Abstract
Subacute combined degeneration (SCD), caused by vitamin B12 disorders, leads to severe degeneration of the spinal cord. Thus, it is significant to make timely diagnosis and treatment options of SCD. The objectives were to summarize clinical features of different sate SCD. Clinical data of 42 SCD patients of spinal cord were retrospectively analyzed, which were classified into early stage, middle stage and late stage SCD. Among the patients, 9 were classified into early stage, 22 into middle stage, and 11 into late stage SCD. Total cholesterol and hemoglobin levels were relatively higher in late stage SCD. In contrast, mean corpusular volume (MCV) level was higher in early stage SCD. There were typical abnormalities only in 8 patients on magnetic resonance imaging (MRI), and a dynamia was a common neurological abnormality in all patients. Importantly, the differences in abnormal findings in anti-nuclear antibodies (ANA) testing, visual acuity and fundus testing were statistically significant in different stage SCD (P < .05). There were correlation between most variances with SCD stage. Strikingly, there existed close relationship between enhanced levels of blood glucose (r = −0.289, P = .066), glycated hemoglobin (GHB) (r = −0.288, P = .068) and homocysteine (r = −0.563, P = .000), abnormal visual findings (r = 0.309, P = .049) and megaloblastic anemia (r = −0.295, P = .061) with different SCD stage, among which abnormal visual findings were closely associated with middle stage SCD. Moreover, levels of total cholesterol, blood glucose, homocysteine and abnormal finding of visual acuity were significant in diagnosis and clinical staging of SCD (P < .05). Although MRI scanning and serum vitamin B12 level were widely used for SCD diagnosis, neurological examination and homocysteine level may be more potentially valuable indexes for SCD diagnosis and staging.
Keywords: clinical features, diagnosis, stage, subacute combined degeneration
1. Introduction
Subacute combined degeneration (SCD), as a rare neurological complication, is caused by the intake, absorption, binding, transport or metabolic disorders of vitamin B12, thus bringing about progressive degeneration of the spinal cord.[1,2] SCD continues to be a serious public health issue of global sanitary and economic importance, with the highest incidence observed in the elderly individuals.[3] Clinically, most patients with SCD mainly presented as abnormalities of gastrointestinal, hematologic, nervous and mental system. In the early stage, atypical manifestations of fatigue, anemia, glossitis, diarrhea were observed in some patients. Moreover, abnormality of nervous symptoms, including clumsy movement of lower limbs, feeling of stepping on cotton, unsteady walking, abnormal feeling of peripheral limbs, symmetrical tingling, numbness and burning sensation, were also common.[4,5] Relatively, there occurred visual abnormalities, sphincter dysfunction, cognitive dysfunction, mental disorders, postural hypotension, bronchospasm and arrhythmia in later stage SCD, and some patients even may lose the ability of walk and become blind.[6,7]
To date, imaging and serological testing of vitamin B12 are used for SCD diagnosis and long-term follow-up. However, Spence has demonstrated that vitamin B12 was at the normal level in some SCD patients, suggesting that serum vitamin B12 detection was not an appropriate diagnostic approach in certain conditions.[8,9] In addition, electromyography, as an objective diagnostic tool, was widely applied to determine the location of SCD abnormalities.[10] Enhanced magnetic resonance imaging (MRI) may show isointense or slight hypointense on T1WI, but significant hyperintense on T2-weighed images, which was a reliable method for SCD diagnosis and widely used for follow-up after treatment. Unfortunately, it has also shown that there may occur brain white matter abnormality on MRI findings and the sensitivity of MRI was not available for predicting SCD diagnosis at later stage.[11,12] Therefore, it is of great significance to make timely diagnosis and treatment options of SCD in clinical settings. Herein, this study aimed to summarize clinical features of 42 patients with different stage SCD, which may provide valuable references for medical professionals in their clinical work.
2. Materials and Methods
2.1. Ethics review
Design of this study was in accordance with the Helsinki Declaration and were approved by the Human Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. Informed consents were obtained from the patients for anonymous collection, analysis and publication of their clinical data. All research was performed in accordance with relevant guidelines and regulations. Patients under the age of 18 were not included in this study.
2.2. Inclusion and exclusion criteria
With reference to the previous studies,[7,13] inclusion criteria for SCD patients were included all the following aspects: Patients with subacute course presented as the clinical manifestations and physical signs of posterior and lateral columns; those with peripheral nerve impairment; clinical symptoms improved greatly after cobalamin (vitamin B12); evidence of vitamin B12 deficiency; typical lesions of the spinal cord on MRI.
Exclusion criteria were as follows: Patients with other spinal cord or peripheral nerve diseases; those with previous history of hypertension, hypoxia, inflammation, diabetes, rheumatic immune-associated diseases.
2.3. Study subjects
A retrospective study involving a total of 42 patients who were diagnosed with SCD at the First Affiliated Hospital of Xinjiang Medical University from January 2018 to January 2021 were conducted in this study. There were 25 males and 17 females, whose age ranged from 19 to 79 years, with the average age of 44.76 ± 12.56 years. According to the disease course, the patients were classified into 3 stages: Patients with early stage SCD: the disease course ranged from 1 to 10 month, and there were 9 cases; patients with middle stage SCD: the disease course ranged from 11 to 17 month, and there were 22 cases; patients with late stage SCD: the disease course ranged from 18 to 25 month, and there were 11 cases.
