Abstract
Sarcomatoid carcinoma is an extremely rare aggressive tumor variant comprising about 0.3% of all primary tumors of the urinary bladder. We report a rare case of giant bladder sarcomatoid tumor arising from a bladder diverticulum. A 60-year-old male on evaluation for long-standing obstructive voiding symptoms with recurrent pyuria found to have renal failure and bladder mass with bilateral hydroureteronephrosis (HDUN) on ultrasound. Further radiologic evaluation revealed multiple bladder diverticulae and anteriorly displaced bladder with a large mass arising from one of the posteriorly located bladder diverticulum with extrinsic compression of both the distal ureters leading to severe bilateral HDUN. Rigid cystourethroscopy was not successful due to anteriorly displaced urethra. Tissue biopsy taken with flexible cystoscope revealed low-grade, noninvasive transitional cell carcinoma. After staging workup, bilateral percutaneous nephrostomy was performed initially for stabilization of renal function. This was followed by radical cystoprostatectomy with bilateral extended pelvic lymphadenectomy with ileal conduit diversion. Histopathology revealed high-grade muscle-invasive sarcomatoid variant of urothelial carcinoma with osseous metaplasia. It is imperative to recognize the rare variants of bladder tumors with different therapeutic and prognostic considerations and hence tailor the management of individual variant.
Keywords: Bladder cancer, Sarcomatoid, Radical cystoprostatectomy
Introduction
Bladder cancer is classified histologically as urothelial or non-urothelial. Urothelial cancer has a propensity for divergent differentiation. The divergent differentiation patterns include, among others, squamous, glandular, micropapillary, nested, lymphepithelioma-like, plasmacytoid, and sarcomatoid variants. These are usually associated with poor prognosis. Sarcomatoid carcinoma also known as malignant mixed mesodermal carcinoma, pseudo sarcoma, or spindle cell carcinoma is a rare variant and constitutes 0.3% of all primary tumors of the bladder. It usually presents with hematuria and management involves a multimodal approach [1–4]. Herein, we report a case of a giant sarcomatoid carcinoma of urinary bladder arising in a bladder diverticulum and review the literature regarding the same.
Material and Methods
A 60-year-old male presented with obstructive lower urinary tract symptoms and intermittent turbiduria. On examination, there was large suprapubic hard mass with normal external genitalia and prostate. Screening ultrasound showed large mass in pelvis along with bilateral gross hydroureteronephrosis.
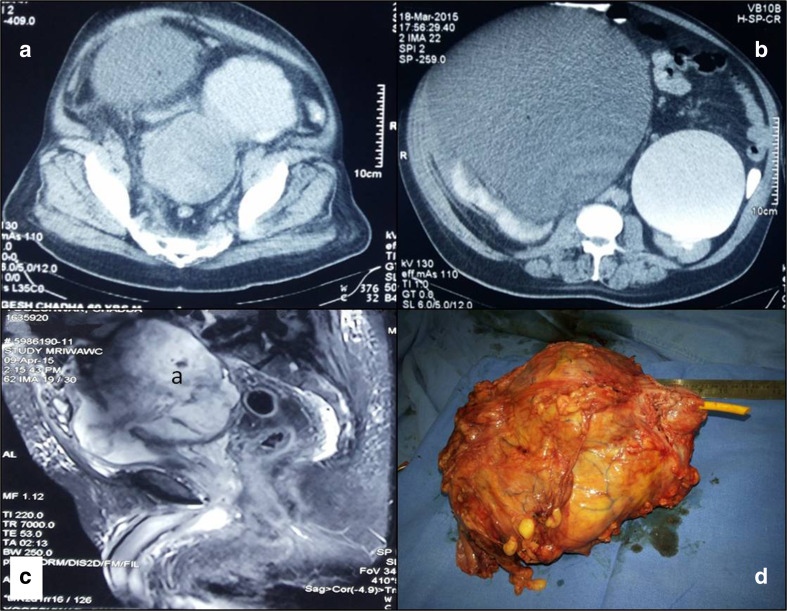
Subsequent CT and MRI revealed bilateral gross hydroureteronephrosis. Excretory phase of CT urogram showed multiple bladder diverticulae and anterior displacement of the bladder by a heterogenously enhancing and irregular nodular intravesical mass arising from one of the posteriorly located bladder diverticulum, Fig. 1. The mass was causing extrinsic compression of both the distal ureters and sigmoid colon but with preserved fat planes. Prostate and bilateral seminal vesical seemed normal on MRI, Fig. 1. Urine cytology was negative. Rigid cystourethroscopy was not successful due to elongated and anteriorly displaced posterior urethra. Flexible cystoscopy with biopsy was performed which revealed large solid tumor arising from the posterior and right lateral wall bladder diverticulum. Biopsy from lesion showed inflammatory and necrotic cells with occasional spindle cell with features of low-grade, noninvasive transitional cell carcinoma, (papillary architecture without lamina propria invasion with focal loss of polarity). PET CT showed no abnormal uptake other than the tumor.
