Abstract
We conducted a 16-week randomized controlled trial in psychiatric outpatients with a lifetime diagnosis of a mood and/or anxiety disorder to measure the impact of a first-of-its-kind precision digital intervention software solution based on social rhythm regulation principles. The full intent-to-treat (ITT) sample consisted of 133 individuals, aged 18–65. An exploratory sub-sample of interest was those individuals who presented with moderately severe to severe depression at study entry (baseline PHQ-8 score ≥15; N = 28). Cue is a novel digital intervention platform that capitalizes on the smartphone's ability to continuously monitor depression-relevant behavior patterns and use each patient's behavioral data to provide timely, personalized “micro-interventions,” making this the first example of a precision digital intervention of which we are aware. Participants were randomly allocated to receive Cue plus care-as-usual or digital monitoring only plus care as usual. Within the full study and depressed-at-entry samples, we fit a mixed effects model to test for group differences in the slope of depressive symptoms over 16 weeks. To account for the non-linear trajectory with more flexibility, we also fit a mixed effects model considering week as a categorical variable and used the resulting estimates to test the group difference in PHQ change from baseline to 16 weeks. In the full sample, the group difference in the slope of PHQ-8 was negligible (Cohen's d = −0.10); however, the Cue group demonstrated significantly greater improvement from baseline to 16 weeks (p = 0.040). In the depressed-at-entry sample, we found evidence for benefit of Cue. The group difference in the slope of PHQ-8 (Cohen's d = −0.72) indicated a meaningfully more rapid rate of improvement in the intervention group than in the control group. The Cue group also demonstrated significantly greater improvement in PHQ-8 from baseline to 16 weeks (p = 0.009). We are encouraged by the size of the intervention effect in those who were acutely ill at baseline, and by the finding that across all participants, 80% of whom were receiving pharmacotherapy, we observed significant benefit of Cue at 16 weeks of treatment. These findings suggest that a social rhythm-focused digital intervention platform may represent a useful and accessible adjunct to antidepressant treatment (https://clinicaltrials.gov/ct2/show/NCT03152864?term=ellen+frank&draw=2&rank=3).
Keywords: depression, treatment, digital intervention platform, passive monitoring, depressive symptoms, social rhythm disruption, social rhythm regularity
Introduction
The chronic, global problem of inadequate access to mental healthcare has been described by many. However, the lack of accurate, consistent measurement in mental health has historically received less attention. Subjective, episodic collection of patient and clinician-reported survey data has been the only option available to clinicians since the field began. Digital behavioral phenotyping uses data collected from sensor-enabled devices (e.g., smartphones) to create a personalized and precise picture of an individual's behavior. For the first time, shortcomings of the legacy approach to measurement are avoided, by enabling continuous, objective, ecologically valid measurement (1). In addition to behavioral phenotyping, digital interventions, such as computerized versions of evidence-based approaches like cognitive therapy, have been developed to improve access to care and augment standard treatment (2).
We report on a randomized clinical trial of a digital phenotyping-based intervention platform for individuals with depression. To the best of our knowledge, this is the first example of a “precision digital intervention,” i.e., one that leverages the continuous data produced by the smartphone to generate inferences about the patient's clinical state and then present the evidence-based micro-intervention that is most relevant to that patient at that time.
Cue is a smartphone sensor-based digital monitoring platform enhanced with psychoeducation on social rhythms and micro-interventions to increase social rhythm regularity, coupled with a clinician or study manager dashboard that displays the collected sensor data and patient self-report responses. Cue continuously monitors behavioral biomarkers and collects patient-reported data that provide an additional measurement of patients' daily routines or “social rhythms” such as sleep timing and daily self-rating of the user's mood and energy. Cue represents the first digital intervention based on the social rhythm regulation conceptual model (3, 4). According to this model, chaotic, irregular daily routines are associated with the onset and maintenance of mental disorder symptomatology (5, 6). A therapeutic approach that helps patients achieve more regular routines has been shown to improve mood disorder symptoms and maintain an asymptomatic state (7–12). We viewed the commercial smartphone as the ideal vehicle for measuring the extent of social rhythm disruption patients might be experiencing and for providing just-in-time micro-interventions to move them in the direction of greater social rhythm regularity. This constitutes a somewhat different concept of just-in-time interventions, most of which have been based until recently on ecological momentary assessment (EMA) and virtually immediate interventions to address the information provided via patient self-report (13) although Spruijt-Metz and colleagues have been advocating for more dynamic models that capitalize on the multiple capabilities of today's smartphones (14). Cue represents one such example of this approach by using passively and continuously collected behavioral data to determine patients' clinical status over the previous 3–4 days and then provides what a specific algorithm determines would be the most appropriate micro-intervention/behavior change suggestion at the point in time.
Originally conceptualized as a feasibility and acceptability study, the National Institute of Mental Health encouraged the investigators to use the planned clinical population (psychiatric outpatients with a lifetime diagnosis of a depressive and/or anxiety disorder and any level of current symptomatology) to explore the potential efficacy of such a digital intervention platform. We evaluated the efficacy of Cue relative to a sensor-based monitoring application (Measure) in a group of individuals who were concomitantly receiving outpatient psychiatric care as usual. We hypothesized that such a digital intervention platform would be associated with greater improvement in depressive symptoms than the comparator condition consisting of smartphone-based behavioral monitoring alone.
Materials and methods
Study designs and participants
Outpatients being treated at the University of Utah Department of Psychiatry who had indicated at the outset of their treatment that they were willing to be approached in the future about participating in research studies were contacted by phone on a sequential basis. Patients who appeared to be eligible and indicated an interest in the study were brought to the Department of Psychiatry for a single in-person visit to confirm eligibility and to obtain informed consent. All future contact with study participants occurred via email, text, or phone.
