Abstract
Citrate uptake in Bacillus subtilis is stimulated by a wide range of divalent metal ions. The metal ions were separated into two groups based on the expression pattern of the uptake system. The two groups correlated with the metal ion specificity of two homologous B. subtilis secondary citrate transporters, CitM and CitH, upon expression in Escherichia coli. CitM transported citrate in complex with Mg2+, Ni2+, Mn2+, Co2+, and Zn2+ but not in complex with Ca2+, Ba2+, and Sr2+. CitH transported citrate in complex with Ca2+, Ba2+, and Sr2+ but not in complex with Mg2+, Ni2+, Mn2+, Co2+, and Zn2+. Both transporters did not transport free citrate. Nevertheless, free citrate uptake could be demonstrated in B. subtilis, indicating the expression of at least a third citrate transporter, whose identity is not known. For both the CitM and CitH transporters it was demonstrated that the metal ion promoted citrate uptake and, vice versa, that citrate promoted uptake of the metal ion, indicating that the complex is the transported species. The results indicate that CitM and CitH are secondary transporters that transport complexes of divalent metal ions and citrate but with a complementary metal ion specificity. The potential physiological function of the two transporters is discussed.
Citrate is abundant in nature and a natural constituent of all living cells. Most bacteria have transport systems in the cytoplasmic membrane that mediate the uptake of citrate. Internalized citrate can be utilized as a carbon and energy source under aerobic as well as under anaerobic conditions. Under aerobic conditions citrate dissimilation occurs via the tricarboxylic acid cycle, while under anaerobic conditions three different fermentative pathways have been described (reviewed in reference 7). All known bacterial citrate transporters are secondary transporters that use the energy stored in electrochemical gradients of protons or sodium ions to drive uptake. Mechanistically, the transporters couple the uptake of citrate to the uptake of one or more protons or sodium ions (25, 26, 27). A special case are the citrate transporters found in lactic acid bacteria that catalyze heterologous exchange of citrate and lactate (precursor-product exchange) (4, 19). These transporters are involved in secondary proton motive force generation by translocation of net negative charge into the cell (20). A similar precursor-product exchange mechanism has been proposed for CitT, a citrate transporter of Escherichia coli that is induced under anaerobic conditions (23).
Citrate forms stable complexes with divalent metal ions. Most citrate transporters are inhibited by the addition of divalent cations because they do not recognize the metal-citrate complex (18, 25, 28). However, in strains of the genera Pseudomonas, Klebsiella, Citrobacter, and Bacillus citrate transporters have evolved that specifically recognize citrate in complex with a divalent metal ion (5, 13, 17). It is believed that these organisms take up complexed citrate because it is available as such in their habitat.
To date, the best-studied system for metal-citrate transport is the Mg2+-dependent citrate transporter CitM of Bacillus subtilis (6). CitM is a proton motive force-driven secondary citrate transporter that is strictly dependent on the presence of Mg2+. Regulation of expression of the transporter is under strict control of the medium composition. Expression requires the presence of citrate in the medium that activates a two-component signal transduction pathway (9, 32) and is under control of catabolite repression by rapidly metabolized carbon sources like glucose, inositol, and succinate (30a). CitM belongs to a novel family of secondary transporters that contains only six known members, three of which are found in B. subtilis. One of these, termed CitH, was also shown to be a citrate transporter. Since uptake of citrate catalyzed by CitH was inhibited by the presence of Mg2+ in the assay buffer, it was reported to transport free citrate (6). The function of the third B. subtilis homolog encoded by the yraO gene is unknown. Other uncharacterized members of the family are found in Streptomyces coelicolor A3, Campylobacter jejuni, and Neisseria meningitidis.
In this study we report on the metal ion specificity of the two homologous B. subtilis citrate transporters CitM and CitH. The latter was erroneously reported to be a transporter for free citrate. It is demonstrated that both transporters transport citrate in complex with divalent metal ions but that the metal ion specificity is complementary. CitM transports the complex of citrate with Mg2+, Ni2+, Co2+, Mn2+, and Zn2+, and CitH transports the complex of citrate with Ca2+, Sr2+, and Ba2+. These findings pose new questions about the physiological role of metal-citrate transporters in bacteria.
MATERIALS AND METHODS
Strains and growth conditions.
B. subtilis strain 168 was used as the wild-type strain. B. subtilis was grown on C medium (2) in which ferric ammonium citrate was omitted. The C medium was supplemented with 6 g of sodium succinate and 8 g of potassium glutamate per liter (CSE-medium) and either 10 mM tri-sodium citrate (CSEC) or 10 mM glucose (CSEG) as an additional carbon source (Warner et al., unpublished data). Auxotrophic requirements were added at a final concentration of 20 μg/ml. Overnight cultures of B. subtilis grown on CSEC were used to inoculate 25 ml of either CSEG or CSEC, yielding an optical density measured at 660 nm of 0.1, after which the cultures were allowed to grow for 6 h under continuous shaking at 150 rpm and at 37°C.
