OBJECTIVES:
For critically ill adults, oxygen saturation is continuously monitored using pulse oximetry (Spo2) as a surrogate for arterial oxygen saturation (Sao2). Skin pigmentation may affect accuracy of Spo2 by introducing error from statistical bias, variance, or both. We evaluated relationships between race, Spo2, Sao2, and hypoxemia (Sao2 < 88%) or hyperoxemia (Pao2 > 150 mm Hg) among adults receiving mechanical ventilation in a medical ICU.
DESIGN:
Single-center, observational study.
SETTING:
Medical ICU at an academic medical center.
PATIENTS:
Critically ill adults receiving mechanical ventilation from July 2018 to February 2021, excluding patients with COVID-19, with race documented as Black or White in the electronic medical record, who had a pair of Spo2 and Sao2 measurements collected within 10 minutes of each other.
INTERVENTIONS:
None.
MEASUREMENTS:
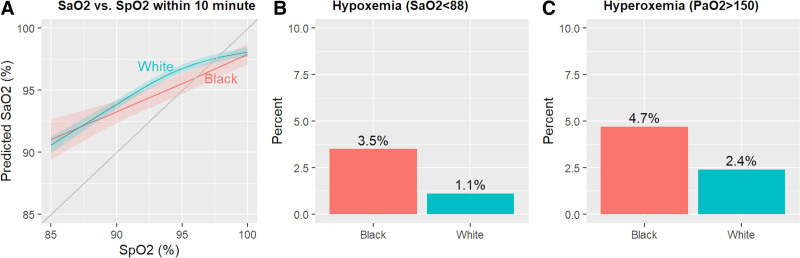
We included 1,024 patients with 5,557 paired measurements within 10 minutes, of which 3,885 (70%) were within 1 minute. Of all pairs, 769 (14%) were from Black patients and 4,788 (86%) were from White patients. In analyses using a mixed-effects model, we found that across the range of Spo2 values of 92–98%, the associated Sao2 value was approximately 1% point lower for Black patients compared with White patients. Among patients with a Spo2 value between 92% and 96%, Black patients were more likely to have both hypoxemia (3.5% vs 1.1%; p = 0.002) and hyperoxemia (4.7% vs 2.4%; p = 0.03), compared with White patients.
CONCLUSIONS:
Among patients with a measured Spo2 of 92–96%, greater variation in Sao2 values at a given Spo2 resulted in a higher occurence rate of both hypoxemia and hyperoxemia for Black patients compared with White patients.
For critically ill adults receiving invasive mechanical ventilation, clinicians set a Fio2 and measure arterial oxygen saturation (Sao2), commonly using a pulse oximeter device (Spo2) to approximate direct laboratory measurement of hemoglobin saturation (Sao2) (1). Skin pigmentation may affect accuracy of pulse oximetry (Spo2) by introducing error from statistical bias (e.g., consistently higher Spo2 values at a given Sao2), variance (e.g. wider range of Spo2 values at a given Sao2), or both (2). These limitations of Spo2 can prevent timely detection of both hypoxemia (low Sao2) and hyperoxemia (high Po2 in blood [Pao2]). Recent studies have demonstrated a higher incidence of hypoxemia at the time of a normal Spo2 value among Black patients, compared with White patients (3–6). Prior studies have focused on the incidence of hypoxemia but have not evaluated whether the difference was related to a systematic bias (difference in the average error of estimates by race) or increased variability in the measurements (difference in the precision of estimates by race). Further, no prior studies have evaluated whether race modifies the incidence of hyperoxemia at the time of a normal Spo2 value.
METHODS
We analyzed prospectively collected data from all adults receiving invasive mechanical ventilation in the medical ICU at Vanderbilt University Medical Center between July 2018 and August 2021. Data were collected as part of a randomized trial (NCT03537937), which excluded patients with COVID-19.
As a surrogate for skin pigmentation, we used patients’ race as recorded in the electronic health record. Our analysis compared patients with a recorded race of Black to patients with a recorded race of White. Patients with other values for race were not included due to an inadequate number of patients for comparison. Values for Spo2 were obtained from Nellcor pulse oximeters (Medtronic, Watford, United Kingdom) with values directly transferred from the bedside Phillips monitor into the institutional data warehouse every 1 minute (7). Measurements of Sao2 and Pao2 were obtained from a Werfen GEM Premier 5000 blood gas analyzer (Instrumentation, Werfen,Germany) which measures Sao2 using co-oximetry and is located in the ICU to minimize time between sampling and analysis.