2.4. Evaluation methods
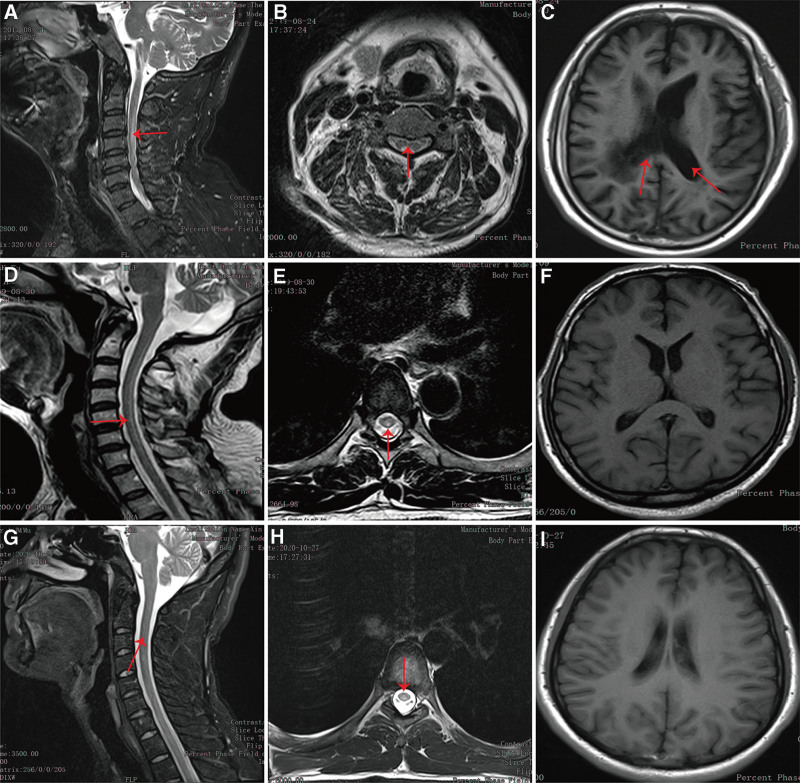
Routine blood tests, serum vitamin B12 and homocysteine level, neurological examination, MRI scanning were performed in all patients, which were conducted according to previously published studies.[14,15] In detail, serum vitamin B12 level were detected by chemiluminescence immunoassay using IMMULITI kit (HUAYUEYANG Biotech Co., Ltd., Beijing, China) in the First Affiliated Hospital of Xinjiang Medical University, whose normal rage was 180 to 914 pg/mL. An enzymatic method was used for measurement of homocysteine level. Neurological examination was performed by professional neurologists independently to identify impairment severity. MRI results were read by professional radiologists and radiologists. On MRI scanning, multifocal and symmetrical abnormal signals were observed in white manner. On T2-weighed images, long and high signals with “strip” and “inverted V” sign were observed. Representative imaging for the patients were shown in Figure 1.
Figure 1.
Representative MRI imaging. (A) On the sagittal position of T2WI, there were abnormal high signals (red arrow) at the third and forth cervical spinal cord of early stage SCD. (B) On the horizontal axis position of T2WI, high signals with “eight” sign (red arrow) were found at the third cervical spinal cord of early stage SCD. (C) Brain MRI showed enlarged ventricles (red arrow) in the early stage SCD. (D) On the sagittal position of T2WI, there were long and high signals (red arrow) in the spinal cord of middle stage SCD. (E) On the horizontal axis position of T2WI, abnormal high signals with “inverted V” sign (red arrow) were found at the third thoracic spinal cord of middle stage SCD. (F) Brain MRI showed no obvious abnormalities in the middle stage SCD. (G) On the sagittal position of T2WI, there were long and high signals (red arrow) from the second cervical spinal cord to the second thoracic spinal cord of late stage SCD. (H) On the horizontal axis position of T2WI, abnormal high signals with “round dot” sign (red arrow) were found at the third thoracic spinal cord of late stage SCD. (I) Brain MRI showed no obvious abnormalities in the late stage SCD. MRI = magnetic resonance imaging, SCD = subacute combined degeneration, T2WI = T2-weighed images.