The B. subtilis CitM and CitH transporters were cloned and expressed in E. coli DH5α. Cells were grown at 37°C in Luria-Bertani medium (21) with 50 μg of carbenicillin per ml and 1 mM isopropylthiogalactopyranoside. An overnight culture was used to inoculate 25 ml of fresh Luria-Bertani medium in 100-ml flasks. Cells were grown at 37°C on a rotary shaker operated at 150 rpm.
Construction of expression vectors.
Vector pET324 is a pTrc99A derivative containing an engineered NcoI site (CCATGG) around the lacZ start codon (24). The multiple cloning site of the low-copy vector pWSK29 (30) was replaced with the multiple cloning site of pET324 by digestion with PvuII, yielding vector pBK29.
Plasmid pWSKcitM contains the B. subtilis citM gene downstream of the lac promoter on the low-copy vector pWSK29 and the original B. subtilis ribosome binding site as described previously (6). The sequence XXGTGX, containing the GTG start codon of citM, was mutated to CCATGG, thereby replacing the GTG initiation codon with an ATG initiation codon and introducing a NcoI site around the start codon. The NcoI sites in the citM sequence were removed by silent mutations using PCR mutagenesis, after which the sequence was verified by sequencing. The plasmid was digested with NcoI and XbaI, and the fragment containing the citM gene was ligated into pBK29. In the resulting plasmid, pBKCitM, the citM gene is cloned immediately behind the lacZ ribosome binding site under control of the lac promoter.
Plasmid pWSKcitH contains the B. subtilis citH gene downstream of the T7 promoter and the B. subtilis ribosome binding site (6). The multiple cloning site of pWSKcitH was inverted using the BssHII restriction sites yielding plasmid pWKScitH with the citH gene under control of the lac promoter and the original B. subtilis ribosome binding site.
Transport assay.
The cells were harvested by centrifugation and washed once with cold 50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid), pH 6.5. Cells were resuspended in the same buffer to yield an optical density at 660 nm of 10. Transport activity was determined by the rapid filtration method (15). Briefly, cells were diluted 10-fold in 50 mM PIPES, pH 6.5, and 100 μl samples were incubated for 8 min at 30°C while being magnetically stirred. At time zero, [1,5-14C]citrate (4.4 μM final concentration at 114 mCi/mmol) or l-[U-14C]proline (3.6 μM final concentration at 260 mCi/mmol) was added. Radiolabeled 63NiCl2 was used at 12.5 μM and 45CaCl2 was used at a 24.7 μM final concentration. Routinely divalent cations were present in the cell suspensions during the 8-min preincubation time. Control experiments in which the metal ions were added together with the radiolabeled citrate at the zero time point did not result in significant differences. Uptake was stopped by the addition of 2 ml of ice-cold 0.1 M LiCl solution, immediately followed by filtering through a 0.45-μm-pore-size nitrocellulose filter. The filters were washed once with 2 ml of ice-cold 0.1 M LiCl, after which the filters were submerged in scintillation fluid and the retained radioactivity was counted in a liquid scintillation counter. Uptake at the zero time point was estimated by adding the radiolabeled substrate to the cell suspension after the addition of 2 ml of ice-cold LiCl, followed by immediate filtering. Experiments were performed at least in triplicate, using cells of independent cultures. Shown are typical uptake curves. Kinetic parameters were determined from the linear parts of the uptake curves. Initial rates were measured in duplicate at the 10- and 20-s time points using metal-citrate concentrations in the range of 4.4 μM to 1 mM.
Speciation of the divalent cations in the transport buffer was calculated using the MINTEQA2 program (13), with the exception of Co2+ and Sr2+, for which the citrate binding constants were not available. All metal ions were added as metal-Cl2 salts dissolved in Milli-Q purified water.
RESULTS
Uptake of divalent metal-citrate complexes in Bacillus subtilis.