To examine whether patients’ race modified the association between simultaneous Spo2 and Sao2 or Pao2 measurements, we first developed generalized linear mixed-effect models, with the outcome of either Sao2 or Pao2, a random effect for patient, and fixed effects for race, Spo2, and the interaction between the two. To adjust for confounders, we included age, sex, and receipt of vasopressors as covariates. Second, we compared the incidence of hypoxemia (Sao2 < 88%) and hyperoxemia (Pao2 > 150 mm Hg) between Black and White patients with Spo2 values of 92–96%. We performed sensitivity analyses: 1) restricted to Spo2 values available within 5 minutes or 1 minute of the blood gas measurement, 2) using the median rather than the closest Spo2 within 10 minutes of the blood gas measurement, and 3) excluding patients receiving extracorporeal membrane oxygenation. This secondary analysis used data collected as part of a clinical trial, for which the Institutional Review Board for Vanderbilt University Medical Center gave ethical approval (no. 171272, August 11, 2017), and all study procedures were conducted in accordance with the ethical standards of that committee and the Helsinki Declaration.
RESULTS
A total of 1,083 patients had at least one arterial blood gas measured within 10 minutes of a Spo2 recording. Of these, 1,024 had race recorded as White or Black. These patients had 5,557 Sao2 measurements with a Spo2 recorded within 10 minutes. For each pair, the Spo2 values closest in time was used, with 3,885 (70%) occurring within 1 minute. The median number of Spo2-Sao2 pairs per patient was 2. A total of 769 pairs (14%) were from Black patients, and 4,788 (86%) were from White patients (Table 1).
TABLE 1.
Baseline Characteristics of Pulse Oximetry and Arterial Oxygen Saturation Pairs, Measured Within 10 Minutes of Each Other
| Characteristics | Spo2 and Sao2 Pairs | |
|---|---|---|
| Black | White | |
| N = 769 | N = 4,788 | |
| Age, yr, median (IQR) | 54 (42–63) | 58 (46–66) |
| Female, n (%) | 364 (47) | 2,060 (43) |
| Vasopressor usea, n (%) | 584 (76) | 3,547 (74) |
| Extracorporeal membrane oxygenationa, n (%) | 39 (5) | 1,605 (34) |
| Sao2, %, median (IQR) | 98 (95–100) | 98 (95–99) |
| Pao2, mm Hg, median (IQR) | 85 (69–121) | 80 (67–105) |
| Spo2, %, median (IQR) | 96 (92–99) | 95 (92–98) |
| 92–96%, n (%) | 254 (33) | 2,058 (43) |
| Time from Sao2 to Spo2, min | 0 (–0.4 to 0.4) | 0 (–0.4 to 0.4) |
| < 5, n (%) | 716 (93) | 4,417 (92) |
| < 1, n (%) | 550 (72) | 3,335 (70) |
| SaO2 value, %, median (IQR) | ||
| At Spo2 of 89% | 93 (91–97) | 94 (92–96) |
| At Spo2 of 90% | 95 (91–98) | 94.5 (92–97) |
| At Spo2 of 91% | 94 (91.5–97.5) | 95 (94–97) |
| At Spo2 of 92% | 95 (93–97) | 96 (94–98) |
| At Spo2 of 93% | 96.5 (94–98) | 96 (94–98) |
| At Spo2 of 94% | 97 (94.5–98) | 97 (95–99) |
| At Spo2 of 95% | 97 (95.5–98) | 98 (96–99) |
| At Spo2 of 96% | 98 (95–99) | 98 (97–99) |
| At Spo2 of 97% | 98 (97–99) | 99 (97–100) |
| At Spo2 of 98% | 99 (97–100) | 99 (98–100) |
IQR = interquartile range, Sao2 = arterial oxygen saturation, Spo2 = pulse oximetry.
aVasopressor use and extracorporeal membrane oxygenation are defined by pair as whether the patient was receiving the therapy at the time of Spo2 measurement.
