Abstract
Testing for antibody against hepatitis C virus (anti-HCV) is a low-cost diagnostic method worldwide; however, an optimal screening test for HCV in patients with cancer has not been established. We sought to identify an appropriate screening test for HCV infection in patients with hematologic malignancies and/or hematopoietic cell transplants (HCT). Patients in our center were simultaneously screened using serological (anti-HCV) and molecular (HCV RNA) assays (February 2019–November 2019).
In total, 214 patients were enrolled in this study. Three patients (1.4%) were positive for anti-HCV, and 2 (0.9%) were positive for HCV RNA. The overall percentage agreement was 99.5% (95% CI: 97.4–99.9). There were no cases of seronegative HCV virus infection. The positive percentage agreement was 66.7% (95% CI: 20.8–93.9), and the negative percentage agreement was 100.0% (95% CI: 98.2–100.0). Cohen kappa coefficient was 0.80 (95% CI: 0.41–1.00, P < .0001).
The diagnostic yield of screening for chronic HCV infection in patients with cancer is similar for serologic and molecular testing.
Keywords: hematologic malignancies, hematopoietic cell transplant recipients, hepatitis C virus, screening
1. Introduction
The prevalence of chronic hepatitis C virus (HCV) infection in patients with cancer has been reported to be 1.5% overall and up to 10.6% in specific subgroups.[1] Chronic HCV infection causes significant morbidity and mortality in patients with cancer and can interfere with cancer treatment.[2] However, little is known about the optimal screening test for HCV in cancer patients. Two types of assays are approved for the diagnosis of HCV infection: serologic assays that detect antibody to HCV (anti-HCV) and confirmatory molecular assays that detect viral nucleic acids (HCV RNA).[3] Serologic assays cost less expensive than molecular assays (US$ 0.50–1.70 vs US$ 30–200).[4] US national guidelines recommend screening with anti-HCV in both immunocompetent patients and immunocompromised patients, such as those with human immunodeficiency virus (HIV) coinfection.[5] However, anti-HCV-based screening may be suboptimal in some immunocompromised patients,[6] including those with HIV infection[7] and hematopoietic cell transplant (HCT) recipients.[8] In the study reported here, we sought to identify the most reliable screening test for chronic HCV infection in patients with underlying hematologic malignancies with and without HCT.
2. Methods
Patients with cancer who were seen at the Lymphoma/Myeloma, Leukemia, and Stem Cell Transplant clinics at the University of Texas MD Anderson Cancer Center between February 11, 2019, and November 5, 2019, were enrolled prospectively. This study was approved by the MD Anderson Institutional Review Board and conformed to the standards set by the Declaration of Helsinki for human studies. Informed consent was obtained from all eligible participants. We included patients aged ≥ 18 years with any type of hematologic malignancy, with or without HCT, and who had never been screened for HCV. Anti-HCV and HCV-RNA tests were simultaneously performed using the same blood samples. Anti-HCV testing was performed by using the ARCHITECT Anti-HCV assay (Abbott Laboratories), which has a specificity of 99.60% (95% confidence interval [CI]: 99.45–99.71) and a sensitivity of 99.10% (95% CI: 96.77–99.89).[9] HCV-RNA testing was performed by using the Cobas HCV test (Roche Molecular Systems, Inc.) with the Cobas 6800 instrument system. The quantification range of this assay was 15 to 100,000,000 IU/mL (1.18 log IU/mL to 8.00 log IU/mL). Seronegative HCV infection was defined as a negative anti-HCV and positive HCV-RNA test results. Resolved HCV infection or false-positive serological test results were defined as positive anti-HCV and negative HCV-RNA test results.
This study was powered by the diagnostic performances of the 2 tests. In a previous study of HIV-infected individuals who underwent both tests, 6.9% of patients tested negative for anti-HCV but positive for HCV RNA, while 0.8% had the opposite results.[10] Assuming these discordant proportions, 214 patients would need to be enrolled to yield 90% power to detect a significant difference (P < .05) in diagnostic performance between the 2 tests using McNemar test. Descriptive statistics were used to summarize patient characteristics. Numerical data were described as medians and ranges, and categorical data were described as frequencies and percentages. The diagnostic agreement between the anti-HCV and HCV-RNA tests was assessed. First, the overall percentage agreement and the positive and negative percentage agreements were estimated. The agreement between the 2 tests was evaluated using Cohen kappa statistic and McNemar test. All tests were 2-sided, with a significance level of 0.05. Data analyses were performed using SAS version 9.3 (SAS Institute Inc.).
