Background:
Malnutrition is a relatively common and often unrecognized condition in stroke survivors, which may negatively affect functional recovery and survival. Though previous studies have indicated significant role of nutrition supplement for rehabilitation of patients with stroke, the results still remain controversy.
Objective:
The present analysis was designed to systematically review effective evidence of nutrition supplement on rehabilitation for patients with stroke.
Methods:
A systematic search of PubMed, EMBASE, the Cochrane Library, and Web of Science up to August 1, 2021 was performed to find relevant studies that analyzed the effect of nutrition supplement on rehabilitation of patients with stroke. The primary outcome was functional outcomes and activities of daily living (ADL). The secondary outcomes included disability, all-cause mortality, infections, pneumonia, walking ability, stroke recurrence, and laboratory results indicating nutrition status of patients. All statistical analyses were performed using standard statistical procedures with Review Manager 5.2.
Results:
Ultimately, 16 studies including 7547 patients were identified. Our pooled results found no significant difference in total, cognitive and motor FIM score between nutrition supplement and placebo groups, with pooled MDs of 7.64 (95% CI − 1.67 to 16.94; P = .11), 0.74 (95% CI − 1.33 to 2.81; P = .48), 1.11 (95% CI − 1.68 to 3.90; P = .44), respectively. However, our result showed that nutritional interventions had significant effect on ADL for patients with stroke (MD 3.26; 95% CI 0.59 to 5.93; P = .02). In addition, nutrition supplement reduced the incidence of infections for patients with stroke, with a pooled RR of 0.65 (95% CI 0.51 to 0.84; P = .0008). No significant results were found in disabilities, complication and laboratory outcomes.
Conclusions:
The present meta-analysis indicated no statistically significant effect of nutrition supplement on functional outcomes as well as disabilities, complication and laboratory outcomes for patients with stroke. However, it increased ADL and reduced the incidence of infections.
Keywords: function, nutrition, rehabilitation, stroke
1. Introduction
Stroke is one of the leading causes of death in industrialized countries ranging just behind cardiac- and cancer-related causes and is currently the leading cause of disability in Western countries.[1] Stroke incidence varies across Europe between 100 and 700 events per 100,000 inhabitants being highest in Eastern European countries (Lithuania and Poland) and lowest in south Europe (Italy and Spain).[2] The poststroke mortality rate is 20% during the acute period (within 30 days) and 30% at 1 year. Furthermore, stroke is one of most important causes of disability.[3,4] In Italy, about one-third of patients surviving 1 year after a stroke are totally dependent on other people, creating an enormous socioeconomic burden.[5]
Malnutrition is frequently observed in patients with stroke, and dysphagia contributes to malnutrition risk.[6] The prevalence of malnutrition after stroke has widely varied from 8% to 34% among published reports. It is estimated that about fifth of patients with acute stroke are malnourished on admission.[7,8] This variation is probably due to patient selection, the definitions of malnutrition, and the method and timing of assessments. In a small study in geriatric patients with severe stroke, 56.3% were malnourished at some point during a hospital stay of > 3 weeks.[9] On the other hand, in poststroke patients residing in an infirmary, the prevalence of malnutrition was 61%.[10]
In patients who undergo rehabilitation after ischemic stroke, nutrition strategies are adopted to provide tube-fed individuals with adequate nutrition and/or to avoid the body wasting responsible for poor functional outcome and prolonged stay in the hospital.[6,9,11] From 2000 to 2009, a focus on connections between nutrition and the brain developed with interventions planned to target some brain metabolic alterations primed by acute brain ischemia, such as impaired protein synthesis, excess free radical production, and deficiencies of minerals, including zinc.[9] Investigations have documented that recovery of neurocognitive function in individuals with ischemic stroke may indeed be enhanced by nutrition interventions.[12–14] In addition, the observational data in a large prospective cohort enrolled in the Feed Or Ordinary Diet (FOOD) provide reliable evidence that nutritional status early after stroke is independently associated with long-term outcome.[15]
However, though previous studies have indicated significant role of nutrition supplement for rehabilitation of patients with stroke, the results still remain controversy. The present analysis was designed to systematically review effective evidence of nutrition supplement on rehabilitation for patients with stroke.
2. Methods and Materials
2.1. Search strategy
A systematic search of the PubMed, Cochrane, Web of Science, and Embase were performed up until August 1, 2021; no date restrictions were applied and the reference lists of all relevant articles and reviews were manually searched. One author performed the literature search and another reviewed ambiguous cases. In cases where the authors had a difference of opinion, consensus was reached following discussion. The search, which was restricted to articles published in English and performed according to major Medical Subject Headings (MeSH) or the title and abstract, used the following keywords: (“stroke” OR “poststroke”) AND (“nutrition” OR “malnutrition” OR “protein” OR “amino acid” OR “energy”) AND (“recovery” OR “function” OR “rehabilitation” OR “outcome*” OR “complication*”).
2.2. Study selection
Relevant randomized control trials were included in the present analysis if they: (1) patients with stroke who received nutritional supplement during their rehabilitation; (2) studies that comparing intensive nutritional supplementation to routine nutritional supplementation; (3) studies collected and evaluated functional outcomes, activities of daily living (ADL) or other relevant outcomes including disability, all-cause mortality, infections, pneumonia, walking ability, stroke recurrence, length of stay (LOS) and laboratory results.
Studies will be excluded if they meet the following criteria: (1) patients suffered other disease that may influence the outcomes; (2) experimental trials on animals or nonhuman studies; (3) abstracts, letters, editorials, expert opinions, reviews, case reports were excluded; (4) studies without sufficient data or did not meet our including criteria were excluded.