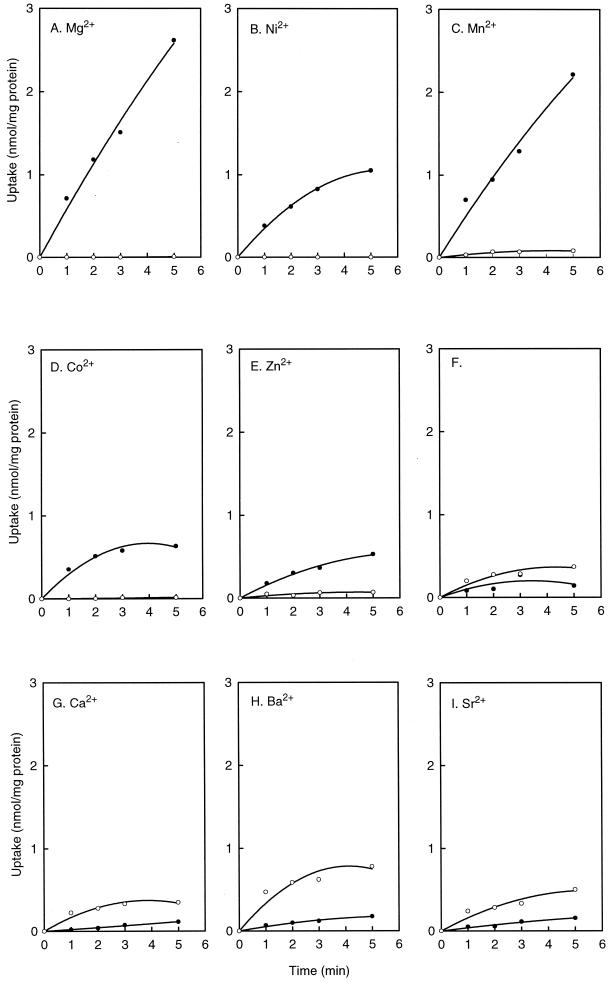
The Mg2+-dependent citrate transporter CitM of B. subtilis is induced by the presence of citrate in the medium and repressed by the presence of glucose (Warner et al., unpublished data). Consequently, B. subtilis cells grown in the presence of citrate readily took up [14C]citrate in the presence of Mg2+, while cells grown in the presence of glucose did not (Fig. 1A). When Mg2+ was omitted from the uptake experiment, very little [14C]citrate uptake was observed in the cells grown on citrate (Fig. 1F), confirming that the Mg2+-citrate complex is the substrate of CitM (6). In contrast, the uptake by cells grown on glucose was inhibited by the presence of Mg2+, suggesting that the Mg2+-citrate complex is not a substrate of the transport system expressed under those conditions (compare Fig. 1A and F). The expression pattern of the uptake activity was used to identify other divalent metal ions that induce citrate uptake via the same transport system by replacing Mg2+ in the transport assay buffer with a series of other divalent metal ions. The same pattern of citrate uptake in cells grown in the presence of citrate and the inhibition of uptake in cells grown on glucose was observed with Ni2+, Co2+, Mn2+, and Zn2+, suggesting that the complexes of citrate with these metal ions are substrates of CitM (Fig. 1B to E). The order of initial uptake rates (from greatest to least) was Mg2+, Mn2+, Ni2+, Co2+, Zn2+. The divalent Ca2+, Sr2+, and Ba2+ ions resulted in citrate uptake, but the pattern of expression was opposite. Cells grown in the presence of citrate showed lower uptake activities in the presence of these metal ions than cells grown in the presence of glucose (Fig. 1G to I). The highest uptake was observed with Ba2+, while the uptakes in the presence of Ca2+ and Sr2+ were similar, as observed in the absence of added metal ions. It should be noted that a concentration of 10 mM Ca2+ drives 98% of the citrate present in the assay in the Ca2+-citrate complex, indicating that the complex is the substrate for the transport system. Remarkably, citrate uptake in the absence of added divalent metal ions showed the same expression pattern as that observed for the Ca2+-, Sr2+-, and Ba2+-citrate complexes (Fig. 1F to I). In conclusion, free citrate and the complex of citrate with Ca2+, Sr2+, and Ba2+ were taken up by a different transport system(s) than citrate complexed to Mg2+, Ni2+, Co2+, Mn2+, and Zn2+.
FIG. 1.
Uptake of [14C]citrate by B. subtilis in the presence of different divalent metal ions. The uptake of [14C]citrate was measured in 50 mM PIPES, pH 6.5, in the presence of 1 mM concentrations of Mg2+ (A), Ni2+ (B), Mn2+ (C), Co2+ (D), or Zn2+ (E); without added metal ions (F); and in the presence of 10 mM concentrations of Ca2+ (G), Ba2+ (H), or Sr2+ (I). B. subtilis 168 was grown in CSEC (●) or CSEG (○) medium.
Uptake of free citrate in Bacillus subtilis.
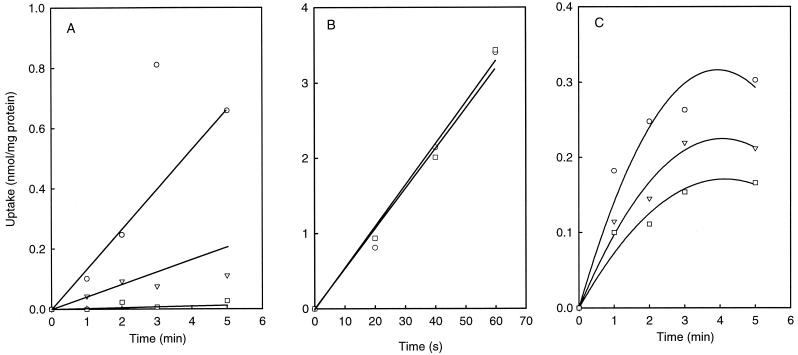
The catalytic activity of B. subtilis CitH was characterized after expression of the protein in E. coli, and it was concluded that CitH transports the free citrate anion (6). In B. subtilis, uptake of free citrate was observed with cells grown in the presence of glucose, and a lower activity was observed in growth medium containing citrate (Fig. 1F). The same pattern of uptake activity was observed in the presence of Ca2+, Ba2+, and Sr2+, which prompted us to reinvestigate the metal ion dependence of CitH in E. coli. In the presence of increasing concentrations of the chelator EGTA the uptake of citrate in E. coli cells expressing CitH decreased to zero uptake at 1 mM concentration of the chelator (Fig. 2A). In a control experiment, the same concentration of EGTA had no effect on l-[U-14C]proline uptake by the cells, indicating no significant effect on the energy status of the cells (Fig. 2B). Apparently, uptake of citrate catalyzed by CitH depends on the presence of residual divalent metal ions in the assay, and it can be concluded that CitH is not a transporter of free citrate.