Fig. 1.
Contrast-enhanced computerized tomography showing diverticular bladder with a large heterogenous mass arising in the diverticulum with bilateral gross hydroureteronephrosis (a, b), T2 weighted sequences in magnetic resonance imaging showing diverticular mass with normal prostate (c), and gross specimen of cystoprostatectomy (d)
Results
Bilateral percutaneous nephrostomy was done before surgery to relive hydroureteronephrosis and stabilize the renal parameters. After 2 weeks, radical cystoprostatectomy with bilateral extended pelvic lymphadenectomy with ileal conduit urinary diversion was done using Bricker type of ureterileal anastomosis. Postoperative course was uneventful.
Grossly tumor was large polypoidal mass arising from large posterior bladder wall diverticulum and replacing almost whole of the bladder measuring 150 × 130 × 110 mm, Fig. 1.
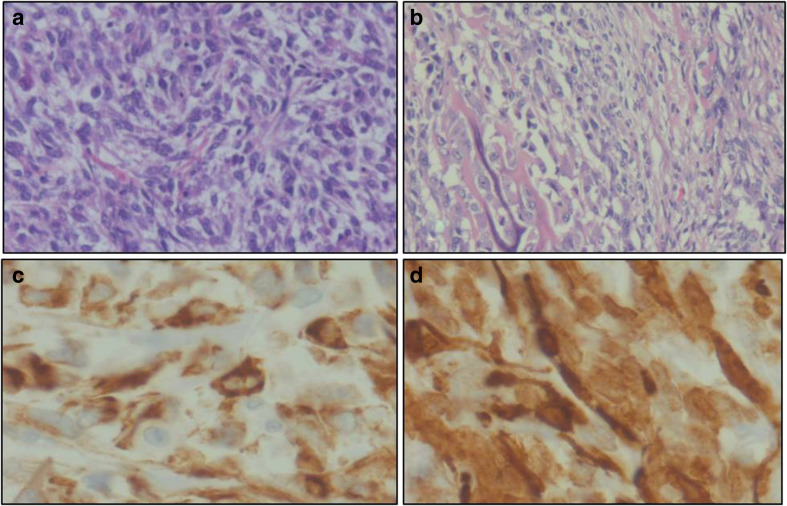
Histopathology showed muscle-invasive transitional cell carcinoma with addition feature like osseous metaplasia and inflammation with large areas of necrosis. There was no perivesical fat infiltration. Seminal vesicles, vas deferens prostate, and both ureteric margins were free of tumor. All pelvic lymph nodes (33) were negative for malignancy (pT2b N0). Immunohistochemistry showed positivity for cytokeratin D, P 63, CK 5/6, vimentin, smooth muscle antigen, and negative for CK 7, CK 20, suggestive of sarcomatoid features, Fig. 2.
Fig. 2.
Plump spindle-shaped cells arranged in fascicles and sheets (a), osseous metaplasia (b). (H&E, ×100), immunohistochemistry revealed positivity cytokeratin D and smooth muscle antigen (c, d)
At 10 weeks, he developed a periumbilical nodule, which on biopsy showed metastatic carcinoma. Further evaluation with PET scan revealed multiple hypermetabolic lesions in omentum and peritoneum. He was subjected to palliative chemotherapy with gemcitabin and carboplatin. CT after three cycles showed significantly increased soft tissue deposits in bowel wall and presacral area. In view of progressive disease, patient was planned for second-line palliative chemotherapy with associated risks involved but patient lost to follow-up.
Discussion
Transitional cell carcinoma is the most common urothelial bladder neoplasm. It has many known pathologic variants like micropapillary, squamous differentiation, glandular differentiation, sarcomatoid differentiation, and inverted or nested pattern. Sarcomatoid differentiation is a rare variant with incidence ranging from 0.1 to 0.3% [1]. Molecular studies showed the same clonal origin and prognosis for the carcinomatous and sarcomatous components in tumors. On histopathology, sarcomatoid areas can be seen merge with foci of urothelial carcinoma, squamous cell carcinoma, adenocarcinoma, or small-cell carcinoma and most commonly resemble a high-grade sarcoma with poor differentiation. In decreasing order of frequency areas of osteosarcoma, chondrosarcoma, rhabdomyosarcoma, liposarcoma, and angiosarcoma may be seen in sarcomatoidurothelial carcinoma [2].
It usually presents at a younger age with mean age of 66 years and is more common in males with male to female ratio of 3:1 [3, 4]. Exact etiology is not known, but association with radiation and cyclophosphamide exposure has been described [5]. It usually presents in the same manner as other urothelial carcinomas of the bladder with hematuria being the most common presenting symptom. Due to aggressive nature of disease, it usually diagnosed at advanced stage. Most common location is lateral walls of the bladder [5], macroscopically seen as polypoidal or large broad-based mass with edematous, hemorrhagic, or cystic component. On histology, it is biphasic with malignant epithelial and predominant mesenchymal elements. Spindle cell component is typically seen in high-grade disease as seen in the present case. Diagnosis is confirmed on IHC markers, including vimentin, cytokeratin, epithelial membrane antigen, and or smooth muscle antigen [5].