These criteria were used to screen the titles and abstracts of publications to determine whether they were eligible for inclusion. Studies deemed eligible upon title and abstract screening were screened in full-text. Publications were reviewed in duplicate at each stage and discrepancies were resolved by their consensus, or by adjudication by a third reviewer. The present study was approved by the Ethics Committee of Wangjing Hospital.
2.3. Quality assessment and data extraction
Quality assessments of the studies were performed independently by 2 researchers and all disagreements were resolved following discussion in conjunction with the input of a third researcher. The previously validated 5-point Jadad scale was used to assess the quality of each RCT.[16] Studies with scores of 0–1 were considered low quality; scores of 2–3 were considered moderate quality; scores of 4 or more were considered high quality. In addition, the risk of bias for each studies and the risk of bias across all studies were evaluated and shown with figures generated by RevMan 5.2 software.[17]
Baseline characteristics and outcomes from the included studies were extracted using a standardized extraction form. Key study characteristics included the following data: first author’s name, publication year, country, sample size, mean age and/or age range of the participants, follow-up time, interventions, and main outcomes. Data were extracted by one reviewer and then examined for accuracy and completeness by a second reviewer.
2.4. Statistical analysis
Data comparing the outcomes in intervention and control groups were analyzed using standard statistical procedures provided in RevMan 5.2.[17] Dichotomous data were measured with risk ratio (RR) and continuous variable data were measured with mean difference (MD). The heterogeneity between studies was evaluated by the chi-square-based Q statistical test,[18] with Ph value and I2 statistic, ranging from 0% to 100 %, to quantify the effect of heterogeneity. Ph ≤ 0.10 was deemed to represent significant heterogeneity,[19] and pooled estimates were estimated using a random-effect model (the DerSimonian and Laird method[20]). On the contrary, if statistical study heterogeneity was not observed (Ph > 0.10), a fixed effects model (the Mantel–Haenszel method[21]) was used. The effects of outcome measures were considered to be statistically significant if pooled RRs with 95% CI did not overlap with 1 or pooled MDs with 95% CI did not overlap with 0. Finally, publication bias was assessed by contour-enhanced funnel plots. If the shape of funnel plots revealed no obvious evidence of asymmetry, we considered that there was no obvious publication bias.
3. Results
3.1. Included studies, study characteristics, and quality assessment
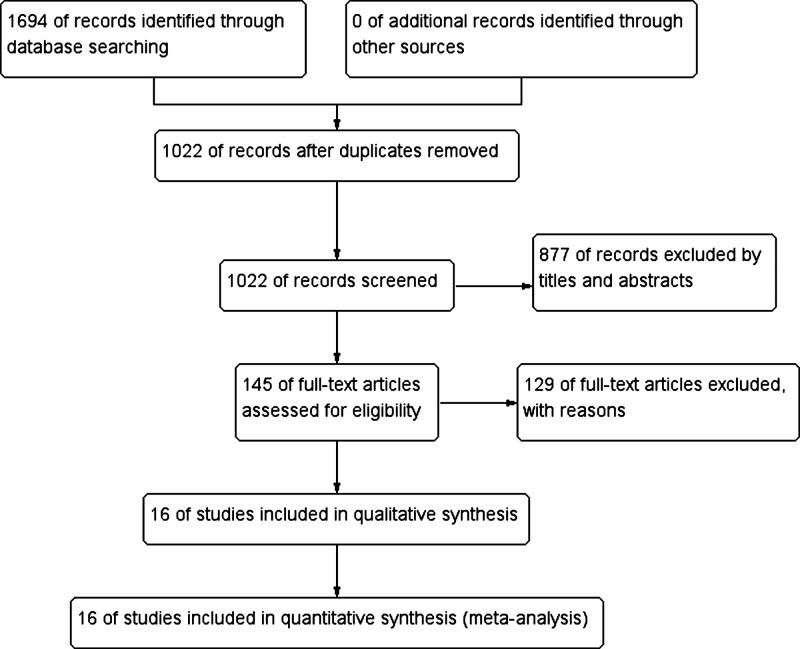
At the beginning of the search, a total of 1964 records of citations were obtained; 1022 of records were reviewed further after duplicates were removed. Via screening the titles and abstracts, 877 studies were excluded preliminarily and then 145 studies were chosen to get full texts for further evaluation. After reading the full texts, 129 studies were excluded further. Eventually, 16 RCTs[22–37] (N = 7547 participants) were included in this systematic review and meta-analysis. Of these studies, 2 were designed as multicenter randomized controlled trials.[25,26] The detailed search process and summary of studies are shown in the study flow diagram (Fig. 1). The other characteristics of each study are shown in Table 1.
Figure 1.
Flow diagram following the PRISMA template of the search strategy for the effects nutrition supplement on the rehabilitation of patients with stroke.
Table 1.
The characteristics of included studies in this meta-analysis.
