Abstract
Renal cell carcinoma (RCC) is the most lethal urological cancer. It is estimated that one thirds of the patients with localized cancer will develop distant metastasis after radical treatment. Adrenal metastasis of RCC are relatively rare and can be either synchronous or metachronous; ipsilateral, contralateral or bilateral; solitary or part of a massive metastatic spread. Contralateral adrenal metastasis are uncommon. It is well-known that some patients with isolated metastasis may benefit from surgical treatment. However, the optimal diagnosis and treatment of the contralateral adrenal metastasis from RCC has not yet been well defined. Since it was first described, laparoscopic adrenalectomy has become the gold standard for the surgical treatment of most adrenal conditions. The benefits of a minimally invasive approach to adrenal resection such as decreased hospital stay, shorter recovery time, and improved patient satisfaction are widely accepted. We report our experience with laparoscopic management of contralateral, metachronous adrenal metastases from RCC. Patients undergoing radical/partial nephrectomy for RCC were prospectively followed and evaluated regularly for general health status, local recurrence of tumor, and distant metastases. Patients identified to have had adrenal lesion/mass during the follow-up period were evaluated in detail both with imaging as well as endocrinal evaluation for assessment of functional status of these lesions. All these patients underwent laparoscopic adrenalectomy under general anesthesia. During the study period Jan 2006–Dec 2015, 8 patients (7 male and 1 female) with a mean age of 57.8 years underwent laparoscopic adrenalectomy. The mean operating time was 111.2 ± 32.5 min, blood loss was 45 ± 8.6 cm3 and postoperative stay was 37.5 ± 9.3 h. None of the patients had any major complications both early and delayed. The overall survival was 44.62 months. Metachronous, solitary, and contralateral adrenal metastasis from RCC is an extremely rare clinical complication that can occur very late after the radical/partial nephrectomy. Increased use of imaging modalities has led to more efficient and early detection of these lesions. Aggressive surgery remains the treatment of choice in these cases. Laparoscopic adrenalectomy remains a good, safe option with minimal morbidity and short hospital stay.
Keywords: Adrenalectomy, Adrenal gland, Laparoscopy, Surgical technique, Metastases, Renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) is known to metastasize via venous and lymphatic routes to almost any organ, including the lungs, liver, kidneys, bones, and brain. Metastases to the adrenals from RCC are uncommon, with autopsy studies showing an incidence of 6 to 23% [1, 2]. In patients undergoing radical nephrectomy, the incidence of solitary adrenal metastasis to the ipsilateral gland is 3% and contralateral gland is 0.7%, respectively [3]. Even with early stages of RCC, metastases can occur much later after complete resection. Development of metastasis is associated with poor prognosis, as non-operative modalities for advanced renal carcinoma have failed to improve survival significantly [4].
Surgical removal is the only known effective treatment in patients with solitary adrenal metastasis, with 29 to 35% of patients subsequently surviving 5 years or more [3]. Till about two decades back open surgical adrenalectomy remained the only known surgical procedure. Yuvaraja et al. [4] reported on 43 patients with solitary metastasis to different sites from renal cell carcinoma who underwent either surgical excision or radiotherapy between 1988 and 2001. All solitary metastatic lesions were treated with intent of cure either by open surgical excision or radiotherapy. Of these, 13 patients had solitary metastasis at the time of presentation in whom 3-year overall median survival was 26 months. The survival of those who developed solitary metastases during follow-up after nephrectomy for primary was 45 months. The patients with long interval between diagnosis and development of metastasis, early stage and low grade of the primary tumor had better prognosis. The authors concluded that complete resection of either synchronous or metachronous solitary metastases from renal cell carcinoma was justified and contributed to a long-term survival in this select group of patients.
Laparoscopic adrenalectomy for metastases though controversial could be performed with acceptable outcomes in carefully selected patients with small, organ-confined, solitary metastasis [5]. Therefore, aggressive treatment in the form of laparoscopic excision/metastatectomy in such lesions is thought to be effective. We report on our laparoscopic experience in the management of adrenal metastases from renal cell carcinoma.