2.5. Treatment and follow-up
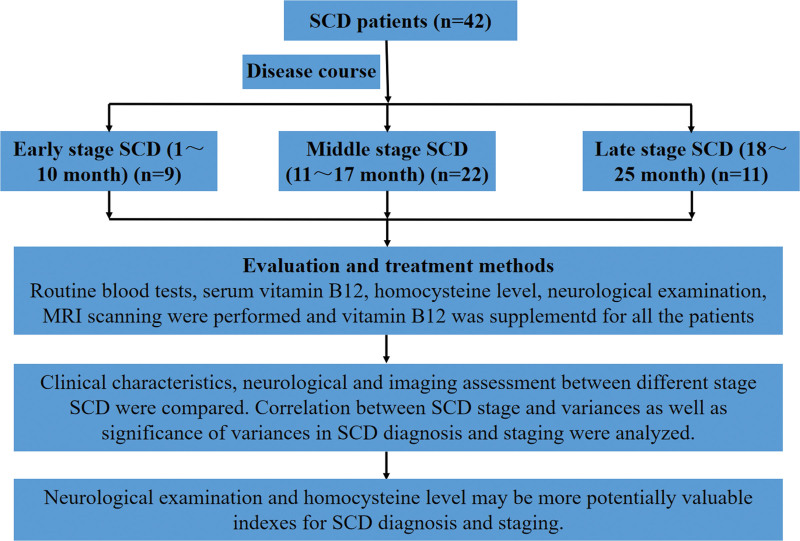
All patients received cobalamin (vitamin B12, Fujian Gutian Pharma Co., Ltd., Fujian, China) as treatment in Rehabilitation Department of the First Affiliated Hospital of Xinjiang Medical University, whose dosage and medication time were determined based on patient’s specific condition. Generally, cobalamin (vitamin B12) was given 2 mg per day for 1 week intravenously and then 1.5 mg per day for 4 weeks orally. All the patients were successfully followed up through the outpatient or telephone consultation. During follow-up, clinical symptoms and physical signs were greatly improved. A schematic diagram was shown in Figure 2.
Figure 2.
A schematic diagram used for the study. The diagram showing main methodological aspect of SCD patients in this current study. SCD = subacute combined degeneration.
2.6. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science version 21.0 (Statistical Package for Social Science Inc, Chicago, IL) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA). First, a normality test was performed. Then, all quantitative data were presented as mean ± standard deviation. Statistical comparisons of parameters between groups were made by Student t test and repeated measures ANOVA was used for comparison between groups. Qualitative data were tested by Pearson Chi-squared test or Fisher exact test. Correlation between influencing factors and different stage SCD were evaluated using the correlation and Multinominal Logistic Regression Analysis. Statistical significance was set at the 5% level and P < .05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of the participants
The diagnosis of all the patients included in this study was clearly confirmed and baseline characteristics were shown in Table 1. The etiology of B12 deficiency in most patients was as follows: unbalanced diet behavior, calcium deficiency caused by unwillingness of sun-bathe and loss of appetite because of psychological stress and getting older. Among the patients, 9 cases were classified into early stage SCD, and there were 6 males and 3 females, whose average age was (47.67 ± 18.25) year. Relatively, 22 patients were classified into middle stage SCD, and there were 13 males and 9 females, whose average age was (45.18 ± 11.44) year. In addition, there was 11 patients who classified into late stage SCD. There were 6 males and 5 females, whose average age was (41.55 ± 9.30) year. Although there were no significant differences in the baseline characteristics between different stage SCD, the differences in total cholesterol, mean corpusular volume (MCV) and hemoglobin levels were statistically significant (P < .05), among which total cholesterol and hemoglobin levels were relatively higher in late stage SCD. However, MCV level was at relatively higher level in early stage SCD, suggesting that these above variance may be potentially used for identifying different stage SCD in clinical settings.
Table 1.
Characteristics of the included participants.
| Variances | Early stage SCD (n = 9) | Middle stage SCD (n = 22) | Late stage SCD (n = 11) | P value |
|---|---|---|---|---|
| Age (yr) | 47.67 ± 18.25 | 45.18 ± 11.44 | 41.55 ± 9.30 | .553 |
| Sex (male:female) | 9:6 | 13:9 | 6:5 | .858 |
| Smoking | 2 | 8 | 2 | .221 |
| Triglyceride | 0.97 ± 0.52 | 1.31 ± 0.70 | 0.99 ± 0.23 | .203 |
| Total cholesterol | 2.93 ± 0.77 | 3.78 ± 1.02 | 3.94 ± 0.68 | .033 |
| LDL | 1.94 ± 0.61 | 2.55 ± 0.92 | 2.71 ± 0.58 | .080 |
| HDL | 0.81 ± 0.24 | 1.02 ± 0.25 | 0.99 ± 0.31 | .102 |
| Urea | 5.24 ± 0.82 | 5.64 ± 1.70 | 5.29 ± 1.51 | .203 |
| Creatinine | 58.87 ± 13.07 | 55.12 ± 24.28 | 67.97 ± 10.80 | .218 |
| Uric acid | 262.15 ± 92.71 | 298.60 ± 104.54 | 243.65 ± 41.73 | .233 |
| Apo-α | 0.86 ± 0.19 | 1.10 ± 0.29 | 1.07 ± 0.24 | .071 |
| Apo-β | 0.69 ± 0.22 | 1.66 ± 2.87 | 0.81 ± 0.29 | .385 |
| Total protein | 64.28 ± 6.48 | 65.35 ± 4.03 | 67.27 ± 6.22 | .425 |
| Albumin | 40.28 ± 3.56 | 39.87 ± 3.18 | 41.62 ± 3.24 | .360 |
| Globulin | 24.00 ± 5.34 | 25.65 ± 4.19 | 25.51 ± 6.18 | .699 |
| Blood glucose | 4.92 ± 2.47 | 4.73 ± 0.93 | 4.65 ± 0.60 | .896 |
| GHB (%) | 5.72 ± 1.20 | 5.33 ± 0.98 | 5.25 ± 0.33 | .469 |
| Hemoglobin | 113.67 ± 21.16 | 127.95 ± 10.45 | 135.91 ± 14.58 | .005 |
| MCV | 100.76 ± 17.30 | 98.27 ± 15.04 | 86.47 ± 5.00 | .041 |
| Folic acid | 23.45 ± 14.57 | 21.75 ± 15.05 | 10.57 ± 10.40 | .066 |
| Ferritin | 144.04 ± 171.81 | 145.60 ± 89.45 | 166.27 ± 95.69 | .867 |
| Erythropoietin | 81.10 ± 193.16 | 25.07 ± 42.90 | 7.19 ± 3.19 | .195 |
| D-dimer | 425.44 ± 363.00 | 297.41 ± 325.20 | 159.99 ± 193.62 | .166 |
| Homocysteine | 34.79 ± 57.98 | 27.47 ± 36.54 | 47.95 ± 94.53 | .199 |
| Fibrinogen | 3.11 ± 0.75 | 3.36 ± 2.78 | 3.14 ± 2.08 | .716 |
| Vitamin B12 | 543.01 ± 380.35 | 523.83 ± 446.03 | 665.81 ± 448.92 | .668 |
| Cerebrospinal fluid protein (g/L) | 0.35 ± 0.155 | 0.311 ± 0.09 | 0.42 ± 0.25 | .177 |
P level of test conducted 5% and quantitative data were represented mean ± standard deviation.