| Study ID | Country | Sample size | Stroke subtype | Age (mean ± SD, range) | Follow-up time | Interventions | Main outcomes |
|---|---|---|---|---|---|---|---|
| Aquilani R (2014) | Italy | 38 | Ischemic and hemorrhagic | 68 ± 13.2 71.3 ± 10 |
Discharge | Nutritional mixture supplement that provided 8 g of EAAs/d (4 g in the morning + 4 g in the afternoon diluted in half a glass of water), until discharge | Arterial amino acid concentrations and muscle amino acid arteriovenous difference. |
| Bellone JA (2019) | USA | 16 | Ischemic and hemorrhagic | 58.13 ± 13.62 59.63 ± 13.48 |
Discharge | Pomegranate (1 g of a concentrated blend of polyphenols, equivalent to levels in approximately 8 oz of juice (approximately 755 mg of gallic acid equivalents: profile includes ellagitannins, gallotannins, ellagic acid, and flavonoids)) supplement twice per day (morning and night) for 1 week. | Neuropsychological testing (primary outcome: Repeatable Battery for the Assessment of Neuropsychological Status) and functional independence scores |
| Boselli M (2012) | Italy | 136 | Ischemic and hemorrhagic | 62.7 ± 12.4 63.9 ± 17 |
60 days | Nutritional mixture supplement with 8 g of EAAs/day (4 g in the morning + 4 g in the afternoon diluted in half a glass of water), 60 days | The incidence of infections, Relationship Between Measured Variables and Functional Independence, Risk-Identifying Variables |
| Dennis MS (2005a) | Multi-countries | 4023 | NR | 78 ± 10 80 ± 7 |
6 months | Regular hospital diet plus oral nutritional supplements (360 mL at 6.27 kJ/mL and 62·5 g/L in protein every day), Until discharge | Death or poor outcome (modified Rankin scale [MRS] grade 3–5) |
| Dennis MS (2005b) | Multi-countries | 859 | NR | 76 ± 11 76 ± 11 |
6 months | Starting enteral tube feeding (via the clinician’s preferred tube) as soon as possible, for > 7 days |
Risk of death |
| Gariballa SE (1998) | UK | 42 | Ischemic | 78 ± 10 80 ± 7 |
2, 4, 12 weeks, discharge | Regular hospital diet plus a twice daily oral nutritional supplement of ≥ 400 mL containing 600 kcal, 4 weeks or until death or discharge | Energy and protein intakes, change in nutritional status, disability, infective complications, length of stay, and mortality |
| Ha L (2010) | Norway | 124 | Ischemic and hemorrhagic | 79.7 ± 6.8 78.5 ± 7.4 |
3 months | Individualized nutritional care using established oral energy- and protein rich feedings or enteral tube feeding, Until discharge | The percentage of patients with weight loss ≥ 5%; QoL, handgrip strength and length of hospital stay |
| Irisawa H (2020) | Japan | 179 | NR | 79.7 ± 11.5 | 4 weeks | NR | Muscle mass and the nutritional status, activities of daily living |
| Mizushima T (2020) | Japan | 668 | Ischemic | 74 ± 66-80 78 ± 72-84 |
90 days | NR | Transfer to acute care and death; 2-year mortality; FIM-motor effectiveness |
| Nishioka S (2017) | Japan | 264 | NR | 78.5 ± 7.5 78.3 ± 7.2 |
3 weeks | oral protein supplementation of 20 g/d for 21 d | the ability of participants; Achievement of full oral intake, malnutrition risk |
| Nishioka S (2020a) | Japan | 420 | Ischemic and hemorrhagic | 80.1 ± 8.0 77.2 ± 7.6 |
NR | NR | Recurrent stroke, prestroke ADL, Total FIM, Comorbidities, Disabilities |
| Nishioka S (2020b) | Japan | 113 | Ischemic and hemorrhagic | 77 (66.5–84) 77.5 (63.5–82) |
3 months | Protein 1.5 g/kg (= 24% energy), carbohydrate 3.12 g/kg (= 50% energy), carbohydrate–protein ratio 2.08, lipids 0.72 g/kg (= 26% energy) | Malnutrition, muscle mass and oral status, and swallowing function recovery |
| Nishiyama A (2019) | Japan | 290 | Ischemic and hemorrhagic | 76.5 ± 7.5 80.2 ± 7.8 |
3 weeks | 21 days of daily supplementation with a formula providing 20 g of protein and 250 kcal (carbohydrate 28.2 g, lipids 7 g in addition to the 20 g of protein) | ADL was evaluated using FIM, and nutritional status |
| Rabadi MH (2008) | United States | 116 | Ischemic and haemorrhagic | 75 ± 10.58 73.58 ± 13.02 |
Discharge | Intensive nutritional supplement every 8 h by mouth (120 ml, 240 calories, 11 g of proteins, 90 mg of vitamin C), Until discharge | Change in total score on the FIM, the FIM motor and cognitive subscores, length of stay, 2-minute and 6-minute timed walk tests |
| Yoshimura Y (2019) | Japan | 113 | Ischemic and hemorrhagic | 80.8 + 7.1 78.9 + 6.3 |
90 days | Early nasogastric nutrition using a solution with high nutritional content, 21 days | Physical function, appendicular muscle mass, muscle strength |
| Zheng T (2015) | China | 146 | Ischemic and hemorrhagic | 71.4 ± 9.3 71.8 ± 10.1 |
21 days | Either Nutrison fiber, Swiss High (RAE; 4.18–6.27 kJ/ml), or a solution with high nutrition content made by nutritionists and based on condition, body weight, and nutritional status. Energy requirements were in the range of 83.68–125.52 kJ/kg/day | Nutritional status, nosocomial infection, and mortality rates |
FIM = functional independence measurement, NR = no report, QoL = quality of life.