Materials and Methods
Patients operated in our department for renal cell carcinoma formed the study group. All patients were prospectively followed up and evaluated regularly for general health status, local recurrence of tumor, and distant metastases. During the period Jan. 2006 and Dec. 2015, 8 patients (7 male and 1 female) presented with metachronous solitary metastases to the contralateral adrenal gland. The location and extent of the metastatic disease was evaluated by various diagnostic methods, which included chest radiograph, isotope bone scan, liver function tests, and serum alkaline phosphatase. All patients underwent ultrasound (USG) and/or computerized tomography (CT) of abdomen to rule out local recurrence and other metastases. Patients with adrenal lesion/mass also underwent a thorough endocrinal evaluation for assessment of functional adrenal lesions.
Surgical Technique
We have routinely used our own technique of laparoscopic adrenalectomy [6, 7] under general anesthesia and endotracheal intubation. The patient was rolled-up to a full-flank position on a beanbag with the affected adrenal side up. The shoulders were then dropped back ≈30° into a modified flank position. The upside arm was supported by an arm board; the legs were bent slightly and padded. The patient was secured to the table by wide adhesive tape over the hips and shoulders. In a modified flank position, the ports were placed in a subcostal arrangement. The initial access was identified ≈2 cm below the costal margin and lateral to the rectus abdominis border. An open (Hasson) entrance was made at this site and pneumoperitoneum created in a standard fashion.
On the left side, three or four ports were placed. The optional lateral port depended on the need for an additional retractor for the spleen or kidney. On the right side, four ports were always used because of the need for a liver retractor. Laparoscopic right adrenalectomy relied on the inferior vena cava (IVC) as the main landmark as the right adrenal vein joins the IVC in a consistent location. After initial inspection of the peritoneal cavity, the liver was lifted up with a fan retractor through the most medial port. The posterior peritoneum was incised below the liver edge and a lateral incision along the liver border was made for adequate exposure. This peritoneal incision was continued caudally along the white line of Toldt to mobilize the colon. The IVC was identified and exposed. The overlying tissue along the anterolateral IVC was split in a cephalad direction. The right adrenal vein was identified, controlled, and divided. Once the adrenal vein was secured, the remainder of the dissection involved mobilizing the adrenal gland. The specimen was removed using a retrieval pouch and all ports closed with an absorbable fascial suture. Laparoscopic left adenalectomy required mobilization of adjacent structures to expose the left adrenal gland. The descending colon was mobilized by incising the white line of Toldt. This incision was carried high up along the spleen, allowing the spleen to fall by gravity away from the suprarenal area. The left renal and adrenal veins were identified and controlled. The numerous arterial supplies of the adrenal gland were divided using cautery for hemostasis. The adrenal gland was freed from the surrounding tissues and retrieved using the retrieval pouch (Fig. 1).
Fig. 1.
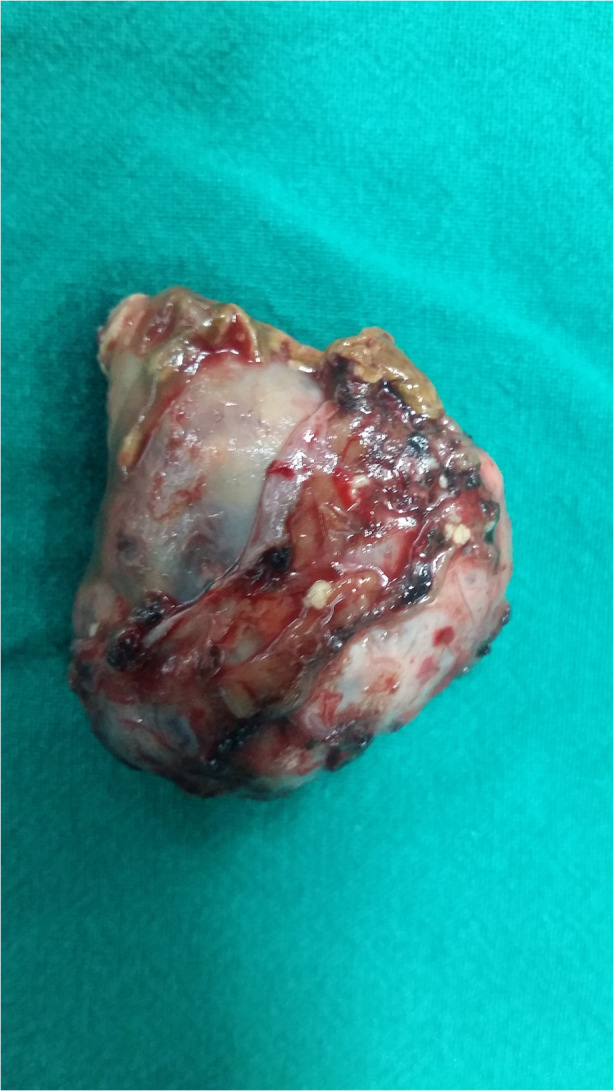
Excised specimen of adrenal gland
Data in relation to the age of the patient at diagnosis of the adrenal metastases, duration following primary treatment of RCC, histopathology, size of the adrenal lesion, operating time, intra and immediate postoperative complications were noted and analyzed.