Patients were included if they had a lifetime diagnosis of a mood or anxiety disorder as assessed by the Mini International Neuropsychiatric Interview (15), were currently receiving treatment for their psychiatric disorder, were age of 18–65, if on psychotropic medication, were on a stable dose, owned and used an iPhone (5s or later) and were able and willing to provide written informed consent. Patients were excluded if they had a lifetime diagnosis of schizophrenia, antisocial personality disorder, primary obsessive-compulsive disorder, were currently psychotic or actively suicidal, met criteria for current alcohol, substance use or eating disorder. Those with a lifetime diagnosis of a bipolar disorder were not excluded if they were not currently psychotic. Individuals who had a poorly controlled medical condition that might cause confounding depressive symptoms (e.g., untreated thyroid disorder or lupus) or required medications that could cause depressive symptoms (e.g., high doses of beta blockers or alpha interferon) were also excluded. Individuals who were taking psychotropic medication at the time of study entry and required a change in dose or in medication(s) prescribed were discontinued from the protocol at the time of medication change. All participants provided informed consent according to procedures approved by the University of Utah Institutional Review Board.
Participants downloaded either Measure which was used to collect both passively sensed data (e.g., pedometer, display status, GPS location) and self-reported measures of clinical status; or the full digital intervention Cue app with (1) the identical monitoring component, (2) a series of psychoeducational Learning Modules and (3) personalized micro-interventions that were selected based on the user's sensed behavioral data and presented to the user via his/her phone every two to three days. Following download, participants were contacted by study personnel (typically only via email) only if there appeared to be a problem in the receipt of their sensed or self-reported data.
Randomization and patient allocation
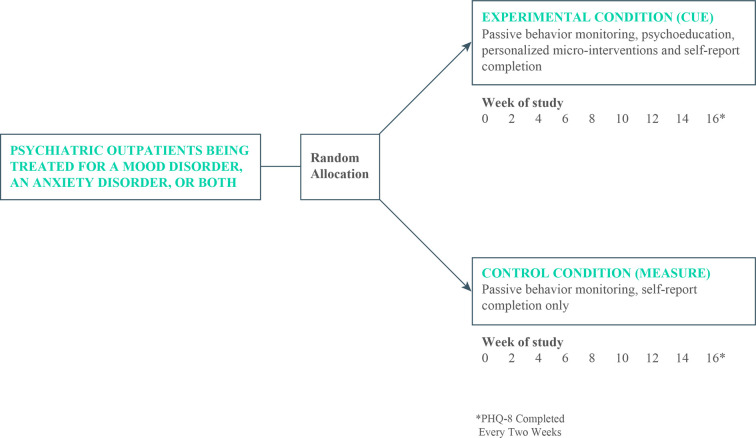
Participants were allocated on a 1:1 basis to either the control condition that consisted of treatment as usual by University of Utah faculty psychiatrists plus passive monitoring only or to an experimental group that received treatment as usual plus monitoring, psychoeducation, and personalized micro-interventions for a period of 16 weeks (see Figure 1).
Figure 1.
Design of RCT comparing a digital intervention platform with monitoring only.
Experimental intervention
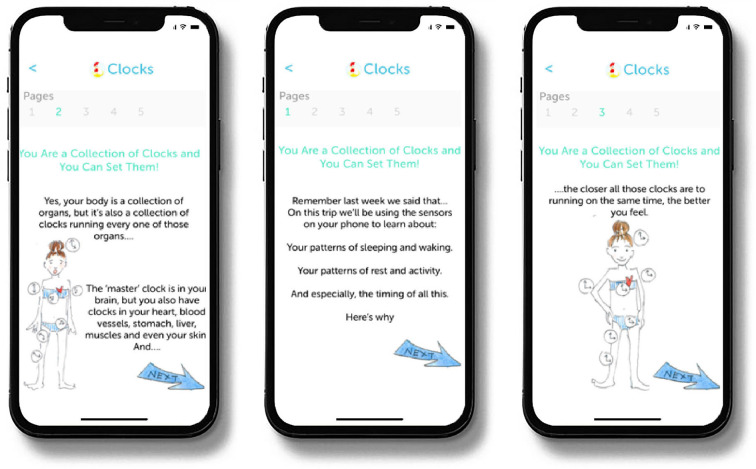
At the outset of the treatment period Cue provides patients with psychoeducational material, referred to as Learning Modules. The Learning Modules provide the rationale for the regular routines that the intervention is intended to help patients achieve and why such regularity can help reduce the severity of depressive symptoms and maintain mood stability. The 10 Learning Modules are released to the patient on a timed basis over the first three weeks of treatment but are also available for patients to refer to later should they choose to review them (see Figure 2).
Figure 2.
Sample learning module screens.
The digital intervention platform evaluated here is built on a well-validated conceptual framework (6, 7) that links irregularity of behavioral rhythms to illness onset (8, 9) and stability of behavioral rhythms to recovery and sustained wellness (10–14, 16). Using this conceptual framework, the platform interprets data gathered through phone sensors and patient self-reports to generate micro-interventions to improve patients' depression symptoms. In the present study, the intervention platform followed a 16-week timeline for providing such micro-interventions that were sent as push notifications to the patient's phone approximately every two to three days. An example of such a behavior change suggestion is displayed in Figure 3.
Figure 3.
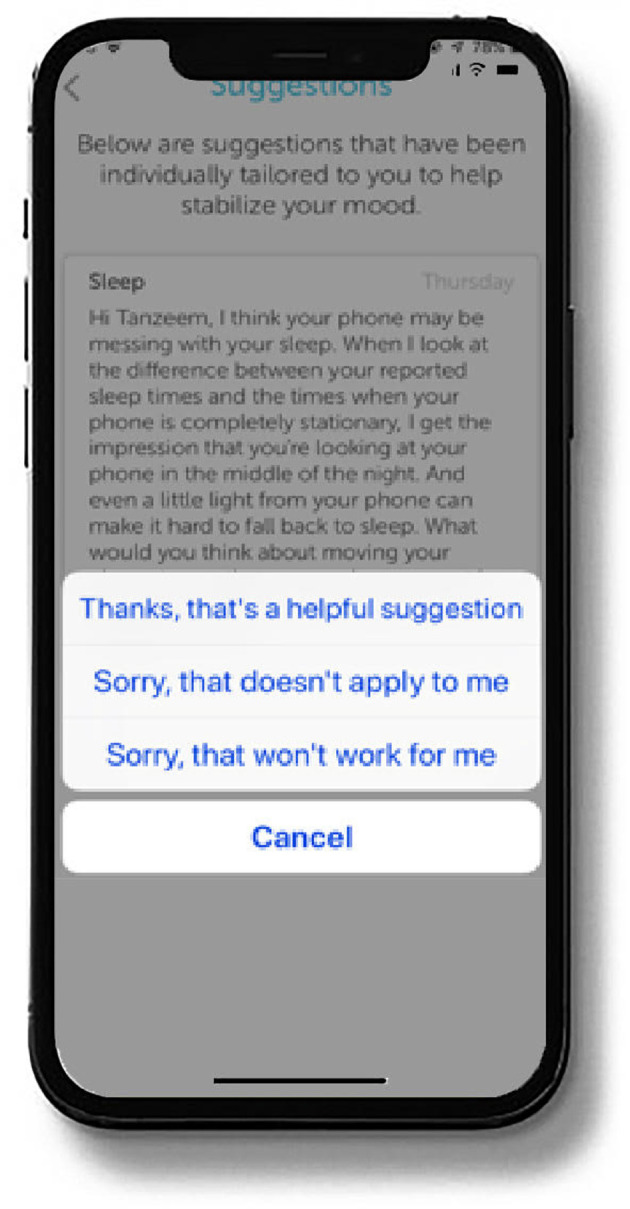
Sample micro-intervention/behavior change suggestion.