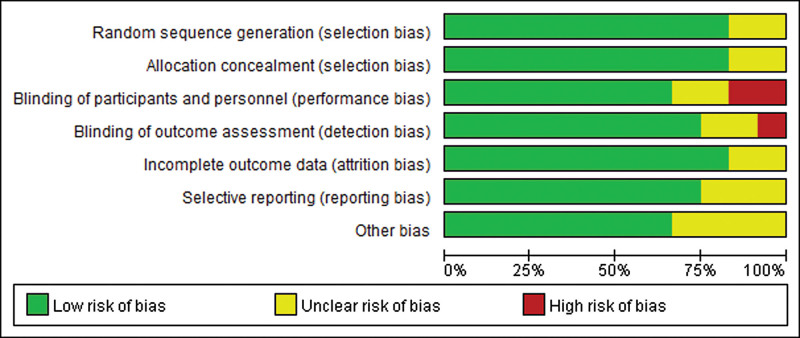
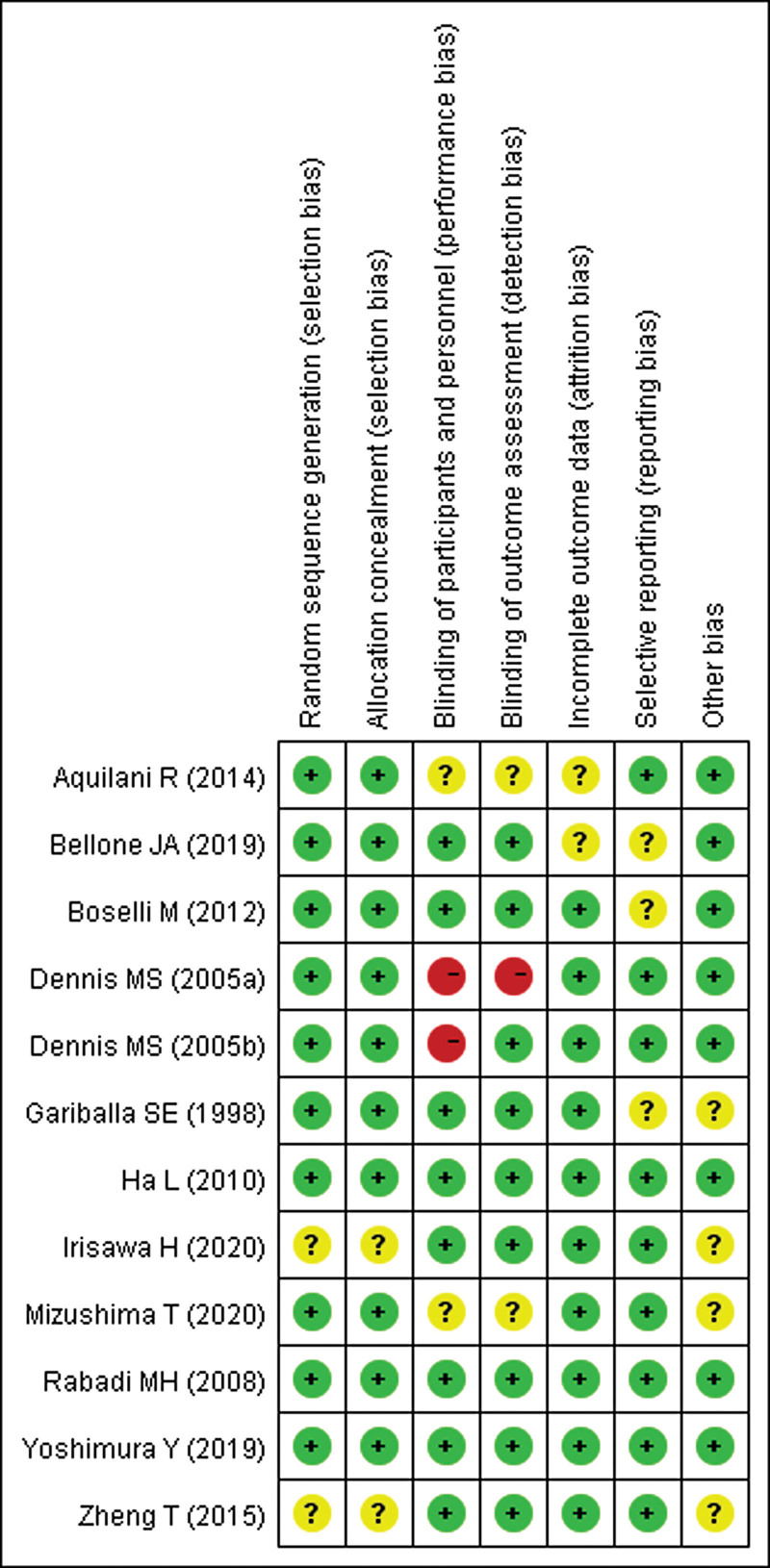
According to our definitions, there was no low quality studies included in this analysis. Except 4 studies[22,29,30,37] evaluated as moderate quality, the other studies were rated as high quality (75%). Additionally, risk-of-bias graphs were generated to further identify the risk of bias of the including studies. The risk of bias for each RCT was presented as percentages across all included studies, and the risk-of-bias item for each included study was displayed (Figs. 2 and 3). The risk-of-bias graphs indicated generally low risk of selection, performance, attrition, detection, reporting and other bias. High risk of bias was mainly observed in performance and detection bias in 2 studies.[25,26] Unclear risk of bias was dispersedly observed in each items.
Figure 2.