Apo-α = apolipoprotein-α, Apo-β = apolipoprotein-β, GHB = glycated hemoglobin, LDL = low-density lipoprotein, HDL = high-density lipoprotein, MCV = mean corpusular volume, SCD = subacute combined degeneration.
3.2. Neurological and imaging assessment
Although MRI scanning of the spinal was widely applied for SCD diagnosis, typical abnormalities were only found 8 patients on MRI scanning, including 3 early stage, 4 middle stage and one late stage SCD. Moreover, there were different degree of neurological disorders in different stage SCD, including incontinence and abnormal findings in electromyogram, visual acuity, anti-nuclear antibodies (ANA), fundus testing, activities of daily living and venous thromboembolism. Importantly, adynamia was found in all patients, which may be valuable for SCD diagnosis. Moreover, speech and swallow disorders as well as abnormal thyroid function were also common abnormalities in most SCD patients. Strikingly, there were significant differences in ANA testing, visual acuity and fundus testing between different stage SCD (P < .05), suggesting that theses neurological examinations were of great significance in identifying different stage SCD and making SCD diagnosis. Neurological and imaging details were shown in Table 2.
Table 2.
Neurological and imaging abnormalities in different stage SCD.
| Abnormal findings | Early stage SCD (n = 9) | Middle stage SCD (n = 22) | Late stage SCD (n = 11) | P value |
|---|---|---|---|---|
| MRI | 3 | 4 | 1 | .385 |
| ANA | 5 | 3 | 0 | .005 |
| Incontinence | 6 | 15 | 6 | .580 |
| EMG | 8 | 15 | 7 | .413 |
| Visual acuity | 2 | 16 | 7 | .028 |
| Fundus testing | 0 | 9 | 2 | .024 |
| Adynamia | 9 | 21 | 11 | 1.000 |
| Sensory impairment | 9 | 21 | 10 | .236 |
| Speech and swallow disorder | 1 | 3 | 4 | .727 |
| Megaloblastic anemia | 2 | 3 | 1 | .708 |
| Abnormal thyroid function | 0 | 5 | 1 | .221 |
| ADL | 3 | 7 | 6 | .657 |
| VTE | 2 | 2 | 0 | .448 |
P level of test conducted 5%.
ADL = activities of daily living, ANA = anti-nuclear antibodies, EMG = electromyogram, MRI = magnetic resonance imaging, SCD = subacute combined degeneration, VTE = venous thromboembolism.
3.3. Correlation analysis between SCD stage and significant variances
Correlation analysis showed that although there was positive correlation between SCD stage and most of the variances (correlation index > 0), there was still negative correlation between SCD stage and some variances including previous history of smoking, levels of creatinine, blood glucose, glycated hemoglobin (GHB), ferritin, erythropoietin, D-dimer, homocysteine, incontinence or not, abnormal electromyogram findings and megaloblastic anemia or not (correlation index < 0). Importantly, there were statistical differences in the correlation analysis of blood glucose level, HbAc level, homocysteine level, visual acuity and megaloblastic anemia or not with different stage SCD (P < .05, Table 3), suggesting their potential value in identifying SCD stage.
Table 3.
