Abstract
Enterovirus 71 (EV71) vaccine for hand-foot-and-mouth disease (HFMD) prevention has been available for several years. However, as a new vaccine, the impact of EV71 vaccination on the epidemiology and etiology of HFMD is currently unclear. The purpose of this study was to compare and analyze the changes of epidemiological characteristics and etiology of HFMD patients after the introduction of EV71 vaccine. The data of hospitalized children with HFMD from 2014 to 2020 were collected from the case record department of a tertiary children hospital of Anhui Province. The changes of epidemiological characteristics, time distribution, disease severity and enterovirus serotypes in hospitalized children were analyzed. A total of 7373 cases of HFMD were reported during 2014 to 2020, including 634 (8.6%) severe cases. The number of cases reached the peak in 2016 (n = 1783) and decreased gradually after EV71 vaccination. The results of etiological test showed the positive rate was 80.5%, in which EV71 accounted for 1599 (21.7%) and CV-A16 accounted for 1028 (13.9%) respectively. The number of patients showed a bimodal distribution throughout the year, which were April to June and October to November. The age distribution changed significantly following the introduction of EV71 vaccine. The proportion of 1-year-old group of post-vaccination was significantly higher than that of pre-vaccination (61.9% vs 50.8%, P < .001). The proportion of HFMD caused by EV71 and severe cases decreased significantly after the vaccination (P < .001 for both). While the comparison of epidemiological characteristics and enterovirus serotypes between unvaccinated and vaccinated cases during 2017 to 2020 showed no significant difference. The dominant enterovirus serotypes of hospitalized HFMD changed significantly after the introduction of EV71 vaccine. The proportion of severe cases decreased significantly after the vaccination, but EV71 was still a major pathogen in patients with severe HFMD. More age-appropriate children are recommended to get vaccinated to establish stronger herd immunity in the population.
Keywords: enterovirus 71 (EV71), epidemiology, etiology, hand-foot-and-mouth disease (HFMD), vaccination
1. Introduction
As a common infectious disease among children, hand-foot-and-mouth disease (HFMD) is typically characterized by fever, mouth ulcers and vesicles on the hands, feet, or buttocks.[1] It is caused by a variety of enterovirus infection, mainly including enterovirus 71 (EV71) and coxsackievirus A16 (CV-A16).[2,3] It is usually mild and self-limiting in most cases. However, some severe cases, especially those due to EV71, may develop fatal neurological, respiratory and circulatory complications quickly.[4–8] That is why EV71 has captured more attention than others.
Owing to the lack of effective antiviral drugs for HFMD, clinical treatment is mainly symptomatic support to date. EV71 vaccination was considered to be the most effective method to prevent this condition. Therefore, a vaccine against EV71 was urgently needed. Since December 2015, 3 inactivated EV71 vaccines were licensed in China for children under 5 years of age. In general, the vaccine was administrated intramuscularly into the anterolateral side of thigh or the deltoid muscle according to a 2-dose schedule (on day 0 and day 28) for children 6 to 59 months of age. Previous study had showed a high degree of protective immunity for HFMD caused by EV71 but no cross-protection against other serotypes.[9–11] That is to say, though EV71 vaccine is available and effective, children in susceptible ages are likely to suffer from HFMD as before.
Anhui Province is a region located in the mid-eastern of China, which has a large population over 60 million. In the last decade, Anhui was one of the districts affected by the epidemics of HFMD.[12] EV71 vaccine had been available in Anhui since August 2016, while there was a paucity of data concerning the impact of vaccination. There was no doubt that the marketing of EV71 vaccine had beneficial effects on the control of HFMD. Furthermore, it brought a series of problems and challenges as well. For example, what happened to the epidemiological characteristics of HFMD and what happened to the enterovirus serotype distribution of HFMD? All these are still open questions.
Therefore, we compared and analyzed the changes of epidemiological characteristics and enterovirus serotypes of HFMD inpatients during 2014 to 2020. This current study aimed to further understand the impact of EV71 vaccination on the epidemiology and etiology of HFMD, so as to provide scientific basis for future vaccine-related studies to prevent and treat HFMD better.
2. Patients & Methods
2.1. Data collection
Anhui Provincial Children’s Hospital is the only designated tertiary hospital for HFMD treatment in Anhui. The data of hospitalized patients with HFMD during 2014 to 2020 were obtained from the Medical Record Department. All individual identifying information such as name, address, and telephone number, etc was anonymized and de-identified prior to analysis. The main information including time, age, sex, etiology, severity and vaccination history was collected. Informed consent was waived due to the retrospective nature of the study. All the study procedures were reviewed and approved by the Ethics Committee of Anhui Provincial Children’s Hospital.
2.2. Case definitions
The clinical and laboratory diagnoses of mild and severe cases were in accordance with the diagnostic criteria in the Guidelines for the Diagnosis and Treatment of HFMD (2018 Edition) issued by the National Health Commission of China.[13] Patients with HFMD, whether probable or confirmed, were classified as severe if they had any neurological complications or cardiopulmonary complications, or both. Otherwise, patients were categorized as mild cases.