All participants completed a daily rating of mood on a visual analogue scale (scored from 0 = worst I've ever felt to 10 = best I've ever felt) and the PHQ-8, a well-accepted measure of depression severity (15) every two weeks throughout the study. We chose to use the PHQ-8 rather than the PHQ-9 which includes a suicidality item because the entirely virtual nature of the study did not allow for adequately rapid attention to reports of suicidality. Study participants were compensated on an escalating basis with gift cards valued respectively at $50, $75, $100, and $125 for the completion of the self-report questionnaires after each of the four months of participation.
In the present study, each participant's sensed behavioral and self-reported data were securely uploaded continuously to remote servers and then summarized on a dashboard that enabled an ongoing record of patient status. Personalized micro-interventions were crafted by the first author and Ms. Moore, both of whom are well-versed in the social rhythm regulation model. Reading of these personalized micro-interventions required minimal patient time (generally between 30 s and one minute) and reflected what was currently happening in the individual patient's life as indicated in their sensed data. For example, a patient who has a regular wake time during the week but tends to “sleep in” on Saturdays and Sundays might receive a micro-intervention pointing this out, reminding the patient that one of the best ways to keep the body's clock running efficiently is to stick to approximately the same wake time seven days a week. To facilitate this behavior change, the micro-intervention would typically suggest a means of achieving this goal such as considering meeting a friend for a walk or a coffee at 8:00 AM on Saturday and Sunday, so that their weekend wake times would approximate their weekday wake times. The micro-intervention would then encourage the patient to do so by pointing out that they will probably find it easier to get up on time and feel better on Monday if they try this.
Control intervention
The control condition consisted of Measure, a digital platform for sensed and self-reported behavioral monitoring. Participants allocated to the Measure monitoring only condition completed the same daily and bi-weekly self-report measures as those allocated to the experimental condition but did not receive any of the psychoeducational material or any behavior change micro-interventions.
Full study sample
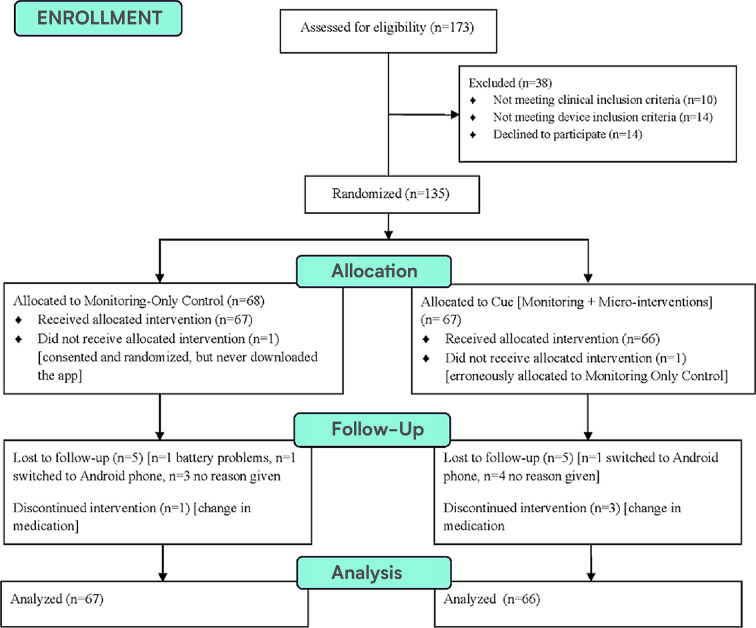
We consented 135 patients with a lifetime diagnosis of a mood and/or anxiety disorder to the 16-week trial. Two participants did not receive the allocated intervention (one who consented but never downloaded the platform and thus provided no PHQ data and another who inserted an incorrect participant code and thus received the monitoring alone instead of the full platform to which this individual was assigned), leaving 133 analyzable participants for the full study. Ten patients (7.4%) dropped out of the study prior to study week 16, and 4 (3%) were discontinued from the study according to the protocol because they experienced a change in medication (see Figure 4). The 133 analyzed participants were between the ages of 18 and 65, with a mean age of 33(±11). Seventy-four percent (n = 98) were female. Nineteen percent (n = 25) had a lifetime mood disorder diagnosis only, 11% (n = 15) had a lifetime anxiety diagnosis only, and 70% (n = 93) met lifetime criteria for both a mood disorder and an anxiety disorder. Eighty percent (n = 107) of participants were receiving pharmacotherapy prescribed by a University of Utah Department of Psychiatry physician (see Table 1). Data quality was excellent; the median coverage of 24/7 sensed behavioral data was 99.09% across all study days and the mean was 93.32%.
Figure 4.
CONSORT diagram.
Table 1.
