Abstract
Introduction
Neuropathic pain is a common complication of spinal cord injury (SCI), and is notoriously difficult to adequately treat. Gunshot wounds (GSW) near the spinal cord may cause intractable chronic pain through spinal/nerve root transection, or reactive tissue formation resulting in nerve root compression from retained bullet fragments (RBF).
Case presentation
This case report describes a 30-year-old man with a T12 AIS B incomplete spinal cord injury with paraplegia secondary to multiple GSW who presented with severe bilateral lower extremity dysesthesias and muscle spasms. Symptoms failed to improve with oral antispasmodic medications. After being diagnosed with Complex regional pain syndrome (CRPS) type I secondary to an SCI via GSW, he underwent a spinal cord stimulator (SCS) trial, which improved his symptoms by greater than 80%.
Discussion
Neuropathic pain refractory to conservative treatment may benefit from SCS. Effects of therapy go beyond gate-theory in SCI patients, and may benefit patients at the cellular and molecular level. Our case demonstrates the effectiveness of SCS treatment in a patient who developed CRPS type 1 after GSW resulting in SCI.
Subject terms: Pain management, Neuropathic pain, Spinal cord diseases
Introduction
Chronic pain is a difficult diagnosis to manage, and this is even more true for those with spinal cord injury (SCI). The incidence of SCI is approximately 54 cases per one million people in the United States, or about 17,810 new cases each year, and the estimated number of people with SCI living in the United States is approximately 294,000 persons, with a range from 250,000 to 368,000 persons [1]. Chronic pain is commonly reported in individuals with SCI, with recent prevalence reported as high as 80%, 1/3 of which describe the pain as severe [2].
Specifically, SCI due to gunshot wounds (GSW) has a variable incidence depending on which region of the world but varies from 13 to 14% [3]. The mechanism of which GSW cause SCI is multifactorial and includes direct impact from the bullet, concussive effect of the bullet impaction and temporary cavitations [4]. Further, thoracic SCI due to GSW is more likely to be complete due to the high energy transfer, narrower spinal canal and relative watershed area of spinal cord circulation due to vascular steal phenomenon [5, 6]. GSW near the spinal cord may cause intractable chronic pain through spinal/nerve root transection, or reactive tissue formation resulting in nerve root compression from retained bullet fragments (RBF).
According to the International Association for the Study of Pain, neuropathic pain is defined as “pain caused by a lesion or disease of the somatosensory nervous system”. For patients with SCI, those reporting neuropathic pain in the subacute period (3–6 months) after injury are likely to continue experiencing pain at 3–5 years following their injury, and it is much more likely to be described as severe or excruciating [7]. Neuropathic pain remains one of the most difficult conditions to treat as it is largely refractory to most pharmacologic agents—only 1/3 of patients report 50% reduction in neuropathic pain with treatment. During the 1990 Sixth World Congress of Pain Plenary Address, Ronald Melzack stated, “There is a set of observations on pain in paraplegics that just does not fit the theory”. This case aims to demonstrate one modality that may help address this challenging issue. This case describes the management of complex regional pain syndrome (CRPS) by SCS in a patient with an incomplete spinal cord injury due to GSW with a RBF.
Case description
A male patient between 25 and 30 years old with a past medical history of anemia, diabetes mellitus type 2, history of deep venous thrombosis, hypertension, mood disorder, thrombocytosis and neuropathy who sustained a T12 AIS B incomplete paraplegia secondary to multiple GSW three years prior (Figs. 1–3) presented to the outpatient pain management clinic with severe bilateral lower extremity dysesthesias and muscle spasms. The dysesthesias were described as “burning” and “stabbing” located at the anterior thighs that typically extended to the knee and occasionally into the foot and toes with greater intensity on his left lower extremity, described as a 10 on a 0–10 numeric rating scale. Furthermore, the episodes were intermittent and occurred suddenly, lasting about 5 min, around 4–5 times an hour per day, and resolved spontaneously. Associated symptoms included muscle spasms that occurred with about 10% of these episodes, mainly in his quadriceps. He had tried venlafaxine and gabapentin but neither of those medications alleviated his symptoms.