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Figure 3.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.2. The effect of nutrition supplement on FIM score
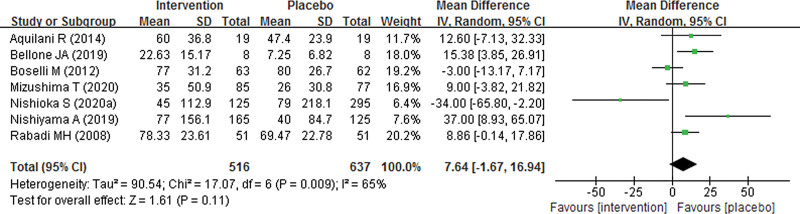
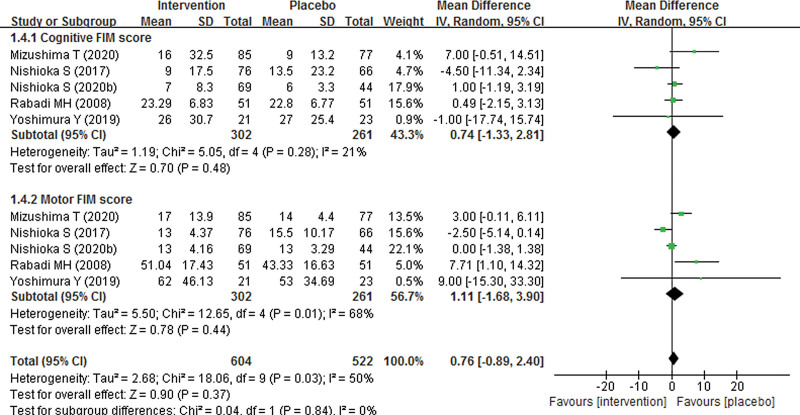
Seven studies with 1153 participants were included to assess the effect of nutrition supplement on FIM score. As shown in Figure 4, our pooled result found no significant difference in total FIM score between nutrition supplement and placebo groups, with a pooled MD of 7.64 (95% CI − 1.67 to 16.94; P = .11). In addition, we also explored subgroup FIM scores including cognitive and motor FIM score between nutrition supplement and placebo groups. However, the pooled results also showed no significant difference in cognitive and motor FIM score, with pooled MDs of 0.74 (95% CI − 1.33 to 2.81; P = .48) and 1.11 (95% CI − 1.68 to 3.90; P = .44), respectively (Fig. 5). As significant heterogeneity was found, the pooled results were analyzed using random effect models.
Figure 4.
Forest plot of total FIM score of nutrition supplement for patients with stroke.
Figure 5.
Forest plot of subgroup FIM scores of nutrition supplement for patients with stroke.
3.3. The effect of nutrition supplement on disabilities
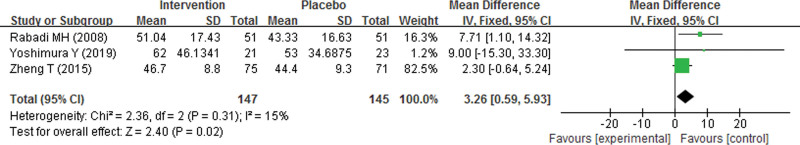
Three studies explored the effect of nutrition supplement on ADL of patients with stroke. As Figure 6 shows, the pooled result indicated that nutritional interventions had significant effect on ADL for patients with stroke (MD 3.26; 95% CI 0.59 to 5.93; P = .02). As no significant heterogeneity was found (P = .31, I2 = 15%), the pooled result was analyzed using random effect model.
Figure 6.
Forest plot of ADL of nutrition supplement for patients with stroke.
In addition, we also explored the effect of nutrition supplement on other disabilities. However, the pooled results indicated no significant difference between nutrition supplement and placebo groups in the incidence of disability (RR 1.08; 95% CI 0.99 to 1.19; P = .09), dysphagia (RR 1.59; 95% CI 0.88 to 2.87; P = .12), aphasia (RR 1.16; 95% CI 0.92 to 1.45; P = .21), neglect (RR 0.68; 95% CI 0.30 to 1.55; P = .36), and dysarthria (MD 0.88; 95% CI 0.67 to 1.14; P = .33) respectively (Table 2).
Table 2.
The pooled results of the effect of nutrition supplement on disabilities.
| Disabilities | No. of study | Sample size | Pooled results | Analytic effect model | ||
|---|---|---|---|---|---|---|
| RR | 95% CI | P value | ||||
| Disability | 4 | 5624 | 1.08 | 0.99, 1.19 | 0.09 | Fixed effect model |
| Dysphagia | 2 | 582 | 1.59 | 0.88, 2.87 | 0.12 | Random effect model |
| Aphasia | 2 | 582 | 1.16 | 0.92, 1.45 | 0.21 | Fixed effect model |
| Neglect | 2 | 162 | 0.68 | 0.30, 1.55 | 0.36 | Fixed effect model |
| Dysarthria | 2 | 170 | 0.88 | 0.67, 1.14 | 0.33 | Fixed effect model |
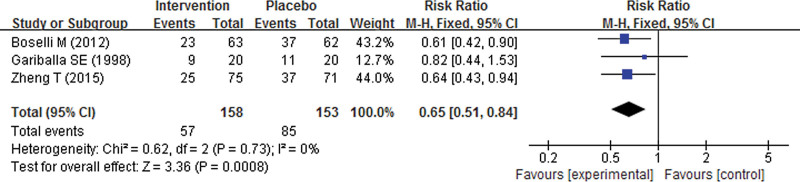
3.4. The effect of nutrition supplement on complications
Our pooled result indicated that patients with stroke receiving nutrition supplement during their rehabilitation experienced significantly lower incidence of infections, with a pooled RR of 0.65 (95% CI 0.51 to 0.84; P = .0008) (Fig. 7). However, no significant difference was found in other complications such as mortality (RR 0.90; 95% CI 0.80 to 1.00; P = .06), pneumonia (RR 0.97; 95% CI 0.84 to 1.13; P = .71) and stroke recurrence (RR 0.94; 95% CI 0.74 to 1.21; P = .64) respectively (Table 3).
Figure 7.
Forest plot of the incidence of infections for patients with stroke.
Table 3.