Results
During the 10 year study period (Jan. 2006–Dec. 2015), 8 patients (7 male and 1 female) with a mean age of 57.8 years underwent laparoscopic adrenalectomy for an adrenal lesion appearing on the contralateral side and identified during the follow-up imaging after radical/partial nephrectomy for RCC (Table 1).
Table 1.
Results of patients undergoing laparoscopic adrenalectomy after previous radical/partial nephrectomy
| No./sex | Age (years) at presentation of adrenal lesion | Stage/Fuhrman grade | Previous Surgery for RCC | HPR | Diagnosis of adrenal lesion (months) after primary treatment | Size of adrenal lesion (cm) |
|---|---|---|---|---|---|---|
| 1/M | 63 | pT1N0M0G2 | Lt rad neph | Clear cell | 36 | 4 × 4 |
| 2/M | 59 | pT2N0M0G2 | Lt rad neph | Clear cell | 58 | 4 × 5 |
| 3/M | 57 | pT1N0M0G3 | Rt rad neph | Clear cell | 24 | 6 × 7 |
| 4/M | 46 | pT1aN0M0G2 | Lt partial neph | Clear cell | 30 | 3 × 4 |
| 5/M | 58 | pT1aN0M0G3 | Rt partial neph | Clear cell | 42 | 4 × 5 |
| 6/F | 62 | pT2N0M0G2 | Rt rad neph | Clear cell | 60 | 4 × 4 |
| 7/M | 54 | pT3aN0M0G2 | Lt rad neph | Clear cell | 36 | 5 × 4 |
| 8/M | 64 | pT1bN0M0G3 | RT rad neph | Clear cell | 24 | 4 × 3 |
| 57.8 ± 5.4 | 38.7 ± 13.03 |
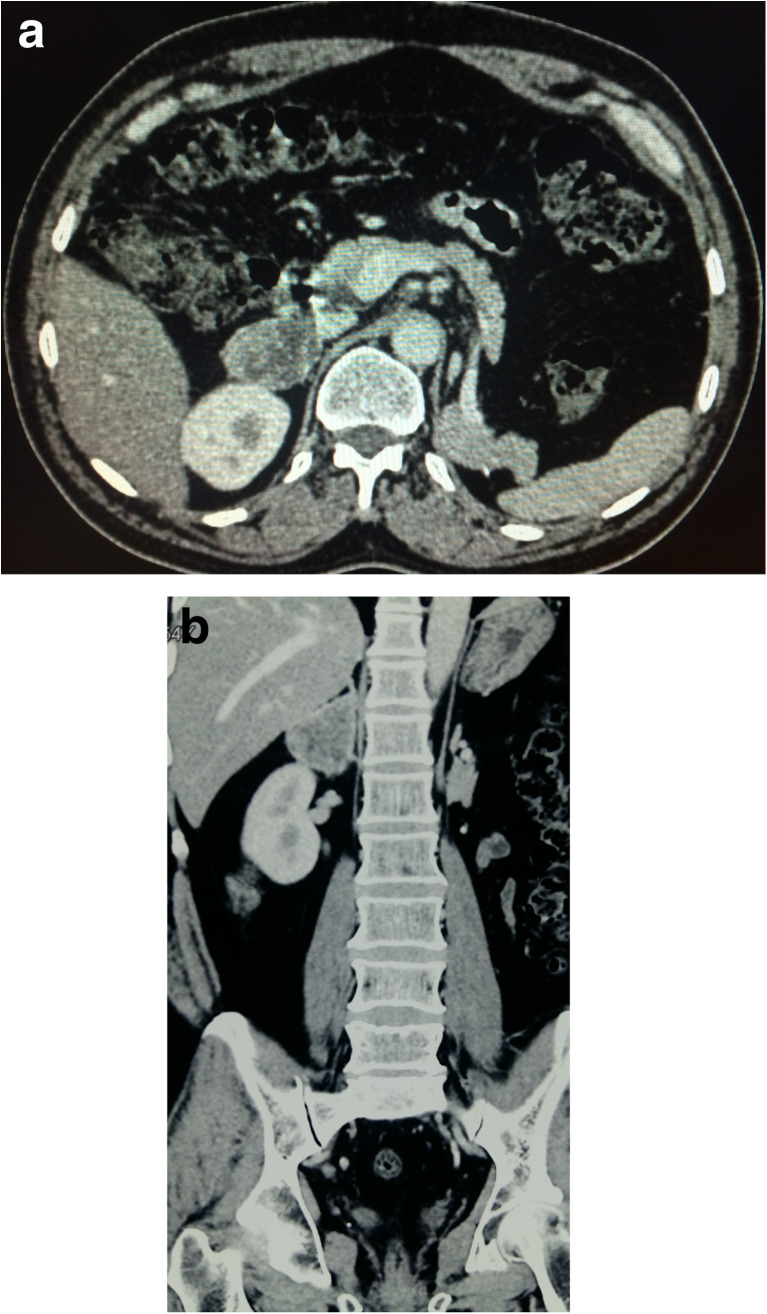
All the adrenal lesions were identified on follow-up ultrasonography examination and further confirmed on CT imaging (Fig. 2). None of the patients were symptomatic for these lesions. All biochemical studies done to assess the functional status of the adrenal glands were within normal levels including serum cortisol, urinary cortisol, and urinary VMA. All patients were counseled regarding the surgery, outcome, prognosis, and need for further treatment if necessary.
Fig. 2.