Fig. 2. Axial CTof the lumbar spine.
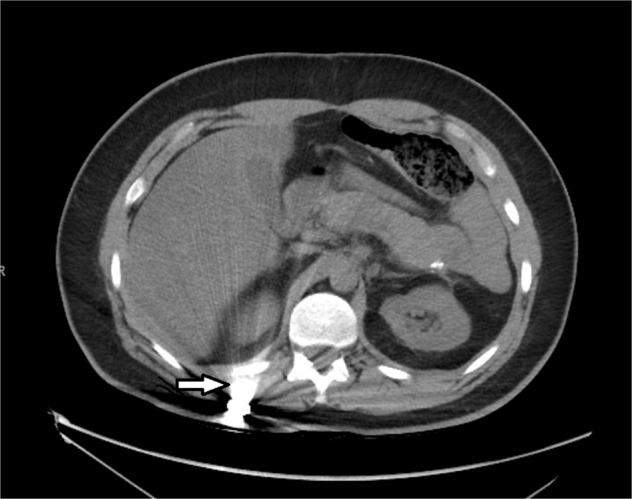
This demonstrates the retained bullet fragment near the T12 vertebra indicated by the white arrow.
Fig. 1. Axial CT of the lumbar spine.
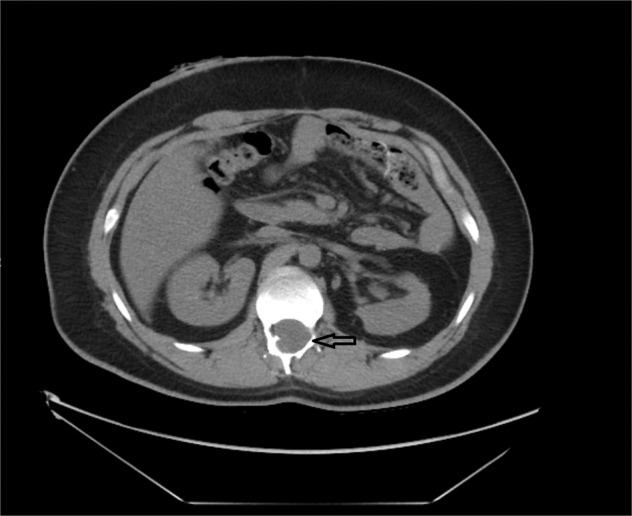
This demonstrates a bony defect of the posterior column of the L1 vertebra as indicated by the clear arrow.
Fig. 3. Sagittal CT of the lumbar spine.
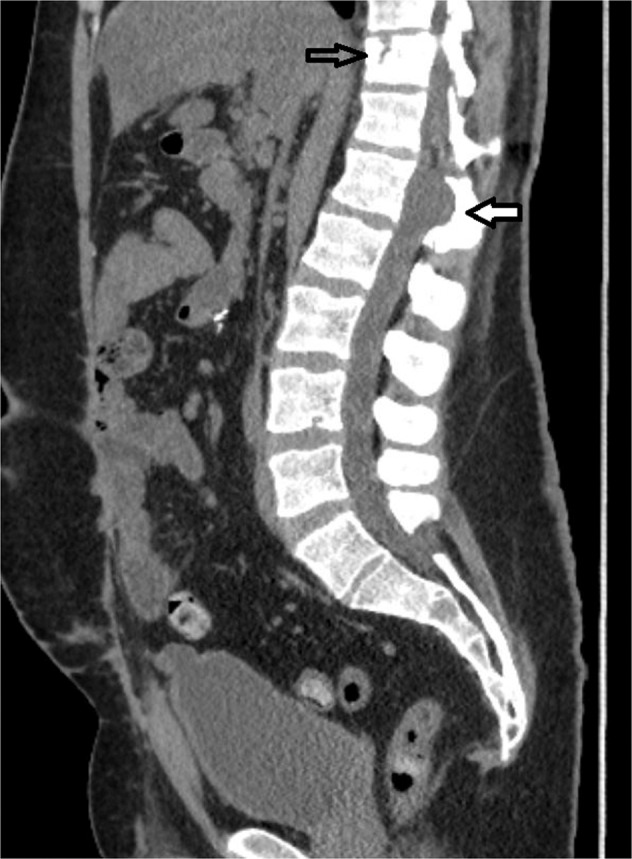
This demonstrates an abnormality of the spinal cord at L1 with posterior extrusion indicated by the white arrow. A T11 vertebral body fracture is also evident noted with the clear arrow.