Demographic and clinical characteristics for the full study sample – Cells show mean (SD) for continuous and % (N) for categorical measures.
| All (N = 133) | Cue (N = 66) | Measure (N = 67) | Comparison | |
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Age | 32.94 (11.39) | 33.85 (11.64) | 32.04 (11.15) | t = 0.91, p = 0.36 |
| Female | 73.68 (98) | 72.73 (48) | 74.63 (50) | X2 < 0.01, p = 0.96 |
| Employment Status | X2 = 2.38, p = 0.31 | |||
| Full Time Work | 60.15 (80) | 65.15 (43) | 55.22 (37) | |
| Part Time Work | 25.56 (34) | 19.7 (13) | 31.34 (21) | |
| Not Working | 14.29 (19) | 15.15 (10) | 13.43 (9) | |
| Living Situation | X2 = 5.38, p = 0.07 | |||
| Living Alone | 17.29 (23) | 22.73 (15) | 11.94 (8) | |
| Living with Family | 63.91 (85) | 65.15 (43) | 62.69 (42) | |
| Living with Unrelated People | 18.8 (25) | 12.12 (8) | 25.37 (17) | |
| Clinical characteristics | ||||
| First PHQ | 11.02 (4.74) | 11.53 (5.14) | 10.52 (4.28) | t = 1.23, p = 0.22 |
| First PHQ ≥15 | 21.05 (28) | 24.24 (16) | 17.91 (12) | X2 = 0.47, p = 0.49 |
| Depression or anxiety medication | 80.45 (107) | 80.3 (53) | 80.6 (54) | X2 < 0.01, p > 0.99 |
| Lifetime diagnosis | X2 = 1.97, p = 0.37 | |||
| Mood disorder only | 18.8 (25) | 18.18 (12) | 19.4 (13) | |
| Anxiety disorder only | 11.28 (15) | 7.58 (5) | 14.93 (10) | |
| Mood and anxiety disorder | 69.92 (93) | 74.24 (49) | 65.67 (44) | |
Exploratory subsample: patients entering in a major depressive episode
To assess the effect of the digital intervention on individuals suffering from a fully symptomatic episode of major depression at study entry, we selected those individuals who scored ≥15 on the PHQ-8 at their baseline assessment, an indicator of moderately severe or severe depression (15). This group consisted of 28 individuals, 12 of whom were allocated to monitoring only and 16 of whom were allocated to the full digital intervention platform. Twenty-five (89%) of these individuals were receiving antidepressant pharmacotherapy prescribed by University of Utah faculty psychiatrists at the time of study entry. Twenty-seven of these 28 participants completed the full 16-week trial. The one non-completer was withdrawn per protocol because of a change in medication, leaving 27 analyzable participants (see Table 2).
Table 2.
Demographic and clinical characteristics for the depressed-at-entry exploratory subsample (baseline PHQ ≥ 15) – Cells show mean (SD) for continuous and % (N) for categorical measures.
| All (N = 28) | Cue (N = 16) | Measure (N = 12) | Comparison | |
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Age | 34.04 (10.37) | 34.31 (10.28) | 33.67 (10.93) | t = 0.16, p = 0.88 |
| Female | 78.57 (22) | 68.75 (11) | 91.67 (11) | X2 = 0.99, p = 0.32 |
| Employment Status | X2 = 1.80, p = 0.41 | |||
| Full Time Work | 75 (21) | 75 (12) | 75 (9) | |
| Part Time Work | 10.71 (3) | 6.25 (1) | 16.67 (2) | |
| Not Working | 14.29 (4) | 18.75 (3) | 8.33 (1) | |
| Living Situation | X2 = 5.38, p = 0.07 | |||
| Living Alone | 17.86 (5) | 25 (4) | 8.33 (1) | |
| Living with Family | 71.43 (20) | 68.75 (11) | 75 (9) | |
| Living with Unrelated People | 10.71 (3) | 6.25 (1) | 16.67 (2) | |
| Clinical characteristics | ||||
| First PHQ | 17.57 (2.33) | 18.56 (2.42) | 16.25 (1.42) | t = 3.16, p = 0.004 |
| Depression or Anxiety Medication | 89.29 (25) | 87.5 (14) | 91.67 (11) | X2 < 0.01, p > 0.99 |
| Lifetime diagnosis | X2 = 1.40, p = 0.41 | |||
| Mood disorder only | 17.86 (5) | 12.5 (2) | 25 (3) | |
| Anxiety disorder only | 10.71 (3) | 6.25 (1) | 16.67 (2) | |
| Mood and Anxiety disorder | 71.43 (20) | 81.25 (13) | 58.33 (7) | |
Outcome analysis
Our original intention had been to use PROMIS (17) measures of depression, anxiety and sleep which were pushed to all patients in the University of Utah outpatient psychiatry clinic on a monthly basis as the primary outcomes of the present study. To our surprise, based on studies showing comparability with legacy measures such as the PHQ (18), the PROMIS measures performed poorly in this sample, demonstrating almost no variability over the course of the 16-week study. We therefore chose to focus the present report on the PHQ-8 which has been shown to be a valid measure of depression severity in outpatient psychiatric settings (19). The PHQ-8 was pushed to all participants in our trial on a bi-weekly basis through the Measure or Cue platform. The primary outcome of interest in the present report is differences in the slope of PHQ change over the course of the 16-week study, first in the full study sample and then in the exploratory depressed at entry subsample. A secondary outcome of interest is change in PHQ score from baseline to 16 weeks in the full study and depressed at entry subsample.
In the full study sample (N = 133) and then in the depressed-at-entry subsample (PHQ-8 ≥ 15; N = 28), we fit a mixed effects model with a random intercept and slope to test for group (experimental vs. control) differences in the slope of depressive symptoms over 16 weeks. Because there was a non-linear effect of week such that initial reductions in PHQ-8 were steeper than those later in follow-up, we used a square-root transformation of week. Evaluation of model fit criteria (AIC, BIC) indicated this was an optimal transformation (as compared to no transformation or a log transformation). Thus, these models included repeatedly measured PHQ-8 as the outcome with square root of week, group (Cue versus Measure), the square root week by group interaction, and covariates. Random intercept and time effects were included. From this model we also extracted the predicted random slope for each individual and used this to compute Cohen's d effect sizes for group differences in slope over 16 weeks. Secondarily, to allow further flexibility in the trajectory of PHQ-8 over follow-up, we fit a mixed effects model with categorical time (baseline, 2, 4, 6, 8, 10, 12, 14, 16 weeks), group, and the categorical time by group interaction, with specific contrasts to test group differences in changes from baseline to 16 weeks. All models covaried for age, sex, living status, employment status, pharmacotherapy use, and lifetime diagnosis.