2.3. Specimen collection and detection
Clinical specimens including peripheral blood, throat swabs, fecal samples or rectal swabs were collected on the first day of admission by hospital trained medical staff. These specimens underwent virus isolation analysis to determine the enterovirus serotypes. Pan-enteroviruses, EV71, and CV-A16 were tested simultaneously, and only positive results were reported with categorization as EV71, CV-A16, or other enteroviruses.
2.4. Statistics
Because EV71 vaccination was implemented since 2016 in Anhui Province, 2016 was regarded as the transition year. Thus, the rest of years were classified into 2 time periods: 2014 to 2015 as pre-vaccination, and 2017 to 2020 as post-vaccination to reflect the changes of epidemiology and etiology. Pearson’s chisquare test was used to analyze categorical data. P values <.05 were regarded as statistically significant. All statistical analyses were performed by SPSS 17.0 software (SPSS, Inc., Chicago, IL).
3. Results
3.1. Demographic characteristics of HFMD
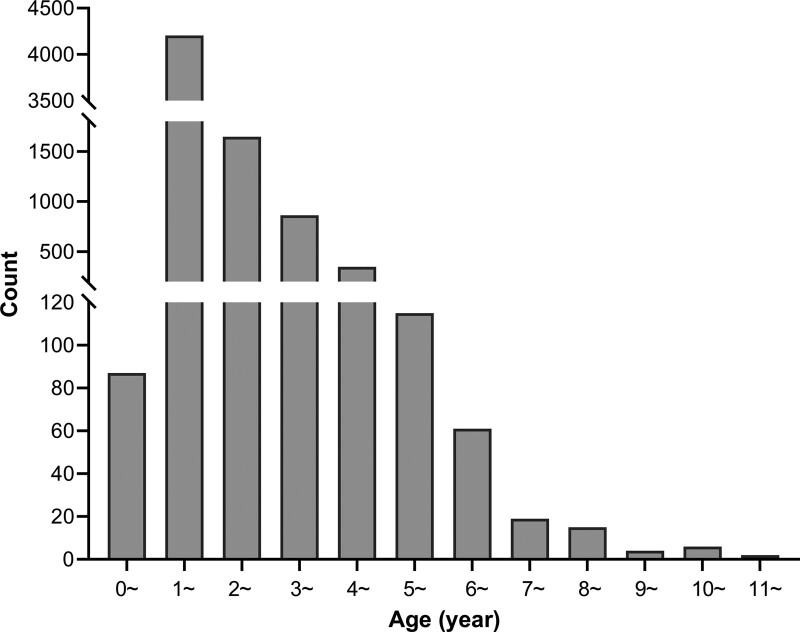
From 2014 to 2020, there were 1175, 1048, 1783, 1277, 1081, 622, and 387 hospitalized children with HFMD, respectively, 7373 cases in total (Table 1). The number of cases reached the peak in 2016 and decreased gradually since then. The age distribution ranged from 6 months to 11 years old, with 7151 (97.0%) cases younger than 5 years old (Fig. 1). Among them, the 1-year-old group accounted for 4204 (57.0%) cases, and the number decreased with the increase of age. The median age was 21 months (IQR: 16–31 months). The proportion of male cases was higher than that of female cases, with a sex ratio of approximately 1.73:1. The cases of HFMD were mainly scattered children, totally 5396 (73.2%). Among 7373 hospitalized children with HFMD, 6739 (91.4%) cases were mild and 634 (8.6%) cases were severe. From 2014 to 2020, the annual percentage of severe cases was 15.1%, 10.4%, 9.4%, 6.3%, 4.7%, 5.1%, and 4.4%, respectively, which showed a downward trend year by year, except for a fluctuation in 2019 (5.1%).
Table 1.
Epidemiological characteristics and etiologic data of inpatients with HFMD in 2014 to 2020.