He was referred by his SCI physician with the intention of receiving a baclofen pump after symptoms failed to improve with oral baclofen that was titrated up to 40 milligrams twice a day, his maximum dose. Constitutional symptoms, including fever, chills, and weight loss were not associated with his current symptoms. His bowel and bladder habits were non-contributory as he had a suprapubic catheter and colostomy in place. He also denied saddle anesthesia. At baseline, the patient uses an electric wheelchair for community ambulation and has bilateral equinovarus contractures.
On physical exam, his motor strength was 5/5 in hip flexors and knee extenders bilaterally but 0/5 in dorsiflexors, plantar flexors and great toe extensors bilaterally, with diminished crude touch sensation at the L1 dermatome and below with partial sacral sparing, intact above. Patellar reflexes were 1+ and Modified Ashworth Score (MAS) was 0 at bilateral hip flexors, quadriceps, hamstrings, dorsiflexors and plantar flexors bilaterally. Of note, his lumbar spine exam was benign, but he did experience one of his painful episodes when going back to rest during the “Slump Test”. His CT imaging was reviewed and evidenced trauma at the L1 level and RBF in the right lower paraspinal area. The patient was ultimately diagnosed with CRPS type I secondary to SCI via GSW.
The patient underwent an SCS trial, yielding an improvement of symptoms by greater than 80% (pain rated at 1/10) at one week follow up, and subsequently underwent SCS implantation which produced equivalent results at one week follow up (Figs. 4 and 5). On his post-operative follow up, he endorsed no adverse effects and was followed closely by the representative of the device company to monitor adherence and continued effectiveness of the device. At six months follow up the patient reported sustained relief from the device with at least 50% (4/10) reduction in pain. The patient granted consent for this case to be published.
Fig. 4. Intra-procedural lateral fluoroscopic image of the lower thoracic spine.
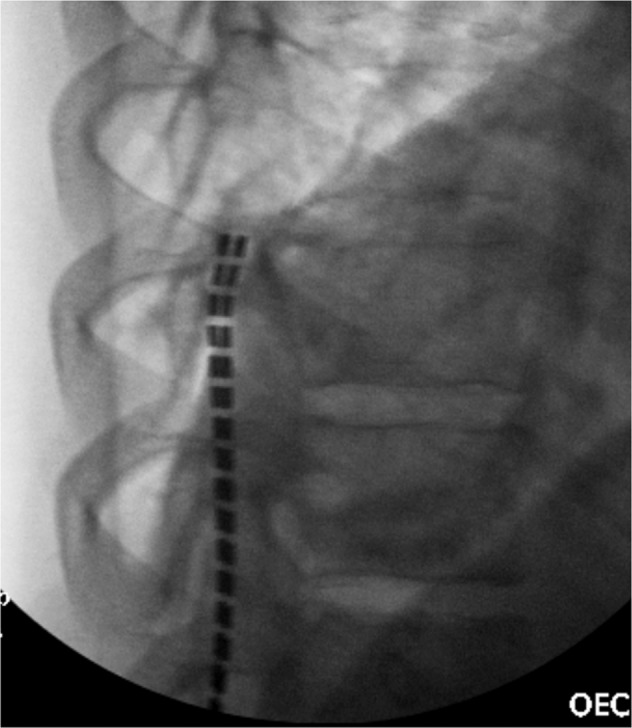
This demonstrates appropriate placement of the spinal cord stimulator leads in the posterior aspect of the epidural space.