Results
Full study sample
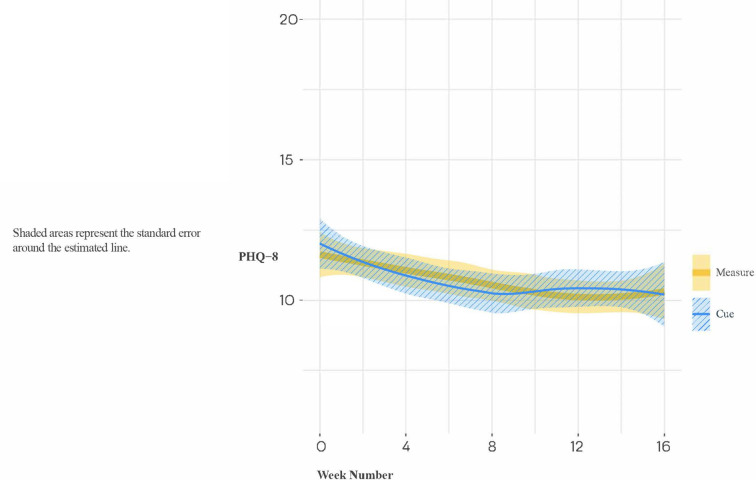
In the full study sample, as indicated in Figure 5, the slopes of change in PHQ scores did not differ significantly for Cue versus Measure (B[SE] = −0.15 [0.22], p = 0.507; Cohen's d [95% CI] = −0.10 [−0.45, 0.24]. However, there were significant group differences in change from baseline to 16 weeks (Estimate [SE] = −1.50[0.73], p = 0.040), with the group assigned to the digital intervention platform experiencing the greater improvement (see Figure 6).
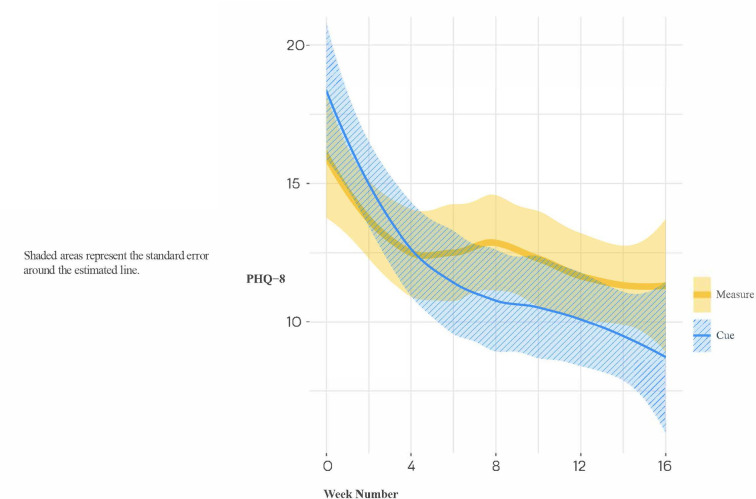
Figure 5.
Loess trajectories of PHQ-8 for Cue and measure (control) conditions in full study sample.
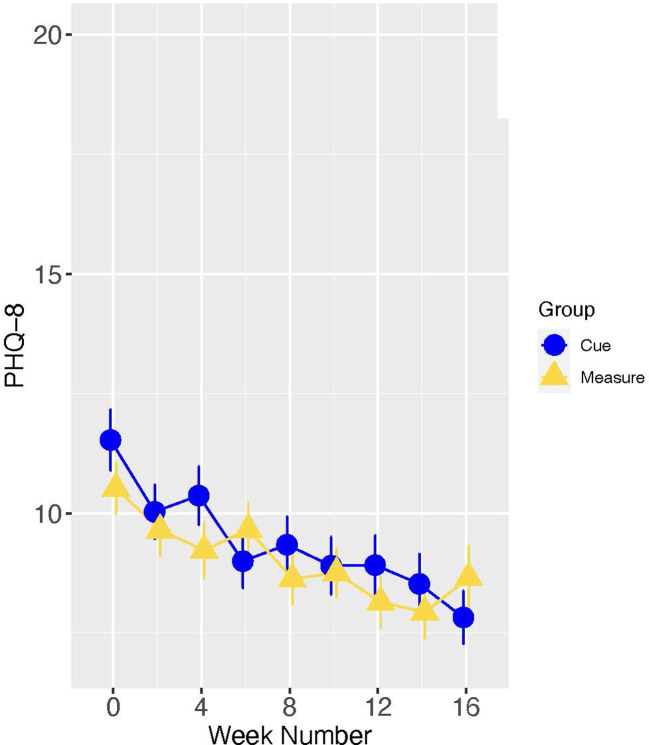
Figure 6.
Means and standard errors of PHQ scores by study week-full sample.