a CT scan showing the right adrenal mass measuring 4 cm. b CT scan showing the right adrenal mass superior to the right kidney
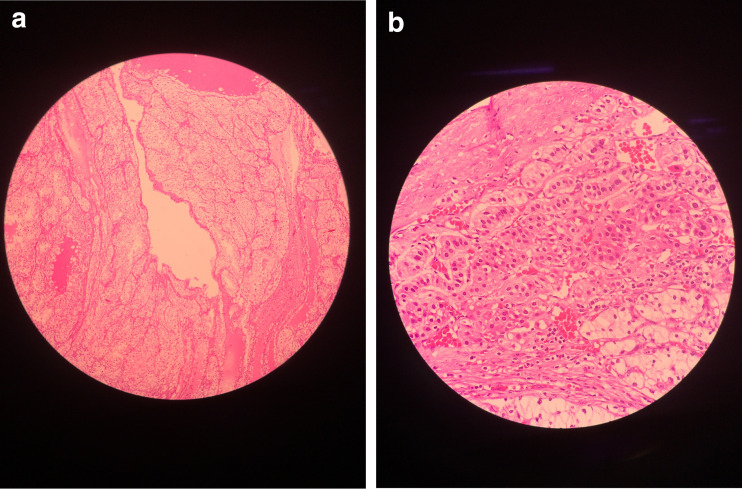
The details of laparoscopic adrenalectomy were shown in Table 2. None of the patients needed conversion to open surgery. The operating time for right side lesions was more than the left side lesions, because of the dissection of the right adrenal vein. The mean operating time was 111.2 ± 32.5mins, blood loss was 45 ± 8.6 cm3, and postoperative stay was 37.5 ± 9.3 h. None of the patients had any major complications both early and delayed. Histopathological examination confirmed metastases from the RCC and all were of clear cell variety (Fig. 3).
Table 2.
Outcomes of patients undergoing laparoscopic adrenalectomy
| Patient no. | Operating time (min) | Blood loss (cc) | Post-op hospital stay (h) | Complications (early) | Complications (delayed) | Patient status dead (months) | Patient status alive (months) |
|---|---|---|---|---|---|---|---|
| 1 | 140 | 40 | 36 | – | – | 53 | |
| 2 | 120 | 50 | 24 | – | – | 65 | |
| 3 | 90 | 40 | 36 | – | – | 47 | |
| 4 | 110 | 60 | 48 | Prolonged ileus | – | 72 | |
| 5 | 80 | 50 | 36 | – | – | 37 | |
| 6 | 80 | 50 | 48 | – | – | 38 | |
| 7 | 180 | 40 | 48 | Post-op vomiting | – | 33 | |
| 8 | 90 | 30 | 24 | – | – | 12 | |
| 111.2 ± 32.57 | 45 ± 8.6 | 37.5 ± 93 |
Fig. 3.
a Central portion tumor proper consisting of clear cell carcinoma of the kidney. b Microscopic picture showing capsule, adrenal gland, and tumor in lower portion of the picture
Four of the patients died during the follow-up period, two because of the disease and the other two because of other causes. The remaining four are alive with no evidence of obvious disease. The overall survival was 44.62 ± 17.8 months and disease specific survival was 40.83 ± 18.41 months.