Fig. 5. Intra-procedural AP fluoroscopic image of the lower thoracic spine.
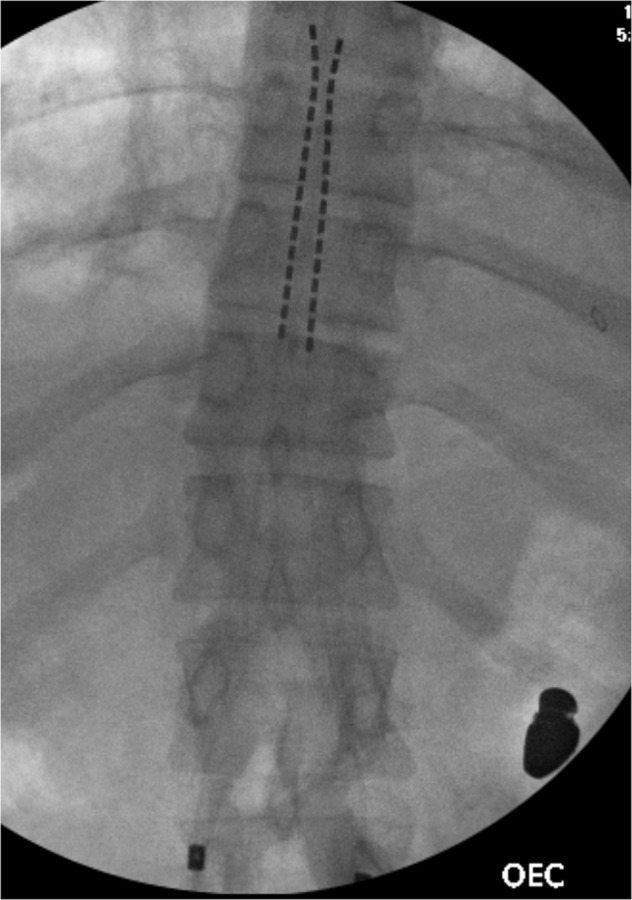
This demonstrates placement of the spinal cord stimulation leads starting at the superior aspect of the T11 vertebral body, and terminating at the inferior aspect of the T8 vertebral body. A bullet fragment is also visualized.
Discussion
Neuropathic pain is more common in incomplete injuries, which may be due to transmission through intact tracts in the spinal cord. Some experimental findings indicate that SCI-related neuropathic pain may originate in the spinal cord near the site of damage and involve secondary changes in damaged nerve roots and brain structures [8]. However, people with clinically complete injuries may have residual sensory transmission through the cord that is not detectable using standard physical examination techniques, sometimes referred to as a ‘sensory discomplete’ lesion. This implies the mechanism of action for neuropathic pain in SCI may be more complex than simply transmission through residual spinal pathways.
There have been mechanisms described in the literature that discuss a cascade of events following spinal injury. Anatomically, there is necrosis, apoptosis, demyelination, cytoskeletal damage, gliosis, and collateral sprouting that occurs after injury. An imbalance between excitatory glutamate and inhibitory GABA leads to neural excitation and an increase in peptides, second messengers, kinases, enzymes, and caspases. Ultimately, an increase in free radicals and reactive oxygenation leads to cell death [8]. This interplay of neurochemical and inflammatory processes results in allodynia, hyperalgesia, and pain.