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 1175(%) | n = 1048(%) | n = 1783(%) | n = 1277(%) | n = 1081(%) | n = 622(%) | n = 387(%) | N = 7373(%) | |||||||||
| Age group (yr) | ||||||||||||||||
| <1 | 43 | (3.7) | 19 | (1.8) | 8 | (0.4) | 4 | (0.3) | 2 | (0.2) | 6 | (1.0) | 5 | (1.3) | 87 | (1.2) |
| 1~ | 559 | (47.6) | 571 | (54.5) | 991 | (55.6) | 765 | (59.9) | 703 | (65.0) | 379 | (60.9) | 236 | (61.0) | 4204 | (57.0) |
| 2~ | 331 | (28.2) | 221 | (21.1) | 429 | (24.1) | 249 | (19.5) | 201 | (18.6) | 133 | (21.4) | 84 | (21.7) | 1648 | (22.4) |
| 3~ | 139 | (11.8) | 144 | (13.7) | 217 | (12.2) | 158 | (12.4) | 102 | (9.4) | 67 | (10.8) | 37 | (9.6) | 864 | (11.7) |
| 4~ | 62 | (5.3) | 55 | (5.2) | 77 | (4.3) | 66 | (5.2) | 46 | (4.3) | 22 | (3.5) | 20 | (5.2) | 348 | (4.7) |
| >5 | 41 | (3.5) | 38 | (3.6) | 61 | (3.4) | 35 | (2.7) | 27 | (2.5) | 15 | (2.4) | 5 | (1.3) | 222 | (3.0) |
| Age (mo) | ||||||||||||||||
| Median (IQR) | 21(15-32) | 21(16-34) | 21(16-32) | 22(17-32) | 20(15-28) | 20(15-29) | 20(16-30) | 21(16-31) | ||||||||
| Mean ± SD | 26.00 ± 14.37 | 26.65 ± 14.85 | 26.38 ± 14.16 | 25.55 ± 14.16 | 24.41 ± 13.98 | 24.51 ± 14.23 | 24.40 ± 12.21 | 25.66 ± 14.20 | ||||||||
| Gender | ||||||||||||||||
| Male | 772 | (65.7) | 633 | (60.4) | 1145 | (64.2) | 815 | (63.8) | 695 | (64.3) | 374 | (60.1) | 235 | (60.7) | 4669 | (63.3) |
| Female | 403 | (34.3) | 415 | (39.6) | 638 | (35.8) | 462 | (36.2) | 386 | (35.7) | 248 | (39.9) | 152 | (39.3) | 2704 | (36.7) |
| M/F ratio | 1.92:1 | 1.53:1 | 1.79:1 | 1.76:1 | 1.80:1 | 1.51:1 | 1.55:1 | 1.73:1 | ||||||||
| Hosting classification | ||||||||||||||||
| Scatter children | 849 | (72.3) | 716 | (68.3) | 1305 | (73.2) | 951 | (74.5) | 830 | (76.8) | 452 | (72.7) | 293 | (75.7) | 5396 | (73.2) |
| Childcare | 311 | (26.5) | 309 | (29.5) | 441 | (24.7) | 306 | (24.0) | 235 | (21.7) | 156 | (25.1) | 89 | (23.0) | 1847 | (25.1) |
| In school | 12 | (1.0) | 22 | (2.1) | 34 | (1.9) | 16 | (1.3) | 15 | (1.4) | 13 | (2.1) | 3 | (0.8) | 115 | (1.6) |
| Others | 3 | (0.3) | 1 | (0.1) | 3 | (0.2) | 4 | (0.3) | 1 | (0.1) | 1 | (0.2) | 2 | (0.5) | 15 | (0.2) |
| Etiology | ||||||||||||||||
| Negative | 238 | (20.3) | 225 | (21.5) | 388 | (21.8) | 211 | (16.5) | 186 | (17.2) | 122 | (19.6) | 67 | (17.3) | 1437 | (19.5) |
| EV71 | 363 | (30.9) | 302 | (28.8) | 493 | (27.7) | 210 | (16.4) | 113 | (10.5) | 79 | (12.7) | 39 | (10.1) | 1599 | (21.7) |
| CV-A16 | 194 | (16.5) | 164 | (15.6) | 319 | (17.9) | 190 | (14.9) | 81 | (7.5) | 44 | (7.1) | 36 | (9.3) | 1028 | (13.9) |
| Others | 380 | (32.3) | 357 | (34.1) | 583 | (32.7) | 666 | (52.2) | 701 | (64.8) | 377 | (60.6) | 245 | (63.3) | 3309 | (44.9) |
| Severity | ||||||||||||||||
| Mild | 998 | (84.9) | 939 | (89.6) | 1615 | (90.6) | 1197 | (93.7) | 1030 | (95.3) | 590 | (94.9) | 370 | (95.6) | 6739 | (91.4) |
| Severe | 177 | (15.1) | 109 | (10.4) | 168 | (9.4) | 80 | (6.3) | 51 | (4.7) | 32 | (5.1) | 17 | (4.4) | 634 | (8.6) |
CV-A16 = coxsackievirus A16, EV71 = enterovirus 71, HFMD = hand-foot-and-mouth disease, IQR = inter-quartile range, SD = standard deviation.
Figure 1.
Age distribution of hospitalized children with HFMD from 2014 to 2020. HFMD = hand-foot-and-mouth disease.