Depressed-at-entry subsample
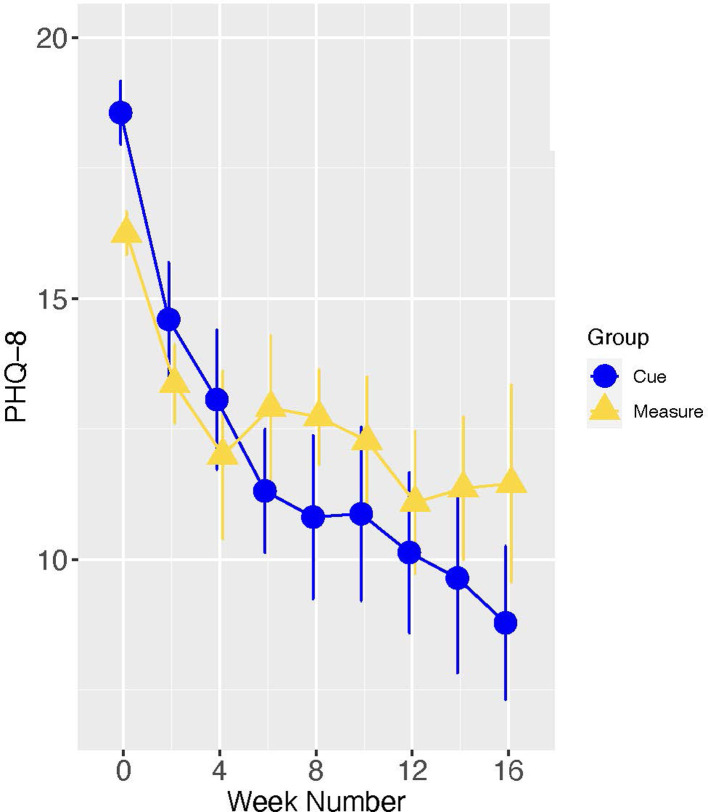
We then focused on the subsample of 28 study participants who entered the trial in a fully symptomatic episode of major depression, as these results have the greatest relevance for the ultimate utility of the Cue platform. In this subsample, the group by time interaction did not meet the conventionally accepted level of statistical significance (B[SE] = −1.18[0.62], p = 0.059). Notably, however, the effect size of the group difference (Cue versus Measure) in slope was moderate to large (Cohen's d [95% CI] = −0.72 [−1.53, 0.09]), indicating that acutely depressed individuals allocated to the experimental condition had a meaningfully steeper rate of improvement in PHQ-8 than those allocated to monitoring alone (see Figure 7). When treating time as categorical in secondary analyses, post-hoc analyses indicated a significant group difference in the PHQ-8 change from baseline to 16 weeks (Estimate [SE] = −4.74 [1.81], t = −2.63, p = 0.009) (see Figure 8).
Figure 7.
Loess trajectories of PHQ-8 for Cue and measure (control) conditions in depressed-at entry participants (initial PHQ-8 ≥ 15).
Figure 8.
Means and standard errors of PHQ scores by study week – depressed-at-entry sample.
Discussion
In the full study sample, consisting of psychiatric outpatients, the majority of whom had a lifetime diagnosis of major depression and an anxiety disorder, who were receiving care as usual and who had with any level of symptomatology at baseline, we did not observe a significant difference in the slopes of change between those assigned to the experimental digital intervention condition (Cue) and those assigned to the control digital monitoring condition (Measure). However, we did observe a significant difference between the two study conditions in change in PHQ-8 scores from baseline to 16 weeks, with the experimental group demonstrating the greater improvement.
Among the exploratory subsample of patients who were experiencing a moderately severe to severe episode of depression at baseline, we found preliminary evidence for a clinically meaningful benefit of a digital therapeutic based on social rhythm regulation principles when combined with outpatient psychiatric care consisting primarily of antidepressant pharmacotherapy. However, as this finding was exploratory and based on a relatively small sample size, this result will need to be replicated in an adequately powered trial.
It is noteworthy that the pattern of change displayed in Figure 8 is typical of that observed in many studies of depression treatment, i.e., one in which there is initial improvement in both conditions, probably as a result of the optimism and clinical attention associated with entering a treatment research program, that is then followed by a clinically meaningful separation between the active and control conditions.
Several other aspects of this trial are worthy of comment. The majority (80%) of study participants were receiving pharmacotherapy prescribed by University of Utah faculty psychiatrists throughout the course of the study. Thus, the observed effects of the digital intervention platform evaluated here are over and above the effects of standard of care pharmacotherapy. It has been rare for any psychosocial intervention to add significantly to evidence-based pharmacotherapy. Indeed, the only instance of this of which we are aware was the study of Cognitive Behavioral-Analysis System of Psychotherapy (CBASP) in patients with chronic depression (20). Furthermore, as part of a meta-analysis, Linardon et al. (21) reported on four trials that examined whether adding a smartphone intervention to a standard intervention (face-to-face, computerized, or pharmacotherapy) for depression was superior to a standard intervention-only condition. The pooled effect size for the four comparisons between smartphone intervention plus standard intervention vs. the standard intervention-only arm was g = 0.26 (95% CI: −0.09 to 0.61). These findings suggest that the effect size observed for Cue, when added to standard outpatient care in an academic department of psychiatry for patients with major depression, is substantially larger than those reported to date.
This is particularly relevant because the context in which we anticipate Cue being used is likely to be one in which most users are receiving medication. It is also worth noting that the proportion dropping out of the trial (7.4%) is among the lowest ever observed in a clinical trial of a depression intervention where drop-out rates in the 17.5%–47.8% range are common (22). Furthermore, both Cooper & Conklin (22) and Torous et al. (23) reported that dropout rates approaching 50% were typical of digital intervention trials for depression.
Much work has been done on the question of how best to deliver mobile health interventions (24–26). Much has also been written on developing behavioral biomarkers for various conditions based on specific sensors and variables, such as sleep, social media engagement etc. (27–37). In contrast, we are not aware of any previous effort to combine the benefits of continuous capture of objective behavioral biomarkers with a platform for the delivery of a digital intervention based on each user's own behavioral data. Nor are we aware of another digital therapeutic based on social rhythm regulation principles, an approach to which smartphone monitoring is especially well-suited because these devices can capture rhythm-relevant behaviors in a completely passive manner on a continuous 24/7 basis. We believe this combination of the novel capacities of the commercial smartphone and a therapeutic model uniquely suited to those capacities may partially explain the high levels of treatment adherence and the positive outcomes observed in those who entered the trial in a major depressive episode.
The present study is limited in several respects. The study population consisting of psychiatric outpatients with any level of symptom severity at baseline was not ideal for addressing the question of the efficacy of the Cue platform; nonetheless, the subset of patients experiencing at least moderately severe depression at baseline provided an opportunity to explore the potential therapeutic effects of this approach to digital intervention. The relatively small number of participants in the depressed-at-entry sample represents another limitation; however, the large effect size observed in this subgroup is encouraging. It is possible the some of the improvement observed in both the full study sample and in the depressed at entry sample may be attributable to pharmacotherapy. Unfortunately, the relatively small study samples and the wide variety of medication being taken at a wide variety of doses by the participants did not permit a meaningful evaluation of the role of pharmacotherapy in patient improvement. However, since a requirement of the protocol was that patients on pharmacotherapy remain on the same medication and the same dose throughout the trial, this somewhat reduces the likelihood that the improvements observed over the 16-week study period was a primarily a function of their pharmacotherapy. Finally, the results reported here emerge from a clinical trial setting in which participants were compensated for completion of the self-report instruments included in the protocol and may not be generalizable to clinical practice. The amount of human attention given to the participants following the initial evaluation visit was minimal. Once consented to the study, participants were only contacted by study personnel (typically only via email) if there appeared to be a problem in the receipt of their sensed or self-reported data and most participants had no contact with study personnel after the baseline/consent visit.