The central sensitization in SCI patients can be simplified to an eight-step process: loss of inhibitory spinal mechanisms, spinal and supraspinal generators and amplifiers of pain, NK-1 receptor expressing neurons in lamina 1, longitudinal progression of the injury cascade, injury-induced activation of cell signaling pathways, increased sodium channel expression, and glial activation, all leading to central pain from deafferentiation, reorganization, abnormal input, sensitization, bursting, and hyperactivity at the level of the thalamus [9]. This has been demonstrated with an increase in blood flow to the thalamus on fMRI, and a decrease in concentration of N-acetyl in the thalamus (corresponding to thalamic hyperactivity and disinhibition) on proton magnetic resonance spectroscopy in patients with SCI [10]. This may be why deep brain stimulation has been effective in treating some patients with neuropathic pain after SCI [11].
Currently, there is little evidence in the literature to support the use of SCS in SCI with neuropathic pain. The CanPain SCI Clinical Practice Guidelines for Rehabilitation Management of Neuropathic Pain after Spinal Cord: Recommendations for treatment describes the highest level of evidence for pharmacological agents, though it notes pain is often refractory to treatment [12]. First line treatment is pregabalin, gabapentin, and amitriptyline, and authors noted insufficient evidence to comment on the effectiveness of spinal cord stimulation.
Other authors have described mechanisms of action of SCS beyond gate-theory in reducing pain specifically in patients with SCS. Caylor et al. [13], discussed SCS resulting in the release of neurotransmitters that are involved in spinal cord pain modulation (eg, serotonin, GABA, substance P).
In addition, a direct inhibition of glial cells (microglia and astrocytes) in the dorsal horn has been described with SCS. Microglia and astrocytes become activated early after SCI to help remove debris and damaged cells. However, persistent activation leads to a release of chemicals including glutamate, pro‐inflammatory cytokines, and reactive oxygen species. Combined, this results in the modulation of supraspinal neuronal activities, thus breaking the cycle of central sensitization previously described in SCI patients.
Currently there are no randomized controlled trials for SCS in SCI patients. There is one recent narrative review by Dombovy-Johnson et. al. in which twenty‐two reports were identified that included at least 1 patient with neuropathic pain from SCI treated with SCS [14]. Five were published case reports. One was a prospective nonrandomized, non-controlled study, and the remaining 16 were case series or reports published as conference abstracts. A total of 69 individuals with traumatic and non-traumatic SCI were included. Of the nine patients for whom both pre‐ and post‐SCS values were reported, all had documented improvement post‐SCS. Post‐SCS pain data were mentioned for 38 patients, of whom only 4 did not receive benefit. Three reports discussed improvement in spasticity based on MAS, and 8 Reports discussed decrease in pain medication use.
Patients with SCI may have sequalae including spasticity and neuropathy that may require interventions. Our patient was initially referred to the pain management clinic for baclofen pump evaluation for spasticity management, but in this case, the main symptom was his neuropathic pain, likely caused by CRPS type 1. Studies suggest that patients with SCI who benefit from SCS have incomplete paraplegia with most pain below the lesioned level [15]. Furthermore, SCS is an effective treatment for chronic radicular pain secondary to RBF [16]. Even in cases of severe spasticity in patients with SCI, SCS has been suggested to be superior to intrathecal baclofen therapy as lowered MAS scores have been reported in patients with SCS compared to Intrathecal Baclofen at 12-month intervals [17]. Although this case exemplifies the efficacy of SCS treatment in a patient who developed CRPS type 1 after GSW resulting in SCI, it is limited by being a retrospective single case. More cases and prospective, controlled studies are imperative to demonstrate the consistency of these results and add to the growing body of literature.
Supplementary information
Author contributions
RR was contributed the conception of the work, data collection, drafting the article, critical revisions, and final approval of the version to be published. EA contributed data collection, drafting the article, and critical revisions. MA contributed critical revisions of the article. DG contributed critical revisions of the article. CP contributed critical revisions of the article.
Data availability
All data underlying the results are available as part of the article. Data was derived directly from the patient’s medical record and no additional databases were used.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41394-022-00546-2.