FIG. 2.
Effect of EGTA on [14C]citrate uptake. (A) Uptake of citrate by E. coli cells expressing CitH in 50 mM PIPES, pH 6.5 (○), and in the presence of 100 μM (▿) and 1 mM EGTA (□). (B) Uptake of [14C]proline in the same cells in the presence (□) or absence (○) of 1 mM EGTA. (C) Citrate uptake in 50 mM PIPES, pH 6.5, by B. subtilis 168 cells grown in CSEG medium measured in the absence (○) and in the presence (□) of 1 mM EGTA and in the presence of 1 mM EGTA–10 mM Ca2+ (▿).
To investigate the presence of a transporter for free citrate, the same experiments were repeated with B. subtilis. In contrast to what was observed for E. coli expressing CitH, 1 mM EGTA decreased the uptake rate by a factor of about 2, leaving a significant level of uptake (Fig. 2C). Therefore, part of the citrate uptake activity observed in B. subtilis without the addition of divalent metal ions was due to the presence of residual metal ions and in part was catalyzed by a transporter for free citrate that, however, is not CitH. Addition of 10 mM Ca2+ in addition to 1 mM EGTA drives the free citrate in the Ca2+-citrate complex (98% complex formation) and resulted in a slightly higher uptake activity, showing that Ca2+-citrate is taken up by B. subtilis grown in the presence of glucose.
Metal ion specificity of CitM and CitH.
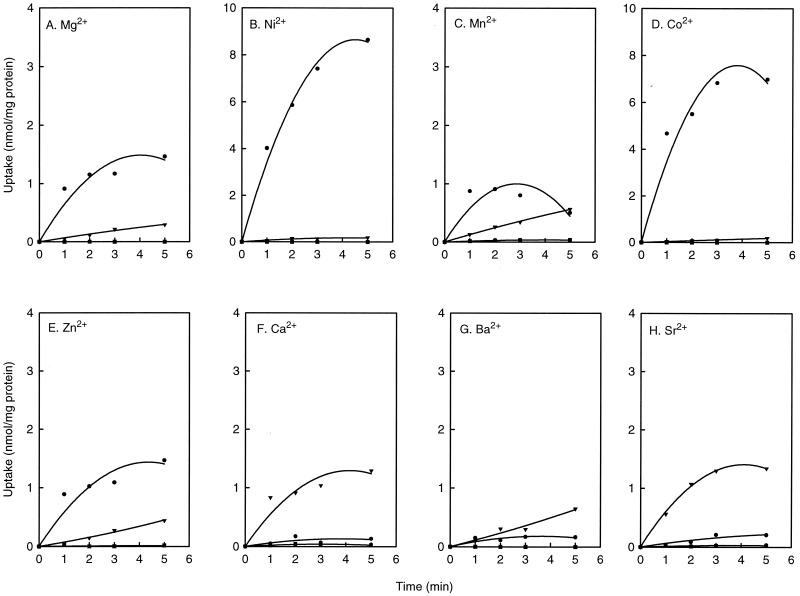
The metal ion specificity of CitM and CitH was determined by expressing the transporters in E. coli. Unlike most enterobacteria, E. coli does not express an endogenous citrate transporter when grown aerobically (14). Accordingly, cells harboring plasmid pWSK29 without the citM or citH inserts did not show any citrate uptake in the presence or absence of divalent metal ions (Fig. 3A to H). E. coli cells expressing CitM transported citrate in the presence of Mg2+, Ni2+, Mn2+, Co2+, and Zn2+ but transported none or very little in the presence of Ca2+, Ba2+, and Sr2+ (Fig. 3A to H). In contrast, E. coli cells expressing CitH took up citrate in the presence of Ca2+, Ba2+, and Sr2+ and took up none or very little in the presence of Ni2+, Mn2+, Co2+, and Zn2+ (Fig. 3B to H). Significant uptake was observed in the presence of 1 mM Mg2+ (Fig. 3A). However, at this concentration, only 42.5% of the citrate in the assay was in the complexed state. Increasing the concentration 10-fold (70% complex formation) further decreased the uptake activity (not shown) showing that the Mg2+-citrate complex is not a substrate of CitH, consistent with what has been observed before (6). The residual citrate uptake in the presence of 1 mM Mg2+ was due to the fraction of citrate not complexed to Mg2+ (Fig. 2). In conclusion, within the series of divalent metal ions tested, the citrate transporters CitM and CitH have complementary metal ion specificities. The metal ion specificity correlated with the expression patterns obtained in B. subtilis, suggesting that both CitM and CitH were expressed but that the expression of each is under different control.