The pooled results of the effect of nutrition supplement on complications.
| Complications | No. of study | Sample size | Pooled results | Analytic effect model | ||
|---|---|---|---|---|---|---|
| RR | 95% CI | P value | ||||
| Mortality | 6 | 5346 | 0.90 | 0.80, 1.00 | 0.06 | Fixed effect model |
| Pneumonia | 5 | 5285 | 0.97 | 0.84, 1.13 | 0.71 | Fixed effect model |
| Stroke recurrence | 2 | 5292 | 0.94 | 0.74, 1.21 | 0.64 | Fixed effect model |
3.5. The effect of nutrition supplement on laboratory outcomes
We evaluated the effect of nutrition supplement on laboratory outcomes. However, our pooled results indicated no significant difference between nutrition supplement and placebo groups in hemoglobin (MD 0.50 g/dL; 95% CI –0.23 to 1.23; P = .18), Creatinine (MD –0.04 mg/dL; 95% CI –0.11 to 0.04; P = .35), BUN (MD 0.96; 95% CI –1.99 to 3.90; P = .52) and albumin (MD –0.04; 95% CI –0.48 to 0.39; P = .85), respectively (Table 4).
Table 4.
The pooled results of the effect of nutrition supplement on laboratory outcomes.
| Complications | No. of study | Sample size | Pooled results | Analytic effect model | ||
|---|---|---|---|---|---|---|
| MD | 95% CI | P value | ||||
| Hemoglobin (g/dL) | 4 | 453 | 0.50 | –0.23, 1.23 | 0.18 | Random effect model |
| Creatinine (mg/dL) | 3 | 325 | –0.04 | –0.11, 0.04 | 0.35 | Fixed effect model |
| BUN (mg/dL) | 2 | 325 | 0.96 | –1.99, 3.90 | 0.52 | Fixed effect model |
| Albumin (g/dL) | 8 | 806 | –0.04 | –0.48, 0.39 | 0.85 | Random effect model |
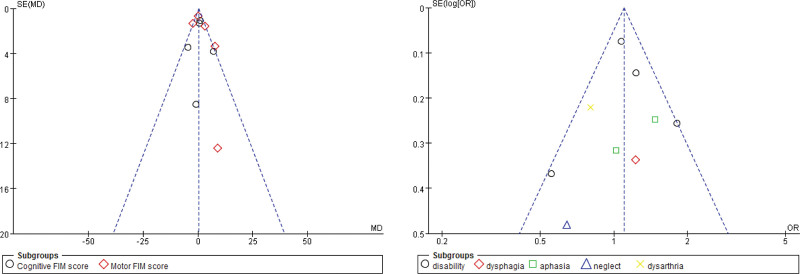
3.6. Publication bias
Begg funnel plots were generated to assess publication bias in the included studies. As Figure 8 shows, no obvious asymmetry which indicated no clear evidence of publication bias of comparison between nutrition supplement and placebo for patients with stroke regarding to FIM score and disability.
Figure 8.
Funnel plot for publication bias of comparison between nutrition supplement and placebo for patients with stroke regarding to FIM score and disability.
4. Discussion and Conclusion
4.1. Malnutrition and stroke
Malnutrition is a relatively common and often unrecognized condition in stroke survivors, which may negatively affect functional recovery and survival. Mizushima T (2020) reported the prevalence of moderate and severe malnutrition was 12.7% and 11.5%, respectively.[30] The author also indicated severe malnutrition did not provide additional prognostic information concerning risk of the combined outcome or 2-year mortality. Conversely, severe malnutrition was associated with poorer functional outcome as expressed by FIM-motor effectiveness.[30]
The association between nutrition status and rehabilitation outcomes has not been well studied. From 2000 to 2009, a focus on connections between nutrition and the brain developed with interventions planned to target some brain metabolic alterations primed by acute brain ischemia, such as impaired protein synthesis, excess free radical production, and deficiencies of minerals, including zinc.[8,38] Investigations have documented that recovery of neurocognitive function in individuals with ischemic stroke may indeed be enhanced by nutrition interventions. This is particularly important in light of the following considerations[6]: (1) no drug acting on damaged brain structures is available in clinical practice for the rehabilitation of patients with ischemic stroke; (2) 80% of the recovery of neurological impairment occurs within the first 30 days after acute ischemia,[10,39] which suggests that every effort should be made to obtain the best functional outcomes during this period of rehabilitation; (3) a large proportion of patients with stroke may have a catabolic state[40]; (4) 30 to 35 days after a stroke, patients tend to have the same nutrition deficits as at the time of their admission to a rehabilitation center[40]; (5) important calorie-protein deficits have been found 6 months after an acute stroke[9]; (6) patients with ischemic stroke have reduced plasma levels of tyrosine, the amino acid precursor of brain adrenergic neurotransmitters (epinephrine, norepinephrine, dopamine).[41]
4.2. The effect of nutrition supplement on rehabilitation
The present analysis systematically review and assessed the effect of nutrition supplement on rehabilitation for patients with stroke, 16 studies including 7547 patients. Our results supported the points that nutrition supplement could reduce the incidence of infections and could increase ADL of patients with stroke. However, we failed to observed significant effect on function and disability recovery as well as other complications, stroke recurrence, and laboratory results. Nishiyama A (2019)[34] found that FIM score was significantly higher in the high energy intake group compared with the low group (median 113 vs 71, P < .001). FIM efficiency was also higher in the high group (median 0.31 vs 0.22, P < .001) and thought that high energy intake was associated with higher FIM efficiency.[34] Yoshimura Y (2019) The FIM-M score increased significantly in leucine-enriched amino acid supplement group compared with the control group (P < .045).[36]
In addition, FOOD Trial Collaboration reliably assessed the importance of baseline nutritional status as an independent. The trial included a total of 3012 patients, and 2955 (98%) were followed up. Of the 275 undernourished patients, 102 (37%) were dead by final follow-up compared with only 445 (20%) of 2194 patients of normal nutritional status (OR 2.32; 95% CI 1.78–3.02). After adjustment for age, prestrike functional state, and stroke severity, this relationship, although weakened, still held (OR 1.82; 95% CI 1.34–2.47). In addition, it was indicated that undernourished patients were more likely to develop pneumonia, other infections, and gastrointestinal bleeding during their hospital admission than other patients. Some of the results were also demonstrated in our pooled results. For example, we found that nutrition supplement could decrease the incidence of infections.