3.2. Etiology of HFMD
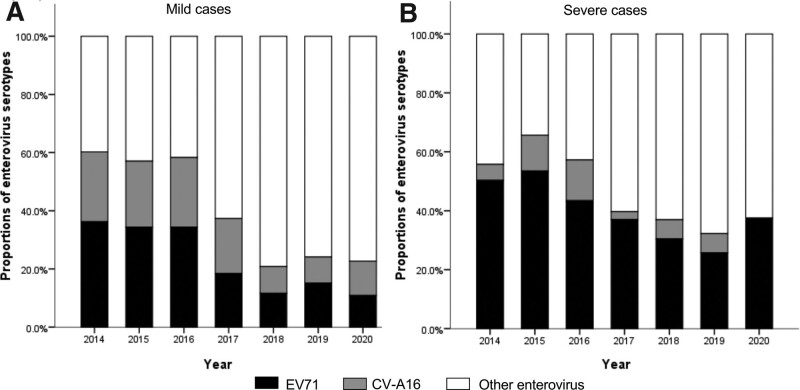
All the hospitalized children diagnosed as HFMD were detected for enterovirus serotypes. Among the 7373 cases, the positive rate was 80.5%, in which 1599 (21.7%) and 1028 (13.9%) cases were associated with EV71 and CV-A16, respectively, and the remaining 3309 (44.9%) were detected as positive for other enteroviruses (non-EV71/non-CV-A16). Since 2014, other enteroviruses occupied the largest proportion among laboratory-confirmed mild cases annually (Fig. 2A). The proportion of mild cases attributable to EV71 decreased from 36.3% in 2014 to 10.9% in 2020. For severe cases, EV71 was the predominant serotype during 2014-2016, accounting for 50.3%, 53.5%, and 43.4% of the cases, respectively (Fig. 2B). While other enteroviruses was predominant since 2017, accounting for 60.3%, 63.0%, 67.7%, and 62.5% of severe cases, respectively. In a word, the proportion of HFMD cases caused by EV71 decreased among both mild and severe cases.
Figure 2.
Constituent ratios of enterovirus serotypes in laboratory-confirmed cases of HFMD by clinical severity during 2014 to 2020. (A) mild cases. (B) severe cases. HFMD = hand-foot-and-mouth disease.
3.3. Time distribution
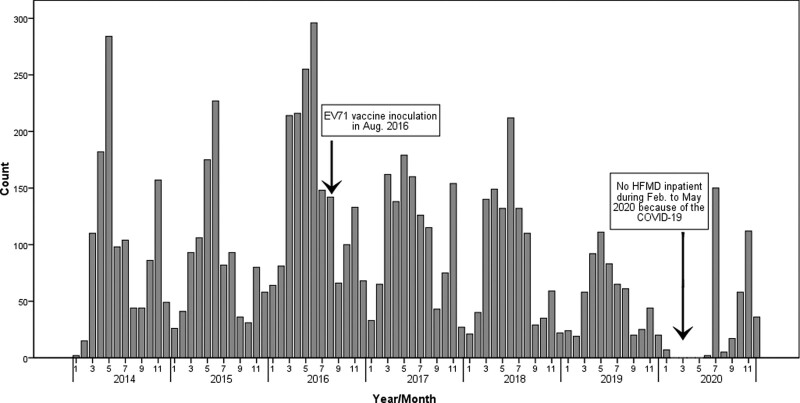
Monthly distribution of patients showed 2 peaks of HFMD activity, with the first peak occurring from April to June and the second from October to November (Fig. 3). Notably, the first peak was higher than the second peak every year, even after the introduction of EV71 vaccine since August 2016. But the results showed that the peak value was significantly decreased after the vaccination. The number of inpatients reached the lowest point in January and September. Due to the rapid spread of the corona virus disease 2019 (COVID-19) worldwide, the present center was conducted as the designated hospital for pediatric patients with COVID-19. As a result, the relevant data of HFMD was absent during February to May in 2020.
Figure 3.
Time distribution of hospitalized children with HFMD from 2014 to 2020. HFMD = hand-foot-and-mouth disease.
3.4. Comparison between cases pre-vaccination and post-vaccination
The epidemiological characteristics and etiology between cases pre-vaccination and post-vaccination were compared in Table 2. The age distribution of hospitalized patients before and after the introduction of EV71 vaccine was significantly different (P < .001). The proportion of 1-year-old group in post-vaccination period was significantly higher than that in pre-vaccination period (61.9% vs 50.8%, P < .001). On the contrary, the proportion of patients aged <1, 2, 3 and >5 years was lower than that in pre-vaccination period. The sex ratio of HFMD patients showed no significant difference between the 2 periods. Compared with that of pre-vaccination, the proportion of scatter children post-vaccination increased significantly (75.0% vs 70.4%, P < .001), while the proportion of children in childcare decreased significantly (23.3% vs 27.9%, P < .001).
Table 2.
The comparison of epidemiology and etiology of HFMD before and after EV71 vaccination.