FIG. 3.
Metal ion specificity of CitM and CitH. [14C]citrate uptake in E. coli cells harboring plasmid pWSK29 (■), expressing CitM (●), and expressing CitH (▾) in the presence of 1 mM concentrations of Mg2+ (A), Ni2+ (B), Mn2+ (C), Co2+ (D), and Zn2+ (E) and 10 mM concentrations of Ca2+ (F), Ba2+ (G), and Sr2+ (H).
The kinetic parameters for citrate uptake catalyzed by CitM and CitH in the presence of the different divalent metal ions were estimated from the initial rates of uptake in cells of E. coli expressing CitM and CitH. Both transporters had a remarkably similar affinity for the complexes, with an affinity constant around 45 μM (Table 1). Larger differences were observed in the maximal uptake rates for both transporters. The highest and lowest maximal rates catalyzed by CitM were observed with Co2+ and Mn2+ (600 and 214 pmol/min · mg of protein, respectively). CitH had the highest activity with Ca2+ and three- to fivefold less so with Ba2+ and Sr2+.
TABLE 1.
Kinetic parameters of CitM and CitH for uptake of divalent metal-citrate complexes in whole cells of E. coli
| Divalent metal | Transporter | Km (μM) | Vmax (pmol/min · mg of protein) | Ionic radiusa (Å) |
|---|---|---|---|---|
| Mg2+ | CitM | 63 | 344 | 0.66 |
| Co2+ | CitM | 35 | 661 | 0.72 |
| Ni2+ | CitM | 43 | 460 | 0.69 |
| Mn2+ | CitM | NDb | 214 | 0.80 |
| Zn2+ | CitM | 40 | 293 | 0.74 |
| Ca2+ | CitH | 33 | 155 | 0.99 |
| Sr2+ | CitH | 50 | 58 | 1.12 |
| Ba2+ | CitH | 42 | 31 | 1.34 |
Ionic radius of divalent metal, taken from reference 31.
ND, not determined.
Role of the metal ions.
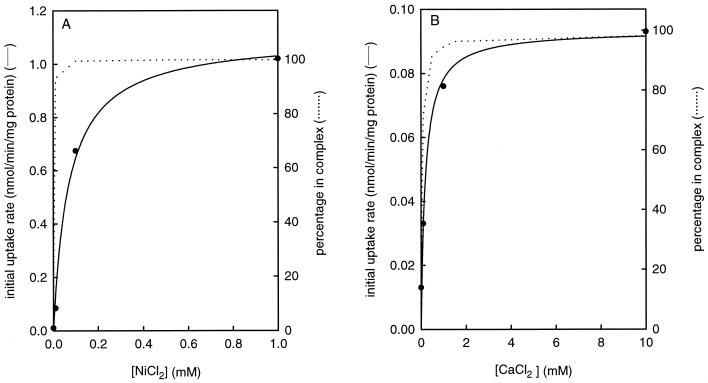
The initial rate of citrate uptake catalyzed by CitM expressed in E. coli at increasing Ni2+ concentration became saturated at a concentration of approximately 1 mM Ni2+ (Fig. 4A). Calculation of the fraction of citrate in the Ni2+ complexed state in this concentration range using a formation constant of log K = 5.4 (10) suggested that much more Ni2+ was needed to saturate the rate than would be required to complex all citrate. The same, but less extreme, was observed for the uptake of citrate catalyzed by CitH for the Ca2+-citrate complex (log K = 3.5). For both transporters there is a clear dependence on the divalent metal ion, but the relation does not seem to correlate with the formation of the metal-citrate complex. This raises some doubt about whether the metal-citrate complex is the actual species transported by the transporters or whether the metal ion is needed to activate the transporter. To resolve the issue, citrate-dependent uptake of the radiolabeled cations 63Ni2+ and 45Ca2+ was studied.
FIG. 4.
Relation between initial rate of citrate uptake and metal ion concentration. The initial rate of uptake of citrate in E. coli cells expressing CitM (A) and CitH (B) was measured at the indicated concentrations of Ni2+ (A) and Ca2+ (B). The dotted lines indicate the calculated fraction of citrate in the metal-citrate complex using formation constants of log K = 5.4 and 3.5 for Ni2+ (A) and Ca2+ (B), respectively.
E. coli cells harboring plasmid pWSK29 without the citM or citH insert took up 63Ni2+ at an initial rate of about 300 pmol/min · mg of protein (Fig. 5A). This endogenous uptake is most likely catalyzed by the CorA Mg2+ uptake system, which has been reported to have affinity for other divalent metal ions as well (reviewed in reference 29). Addition of 100 μM citrate greatly reduced the endogenous uptake by chelating the 63Ni2+ in the buffer. In the cells expressing CitM the endogenous uptake of 63Ni2+ was somewhat lower, but, most importantly, addition of citrate resulted in a strong stimulation of 63Ni2+ uptake (Fig. 5B). Therefore, Ni2+ induces citrate uptake (Fig. 4A) and citrate induces Ni2+ uptake in E. coli cells expressing CitM, showing that CitM transports the complex of Ni2+ and citrate. The endogenous uptake of Ni2+ in the cells and binding of Ni2+ to cell material are likely to account for the discrepancy between the theoretical complex formation of Ni2+-citrate and the Ni2+ dependence of the uptake rate observed in Fig. 4A.