There is no consensus on standard treatment because of its rarity and lack of randomized controlled trials and multimodal approach including surgery and chemoradiotherapy is needed. As the tumor is aggressive and usually presents at advanced stage, transurethral resection of the bladder tumor or partial cystectomy is inappropriate. Sanfrancesco et al. recently evaluated 28 patients with sarcomatoid variant of urothelial carcinoma. Twenty-two were treated with radical cystectomy and two received neoadjuvant chemotherapy and remaining eight received adjuvant chemotherapy. Follow-up ranged from 0.5 to 72 months and 46% showed metastasis. Sixteen patients (67%) died of disease, with an estimated median survival of 10.2 months and a mean survival of 9.1 months [6].
The overall survival is better for those who treated with radical cystectomy [3, 4]. Radical cystoprostatectomy is considered as mainstay of treatment, but recurrences still tend to develop after surgery. And hence, adjuvant therapy in form of radiotherapy or chemotherapy is given to prevent recurrences. They are especially indicated in high-risk cases like those with high-grade, margin positivity, lymph node positivity, and extravesical spread. Role of neoadjuvant therapy in advanced cases was studied in few studies [3, 5, 6]. Prognosis is even worse than high-grade urothelial carcinoma. Five-year survival is 17% as compared to 47% in urothelial carcinoma [4].
Conclusion
Sarcomatoid carcinoma is a rare variant with different therapeutic and diagnostic implications. Importance of early diagnosis of one of these rare variants lies in the prognostic and therapeutic considerations. Outcomes associated with chemotherapy and radiotherapy has been inconsistent in most of the series, so treatment should be aggressive and multimodal. Multi-institutional prospective clinical trials are needed to establish treatment protocols for sarcomatoid variant.
Acknowledgements
Medanta, The Medicity Hospital, Gurgaon is gratefully acknowledged by the authors.
Contribution details
| Author 1 | Author 2 | Author 3 | Author 4 | |
| Concepts | ✓ | ✓ | ||
| Design | ✓ | ✓ | ✓ | |
| Definition of intellectual content | ✓ | ✓ | ✓ | |
| Literature search | ✓ | ✓ | ||
| Clinical studies | ✓ | ✓ | ||
| Experimental studies | ||||
| Data acquisition | ✓ | ✓ | ||
| Data analysis | ✓ | ✓ | ✓ | |
| Statistical analysis | ✓ | |||
| Manuscript preparation | ✓ | ✓ | ✓ | ✓ |
| Manuscript editing | ✓ | ✓ | ||
| Manuscript review | ✓ | ✓ | ✓ | |
| Guarantor | ✓ |
Contributor Information
Varun Mittal, Phone: +91-9650126095, Email: varunshonu512@gmail.com.
Ketan K. Rupala, Email: ketanrupala10@gmail.com
Rajiv Yadav, Email: drrajivyadav@gmail.com.
Manav Suryavanshi, Email: manavsuryavanshi@gmail.com.
References
- 1.Mallik AU, Rahman MZ, Sarker MM. Sarcomatoid carcinoma of the urinary bladder: a case report. Pulse. 2011;4:28–29. doi: 10.3329/pulse.v4i1.6961. [DOI] [Google Scholar]
- 2.Venyo AK, Titi S. Sarcomatoid variant of urothelial carcinoma (carcinosarcoma, spindle cell carcinoma): a review of the literature. ISRN urology. 2014;22:2014. doi: 10.1155/2014/794563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal A, Kumar N, Sharma SC. Sarcomatoid variant of urothelial carcinoma of the urinary bladder. J Cancer Res Ther. 2013;9:571. doi: 10.4103/0973-1482.126449. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Gillaspie C, Kunadharaju R, Talmon GA, Enke C. Sarcomatoid urothelial carcinoma: a single cancer center experience. World Journal of Oncology. 2011;2:175–180. doi: 10.4021/wjon370w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez JE, Figueira YR, Gonzalez RT, Lopez RM, Tejeda LM. Sarcomatoid carcinoma of the urinary bladder: a case report and review of the literature. Journal of Medical Cases. 2014;5:116–119. [Google Scholar]
- 6.Sanfrancesco J, McKenney JK, Leivo MZ, Gupta S, Elson P, Hansel DE. Sarcomatoid urothelial carcinoma of the bladder: analysis of 28 cases with emphasis on clinicopathologic features and markers of epithelial-to-mesenchymal transition. Archives of pathology & laboratory medicine. 2016;140:543–551. doi: 10.5858/arpa.2015-0085-OA. [DOI] [PubMed] [Google Scholar]