3. Results
3.1. Demographics
In total, 214 patients were enrolled in this study. Of these, 127 (59%) were men, and 180 (84%) were White. One hundred forty-nine patients (70%) had lymphoid neoplasms, 65 (30%) had myeloid neoplasms, and 15 (7%) had undergone HCT (Table 1). One hundred one patients (47%) had stable disease, and 93 (43%) had progressive disease. Twenty patients (9%) were newly diagnosed with hematologic malignancies at the time of enrollment; therefore, their cancer status could not be determined.
Table 1.
Study population characteristics.
| Characteristic | Value |
|---|---|
| Median age (range, yrs) | 64 (27–84) |
| Male sex | 127 (59%) |
| Race | |
| White | 180 (84%) |
| Black | 18 (8%) |
| Asian | 9 (4%) |
| Native American | 1 (0.5%) |
| Other | 6 (3%) |
| Hematologic neoplasm | |
| Lymphoid* | 149 (70%) |
| Myeloid† | 65 (30%) |
| HCT | 15 (7%) |
| Allogeneic | 3/15 (20%) |
| Autologous | 12/15 (80%) |
| HCV genotype | |
| 1b | 2/2 (100%) |
Data are median (range) or n (%).
HCT = hematopoietic cell transplant; HCV = hepatitis C virus.
Lymphoid neoplasms included the following categories based on the 2016 World Health Organization classification: mature B-cell neoplasms and Hodgkin lymphoma.
Myeloid neoplasms included the following categories based on the 2016 World Health Organization classification: myeloproliferative neoplasms, myelodysplastic/myeloproliferative neoplasms, myelodysplastic syndromes, acute myeloid leukemia, and related neoplasms, and B-lymphoblastic leukemia/lymphoma.
3.2. Diagnostic performance
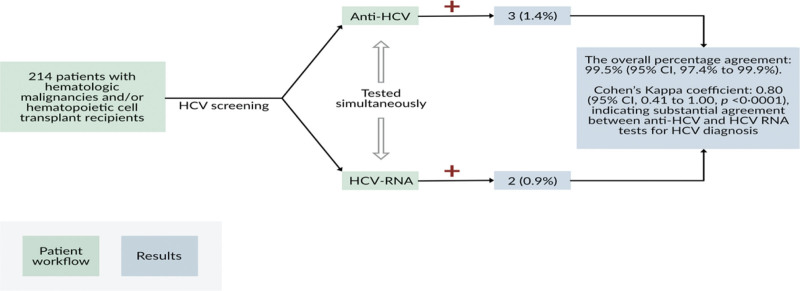
Three patients (1.4%) had positive anti-HCV test results and 2 (0.9%) had positive HCV-RNA test results (Table 1). The overall percentage agreement was 99.5% (95% CI: 97.4–99.9). Of the 3 patients with positive anti-HCV test results, 2 were positive and 1 had negative HCV-RNA test results. There were no cases of seronegative HCV infection, that is, of the 211 patients with negative anti-HCV test results, all had negative HCV-RNA test results. The positive percentage agreement was 66.7% (95% CI: 20.8–93.9), and the negative percentage agreement was 100.0% (95% CI: 98.2–100.0). Cohen kappa coefficient was 0.80 (95% CI: 0.41–1.00, P < .0001), indicating substantial agreement between anti-HCV and HCV-RNA tests for the diagnosis of HCV infection (Fig. 1). Consistent with this, McNemar test showed no significant difference in overall performance between the 2 tests (P = .32). One patient with a negative anti-HCV test result had an inconclusive HCV-RNA test result; however, a repeated HCV-RNA test produced a negative result.
Figure 1.
Serologic vs molecular testing for HCV screening in patients with hematologic malignancies. anti-HCV = antibody to hepatitis C virus, HCV = hepatitis C virus.
4. Discussion
To our knowledge, this is the first study to prospectively compare different HCV screening methods in heavily immunocompromised patients with cancer. We found that serological and molecular testing had similar diagnostic performance in this patient population. There were no cases of seronegative (false-negative for anti-HCV) infections. The findings of this prospective study reflect our clinical practice, where cases of seronegative HCV have not been identified for many years in our center.