FIG. 5.
Uptake of radiolabeled 63Ni2+ and 45Ca2+. 63Ni2+ uptake was measured in E. coli cells harboring plasmid pWSK29 (A) and expressing CitM (B) in the presence (▿) and absence (○) of 100 μM citrate. 45Ca2+ uptake was measured in E. coli cells harboring plasmid pWSK29 (C) and expressing CitH (D) in the presence (▿) and in the absence (○) of the same concentration of citrate.
Endogenous uptake of 45Ca2+ in E. coli cells harboring plasmid pWSK29 was low and may even reflect binding to cell wall constituents (Fig. 4C). In the presence of citrate, the amount of 45Ca2+ retained by the cells was not significantly different. Cells expressing CitH showed a significantly increased uptake of 45Ca2+ in the presence of 100 μM citrate (Fig. 4D). Clearly CitH catalyzes the transport of the 45Ca2+-citrate complex.
DISCUSSION
In the early 1980s, it was demonstrated that citrate uptake in B. subtilis was stimulated by the presence of a wide range of divalent metal ions, but the transporters responsible for the uptake activity were not known (5). Much later, a secondary transporter, termed CitM, of B. subtilis was cloned in E. coli and shown to transport citrate in the presence of Mg2+ but not in its absence (6). The present study demonstrates that CitM transports citrate not only in complex with Mg2+ but also with Mn2+, Ni2+, Zn2+, and Co2+, although it does not transport citrate in complex with Ca2+, Ba2+, or Sr2+. A paralog of CitM, termed CitH, was also cloned and characterized in E. coli. It was thought to transport free citrate since uptake was observed without added metal ions and was inhibited by the addition of Mg2+ (6). The conclusion was unfortunate, since it is now demonstrated that CitH transports citrate in complex with Ca2+, Ba2+, and Sr2+ but not with Mg2+, Mn2+, Ni2+, Zn2+, or Co2+. Therefore, both transporters are metal-citrate transporters with complementary metal ion specificity. The uptake observed in E. coli cells expressing CitH without adding metal ions was due to residual metal ions, most likely Ca2+, present in the cell suspension that could effectively be scavenged with EGTA. Both CitM and CitH do not transport the free citrate anion. Nevertheless, citrate uptake in B. subtilis was observed in the presence of the chelator EGTA, indicating that at least one additional citrate transporter is expressed in B. subtilis that is specific for free citrate. Possible candidates are the product of open reading frame yraO that is homologous to both CitM and CitH, and a protein encoded by the yxkJ gene that is a member of the 2-hydroxycarboxylate transporter (3) family, a family of citrate and malate transporters. Preliminary experiments have demonstrated that the gene product of yxkJ indeed is a citrate transporter (B. P. Krom, R. Aardema, J. B. Warner, W. N. Konings, and J. S. Lolkema, unpublished data). At any rate, B. subtilis seems to express a multitude of transporters for citrate but not under the same control.
Expression of CitM in B. subtilis is under strict control of the medium composition. The transporter is induced by citrate in the medium and is repressed by other, more easily metabolized carbon sources (14, 30a) Such an expression pattern suggests that CitM is the transporter responsible for growth on citrate as the carbon and energy source that is only needed in the absence of better growth substrates. The transporter would be specific for the metal-citrate complex because citrate is present as such in the medium, but the physiological function would be citrate uptake. Nevertheless, in the presence of other divalent metal ions, these will also be taken up and will affect the usually delicate balance of the metal ions in the cell. For example, Ni2+ is an essential cofactor for a number of enzymatic reactions but becomes toxic at elevated intracellular concentrations. Therefore, bacterial cells contain transporters that take up Ni2+ as well as transporters that expel the metal ion to keep the intracellular concentration within certain limits (for a review, see reference 8). In specific medium compositions, the uptake of Ni2+ in complex with citrate may be an important factor in the homeostasis of the metal ion.
The physiological role of CitH is not clear. Possibly, CitH functions under those conditions where much of the citrate is complexed to f.i. Ca2+. Alternatively, CitH activity might be relevant to the uptake of the Ca2+ ion rather than citrate. Recently, the role of Ca2+ as a signaling ion in bacteria (22), and in particular in B. subtilis (12), has become evident. Ca2+ is implicated in a number of bacterial functions, including heat shock response, pathogenicity, differentiation, and cell cycle. Ca2+ signaling requires a strict control of the intracellular concentration via uptake and extrusion systems. A number of secondary calcium exchangers have been identified in bacteria, which extrude Ca2+ from the cytosol driven by the proton or sodium ion motive force. Uptake of Ca2+ is believed to be mediated by channel activity or transporters that take up Ca2+ complexed to inorganic phosphate or DNA. Possibly, CitH plays an important role in the Ca2+ signaling pathways as well. Clearly, the physiological role of CitH needs further investigation.