Table 1 displays the raw Sao2 value at each Spo2 for Black patients compared with White patients. Because 1) the median value for Sao2 was higher than the associated Spo2 value among all patients and 2) the median value for Sao2 at a given Spo2 was approximately 1% point lower for Black patients compared with White patients, the difference between patients’ contemporaneous Sao2 and Spo2 measurements was slightly less for Black patients compared with White patients (Supplemental eFig. 1, http://links.lww.com/CCX/B56).
In the primary analysis using a mixed-effect model, at an Spo2 of 89%, the mean Sao2 was 92.8% for Black patients and 93.2% for White patients (mean difference, –0.4; 95% CI, –1.5 to 0.7), at an Spo2 of 93% was 94.6% and 95.8% (–1.1; –0.2 to –2.0), at an Spo2 of 95% was 95.5% and 96.8% (–1.2; –0.3 to –2.1), and at an Spo2 of 97% was 96.5% and 97.4% (–1.0; –0.1 to –1.8) (Fig. 1A). Results were similar in the model without covariates and in all sensitivity analyses. We found no statistically significant difference in Pao2 between Black patients and White patients at any Spo2 in the range 88–100%.
Figure 1.
A, Association between pulse oximetry (Spo2) and arterial oxygen saturation (Sao2) by patient race. Results of a generalized linear mixed effects model show that at Spo2 values between 92% and 98%, Sao2 values were an average of 1% point lower for Black patients compared with White patients. B and C, Frequency of hypoxemia and hyperoxia in patients with Spo2 values between 92% and 96%, by race. Results demonstrate that, compared with White patients, Black patients had a higher incidence of both hypoxemia (9/254 [3.5%] vs 23/2,058 [1.1%]; p = 0.002) and hyperoxemia (12/254 [4.7%] vs 50/2,058 [2.4%]; p = 0.03).
Of all pairs, 2,058 (37%) had a Spo2 within the range of 92–96%. Among these, hypoxemia occurred in nine of 254 arterial blood gas measurements (3.5%; 95% CI, 1.6–6.6) among Black patients and in 23 of 2,058 measurements (1.1%; 0.7–1.7) among White patients (p = 0.002). Hyperoxemia occurred in 12 of 254 measurements (4.7%; 2.5–8.1) among Black patients and 50 of 2,058 measurements among (2.4%; 1.8–3.2) among White patients (p = 0.03) (Fig. 1, B and C).
DISCUSSION
This study comparing Spo2, Sao2, and Pao2 values among adults receiving invasive mechanical ventilation in a single ICU found that for patients with an Spo2 of 92–96%, the incidence of hypoxemia was three times greater among Black patients compared with White patients. A small statistical bias contributed to this difference by race, but hyperoxemia was also present twice as often among Black patients as White patients. This dual risk of more hypoxemia and more hyperoxemia illustrates that differences in error from Spo2 result largely from greater variability in Spo2 measurements for Black patients compared with White patients.
Our analysis confirms findings of prior studies that Black patients experience hypoxemia not detected on Spo2 more often than White patients. The incidence of hypoxemia with an Spo2 of 92–96% in this study (3.5% among Black patients and 1.1% among White patients) was lower than in prior studies (11.7–17.0% among Black patients and 3.6–6.2% among White patients) (3). Assessing for statistical bias, we found, at Spo2 values of 92–98%, the associated Sao2 value was an average of 1% point lower for Black patients compared with White patients. This difference was similar in direction to prior studies (4, 5). Unlike prior studies (6), in our population, the values for Sao2 were generally higher than the associated value for Spo2, and as a result, the difference between contemporaneous Spo2 and Sao2 values was less for Black patients compared with White patients.
This study is the first to show that, among patients with Spo2 values of 92–96%, hyperoxemia (Pao2 > 150 mm Hg) was more common than hypoxemia and occurred approximately twice as often for Black patients compared with White patients. Hyperoxemia may worsen clinical outcomes among mechanically ventilated patients through oxidative damage to tissues (8).