| Total | Post-vaccination (2017–2020) | Pre-vaccination(2014-2015) | P | OR(95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| N = 5590(%) | n = 3367(%) | n = 2223(%) | ||||||
| Age group (yr) | <.001 | |||||||
| <1 | 79 | (1.4) | 17 | (0.5) | 62 | (2.8) | <.001 | 0.18(0.10-0.30) |
| 1~ | 3213 | (57.5) | 2083 | (61.9) | 1130 | (50.8) | <.001 | 1.57(1.41-1.75) |
| 2~ | 1219 | (21.8) | 667 | (19.8) | 552 | (24.8) | <.001 | 0.75(0.66-0.85) |
| 3~ | 647 | (11.6) | 364 | (10.8) | 283 | (12.7) | .028 | 0.83(0.70-0.98) |
| 4~ | 271 | (4.8) | 154 | (4.6) | 117 | (5.3) | .240 | 0.86(0.67-1.10) |
| >5 | 161 | (2.9) | 82 | (2.4) | 79 | (3.6) | .014 | 0.68(0.50-0.93) |
| Gender | ||||||||
| Male | 3524 | (63.0) | 2119 | (62.9) | 1405 | (63.2) | .839 | 0.99(0.89-1.11) |
| Female | 2066 | (37.0) | 1248 | (37.1) | 818 | (36.8) | Referent | |
| Hosting classification | .002 | |||||||
| Scatter children | 4091 | (73.2) | 2526 | (75.0) | 1565 | (70.4) | <.001 | 1.26(1.12-1.42) |
| Childcare | 1406 | (25.2) | 786 | (23.3) | 620 | (27.9) | <.001 | 0.79(0.70-0.89) |
| In school | 81 | (1.4) | 47 | (1.4) | 34 | (1.5) | .683 | 0.91(0.58-1.42) |
| Others | 12 | (0.2) | 8 | (0.2) | 4 | (0.2) | .648 | 1.32(0.40-4.39) |
| Etiology | <.001 | |||||||
| EV71 | 1106 | (19.8) | 441 | (13.1) | 665 | (29.9) | <.001 | 0.35(0.31-0.40) |
| CV-A16 | 709 | (12.7) | 351 | (10.4) | 358 | (16.1) | <.001 | 0.61(0.52-0.71) |
| Others | 2726 | (48.8) | 1989 | (59.1) | 737 | (33.2) | <.001 | 2.91(2.60-3.26) |
| Negative | 1049 | (18.8) | 586 | (17.4) | 463 | (20.8) | .001 | 0.80(0.70-0.92) |
| Severity | ||||||||
| Severe | 466 | (8.7) | 180 | (5.3) | 286 | (12.9) | <.001 | 0.38(0.32-0.47) |
| Mild | 5124 | (91.7) | 3187 | (94.7) | 1937 | (87.1) | Referent | |
CI = confidence interval, CV-A16 = coxsackievirus A16, EV71 = enterovirus 71, HFMD = hand-foot-and-mouth disease, OR = odds ratio.
In the meanwhile, the enterovirus serotypes distribution changed significantly following the introduction of EV71 vaccine. A significant decrease of EV71 and CV-A16 related HFMD was reported (P < .001 for both) in post-vaccination period compared with that of pre-vaccination. In contrast, the proportion of HFMD caused by other enteroviruses was significantly increased after vaccination (P < .001). In addition, the proportion of severe cases in post-vaccination period was significantly lower than that in pre-vaccination period (5.3% vs 12.9%, P < .001).
3.5. Comparison between unvaccinated and vaccinated cases during 2017 to 2020
Table 3 showed the epidemiological characteristics and etiology of unvaccinated and vaccinated patients during 2017 to 2020. About 2478 (60.2%) patients were vaccinated since the available of EV71 vaccine. There was no significant difference in different age gender, and enterovirus serotypes distribution between the 2 groups. Severe cases occurred in 54 (6.1%) of unvaccinated patients and 126 (5.1%) of vaccinated patients, and the difference was not statistically significant as well.
Table 3.
The comparison of epidemiology and etiology of HFMD between vaccinated and unvaccinated cases during 2017 to 2020.
| Total N = 3367(%) |
Vaccinated n = 2478(%) |
Unvaccinated n = 889(%) |
P | OR(95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Age group (yr) | .905 | |||||||
| <1 | 17 | (0.5) | 12 | (0.5) | 5 | (0.6) | .778 | 0.86(0.30-2.45) |
| 1~ | 2083 | (61.9) | 1529 | (61.7) | 554 | (62.3) | .746 | 0.97(0.83-1.14) |
| 2~ | 667 | (19.8) | 491 | (19.8) | 176 | (19.8) | .991 | 1.00(0.83-1.21) |
| 3~ | 364 | (10.8) | 272 | (11.0) | 92 | (10.3) | .605 | 1.07(0.83-1.37) |
| 4~ | 154 | (4.6) | 110 | (4.4) | 44 | (4.9) | .532 | 0.89(0.62-1.28) |
| ≥5 | 82 | (2.4) | 64 | (2.6) | 18 | (2.0) | .354 | 1.28(0.76-2.18) |
| Gender | ||||||||
| Male | 2119 | (62.9) | 1545 | (62.3) | 574 | (64.6) | .240 | 0.91(0.78-1.07) |
| Female | 1248 | (37.1) | 933 | (37.7) | 315 | (35.4) | Referent | |
| Hosting classification | .902 | |||||||
| Scatter children | 2526 | (75.0) | 1857 | (74.9) | 669 | (75.3) | .853 | 0.98(0.82-1.17) |
| Childcare | 786 | (23.3) | 581 | (23.4) | 205 | (23.1) | .815 | 1.02(0.85-1.23) |
| In school | 47 | (1.4) | 35 | (1.4) | 12 | (1.3) | .891 | 1.05(0.54-2.03) |
| Others | 8 | (0.2) | 5 | (0.2) | 3 | (0.3) | .756 | 0.60(0.14-2.50) |
| Etiology | .291 | |||||||
| EV71 | 441 | (13.1) | 319 | (12.9) | 122 | (13.7) | .519 | 0.93(0.74-1.16) |
| CV-A16 | 351 | (10.4) | 245 | (9.9) | 106 | (11.9) | .088 | 0.81(0.64-1.03) |
| Others | 1989 | (59.1) | 1476 | (59.6) | 513 | (57.7) | .334 | 1.08(0.92-1.26) |
| Negative | 586 | (17.4) | 438 | (17.7) | 148 | (16.6) | .488 | 1.08(0.88-1.32) |
| Severity | ||||||||
| Severe | 180 | (5.3) | 126 | (5.1) | 54 | (6.1) | .261 | 0.83(0.60-1.15) |
| Mild | 3187 | (94.7) | 2352 | (94.9) | 835 | (93.9) | Referent | |
CI = confidence interval, CV-A16 = coxsackievirus A16, EV71 = enterovirus 71, HFMD = hand-foot-and-mouth disease, OR = odds ratio.