Correlation analysis between different stage SCD and variances.
| Variances | Correlation index | P value |
|---|---|---|
| Age (yr) | 0.216 | .176 |
| Sex (male:female) | 0.001 | .997 |
| Smoking | −0.104 | .516 |
| Triglyceride | 0.057 | .724 |
| Total cholesterol | 0.238 | .133 |
| LDL | 0.169 | .290 |
| HDL | 0.237 | .136 |
| Urea | 0.045 | .780 |
| Creatinine | −0.026 | .876 |
| Uric acid | 0.116 | .471 |
| Apo-α | 0.250 | .114 |
| Apo-β | 0.116 | .469 |
| Total protein | 0.202 | .205 |
| Albumin | 0.031 | .845 |
| Globulin | 0.201 | .208 |
| Blood glucose | −0.289 | .066 |
| GHB (%) | −0.288 | .068 |
| Hemoglobin | 0.248 | .117 |
| MCV | −0.209 | .190 |
| Folic acid | 0.028 | .864 |
| Ferritin | −0.106 | .509 |
| Erythropoietin | −0.251 | .113 |
| D-dimer | −0.267 | .091 |
| Homocysteine | −0.563 | .000 |
| Fibrinogen | 0.052 | .745 |
| Vitamin B12 | 0.163 | .309 |
| Cerebrospinal fluid protein (g/L) | 0.039 | .810 |
| MRI | 0.088 | .582 |
| ANA | 0.030 | .853 |
| Incontinence | −0.141 | .378 |
| EMG | −0.016 | .922 |
| Visual acuity | 0.309 | .049 |
| Fundus testing | 0.195 | .221 |
| Sensory impairment | 0.053 | .744 |
| Speech and swallow disorder | 0.080 | .617 |
| Megaloblastic anemia | −0.295 | .061 |
| Abnormal thyroid function | 0.127 | .429 |
| ADL | 0.014 | .930 |
| VTE | 0.066 | .680 |
P level of test conducted 5%.
ADL = activities of daily living, ANA = anti-nuclear antibodies, Apo-α = apolipoprotein-α, Apo-β = apolipoprotein-β, EMG = electromyogram, GHB = glycated hemoglobin, HDL = high-density lipoprotein, LDL = low-density lipoprotein, MCV = mean corpusular volume, MRI = magnetic resonance imaging, SCD = subacute combined degeneration, VTE = venous thromboembolism.
3.4. Multinominal logistic regression analysis of significant variances
To further exclude the multi-collineality, Multinominal Logistic Regression Analysis of these variance (blood glucose level, GHB level, homocysteine level, visual acuity and megaloblastic anemia or not) that had significant correlation between SCD stage was applied (Table 4). The reference category was early stage SCD. The results suggested that visual acuity was closely associated with middle stage SCD. However, there were no statistical difference in other variance with middle or late stage SCD.
Table 4.
Multinominal logistic regression analysis of significant variances.
| P value | OR value | 95% confidence interval | ||
|---|---|---|---|---|
| Upper bound | Lower bound | |||
| Middle stage SCD | ||||
| Blood glucose | .497 | 1.432 | 0.508 | 4.032 |
| GHB (%) | .584 | 0.682 | 0.173 | 2.689 |
| Homocysteine | .294 | 0.994 | 0.982 | 1.006 |
| Visual acuity | .044 | 7.754 | 1.061 | 56.669 |
| Megaloblastic anemia | .375 | 4.182 | 0.178 | 98.342 |
| Late stage SCD | ||||
| Blood glucose | .590 | 1.329 | 0.472 | 3.738 |
| GHB (%) | .464 | 0.571 | 0.127 | 2.562 |
| Homocysteine | .367 | 0.991 | 0.972 | 1.011 |
| Visual acuity | .246 | 3.413 | 0.429 | 27.157 |
| Megaloblastic anemia | .785 | 1.642 | 0.047 | 57.628 |
Note: The reference category was early stage SCD and P level of test conducted 5%.
GHB = glycated hemoglobin, OR = odds ratio, SCD = subacute combined degeneration.
3.5. Receiver operator characteristic curve analysis of significant variances
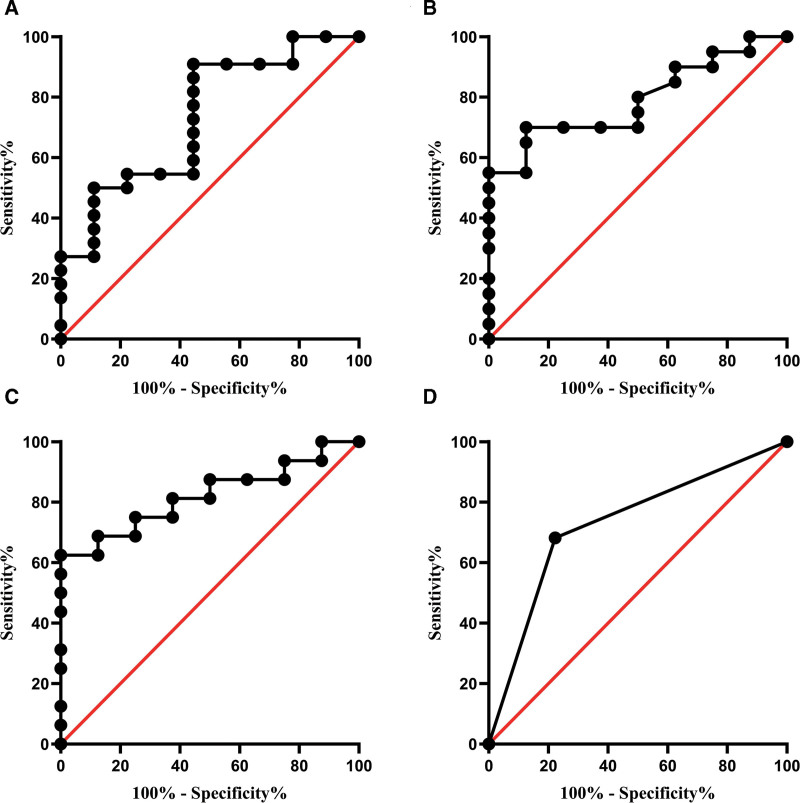
Receiver operator characteristic curve analysis was performed to estimate the value of significant variances including total cholesterol level, blood glucose level, homocysteine level and abnormal finding of visual acuity in SCD diagnosis and identifying SCD stage. The area under curve was 0.732, 0.791, 0.820, and 0.730, respectively for total cholesterol level (Fig. 2A), blood glucose level (Fig. 2B), homocysteine level (Fig. 2C) and abnormal finding of visual acuity (Fig. 2D), and the differences were statistically significant (P < .05, Table 5), suggesting their great significance in diagnosis and clinical staging of SCD.