The reported prevalence of seronegative HCV infection (negative for anti-HCV but positive for HCV RNA) ranges from 3.2% to 13.2% in HIV/HCV co-infected patients, from 1% to 15% in patients undergoing hemodialysis, from 0.2% to 0.9% in solid organ donors, and from 0.0004% to 0.08% in blood donors.[11]
The occurrence of seronegative HCV infection has significant implications in cancer patients, as unaddressed chronic HCV infection might lead to liver disease progression, increased mortality in patients with non-Hodgkin lymphoma or prior HCT, development of hepatocellular carcinoma and/or non-Hodgkin lymphoma as a second primary malignancy, or the need for burdensome adjustments in cancer treatment due to HCV reactivation.[1,2] One proposed pathophysiological mechanism for seronegative HCV infection is delayed seroconversion,[12] a phenomenon reported in immunosuppressed patients and persons who inject drugs.[9,13] In our study, which included heavily immunocompromised patients with hematologic malignancies, we observed no seronegative HCV infection. An explanation for this finding is the use of sensitive diagnostic serological tests in our study.
Because of the significant public health burden of viral hepatitis, the World Health Organization (WHO) has set a goal of eliminating hepatitis by 2030 (WHO, 2017).[4] The most efficient strategy to achieve the WHO’s goal of eliminating HCV by 2030 is to expand HCV testing such that 90% of all HCV-positive people are diagnosed and offered treatment.[4,6,14] Our findings favor the use of anti-HCV, a relatively affordable test in use worldwide, for HCV screening in most cancer patients.
It should be noted that our findings are applicable to 1-time screening for chronic HCV infection in patients at a low risk of infection. Patients at a high risk for HCV infection with suspected acute HCV infection, including reinfection, should be tested for HCV RNA more than once.[15] Likewise, all HCT donors should be screened for HCV within 30 days before cell harvest with Food and Drug Administration (FDA)-approved anti-HCV and HCV RNA testing in accordance with the Foundation for the Accreditation of Cellular Therapies standards and FDA guidance.[8]
Our study had several limitations. First, the statistical power was low due to the small number of patients with HCV infection. The sample size calculated for this study was based on a study evaluating the diagnosis of HCV infection in HIV-positive individuals,[10] in which 23.7% of the patients tested positive for anti-HCV and 29.8% tested positive for HCV RNA. Unlike patients with cancer, HIV-infected individuals are a high-risk population for HCV infection. The HCV infection rate in the study population was much lower (1.4%). This difference in HCV infection rates between the 2 studies led to the underpower of our study. Second, the small number of patients who tested positive for HCV may limit the generalizability of our findings. Third, future studies may yield different diagnostic outcomes if other serological and molecular assays that were not used in our study were compared. Fourth, we did not perform a cost-effectiveness analysis of serologic versus molecular testing, as our center is granted special pricing for laboratory testing and does not reflect the true market price.
In conclusion, the diagnostic yield of screening for chronic HCV infection in heavily immunocompromised cancer patients seems to be similar for serological and molecular testing. The use of low-cost diagnostic methods, such as anti-HCV, would contribute to the long-term goal of eliminating HCV infection in the U.S. and globally.
Acknowledgments
We thank Stephanie Deming of the Research Medical Library at MD Anderson for the editorial assistance. This study was presented in part at IDWeek 2020 as Poster No.1052. Abstract available at Open Forum Infectious Diseases, 2020 [https://doi.org/10.1093/ofid/ofaa4].
Author contributions
Harrys A. Torres: formal analysis, conceptualization, writing—original draft, writing-reviewing and editing, supervision; Georgios Angelidakis: formal analysis, investigation, data curation, writing—original draft; Ying Jiang: software-formal analysis; minas economides: visualization, investigation, writing-reviewing and editing; Khalis Mustafayev: investigation, writing-reviewing and editing; Marcel Yibirin: investigation, writing-reviewing and editing; Robert Orlowski: investigation, writing-reviewing and editing; Richard Champlin: investigation, writing-reviewing and editing; Srdan Verstovsek: investigation, writing-reviewing and editing; Issam Raad: investigation, writing–review and editing.
Abbreviations:
- anti-HCV =
- antibody to hepatitis C virus
- HCT =
- hematopoietic cell transplant
- HCV =
- hepatitis C virus
- WHO =
- World Health Organization
Dr. Torres is or has been the principal investigator for research grants from the National Cancer Institute, Gilead Sciences and Merck & Co., Inc., with all funds paid to the MD Anderson Cancer Center. Dr. Torres is or has been a paid scientific advisor for AbbVie, Inc., Gilead Sciences, Merck & Co., Inc., and Dynavax Technologies; the terms of these arrangements are being managed by MD Anderson Cancer Center in accordance with its conflict-of-interest policies. All other authors declare no competing interests.