4. Discussion
EV71 vaccine is independently developed by China, and EV71 vaccination is the only effective way to prevent EV71 related HFMD.[10,11] As a new vaccine, EV71 vaccine has been used for more than 4 years in Anhui. Although it is not administered as part of the expanded programme on immunization in China, more and more children have been vaccinated with EV71 vaccine with the improvement of the guardians’ acceptance.[14] This condition is bound to cause major changes to the epidemic of HFMD. Accordingly, in order to provide a comprehensive analysis of the effect of EV71 vaccination, we conducted the present retrospective case-study based on the data of hospitalized children from 2014 to 2020 for the first time in Anhui. Our findings would have great values to guide the prevention and treatment of HFMD in other areas of the world.
At first, our age distribution of HFMD was consistent with those reported in other countries.[12,15,16] Maternal immunization experience protected newborns from HFMD infection, so there were no patients younger than 6 months of age in our study.[17] Previous serological study showed the positive rate of maternal EV71 neutralizing antibody at birth was 48% to 85%, and decreased to 1% to 7% at the age of 6 months.[18,19] The proportion of patients younger than 1-year-old was quite low in our study. On the one hand, there is a small number of maternally-derived EV71 neutralizing antibody left. On the other hand, children under 1 years old have less contact with outside due to their limited activities.[20] Since then, with the increase of age, maternally-derived EV71 neutralizing antibody could not be detected at the age of 12 months.[21] Our results suggested the number of patients peaked at 1-year-old group and declined with the increase of age. Therefore, if a vaccine is available, the most pragmatic time to vaccinate would be when the infant is 6 months to 12 months of age. However, children aged 2 to 4 years old still accounted for a large proportion of the cases after EV71 vaccination, suggesting children in this age range were the second population for HFMD prevention and control as well. The antibody level in children above 6 years old is stable at 58-80%, and generally has immunity to EV71.[18,21,22] Our results also confirm this point that only 222 (3%) cases were over 5 years old. In the meanwhile, the change trend of CV-A16 neutralizing antibody is similar to that of EV71.[23]
The results of this study showed HFMD occurred with obvious and typical seasonal characteristics and presented a bimodal distribution throughout the year. The main peak occurred from April to June and the secondary from October to November. This distribution may be related to different climatic conditions.[24,25] Warm climate could prolong the survival of enteroviruses, improve the pathogenicity and increase the probability of infection.[26] In addition, children would participate in more outdoor activities during warm seasons, which may increase the chance of contracting virus through direct contact with contaminated toys or aerosol.[27,28] Moreover, there was a significant decrease during July to August which was the summer holidays in China, suggesting that the gathering of school children was a risk factor for HFMD outbreaks. The proportion of male cases was more than that of females in both pre- and post-vaccination. One explanation may be that boys are more active, and spend more time in public places. After EV71 vaccination, the proportion of severe cases decreased significantly, while EV71 remained one of the most common pathogens in severe cases, suggesting the prevention of EV71 infection was still the focus of HFMD.
The introduction of EV71 vaccine not only reduced the number of HFMD inpatients, but brought benefits to the unvaccinated population indirectly. With the increase of the vaccination rate during 2017 to 2020, the comparison of epidemiological characteristics and enterovirus serotypes distribution between vaccinated and unvaccinated patients showed no significant difference. With the increase of EV71 vaccination, an immune barrier can be formed in the population, which is called herd immunity. It means that when a certain proportion of the population obtains immunity through vaccination, it can reduce the spread of the EV71 and protect the unvaccinated population.[29] A previous study showed that herd immunity threshold for EV71 related HFMD was about 90.0%.[30] This phenomenon was also observed after the use of rotavirus vaccine.[31,32] The incidence rate of rotavirus associated gastroenteritis dropped by more than 70% in American hospitals after the use of rotavirus vaccine. However, this significant decline cannot be fully attributed to the direct effect of the vaccination, as the vaccination rate did not exceed 50%. Another study from Australia found that besides the reduction in the incidence of gastroenteritis among vaccinated population, the incidence among non-vaccinated population or children who did not receive full doses of vaccine decreased as well.[33] Similarly, the effect of herd immunity was also observed after the use of pertussis vaccine and streptococcus pneumoniae vaccine.[34,35] These results suggested that herd immunity after EV71 vaccination worked in the prevention of HFMD.