Table 5.
ROC curve analysis of significant variances.
| Variances | P value | AUC | 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Total cholesterol | .045 | 0.732 | 0.535 | 0.930 |
| Blood glucose | .018 | 0.791 | 0.624 | 0.957 |
| Homocysteine | .012 | 0.820 | 0.654 | 0.986 |
| Visual acuity | .048 | 0.730 | 0.532 | 0.927 |
P level of test conducted 5%.
AUC = area under curve, ROC = receiver operator characteristic.
4. Discussion
SCD, as a demyelinating disease, is intimately associated with vitamin B12 deficiency and metabolic disorders, which is mainly caused by malnutrition, inherited disorders and nitrous oxide misuse.[16,17] Vitamin B12 is an indispensable coenzyme for the synthesis of nucleoprotein and myelin. Thus, lack of vitamin B12 usually results in myelin synthesis disorders, then further leading to neuropathy. In clinical settings, vitamin B12 deficiency was chiefly caused by the following aspects: insufficient intake: mostly found in long-term vegetarians; malabsorption: usually observed in patients who underwent subtotal gastrectomy, ileectomy and atrophic gastritis induced by excessive drinking; combination obstacle: effects of anti-internal factor antibodies and anti-parietal cell antibodies on the combination of vitamin B12 and internal factors; transcobalamin deficiency or its abnormal function obviously decreased vitamin B12 utilization rate. Collectively, any abnormal segment in the process of vitamin B12 uptake, absorption, combination and transportation may bring about vitamin B deficiency.[18,19] For these patients, standardized application of large dose of vitamin B12 was the key treatment modality to obtain significant curative effects at earlier stage.[20] In this present study, all patients received methycobal treatment and their clinical symptoms obviously improved after treatment.
Due to the complexity and diversity of clinical features, SCD patients may be presented as different clinical symptoms based on the degree and duration of vitamin B12 deficiency.[15] The spinal cord and peripheral nerves were also affected in some patients, which may be featured as symmetrical movements or sensory disorders including ataxia, spastic paralysis. In addition, cognitive impairment was found most SCD patients, but only few patients were manifested as memory loss. Relatively, mental disorders, dementia-like performance and coma were common clinical manifestations in the late stage SCD.[21,22] In this current study, different stage SCD presented as different degrees of neurological abnormalities, among which adynamia, sensory impairment and incontinence were observed in most patients. In comparison, there were more visual abnormalities in middle and late stage SCD compared with the early stage SCD, suggesting their diagnostic value in relatively later stage SCD. These above clinical features were consistent with a previous study that dizziness, sensory disturbance and visual abnormalities were the main symptoms of SCD patients.[23]
Although serum vitamin B12 detection was a valuable laboratory examination for SCD diagnosis, some patients showed normal vitamin B12 level and no signs of megaloblastic anemia. In practical clinical work, there may be deficiency of intracellular vitamin B12 or vitamin B12 utilization disturbance. Therefore, normal vitamin B12 level in the serum was not representative of the precise and timely vitamin B12 availability.[7,21,24] In our study, serum vitamin B12 level was at normal level and there was no megaloblastic anemia in some SCD patients. Thus, identifying other biological markers that may indeed reflect cellular availability of vitamin B12 is of greater clinical significance. Strikingly, serum levels of total cholestrol, hemoglobin and MCV were significantly increased in SCD patients, and there was also obvious difference in different stage SCD, suggesting that these above markers may represent cellular vitamin B12 level, which were not only used for SCD diagnosis, but they also reflect the degree and duration of cellular vitamin B12 deficiency. Previous studies have also demonstrated that elevated serum levels of hemoglobin and methylmalonic acid indicated the real cellular vitamin B12 deficiency, which were further applied for SCD diagnosis in patients with normal vitamin B12 level.[19,25]