Investigator-initiated study sponsored by Merck & Co., Inc. Also supported in part by the National Cancer Institute under award number P30CA016672, which supports the MD Anderson Cancer Center Clinical Trials Support Resource.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Torres HA, Angelidakis G, Jiang Y, Economides M, Mustafayev K, Yibirin M, Orlowski R, Champlin R, Verstovsek S, Raad I. Serologic versus molecular testing for screening for hepatitis C virus infection in patients with hematologic malignancies. Medicine 2022;101:37(e30608).
Contributor Information
Georgios Angelidakis, Email: gangelidakis@mdanderson.org.
Ying Jiang, Email: yijiang@mdanderson.org.
Minas Economides, Email: Minas.Economides@nyulangone.org.
Khalis Mustafayev, Email: kmustafayev@mdanderson.org.
Marcel Yibirin, Email: marceljose23@gmail.com.
Robert Orlowski, Email: ROrlowski@mdanderson.org.
Richard Champlin, Email: rchampli@mdanderson.org.
Srdan Verstovsek, Email: sverstov@mdanderson.org.
Issam Raad, Email: iraad@mdanderson.org.
References
- [1].Torres HA, Shigle TL, Hammoudi N, et al. The oncologic burden of hepatitis C virus infection: a clinical perspective. CA Cancer J Clin. 2017;67:411–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Torres HA, Pundhir P, Mallet V. Hepatitis C virus infection in patients with cancer: impact on clinical trial enrollment, selection of therapy, and prognosis. Gastroenterology. 2019;157:909–16. [DOI] [PubMed] [Google Scholar]
- [3].Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization, 2017. Available at: https://www.ncbi.nlm.nih.gov/books/NBK442261/table/ch9.t1/ [access January 25, 2022]. [Google Scholar]
- [5].Tien PC, Veterans Affairs Hepatitis C Resource Center Program. Management and treatment of hepatitis C virus infection in HIV-infected adults: recommendations from the veterans affairs hepatitis C resource center program and national hepatitis C program office. Am J Gastroenterol. 2005;100:2338–54. [DOI] [PubMed] [Google Scholar]
- [6].Macedo de Oliveira A, White KL, Beecham BD, et al. Sensitivity of second-generation enzyme immunoassay for detection of hepatitis C virus infection among oncology patients. J Clin Virol. 2006;35:21–5. [DOI] [PubMed] [Google Scholar]
- [7].George SL, Gebhardt J, Klinzman D, et al. Hepatitis C virus viremia in HIV-infected individuals with negative HCV antibody tests. J Acquir Immune Defic Syndr. 2002;31:154–62. [DOI] [PubMed] [Google Scholar]
- [8].Torres HA, Chong PP, De Lima M, et al. Hepatitis C virus infection among hematopoietic cell transplant donors and recipients: american society for blood and marrow transplantation task force recommendations. Biol Blood Marrow Transplant. 2015;21:1870–82. [DOI] [PubMed] [Google Scholar]
- [9].Beld M, Penning M, van Putten M, et al. Low levels of hepatitis C virus RNA in serum, plasma, and peripheral blood mononuclear cells of injecting drug users during long antibody-undetectable periods before seroconversion. Blood. 1999;94:1183–91. [PubMed] [Google Scholar]
- [10].Clifford DB, Smurzynski M, Park LS, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology. 2009;73:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kaźmierczak J, Pawełczyk A, Cortes KC, Radkowski M. Seronegative hepatitis C virus infection. Arch Immunol Ther Exp (Warsz). 2014;62:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stapleton JT, Klinzman D, Schmidt WN, et al. Prospective comparison of whole-blood- and plasma-based hepatitis C virus RNA detection systems: improved detection using whole blood as the source of viral RNA. J Clin Microbiol. 1999;37:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arrojo IP, Pareja MO, Orta MD, et al. Detection of a healthy carrier of HCV with no evidence of antibodies for over four years. Transfusion. 2003;43:953–7. [DOI] [PubMed] [Google Scholar]
- [14].Wiktor S. How feasible is the global elimination of HCV infection? Lancet. 2019;393:1265–7. [DOI] [PubMed] [Google Scholar]
- [15].Page K, Osburn W, Evans J, et al. Frequent longitudinal sampling of hepatitis C virus infection in injection drug users reveals intermittently detectable viremia and reinfection. Clin Infect Dis. 2013;56:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]