CitM and CitH are homologous proteins that share 52% identical amino acid residues and very similar hydropathy profiles, suggesting a similar three-dimensional folding (6, 16). Both transporters transport citrate complexed to divalent metal ions, but their metal ion specificities are complementary. They specifically recognize the metal ion in the complex with citrate, which demonstrates that complexes of citrate with Ca2+, Ba2+, and Sr2+ are different from complexes with Mg2+, Mn2+, Ni2+, Zn2+, or Co2+. The differences between the two groups are reflected by, most likely, subtle differences in the binding site of the transporters. Within the two groups, the affinity of the transporters is more or less the same (Table 1), indicating that the initial recognition is similar. In the translocation step, the differences are more prominent, indicating that the actual catalytic step is more sensitive to differences within a group of complexes. Bidentate and tridentate complex formation has been suggested to be the basis of biological recognition of complexes of citrate with different metal ions (13). However, the series of metal ions used in this study are all expected to form bidentate complexes with citrate. If so, the conformation of the citrate anion is mainly determined by the size of the metal ion in the complex. In crystal structures of metal ion citrate complexes, two major conformations were identified (11). The backbone of the citrate molecule can form a linear molecule, or the backbone is bent backwards. Some metal ions, like Co2+ or Ni2+, show up in both conformations, while others are predominantly found in one of the two conformations. Ca2+ complexes of citrate have the bent conformation, while Mg2+ and Mn2+ complexes have the linear conformation. Clearly, the group of metal ions transported by CitM are the smaller cations, with a Pauling radius of less than 0.80 Å, while the ions transported by CitH have radii that are larger than 0.98 Å (Table 1). These conformations and radii follow from crystallographic studies, and their relevance to the discrimination between the two groups of metal-citrate complexes by CitM and CitH in solution remains to be established.
ACKNOWLEDGMENT
This research was supported by the Ministry of Economic Affairs, in the framework of the IOP Milieutechnologie/Zware metalen IZW97404.
REFERENCES
- 1.Asai K, Baik S-H, Kasahara Y, Moriya S, Ogasawara N. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology. 2000;146:263–271. doi: 10.1099/00221287-146-2-263. [DOI] [PubMed] [Google Scholar]
- 2.Aymerich S, Gonzy-Trebou G, Steinmetz M. 5′-Noncoding region sacR is the target of all identified regulation affecting the levansucrase gene in Bacillus subtilis. J Bacteriol. 1986;166:993–998. doi: 10.1128/jb.166.3.993-998.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandell M, Ansanay V, Rachidi N, Dequin S, Lolkema J S. Membrane potential-generating malate (MleP) and citrate (CitP) transporters of lactic acid bacteria are homologous proteins. J Biol Chem. 1997;272:18140–18146. doi: 10.1074/jbc.272.29.18140. [DOI] [PubMed] [Google Scholar]
- 4.Bandell M, Lhotte M E, Marty-Teysset C, Veyrat A, Prevost H, Dartois V, Divies C, Konings W N, Lolkema J S. Mechanism of the citrate transporters in carbohydrate and citrate cometabolism in Lactococcus and Leuconostoc species. Appl Environ Microbiol. 1998;64:1594–1600. doi: 10.1128/aem.64.5.1594-1600.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsma J, Konings W N. The properties of citrate transport in membrane vesicles from Bacillus subtilis. Eur J Biochem. 1983;134:151–156. doi: 10.1111/j.1432-1033.1983.tb07545.x. [DOI] [PubMed] [Google Scholar]
- 6.Boorsma A, van der Rest M E, Lolkema J S, Konings W N. Secondary transporters for citrate and the Mg2+-citrate complex in Bacillus subtilis are homologous proteins. J Bacteriol. 1996;178:6216–6222. doi: 10.1128/jb.178.21.6216-6222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bott M. Anaerobic citrate metabolism and its regulation in enterobacteria. Arch Microbiol. 1997;167:78–88. [PubMed] [Google Scholar]
- 8.Eitinger T, Mandrand-Berthelot M A. Nickel transport systems in microorganisms. Arch Microbiol. 2000;173:1–9. doi: 10.1007/s002030050001. [DOI] [PubMed] [Google Scholar]
- 9.Fabret C, Feher V A, Hoch J A. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis A J, Dodge C J, Gillow J B. Biodegradation of metal-citrate complexes and implications for toxic-metal mobility. Nature. 1992;356:140–142. [Google Scholar]
- 11.Glusker J P. Citrate conformation and chelation: enzymatic implications. Acc Chem Res. 1980;13:345–352. [Google Scholar]