Encouraged by these results and recognizing that a platform that requires human curation of behavior change suggestions is not sustainable or scalable, we are in the process of developing and validating an automated micro-intervention generation engine based for an adaptation of Cue that is focused on patients with sleep problems. We will shortly be applying this automated approach to Cue for depression as well and are hopeful that these fully automated platforms will represent practical, scalable, and cost-effective approaches to addressing a variety of mental health problems.
Contribution to the field
Digital interventions represent one of the most promising approaches to increasing access to mental healthcare. Several examples of digital interventions have been developed and disseminated to patients via their computers and smartphones. These interventions benefit from their limitless, low-cost scalability, and evidence continues to grow that they are effective. However, they have historically been one-size-fits-all applications, which may account for a problematic record of poor engagement, with patients rapidly losing interest in static content.
Cue, a digital monitoring, psychoeducation, and intervention platform for depression, may be considered the first example of a precision digital intervention. Cue's AI-based solution translates passively acquired normal smartphone sensor data into detailed, continuous behavioral information about the patient's mental health status. Cue then uses that information to provide just-in-time micro-interventions sent to patients through the phone based on their own behavioral data, exposing patients to the intervention that is most relevant to them at any given moment.
We compared outcomes for Cue compared to behavioral monitoring alone among patients receiving treatment, primarily antidepressant medication, at the University of Utah Department of Psychiatry. Depressed patients who received Cue, in addition to outpatient treatment, improved approximately two times more than patients who received only outpatient treatment plus behavioral monitoring.
Acknowledgments
The authors would like to acknowledge Allison Bustamante, Sarah Commesso, and Paul Gilbert of HealthRhythms, Inc. for their dedication and support of this work, as well as Denzil Ferreira for his comments on a previous version of this manuscript. They would also like to thank Adam Haim, PhD of the National Institute of Health for his support and encouragement throughout the process of developing and testing of the digital intervention described here.
Funding Statement
Research reported in this publication was supported by a Small Business Innovation Research (SBIR) award from the National Institute of Mental Health, Award Number R44MH113520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by University of Utah Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
EF, MJM, TM, JK, TC and DK contributed to conception, design and conduct of the study. MJM, GP, and SAB developed the digital platforms to be tested. GP and JL organized the database. MLW and JL performed the statistical analysis. EF, NS and ZF wrote the first draft of the manuscript. EF, MLW, and GA wrote additional sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare the following commercial and/or financial relationships with HealthRhythms, Inc., the developer of the digital intervention platform that is the focus of this report. Frank, Matthews, Leach, and Aranovich, Moore, Posey and Burgess are employees of and hold equity in HealthRhythms, Inc. Choudhury, Shah and Kupfer are advisors to and hold equity in HealthRhythms, Inc. Wallace is employed as a part-time consultant to HealthRhythms, Inc. and is also a statistical consultant for Noctem Health and Sleep Number Bed Corporation. Framroze declares that she has no commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Hidalgo-Mazzei D, Young AH, Vieta E, Colom F. Behavioural biomarkers and mobile mental health: a new paradigm. Int J Bipolar Disord. (2018) 6(1):9. 10.1186/s40345-018-0119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apolinário-Hagen J, Druge M, Fritsche L. Cognitive behaviorla therapy, minfulness-based cognitive therapy and acceptance commitment therapy for anxiety disorders: integrating tradiontal with digital treatment approaches. Adv Exp Med Biol. (2020) 1191:291–329. 10.1007/978-981-32-9705-0_17 [DOI] [PubMed] [Google Scholar]
- 3.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. Arch Gen Psychiatry. (1988) 45:948–52. 10.1001/archpsyc.1988.01800340076012 [DOI] [PubMed] [Google Scholar]
- 4.Ehlers CL, Kupfer DJ, Frank E, Monk TH. Biological rhythms and depression: the role of zeitgebers and zeitstorers. Depression. (1993) 1:285–93. 10.1002/depr.3050010602 [DOI] [Google Scholar]
- 5.Malkoff-Schwartz S, Frank E, Anderson BP, Sherrill JF, Siegel L, Patterson D, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes. Arch Gen Psychiatry. (1998) 55:702–7. 10.1001/archpsyc.55.8.702 [DOI] [PubMed] [Google Scholar]
- 6.Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, et al. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol Med. (2000) 30:1005–16. 10.1017/s0033291799002706 [DOI] [PubMed] [Google Scholar]
- 7.Frank E, Kupfer DJ, Thase ME, Malinger AG, Swartz HA, Fagiolini A, et al. Two-year outcomes for individuals with bipolar I disorder. Arch Gen Psychiatry. (2005) 62:996–1004. 10.1001/archpsyc.62.9.996 [DOI] [PubMed] [Google Scholar]
- 8.Frank E. Treating bipolar disorder: A Clinician's guide to interpersonal and social rhythm therapy. New York: Guilford Press; (2005). [Google Scholar]
- 9.Goldstein TR, Fersch-Podrat R, Axelson DA, Gilbert A, Hlastala SA, Birmaher B, et al. Early intervention for adolescents at high risk for the development of bipolar disorder: pilot study of interpersonal and social rhythm therapy (IPSRT). Psychotherapy. (2014) 1:180–9. 10.1037/a0034396 [DOI] [PubMed] [Google Scholar]
- 10.Inder ML, Crowe MT, Luty SE, Carter JD, Moor S, Frampton CM, et al. Randomized, controlled trial of interpersonal and social rhythm therapy for young people with bipolar disorder. Bipolar Disord. (2015) 2:128–38. 10.1111/bdi.12273 [DOI] [PubMed] [Google Scholar]