References
- 1.National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2021.
- 2.Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33:62–68. doi: 10.1038/sc.1995.16. [DOI] [PubMed] [Google Scholar]
- 3.de Barros Filho TE, Cristante AF, Marcon RM, Ono A, Bilhar R. Gunshot injuries in the spine. Spinal Cord. 2014;52:504–10. doi: 10.1038/sc.2014.56. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu GS, Ghag A, Prokuski V, Vaccaro AR, Radcliff KE. Civilian gunshot injuries of the spinal cord: a systematic review of the current literature. Clin Orthop Relat Res. 2013;471:3945–55. doi: 10.1007/s11999-013-2901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019;30:683–99. doi: 10.3171/2018.10.SPINE18802. [DOI] [PubMed] [Google Scholar]
- 6.Waters RL, Adkins RH. The effects of removal of bullet fragments retained in the spinal canal: a collaborative study by the national spinal cord injury. Spine. 1991;16:934–9. [DOI] [PubMed]
- 7.Siddall PJ. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord. 2009;47:352–9. doi: 10.1038/sc.2008.136. [DOI] [PubMed] [Google Scholar]
- 8.D’Angelo R, Morreale A, Donadio V, Boriani S, Maraldi N, Plazzi G, et al. Neuropathic pain following spinal cord injury: what we know about mechanisms, assessment and management. Eur Rev Med Pharm Sci. 2013;17:3257–326. [PubMed] [Google Scholar]
- 9.Yezierski RP. Spinal cord injury pain: spinal and supraspinal mechanisms. J rehabilitation Res Dev. 2009;46:95–107. doi: 10.1682/JRRD.2008.06.0074. [DOI] [PubMed] [Google Scholar]
- 10.Pattany PM, Yezierski RP, Widerström-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Prévinaire JG, Nguyen JP, Perrouin-Verbe B, Fattal C. Chronic neuropathic pain in spinal cord injury: efficiency of deep brain and motor cortex stimulation therapies for neuropathic pain in spinal cord injury patients, Ann of Phys and Rehabil Med. 2009;52:188–93. 10.1016/j.rehab.2008.12.002. [DOI] [PubMed]
- 12.Guy S, Mehta S, Casalino A, Cote I, Kras-Dupuis A, Moulin DE, et al. The CanPain SCI clinical practice guidelines for rehabilitation management of neuropathic pain after spinal cord: recommendations for treatment. Spinal Cord. 2016;54:S14–S23. doi: 10.1038/sc.2016.90. [DOI] [PubMed] [Google Scholar]
- 13.Caylor J, Reddy R, Yin S, Cui C, Huang M, Huang C, et al. Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectron Med. 2019;5:12.. doi: 10.1186/s42234-019-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dombovy‐Johnson ML, Hunt CL, Morrow MM, Lamer TJ, Pittelkow TP. Current evidence lacking to guide clinical practice for spinal cord stimulation in the treatment of neuropathic pain in spinal cord injury: a review of the literature and a proposal for future study. Pain Pract. 2020;20:325–35. doi: 10.1111/papr.12855. [DOI] [PubMed] [Google Scholar]
- 15.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain-some predictors of success. A 15-year experience. Surg Neurol. 1998;50:110–20. doi: 10.1016/S0090-3019(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 16.Keel JC, Lau ME, Gulur P. Spinal cord stimulation for radicular pain following retained bullet in the spinal canal. Pain Physician. 2013;16:E103–E106. [PubMed] [Google Scholar]
- 17.Biktimirov A, Bryukhovetskiy I, Sharma A, Sharma HS. Spinal cord stimulation and intrathecal baclofen therapy for patients with severe spasticity after spinal cord injury. Prog Brain Res. 2020;258:79–99. doi: 10.1016/bs.pbr.2020.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying the results are available as part of the article. Data was derived directly from the patient’s medical record and no additional databases were used.