4.3. The limitations of this work
This work has several limitations, which need to be taken into account when designing future studies. First, the follow-up time of the studies included in our analysis was much too various, which may lead to any risk bias of our results. However, because of significant heterogeneity in follow-up time of these studies, we failed to conducted subgroup analysis according to follow-up time. Second, the nutrition supplement composition was various. Reports of nutrition supplement for patients with stroke including the antioxidant vitamins E and C, energy, protein and zinc intake. Pellicane AJ (2013) indicated that number of complications, length of stay, and functional outcomes in this patient were not affected by prealbumin levels, protein intake, or calorie intake.[15] However, Boselli M (2012) demonstrated that supplementation with oral essential amino acids (EAAs) may reduce the occurrence of nosocomial infection among patients with brain injury (stroke, trauma, anoxic coma),[24] which was supported by our results. Third, the majority of our included studies included both ischemic and hemorrhagic stroke patients and did not perform subgroup analysis. The authors explained that they included both ischemic and hemorrhagic individuals because, in the rehabilitative phase of stroke, these 2 groups have similar metabolic, nutritional, functional profile. Thus, we failed to conducted subgroup analysis according to stroke subtypes. Fourth, we failed to perform subgroup analysis according to the neurological deficits of the patients as the original studies did not evaluate the outcomes separately according to the neurological deficits of the patients. This was one of our limitations and we suggested future researches could perform this subgroup analysis as more research comes out. Finally, in the present analysis, we did not define the age of trial participants. However, we selected trials in which the participants’ mean age was over 65 years because all the trials had no restriction on age. Considering the majority of participants were old patients, the effect of nutrition supplement may be impaired by their poor intestinal absorptive or metabolic capability. This may be another potential bias of our work.
5. Conclusions
The present meta-analysis indicated no statistically significant effect of nutrition supplement on functional outcomes as well as disabilities, complication and laboratory outcomes for patients with stroke. However, it increased ADL and reduced the incidence of infections.
Author contributions
JH Liu and JG Dong searched the studies for including and extracted the study data. JZ Guo wrote the initial manuscript. All authors assessed the quality of included studies and revised the manuscript.
Abbreviations:
- ADL =
- activities of daily living
- FIM =
- functional independence measurement
- LOS =
- length of stay
- MD =
- mean difference
- QoL =
- quality of life
- RR =
- risk ratio
How to cite this article: Liu J, Dong J, Guo J. The effects of nutrition supplement on rehabilitation for patients with stroke: analysis based on 16 randomized controlled trials. Medicine 2022;101:37(e29651).
Ethical approval: Not applicable.
The authors have no funding or conflict of interest to disclose.
All data generated or analyzed during this study are included in this published article.
Contributor Information
Jianhua Liu, Email: xlzhu1026@163.com.
Jige Dong, Email: yqdiao1026@163.com.
References
- [1].Scherbakov N, Doehner W. Sarcopenia in stroke-facts and numbers on muscle loss accounting for disability after stroke. J Cachexia Sarcopenia Muscle. 2011;2:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heuschmann PU, Di Carlo A, Bejot Y, et al. ; European Registers of Stroke (EROS) Investigators. Incidence of stroke in Europe at the beginning of the 21st century. Stroke. 2009;40:1557–63. [DOI] [PubMed] [Google Scholar]
- [3].Abbott RD, Donahue RP, MacMahon SW, et al. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA. 1987;257:949–52. [PubMed] [Google Scholar]
- [4].Mathers CD, Vos ET, Stevenson CE, et al. The Australian burden of disease study: measuring the loss of health from diseases, injuries and risk factors. Med J Aust. 2000;172:592–6. [DOI] [PubMed] [Google Scholar]
- [5].Scholte op Reimer WJ, de Haan RJ, Rijnders PT, et al. The burden of caregiving in partners of long-term stroke survivors. Stroke. 1998;29:1605–11. [DOI] [PubMed] [Google Scholar]
- [6].Aquilani R, Sessarego P, Iadarola P, et al. Nutrition for brain recovery after ischemic stroke: an added value to rehabilitation. Nutr Clin Pract. 2011;26:339–45. [DOI] [PubMed] [Google Scholar]
- [7].Poungvarin N. Stroke in the developing world. Lancet. 1998;352(suppl III):sIII19–21. [DOI] [PubMed] [Google Scholar]
- [8].Unosson M, Ek AC, Bjurulf P, et al. Feeding dependence and nutritional status after acute stroke. Stroke. 1994;25:366–71. [DOI] [PubMed] [Google Scholar]
- [9].Gariballa SE, Parker SG, Taub N, et al. Influence of nutritional status on clinical outcome after acute stroke. Am J Clin Nutr. 1998;68:275–81. [DOI] [PubMed] [Google Scholar]
- [10].Perry L. Eating and dietary intake in communication-impaired stroke survivors: a cohort study from acute-stage hospital admission to 6 months post-stroke. Clin Nutr. 2004;23:1333–43. [DOI] [PubMed] [Google Scholar]
- [11].Finestone HM, Greene-Finestone LS, Wilson ES, et al. Malnutrition in stroke patients on the rehabilitation service and at follow-up: prevalence and predictors. Arch Phys Med Rehabil. 1995;76:310–6. [DOI] [PubMed] [Google Scholar]