- 11.Frank E, Soreca I, Swartz HA, Fagiolini AM, Mallinger AG, Thase ME, et al. The role of interpersonal and social rhythm therapy in improving occupational functioning in patients with bipolar I disorder. Am J Psychiatry. (2008) 165(12):1559–65. 10.1176/appi.ajp.2008.07121953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corruble E, Swartz HA, Bottai T, Vaiva G, Bayle F, Llorca PM, et al. Telephone-administered psychotherapy in combination with antidepressant medication for the acute treatment of major depressive disorder. J Affect Disord. (2016) 190:6–11. 10.1016/j.jad.2015.07.052 [DOI] [PubMed] [Google Scholar]
- 13.Coppersmith DD, Dempsey W, Kleiman E, Bentley K, Murphy S, Nock M. Just-in-time adaptive interventions for suicide prevention: promise, challenges, and future directions. (2021) 84:1–17. 10.31234/osf.io/eg9fx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hekler EB, Michie S, Pavel M, Rivera DE, Collins LM, Jimison HB, et al. Advancing models and theories for digital behavior change interventions. Am J Prev Med. (2016) 51(5):825–32. 10.1016/j.amepre.2016.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. (2009) 114(1-3):163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 16.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 17.www.nihpromis.org Patient-Reported Outcomes Measurement Information System (PROMIS). Available at:
- 18.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess. (2014) 26(2):513–27. 10.1037/a0035768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Levis B, Riehm K, Saadat N, Levis A, Azar M, et al. Equivalency of the diagnostic accuracy of the PHQ-8 and PHQ-9: a systematic review and individual participant data meta-analysis. Psychol Med. (2020) 50(8):1368–80. 10.1017/S0033291719001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg SB, Lam SU, Simonsson O, Torous J, Sun S. Mobile phone-based interventions for mental health: a systematic meta-review of 14 meta-analyses of randomized controlled trials. PLOS Digital Health. (2022) 1(1):e0000002. 10.1371/journal.pdig.0000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manber R, Kraemer HC, Arnow BA, Trivedi MH, Rush AJ, Thase ME, et al. Faster remission of chronic depression with combined psychotherapy and medication than with each therapy alone. J Consult Clin Psychol. (2008) 76(3):459–67. 10.1037/0022-006X.76.3.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AA, Conklin LR. Dropout from individual psychotherapy for major depression: a meta-analysis of randomized clinical trials. Clin Psychol Rev. (2015) 40:57–65. 10.1016/j.cpr.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 23.Torous J, Lipschitz J, Ng M, Firth J. Dropout rates in clinical trials of smartphone apps for depressive symptoms: a systematic review and meta-analysis. J Affect Disord. (2020) 263:413–9. 10.1016/j.jad.2019.11.167 [DOI] [PubMed] [Google Scholar]
- 24.Bucci S, Schwannauer M, Berry N. The digital revolution and its impact on mental health care. Psychol Psychother. (2019) 92(2):277–97. 10.1111/papt.12222 [DOI] [PubMed] [Google Scholar]
- 25.Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. (2016) 18(6):e135. 10.2196/jmir.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp J, Zhang T, Inglis F, Wiljer D, Sockalingam S, Crawford A, et al. Delivery of compassionate mental health care in a digital technology-driven age: scoping review. J Med Internet Res. (2020) 22(3):e16263. 10.2196/16263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeb S, Cybulski TR, Schueller SM, Kording KP, Mohr DC. Scalable passive sleep monitoring using mobile phones: opportunities and obstacles. J Med Internet Res. (2017) 19(4):e118. 10.2196/jmir.6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naslund JA, Bondre A, Torous J, Aschbrenner KA. Social media and mental health: benefits, risks, and opportunities for research and practice. J Technol Behav Sci. (2020) 5(3):245–57. 10.1007/s41347-020-00134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr DC, Zhang M, Schueller SM. Personal sensing: understanding mental health using ubiquitous sensors and machine learning. Annu Rev Clin Psychol. (2017) 13(13):23–47. 10.1146/annurev-clinpsy-032816-044949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler DA, Ben-Zeev D, Tseng VW, Kane JM, Brian R, Campbell AT, et al. Predicting early warning signs of psychotic relapse from passive sensing data: an approach using encoder-decoder neural networks. JMIR Mhealth Uhealth. (2020) 8(8):e19962. 10.2196/19962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdullah S, Murnane E, Matthews M, Kay M, Keintz J, Gay G, et al. Cognitive rhythms: unobtrusive and continuous sensing of alertness using a mobile phone. Ubicomp’16. (2016). p. 178–89. 10.1145/2971648.2971712 [DOI] [Google Scholar]
- 32.Matthews M, Abdullah S, Murnane E, Voida S, Choudhury T, Gay G, et al. Development and evaluation of a smartphone-based measure of social rhythms for bipolar disorder. Assessment. (2016) 23(4):472–83. 10.1177/1073191116656794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdullah S, Matthews M, Frank E, Doherty G, Gay G, Choudhury T. Automatic detection of social rhythms in bipolar disorder. J Am Med Inform Assoc. (2016) 23(3):538–43. 10.1093/jamia/ocv200 [DOI] [PubMed] [Google Scholar]
- 34.Murnane EL, Cosley D, Chang P, Guha S, Frank E, Gay G, et al. Self-monitoring practices, attitudes, and needs of individuals with bipolar disorder: implications for the design of technologies to manage mental health. J Am Med Inform Assoc. (2016) 23(3):477–84. 10.1093/jamia/ocv165 [DOI] [PubMed] [Google Scholar]
- 35.Ben-Zeev D, Wang R, Abdullah S, Brian R, Scherer EA, Mistler LA, et al. Mobile behavioral sensing for outpatients and inpatients with schizophrenia. Psychiatr Serv. (2016) 67(5):558–61. 10.1176/appi.ps.201500130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews M, Abdullah S, Gay G, Choudhury T. Tracking mental well-being: striking the right balance between rich sensing and patient needs. IEEE Comput. (2014) 47:36–43. 10.1109/MC.2014.107 [DOI] [Google Scholar]
- 37.Berke EM, Choudhury T, Ali S, Rabbi M. Objective measurement of sociability and activity: mobile sensing in the community. Ann Fam Med. (2011) 9(4):344–50. 10.1370/afm.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.