Discussion
RCC is the most common renal tumor. RCC can recur at any time after nephrectomy. Frequent sites of metastasis include the lung parenchyma (50 to 60%), bone (30 to 40%), liver (30 to 40%), and brain (5%) [4]. The incidence of adrenal metastases from RCC is 6–23% in autopsy series [1, 2], but from clinical diagnosis is less frequent, at 2–10% [8]. In particular, the involvement of the ipsilateral adrenal gland is 19% in the autopsy and 5.5% in the surgical series, whereas the contralateral adrenal is involved in up to 11% of the autopsy series, but with slightly fewer than 60 cases reported as of 2003 [1, 2, 8, 9].
The adrenal glands are an uncommon site of metastases from contralateral RCC. But controversy exists as to whether adrenalectomy would improve survival. The pathological mechanisms for secondary involvement of the contralateral adrenal gland are unknown. It is thought that the disease spreads via hematogenous route as in the case of other organ metastases [2]. Explanation of this fact can be a rich blood supply of the adrenal gland and its high blood volume-to-unit weight ratio. It has been speculated that the contralateral adrenal metastasis occur as the adrenal gland has a higher affinity to the RCC cells than other organ tissues. In another words, tumor cells reaching the adrenal gland, the latter acts as a fertile soil and stimulates the growth of these cells [2, 3]. In consistent with this theory, some studies have shown that the adrenal metastases from the contralateral primary RCC can grow to a considerable size without metastasing to other organs [8].
Adrenal metastases are usually anatomically and functionally silent, and patients rarely have symptoms or signs of adrenal insufficiency. Imaging during the follow-up period remains the main means of diagnosing these lesions. However, imaging studies usually cannot verify with certainty that the adrenal masses detected in the patients previously operated for RCC are metastases. It would be difficult to determine whether the mass is a primary adrenal tumor (carcinoma), a benign tumor (i.e., an adrenal cortical adenoma), or a metastasis. The finding of solitary adrenal mass without elevated serum adrenocortical hormones is strongly suggestive of a metastatic lesion. Metastatic adrenal tumors are usually well vascularized as compared with the adrenal cortical adenoma or primary adrenal carcinoma. CT is a highly specific in diagnosing adrenal metastases. In 82% of cases reported in the literature, contralateral adrenal metastases have been diagnosed by abdominal CT. Antonelli et al. [8] reported positive and negative predictive value of CT as 73 and 96% in detecting the adrenal metastases. Remarkably, the positive predictive value of CT in diagnosing the contralateral adrenal metastases was higher.
Several experts believe that favorable outcomes can be achieved in patients with small, metachronous, and isolated metastases from renal-cell carcinoma and in whom a complete resection can be achieved. Moreover, surgical resection of metastases from renal-cell carcinoma remains a valid option in the era of targeted molecular therapies, especially as accumulating data show that complete responses are rare with these new agents [10, 11]. In patients with adrenal metastases after nephrectomy for RCC, open adrenalectomy necessitates a large incision to gain access for removal of a small gland. In addition to the possible benefits of less pain and a quicker recovery, patients may be able to return to systemic therapy for mRCC faster when using laparoscopic techniques compared with open surgery. Laparoscopic adrenalectomy (LA) has been considered the standard of care for patients with small adrenal masses [11]. Abel et al. [11] reported on 54 consecutive patients who underwent LA. Adrenalectomy performed either laparoscopically or by robotic-assisted laparoscopy decreases the surgery-associated morbidity and hospital stay.
Our study clearly shows that metachronous metastases to the contralateral adrenal gland from RCC occur late and are usually asymptomatic. They are identified on imaging procedures during follow-up. The metastases were small and non-functioning in our series. Laparoscopic adrenalectomy seems to be the right option for these patients as the operating time was short, blood loss was minimal, and hospital stay was short. The procedure was associated with minimal morbidity and none of the patients needed conversion to open.