In the meanwhile, we found a significant decrease of HFMD patients in 2019-2020. This result was not only caused by herd immunity, but also due to the outbreak of COVID-19.[36] Researches in the same period also suggested that the incidence of most infectious diseases in China showed a downward trend, one of these diseases with the largest decline was HFMD.[37–39] Interventions such as the wearing of masks, frequent hand-washing, less gathering, or even school closure, played an positive role in the prevention and control of HFMD.[40] These various public health intervention strategies and measures adopted by China to contain COVID-19 can provide a reference for the prevention and control of HFMD and indeed other infectious diseases.
The present study had several limitations. First, the study included a limited number of cases enrolled from a single center, which may cause bias. The findings need to be confirmed by more prospective, multi-center studies. Second, EV71 vaccine coverage also has an impact on HFMD. Unfortunately, we cannot obtain the vaccination coverage of EV71 vaccine among the population in Anhui Province. Another deficiency of this study was that we cannot accurately evaluate the serotypes other than EV71 and CV-A16 due to the limited laboratory technology. It is necessary to carry out more comprehensive detection of enterovirus serotypes in the future. At last but not least, the time so far of post-vaccination surveillance was relatively short, which could just reflect the short-term effect on the epidemic of HFMD. As for the long-term effect, it takes more time to accumulate data for further analysis.
5. Conclusions
In conclusion, the vaccination of EV71 vaccine has significantly changed the epidemiology and etiology of HFMD in Anhui. EV71 vaccine is effective and worthy of popularization and application. More age-appropriate children are recommended to get vaccinated to establish stronger herd immunity in the population. Considering the EV71 vaccine has no protective effect on non-EV71 infection, enhanced surveillance of other enteroviruses, as well as the development of a multi-valent vaccine with broader protection for HFMD, is urgently required.
Acknowledgments
We thank all the doctors and nurses in Anhui Provincial Children’s Hospital for their help in conducting this study and all the parents and children who made it possible.
Author contributions
Conceptualization: Jing Sun, Yuanyuan Li, Zhi Yang, Qingfeng Fang, Biquan Chen.
Data curation: Jing Sun, Yuanyuan Li, Zhi Yang, Qingfeng Fang.
Formal analysis: Jing Sun.
Methodology: Jing Sun.
Software: Jing Sun.
Validation: Biquan Chen.
Writing – original draft: Jing Sun.
Writing – review & editing: Biquan Chen.
Abbreviations:
- COVID-19 =
- corona virus disease 2019
- CV-A16 =
- coxsackievirus A16
- EV71 =
- enterovirus 71
- HFMD =
- hand-foot-and-mouth disease
The authors have no funding and conflicts of interest to disclose.
The study procedures were reviewed and approved by the ethics committee of the hospital. Informed consent was waived due to the retrospective nature of the study.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Sun J, Li Y, Yang Z, Fang Q, Chen B. Effect of enterovirus 71 vaccination on the epidemiological characteristics and etiology in hospitalized children with hand-foot-and-mouth disease: A retrospective study from a tertiary children’s hospital. Medicine 2022;101:37(e30356).
Contributor Information
Jing Sun, Email: jingsun1106@163.com.
Yuanyuan Li, Email: 78699137@qq.com.
Zhi Yang, Email: 767210474@qq.com.
Qingfeng Fang, Email: fang120@yeah.net.
References
- [1].Lu B, Guo H, Lu H. Hand, foot, and mouth disease in mainland China. Lancet Infect Dis. 2014;14:1041. [DOI] [PubMed] [Google Scholar]
- [2].Kadurugamuwa JL, Shaheen E. Inactivation of human enterovirus 71 and coxsackie virus A16 and hand, foot, and mouth disease. Am J Infect Control. 2011;39:788–9. [DOI] [PubMed] [Google Scholar]
- [3].Xu M, Su L, Cao L, et al. Enterovirus genotypes causing hand foot and mouth disease in Shanghai, China: a molecular epidemiological analysis. BMC Infect Dis. 2013;13:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sabanathan S, Tan le V, Thwaites L, et al. Enterovirus 71 related severe hand, foot and mouth disease outbreaks in South-East Asia: current situation and ongoing challenges. J Epidemiol Community Health. 2014;68:500–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wong SS, Yip CC, Lau SK, et al. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol Infect. 2010;138:1071–89. [DOI] [PubMed] [Google Scholar]
- [6].Zhang Y, Zhu Z, Yang W, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li W, Teng G, Tong H, et al. Study on risk factors for severe hand, foot and mouth disease in China. PLoS One. 2014;9:e87603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, Zhao H, Ou R, et al. Epidemiological and clinical characteristics of severe hand-foot-and-mouth disease (HFMD) among children: a 6-year population-based study. BMC Public Health. 2020;20:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wei M, Meng F, Wang S, et al. 2-year efficacy, immunogenicity, and safety of vigoo enterovirus 71 vaccine in healthy Chinese children: a randomized open-label study. J Infect Dis. 2017;215:56–63. [DOI] [PubMed] [Google Scholar]
- [10].Zhu F, Xu W, Xia J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–28. [DOI] [PubMed] [Google Scholar]