Imaging techniques, especially MRI and diffusion-weighted imaging, are not only a significant basis for SCD diagnosis, but they also are applied for evaluating the long-term curative effects after treatment. Abnormal hyperintensity on spinal cord may be observed in patients with SCD.[26,27] Moreover, typical imaging abnormalities, including “inverted V” or “binoculars” signs on MRI in the cervical or spinal cord may be also valuable for the diagnosis of SCD.[28,29] However, because of its poor sensitivity, MRI may not be a ideal tool for SCD diagnosis or predicting SCD severity and staging or having prognostic value.[15,30] Similarly, MRI showed abnormal spinal cord signals in only 8 patients, most of whom were at early and middle stage SCD in this current study. The low sensitivity of MRI may be associated with the following aspects: The disease course: sensitivity of MRI was relatively higher in patients with shorter disease course. In late-stage patients, MRI may not show abnormal signals due to hyperplasia of astrocytes and fibrous tissue. Lesion location: there was no definite sensory plane in most patients. Therefore, convention MRI may not precisely locate the lesion. Magnetic resonance field strength: advanced MRI techniques (diffusion-tensor imaging) may enhance the sensitivity through picking up micro-structural alterations on the spinal cord.[13,31]
In this present study, ANA detection was also of great clinical value for the diagnosis of early and middle stage SCD. In addition, enhanced levels of creatinine, blood glucose, GHB, MCV, ferritin, erythropoietin, homocysteine, visual abnormalities were negatively correlated with SCD stage, among which visual abnormalities were closely related to middle stage SCD. Strikingly, our results also demonstrated that levels of total cholesterol, blood glucose and homocysteine as well as abnormal finding of visual acuity may be applied for SCD diagnosis and identifying different SCD stage, which may give clinical guidance for the medical professionals in their clinical work. Vitamin B12 deficiency was the main cause of SCD, thus vitamin B12 supplement was applied in most patients as suggested,[32] and the follow-up data demonstrated that clinical states of the patients were greatly improved. Due to the retrospective nature, there may exist some recall basis in this study. Moreover, there may be a relationship between severity of vitamin B12 deficiency and disease course or treatment plan, which still needs further investigation. Therefore, long-term follow-up and prospective clinical studies are further required to better evaluate the clinical value of these above biomarkers in SCD diagnosis and staging in our future studies.
In conclusion, SCD of the spinal cord is a curable neurological disorder. If not treated timely and properly, the disease course may continue to progress, and there may still remain irreversible neurological deficit. Therefore, early diagnosis and timely standardized treatment of SCD are of great clinical significance in improving patients’ prognosis. Due to low sensitivity of MRI scanning and serum vitamin B12 detection, neurological examination (such as visual acuity), ANA testing and serum homocysteine level may become significant biomarkers for SCD diagnosis and staging in further clinical settings.
Figure 3.
ROC curve analysis of significant variance. (A) ROC curve analysis of total cholesterol in different stage SCD. (B) ROC curve analysis of blood glucose in different stage SCD. (C) ROC curve analysis of homocysteine level in different stage SCD. (D) ROC curve analysis of visual acuity in different stage SCD. ROC = receiver operator characteristic, SCD = subacute combined degeneration.
Author contributions
Conceptualization: Gu Linazi, Yanling Xi.
Formal analysis: Jingjing Zhang, Abudukadier Wulamu, Miyesier Maimaitiaili, Baolan Wang, Banu Bakeer.
Validation: Yanling Xi.
Writing – review & editing: Gu Linazi, Shajidan Abudureyimu.
Abbreviations:
- ANA =
- anti-nuclear antibodies
- GHB =
- glycated hemoglobin
- MCV =
- mean corpusular volume
- MRI =
- magnetic resonance imaging
- ROC =
- receiver operator characteristic
- SCD =
- subacute combined degeneration
GL and SA contributed equally to this work.
This study was supported by the Regional Project of National Science Foundation of China (Grant No. 81860407, 81660379).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Linazi G, Abudureyimu S, Zhang J, Wulamu A, Maimaitiaili M, Wang B, Bakeer B, Xi Y. Clinical features of different stage subacute combined degeneration of the spinal cord. Medicine 2022;101:37(e30420).
Contributor Information
Gu Linazi, Email: 2195291582@qq.com.
Shajidan Abudureyimu, Email: 1136783325@qq.com.
Jingjing Zhang, Email: 28958562@qq.com.
Abudukadier Wulamu, Email: 813227097@qq.com.
Miyesier Maimaitiaili, Email: 3293485775@qq.com.
Baolan Wang, Email: wbl0308@163.com.