The available data on the outcome of the surgical treatment of the contralateral adrenal metastases from RCC are limited and controversial. Lau WK et al. [1] from the Mayo clinic reported in 2003, that 82% of the metastases were metachronous, diagnosed at the mean 4.2 years (0–9.2 years) after the nephrectomy. The majority (62%) of metastases developed on the left side. Abdominal CT was the preferred method of diagnosis in the vast majority of cases (82%) followed-up by arteriography (6%) and IVP (2%) [12]. At the mean follow-up of 6.8 years (0.3–14.3 years) 55% were alive without evidence of disease and 34% were dead of disease. Surgical removal of the adrenal gland was the only treatment used in these patients. None of the patients received any form of adjuvant systemic therapy [12].
Conclusions
Solitary contralateral metachronous adrenal metastasis from RCC is an extremely rare clinical situation that can occur late after the primary treatment, i.e., radical/partial nephrectomy. Increased application of imaging techniques has led to a more efficient means of detecting these lesions. Aggressive surgery remains a valid treatment option in these cases and help in improving prognosis. Laparoscopic adrenalectomy is feasible, safe, and associated with decreased morbidity and hospital stay.
References
- 1.Lau WK, Zincke H, Blute ML, et al. Contralateral adrenal metastasis of renal cell carcinoma: treatment, outcome, and a review. BJU Int. 2003;91:775–779. doi: 10.1046/j.1464-410X.2003.04237.x. [DOI] [PubMed] [Google Scholar]
- 2.Kessler OJ, Mukamel E, Servadio C, et al. Metachronous renal cell carcinoma metastasis to the contralateral adrenal gland. J Urol. 1998;51:539–543. doi: 10.1016/S0090-4295(97)00698-5. [DOI] [PubMed] [Google Scholar]
- 3.Plawner J. Results of surgical treatment of kidney cancer with solitary metastasis to contralateral adrenal. Urology. 1991;37:233–236. doi: 10.1016/0090-4295(91)80291-E. [DOI] [PubMed] [Google Scholar]
- 4.Yuvaraja BT, Mahantshetty U, Chamarajanagar RS, Raibhattanavar SG, Tongaonkar HB. Management of renal cell carcinoma with solitary metastasis. World Journal of Surgical Oncology. 2005;3:48. doi: 10.1186/1477-7819-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakoji H, Miyamoto T, Inuzuka H, Sawada N, Takeda M. Contralateral adrenal metastasis of renal cell carcinoma arising from a horseshoe kidney: an initial case report. Urology Case Reports. 2014;2:131–133. doi: 10.1016/j.eucr.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerli RB, Reddy MN, Guntaka A, Patil SM, Hiremath MB. Laparoscopic adrenalectomy for adrenal masses in children. J Paed Urol. 2010;4:6. doi: 10.1016/j.jpurol.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Ravish IR, Nerli RB, Reddy MN, Suresh N, Thakkar R. Laparoscopic adrenalectomy for large pheochromocytoma. BJU Int. 2007;100:1126–1129. doi: 10.1111/j.1464-410X.2007.07179.x. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli A, Cozzoli A, Simeone C, Zani D, Zanotelli T, Portes IE, Cunico SC. Surgical treatment of adrenal metastasis from renal cell carcinoma: a single-centre experience of 45 patients. BJU Int. 2006;97:505–508. doi: 10.1111/j.1464-410X.2006.05934.x. [DOI] [PubMed] [Google Scholar]
- 9.Dieckmann KP, Wullbrand A, Kroizig G. Contralateral adrenal metastasis in renal cell cancer. Scand J Urol Nephrol. 1996;30:139–143. doi: 10.3109/00365599609180905. [DOI] [PubMed] [Google Scholar]
- 10.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 11.Jason Abel E, Karam JA, Carrasco A, Matin SF. Laparoscopic adrenalectomy for metachronous metastases after ipsilateral nephrectomy for renal-cell carcinoma. J Endourol. 2011;25:1323–1327. doi: 10.1089/end.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chkhotua A, Managadze L, Pertia A (2013) Surgical and oncological results of treatment of metastases of renal cell carcinoma to the contralateral adrenal gland. doi:10.5772/53745 [PubMed]