- [11].Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–37. [DOI] [PubMed] [Google Scholar]
- [12].Xing W, Liao Q, Viboud C, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. 2014;14:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Health Commission of China. Diagnosis and treatment guideline on hand, foot and mouth disease (2018 Version). Chin J Clin Infect Dis. 2018;11:161–6. [Google Scholar]
- [14].Li T, Wang H, Lu Y, et al. Willingness and influential factors of parents to vaccinate their children with novel inactivated enterovirus 71 vaccines in Guangzhou, China. Vaccine. 2018;36:3772–8. [DOI] [PubMed] [Google Scholar]
- [15].Momoki ST. Surveillance of enterovirus infections in Yokohama city from 2004 to 2008. Jpn J Infect Dis. 2009;62:471–3. [PubMed] [Google Scholar]
- [16].Liu B, Luo L, Yan S, et al. Clinical features for mild hand, foot and mouth disease in China. PLoS One. 2015;10:e0135503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cinicola B, Conti MG, Terrin G, et al. The protective role of maternal immunization in early life. Front Pediatr. 2021;9:638871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luo ST, Chiang PS, Chao AS, et al. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis. 2009;15:581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhu FC, Liang ZL, Meng FY, et al. Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PLoS One. 2012;7:e37206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang B, Wu P, Wu JT, et al. Seroprevalence of enterovirus 71 antibody among children in China: a systematic review and meta-analysis. Pediatr Infect Dis J. 2015;34:1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tran CB, Nguyen HT, Phan HT, et al. The seroprevalence and seroincidence of enterovirus71 infection in infants and children in Ho Chi Minh City, Viet Nam. PLoS One. 2011;6:e21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhu Z, Zhu S, Guo X, et al. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J. 2010;7:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rabenau HF, Richter M, Doerr HW. Hand, foot and mouth disease: seroprevalence of Coxsackie A16 and Enterovirus 71 in Germany. Med Microbiol Immunol. 2010;199:45–51. [DOI] [PubMed] [Google Scholar]
- [24].Lin H, Zou H, Wang Q, et al. Short-term effect of El Niño-Southern Oscillation on pediatric hand, foot and mouth disease in Shenzhen, China. PLoS One. 2013;8:e65585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li T, Yang Z, Di B, et al. Hand-foot-and-mouth disease and weather factors in Guangzhou, southern China. Epidemiol Infect. 2014;142:1741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Altizer S, Dobson A, Hosseini P, et al. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006;9:467–84. [DOI] [PubMed] [Google Scholar]
- [27].Robinson M, Drossinos Y, Stilianakis NI. Indirect transmission and the effect of seasonal pathogen inactivation on infectious disease periodicity. Epidemics. 2013;5:111–21. [DOI] [PubMed] [Google Scholar]
- [28].Wang Y, Feng Z, Yang Y, et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology. 2011;22:781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ashby B, Best A. Herd immunity. Curr Biol. 2021;31:R174–7. [DOI] [PubMed] [Google Scholar]
- [30].Han Y, Chen Z, Zheng K, et al. Epidemiology of hand, foot, and mouth disease before and after the introduction of enterovirus 71 vaccines in Chengdu, China, 2009-2018. Pediatr Infect Dis J. 2020;39:969–78. [DOI] [PubMed] [Google Scholar]
- [31].Centers for Disease Control and Prevention. Delayed onset and diminished magnitude of rotavirus activity--United States, November 2007-May 2008. MMWR Morb Mortal Wkly Rep. 2008;57:697–700. [PubMed] [Google Scholar]
- [32].Tate JE, Panozzo CA, Payne DC, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–71. [DOI] [PubMed] [Google Scholar]
- [33].Paulke-Korinek M, Rendi-Wagner P, Kundi M, et al. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in Austrian children. Pediatr Infect Dis J. 2010;29:319–23. [DOI] [PubMed] [Google Scholar]
- [34].Préziosi MP, Yam A, Wassilak SG, et al. Epidemiology of pertussis in a West African community before and after introduction of a widespread vaccination program. Am J Epidemiol. 2002;155:891–6. [DOI] [PubMed] [Google Scholar]
- [35].Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. [DOI] [PubMed] [Google Scholar]
- [36].Ochani R, Asad A, Yasmin F, et al. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29:20–36. [PubMed] [Google Scholar]
- [37].Chen B, Wang M, Huang X, et al. Changes in incidence of notifiable infectious diseases in China under the prevention and control measures of COVID-19. Front Public Health. 2021;9:728768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Geng MJ, Zhang HY, Yu LJ, et al. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun. 2021;12:6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Song S, Wang P, Li J, et al. The indirect impact of control measures in COVID-19 pandemic on the incidence of other infectious diseases in China. Public Health Pract (Oxf). 2022;4:100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shen L, Sun M, Song S, et al. The impact of anti-COVID-19 nonpharmaceutical interventions on hand, foot, and mouth disease-a spatiotemporal perspective in Xi’an, northwestern China. J Med Virol. 2022;94:3121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]