The finding that both hypoxemia and hyperoxemia at Spo2 values of 92–96% were more common among Black patients highlights the contributions of two separate sources of error in Spo2 measurement: statistical bias (a directional deviation between Sao2 values and Spo2 values) and variation (a wider range of Sao2 values at a given Spo2 value for Black patients compared with White patients). Recognizing these two separate sources of error has important implications for addressing the problem. Quantitatively correcting an Spo2 value based on the expected effect of skin pigmentation has been proposed for addressing statistical bias in the direction of Spo2 values (6, 9). This approach, however, would not address the error introduced by greater variation (noise). Fixing both the statistical bias and the difference in variation will require redesign and reassessment of the Spo2 devices themselves (2, 10). The finding that the relationship between Spo2 and Pao2 did not differ by race in our study, despite differences in Spo2 and Sao2, may be due to the wider statistical distribution of Pao2 values. The relationships between race, skin pigmentation, Sao2, and Pao2 among critically ill adults requires additional evaluation in future research.
Our study has several strengths. We evaluated a population of critically ill patients on mechanical ventilation for whom titration of oxygen therapy is universally important to avoid hypoxemia and hyperoxemia. Further, we used prospectively collected data and a validated sampling model for continuous Spo2 monitoring (7), allowing us to compare Spo2 and laboratory results assessed at a similar time point, typically within 1 minute.
These findings have multiple limitations. Arterial blood gas measurements are typically requested by clinicians to assess unstable oxygenation or ventilation, which introduces a selection bias. Although skin pigmentation is hypothesized to affect the performance of Spo2 due to limitations of pulse oximeters not calibrated to account for skin differences, we did not directly measure skin pigmentation. We instead used patients’ race recorded in the electronic medical record, which is an imperfect proxy measure.
Future research should 1) develop methods of noninvasive assessment of Sao2 unaffected by skin pigmentation or race; 2) determine optimal oxygen saturation targets for individual patients based on their chronic comorbidities, acute conditions, and other determinants of health; and 3) systematically identify and correct sources of healthcare disparities and suboptimal outcomes related to medical devices, software, and systems of healthcare delivery.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Casey was supported in part by the National Institutes of Health (NIH) (K23HL153584). Dr. Qian was supported by the NIH (T32HL087738). Dr. Rice was supported in part by the NIH (U01HL123009) and has received unrelated consulting fees from Cumberland Pharmaceuticals, Cytovale, and Sanofi. Dr. Semler was supported in part by the National Heart, Lung, and Blood Institute (K23HL143053). Data collection used the Research Electronic Data Capture tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from National Center for Advancing Translational Sciences/NIH).
REFERENCES
- 1.Jubran A, Tobin MJ: Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator-dependent patients. Chest 1990; 97:1420–1425 [DOI] [PubMed] [Google Scholar]
- 2.Bickler PE, Feiner JR, Severinghaus JW: Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology 2005; 102:715–719 [DOI] [PubMed] [Google Scholar]
- 3.Sjoding MW, Dickson RP, Iwashyna TJ, et al. : Racial bias in pulse oximetry measurement. N Engl J Med 2020; 383:2477–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valbuena VSM, Barbaro RP, Claar D, et al. : Racial bias in pulse oximetry measurement among patients about to undergo ECMO in 2019-2020, a retrospective cohort study. Chest 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong AI, Charpignon M, Kim H, et al. : Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw Open 2021;4:e2131674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry NR, Hanson AC, Schulte PJ, et al. : Disparities in hypoxemia detection by pulse oximetry across self-identified racial groups and associations with clinical outcomes. Crit Care Med 2022; 50:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buell KG, Casey JD, Wang L, et al. : Big data for clinical trials: Automated collection of SpO2 for a trial of oxygen targets during mechanical ventilation. J Med Syst 2020; 44:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girardis M, Busani S, Damiani E, et al. : Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The oxygen-ICU randomized clinical trial. JAMA 2016; 316:1583–1589 [DOI] [PubMed] [Google Scholar]
- 9.Jubran A, Tobin MJ: Chapter 48. Monitoring during mechanical ventilation. In: Tobin MJ, ed. Principles and Practice of Mechanical Ventilation, 3e. New York: The McGraw Hill Companies, 2013. Accessed April 4, 2022. Available at: accessmedicine.mhmedical.com/content.aspx?aid=57077186 [Google Scholar]
- 10.Martin GS, Wathen BA, Schulman DA, et al. : Critical Care Societies Collaborative Reply to the U.S. Food and Drug Administration. Available at: https://www.chestnet.org/Newsroom/CHEST-News/2022/02/Inaccuracy-of-pulse-oximeters-based-on-skin-pigmentation-requires-additional-FDA-regulation. Accessed February 7, 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.