves was determined using log-rank tests. A p value <0.05 was considered to indicate statistical significance.
Results
The median survival time (MST) and 2-year overall survival (OS) of the 61 patients was 9 months (range, 1–96 months) and 23%, respectively. The patients could be divided into four groups according to their mode of treatment. In group 1, 24 patients were treated with surgical intervention plus chemotherapy (21 patients with surgical intervention followed by chemotherapy, 3 patients with chemotherapy followed by surgical intervention); in group 2, 16 patients were treated chemotherapy alone; in group 3, 13 patients were treated only with surgical intervention; in group 4, 8 patients received best supportive care (Table 2). Thus, surgical intervention was performed in 37 patients (artificial anus reconstruction, 27 patients; bypass operation, 8; and artificial anus reconstruction plus bypass operation, 2) and chemotherapy in 40 patients. Chemotherapy was ceased after surgical intervention in 13 patients because of poor performance status or rapid tumor growth after surgery. MST and 2-year OS were significantly longer in the 40 patients who underwent chemotherapy than in the remaining 21 patients (10 months and 32.5% vs. 3 months and 4.8%, p = 0.0002). Primary tumors could be removed in 3 of the 61 patients after intensive chemotherapy. One patient underwent complete resection of both the distant metastatic sites (lung and liver) and the primary tumor after bypass surgery followed by intensive chemotherapy. This patient was alive 96 months after the initial visit to our hospital, without recurrence or additional treatment. In another 2 patients, one died at 35 months as a result of progression of liver metastasis, and the other was alive at 33 months with chemotherapy. Thus, chemotherapy was key to the favorable prognosis of patients with unresectable CRC.
Table 2.
Treatment strategy and patient survival
| Number | MST (months) | 2-year OS (%) | |
|---|---|---|---|
| Surgical intervention plus chemotherapy | 24 | 9 | 20.8 |
| Chemotherapy only | 16 | 15 | 43.8 |
| Surgical intervention only | 13 | 5 | 7.7 |
| Best supportive care | 8 | 2 | 0 |
MST median survival time, OS overall survival
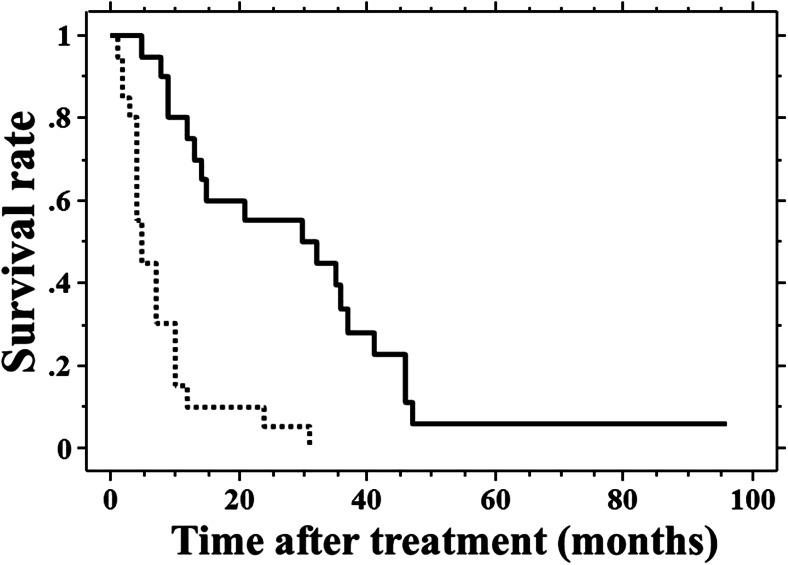
The pretreatment serum CRP and ALB levels were used to select those patients likely to survive after intensive treatment. The mean pretreatment serum CRP, ALB, and CEA levels of the 61 patients were as follows: 3.2 (median, 2.2; range, 0.01–13.9) mg/dL, 3.4 (median, 3.5; range, 1.8–4.7) g/dL, and 567 (median, 20.6; range, 1.2–20,157) ng/mL, respectively. The mean CAR of these patients was 1.1 (median, 0.65; range, 0.01–6.94). There was no correlation between CEA and CAR (ρ = −0.024, p = 0.855). The 61 patients were divided into two sub-groups according to the median CAR (high CAR >0.65 and low CAR ≤0.65). The median value of CAR (0.65) of the 61 patients was used as the cut-off. The MST and 2-year OS were significantly lower in the 30 high-CAR patients (4 months, 6.7%) than in the 31 low-CAR patients (13 months, 38.7%, p < 0.001). Similarly, the 61 patients were also divided into two sub-groups based on the serum CEA level (cut-off=10 ng/mL). The MST and 2-year OS did not differ between the 33 high-CEA patients (>10 ng/mL, 8 months, 27.3%) and the 28 low-CEA patients (≤10 ng/mL, 9 months, 14.3%, p = 0.525). The prognostic significance of the pretreatment CAR was investigated in the 40 patients who were treated with chemotherapy. Both MST and 2-year OS were significantly lower in the 20 high-CAR patients (5 months, 5%) than in the 20 low-CAR patients (30 months, 55%, p < 0.001, Fig. 1). These results indicate that the pretreatment CAR is a useful parameter for selecting patients with initially unresectable advanced CRC who have a greater likelihood of survival.