- 12.Herbaud M L, Guiseppi A, Denizot F, Haiech J, Kilhoffer M C. Calcium signalling in Bacillus subtilis. Biochim Biophys Acta. 2000;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 13.Joshi-Tope G, Francis A J. Mechanisms of biodegradation of metal-citrate complexes by Pseudomonas fluorescens. J Bacteriol. 1995;177:1989–1993. doi: 10.1128/jb.177.8.1989-1993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay W W, Sweet G D, Widenhorn K, Somers J M. Transport of organic acids in prokaryotes. In: Rosen B P, Silver S, editors. Ion transport in prokaryotes. San Diego, Calif: Academic Press; 1987. pp. 269–302. [Google Scholar]
- 15.Lolkema J S, Enequist H, van der Rest M E. Transport of citrate catalyzed by the sodium-dependent citrate carrier of Klebsiella pneumoniae is obligatorily coupled to the transport of two sodium ions. Eur J Biochem. 1994;220:469–475. doi: 10.1111/j.1432-1033.1994.tb18645.x. [DOI] [PubMed] [Google Scholar]
- 16.Lolkema J S, Slotboom D J. Hydropathy profile alignment: a tool to search for structural homologues of membrane proteins. FEMS Microbiol Rev. 1998;22:305–322. doi: 10.1111/j.1574-6976.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 17.Madsen E L, Alexander M. Effects of chemical speciation on the mineralization of organic compounds by microorganisms. Appl Environ Microbiol. 1985;50:342–349. doi: 10.1128/aem.50.2.342-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magni C, Lopez P, de Mendoza D. The properties of citrate transport catalyzed by CitP of Lactococcus lactis ssp. lactis biovar diacetylactis. FEMS Microbiol Lett. 1996;142:265–269. [Google Scholar]
- 19.Marty-Teysset C, Lolkema J S, Schmitt P, Divies C, Konings W N. Membrane potential-generating transport of citrate and malate catalysed by CitP of Leuconostoc mesenteroides. J Biol Chem. 1995;270:25370–25376. doi: 10.1074/jbc.270.43.25370. [DOI] [PubMed] [Google Scholar]
- 20.Marty-Teysset C, Posthuma C, Lolkema J S, Schmitt P, Divies C, Konings W N. Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J Bacteriol. 1996;178:2178–2185. doi: 10.1128/jb.178.8.2178-2185.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 22.Norris V, Grant S, Freestone P, Canvin J, Sheikh F N, Toth I, Trinei M, Modha K, Norman R I. Calcium signalling in bacteria. J Bacteriol. 1996;178:3677–3682. doi: 10.1128/jb.178.13.3677-3682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pos K M, Dimroth P, Bott M. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J Bacteriol. 1998;180:4160–4165. doi: 10.1128/jb.180.16.4160-4165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Does C, den Blaauwen T, de Wit J G, Manting E H, Groot N A, Fekkes P, Driessen A J M. SecA is an intrinsic subunit of the Escherichia coli preprotein translocase and exposes its carboxyl terminus to the periplasm. Mol Microbiol. 1996;22:619–629. doi: 10.1046/j.1365-2958.1996.d01-1712.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Rest M E, Abee T, Molenaar D, Konings W N. Mechanism and energetics of a citrate-transport system of Klebsiella pneumoniae. Eur J Biochem. 1991;195:71–77. doi: 10.1111/j.1432-1033.1991.tb15677.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Rest M E, Molenaar D, Konings W N. Mechanism of Na+-dependent citrate transport in Klebsiella pneumoniae. J Bacteriol. 1992;174:4893–4898. doi: 10.1128/jb.174.15.4893-4898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Rest M E, Schwarz E, Oesterhelt D, Konings W N. DNA sequence of a citrate carrier of Klebsiella pneumoniae. Eur J Biochem. 1990;189:401–407. doi: 10.1111/j.1432-1033.1990.tb15502.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Rest M E, Siewe R M, Abee T, Schwarz E, Oesterhelt D, Konings W N. Nucleotide sequence and functional properties of a sodium-dependent citrate transport system from Klebsiella pneumoniae. J Biol Chem. 1992;267:8971–8976. [PubMed] [Google Scholar]
- 29.Smith R L, Maguire M E. Microbial magnesium transport: unusual transporters searching for identity. Mol Microbiol. 1998;28:217–226. doi: 10.1046/j.1365-2958.1998.00810.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 30a.Warner J B, Krom B P, Magni C, Konings W N, Lolkema J S. Catabolite repression and induction of the Mg2+-citrate transporter CitM of Bacillus subtilis. J Bacteriol. 2000;182:6099–6105. doi: 10.1128/jb.182.21.6099-6105.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weast R C. Handbook of chemistry and physics. Boca Raton, Fla: CRC Press; 1973. [Google Scholar]
- 32.Yamamoto H, Uchiyama S, Nugroho F A, Sekiguchi J. Cloning and sequencing of a 35.7 kb in the 70 degree-73 degree region of the Bacillus subtilis genome reveal genes for a new two-component system, three spore germination proteins, an iron uptake system and a general stress response protein. Gene. 1997;194:191–199. doi: 10.1016/s0378-1119(97)00130-3. [DOI] [PubMed] [Google Scholar]