References
- [1].Green R, Allen LH, Bjørke-Monsen AL, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040. [DOI] [PubMed] [Google Scholar]
- [2].Sun HY, Lee JW, Park KS, et al. Spine MR imaging features of subacute combined degeneration patients. Eur Spine J. 2014;23:1052–8. [DOI] [PubMed] [Google Scholar]
- [3].Spence JD. Nutrition and stroke prevention. Nutrients. 2019;11:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang YH, Yan F, Zhang WB, et al. An investigation of vitamin Bl2 deficiency in elderly inpatients in neurology department. Neumsci Bul. 2009;25:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lim CC. Neuroimaging in postinfectious demyelination and nutritional disorders of the central nervous system. Neuroimaging Clin N Am. 2011;21:843–58, viii. [DOI] [PubMed] [Google Scholar]
- [6].Puntambekar P, Bssha MM, Zak IT, et al. Rare sensory and automatic disturbances associated with vitamin B12 deficiency. J Neurol Sci. 2009;287:285–7. [DOI] [PubMed] [Google Scholar]
- [7].Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5:4521–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Spence JD. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementiaand stroke. Nutr Res. 2016;36:109–16. [DOI] [PubMed] [Google Scholar]
- [9].Makdsi F, Kadrie T. Sub-acute combined degeneration with an initially normal level of vitamin B12: a case report. Cases J. 2009;2:6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency—an update. Haematologica. 2006;2:1506–12. [PubMed] [Google Scholar]
- [11].Ravina B, Loevner LA, Bank W. MR findings in subacute combined degeneration of the spinal cord: a case of reversible cervical myelopathy. AJR Am J Roentgenol. 2000;174:863–5. [DOI] [PubMed] [Google Scholar]
- [12].Tangney CC, Aggarwal NT, Li H, et al. Vitamin B12, cognition, and brain MRI measures: a cross-sectional examination. Neurology. 2011;77:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li J, Ren M, Dong A, et al. A retrospective study of 23 cases with subacute combined degeneration. Int J Neurosci. 2016;126:872–7. [DOI] [PubMed] [Google Scholar]
- [14].Bi Z, Cao J, Shang K, et al. Correlation between anemia and clinical severity in subacute combined degeneration patients. J Clin Neurosci. 2020;80:11–5. [DOI] [PubMed] [Google Scholar]
- [15].Jain KK, Malhotra HS, Garg RK, et al. Prevalence of MR imaging abnormalities in vitamin B12 defificiency patients presenting with clinical features of subacute combined degeneration of the spinal cord. J Neurol Sci. 2014;342:162–6. [DOI] [PubMed] [Google Scholar]
- [16].Massey TH, Pickersgill TT, Peall KJ. Nitrous oxide misuse and vitamin B12 deficiency. BMJ Case Rep. 2016;2016:bcr2016215728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. [DOI] [PubMed] [Google Scholar]
- [18].Divate PG, Patanwala R. Neurological manifestations of Bl2 deficiency with emphasis on its aetiology. J Assoc Physicians India. 2014;62:400–5. [PubMed] [Google Scholar]
- [19].Li J, Zhang L, Zhang Y, et al. Misdiagnosis of spinal subacute combined degeneration in a patient with elevated serum B12 concentration and sensory deficiency level. Neurol Sci. 2016;37:1577–8. [DOI] [PubMed] [Google Scholar]
- [20].Vasconcehis OM, Poehm EH, Mccarter RJ, et al. Potential outcome factors in subacute combined degeneration: review of ohservational studies. J Gen Intern Med. 2006;21:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol. 2010;148:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grober U, Kisters K, Schmidt J. Neuroenhancement with vitamin B12 underestimated neurological significance. Nutrients. 2013;5:5031–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen H, Li HY, Li YC, et al. Clinical and imaging characteristics of subacute combined degeneration complicated with white matter lesions in the brain: a report of five cases. Somatosens Mot Res. 2018;35:119–23. [DOI] [PubMed] [Google Scholar]
- [24].Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166:496–513. [DOI] [PubMed] [Google Scholar]
- [25].Schrempf W, EuliIz M, Neumeister V, et al. Utility of measuring vitamin B12 and its active fraction, holotranscobalamin in neurological vitamin B12 deficiency syndromes. J Neurol. 2011;258:393–401. [DOI] [PubMed] [Google Scholar]
- [26].Tian C. Hyperintense signal on spinal cord diffusion-weighted imaging in a patient with subacute combined degeneration. Neurol India. 2011;59:429–31. [DOI] [PubMed] [Google Scholar]
- [27].Kim EY, Lee SY, Cha SH, et al. Subacute combined degeneration revealed by diffusion-weighted imaging: a case study. Clin Neuroradiol. 2013;23:157–9. [DOI] [PubMed] [Google Scholar]
- [28].Narra R, Mandapalli A, Jukuri N, et al. ‘‘Inverted V sign” in sub-acute combined degeneration of cord. J Clin Diagn Res. 2015;9:TJ01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun HY, Lee JW, Park KS, et al. Spine MR imaging features of subacute combined degeneration patients. Eur Spine J. 2014;23:1052–8. [DOI] [PubMed] [Google Scholar]
- [30].Srikanth SG, Jayakumar PN, Vasudev MK, et al. MRI in subacute combined degeneration of spinal cord: a case report and review of literature. Neurol India. 2002;50:310–2. [PubMed] [Google Scholar]
- [31].Xiao CP, Ren CP, Cheng JL, et al. Conventional MRI for diagnosis of subacute combined degeneration (SCD) of spinal cord due to vitamin B12 deficiency. Asia Pac J Clin Nutr. 2016;25:34–8. [DOI] [PubMed] [Google Scholar]
- [32].Zhang N, Li RH, Ma L, et al. Subacute combined degeneration, pernicious anemia and gastric neuroendocrine tumor occurred simultaneously caused by autoimmune gastritis. Front Neurosci. 2019;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]