- [12].Chen HJ, Chen JL, Chen CY, et al. Effect of an oral health programme on oral health, oral intake, and nutrition in patients with stroke and dysphagia in Taiwan: a randomised controlled trial. Int J Environ Res Public Health. 2019;16:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lieber AC, Hong E, Putrino D. Nutrition, energy expenditure, dysphagia, and self-efficacy in stroke rehabilitation: a review of the literature. 2018;8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nip WF, Perry L, McLaren S, et al. Dietary intake, nutritional status and rehabilitation outcomes of stroke patients in hospital. J Hum Nutr Diet. 2011;24:460–9. [DOI] [PubMed] [Google Scholar]
- [15].Trial Collaboration FOOD. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. 2003;34:1450e–6. [DOI] [PubMed] [Google Scholar]
- [16].Clark HD, Wells GA, Huët C, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20:448–52. [DOI] [PubMed] [Google Scholar]
- [17].Review Manager (RevMan) [Computer Program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012. [Google Scholar]
- [18].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [19].University of York Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: CRD, University of York, 2009. [Google Scholar]
- [20].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- [22].Aquilani R, Boselli M, D’Antona G, et al. Unaffected arm muscle hypercatabolism in dysphagic subacute stroke patients: the effects of essential amino acid supplementation. Biomed Res Int. 2014;2014:964365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bellone JA, Murray JR, Jorge P, et al. Pomegranate supplementation improves cognitive and functional recovery following ischemic stroke: a randomized trial. Nutr Neurosci. 2019;22:738–43. [DOI] [PubMed] [Google Scholar]
- [24].Boselli M, Aquilani R, Baiardi P, et al. Supplementation of essential amino acids may reduce the occurrence of infections in rehabilitation patients with brain injury. Nutr Clin Pract. 2012;27:99–113. [DOI] [PubMed] [Google Scholar]
- [25].Dennis MS, Lewis SC, Warlow C. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:755–63. [DOI] [PubMed] [Google Scholar]
- [26].Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:764–72. [DOI] [PubMed] [Google Scholar]
- [27].Gariballa SE, Parker SG, Taub N, et al. A randomized, controlled, a single-blind trial of nutritional supplementation after acute stroke. JPEN J Parenter Enteral Nutr. 1998;22:315–9. [DOI] [PubMed] [Google Scholar]
- [28].Ha L, Hauge T, Spenning AB, et al. Individual, nutritional support prevents undernutrition, increases muscle strength and improves QoL among elderly at nutritional risk hospitalized for acute stroke: a randomized, controlled trial. Clin Nutr. 2010;29:567–73. [DOI] [PubMed] [Google Scholar]
- [29].Irisawa H. Correlation of body composition and nutritional status with functional recovery in stroke rehabilitation patients. Nutrients. 2020;12:1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mizushima T, et al. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. Nutrients. 2020;101:852–60. [DOI] [PubMed] [Google Scholar]
- [31].Nishioka S, Okamoto T, Takayama M, et al. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: secondary analysis of a multicentre survey (the APPLE study). Nutr Neurosci. 2017;36:1089–96. [DOI] [PubMed] [Google Scholar]
- [32].Nishioka S, Omagari K, Nishioka E, et al. Concurrent and predictive validity of the mini nutritional assessment short-form and the geriatric nutritional risk index in older stroke rehabilitation patients. J Hum Nutr Diet. 2020;33:12–22. [DOI] [PubMed] [Google Scholar]
- [33].Nishioka S, Yamasaki K, Ogawa K, et al. Impact of nutritional status, muscle mass and oral status on recovery of full oral intake among stroke patients receiving enteral nutrition: a retrospective cohort study. Nutr Diet. 2020;77:456–66. [DOI] [PubMed] [Google Scholar]
- [34].Nishiyama A, Wakabayashi H, Nishioka S, et al. Energy intake at admission for improving activities of daily living and nutritional status among convalescent stroke patients. Neurol Med Chir (Tokyo). 2019;59:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rabadi MH, Coar PL, Lukin M, et al. Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology. 2008;71:1856–61. [DOI] [PubMed] [Google Scholar]
- [36].Yoshimura Y, Bise T, Shimazu S, et al. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: a randomized controlled trial. Brain Sci. 2019;58:1–6. [DOI] [PubMed] [Google Scholar]
- [37].Zheng T, Zhu X, Liang H, et al. Impact of early enteral nutrition on short term prognosis after acute stroke. J Clin Neurosci. 2015;22:1473–6. [DOI] [PubMed] [Google Scholar]
- [38].Aquilani R, Galli M, Guarnaschelli C, et al. Prevalence of malnutrition and inadequate food intake in self-feeding rehabilitation patients with stroke. Eur Medicophys. 1999;35:75–81. [Google Scholar]
- [39].Lee JM, Zipfel GJ, Park KH, et al. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–8. [DOI] [PubMed] [Google Scholar]
- [40].Aquilani R, Scocchi M, Iadarola P, et al. Protein supplementation may enhance the spontaneous recovery of neurological alterations in patients with ischaemic stroke. Clin Rehabil. 2008;22:1042–50. [DOI] [PubMed] [Google Scholar]
- [41].Aquilani R, Verri M, Iadarola P, et al. Plasma precursors of brain catecholaminergic and serotonergic neurotransmitters in rehabilitation patients with ischemic stroke. Arch Phys Med Rehabil. 2004;85:779–84. [DOI] [PubMed] [Google Scholar]