Fig. 1.
The survival curves of the 40 study patients who received chemotherapy. Survival was significantly lower in the 20 patients with a high C-reactive protein/albumin ratio (CAR; dotted line) than in the 20 patients with a low CAR (solid line, p < 0.001)
Discussion
In patients with unresectable CRC and distant metastasis, chemotherapy is essential to improving survival. However, many patients with unresectable CRC also suffer from tumor obstruction, bleeding, or local pain and should therefore undergo palliative surgery (bypass surgery or artificial anus reconstruction). Intensive treatment may allow complete resection of both the primary tumor and its metastatic foci. This so-called conversion therapy has been associated with a favorable long-term survival [6–8]. In the present study, one of the 61 (1.6%) study patients has survived for over 5 years after conversion therapy, without cancer recurrence. In two other patients, the primary tumor could be removed. Nonetheless, many patients recommended for chemotherapy will suffer from its severe side effects, and some may not be candidates for chemotherapy after palliative surgery because of poor postoperative performance status. Thus, the identification of prognostic factors would avoid unnecessary intensive treatment for patients with unresectable CRC.
Pretreatment serum CRP and ALB levels are indicative of inflammation and nutritional status. Both the Glasgow prognostic score (GPS) and the modified GPS (mGPS) include CRP and ALB as inflammatory and nutritional prognostic parameters in assessing various cancers [9–11]. Recently, Chen et al. [5] and Kinoshita et al. [12] reported that the CAR was better than the GPS or mGPS in predicting the prognosis of patients with renal cell or hepatocellular carcinoma. Thus, in this study, we examined the prognostic value of the CAR in patients with unresectable CRC. Our results showed that high vs. low CAR well represented the difference in survival of patients who received chemotherapy. Among chemotherapy treated patients with a high CAR, the MST (5 months) was almost the same as that of patients who did not undergo chemotherapy (3 months). These findings indicate that, among patients with unresectable CRC, those with a low preoperative CAR may be candidates for conversion therapy after intensive chemotherapy, and some of them will have greatly improved survival. Conversely, among patients with unresectable, locally advanced CRC with distant metastasis and a high CAR, an alternative treatment strategy should be considered to ensure a good quality of life rather than compromising it to prolong survival.
We also found that high serum CEA levels did not reflect the survival of patients with locally advanced CRC. Thus, although the serum CEA level is a good marker of tumor progression, it may not be a reliable indicator of chemosensitivity.
Conclusion
Patients with locally advanced CRC and distant metastasis who have a low CAR may benefit from intensive treatment, in terms of survival, even if prior to treatment their disease was considered to be unresectable.
References
- 1.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 3.Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006;12:3746–3750. doi: 10.3748/wjg.v12.i23.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, Bae WK, Hwang EC, Cho SH, Chung IJ. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011;11:489. doi: 10.1186/1471-2407-11-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Shao Y, Fan M, Zhuang Q, Wang K, Cao W, Xu X, He X. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:14893–14900. [PMC free article] [PubMed] [Google Scholar]
- 6.Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532–535. doi: 10.1007/s00595-011-0061-0. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Shibutani M, Otani H, Nagahara H, Sugano K, Ikeya T, Kubo N, Amano R, Kimura K, Muguruma K, Tanaka H, Hirakawa K. Low nutritional prognostic index correlates with poor survival in patients with stage IV colorectal cancer following palliative resection of the primary tumor. World J Surg. 2014;38:1217–1222. doi: 10.1007/s00268-013-2386-x. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CW, King TM, Wang HT, Wang JH. Factors that influence survival in unresectable metastatic or locally advanced colorectal cancer. Int J Color Dis. 2011;26:1559–1566. doi: 10.1007/s00384-011-1231-7. [DOI] [PubMed] [Google Scholar]
- 9.Kishiki T, Masaki T, Matsuoka H, Kobayashi T, Suzuki Y, Abe N, Mori T, Sugiyama M. Modified Glasgow prognostic score in patients with incurable stage IV colorectal cancer. Am J Surg. 2013;206:234–240. doi: 10.1016/j.amjsurg.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 10.La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The Glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 11.Hirashima K, Watanabe M, Shigaki H, Imamura Y, Ida S, Iwatsuki M, Ishimoto T, Iwagami S, Baba Y, Baba H. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49:1040–1046. doi: 10.1007/s00535-013-0855-5. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita A, Onoda H, Imai A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]